Abstract
Background:
Along with the rising incidence of obesity, there has been an increase in patients diagnosed with early-onset colorectal cancer (EOCRC)(<50 yo). In CRC, worse patient survival is associated with certain cytokine expression and downregulation of peroxisome proliferator activated receptor gamma (PPARγ) expression. The effects of the obesity-hormone leptin and macrophage-specific metabolite itaconate on these mechanisms are poorly understood. We investigated their impact on PPARγ and macrophage cytokine expression in vitro.
Methods:
M2-like macrophages were treated with either leptin, 4-octyl itaconate (OI) or dimethyl itaconate (DI) in a dose and time dependent manner. Gene expression after treatment with four doses(D1-4) of each compound was analyzed at four time points (3, 6, 18 and 24h).
Results:
PPARγ was downregulated following OI treatment at 18h (FC −32.67,p=<0.001). Interleukin-8 was upregulated after leptin and DI treatment at 6h (FC 26.35 at D4,p=<0.001 and FC 23.26 at D3,p=0.006). DI upregulated IL-1β at 24h (FC 18.00 at D4,p=<0.001). Tumor Necrosis Factor-α showed maximum downregulation after OI at 18h (FC −103.25 at D4,p=<0.001).
Conclusions:
Itaconate downregulates PPARγ as a tumor suppressing factor and upregulates anti-inflammatory cytokines in M2-like macrophages. Itaconate provides a link between obesity and CRC and may be a key regulator in EOCRC.
Article summary
The aim of this study was to investigate the effect of the obesity hormone leptin, and the membrane-permeable itaconate derivatives 4-octyl itaconate (4-OI) and dimethyl itaconate (DI) on M2-like macrophages, their cytokine expression profiles and on PPARγ expression in vitro. Itaconate affects PPARγ gene expression in M2-like macrophages and can thereby promote carcinogenic mechanisms in colorectal cancer.
Introduction
The rising incidence of obesity and metabolic dysfunction is accompanied by an increasing number of patients that are diagnosed with early-onset colorectal cancer (EOCRC) (1-4). EOCRC is generally defined as colorectal cancer (CRC) occurring in individuals <50 years of age. In patients younger than 50 years of age, incidence rates in the United States increased by 22% from 2000 to 2013 and mortality increased by 13% (5). This development recently led to a significant change in recommendations for CRC screening by the US Preventive Services Task Force (USPSTF), that reduced the recommended starting age for average-risk CRC screening from 50 to 45 years (6). While inherited conditions including Lynch syndrome or familial adenomatous polyposis can cause an early cancer onset, more than 80% of patients with EOCRC are diagnosed with sporadic cancers (7). The Nurses’ Health Study II also showed an association between both body mass index (BMI) and weight gain since 18 years of age and an increased risk for EOCRC (2). Furthermore, CRC is the only gastrointestinal cancer associated with an increase in the number of surgical procedures among patients that are young and also obese (8). In addition, recent studies have shown a link between EOCRC and prenatal stress, which is a risk factor for obesity in the offspring, mediated by inflammatory immune responses and metabolic dysfunction (1).
A systemic proinflammatory state and local immune mechanisms on a tissue level may link obesity to early cancer onset and tumor progression in CRC (9). Current research focuses on the tumor microenvironment (TME) surrounding the tumor which consists of cancer cells, stromal cells, immune cells and extracellular matrix (10). Tumor-associated macrophages (TAM) are key regulators of the dynamic pro- and anti-inflammatory signaling within the TME that play a central role in CRC progression and patient survival (11, 12). TAM are highly plastic cells that can switch dynamically between a predominantly proinflammatory M1-like subtype and a more anti-inflammatory M2-like state (13). Since macrophage polarization states are driven by macrophage metabolism, M1-like macrophages prefer aerobic glycolysis as their main source of cellular energy, while M2-like macrophages mostly rely on oxidative phosphorylation (14). Advanced tumor stage, decreased overall and progression-free survival are associated with TAM with a high M2/M1 ratio, with expression of certain pro-and anti-inflammatory cytokines, such as IL-1β, IL-8 or IL-10, and with downregulation of peroxisome proliferator activated receptor gamma (PPARγ) gene expression (15-18). Cancer cells and TAM both contribute to these expression patterns.
The metabolic states of cells within the TME can be affected by paracrine signaling and the accumulation of systemic mediators. Obesity-related hormones are secreted by adipocytes and have a direct impact on cytokine expression in CRC cells as well as on macrophage polarization. Leptin acts as a central mediator of inflammation in CRC, inducing proinflammatory cytokine production in macrophages (19). With respect to obesity and CRC, leptin has been the subject of metabolic research for decades, but there is as of yet no defined association between leptin and obesity-specific tumor-promoting effects (20).
Recent studies have focused on the macrophage-specific metabolite itaconate as a key mediator of metabolic reprogramming and inflammation in cancer (9, 21). Itaconate is produced within the Krebs cycle and has been demonstrated to have carcinogenic effects in several types of cancers including ovarian carcinoma and glioma (21-23). M1-like macrophages supposedly produce this anti-inflammatory metabolite to prevent excessive inflammatory stress responses, but M2-like macrophages can also produce itaconate under certain conditions (21). Itaconate increases reactive oxygen species (ROS) and affects gene transcription through several pathways that are also regulated by leptin. Our own investigations in human colon cancer samples have shown that the macrophage-specific metabolite itaconate plays a role in CRC (unpublished observations). As a regulator of inflammation and by its presence in cancer, itaconate may provide a link between obesity, chronic inflammation and EOCRC.
The aim of this study was to investigate the effect of the obesity hormone leptin, and the membrane-permeable itaconate derivatives 4-octyl itaconate (4-OI) and dimethyl itaconate (DI) on M2-like macrophages, their cytokine expression profiles and on PPARγ expression in vitro.
Background from the bench – leptin, itaconate and a new potential link in early-onset colon cancer
The obesity-related hormone leptin circulates systemically as a 146-amino acid glycoprotein that is primarily produced and released by adipocytes (24). Its role in central appetite and energy regulation is only one facet of this hormone’s functions and does not describe its complexity due to the numerous effects of leptin in obesity-related complications, such as type 2 diabetes, cardiovascular disease and hypertension (20). In CRC, leptin has a metabolic impact, affecting cytokine expression in CRC, as well as macrophage polarization (25, 26). Leptin acts as a central mediator of inflammation in colorectal cancer inducing proinflammatory cytokine production of Tumor Necrosis Factor Alpha (TNFα) and/or Interleukin 6 (IL-6) in macrophages and lymphocytes (27). In mouse models, leptin treatment results in reduced colon cancer growth, an increased proportion of proinflammatory M1-like macrophages in colon cancer and increased proinflammatory cytokine production (26, 28). An indirect relationship between obesity and CRC has been reported due to the association of the function of leptin with several known risk factors of CRC, such as energy intake, sex hormone levels, stress, and inflammatory immune responses (20). A causal link between obesity and CRC through leptin, however, has never been demonstrated (20).
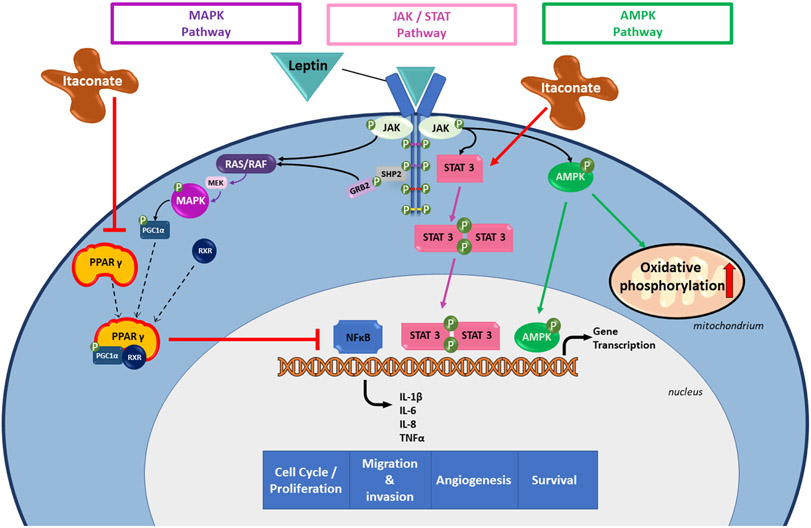
Acting through the JAK2-STAT3 pathway, a key pathway in tumorigenesis and metastasis, leptin increases cell survival and cell growth in colon cancer cells, therefore promoting colon cancer progression (18, 25, 27) (Figure 1). The MAPK and the AMPK pathways are also regulated by leptin, both altering gene transcription and inflammatory responses of cells. All three of these pathways affect Nuclear Factor-kappa B (NFκB) activity. An excessive activation of NFκB plays a key role in colorectal carcinogenesis (29).
Fig. 1. Effects of leptin and itaconate on cellular pathways altering gene transcription, including the MAPK, the JAK/STAT and the AMPK pathway.
The three molecular pathways MAPK (purple), JAK/STAT (pink) and AMPK (green) and their regulation through leptin and its receptor. Molecules in the cytoplasm (light blue) and the nucleus (grey) are shown.
All three of these pathways alter gene transcription affecting inflammatory responses induced by the NFκB system. MAPK and STAT3 initiate induction of NFκB mediated gene expression, enhancing pro-proliferative and anti-apoptotic mechanisms. On the other hand, AMPK signaling can indirectly inhibit NFκB activity by induction of several downstream factors, such as p53. Furthermore, AMPK plays a key role in macrophage polarization by regulating mitochondrial oxidative phosphorylation and glycolysis. Itaconate can enhance mechanisms mediated by leptin through activation of the JAK/STAT pathway and downregulation of PPARγ (red arrows).
MAPK = mitogen-activated protein kinase; JAK/STAT = Janus kinase-signal transducer and activator of transcription; AMPK = AMP-activated protein kinase; NFκB = Nuclear factor kappa light chain enhancer of activated B cells; p53 = tumor protein p53; PPARγ = peroxisome proliferator activated receptor gamma; SHP2 = SH2 containing protein tyrosine phosphatase-2; GRB2 = Growth Factor Receptor Bound Protein 2; RAS/RAF = Rapidly Accelerated Fibrosarcoma-Rat sarcoma protein; MEK = MAPK/extracellular signal–regulated kinase kinase; PGC1α = peroxisome proliferator-activated receptor gamma coactivator 1-alpha; RXR = retinoid X receptor; IL = interleukin; TNFα = Tumor Necrosis Factor Alpha
NFκB is a ubiquitous transcription factor that regulates cytokine, cytokine receptor and adhesion molecule expression in an inflammatory setting, affecting both cancer cells and tumor-associated macrophages (TAM) (29). The anti-apoptotic effect of leptin on cancer cells is also mediated through NFκB, inducing proliferation, differentiation, metastasis, angiogenesis and chemoradiotherapy resistance in cancer cells (30). PPARγ is an established target in metabolic dysfunction and insulin resistance, also known as the glitazone receptor (31). This protein is a potent inhibitor of NFκB. Low PPARγ expression in CRC is associated with worse clinical outcomes (16, 17). Leptin slightly downregulates PPARγ expression in human macrophages thereby contributing to this expression pattern (32).
As immune responses and inflammation play an increasingly important role in CRC research, macrophages and their metabolic states are coming into focus of carcinogenic mechanisms and clinical outcomes. The macrophage-specific metabolite itaconate is a dicarboxylic acid derived from cis-aconitate in the Krebs cycle in macrophages (21). Macrophage activation with lipopolysaccharide (LPS) or other cytokines, such as interferons, can induce itaconate production by the enzyme aconitate decarboxylase 1 (ACOD1) in the mitochondrial matrix (21). Itaconate is highly polar and is therefore not able to cross cell membranes easily (33). Due to this limitation, membrane-permeable itaconate derivatives, such as 4-OI and DI, are commonly used for in vitro experiments (34, 35). In addition to the effects of leptin, itaconate can enhance NF-κB activity by inducing succinate accumulation and thereby increasing mitochondrial production of ROS (22, 36). The association between itaconate production and PPARγ expression in macrophages and CRC cells are widely unknown.
Methods
Cell culture and treatment of M2-like macrophages
THP-1 monocyte-like cells were differentiated and polarized into a distinct M2-like macrophage subtype as previously described (37). Phenotypical characterization of cells derived from this model has confirmed an M2-like macrophage profile and cell characteristics mimicking human primary cells (manuscript under review). Dose-response experiments were performed for each treatment by incubating cells with either leptin (n=20), 4-OI (n=20) or DI (n=20) (Sigma-Aldrich, St. Louis, USA). Cellular cytokine expression after treatment with four different doses of each compound (n=5) was determined at four time points including 3, 6, 18 and 24 hours of cell treatment.
Gene expression analysis using quantitative real-time PCR
Total RNA was extracted from cells using the RNeasy purification kit (Qiagen, Maryland, USA). RNA was quantified with spectrophotometry (NanoDrop 1000, Thermo Scientific, Massachusetts, USA) and 20ng of total RNA was used to perform reverse transcription to cDNA according to the manufacturer’s protocol. TaqMan Gene Expression Assays (PPARγ: HS01115513_m1, TNFα: HS00174128_m1, IL-1β: HS01555410_m1, IL-6: HS00174131_m1, IL-8/CXCL8: HS00174103_m1, IL-10: HS00961622_m1, CCL18: HS00268113_m1, RNA18S5: Hs03928990_g1) (Applied Biosystems, California, USA) and Fast Advanced Master Mix (Applied Biosystems, California, USA) were used for quantitative real-time PCR (qRT-PCR) using StepOne Real-Time PCR systems (Applied Biosystems, California, USA). 18S was used as a housekeeping gene to normalize results.
Statistical analysis
The descriptive statistics for ΔCt values were compared among the treatment dose groups for each respective gene by treatment and time point. The mean ΔCt values with 95% confidence intervals were presented (38). Maximum fold changes (FC) over all doses at the various time points are reported.
A one-way Analysis of Variance (ANOVA) test was performed for each respective gene, treatment and time point. Following a contrast analysis, the p-values were reported for the comparisons between dose groups, with a treatment confirmed as significant in cases of at least 2 significant changes in gene expression at 2 consecutive doses and 2 different time points (39).
All calculations are performed using SAS System V9 statistical software (SAS Institute Inc., North Carolina, USA) (40).
Results
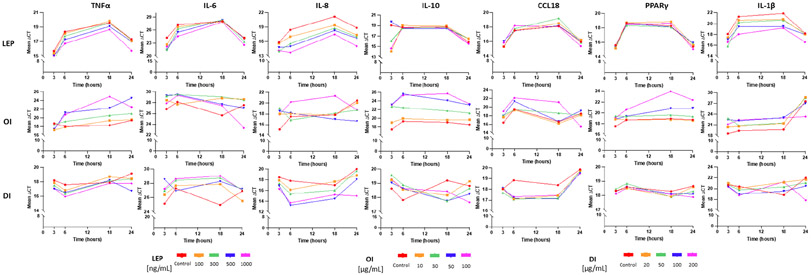
An overview of the raw gene expression data, demonstrated as normalized PCR cycles (ΔCT values), is shown in Table 1. Mean ΔCT values for all doses (D1, D2, D3, D4) of all treatments with leptin, 4-OI and DI and for each respective gene at all four time points are shown in Figure 2.
Tab. 1.
Maximum fold changes (FC) and associated mean ΔCT values for the respective treatment and gene
| Gene | Treatment | Time [hours] |
Control [mean ΔCT (95% CI)] |
D1 [mean ΔCT (95% CI)] |
D2 [mean ΔCT (95% CI)] |
D3 [mean ΔCT (95% CI)] |
D4 [mean ΔCT (95% CI)] |
max. FC (dose) |
p-value |
|---|---|---|---|---|---|---|---|---|---|
| PPARγ | leptin | 18 | 18.25 (17.85 - 18.64) | 18.84 (18.48 - 19.20) | 18.08 (17.40 - 18.77) | 18.11 (17.76 - 18.46) | 18.64 (18.28 - 19.00) | −1.51 (D1) | ns |
| PPARγ | 4-octyl itaconate | 18 | 18.92 (18.50 - 19.34) | 18.75 (18.40 - 19.11) | 19.60 (18.75 - 20.46) | 20.77 (19.80 - 21.74) | 23.95 (23.72 - 24.17) | −32.67 | <0.001 |
| PPARγ | dimethyl itaconate | 24 | 18.66 (18.28 - 19.04) | 18.60 (18.36 - 18.83) | 18.31 (18.02 - 18.60) | 18.11 (17.76 - 18.46) | 17.89 (17.49 - 18.29) | 1.71 (D4) | ns |
| IL-8 | leptin | 6 | 18.45 (18.31 - 18.58) | 17.00 (16.68 - 17.32) | 15.58 (14.31 - 16.84) | 14.97 (13.86 - 16.07) | 13.73 (12.62 - 14.84) | 26.35 (D4) | <0.001 |
| IL-8 | leptin | 6 | 18.45 (18.31 - 18.58) | 17.00 (16.68 - 17.32) | 15.58 (14.31 - 16.84) | 14.97 (13.86 - 16.07) | 13.73 (12.62 - 14.84) | 26.35 | <0.001 |
| IL-8 | dimethyl itaconate | 6 | 17.80 (16.66 - 18.95) | 16.13 (14.62 - 17.64) | 15.37 (13.73 - 17.01) | 13.26 (11.01 - 15.50) | 13.71 (12.44 - 14.97) | 23.26 | 0.006 |
| CCL18 | leptin | 18 | 18.15 (17.86 - 18.44) | 18.54 (17.60 - 19.48) | 19.22 (18.28 - 20.16) | 18.20 (17.74 - 18.65) | 18.07 (17.73 - 18.42) | −2.10 (D2) | ns |
| CCL18 | 4-octyl itaconate | 18 | 16.61 (10.33 - 22.88) | 16.19 (10.21 - 22.18) | 18.60 (10.88 - 26.33) | 16.67 (10.38 - 22.95) | 21.14 (14.25 - 28.04) | −21.10 (D4) | ns |
| CCL18 | dimethyl itaconate | 6 | 18.85 (18.04 - 19.66) | 17.12 (15.97 - 18.26) | 17.09 (15.81 - 18.37) | 17.19 (16.23 - 18.15) | 17.32 (16.48 - 18.17) | 3.39 (D2) | ns |
| IL-10 | leptin | 3 | 19.05 (18.16 - 19.94) | 14.09 (13.71 - 14.47) | 16.10 (13.86 - 18.33) | 19.71 (19.37 - 20.05) | 14.64 (12.28 - 17.01) | 31.12 (D1) | ns |
| IL-10 | 4-octyl itaconate | 18 | 19.06 (16.94 - 21.19) | 19.58 (17.92 - 21.24) | 21.72 (19.42 - 24.02) | 24.12 (22.58 - 25.65) | 25.72 (23.42 - 28.03) | −101.13 | <0.001 |
| IL-10 | dimethyl itaconate | 18 | 19.19 (17.18 - 21.19) | 18.16 (17.00 - 19.32) | 17.76 (16.29 - 19.24) | 17.78 (16.25 - 19.30) | 18.43 (17.62 - 19.23) | 2.69 | ns |
| IL-1β | leptin | 6 | 21.28 (20.74 - 21.82) | 20.61 (19.88 - 21.35) | 20.20 (19.34 - 21.05) | 19.41 (18.10 - 20.73) | 18.04 (16.74 - 19.33) | 9.45 (D4) | ns |
| IL-1β | 4-octyl itaconate | 3 | 18.58 (15.99 - 21.16) | 20.41 (20.17 - 20.65) | 22.80 (22.46 - 23.14) | 22.47 (22.07 - 22.88) | 21.18 (20.08 - 22.27) | −18.64 (D2) | ns |
| IL-1β | dimethyli taconate | 24 | 22.01 (21.75 - 22.27) | 21.70 (20.71 - 22.70) | 21.08 (20.85 - 21.30) | 20.48 (19.90 - 21.06) | 17.84 (17.46 - 18.21) | 18.00 (D4) | <0.001 |
| IL-6 | leptin | 3 | 24.23 (20.46 - 28.00) | 22.64 (19.11 - 26.17) | 21.51 (16.84 - 26.18) | 21.39 (18.55 - 24.24) | 22.19 (19.26 - 25.12) | 7.16 (D3) | ns |
| IL-6 | 4-octyl itaconate | 24 | 27.56 (26.59 - 28.53) | 28.65 (28.02 - 29.29) | 28.50 (27.60 - 29.40) | 27.01 (24.66 - 29.36) | 23.31 (18.97 - 27.66) | 19.03 (D4) | ns |
| IL-6 | dimethyl itaconate | 18 | 24.99 (20.99 - 29.00) | 27.86 (26.86 - 28.87) | 28.72 (27.93 - 29.51) | 28.27 (27.52 - 29.03) | 29.03 (28.25 - 29.80) | −16.45 (D4) | ns |
| TNFα | leptin | 6 | 18.38 (18.15 - 18.60) | 18.15 (17.99 - 18.30) | 17.77 (17.53 - 18.01) | 17.27 (16.73 - 17.80) | 16.72 (16.01 - 17.43) | 3.16 (D4) | ns |
| TNFα | 4-octyl itaconate | 18 | 18.25 (17.15 - 19.36) | 19.36 (18.80 - 19.93) | 20.56 (19.49 - 21.62) | 22.28 (21.17 - 23.40) | 24.94 (23.05 - 26.83) | −103.25 (D4) | <0.001 |
| TNFα | dimethyl itaconate | 24 | 19.17 (18.59 - 19.76) | 18.45 (17.48 - 19.43) | 18.40 (17.88 - 18.93) | 16.76 (16.49 - 17.03) | 17.78 (17.56 - 18.00) | 5.31 (D3) | ns |
Leptin treatment [ng/ml]:
D1: 100
D2: 300
D3: 500
D4: 1000
4-octyl itaconate [μg/ml]:
D1: 10
D2: 30
D3: 50
D4: 100
Dimethyl itaconate [μg/ml]
D1: 20
D2: 50
D3: 100
D4: 200
LEP = leptin; OI = 4-octyl itaconate; DI = dimethyl itaconate; IL = interleukin; PPARγ = peroxisome proliferator activated receptor gamma
ns = not significant
Fig. 2. Dose and time response after cell treatment with leptin, 4-octyl itaconate (OI) and dimethyl itaconate (DI).
Mean PCR gene expression values (ΔCT values) for each cell treatment and for each respective gene, including four different doses (see legend within figure) and four time points (3h, 6h, 18h, 24h).
LEP = leptin; OI = 4-octyl itaconate; DI = dimethyl itaconate; IL = interleukin; TNFα = Tumor Necrosis Factor Alpha; PPARγ = peroxisome proliferator activated receptor gamma
PPARγ gene expression was not altered in M2-like macrophages treated with either leptin or DI. In contrast, OI treatment resulted in a significant downregulation of PPARγ at the highest dose (D4) at 6h (FC −3.78, p=<0.001), 18h (FC −32.67, p=<0.001) and 24h (FC −12.55, p=<0.001).
Leptin and DI had no significant effect on anti-inflammatory IL-10 expression among doses and over time. OI treatment induced a clear downregulation of IL-10 using the two highest doses (D3 and D4) over all time points (at 3h [FC −54.57 at D3, p=<0.001], at 6h [FC −80.45 at D3, p=<0.001], at 18h [FC −101.13 at D4, p=<0.001] and at 24h [FC −28.84 at D4, p=<0.001]).
Treatment of cells with leptin consistently upregulated IL-8 expression among doses with a maximum fold change using the highest dose at 6h (FC 26.35 at D4, p=<0.001) and 18h (FC 12.82 at D4, p=0.006). DI showed this effect as an early response at 3h (FC 4.44 at D4, p=<0.001) and at 6h (FC 23.26 at D3, p=0.006). OI had no significant effect on IL-8 expression.
Investigating CCL18 expression, there was no significant effect pattern among doses and time points for leptin, DI or OI treatments.
Proinflammatory IL-1β and IL-6 expression also were not affected in a time- and dose-dependent manner by leptin or OI. DI showed a significant upregulation of IL-1β at 6h (FC 3.14 at D4, p=0.001) and at 24h (FC 18.00 at D4, p=<0.001) and no significant effect on IL-6.
A significant downregulation of proinflammatory TNFα gene expression was demonstrated following OI treatment for 6h (FC −9.06 at D3, p=<0.001), 18h (FC −103.25 at D4, p=<0.001) and for 24h (FC 36.00 at D3, p=<0.001).
Discussion
The expression of the metabolic nuclear transcription factor PPARγ in CRC has a key role in cellular lipid and glucose metabolism and mediates several antineoplastic mechanisms in CRC (16, 41). PPARγ is understood to function as a tumor suppressor, that can serve as a marker of an indolent subset of CRC (17). Itaconate is a metabolic product of the Krebs cycle in TAM and can alter macrophage cytokine expression as well as their polarization state (21). In metabolic dysfunction and obesity, itaconate can provide a link to early-onset CRC by enhancing carcinogenic mechanisms that are regulated by obesity-related hormones such as leptin. Identifying this link is the basis for developing new immunotherapeutic targets in CRC. In this study, we provide evidence that itaconate promotes significant downregulation of the tumor suppressor PPARγ in anti-inflammatory M2-like macrophages and upregulation of M2-like macrophage cytokines. In this manner, itaconate has the potential to mediate tumor-promoting mechanisms in EOCRC and obesity-affected pathways through PPARγ.
The itaconate derivatives that have been used in this study demonstrated disparate effects on gene expression in M2-like macrophages. While DI did not seem to alter PPARγ expression, OI resulted in significant downregulation. Furthermore, OI induced a clear downregulation of anti-inflammatory IL-10 expression as well as of proinflammatory TNFα expression. In contrast, DI had a clear impact on IL-8 expression, while OI showed no significant effects. The membrane-permeable derivatives of itaconate that are established in in vitro investigation, have been reported to not necessarily show similar effects (34, 42). OI and DI are the most commonly used forms of this macrophage-metabolite that are used to provide itaconate intracellularly (42). Intracellular modification mechanisms of these derivatives altering their function and potential transmembrane transport mechanisms of itaconate itself, however, are not well understood and are therefore important subjects of current research (34, 35).
Treatment of cells with either leptin, OI or DI led to significant effects on gene expression among doses over time. Variation of PCR cycle values between plate replicates was shown depending on the respective gene analyzed and upon the compound added for cell treatment. The variability of cellular responses of M2-like polarized macrophages in cell culture demonstrates the high plasticity of this cell type with dynamic and continuous switching between either more M1-like or more M2-like marker expression. Characterization of the distinct anti-inflammatory M2-like macrophage subtype that was used in this study, however, revealed a clear M2-like polarization state. Despite variability of PCR cycle values between cell plates, a clear pattern of cellular responses could be demonstrated for each respective treatment.
A limitation of this study is the focus on TAM and therefore on only one cell type within the TME. On the other hand, the investigation of individual responses of the macrophage population is important to define their individual contribution to gene expression patterns in CRC. Cell line co-culture models or human CRC organoid cultures are necessary to analyze intercellular mechanisms and paracrine signaling following treatment with leptin and itaconate. Furthermore, this study is limited to the gene expression levels of pathway-related cytokines and PPARγ. After investigating dose- and time-dependent effects in detail, protein expression and further macrophage subtype markers should be determined to identify protein activation and TAM polarization due to leptin and itaconate. In addition, the effects of leptin and itaconate on TAM should be analyzed using macrophages in different polarization states. Anti-inflammatory M2-like macrophages are the most common subtype of macrophage in more advanced tumor stages and have been shown to be associated with worse clinical outcomes in CRC, such as overall or progression free survival (15). This study limited to TAMs of this M2-like subtype, but the effects on macrophages of other metabolic polarization states, such as M0 or proinflammatory M1-like macrophages, should be evaluated in future experiments.
In conclusion, this study provides evidence that the macrophage-specific metabolite itaconate can exert cancer-promoting effects through cellular pathways that are regulated by the obesity-related hormone leptin. Itaconate affects PPARγ gene expression in M2-like macrophages and can thereby contribute to downregulation of PPARγ as a tumor suppressing factor in CRC. Furthermore, itaconate promotes anti-inflammatory cytokine expression in M2-like macrophages. These expression patterns, including downregulation of PPARγ and upregulation of anti-inflammatory cytokines representing M2-like polarization of macrophages, are associated with worse clinical outcomes in patients with CRC. Itaconate provides a link between metabolic dysfunction in obese patients and CRC and may thereby function as a key regulator in EOCRC. Further studies are necessary to demonstrate the pathway mechanisms of itaconate that directly mediate tumor-promoting effects, and the specific role of itaconate in EOCRC.
Funding
The Price Institute of Surgical Research at the University of Louisville is financially supported by the John W. Price and Barbara Thruston Atwood Price Trust. MNW and CRF were supported by the University of Louisville Cancer Education Program (National Institutes of Health, National Cancer Institute R25CA134283). The funding sources had no role in the design and conduct of the study as well as on the collection, management, analysis, and interpretation of the data.
Biography
EMRE GORGUN: Young onset colorectal cancer is indeed a rising healthcare issue and an increasing number of centers in the United States, including ours, are building multidisciplinary care groups in order to provide better care to this young group of patients. According to your results, you were able to find that itaconate provides a link between metabolic dysfunction in obese patients and early-onset colorectal cancer. My questions are following: Did itaconate only provide a link in obese patients, or can this also be found in sporadic non-obese colorectal patients as well? Second, is there a similar link also found in non-colorectal cancer patients? And third and lastly, how do you think this information in molecular data could be utilized in real clinical settings and daily practice.
KATHARINA SCHEURLEN: Whether itaconate can only be found in obese patients, and whether it is specific for colorectal cancer are very good questions. Itaconate may play a role in certain different types of cancers. It has been found in ovarian carcinoma macrophages, tumor-associated macrophages, and has been associated with cancer progression, so advanced stages showed higher level of itaconate. We actually measured itaconate in our own patients and compared cancer samples versus normal adjacent colon tissue, and we found that the expression of the enzyme producing itaconate is actually specific for the cancer tissue. Is it specific for colorectal cancer? Probably not. It might play a role in several types of cancers. As to the role it plays in cancers in obesity - the interesting thing that we found in this study was that it might have an effect on the pathways that are actually regulated by obesity-related hormones. There has been a discussion going on for years now where the leptin has a direct effect on colon cancer progression and what the effect of adiponectin is, which is kind of like the counterpart, with an antiinflammatory role as well. And if there is a mechanism that can actually enhance the effects of those obesity-related hormones, this would provide a direct link between obesity and at least colon cancer in this case. It might be specific for colon cancer when it comes to those hormones, but it might not be exclusive.
EMRE GORGUN: Right, the clinical use - since it’s not specific for a certain type of cancer, it might not have a big diagnostic value, but it potentially can provide a therapeutic target for a more targeted immunotherapy in obese patients with colon cancer, because the view of colon cancer as a genetic predisposition that causes it would switch towards a more metabolic entity of those cancers, at least in patients that are young, and this could provide a new point of view for actually treating those patients.
JOAL BEANE: Congratulations on a great talk. Is this pathway important in say, mismatch repair sufficient colon cancers, where they’re not as reliant on the host immune response for therapy? And then the second question: Kupffer cells in the liver, tons of macrophages in the liver. Has this pathway been looked at in a metastatic model?
KATHARINA SCHEURLEN: Thank you for those questions. That’s a very good question about the microsatellite instability, and whether that is the same mechanism. That’s something that’s worth having a look at. And your second question was whether that has been tested in a metastatic model… no, definitely not. So that would also be something that should be tested in the future. First, we have to have a look whether clinical parameters and outcomes in patients are actually related to itaconate levels.
SUSAN TSAI: You showed some nice data on how itaconate can change the macrophage cytokine expression. Have you looked at the tumor itself or the tumor marker environment, to see how that might affect that? Is it different in high-expressing itaconate versus low tumors, and then as you have mentioned before, the leptin has a tumor-specific effect. Do you see that with itaconate as well - independent of the immunologic effect?
KATHARINA SCHEURLEN: Yes, the answer to the first question… have we tested that with different itaconate levels? No, we haven’t. This might be something that’s worth having a look at in a co-culture model or with primary patient samples as well. And the second question was with the leptin can you use those pro-carcinogenic mechanisms independently from itaconate? Yes, it can. So that’s something that’s known, colon cancers have those leptin receptors, they express it on their surface, so… and there is a pro-carcinogenic effect, meaning cells proliferating faster if you incubate them with leptin.
SUSAN TSAI: My question was does itaconate have a tumor-specific effect in and of itself independent of the immunologic changes that you see? So if you just gave itaconate to tumor cells, is it tumor-genic, or do you see increased proliferation?
KATHARINA SCHEURLEN: In colon cancer, that hasn’t been shown yet. In other types of cancer, it actually has (applause).
Footnotes
Disclosures
The authors have no related conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hofseth LJ, Hebert JR, Chanda A, Chen H, Love BL, Pena MM, et al. Early-onset colorectal cancer: initial clues and current views. Nat Rev Gastroenterol Hepatol. 2020;17(6):352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu PH, Wu K, Ng K, Zauber AG, Nguyen LH, Song M, et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol. 2019;5(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Boakye D, Chen X, Hoffmeister M, Brenner H. Association of Body Mass Index With Risk of Early-Onset Colorectal Cancer: Systematic Review and Meta-Analysis. Am J Gastroenterol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93. [DOI] [PubMed] [Google Scholar]
- 6.Force USPST, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965–77. [DOI] [PubMed] [Google Scholar]
- 7.Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol. 2017;3(4):464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussan H, Patel A, Le Roux M, Cruz-Monserrate Z, Porter K, Clinton SK, et al. Rising Incidence of Colorectal Cancer in Young Adults Corresponds With Increasing Surgical Resections in Obese Patients. Clin Transl Gastroenterol. 2020;11(4):e00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheurlen KM, Billeter AT, O'Brien SJ, Galandiuk S. Metabolic dysfunction and early-onset colorectal cancer - how macrophages build the bridge. Cancer Med. 2020;9(18):6679–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YS, Song SJ, Hong HK, Oh BY, Lee WY, Cho YB. The FBW7-MCL-1 axis is key in M1 and M2 macrophage-related colon cancer cell progression: validating the immunotherapeutic value of targeting PI3Kgamma. Exp Mol Med. 2020;52(5):815–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaput N, Svrcek M, Auperin A, Locher C, Drusch F, Malka D, et al. Tumour-infiltrating CD68+ and CD57+ cells predict patient outcome in stage II-III colorectal cancer. Br J Cancer. 2013;109(4):1013–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu SX, Gustafson HH, Jackson DL, Pun SH, Trapnell C. Trajectory analysis quantifies transcriptional plasticity during macrophage polarization. Sci Rep. 2020;10(1):12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batista-Gonzalez A, Vidal R, Criollo A, Carreno LJ. New Insights on the Role of Lipid Metabolism in the Metabolic Reprogramming of Macrophages. Front Immunol. 2019;10:2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C, Wei C, Wang S, Shi D, Zhang C, Lin X, et al. Elevated CD163(+)/CD68(+) Ratio at Tumor Invasive Front is Closely Associated with Aggressive Phenotype and Poor Prognosis in Colorectal Cancer. Int J Biol Sci. 2019;15(5):984–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motawi TK, Shaker OG, Ismail MF, Sayed NH. Peroxisome Proliferator-Activated Receptor Gamma in Obesity and Colorectal Cancer: the Role of Epigenetics. Sci Rep. 2017;7(1):10714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogino S, Shima K, Baba Y, Nosho K, Irahara N, Kure S, et al. Colorectal cancer expression of peroxisome proliferator-activated receptor gamma (PPARG, PPARgamma) is associated with good prognosis. Gastroenterology. 2009;136(4):1242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riondino S, Roselli M, Palmirotta R, Della-Morte D, Ferroni P, Guadagni F. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J Gastroenterol. 2014;20(18):5177–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez-Riejos P, Najib S, Santos-Alvarez J, Martin-Romero C, Perez-Perez A, Gonzalez-Yanes C, et al. Role of leptin in the activation of immune cells. Mediators Inflamm. 2010;2010:568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slattery ML, Wolff RK. Leptin and colorectal cancer: an undefined link. Nat Clin Pract Gastroenterol Hepatol. 2007;4(3):118–9. [DOI] [PubMed] [Google Scholar]
- 21.O'Neill LAJ, Artyomov MN. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat Rev Immunol. 2019;19(5):273–81. [DOI] [PubMed] [Google Scholar]
- 22.Weiss JM, Davies LC, Karwan M, Ileva L, Ozaki MK, Cheng RY, et al. Itaconic acid mediates crosstalk between macrophage metabolism and peritoneal tumors. J Clin Invest. 2018;128(9):3794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan J, Zhao X, Lin C, Xu H, Yin Z, Liu T, et al. Immune responsive gene 1, a novel oncogene, increases the growth and tumorigenicity of glioma. Oncol Rep. 2014;32(5):1957–66. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord. 1998;22(12):1145–58. [DOI] [PubMed] [Google Scholar]
- 25.Jin W Role of JAK/STAT3 Signaling in the Regulation of Metastasis, the Transition of Cancer Stem Cells, and Chemoresistance of Cancer by Epithelial-Mesenchymal Transition. Cells. 2020;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudzinski SO, Beckermann KE, Young K, Hongo R, Giorgio T, Rathmell J. Macrophage repolarization via leptin increases immunotherapy efficacy in obesity. The Journal of Immunology. 2020;204(1 Supplement):240.12–.12. [Google Scholar]
- 27.Perez-Perez A, Sanchez-Jimenez F, Vilarino-Garcia T, Sanchez-Margalet V. Role of Leptin in Inflammation and Vice Versa. Int J Mol Sci. 2020,’21(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naylor C, Petri WA Jr. Leptin Regulation of Immune Responses. Trends Mol Med. 2016;22(2):88–98. [DOI] [PubMed] [Google Scholar]
- 29.Soleimani A, Rahmani F, Ferns GA, Ryzhikov M, Avan A, Hassanian SM. Role of the NF-kappaB signaling pathway in the pathogenesis of colorectal cancer. Gene. 2020,’726:144132. [DOI] [PubMed] [Google Scholar]
- 30.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107(3):241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogacka I, Xie H, Bray GA, Smith SR. The effect of pioglitazone on peroxisome proliferator-activated receptor-gamma target genes related to lipid storage in vivo. Diabetes Care. 2004;27(7):1660–7. [DOI] [PubMed] [Google Scholar]
- 32.Cabrero A, Cubero M, Llaverias G, Alegret M, Sanchez R, Laguna JC, et al. Leptin down-regulates peroxisome proliferator-activated receptor gamma (PPAR-gamma) mRNA levels in primary human monocyte-derived macrophages. Mol Cell Biochem. 2005;275(1-2):173–9. [DOI] [PubMed] [Google Scholar]
- 33.Liao ST, Han C, Xu DQ, Fu XW, Wang JS, Kong LY. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat Commun. 2019;10(1):5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sano M, Tanaka T, Ohara H, Aso Y. Itaconic acid derivatives: structure, function, biosynthesis, and perspectives. Appl Microbiol Biotechnol. 2020;104(21):9041–51. [DOI] [PubMed] [Google Scholar]
- 35.Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature. 2018;556(7699):113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palsson-McDermott EM, O'Neill LAJ. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res. 2020;30(4):300–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheurlen KM, Snook DL, Gardner SA, Eichenberger MR, Galandiuk S. Macrophage Differentiation and Polarization into an M2-Like Phenotype using a Human Monocyte-Like THP-1 Leukemia Cell Line. JoVE. 2021;in press. [DOI] [PubMed] [Google Scholar]
- 38.Steel RGD, Torrie JH. Steel RGD, and Torrie JH: Principles and Procedures of Statistics. New York, 1980, McGraw-Hill. 1980. [Google Scholar]
- 39.Scheffé H. The Analysis of Variance, New York: John Wiley & Sons. 1959. [Google Scholar]
- 40.The SAS System V9. Cary, NC, SAS Institute Inc; 2003. [Google Scholar]
- 41.Auwerx J, Cock TA, Knouff C. PPAR-gamma: a thrifty transcription factor. Nucl Recept Signal. 2003;1:e006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hooftman A, O'Neill LAJ. The Immunomodulatory Potential of the Metabolite Itaconate. Trends Immunol. 2019;40(8):687–98. [DOI] [PubMed] [Google Scholar]