Abstract
Background:
Nicotine vaping among youth has increased, warranting concern from tobacco control proponents. Many youth who vape indicate interest in quitting; however, few empirically supported vaping cessation interventions exist. This pilot feasibility study adapted an established behavioral intervention, contingency management (CM), delivered via telehealth to promote vaping cessation among young adults.
Methods:
Participants (N=27; ages 17-21) vaping nicotine regularly were recruited via social media and digital advertisements from across the US (June 2020 - January 2021). Participants were randomized at approximately 4:1 to CM or Monitoring control (22:5). CM was delivered through DynamiCare Health’s smartphone app for 4 weeks, in which financial incentives were delivered contingent on abstinent cotinine samples after the quit day until the end of treatment (EOT; Days 7-28; 10 expected submissions). Control participants earned incentives for submitting cotinine, regardless of abstinence. Feasibility, acceptability, and abstinence was collected throughout treatment, at EOT, and at 1-month follow-up.
Results:
The majority of enrolled participants completed treatment (Monitoring: 5/5; CM: 20/22), and intervention components were rated favorably overall (>80%). CM participants submitted 112/220 (55%) abstinent cotinine samples throughout the quit attempt, while the Monitoring group submitted 4/50 (8%) negative samples. There were no differences in abstinence between groups at EOT or follow-up.
Conclusion:
This pilot study of a telehealth-based youth vaping cessation intervention demonstrated preliminary feasibility and acceptability. These results suggest that CM for young adult vaping cessation, delivered remotely, is a promising direction for future work and fully powered trials are warranted to assess intervention efficacy.
Keywords: vaping, electronic cigarettes, youth, treatment, telehealth, contingency management
1.0. Introduction
Use of nicotine-containing electronic (e-)cigarettes (i.e., vaping) among adolescents and young adults has increased over the past decade, generating concern (U.S. Department of Health and Human Services, 2018). Estimates from 2020 suggest an increase in vaping prevalence from 2019, with 12.5% of 8th graders, 19.3% of 10th graders, and 24.7% of 12th graders reporting past 30-day vaping (Cornelius et al., 2020; Johnston et al., 2021; Wang et al., 2020). In 2019, 28.5% of college students reported vaping in the past 30 days (Schulenberg et al., 2020). Nicotine vaping among youth has likely been maintained by the heightened popularity of new disposable flavored products into the market (Wang et al., 2021). This trend has garnered concern from public health advocates, schools, and parents (U.S. Department of Health and Human Services, 2018). Estimates from 2021 show a decrease in past 30-day use (11.3% of high school students and 2.8% of middle school students), which is encouraging, but may be a result of restrictions due to the Covid-19 pandemic.
Nicotine vaping among youth has been associated with higher odds of developing nicotine dependence, transitioning to combustible tobacco use (National Academies of Sciences Engineering and Medicine, 2018; Soneji et al., 2017), and/or use of multiple tobacco/nicotine products (Hair et al., 2019; Huh and Leventhal, 2016). Concerning health effects associated with nicotine vaping include respiratory irritation, increased risk or exacerbation of substance use initiation and transitioning to problematic use, and psychiatric symptoms (Audrain-McGovern et al., 2018; Becker and Rice, 2021; Hershberger et al., 2020; Park et al., 2020). Strategies to reduce and prevent youth vaping uptake have included school-based programs, mass media campaigns, and public health policy (e.g., banning certain flavors, raising the minimum age to purchase) (Liu et al., 2020); however, the prevalence of initiation and regular use remains high. Those who transition to regular vaping report feeling dependent on the products and face challenges in quitting (Amato et al., 2021). Nearly half (44%) of adolescents ages 12-17 report interest in vaping cessation, and 25% reported unsuccessful past year quit attempts (Smith et al., 2021). This demonstrates a need for youth-focused vaping cessation interventions.
To date, few empirically rigorous and fully powered vaping cessation intervention trials have been conducted. One prospective trial of adults showed promising results for varenicline to facilitate cessation of both cigarettes and e-cigarettes (Hajek et al., 2019). However, medication trials of varenicline for youth smoking cessation have failed to demonstrate comparable efficacy as in adults (Gray et al., 2019; Gray et al., 2020). This suggests that age-tailored treatment approaches may be required, such as emphasizing behavioral skills and strategies administered via telehealth for greater reach and accessibility. Others have recommended that interventions for this age group incorporate salient elements provided via mobile phones, including text messaging, interactive applications, and gamification (Berg et al., 2021). Recently, a vaping cessation support and skill-building intervention delivered via texting (This is Quitting (Graham et al., 2021; Graham et al., 2019)) achieved a 24.1% self-reported abstinence rate among young adults (ages 18-24) compared to 18.6% in the control group (OR = 1.39; 95% CI 1.15-1.68; p < .001). Other text platforms provide cessation support, but a majority focus on general tobacco use and do not specifically address vaping (Liu et al., 2020). Tailoring treatments to vaping specifically may increase effectiveness by providing messaging and skills related to the unique aspects of vaping, such as the product components, ubiquity of use, and social conditions.
Adapting well-established smoking cessation and other substance use treatment approaches to address vaping is a worthwhile extension to facilitate evidence-based treatment development and dissemination. Contingency management (CM) is an established behavioral intervention that has been used successfully to treat substance use disorders, including tobacco use (Prendergast et al., 2006; Secades-Villa et al., 2020). In CM, reinforcers that maintain drug use are diminished through the reinforcement of abstinence, typically administered as generalized reinforcers in the form of financial incentives. Meta-analyses from contemporary trials of CM delivered through telehealth have demonstrated efficacy for treating substance use disorders (Getty et al., 2019).
CM for smoking cessation has been successfully implemented among youth (Krishnan-Sarin et al., 2006; Reynolds et al., 2008; Reynolds et al., 2015; Roll, 2005), while advancements in telehealth platforms may be utilized to increase treatment accessibility (Dallery et al., 2019). Indeed, a recent single-case design pilot study of CM for vaping cessation (N=8) delivered remotely was shown to be acceptable and promoted abstinence for a brief 2-week duration among a sample of college students (Raiff et al., 2021). Behavioral telehealth interventions are appealing for youth vaping cessation, as they may increase reach by utilizing established platforms that streamline the CM procedures. For example, DynamiCare Health (DynamiCare Health Inc., 2021) is a commercially-available mobile app that promotes recovery from substance use via remote CM delivery. The app is enabled with video capture for the collection of saliva and/or breath drug/alcohol tests for specific substances and then delivers contingent incentives for abstinence shortly thereafter (generally within a few hours). DynamiCare has demonstrated utility for alcohol use disorder (Hammond et al., 2021) as well as smoking cessation in special populations and as part of a substance use disorder outpatient program (DeFulio et al., 2021; Kurti et al., 2020), but has not yet been adapted for youth vaping cessation.
The primary goal of this pilot study was to conduct a feasibility and acceptability trial of CM delivered via telehealth for youth/young adult nicotine vaping cessation. Given the challenges of implementing youth tobacco cessation interventions (e.g., recruitment, retention, missing data, accurate self-report) and treating youth smoking effectively (Diviak et al., 2004; Piper et al., 2019; Sussman, 2002), pilot feasibility and acceptability trials of intervention procedures are necessary to establish rationale for larger trials. Additionally, vaping cessation and reduction outcomes were evaluated to determine the potential therapeutic signal of CM compared to control. This aim, however, was exploratory due to small sample size and imbalanced randomization groups. CM is an established behavioral intervention for promoting abstinence from substances and was used in the current trial to address the public health concern of youth vaping, which currently has limited evidence-based treatment options. Further, the intervention was delivered via telehealth to improve reach and access of the intervention, thus contributing to the novelty of this work.
2.0. Methods
2.1. Participants
Participants were recruited locally in the Charleston, South Carolina (SC) area and from across the United States (US) using social media platforms (Facebook, Instagram) and online advertisements (Craigslist) from June 2020 through January 2021. Advertisement text included the study age range and that this was a remote treatment study for nicotine vaping (e.g., ready to quit, treatment provided, interested in quitting, etc.). Online and social media posts targeted regional cities for recruitment, as well as larger cities in the US. Inclusion criteria were ages 12-21, vaping nicotine at least 25 days/month, interest in quitting vaping (defined as at least a 5 on a 10-point scale), and access to a smartphone or computer with webcam. The age range for this study (12-21) was intentionally broad to be inclusive of those with high rates of current vaping prevalence based on national estimates (Johnston et al., 2021; Schulenberg et al., 2020). Further, the primary goal was feasibility and the defined age range allowed for testing of procedures and the intervention broadly across the age range to inform a larger trial. Developmentally appropriate interventions for vaping are likely needed for this age range but given that the primary aim was feasibility and acceptability, a larger age range was appropriate.
Participants over the age of 18 provided consent through telehealth procedures and assent was obtained from minors. Informed consent included a video visit with the participant (and parent/guardian, if under 18) while reviewing the informed consent via REDCap (Harris et al., 2009) or Doxy.me. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies and can be used to obtain eConsent. Doxy.me is a telemedicine platform that allows for forms to be sent and completed during video visits. Electronic forms were digitally signed on either platform by all applicable parties and PDF copies were emailed to participants upon completion.
Participants were asked to confirm current nicotine vaping via a positive salivary cotinine test during the pre-enrollment period. Participants were excluded if they were using any other nicotine-containing product or had a serious physical or mental health condition that would impact safety or compliance with participation. Participants were compensated $10 for completing the screening visit and $5 for completing the pre-enrollment saliva cotinine sample.
A total of 88 callers responded to study advertisements, of whom, 30 were ineligible (34%). Of the remaining 58, 3 were lost to follow-up before scheduling a screening appointment, 7 were screened after enrollment goals were met, and 20 did not show for their remote screening visit. A total of 28 participants signed consent (32% of callers), and one participant screen failed and was not included (total enrolled N=27).
2.2. Study Design and Randomization
As the primary aim of this pilot study was to assess feasibility and acceptability of the intervention, eligible participants were randomized at approximately 4:1 to either the CM or Control (Monitoring) condition, respectively (22:5). The CM and Monitoring interventions were delivered via the DynamiCare Health mobile app. Participants were mailed saliva test collection kits (instant-read Alere iScreen® Cotinine Oral Fluid Screening Device; cut-off <30 ng/mL) and downloaded the DynamiCare app at their Day 0 (randomization) visit. Every 2-3 days during the treatment period, the app prompted participants to submit videos of the entire saliva cotinine test and result (3-4 times per week). Once prompted, participants had 12 hours to complete the sample. DynamiCare staff reviewed videos within 12 hours of submission (though generally within a few hours when submitted during business hours). Participants were alerted of the result and immediately earned incentives for either abstinence (CM group) or sample submission (Monitoring group) (delivered via reloadable debit cards).
To augment the CM intervention and provide skills-based resources and content during the vaping quit attempt, all participants were provided with information for This is Quitting from the Truth Initiative (Graham et al., 2021; Graham et al., 2020a; Graham et al., 2020b). This SMS text-based program is free of charge but does use the participants’ text messaging service rates. Participants were encouraged to enroll in this program during their first week in the study (Day 0) to support their vaping quit attempt and were emailed information about the service.
The study design is shown in Figure 1. At Days 0, 3, and 6 (pre-quit attempt), participants submitted cotinine samples and were compensated for successful submission ($5 each; $15 total for pre-quit attempt submissions), regardless of the result. Pre-quit cotinine samples were completed to establish competency with procedures prior to the quit attempt. All participants were asked to make a vaping quit attempt on Day 7. During treatment (Days 7-28), participants submitted saliva samples 3-4 times per week (samples prompted every 2-3 days) and at end of treatment (EOT [Day 28]; 10 total quit attempt submissions). Participants in the CM group were compensated $20 per abstinent saliva sample submitted during the quit attempt and EOT (Days 7-28; 10 quit attempt samples; $200 possible), whereas participants in the Monitoring condition were compensated $20 for sample submission, regardless of abstinence (10 quit attempt samples; $200 possible). Participants also completed mobile daily dairies that assessed vaping and use of other tobacco/nicotine products via REDCap (Harris et al., 2009; Tomko et al., 2019) and weekly assessments and questionnaires. Following the EOT (Day 28) visit, participants completed a 1-month follow-up assessment (Day 56). Participants could earn a total of $310 maximum for study participation, including abstinence or submission incentives ($200), pre-quit saliva submissions ($15) and for study visits ($95; screening, weekly phone assessments, follow-up assessments).
Figure 1.
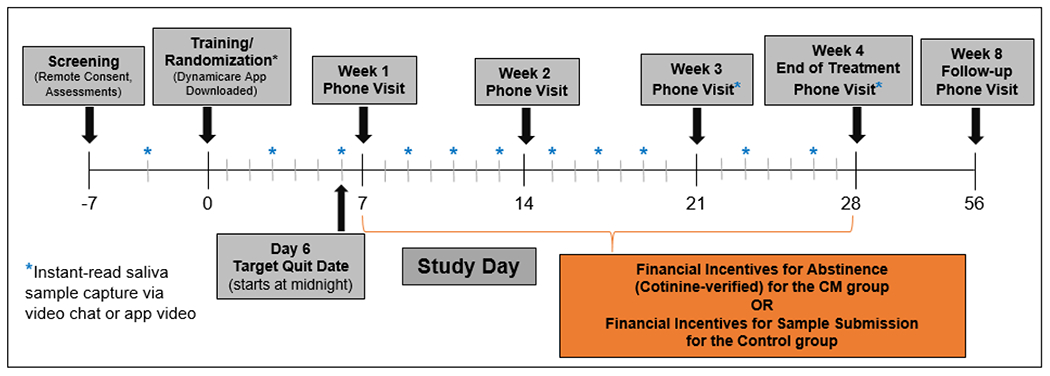
Study design and procedures.
2.3. Outcomes
2.3.1. Feasibility and Acceptability
Feasibility was assessed by; 1) the number of participants who completed the EOT and follow-up visits, 2) the number of saliva samples submitted, and 3) the number of weekly study visits completed. Ease of using the mobile app was rated using the System Usability Scale (SUS) (Sauro, 2011). Total SUS scores range from 0-100 with recommended scores of 84.1 or higher representing highly favorable usability ratings (scores above 68 are above average and any score above 80.3 is among the top 10%). Acceptability was assessed by asking participants to rate agreement with the following items: “I liked using the DynamiCare app”; “The video upload process worked well”; “I would definitely use this app in the future”; “I liked the reloadable debit card as a way to get my study compensation”; “Participation in this study improved my confidence in quitting vaping; “This study made me feel more in control of my vaping”; and “I used the text-based program ‘This Is Quitting’ during my quit attempt” (Graham et al., 2021) on a 1 (strongly disagree) to 5 (strongly agree) Likert scale. Responses of “strongly agree” and “moderately agree” were collapsed to indicate favorable endorsement.
2.3.2. Abstinence and Quit Attempts
Vaping abstinence was determined by a negative saliva cotinine test (cut-off < 30 ng/mL). The cut-off for this particular test is higher than more conservative biochemical recommendations to verify abstinence (Benowitz et al., 2020), but the test is easier to use and better liked by participants than other commercially available instant-read salivary cotinine tests (Raiff et al., 2021). Samples results are also easily verified over video, which was the primary means of sample submission and collection in this study. Quit attempts were determined by self-reported endorsement of the item “I attempted to quit vaping today.” Missing cotinine samples and self-report responses were coded as not abstinent or did not try to quit, respectively. At the 1-month follow-up (Day 56), participants were asked the same questions and reported if they had vaped or reduced their vaping since the Day 28 (EOT) visit (“Have you reduced your e-cig use?” Yes/No), but no cotinine sample was collected at this visit.
2.4. Analysis
Descriptive statistics for sample characteristics, feasibility, and acceptability were computed. Independent samples t-tests were used to determine differences in number of abstinent cotinine samples between treatment conditions following the quit day. Chi-square tests were used to determine differences in proportions of participants who made or did not make quit attempts at the weekly assessments. Given the preliminary nature of intervention development, small sample size, and the oversampling of intervention participants, we emphasize effect sizes (Cohen’s d and Phi (φ)) throughout. Missing data were coded as vaping (not abstinent) or no attempt to quit or reduce vaping.
3.0. Results
3.1. Study Participants
Of the 28 participants who were consented, 27 were eligible (1 did not submit positive cotinine sample at pre-enrollment and was excluded) and randomized to CM (n=22) or Monitoring (n=5). Characteristics of enrolled participants are presented in Table 1. Only one participant (out of 27 enrolled) was under the age of 18. A majority were local (57% Charleston, SC area, 11% elsewhere in SC) and 32% resided in other states. Given that this was a pilot study that oversampled to the intervention condition, we did not formally assess baseline differences, though Table 1 shows demographics separated by treatment group.
Table 1.
Participant demographics and vaping characteristics for the full study sample (N=27) and separated by treatment group (CM or Monitoring).
| Full Sample (N=27) | CM (n=22) | Monitoring (n=5) | |
|---|---|---|---|
| Age – Mean (SD) | 20.3 (1.2) | 19.9 (1.2) | 20.6 (0.5) |
| Gender – N (%) | |||
| Women | 18 (66.7) | 15 (68.2) | 3 (60) |
| Men* | 9 (33.3) | 6 (27.3) | 2 (40) |
| Race – N (%) | |||
| White | 24 (88.9) | 19 (86.4) | 5 (100) |
| Asian | 2 (7.4) | 2 (9.1) | 0 |
| Multi-racial | 1 (3.7) | 1 (4.5) | 0 |
| Ethnicity – N (%) | |||
| Hispanic | 4 (14.8) | 3 (13.6) | 1 (20) |
| Non-Hispanic | 23 (85.2) | 19 (86.4) | 4 (80) |
|
| |||
| Age of vaping initiation – M (SD) | 16.3 (1.2) | 16.2 (1.2) | 16.9 (0.9) |
| Age of regular vaping (>1x/week) – M (SD) | 17.6 (1.5) | 17.34 (1.6) | 18.6 (0.9) |
| Months vaping – M (SD) | 31.1 (16.0) | 33.1 (15.7) | 22 (16.1) |
|
| |||
| E-cigarette used – N (%) | |||
| Cartridge/pod (e.g., JUUL) | 15 (55.6) | 13 (59.1) | 2 (40) |
| Disposable (e.g., Blu) | 8 (29.6) | 5 (22.7) | 3 (60) |
| Tank system or Other | 4 (14.8) | 4 (18.2) | 0 |
|
| |||
| Days vaped in past 30 days – M (SD) | 28.5 (4.3) | 28.2 (4.8) | 29.6 (0.9) |
| # vaping episodes per day in past 30 days – M (SD) | 19.8 (14.9) | 20.4 (15.4) | 20.4 (13.4) |
| Minutes per vaping episode – M (SD) | 6.5 (13.4) | 7.4 (15) | 3.6 (2.6) |
| Puffs per vaping episode – M (SD) | 7.7 (11.7) | 8.7 (13.1) | 3.2 (1.1) |
| ECDI score – M (SD) | 12.4 (3.6) | 12.3 (3.7) | 13.4 (3.9) |
|
| |||
| Interest in quitting 1 (low) - 10 (high) – M (SD) | 8.5 (1.4) | 8.4 (1.3) | 8.8 (1.8) |
| Past year quit attempt – N (%) | 21 (77.8) | 18 (81.8) | 3 (60) |
|
| |||
| Smoked > 100 lifetime cigarettes – N (%) | 6 (21.4) | 5 (22.7) | 1 (20) |
| History of regular (>1x/week) smoking – N (%) | 8 (28.6) | 6 (27.3) | 2 (40) |
Note:
Includes cisgender and transgender men.
ECDI = Penn State Electronic Cigarette Dependence Inventory (Foulds et al., 2015), score range 0 (low) – 20 (high). One participant the CM group was under the age of 18.
Overall, participants reported vaping for approximately 2.5 years (M=31.1 months, SD=16.0) prior to enrollment. The most common vaping devices reported were replaceable cartridge/pod systems (e.g., JUUL®, n=15, 56%) and disposables (e.g., Blu® disposable, n=8, 30%), with 71.4% (n=20) reporting nicotine content as 4.6-5.0%. Most participants reported daily vaping (n=18, 67%), vaping a mean of 19.8 (SD=14.9) episodes per day, with each vaping episode lasting an average of 6.5 minutes (SD=13.4) and 7.7 puffs (SD=11.7) per episode. A majority reported having made a serious vaping quit attempt (at least 24 hours) in the past year (n=21, 78%).
3.2. Feasibility and Acceptability
A majority of participants (25/27; 93%) completed the Day 28 EOT visit, and 89% (24/27) completed the Day 56 follow-up visit. Two participants in the CM group (2/22; 9%) were lost to follow-up during treatment (prior to Day 28) and 1 participant in the CM group (1/22; 5%) was lost during post treatment (prior to Day 56). All participants in the Monitoring group (5/5) completed treatment and follow-up. Overall, 94% (203/216) of all study visits were completed across both groups (8 visits per participant, including the pre-enrollment saliva sample). During treatment, 80% (280/351) of scheduled cotinine samples were completed.
Among treatment completers, the mean SUS score was 83.1 (SD=15.5), indicating that the mobile app was rated as having moderately to highly favorable usability. Although not statistically significant, those in the CM condition rated app usability as higher (86.8 [SD=11.2]) than the control group (69.0 [SD=22.3]; shown in Table 2). The intervention, mobile app, debit card to deliver incentives, and study were favorably rated by participants in both groups (Table 2). Only 36% of participants (7/20 CM and 2/5 Monitoring) reported signing up for and using This is Quitting (Graham et al., 2021).
Table 2.
Acceptability Item Reponses for Day 28 (EOT) Completers.
| Acceptability Item | Txt Completers (N=25) N (%) | CM (N=20) | Monitoring (N=5) |
|---|---|---|---|
| I liked using the DynamiCare app | 21 (84%) | 17 (85%) | 4 (80%) |
| The video upload process worked well | 20 (80%) | 16 (80%) | 4 (80%) |
| I would definitely use this app in the future | 21 (84%) | 17 (85%) | 4 (80%) |
| I liked the reloadable debit card | 22 (88%) | 17 (85%) | 5 (100%) |
| Participation in this study improved my confidence in quitting vaping | 22 (88%) | 17 (85%) | 5 (100%) |
| This study made me feel more in control of my vaping | 20 (80%) | 16 (80%) | 4 (80%) |
Note: Ns correspond to number of participants indicating “Strongly Agree” or “Moderately Agree’ to each item, which were collapsed to indicate favorable.
3.3. Vaping Outcomes
Vaping outcomes are shown in Table 3 for all randomized participants. Out of 10 possible cotinine samples during the treatment period (Days 7-28; 270 expected samples for 27 participants), participants in the CM group provided, on average, 5.5 (SD=4.4; 122/220; 55.4%) negative (i.e., abstinent) samples, whereas those in the control group provided a mean of 0.8 (SD=1.30; 4/50; 8%; t(22.78)=−4.26, p<.001, d=−1.15). At the Day 28 EOT visit, no significant differences were found between the CM and monitoring groups on proportion of abstinent participants (13/22 [59.1%] in CM vs. 2/5 [40%] in Monitoring, with missing data coded as not abstinent; p=0.43; φ=15). When asked if participants had made a quit attempt on Day 7 (target quit day), Day 14, or Day 28 (EOT), chi-square tests did not show significant differences (φs ranged from .03-.34) between quit attempt endorsement for the CM group (n=20/22, 17/22, 14/22, respectively) compared to the Monitoring group (n=3/5, 3/5, 3/5, respectively).
Table 3.
Vaping and usability outcomes by treatment condition.
| CM (n=22) | Monitoring (n=5) | P | d or φ | |
|---|---|---|---|---|
| # Abstinent samples – Mean (SD) (out of 10 possible) | 5.5 (4.4) | 0.8 (1.3) | <.001 | −1.15 |
| Abstinent sample submitted at EOT (Day 28) | 13/22 (59.1%) | 2/5 (40%) | .438 | .15 |
| Endorsed quit attempt1 – N (%) | ||||
| Day 7 | 20/22 (90.9) | 3/5 (60) | .08 | .34 |
| Day 14 | 17/22 (78.3) | 3/5 (60) | .43 | .15 |
| Day 28 (EOT) | 14/22 (63.6) | 3/5 (60) | .87 | .03 |
| Day 56 (Follow-up) | 9/22 (40.9) | 3/5 (60) | .44 | −.15 |
| Quit vaping at follow-up – N (%) | 6/22 (27.3) | 1/5 (20) | .74 | −.06 |
| Reduced vaping at follow-up – N (%) | 10/22 (45.4) | 4/5 (80) | .16 | −.27 |
| App Usability – Treatment Completers | n=20 | n=5 | ||
| SUS2 score at EOT (Day 28) | 86.8 (11.2) | 69.0 (22.3) | .15 | −.128 |
Note: Day 7 was the Quit Day for all participants, and Day 28 was the end of treatment (EOT; Day 28).
Quit attempts were new or continued from the previous attempt.
SUS = System Usability Scale (Sauro, 2011).
Missing data were coded as vaping (not abstinent) or no attempt to quit or reduce vaping.
At the 1-month follow-up (Day 56), 9/22 (41%) in the CM group and 3/5 (60%) participants in the Monitoring group endorsed a vaping quit attempt since EOT (p=.44, φ= −.15). When self-reported abstinence was assessed at follow-up, 6/22 (27%) and 1/5 (20%) reported abstinence from vaping since EOT (p=.72, φ= −.06). This was not biochemically verified, and participants lost to follow-up were considered to be vaping and not having made a quit attempt. Participants reported reductions in vaping since EOT (10/22 [45%] of CM and 4/5 [80%] of Monitoring; p=.16, φ= −.27).
Finally, participants were asked to report on the use of other tobacco/nicotine products through mobile daily diaries throughout the study. During treatment, use of other tobacco/nicotine products was endorsed by 6 participants on 20 out of 715 sampled days (3%). During the follow-up period, other tobacco/nicotine products were endorsed by 2 participants on 24 out of 678 days (4%).
4.0. Discussion
This pilot study aimed to evaluate feasibility and acceptability of a telehealth-delivered CM intervention among young adults who were vaping regularly and were interested in quitting. Even as a small feasibility study, comparisons between those randomized to a CM intervention group (n=22) vs. Monitoring controls (n=5), suggest that: 1) most participants were retained in the study through the Day 56 follow-up (Monitoring: 5/5 and CM: 19/22), 2) many endorsed high ratings of acceptability of the treatment and study procedures, 3) there was preliminary support for the 28-day CM intervention, as evidenced by 55% (122/220) of submitted cotinine samples being negative in the CM group. The present study represents an extension of CM, a well-established treatment (Davis et al., 2016; Notley et al., 2019; Secades-Villa et al., 2020), to address nicotine vaping, which has currently surpassed all other forms of tobacco as the most popular product used by youth (Johnston et al., 2021). Study procedures also addressed a critical research demand (Dallery et al., 2019; Davis et al., 2016) by capitalizing on telehealth technology to broaden reach and facilitate important study measures to improve rigor, including biochemical verification of vaping and abstinence.
Outcomes related to vaping cessation were exploratory and should be interpreted with the utmost caution given the small sample size, imbalanced active and control groups, and the sample of highly motivated older e-cigarette users that were enrolled in the study. This pilot was not powered to detect clinically meaningful group differences in cessation-related outcomes (negative cotinine, quit attempts, days of abstinence, etc.), and overinterpretation of small sample sizes has implications for rigor and reproducibility (Button et al., 2013). Abstinence results, in their current form, likely have minimal impact for application to the greater population of youth who are vaping, though feasibility and acceptability data are promising and may inform future trials. Outcomes suggest that CM for young adult vaping cessation, delivered via telehealth, is a worthwhile intervention to further adapt and properly evaluate in a larger randomized clinical trial with vaping cessation as the primary outcome to produce more statistically reliable estimates of intervention effects.
While CM may motivate quit attempts and promote short-term abstinence, additional intervention components and design features may be needed to maintain sustained abstinence and support intervention durability. One recent qualitative study found that adolescents who considered quitting vaping did so due to acute, proximal physical effects and health concerns (e.g., decreased sports performance). However, barriers to quitting included experiencing withdrawal symptoms as well as social norms and cues that promote vaping (Kong et al., 2021). Future CM vaping cessation interventions may include components that address health concerns, withdrawal, as well as including aspects that address satisfaction from vaping and maintaining abstinence in challenging social situations. Interventions that also address related psychosocial needs of youth may enhance the outcomes of treatment. Further, motivation enhancement techniques may be used to appeal to those who are currently ambivalent about quitting. The sample in the current trial was highly motivated to quit vaping, which should be considered when interpreting outcomes. A larger trial may provide the opportunity to assess the relationship between baseline motivation to quit and outcomes of treatment.
Behavioral interventions that can be delivered via telehealth have appeal for this population, may address barriers that typically hinder youth engagement in interventions (e.g., transportation, privacy concerns, etc.), and may aid in study retention. Overcoming these challenges broadens access to those who live in otherwise hard-to-reach areas. In the present study, most participants resided in-state and within the Charleston area (57%). We speculate that may be due to regional recognition of the health system (MUSC) and a larger, national study would likely have greater representation across the US. Even within this study, we recruited 43% from outside of our immediate geographic area, which is encouraging for future work. Taken together, these findings extend prior work that has shown promise for remotely delivered CM interventions in promoting behavior change (Getty et al., 2019; Raiff et al., 2021) and demonstrates that this is a promising intervention to further pursue for youth vaping.
Beyond the need for a larger and more diversified study sample, there are several challenges and limitations to the present study that may be addressed with a fully powered clinical trial or alternative study design. First, CM tends to be a resource-heavy intervention, both in costs and staff time, which makes adoption and dissemination challenging. Continued improvements in technology, such as those used by the present study, can help bridge this gap to deliver extended support over time, which can increase treatment effectiveness (Davis et al., 2016). Several options exist to make CM interventions potentially more affordable and scalable: 1) “fishbowl” approaches, in which abstinent participants randomly draw from a pool of varying incentives (Branson et al., 2012; Petry and Bohn, 2003), 2) group contingencies, wherein incentives are delivered based on group performance (Dallery et al., 2015; Dallery et al., 2019), or 3) use of non-financial incentives (e.g., special privileges, vouchers). Sustainability of CM interventions require consideration of incentive costs and stakeholders who would cover costs. On a smaller scale, CM could be funded through internal mechanisms (e.g., within a school or institution) or as part of deposits from the individual or family member (Ryan-Pettes et al., 2020). On a larger scale, programs may have to rely more heavily on nonfinancial incentives or collaborate with larger systems (e.g., insurance providers) to cover the cost of incentives and the delivery platform.
Second, our age range for inclusion was broad (12-21), though recruitment was successful among mostly 18-21 year olds, which is consistent with trends that show increased prevalence of vaping with age (Johnston et al., 2021; Schulenberg et al., 2020). Enrollment of participants under the age of 18 was low (n=1 out of 28 enrolled), which could be due to hesitancy to disclose vaping to parents, which was required to consent. The challenge in recruiting minors is consistent with prior adolescent tobacco cessation trials (Diviak et al., 2004), thus representing an area for improvement in future trials. Third, vaping cessation research would benefit from biochemical verification specific to nicotine vaping (Hiler et al., 2020) to indicate abstinence from vaping and differentiate from other tobacco or nicotine product use (combustible cigarettes, nicotine replacement, etc.). Indeed, our cut-off for biochemical verification was slightly higher than what has been recommended (Benowitz et al., 2020), but is preferred (compared to other available tests) among youth engaging in vaping cessation (Raiff et al., 2021). Finally, our control group (Monitoring) was intentionally under sampled to better assess acceptability and feasibility in the CM intervention group.
4.1. Conclusions
A pilot intervention of contingency management for vaping cessation delivered via telehealth was shown to be feasible and acceptable among highly motivated young adults (ages 17-21) who were vaping nicotine regularly. Given potential feasibility, these results provide a foundation from which future research may implement more rigorous methodology to assess efficacy, engagement, reach, while also considering motivation for vaping cessation. Vaping cessation outcomes were explored and showed preliminary evidence of an effect; however, this should be tested in a fully powered trail prior to drawing conclusions regarding the efficacy of CM for young adult vaping cessation. Future research should expand on telehealth-delivered CM platforms in larger clinical trials, while including additional treatment elements to bolster long-term abstinence and improve cost-effectiveness. Effective interventions that promote vaping cessation among youth is an important component of tobacco control that increases public health benefits and warrants further evaluation.
Highlights.
We evaluated a short-term remote vaping cessation intervention for young adults
24/27 (89%) participants completed the 2-month study
Intervention components were rated favorably overall (>80%)
The CM group submitted 112/220 negative cotinine samples during treatment
Telehealth-delivered CM for young adult vaping cessation is a promising future area
Acknowledgments:
The authors would like to thank the research and medical staff of the Addiction Sciences Division of the Medical University of South Carolina for the successful execution of this study protocol. Specifically, we would like to thank Benjamin Laprise, Patrick Cato, Elizabeth Bradley, and Bryttin Boyde. We would also like to thank DynamiCare Health for their collaboration on this project. Finally, we would like to thank the participants for their involvement in this study.
Role of Funding Source:
The funding sources had no role in the development of this manuscript. This study was supported by internal funds at MUSC (Gray, Carpenter, McClure), and in part by pilot research funding from the Hollings Cancer Center’s Cancer Center Support Grant P30 CA138313 at MUSC, specially within the Cancer Prevention and Control program of Hollings. Additional support was provided from the National Institute on Drug Abuse (K01DA047433 to TS and K23DA045766 to JD), National Institute on Alcohol Abuse and Alcoholism (K23AA025399 to LS), National Center for Advancing Translational Sciences (NCATS UL1TR001450, PI Brady) and the NIH Institutional Postdoctoral Training Grant NIH-T32-HL144470 to AP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: Drs. Carpenter, Gray, and Toll have consulted to Pfizer for advisory boards. Dr. Toll testifies as expert witnesses on behalf of plaintiffs who filed litigation against the tobacco industry. Dr. Carpenter received consulting honoraria from Frutarom Pharmaceuticals. Dr. Tomko provides consultation to the American Society of Addiction Medicine. Dr. Dahne is co-owner of Behavioral Activation Tech LLC, a small business that develops digital interventions for the treatment of depression and common comorbidities. No other authors have conflicts of interest to declare.
References
- Amato MS, Bottcher MM, Cha S, Jacobs MA, Pearson JL, Graham AL, 2021. “It’s really addictive and I’m trapped:” A qualitative analysis of the reasons for quitting vaping among treatment-seeking young people. Addict. Behav 112, 106599. [DOI] [PubMed] [Google Scholar]
- Audrain-McGovern J, Stone MD, Barrington-Trimis J, Unger JB, Leventhal AM, 2018. Adolescent E-Cigarette, Hookah, and Conventional Cigarette Use and Subsequent Marijuana Use. Pediatrics 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker TD, Rice TR, 2021. Youth vaping: a review and update on global epidemiology, physical and behavioral health risks, and clinical considerations. Eur. J. Pediatr, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Bernert JT, Foulds J, Hecht SS, Jacob P, Jarvis MJ, Joseph A, Oncken C, Piper ME, 2020. Biochemical Verification of Tobacco Use and Abstinence: 2019 Update. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 22, 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg CJ, Krishnan N, Graham AL, Abroms LC, 2021. A synthesis of the literature to inform vaping cessation interventions for young adults. Addict. Behav 119, 106898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branson CE, Barbuti AM, Clemmey P, Herman L, Bhutia P, 2012. A pilot study of low-cost contingency management to increase attendance in an adolescent substance abuse program. Am. J. Addict 21, 126–129. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR, 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci 14, 365–376. [DOI] [PubMed] [Google Scholar]
- Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ, 2020. Tobacco Product Use Among Adults - United States, 2019. MMWR Morb. Mortal. Wkly. Rep 69, 1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Meredith S, Jarvis B, Nuzzo PA, 2015. Internet-based group contingency management to promote smoking abstinence. Exp. Clin. Psychopharmacol 23, 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, Grabinski MJ, Marsch LA, 2019. Technology-Based Contingency Management in the Treatment of Substance-Use Disorders. Perspect Behav Sci 42, 445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST, 2016. A review of the literature on contingency management in the treatment of substance use disorders, 2009-2014. Prev. Med 92, 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFulio A, Rzeszutek MJ, Furgeson J, Ryan S, Rezania S, 2021. A smartphone-smartcard platform for contingency management in an inner-city substance use disorder outpatient program. J. Subst. Abuse Treat 120, 108188. [DOI] [PubMed] [Google Scholar]
- Diviak KR, Curry SJ, Emery SL, Mermelstein RJ, 2004. Human participants challenges in youth tobacco cessation research: researchers’ perspectives. Ethics Behav 14, 321–334. [DOI] [PubMed] [Google Scholar]
- DynamiCare Health Inc., 2021. DynamiCare Health. Retrived from Apple App Store (http://www.apple.com/app-store/). p. Mobile application software. [Google Scholar]
- Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, Eissenberg T, 2015. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 17, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getty CA, Morande A, Lynskey M, Weaver T, Metrebian N, 2019. Mobile telephone-delivered contingency management interventions promoting behaviour change in individuals with substance use disorders: a meta-analysis. Addiction 114, 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Amato MS, Cha S, Jacobs MA, Bottcher MM, Papandonatos GD, 2021. Effectiveness of a Vaping Cessation Text Message Program Among Young Adult e-Cigarette Users: A Randomized Clinical Trial. JAMA Internal Medicine [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Jacobs MA, Amato MS, 2019. Engagement and 3-month outcomes from a digital e-cigarette cessation program in a cohort of 27,000 teens and young adults. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Jacobs MA, Amato MS, 2020a. Engagement and 3-Month Outcomes From a Digital E-Cigarette Cessation Program in a Cohort of 27 000 Teens and Young Adults. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 22, 859–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Jacobs MA, Amato MS, Cha S, Bottcher MM, Papandonatos GD, 2020b. Effectiveness of a Quit Vaping Text Message Program in Promoting Abstinence Among Young Adult E-Cigarette Users: Protocol for a Randomized Controlled Trial. JMIR Res Protoc 9, e18327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Baker NL, McClure EA, Tomko RL, Squeglia LM, Saladin ME, Carpenter MJ, 2019. Efficacy and Safety of Varenicline for Adolescent Smoking Cessation: A Randomized Clinical Trial. JAMA pediatrics 173, 1146–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Rubinstein ML, Prochaska JJ, DuBrava SJ, Holstein AR, Samuels L, McRae TD, 2020. High-dose and low-dose varenicline for smoking cessation in adolescents: a randomised, placebo-controlled trial. The Lancet Child & Adolescent Health 4, 837–845. [DOI] [PubMed] [Google Scholar]
- Hair EC, Romberg AR, Niaura R, Abrams DB, Bennett MA, Xiao H, Rath JM, Pitzer L, Vallone D, 2019. Longitudinal Tobacco Use Transitions Among Adolescents and Young Adults: 2014-2016. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 21, 458–468. [DOI] [PubMed] [Google Scholar]
- Hajek P, Peerbux S, Phillips-Waller A, Smith C, Pittaccio K, Przulj D, 2019. Are ‘dual users’ who smoke and use e-cigarettes interested in using varenicline to stop smoking altogether, and can they benefit from it? A cohort study of UK vapers. BMJ open 9, e026642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond AS, Sweeney MM, Chikosi TU, Stitzer ML, 2021. Digital delivery of a contingency management intervention for substance use disorder: A feasibility study with DynamiCare Health. J. Subst. Abuse Treat 126, 108425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, 2009. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger A, Argyriou E, Cyders M, 2020. Electronic nicotine delivery system use is related to higher odds of alcohol and marijuana use in adolescents: Meta-analytic evidence. Addict. Behav 105, 106325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiler M, Breland A, Wolf CE, Poklis JL, Nanco CR, Eissenberg T, 2020. Are Urine Propylene Glycol or Vegetable Glycerin Markers of E-cigarette Use or Abstinence? Tobacco Regulatory Science 6, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh J, Leventhal AM, 2016. Progression of Poly-tobacco Product Use Patterns in Adolescents. Am. J. Prev. Med 51, 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME, 2021. Monitoring the Future National Survey Results on Drug Use, 1975-2020: Overview, Key Findings on Adolescent Drug Use. Institute for Social Research [Google Scholar]
- Kong G, Bold KW, Cavallo DA, Davis DR, Jackson A, Krishnan-Sarin S, 2021. Informing the development of adolescent e-cigarette cessation interventions: A qualitative study. Addict. Behav 114, 106720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Duhig AM, McKee SA, McMahon TJ, Liss T, McFetridge A, Cavallo DA, 2006. Contingency management for smoking cessation in adolescent smokers. Exp. Clin. Psychopharmacol 14, 306–310. [DOI] [PubMed] [Google Scholar]
- Kurti AN, Tang K, Bolivar HA, Evemy C, Medina N, Skelly J, Nighbor T, Higgins ST, 2020. Smartphone-based financial incentives to promote smoking cessation during pregnancy: A pilot study. Prev. Med 140, 106201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gaiha SM, Halpern-Felsher B, 2020. A Breath of Knowledge: Overview of Current Adolescent E-cigarette Prevention and Cessation Programs. Curr Addict Rep, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences Engineering and Medicine, 2018. Public health consequences of e-cigarettes. National Academies Press. [PubMed] [Google Scholar]
- Notley C, Gentry S, Livingstone-Banks J, Bauld L, Perera R, Hartmann-Boyce J, 2019. Incentives for smoking cessation. Cochrane Database Syst Rev 7, Cd004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Livingston JA, Wang W, Kwon M, Eiden RD, Chang YP, 2020. Adolescent E-cigarette use trajectories and subsequent alcohol and marijuana use. Addict. Behav 103, 106213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Bohn MJ, 2003. Fishbowls and candy bars: using low-cost incentives to increase treatment retention. Sci Pract Perspect 2, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Mermelstein R, Baker TB, 2019. Progress in Treating Youth Smoking: Imperative, Difficult, Slow. JAMA pediatrics. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J, 2006. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction 101, 1546–1560. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Newman ST, Upton CR, Burrows CA, 2021. The feasibility, acceptability, and initial efficacy of a remotely delivered, financial-incentive intervention to initiate vaping abstinence in young adults. Exp. Clin. Psychopharmacol [DOI] [PubMed] [Google Scholar]
- Reynolds B, Dallery J, Shroff P, Patak M, Leraas K, 2008. A web-based contingency management program with adolescent smokers. J. Appl. Behav. Anal 41, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Harris M, Slone SA, Shelton BJ, Dallery J, Stoops W, Lewis R, 2015. A feasibility study of home-based contingency management with adolescent smokers of rural Appalachia. Exp. Clin. Psychopharmacol 23, 486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, 2005. Assessing the feasibility of using contingency management to modify cigarette smoking by adolescents. J. Appl. Behav. Anal 38, 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan-Pettes SR, Devoto A, DeFulio A, 2020. Acceptability and willingness to pay for contingency management interventions among parents of young adults with problematic opioid use. Drug Alcohol Depend. 206, 107687. [DOI] [PubMed] [Google Scholar]
- Sauro J, 2011. A practical guide to the system usability scale: Background, benchmarks & best practices. Measuring Usability LLC. [Google Scholar]
- Schulenberg J, Johnston L, O’Malley P, Bachman J, Miech R, Patrick M, 2020. Monitoring the Future national survey results on drug use, 1975-2019: Volume II, college students and adults ages 19–60. [Google Scholar]
- Secades-Villa R, Aonso-Diego G, García-Pérez Á, González-Roz A, 2020. Effectiveness of contingency management for smoking cessation in substance users: A systematic review and meta-analysis. J. Consult. Clin. Psychol 88, 951–964. [DOI] [PubMed] [Google Scholar]
- Smith TT, Nahhas GJ, Carpenter MJ, Squeglia LM, Diaz VA, Leventhal AM, Dahne J 2021. Intention to Quit Vaping Among United States Adolescents. JAMA pediatrics 175, 97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneji S, Barrington-Trimis JL, Wills TA, Leventhal AM, Unger JB, Gibson LA, Yang J, Primack BA, Andrews JA, Miech RA, Spindle TR, Dick DM, Eissenberg T, Hornik RC, Dang R, Sargent JD, 2017. Association Between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA pediatrics 171, 788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman S, 2002. Effects of sixty six adolescent tobacco use cessation trials and seventeen prospective studies of self-initiated quitting. Tob. Induc. Dis 1, 35–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomko RL, Gray KM, Oppenheimer SR, Wahlquist AE, McClure EA, 2019. Using REDCap for ambulatory assessment: Implementation in a clinical trial for smoking cessation to augment in-person data collection. Am. J. Drug Alcohol Abuse 45, 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, O.o.S.G., 2018. Surgeon General’s advisory on e-cigarette use among youth [Google Scholar]
- Wang TW, Gentzke AS, Neff LJ, Glidden EV, Jamal A, Park-Lee E, Ren C, Cullen KA, King BA, Hacker KA, 2021. Disposable E-Cigarette Use among U.S. Youth - An Emerging Public Health Challenge. N. Engl. J. Med 384, 1573–1576. [DOI] [PubMed] [Google Scholar]
- Wang TW, Neff LJ, Park-Lee E, Ren C, Cullen KA, King BA, 2020. E-cigarette Use Among Middle and High School Students—United States, 2020. MMWR. Morbidity and Mortality Weekly Report 69. [DOI] [PMC free article] [PubMed] [Google Scholar]


