Abstract
Background:
The simultaneous consumption of cocaine and alcohol results in the production of cocaethylene (CE) in the liver, a highly toxic metabolite. Prior research suggests that cocaine use contributes to liver disease and its concomitant use with alcohol may increase its hepatotoxicity, but studies in humans are lacking. We evaluated the role of cocaine, its simultaneous use with alcohol, and CE on liver fibrosis.
Methods:
We performed a cross-sectional analysis of the Miami Adult Studies on HIV (MASH) cohort. Cocaine use was determined via self-report, urine screen, and blood metabolites, using liquid chromatography with tandem mass spectrometry. Hazardous drinking was determined with the AUDIT-C and liver fibrosis with the Fibrosis-4 Index (FIB-4).
Results:
Out of 649 participants included in this analysis, 281 (43.3%) used cocaine; of those, 78 (27.8%) had CE in blood. Cocaine users with CE had higher concentrations of cocaine metabolites in blood and were more likely to drink hazardously than cocaine users without CE and cocaine non-users. Overall, cocaine use was associated with liver fibrosis. CE in blood was associated with 3.17 (95% CI: 1.61, 6.23; p=0.0008) times the odds of liver fibrosis compared to cocaine non-users, adjusting for covariates including HIV and HCV infection. The effect of CE on liver fibrosis was significantly greater than that of cocaine or alcohol alone.
Conclusions:
CE is a reliable marker of simultaneous use of cocaine and alcohol that may help identify individuals at risk of liver disease and aid in the prevention of its development or progression.
Keywords: Cocaine, Cocaethylene, Substance abuse, Liver disease, Liver fibrosis
1. Introduction
Chronic liver disease is a leading cause of morbidity and mortality worldwide (Cheemerla and Balakrishnan, 2021) and it disproportionately affects people living with HIV (PLWH) (Campa et al., 2016; Rockstroh et al., 2014; Sherman et al., 2017). As advances in antiretroviral therapy (ART) have drastically reduced HIV-related mortality, non-HIV-related morbidities have become increasingly relevant for PLWH, in whom they occur at higher rates than in the general population (Bosh et al., 2020; Marcus et al., 2020). Several factors contribute to liver diseases in PLWH, including liver injury as a result of HIV infection and use of ART (Kaspar and Sterling, 2017). Co-infections with hepatitis viruses continue to affect PLWH despite the introduction of effective vaccines and antivirals (Sherman et al., 2019). In addition, substance misuse, which is disproportionately prevalent among PLWH (Shiau et al., 2017), may pose a substantial risk for liver disease (Pateria et al., 2013). Historically, PLWH have had a high burden of alcohol-associated liver disease (AALD), consistent with the disproportionate prevalence of alcohol use disorders (Duko et al., 2019; Shiau et al., 2017). Yet, much less is known about the impact of non-prescribed recreational drugs on liver disease in PLWH (Pateria et al., 2013). Polysubstance use is also common among PLWH and increases their risk for morbidity and mortality (Hartzler et al., 2016). In a nationally representative sample of United States (U.S.) adults, PLWH were more likely than HIV-uninfected persons to report lifetime cocaine use (57.6% vs. 15.9%, p<0.001) and lifetime alcohol use (94.6% vs. 87.5%, p=0.001), as well as to meet the DSM-IV criteria for cocaine use disorder (4.6% vs. 0.3%, p<0.0001) and alcohol use disorder (6.1% vs. 3.4%, p=0.018) (Shiau et al., 2017).
Cocaine remains one of the most widely used recreational drugs in the United States (Carliner et al., 2017). In vitro and animal models have demonstrated cocaine-induced hepatotoxicity leading to liver fibrosis (Duysen et al., 2008; Labib et al., 2001; Wang et al., 1991), but prior observational studies on human subjects have shown contrasting results (Campa et al., 2016; Martel-Laferriere et al., 2017; Zarini et al., 2020). Liver fibrosis is a pathological consequence of chronic liver insults, such as oxidative stress, that can progress to liver cirrhosis and hepatocellular carcinoma. After ingestion, cocaine is rapidly metabolized largely by hepatic esterase, thus the liver is a primary target for cocaine-induced toxicity (and may be more susceptible to cocaine’s toxicity than the heart) (Wang et al., 2001). Most cocaine in circulation is converted to benzoylecgonine (BE), which has a longer half-life than cocaine and serves as the best biomarker of cocaine use (Chen et al., 2016; Goldstein et al., 2009; Kolbrich et al., 2006). However, cocaine-induced hepatotoxicity is largely mediated by oxidative stress as the remaining cocaine (<5%) undergoes oxidative metabolism to norcocaine (NC) and other minor, but active metabolites (Valente et al., 2012; Wang et al., 2001).
In the presence of ethanol, however, cocaine is transesterified by hepatic carboxylesterase into cocaethylene (CE), which has a plasma half-life 3 to 5 times that of cocaine and its toxicity is about 30% greater than that of cocaine or alcohol alone (McCance-Katz et al., 1993; Ponsoda et al., 1999). Therefore, CE serves as a biomarker of simultaneous/concomitant alcohol and cocaine consumption. Moreover, ethanol potentiates the hepatotoxicity of cocaine by depleting antioxidant defenses and making hepatocytes more susceptible to cocaine (Ponsoda et al., 1999). For people who use cocaine, the combination of cocaine with alcohol is desirable as it prolongs and increases the euphoric effects of cocaine while ameliorating some of its negative withdrawal symptoms (McCance-Katz et al., 1998). Not surprisingly, alcohol consumption is common among cocaine users. In a meta-analytic study, Liu et al. (2018) estimated that about three-quarters of people who use cocaine also use alcohol, either simultaneously (74%) or concurrently (77%).
Prior research suggests that cocaine use may contribute to liver disease progression in PLWH (Campa et al., 2016; Duysen et al., 2008; Labib et al., 2001; Wang et al., 1991; Zarini et al., 2020), and its concomitant use with alcohol may increase its hepatotoxicity (McCance-Katz et al., 1993; Ponsoda et al., 1999). To the best of our knowledge, no study has previously used CE as a marker of simultaneous alcohol-cocaine use to examine its association with liver fibrosis in human subjects. Consequently, the purpose of this study was to investigate the associations between cocaine and CE with liver fibrosis.
2. Methodology
We performed a cross-sectional analysis of data obtained between 2016-2018 from the Miami Adult Studies on HIV (MASH) cohort. The MASH cohort follows people with and without HIV for substance use, comorbidities, and social determinants of health. Participants are recruited from neighboring clinics. HIV and viral hepatitis status are confirmed via medical documentation with participant’s written consent. All participants were 40 years of age or older and seronegative for hepatitis B at enrollment. MASH participants who used heroin (N=121) were excluded from this analysis since we have previously reported on the association of heroin use with liver fibrosis (Baum et al., 2021). The protocols for this study were approved by the Institutional Review Board at Florida International University.
2.1. Assessments
Demographic data were self-reported during interviewer-assisted interviews. Height and weight were obtained by trained research assistants and used to calculate body mass index (BMI) (kg/m2). Fasting blood samples were drawn to obtain routine biomarkers, including high-sensitivity C-reactive protein (hs-CRP) and a liver function panel, among others (LabCorp, Burlington, NC, USA). HIV and HCV serology and viral testing, as well as CD4+ cell counts were abstracted from medical charts with participants’ signed releases. Viral suppression was defined as having an HIV RNA ≤ 50 copies/mL. HCV seropositive participants with detectable HCV viremia were considered to have an active infection; otherwise, they were considered cured or to have spontaneously cleared the infection.
2.1.1. Substance use
Substance use was assessed by questionnaire (use in the last 30 days) and urine drug screen. The Alcohol Use Disorders Identification Test–Consumption (AUDIT-C) was used to assess current alcohol misuse (scores ≥ 4 for men and ≥ 3 for women) (Bradley et al., 2007). Participants also reported how many drinks per week they consumed, on average, in the past 30 days. Urine drug screen was used to test for marijuana, cocaine, opiates (American Bio Medica, Kinderhook, NY, USA), and fentanyl (AllTest, Hangzhou, P.R. China).
2.1.2. Cocaine use
In addition to self-report and urine toxicology, cocaine users were identified by the presence of cocaine (COC) and its metabolites benzoylecgonine (BE), norcocaine (NC), m-hydrobenzoylecgonine (m-HOBE), and CE in blood. Plasma samples of all participants were analyzed using a modified liquid chromatography with tandem mass spectrometry (LC-MS/MS) method (Snozek et al., 2012). The compounds of interest were separated from endogenous interferences on a Agilent Zorbax Eclipse XDB-C8 column using an Agilent 1200 HPLC system (Agilent Technologies Inc., Santa Clara, CA, USA). The target analyte and internal standard were identified and quantified using a triple quadrupole mass spectrometer with a HESI-II probe (TSQ Quantum Ultra, Thermo Fisher Scientific Inc., San Jose, CA, USA).
Participants with ≥0.1 ng/mL of any of these metabolites in blood were considered cocaine users. Given these data, three mutually exclusive groups were formed: cocaine non-using comparisons (COC−), cocaine users who were negative for CE (COC+/CE−), and cocaine users with ≥0.1 ng/mL of CE in blood (COC+/CE+).
2.1.3. Liver fibrosis
Liver fibrosis was determined via the Fibrosis-4 Index (FIB-4) calculated from age, aspartate transaminase (AST), alanine transaminase (ALT) and platelet count (Sterling et al., 2006). A FIB-4 < 1.45 has a negative predictive value of 90% for advanced fibrosis (Ishak fibrosis score 4–6) with a sensitivity of 70%. At ≥3.25, it identifies advanced fibrosis with a sensitivity and specificity of 23% and 96.6%, respectively.
2.2. Statistical analysis
Descriptive statistics are reported as No. (percentage, %) and mean ± standard deviation (SD) or median (interquartile range, IQR). Between-group comparisons for categorical variables consisted of Chi-square tests. Between groups comparisons for normally distributed continuous variables consisted of Independent Samples T-tests and One-way Analysis of Variance (ANOVA) with Tukey post hoc multiple comparison. Non-parametric methods were used for data that violated the assumptions of normality, which included FIB-4 and cocaine metabolite concentrations in blood; these included Wilcoxon Rank-Sum and Kruskal-Wallis tests with the Dwass, Steel, Critchlow-Fligner method for post hoc pairwise comparisons. Binary logistic regressions were performed to calculate the odds ratios and 95% confidence intervals (CI) of having liver fibrosis. Multiple logistic regressions were employed to evaluate the relationship between cocaine use and liver fibrosis after controlling for sociodemographic characteristics (age, race/ethnicity, sex), BMI, hypertension (≥130 mm Hg systolic or ≥85 mm Hg diastolic blood pressure), hyperglycemia (fasting plasma glucose level ≥100 mg/dL), and hypertriglyceridemia (triglycerides ≥150 mg/dL), use of tobacco and alcohol, as well as HIV and HCV infections. Statistical significance was considered as a p-value<0.05 and two-sided p-values are reported unless otherwise specified. All statistical analyses were performed in SAS software, version 9.4, Copyright 2012 SAS Institute Inc.
3. Results
3.1. Participant characteristics
A total of 649 participants were included in this analysis. As seen in Table 1, participants were 53.6±8.3 years old on average, and mostly Black (61.8%) and male (61.3%). Nearly a third of participants were Hispanic (29.0%) and most (76.1%) lived below the federal poverty level. A total of 354 (54.6%) were living with HIV, 205 (31.6%) were HCV seropositive, with 93 (14.3%) who had active HCV and 39 (6.0%) who were actively co-infected with HIV/HCV. The vast majority of PLWH were receiving ART (98.9%) and were virally suppressed (70.9%) with a median CD4 cell count of 513 (332, 767) cells/μL. Over half (54.4%) of participants smoked cigarettes and 222 (34.2%) participants misused alcohol.
Table 1.
Participant characteristicsa
| Total | Cocaine non-user | Cocaine user | COC+/CE− | COC+/CE+ | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N=649 | N=368 (56.7%) | N=281 (43.3%) | P | N=203 (72.2%) | N=78 (27.8%) | P | |
| Age | 53.6 ± 8.3 | 54.4 ± 8.2 | 52.5 ± 8.4 | 0.005 | 52.3 ± 8.5 | 53.3 ± 8.1 | 0.372 |
| Race | |||||||
| Black | 401 (61.8%) | 189 (51.4%) | 212 (75.4%) | <0.0001 | 143 (70.4%) | 69 (88.5%) | - |
| White | 202 (31.1%) | 146 (39.7%) | 56 (19.9%) | 47 (23.2%) | 9 (11.5%) | ||
| Other | 46 (7.1%) | 33 (9.0%) | 13 (4.6%) | 13 (6.4%) | 0 | ||
| Hispanic ethnicity | 188 (29.0%) | 132 (35.9%) | 56 (19.9%) | <0.0001 | 47 (23.2%) | 9 (11.5%) | 0.029 |
| Sex | |||||||
| Female | 251 (38.7%) | 137 (37.2%) | 114 (40.6%) | 0.387 | 77 (37.9%) | 37 (47.4%) | 0.146 |
| Male | 398 (61.3%) | 231 (62.8%) | 167 (59.4%) | 126 (62.1%) | 41 (52.6%) | ||
| Education | |||||||
| Less than High-School | 256 (39.5%) | 138 (37.5%) | 118 (42.0%) | 0.049 | 87 (42.9%) | 31 (39.7%) | 0.199 |
| High-School or GED | 201 (31.0%) | 107 (29.1%) | 94 (33.5%) | 62 (30.5%) | 32 (41.0%) | ||
| More than High-School | 192 (29.6%) | 123 (33.4%) | 69 (24.6%) | 54 (26.6%) | 15 (19.2%) | ||
| Income (below poverty) Metabolic | 494 (76.1%) | 265 (72.0%) | 229 (81.5%) | 0.005 | 160 (78.8%) | 69 (88.5%) | 0.062 |
| BMI (kg/m2) | 29.3 ± 6.6 | 30.3 ± 6.5 | 27.9 ± 6.4 | <0.0001 | 27.8 ± 6.3 | 28.3 ± 6.7 | 0.605 |
| Obese | 259 (39.9%) | 172 (46.7%) | 87 (31.0%) | <0.0001 | 61 (30.1%) | 26 (33.3%) | 0.594 |
| Hypertension | 259 (55.3%) | 189 (51.4%) | 170 (60.5%) | 0.020 | 114 (56.2%) | 56 (71.8%) | 0.016 |
| Hyperglycemia | 141 (21.7%) | 81 (22.0%) | 60 (21.4%) | 0.840 | 46 (22.7%) | 14 (18.0%) | 0.388 |
| Hypertriglyceridemia | 176 (27.1%) | 108 (29.4%) | 68 (24.2%) | 0.144 | 47 (23.2%) | 21 (26.9%) | 0.509 |
| HIV | 354 (54.6%) | 216 (58.7%) | 138 (49.1%) | 0.015 | 105 (51.7%) | 33 (42.3%) | 0.157 |
| On ARTb | 350 (98.9%) | 213 (98.6%) | 137 (99.3%) | 104 (99.1%) | 33 (100%) | ||
| HIV RNA <50 copies/mLb | 251 (70.9%) | 165 (76.4%) | 86 (62.3%) | 0.005 | 66 (62.9%) | 20 (60.6%) | 0.816 |
| CD4+ cell countb | 513 (332, 767) | 544 (347, 792) | 474 (300, 721) | 0.130 | 504 (324, 812) | 433 (247, 582) | 0.042 |
| HCV | 205 (31.6%) | 114 (31.0%) | 91 (32.4%) | 0.703 | 65 (32.0%) | 26 (33.3%) | 0.833 |
| Cleared or cured | 112 (17.3%) | 70 (19.0%) | 42 (15.0%) | 0.085 | 32 (15.8%) | 10 (12.8%) | 0.629 |
| Viremic | 93 (14.3%) | 44 (12.0%) | 49 (17.4%) | 33 (16.3%) | 16 (20.5%) | ||
| HIV/HCV co-infected Substance use | 39 (6.0%) | 19 (5.2%) | 20 (7.1%) | 0.644 | 13 (6.4%) | 7 (9.0%) | 0.762 |
| Cigarette smoker | 353 (54.4%) | 162 (44.0%) | 191 (68.0%) | <0.0001 | 130 (64.0%) | 61 (78.2%) | 0.023 |
| Alcohol misuse | 222 (34.2%) | 95 (25.8%) | 127 (45.2%) | <0.0001 | 78 (38.4%) | 49 (62.8%) | 0.0002 |
| Binge drinking | 113 (17.4%) | 47 (12.8%) | 66 (23.5%) | 0.0004 | 40 (19.7%) | 26 (33.3%) | 0.0160 |
| Drinks per week | 0 (0, 3) | 0 (0, 2) | 1 (0, 8) | <0.0001 | 1 (0, 4) | 5 (1, 15) | <0.0001 |
| Marijuana | 205 (31.6%) | 75 (20.4%) | 46.4%) | <0.0001 | 95 (46.8%) | 35 (45.5%) | 0.841 |
| Hepatic | |||||||
| Alanine transaminase | 19 (14, 28) | 19 (15, 29) | 18 (13, 28) | 0.149 | 18 (13, 27) | 19 (13, 32) | 0.258 |
| Aspartate transaminase | 22 (17, 31) | 22 (18, 29) | 23 (17, 33) | 0.152 | 22 (16, 31) | 29 (19, 36) | 0.002 |
| Alkaline phosphatase | 79 (66, 96) | 79 (66, 96) | 80 (66, 94) | 0.961 | 78 (66, 93) | 84 (67, 106) | 0.133 |
| FIB-4 ≥1.45 | 186 (28.7%) | 92 (25.0%) | 94 (33.5%) | 0.018 | 58 (28.6%) | 36 (46.2%) | 0.005 |
Abbreviations: ART, antiretroviral therapy; COC+/CE−, cocaine users without cocaethylene in blood; COC+/CE+, cocaine users with cocaethylene in blood; FIB-4, Fibrosis 4 Index
Data reported as No. (%), mean ± standard deviation, or median (interquartile range). Between-group differences were tested with Chi-square for categorical outcomes and T-test (or non-parametric Wilcoxon Rank-Sum) for continuous outcomes.
Among HIV+ participants (n=353).
Other than the exclusion of people who use heroin/fentanyl (N=121), the characteristics of this sample are representative of the MASH cohort.
3.1.1. Characteristics of cocaine users
A total of 281 (43.3%) participants used cocaine as determined by self-report, urine, and/or plasma cocaine or metabolites. In total, 138 (21.3%) of participants self-reported use of cocaine, 233 (35.9%) were positive for cocaine in urine screen, and 336 (51.8%) were positive for cocaine via blood metabolites. Participants who self-reported cocaine use, as compared to those who denied cocaine use, were more likely to test positive for cocaine in urine (86.2% vs. 22.3%, respectively; p<0.0001) and to test positive for cocaine metabolites in blood (94.2% vs. 40.3%, respectively; p<0.0001). Compared to cocaine non-users, cocaine users were slightly younger, more likely to be Black but less likely to be Hispanic, had lower educational achievement, and a greater proportion lived in poverty. Cocaine users had lower BMIs and a lower prevalence of obesity, but a higher prevalence of hypertension. Cocaine users were less likely to be HIV-infected than cocaine non-users (p=0.015); yet, cocaine users were less likely to have a suppressed viral load (p=0.005). Cocaine users were also more likely to smoke cigarettes, misuse alcohol, and use marijuana than cocaine non-users. All data shown in Table 1.
3.1.2. Cocaine users with and without CE in blood
Out of 281 participants who used cocaine, 78 (27.8%) had detectable levels of CE in blood (≥0.1 ng/mL). The differences between cocaine users and non-users described above were accentuated in the COC+/CE+ group (Table 1). Among cocaine users, those in the COC+/CE+ group had significantly higher concentrations of COC, BE, NC, and m-HOBE in blood (data shown in Supplementary Table 1). Notably, participants in the COC+/CE+ group were more likely to report alcohol misuse and binge drinking, as well as consuming a greater number of drinks per week than those in the COC+/CE− group (Table 1). Use of marijuana did not differ between the COC+/CE+ and COC+/CE− groups.
Conversely, CE was found in 22.1% of participants who reported alcohol misuse compared to 6.8% of those who reported no alcohol misuse (p<0.0001). Compared to cocaine non-users, the COC+ and COC+/CE+ groups had 1.79 (95% CI: 1.24, 2.59; p=0.002) and 4.86 (95% CI: 2.90, 8.13; P<0.0001) times the odds of alcohol misuse, respectively.
3.1.3. Cocaine use and CE in people living with HIV or HCV
The prevalence of cocaine use was significantly lower for PLWH than HIV-uninfected participants (39.0% vs. 61.0%, respectively; p=0.015), but significantly higher among participants with active HCV infection than those without active HCV (52.7% vs. 41.7%, respectively; p=0.048). On the other hand, among cocaine users, neither HIV nor HCV status were associated with having CE in blood.
Among PLWH, cocaine users had lower rates of viral suppression than cocaine non-users, although use of ART and CD4+ cell counts did not differ (Table 1). Among PLWH who used cocaine, those with CE in blood had significantly lower CD4+ cell counts than those without CE in blood.
3.2. Liver fibrosis
One-hundred and eighty-six (28.7%) participants had a FIB-4 ≥ 1.45 and 23 (3.5%) had a FIB-4 ≥ 3.25. Due to the limited number of participants with FIB-4 ≥ 3.25, we used 1.45 as the cutoff for the presence of liver fibrosis.
3.2.1. Alcohol, cocaine, CE, and liver fibrosis
Alcohol misuse was not independently associated with liver fibrosis (OR=1.01, 95% CI: 0.71, 1.45; p=0.945), but the risk tended to increase by 7% with every 5 drinks per week that were consumed (p=0.077). Overall, cocaine users had greater odds of liver fibrosis than cocaine non-users (OR=1.51, 95% CI: 1.07, 2.12; p=0.019). As shown in Table 1, 33.5% of cocaine users and 25.0% of cocaine non-users had liver fibrosis (p=0.018). Yet, 46.2% of COC+/CE+ participants and 28.6% of COC+/CE− participants had liver fibrosis (p=0.005). The COC+/CE+ group had over two and a half times the odds of liver fibrosis compared to cocaine non-users (OR=2.57, 95% CI: 1.55, 4.26; p=0.0002).
3.2.2. Covariates and liver fibrosis
Univariate analyses were performed to determine whether and how other factors related to liver fibrosis (Supplementary Table 2). Older age, male sex, cigarette smoking, lower BMI, and hypertension were associated with increased risk for liver fibrosis, while being Hispanic was associated with reduced risk. HIV infection was independently associated with increased risk for liver fibrosis (OR=1.80, 95% CI: 1.26, 2.55; p=0.001). The highest risk of liver fibrosis was seen among participants actively infected with HCV (OR=5.16, 95% CI: 3.22, 8.25; p<0.0001), yet those that were cured or spontaneously cleared the infection were also at higher risk (OR=1.81, 95% CI: 1.22, 3.01; p=0.005). HIV/HCV co-infected participants had the highest risk of liver fibrosis compared to HIV/HCV uninfected participants (OR=10.74, 95% CI: 5.07, 22.74; p<0.0001).
3.2.3. CE and liver fibrosis, controlling for covariates
Multiple logistic regression was employed to evaluate the relationship between CE and liver fibrosis after controlling for potential confounders: age, race/ethnicity, sex, cigarette smoking, alcohol consumption, BMI, hypertension, hyperglycemia, hypertriglyceridemia, HIV, and HCV infections (Figure 1). After adjustment for covariates, COC+/CE+ was associated with 3.17 (95% CI: 1.61, 6.23; p=0.0008) times the odds of liver fibrosis as compared to cocaine non-users. In addition to COC+/CE+, older age, male sex, hypertension, HIV, and active HCV infection were significantly associated with increased risk of liver fibrosis after adjustment for covariates. BMI remained inversely associated with the risk of liver fibrosis.
Figure 1. Multiple logistic regression model: Odds of liver fibrosis (FIB-4 ≥ 1.45)a.
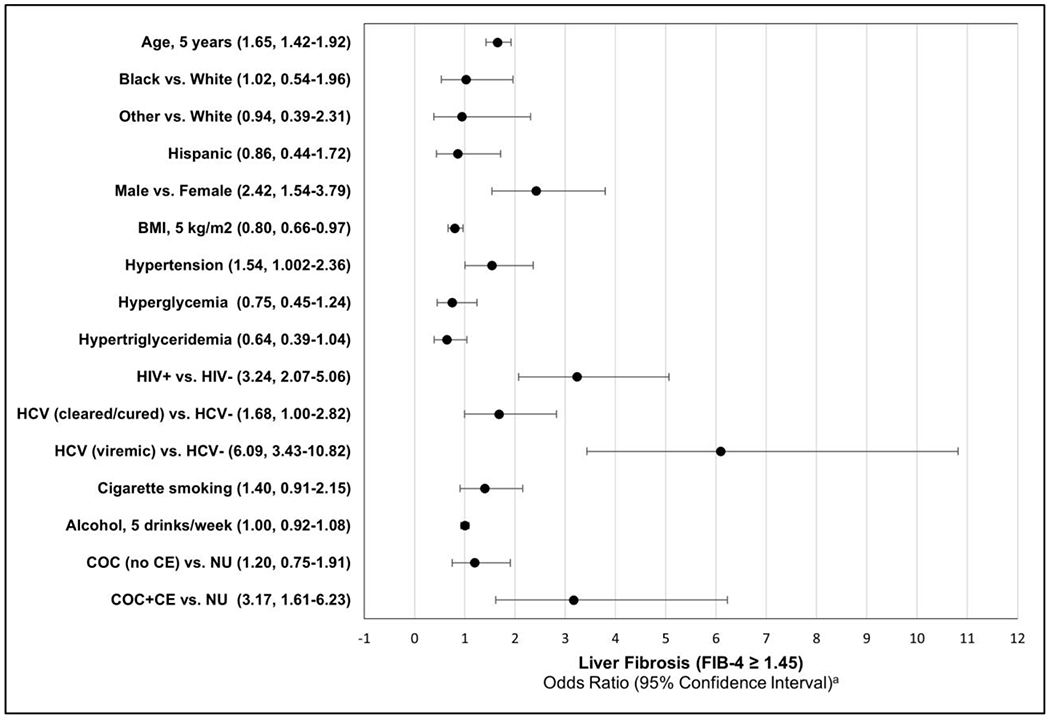
a A multiple logistic regression was performed to calculate odds ratios for liver fibrosis (FIB-4 ≥ 1.45). Odds ratios are adjusted for all of the factors shown.
Abbreviations: COC+/CE−, cocaine users without cocaethylene in blood; COC+/CE+, cocaine users with cocaethylene in blood; FIB-4, Fibrosis-4 Index
3.2.4. Liver fibrosis in PLWH
Since PLWH who used cocaine had lower rates of viral suppression and those with CE in blood had lower CD4+ cell counts (Table 1), we also assessed how cocaine and CE would impact PLWH adjusting for HIV-related covariates (Table 2). Univariate logistic regressions showed that having a non-suppressed viral load (≥50 copies/mL), lower CD4+ cell counts, increased alcohol consumption, and COC+/CE+ were significantly associated with increased odds of liver fibrosis. In multiple regression, additionally adjusting for ART use, viral suppression, and CD4+ counts, only COC+/CE+ continued to be significantly associated with liver fibrosis (OR=4.50, 95% CI: 1.59, 12.71; p=0.005).
Table 2.
Logistic regressions for liver fibrosis (FIB-4 ≥1.45) among people living with HIV (N=354).
| Unadjusted | Adjusteda | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR | 95% CI | Chi-Square | P | OR | 95% CI | Chi-Square | P | |
| ART | 1.55 | (0.16, 15.01) | 0.14 | 0.708 | 1.18 | (0.09, 16.47) | 0.02 | 0.902 |
| HIV RNA<50 copies/mL | 0.52 | (0.32, 0.83) | 7.40 | 0.007 | 0.60 | (0.32, 1.12) | 2.59 | 0.107 |
| CD4+ cell count, 100 copies/μL | 0.87 | (0.81, 0.94) | 13.65 | 0.0002 | 0.91 | (0.83, 1.01) | 3.21 | 0.073 |
| Drinks per week, 5 drinks | 1.17 | (1.02, 1.34) | 4.71 | 0.030 | 1.01 | (0.09, 1.12) | 0.02 | 0.880 |
| COC+/CE− | 1.30 | (0.79, 2.14) | 1.04 | 0.309 | 1.22 | (0.67, 2.24) | 0.39 | 0.533 |
| COC+/CE+ | 4.97 | (2.27, 10.85) | 16.16 | <0.0001 | 4.50 | (1.59, 12.71) | 8.04 | 0.005 |
Abbreviations: ART, antiretroviral therapy; COC+/CE−, cocaine users without cocaethylene in blood; COC+/CE+, cocaine users with cocaethylene in blood
Odds ratios are adjusted for age, race/ethnicity, sex, cigarette smoking, alcohol consumption, BMI, hypertension, hyperglycemia, hypertriglyceridemia, HCV, and all the factors shown.
4. Discussion
In this cross-sectional study, we assessed cocaine use, cocaethylene (CE) in blood—a marker of simultaneous cocaine and alcohol use—and their relationship with liver disease, a major cause of morbidity and mortality globally and particularly for PLWH. Cocaethylene in blood was associated with increased odds of liver fibrosis, a precursor to liver cirrhosis, hepatocellular carcinoma, and numerous extrahepatic complications. The effect of CE persisted after accounting for relevant covariates, including HIV and active HCV infections. Few studies have examined the relationship between cocaine use and liver disease. Yet, this may be particularly relevant for PLWH due to increased substance misuse and vulnerability to liver disease in this population. Our findings therefore provide valuable insights into how substance misuse may contribute to liver disease, particularly among PLWH. Screening for substance misuse in the clinical setting, including the use of CE as a reliable marker of simultaneous cocaine and alcohol use, may help identify individuals at risk of liver disease and aid in the prevention of its development or progression.
In vitro and animal models have demonstrated cocaine-induced hepatotoxicity (Duysen et al., 2008; Labib et al., 2001; Wang et al., 1991). However, evidence of these effects in humans has been limited. One study of HIV/HCV co-infected individuals did not find a significant relationship between the use of cocaine and liver fibrosis as measured by APRI (Martel-Laferriere et al., 2017). However, we have previously reported associations between cocaine use and liver fibrosis as measured by FIB-4 (Campa et al., 2016; Zarini et al., 2020), which has similar performance as APRI (Lemoine et al., 2019; Xiao et al., 2017, 2015; Xu et al., 2014). In this study, CE in blood was associated with increased odds of liver fibrosis as measured by FIB-4 after considering relevant covariates. Other observational studies support the case for cocaine-related hepatotoxicity in humans. In a case-control study of adult liver transplant recipients, graft survival was poorer among 72 recipients of livers from cocaine users than 126 controls whose donors were matched for age and had no history of drug use (Komokata et al., 2006). That said, donors who used cocaine were much more likely than controls to present with a serum ethanol concentration >50 mg/dL at hospitalization. Another study found an inverse association between ever snorting/injecting cocaine and liver fibrosis as measured by FibroScan among 298 individuals who used drugs (Foucher et al., 2009). However, the study was designed to assess the influence of FibroScan use on HCV screening and management, and adequate comparisons (drug non-users) were not included. Ever snorting/injecting cocaine was also associated with a lower prevalence of HCV. Thus, the findings are likely not generalizable.
At least with regards to liver disease, the effect of cocaine may not be entirely exclusive from that of alcohol. For one, among cocaine users, concurrent and simultaneous use with alcohol is exceedingly common (Liu et al., 2018), but also the combination of alcohol and cocaine may be more hepatotoxic than either substance alone. For example, in human hepatocytes, the presence of cocaine and alcohol, together, led to greater oxidative stress than cocaine, alcohol, or CE alone (Ponsoda et al., 1999). It is also possible that cocaine may heighten the hepatotoxicity of alcohol by altering the activity of cytochrome P-450 (Shimomura et al., 2019). Nevertheless, our findings directly implicate CE as an etiologic factor in liver fibrosis with greater hepatotoxicity than cocaine or alcohol alone (McCance-Katz et al., 1993; Ponsoda et al., 1999). In this study, cocaine users had one and a half times higher odds of liver fibrosis compared to cocaine non-users. Yet, the effect of simultaneous use of cocaine and alcohol, as measured by CE, largely explained that relationship. In comparison, cocaine users with CE in blood had over three times the odds of liver fibrosis as cocaine non-users, adjusted for covariates. All in all, our findings reflect CE-related hepatoxicity and/or the effect of concomitant consumption of cocaine and alcohol on the liver.
Prior research has indicated that the majority of people who use cocaine also use alcohol, either simultaneously or concurrently (Liu et al., 2018). Among the MASH cohort participants included in this study, 43% used cocaine; of those, 45% reported alcohol misuse and 28% had CE in blood. People who used cocaine and alcohol simultaneously, as evidenced by CE in blood (COC+/CE+), demonstrated greater alcohol misuse than both cocaine users without CE in blood (COC+/CE−) and cocaine non-users (COC−). Moreover, we found greater plasma concentrations of cocaine and its metabolites among COC+/CE+ than COC+/CE−. These findings are consistent with prior reports. Elevated blood levels of cocaine and norcocaine have been noted when cocaine was consumed with alcohol than when consumed alone (Farré et al., 1997, 1993; McCance-Katz et al., 1998, 1993; Perez-Reyes and Jeffcoat, 1992). Moreover, Gossop, et al. (2006) found that when alcohol and cocaine were used simultaneously, the amounts of alcohol and cocaine that were consumed were higher than when either was consumed alone. That said, this may only be the case among individuals who use powdered cocaine, as the opposite effect was seen among those who used crack-cocaine. CE concentrations in blood are also increased when cocaine is administered orally as compared to smoked (Herbst et al., 2011).
Blood concentrations of CE depend on the amount, order, and timing that cocaine and alcohol are consumed, as well as the route of cocaine administration (Herbst et al., 2011; Jones, 2019). The highest levels of CE are likely the result of consuming cocaine when blood alcohol levels are already high. Conversely, no matter how much alcohol is consumed, low levels of CE in blood would be expected if the concentration of cocaine in blood was low when alcohol was consumed. The available evidence suggests that individuals who use cocaine and alcohol simultaneously are most likely to consume cocaine after alcohol (Gossop et al., 2006a; Macdonald et al., 2015). Reasons for using cocaine after alcohol include a desire for a longer and more intense high, wanting to be more sociable, and increased alertness/reducing fatigue (Macdonald et al., 2015). Alternatively, reasons to consume alcohol after cocaine are mostly related to reducing withdrawal or undesired effects of cocaine (Macdonald et al., 2015). The use of alcohol after cocaine has been associated with cocaine dependence (hence, their desire to ameliorate the adverse effects of cocaine use) and may be more common among people who use crack-cocaine (Gossop et al., 2006a, 2006b; Macdonald et al., 2015).
There are several implications from this study’s findings. Cocaine use, due to its addictive nature and lack of effective treatments for long-term cessation, can become a persistent trigger of liver fibrogenesis. Chronic use of cocaine also extends its half-life in circulation, thereby increasing its toxicity (Jufer et al., 2000; Moolchan et al., 2000). In turn, as liver disease progresses, impaired liver function can delay the metabolism of drugs, such as cocaine, resulting in increased toxicity. Furthermore, these findings are highly relevant for PLWH, in whom the use of cocaine and alcohol are disproportionately prevalent compared to the general population (Shiau et al., 2017), and who have increased vulnerability and susceptibility to liver diseases (Campa et al., 2016; Rockstroh et al., 2014; Sherman et al., 2017). For example, alcohol has a greater impact on hepatic fibrosis in PLWH than HIV-uninfected peers (Lim et al., 2014). Whether the same is true for cocaine or other drugs remains to be understood. Additionally, substance misuse can interfere with ART, accelerate HIV disease progression (Desai et al., 2020), and contribute to the high burden of comorbidities that PLWH experience. Indeed, excessive alcohol consumption and concomitant alcohol-cocaine use are associated with increased HIV viral loads and lower CD4+ cell counts (Baum et al., 2010), which subsequently lead to greater fibrogenesis (Cooper et al., 2015; Maponga et al., 2018). In this study, cocaine use was associated with increased HIV viral loads and CE in blood was associated with lower CD4+ cell counts. Interactions of substances of abuse with ART may also contribute to liver fibrosis. Alcohol use, for example, is associated with greater liver fibrosis among those taking protease inhibitors (Bilal et al., 2016). Liver disease is a leading cause of morbidity and mortality in PLWH with a highly complex etiology (Campa et al., 2016; Rockstroh et al., 2014; Sherman et al., 2017). In an era when the incidence and prevalence of hepatitis infections are in decline, the role of substance misuse on liver disease among PLWH may become more considerable.
4.1. Strengths and limitations
The findings of this study should be interpreted in consideration of its strengths and limitations. The use of urine and blood tests provided objective measures of cocaine use; however, biologic samples can only determine recent use and cannot differentiate between acute and chronic use. Blood CE is a reliable biomarker of simultaneous alcohol and cocaine use, but an objective measure of alcohol consumption was not available. Future studies may consider the use of Phophatidylethanol (Peth) as a biomarker of alcohol consumption (Årving et al., 2021). Additionally, the FIB-4 does not measure liver fibrosis directly; rather, it is an index designed to assess the risk of liver fibrosis. On the other hand, it provides certain advantages such as being non-invasive, cost-effective, and easily applied in clinical settings. The characteristics of the MASH cohort should also be considered with regards to the generalizability of the findings. PLWH in the MASH cohort are not representative of the overall population of adults living with HIV, especially those that are undiagnosed, newly infected, or not receiving HIV care. PLWH in the MASH cohort have been living with HIV for an average of 16±9 years and display excellent rates of engagement with HIV care.
5. Conclusion
Cocaethylene, a metabolite produced from the simultaneous use of cocaine and alcohol, was associated with increased odds of liver fibrosis in Black and Hispanic people living with and without HIV and/or HCV. The association of CE with liver fibrosis remained significant after adjusting for relevant covariates including HIV and active HCV infection. The effect of CE on liver fibrosis was significantly greater than that of cocaine or alcohol alone. These findings are highly significant as most people who use cocaine also use alcohol, and the use of these substances is disproportionately prevalent in PLWH, who are especially vulnerable and susceptible to liver disease. Further research is needed to explore the independent and interactive effects of alcohol, cocaine, and CE. Cocaethylene is a reliable marker of simultaneous use of cocaine and alcohol that may help identify individuals at risk of liver disease and aid in the prevention of its development or progression.
Supplementary Material
Highlights.
In vitro and animal models have demonstrated cocaine-induced hepatotoxicity leading to liver fibrosis, but studies on human subjects are lacking.
Cocaethylene is a highly toxic metabolite of cocaine produced in the liver in the presence of alcohol; thus, it serves as a marker of simultaneous use of cocaine and alcohol.
Using the Fibrosis-4 Index to assess liver fibrosis, we show that the presence of cocaethylene in blood was associated with increased risk of liver fibrosis.
The effect of cocaethylene on liver fibrosis was greater than that of cocaine or alcohol alone.
Role of Funding Source:
This study was supported by the National Institute on Drug Abuse (NIDA) under Award Number U01-DA040381. The authors are solely responsible for the content of this article, which does not necessarily represent the official views of NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No conflicts of interest declared by any of the authors. Dr. RN Mandler, an employee of the National Institute on Drug Abuse, is an author and did review and approve the manuscript as a part of his authorship role as a Scientific Official.
References
- Årving A, Høiseth G, Hilberg T, Trydal T, Husa A, Djordjevic A, Kabashi S, Vindenes V, Bogstrand ST, 2021. Comparison of the Diagnostic Value of Phosphatidylethanol and Carbohydrate-Deficient Transferrin as Biomarkers of Alcohol Consumption. Alcoholism: Clinical and Experimental Research 45, 153–162. 10.1111/ACER.14503 [DOI] [PubMed] [Google Scholar]
- Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A, 2010. Alcohol use accelerates HIV disease progression. AIDS Research and Human Retroviruses 26, 511–518. 10.1089/aid.2009.0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MK, Tamargo JA, Ehman RL, Sherman KE, Chen J, Liu Q, Mandler RN, Teeman C, Martinez SS, Campa A, 2021. Heroin use is associated with liver fibrosis in the Miami Adult Studies on HIV (MASH) cohort. Drug and Alcohol Dependence 220, 108531. 10.1016/j.drugalcdep.2021.108531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal U, Lau B, Lazo M, McCaul ME, Hutton HE, Sulkowski MS, Moore RD, Chander G, 2016. Interaction Between Alcohol Consumption Patterns, Antiretroviral Therapy Type, and Liver Fibrosis in Persons Living with HIV. AIDS Patient Care and STDs 30, 200–207. 10.1089/apc.2016.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosh KA, Johnson AS, Hernandez AL, Prejean J, Taylor J, Wingard R, Valleroy LA, Hall HI, 2020. Vital Signs: Deaths Among Persons with Diagnosed HIV Infection, United States, 2010–2018. MMWR. Morbidity and Mortality Weekly Report 69, 1717–1724. 10.15585/mmwr.mm6946a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, Debenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR, 2007. AUDIT-C as a Brief Screen for Alcohol Misuse in Primary Care. Alcoholism: Clinical and Experimental Research 31, 1208–1217. 10.1111/J.1530-0277.2007.00403.X [DOI] [PubMed] [Google Scholar]
- Campa A, Martinez SS, Sherman KE, Greer JP, Li Y, Garcia S, Stewart T, Ibrahimou B, Williams OD, Baum MK, 2016. Cocaine Use and Liver Disease are Associated with All-Cause Mortality in the Miami Adult Studies in HIV (MASH) Cohort. J Drug Abuse 2. 10.21767/2471-853X.100036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carliner H, Mauro PM, Brown QL, Shmulewitz D, Rahim-Juwel R, Sarvet AL, Wall MM, Martins SS, Carliner G, Hasin DS, 2017. The widening gender gap in marijuana use prevalence in the U.S. during a period of economic change, 2002–2014, Drug and Alcohol Dependence. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. 10.1016/j.drugalcdep.2016.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheemerla S, Balakrishnan M, 2021. Global Epidemiology of Chronic Liver Disease. Clinical Liver Disease 17, 365–370. 10.1002/CLD.1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zheng X, Zhan M, Zhou Z, Zhan CG, Zheng F, 2016. Metabolic Enzymes of Cocaine Metabolite Benzoylecgonine. ACS Chem Biol 11, 2186–2194. 10.1021/acschembio.6b00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C, Rollet-Kurhajec KC, Young J, Vasquez C, Tyndall M, Gill J, Pick N, Walmsley S, Klein MB, Canadian Co-infection Cohort Study, I., 2015. HIV virological rebounds but not blips predict liver fibrosis progression in antiretroviral-treated HIV/hepatitis C virus-coinfected patients. HIV Med 16, 24–31. 10.1111/hiv.12168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N, Burns L, Gong Y, Zhi K, Kumar A, Summers N, Kumar S, Cory TJ, 2020. An update on drug-drug interactions between antiretroviral therapies and drugs of abuse in HIV systems. Expert Opin Drug Metab Toxicol 16, 1005–1018. 10.1080/17425255.2020.1814737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duko B, Ayalew M, Ayano G, 2019. The prevalence of alcohol use disorders among people living with HIV/AIDS: a systematic review and meta-analysis. Subst Abuse Treat Prev Policy 14, 52. 10.1186/s13011-019-0240-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duysen EG, Li B, Carlson M, Li YF, Wieseler S, Hinrichs SH, Lockridge O, 2008. Increased hepatotoxicity and cardiac fibrosis in cocaine-treated butyrylcholinesterase knockout mice. Basic Clin Pharmacol Toxicol 103, 514–521. 10.1111/j.1742-7843.2008.00259.x [DOI] [PubMed] [Google Scholar]
- Farré M, de La Torre R, González ML, Terán MT, Roset PN, Menoyo E, Camí J, 1997. Cocaine and alcohol interactions in humans: Neuroendocrine effects and cocaethylene metabolism. Journal of Pharmacology and Experimental Therapeutics 283, 164–176. [PubMed] [Google Scholar]
- Farré M, de la Torre R, Llorente M, Lamas X, Ugena B, Segura J, Camí J, 1993. Alcohol and cocaine interactions in humans. Journal of Pharmacology and Experimental Therapeutics 266, 1364. [PubMed] [Google Scholar]
- Foucher J, Reiller B, Jullien V, Leal F, di Cesare ES, Merrouche W, Delile JM, de Ledinghen V, 2009. FibroScan used in street-based outreach for drug users is useful for hepatitis C virus screening and management: a prospective study. J Viral Hepat 16, 121–131. 10.1111/j.1365-2893.2008.01050.x [DOI] [PubMed] [Google Scholar]
- Goldstein RA, DesLauriers C, Burda A, Johnson-Arbor K, 2009. Cocaine: history, social implications, and toxicity: a review. Semin Diagn Pathol 26, 10–17. 10.1053/j.semdp.2008.12.001 [DOI] [PubMed] [Google Scholar]
- Gossop M, Manning V, Ridge G, 2006a. Concurrent use and order of use of cocaine and alcohol: behavioural differences between users of crack cocaine and cocaine powder. Addiction 101, 1292–1298. 10.1111/j.1360-0443.2006.01497.x [DOI] [PubMed] [Google Scholar]
- Gossop M, Manning V, Ridge G, 2006b. Concurrent use of alcohol and cocaine: differences in patterns of use and problems among users of crack cocaine and cocaine powder. Alcohol Alcohol 41, 121–125. 10.1093/alcalc/agh260 [DOI] [PubMed] [Google Scholar]
- Hartzler B, Dombrowski JC, Crane HM, Eron JJ, Geng EH, Christopher Mathews W, Mayer KH, Moore RD, Mugavero MJ, Napravnik S, Rodriguez B, Donovan DM, 2016. Prevalence and Predictors of Substance Use Disorders Among HIV Care Enrollees in the United States. AIDS and Behavior 2016 21:4 21, 1138–1148. 10.1007/S10461-016-1584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst ED, Harris DS, Everhart ET, Mendelson J, Jacob P, Jones RT, 2011. Cocaethylene formation following ethanol and cocaine administration by different routes. Exp Clin Psychopharmacol 19, 95–104. 10.1037/a0022950 [DOI] [PubMed] [Google Scholar]
- Jones AW, 2019. Forensic Drug Profile: Cocaethylene. J Anal Toxicol 43, 155–160. 10.1093/jat/bkz007 [DOI] [PubMed] [Google Scholar]
- Jufer RA, Wstadik A, Walsh SL, Levine BS, Cone EJ, 2000. Elimination of cocaine and metabolites in plasma, saliva, and urine following repeated oral administration to human volunteers. J Anal Toxicol 24, 467–477. 10.1093/jat/24.7.467 [DOI] [PubMed] [Google Scholar]
- Kaspar MB, Sterling RK, 2017. Mechanisms of liver disease in patients infected with HIV. BMJ Open Gastroenterol 4, e000166. 10.1136/bmjgast-2017-000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbrich EA, Barnes AJ, Gorelick DA, Boyd SJ, Cone EJ, Huestis MA, 2006. Major and minor metabolites of cocaine in human plasma following controlled subcutaneous cocaine administration. J Anal Toxicol 30, 501–510. 10.1093/jat/30.8.501 [DOI] [PubMed] [Google Scholar]
- Komokata T, Nishida S, Ganz S, Levi DM, Fukumori T, Tzakis AG, 2006. The impact of donor cocaine use on the outcome of adult liver transplantation. Clin Transplant 20, 295–300. 10.1111/j.1399-0012.2006.00481.x [DOI] [PubMed] [Google Scholar]
- Labib R, Turkall R, Abdel-Rahman MS, 2001. Oral cocaine produces dose-related hepatotoxicity in male mice. Toxicol Lett 125, 29–37. 10.1016/s0378-4274(01)00412-x [DOI] [PubMed] [Google Scholar]
- Lemoine M, Assoumou L, de Wit S, Girard PM, Valantin MA, Katlama C, Necsoi C, Campa P, Huefner AD, Schulze Zur Wiesch J, Rougier EL, Bastard JP, Stocker H, Mauss S, Serfaty L, Ratziu V, Menu Y, Schlue J, Behrens G, Bedossa P, Capeau J, Ingiliz P, Costagliola D, 2019. Diagnostic Accuracy of Noninvasive Markers of Steatosis, NASH, and Liver Fibrosis in HIV-Monoinfected Individuals at Risk of Nonalcoholic Fatty Liver Disease (NAFLD): Results From the ECHAM Study. J Acquir Immune Defic Syndr 80, e86–e94. 10.1097/qai.0000000000001936 [DOI] [PubMed] [Google Scholar]
- Lim JK, Tate JP, Fultz SL, Goulet JL, Conigliaro J, Bryant KJ, Gordon AJ, Gibert, Rimland D, Goetz MB, Klein MB, Fiellin DA, Justice AC, lo Re V 3rd, 2014. Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus-infected, and uninfected patients. Clin Infect Dis 58, 1449–1458. 10.1093/cid/ciu097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Williamson V, Setlow B, Cottler LB, Knackstedt LA, 2018. The importance of considering polysubstance use: lessons from cocaine research. Drug and Alcohol Dependence 192, 16–28. 10.1016/j.drugalcdep.2018.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald S, mac Intyre P, Joordens C, Stockwell T, Martin G, 2015. Factors Related to Simultaneous Cocaine and Alcohol Use for Clients in Treatment. J Alcohol Drug Depend 03, 100–193. 10.4172/2329-6488.1000193 [DOI] [Google Scholar]
- Maponga TG, Andersson MI, van Rensburg CJ, Arends JE, Taljaard J, Preiser W, Glashoff RH, 2018. HBV and HIV viral load but not microbial translocation or immune activation are associated with liver fibrosis among patients in South Africa. BMC Infect Dis 18, 214. 10.1186/s12879-018-3115-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JL, Leyden WA, Alexeeff SE, Anderson AN, Hechter RC, Hu H, Lam JO, Towner WJ, Yuan Q, Horberg MA, Silverberg MJ, 2020. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000-2016. JAMA Netw Open 3, e207954. 10.1001/jamanetworkopen.2020.7954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel-Laferriere V, Nitulescu R, Cox J, Cooper C, Tyndall M, Rouleau D, Walmsley S, Wong L, Klein MB, Canadian Co-infection Cohort Study, I, 2017. Cocaine/crack use is not associated with fibrosis progression measured by AST-to-Platelet Ratio Index in HIV-HCV co-infected patients: a cohort study. BMC Infect Dis 17, 80. 10.1186/s12879-017-2196-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P, 1998. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either alone—A multiple-dose study. Biological Psychiatry 44, 250–259. 10.1016/S0006-3223(97)00426-5 [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Price LH, McDougle CJ, Kosten TR, Black JE, Jatlow PI, 1993. Concurrent cocaine-ethanol ingestion in humans: pharmacology, physiology, behavior, and the role of cocaethylene. Psychopharmacology (Berl) 111, 39–46. 10.1007/BF02257405 [DOI] [PubMed] [Google Scholar]
- Midanik LT, Tam TW, Weisner C, 2007. Concurrent and simultaneous drug and alcohol use: results of the 2000 National Alcohol Survey. Drug and alcohol dependence 90, 72–80. 10.1016/j.drugalcdep.2007.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolchan ET, Cone EJ, Wstadik A, Huestis MA, Preston KL, 2000. Cocaine and metabolite elimination patterns in chronic cocaine users during cessation: plasma and saliva analysis. J Anal Toxicol 24, 458–466. 10.1093/jat/24.7.458 [DOI] [PubMed] [Google Scholar]
- Pateria P, de Boer B, MacQuillan G, 2013. Liver abnormalities in drug and substance abusers. Best Pract Res Clin Gastroenterol 27, 577–596. 10.1016/j.bpg.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Perez-Reyes M, Jeffcoat AR, 1992. Ethanol/cocaine interaction: Cocaine and cocaethylene plasma concentrations and their relationship to subjective and cardiovascular effects. Life Sciences 51, 553–563. 10.1016/0024-3205(92)90224-D [DOI] [PubMed] [Google Scholar]
- Ponsoda X, Bort R, Jover R, Gomez-Lechon MJ, Castell J. v., 1999. Increased toxicity of cocaine on human hepatocytes induced by ethanol: role of GSH. Biochem Pharmacol 58, 1579–1585. 10.1016/s0006-2952(99)00249-x [DOI] [PubMed] [Google Scholar]
- Rockstroh JK, Mohr R, Behrens G, Spengler U, 2014. Liver fibrosis in HIV: which role does HIV itself, long-term drug toxicities and metabolic changes play? Curr Opin HIV AIDS 9, 365–370. 10.1097/COH.0000000000000064 [DOI] [PubMed] [Google Scholar]
- Sherman KE, Peters MG, Thomas D, 2017. Human immunodeficiency virus and liver disease: A comprehensive update. Hepatol Commun 1, 987–1001. 10.1002/hep4.1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman KE, Peters MG, Thomas DL, 2019. HIV and the liver. Top Antivir Med 27, 101–110. [PMC free article] [PubMed] [Google Scholar]
- Shiau S, Arpadi SM, Yin MT, Martins SS, 2017. Patterns of drug use and HIV infection among adults in a nationally representative sample. Addictive Behaviors 68, 39–44. 10.1016/j.addbeh.2017.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura ET, Jackson GF, Paul BD, 2019. Chapter 17 - Cocaine, Crack Cocaine, and Ethanol: A Deadly Mix, in: Dasgupta A (Ed.), Critical Issues in Alcohol and Drugs of Abuse Testing (Second Edition). Academic Press, pp. 215–224. 10.1016/B978-0-12-815607-0.00017-4 [DOI] [Google Scholar]
- Snozek CL, Bjergum MW, Langman LJ, 2012. Cocaine and metabolites by LC-MS/MS. Methods Mol Biol 902, 91–103. 10.1007/978-1-61779-934-1_8 [DOI] [PubMed] [Google Scholar]
- Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, M SS, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M, Investigators, A.C., 2006. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43, 1317–1325. 10.1002/hep.21178 [DOI] [PubMed] [Google Scholar]
- Valente MJ, Carvalho F, Bastos M, de Pinho PG, Carvalho M, 2012. Contribution of oxidative metabolism to cocaine-induced liver and kidney damage. Curr Med Chem 19, 5601–5606. 10.2174/092986712803988938 [DOI] [PubMed] [Google Scholar]
- Wang GJ, Som P, Oster ZH, Volkow ND, 1991. Evaluation of Cocaine-Induced Hepatotoxicity. Nuc Compact-European-American Communications in Nuclear Medicine 22, 72–75. [Google Scholar]
- Wang JF, Ren X, DeAngelis J, Min J, Zhang Y, Hampton TG, Amende I, Morgan JP, 2001. Differential patterns of cocaine-induced organ toxicity in murine heart versus liver. Exp Biol Med (Maywood) 226, 52–60. 10.1177/153537020122600108 [DOI] [PubMed] [Google Scholar]
- Xiao G, Yang J, Yan L, 2015. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology 61, 292–302. 10.1002/hep.27382 [DOI] [PubMed] [Google Scholar]
- Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G, 2017. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 66, 1486–1501. 10.1002/hep.29302 [DOI] [PubMed] [Google Scholar]
- Xu XY, Kong H, Song RX, Zhai YH, Wu XF, Ai WS, Liu HB, 2014. The effectiveness of noninvasive biomarkers to predict hepatitis B-related significant fibrosis and cirrhosis: a systematic review and meta-analysis of diagnostic test accuracy. PLoS One 9, e100182. 10.1371/journal.pone.0100182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarini G, Sales Martinez S, Campa A, Sherman K, Tamargo J, Hernandez Boyer J, Teeman C, Johnson A, Degarege A, Greer P, Liu Q, Huang Y, Mandler R, Choi D, Baum MK, 2020. Sex Differences, Cocaine Use, and Liver Fibrosis Among African Americans in the Miami Adult Studies on HIV Cohort. J Womens Health (Larchmt) 29, 1176–1183. 10.1089/jwh.2019.7954 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


