Abstract
Although Black American mothers and infants are at higher risk for morbidity and mortality than their White counterparts, the biological mechanisms underlying these phenomena remain largely unknown. To investigate the role that lifetime stressor exposure, perceived stressor severity, and systemic inflammatory markers might play, we studied how these factors were interrelated in 92 pregnant Black American women. We also compared inflammatory marker levels for women who did versus did not go on to give birth preterm. During the early third trimester, women completed the Stress and Adversity Inventory for Adults to assess the stressors they experienced over their lifetime. Women also provided blood samples for plasma interleukin (IL)-6, IL-8, IL-1β, and tumor necrosis factor (TNF)-α quantification. Preterm births were identified by medical record review. Controlling for relevant covariates, there were significant positive associations between average levels of both overall and acute perceived stressor severity and plasma IL-1β levels. Controlling for perceived stress at assessment and exposure to racial discrimination did not affect these results. Mediation models revealed that exposure to more chronic stressors was related to higher plasma IL-1β levels, as mediated by higher average levels of overall perceived stressor severity. Exposure to fewer acute stressors was related to higher plasma IL-1β levels, as mediated by higher average levels of acute perceived stressor severity. Finally, women who went on to give birth preterm had higher levels of plasma IL-6. These data thus highlight the potential importance of assessing and addressing lifetime stressor exposure among mothers before and during maternal-infant care.
Keywords: African Americans, Stress, Health Disparities, Minority Health, Obstetrics, Premature Birth, Disease
1. Introduction
Black American mothers are at greater risk for maternal morbidity and mortality than their White counterparts (Joseph et al., 2021; Thompson & Suter, 2020). Moreover, Black American infants are more than twice as likely to die as compared to White American infants (Ely & Driscoll, 2020; Thompson & Suter, 2020). Despite increasing acknowledgement that “race as biology is fiction, racism as a social problem is real,” (Smedley & Smedley, 2005) racial disparities in pregnancy outcomes have persisted and even widened. Indeed, racism cuts across structures, systems, and individual interactions, shaping the health of Black Americans (Boyd, Lindo, Weeks, & McLemore, 2020). For example, though largely cross-sectional, available data suggest that Black American women are exposed to more life stressors than White women and White and Black men, including racial and gender discrimination but also many other types of stressors (Assari, 2020; Gordon, Banegas, & Tucker-Seeley, 2020; Gur et al., 2020; Kim, Im, Liu, & Ulrich, 2020; White, Bell, Huang, & Williams, 2020). Black American women are also more likely to be exposed to chronic (i.e., persistent or repeated) stressors, which appear to degrade health more than acute stressors (Kim et al., 2020). Nevertheless, how stressors occurring over the lifetime affect clinically relevant biological processes among Black American women, particularly during pregnancy, remains a largely unanswered question of significant public importance.
The well-known allostatic load framework as well as emerging theories such as Social Safety Theory posit that biological wear and tear accumulates when environmental exposures are perceived as stressful or threatening and an individual’s biobehavioral stress responses are repeatedly activated (McEwen, 1998; McEwen & Seeman, 1999; McEwen, 2007; McEwen & Gianaros, 2010; Slavich, 2020). However, such models have not been fully explored. Comprehensive data documenting the effect of lifetime stressor exposures on health are scarce, and we know of no studies that have investigated associations between cumulative lifetime stressors, perceived stressor severity, and inflammatory biology in the context of pregnancy. Moreover, many of the most used stress measurement instruments are relatively imprecise (Simmons, Winsky, Zehr, & Gordon, 2021; Slavich, 2019). It is therefore not surprising that researchers continue to debate whether stress exerts direct effects on biological processes relevant to health, including immune parameters among expectant mothers (e.g., Coussons-Read et al., 2012; Finy & Christian, 2018; McCormack et al., 2021).
A link between lifetime stressor exposure and the immune system would be important to investigate considering that maternal inflammation is one of the most consistently reported biological correlates of pregnancy complications, including preterm birth (Black & Horowitz, 2018; Gomes et al., 2019). Further, whereas some data suggest that stressors affect maternal risk [e.g., by altering immune processes (reviewed by Christian, 2020)], clinicians remain ill equipped to mitigate this risk. Only expectant mothers diagnosed with depression or anxiety during pregnancy receive mental health support with any consistency (American College of Obstetricians and Gynecologists, 2018). Interestingly, though, even when perinatal mental health conditions are treated, risk for pregnancy complications often remain elevated (Snapper, Hart, Venkatesh, Kaimal, & Perlis, 2018; Venkatesh, Ferguson, Smith, Cantonwine, & McElrath, 2019). Such findings suggest that there could be an added benefit to addressing other effects of lifetime stressor exposure that go unrecognized by healthcare providers within the parameters of current clinical practice.
To address this issue, we examined associations among lifetime stressor exposure, lifetime perceived stressor severity, and systemic inflammatory markers during pregnancy among Black Americans, who experience a disproportionate burden of stressors. We also investigated possible differential effects of chronic versus acute stressors. Grounded in the Allostatic Load Framework (McEwen, 1998; McEwen & Seeman, 1999; McEwen, 2007; McEwen & Gianaros, 2010) and informed by Social Safety Theory (Slavich, 2020), we also tested whether associations among stressor exposures and systemic inflammatory markers were mediated by levels of perceived stressor severity, controlling for pertinent covariates (Figure 1). Finally, we compared levels of prenatal inflammatory markers for women who did versus did not go on to give birth preterm.
Figure 1. Pathways linking lifetime stressor exposure to systemic inflammatory markers as mediated by lifetime perceived stressor severity.
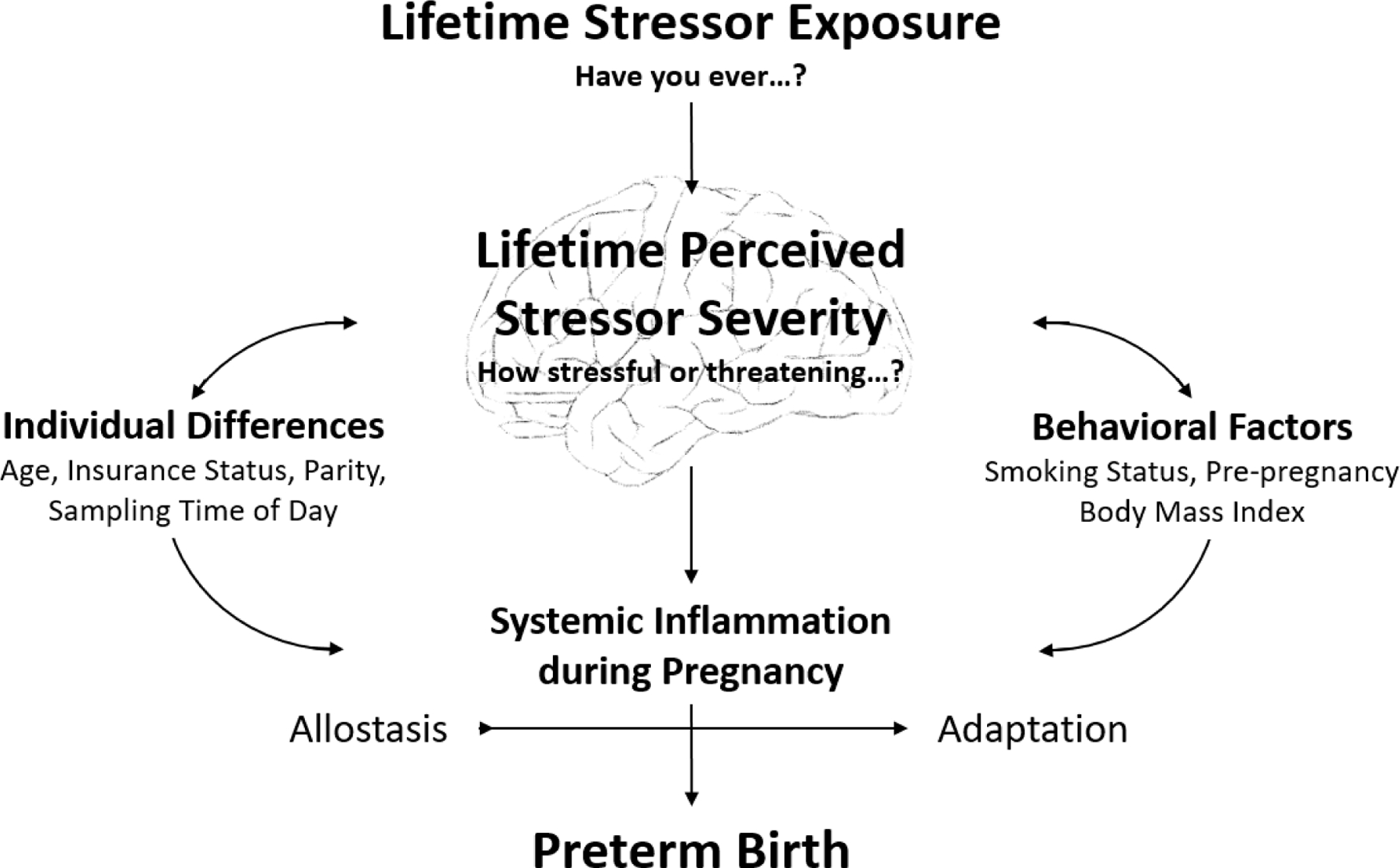
We propose a framework for empirical testing positing that lifetime stressor exposure affects biology relevant to health as mediated by individuals’ perceptions of how stressful or threatening a stressor is perceived. In testing this model in the present study, we focused on systemic inflammatory markers that were assessed during pregnancy and the occurrence of preterm birth, while controlling for relevant covariates.
Based on the literature summarized above, we hypothesized that greater lifetime stressor exposure and greater perceived stressor severity would be associated with higher levels of systemic inflammatory markers. In addition, we hypothesized that these associations would be strongest for chronic stressors, and, moreover, that perceived stressor severity would mediate the link between stressor exposure and levels of systemic inflammation. Finally, we hypothesized that women who went on to give birth preterm would exhibit higher levels of systemic inflammatory markers than those who did not. We tested these hypotheses in a unique observational cohort study of Black American women who were enrolled early in their third trimester of pregnancy and prospectively followed to enable post-birth medical record review.
2. Method
2.1. Participants
This study was conducted from 2013–2015 using a convenience sample of women recruited from a populous Midwestern city during prenatal care. Participants were recruited using direct solicitation by a member of the research team, waiting area flyers, examination room flyers, flyers posted in community locations (e.g., daycares, community events), and through electronic recruitment messages (e.g., ResearchMatch.org, StudySearch, social media). Since a primary goal of this research was to address disparities in pregnancy complications witnessed among Black Americans, participants had to self-report as Black, non-Hispanic, and U.S.-born and raised. Inclusion criteria also included a current singleton pregnancy, completion of dating and anatomy ultrasounds, maternal age 18–34, pre-pregnancy body mass index (BMI) 18.5–39, and self-reported non-smoking status or smoking cessation by the second trimester. At the time of recruitment, women who reported receiving a diagnosis of a chronic immune-related condition (e.g., chronic hypertension) or major complication of pregnancy (e.g., gestational diabetes, a gestational hypertensive disorder) during the assessed pregnancy were not eligible to enroll. However, several women were diagnosed with complications after enrollment and were retained in the sample. Women regularly taking medications with immune implications (e.g., corticosteroids) or self-reporting alcohol or illicit drug use after the first trimester were excluded.
Of the 96 pregnant women enrolled in this prospective cohort study, all had complete lifetime stressor and systemic inflammatory marker data except for three participants. One additional participant was lost to follow up prior to the completion of pregnancy. This left a final analytical sample of 92 participants. Due to the study design and enrollment criteria, all participants were pregnant with one baby [M = 30 weeks, 3 days (SD = 10.27 days) gestation at enrollment and data collection]. All participants (100%) self-reported as Black, non-Hispanic, and U.S.-born. As shown in Table 1, women were primarily in their twenties (68.48%), employed at least part time (73.91%), and did not hold private insurance (67.39%). Some women reported smoking in early pregnancy but quitting by the second trimester (23.91%), with all other women self-reporting as non-smokers. Mean values for pre-pregnancy BMI are also shown in Table 1, with 64.13% of women exhibiting a BMI in the overweight (BMI = 25–29) or obese (BMI > 30) category. Most participants did not experience a major complication of pregnancy [e.g., gestational diabetes (0%), gestational hypertension (9.78%), preeclampsia (3.26%), and preterm birth (7.61%)].
Table 1.
Participant Characteristics (N = 92)
| M ± SD | Count (%) | |
|---|---|---|
| Maternal age | 26.36 ± 4.47 | |
| Married (yes) | 22 (23.91%) | |
| Bachelor’s degree or greater (yes) | 24 (26.09%) | |
| Employed (yes) | 68 (73.91%) | |
| Private insurance (yes) | 30 (32.61%) | |
| Non-smoker (yes) | 70 (76.09%) | |
| Pre-pregnancy body mass index | 28.32 ± 5.71 | |
| Nulliparity (yes) | 29 (31.52%) | |
| Gestational diabetes (yes) | 0 (0%) | |
| Gestational hypertension (yes) | 9 (9.78%) | |
| Preeclampsia (yes) | 3 (3.26%) | |
| Preterm birth (yes) | 7 (7.61%) | |
2.2. Data Collection
Participants completed a study visit between 28 weeks 0 days and 32 weeks 6 days of pregnancy (i.e., the early third trimester), at which time the informed consent process was completed and demographic, behavioral, clinical, and stressor-related data were collected by self-report and entered using direct electronic data capture. At the end of the study visit, whole blood was collected by antecubital or distal venipuncture standardized to the hours of 1100–1600 to reduce potential for confounding by diurnal variation (Nilsonne, Lekander, Akerstedt, Axelsson, & Ingre, 2016). Sampling time of day was recorded and used as a covariate. If a participant reported a cold- or flu-like illness, antibiotic use, or vaccination, the study visit was rescheduled for at least seven days later. Oral temperature was also measured, with no participants showing signs of fever. The protocol for the study was approved by The Ohio State University Biomedical and OhioHealth Institutional Review Boards. Participants received $50 in gift cards at the study visit.
2.3. Measures
2.3.1. Demographic, behavioral, and clinical characteristics
Demographic (e.g., age, marital status, education, employment status, insurance status), behavioral (e.g., smoking status), and clinical (e.g., pre-pregnancy weight, parity) characteristics were determined by self-report using standardized instruments. Height was also measured to enable calculation of pre-pregnancy BMI. Complications of pregnancy were determined by manual abstraction of data from a detailed review of prenatal, labor and delivery, and newborn medical records after the birth of the baby, including all provider notes.
2.3.2. Lifetime stressor exposure and lifetime perceived stressor severity
Lifetime stressor exposure and lifetime perceived stressor severity were assessed using the Stress and Adversity Inventory for Adults (Adult STRAIN) (Slavich & Shields, 2018). The Adult STRAIN is a National Institute of Mental Health/Research Domain Criteria Initiative-recommended electronic instrument developed as an efficient and reliable method for assessing exposure to acute and chronic stressors occurring over the entire lifespan (https://www.strainsetup.com). Unlike investigator-based methods that require several hours for interviewing participants and conducting expert panel consensus ratings, the Adult STRAIN uses extensive intelligent logic to assess stressors experienced from the earliest memories of childhood up to the date of assessment, with an average administration time of approximately 18 minutes. The Adult STRAIN shows great concurrent, discriminant, and predictive validity, and excellent test-retest reliability of up to 0.953 over one month, including in primarily female samples (Cazassa, Oliveira, Spahr, Shields, & Slavich, 2020; Dooley, Slavich, Moreno, & Bower, 2017; Shields, Moons, & Slavich, 2017; Slavich & Shields, 2018).
2.3.2.1. Stressor exposure versus perceived stressor severity.
The Adult STRAIN (Slavich & Shields, 2018) systematically assesses participants’ exposures to 55 different moderate-to-severe stressors that are known to substantially affect health. Follow-up questions assess each reported stressors’ frequency, timing, duration, and perceived severity. The sum of lifetime stressors endorsed was used for analysis, with higher scores indicating greater lifetime stressor exposure (possible range = 0–166, given how frequency is calculated). Participants’ perception of the severity of each stressor experienced was also assessed using a 0 (very slightly or not at all) to 5 (extremely) scale (possible range = 0–265). The sum of perceived stressor severity scores (i.e., “total severity”) and average of perceived stressor severity scores (i.e., “average severity”) were used for analysis, with higher scores indicating greater lifetime perceived stressor severity.
2.3.2.2. Chronic versus acute stressor exposure.
The Adult STRAIN systematically probes for two main types of lifetime stressor exposure: chronic difficulties and acute life events. Chronic difficulties are persistent stressors that include situations such as prolonged housing, financial, or marital problems that typically last for at least one month but are frequently present for several months or years. In contrast, acute life events are episodic in nature and include situations such as receiving bad news or getting into an accident. Twenty-nine questions assess chronic difficulties and 26 questions assess acute life events. Lifetime stressor exposures and lifetime perceived stressor severity variables were created for all exposures (i.e., “overall”), for chronic difficulties (i.e., “chronic”), and for acute life events (i.e., “acute”).
2.3.3. Perceived stress at the time of assessment
Perceived stress levels at the time of assessment were quantified using the Perceived Stress Scale (PSS)-14 and used as a covariate (Cohen, Kessler, & Underwood Gordon, 1995). The PSS-14 asks participants to indicate if, over the past month, they have never, almost never, sometimes, fairly often, or very often had each of a possible 14 experiences (e.g., felt difficulties were piling up so high that you could not overcome them). Items are scored from 0–4, with reverse scoring applied as appropriate. Higher scores indicate greater perceived stress over the past month, which can range from 0–56. The PSS has been widely validated, including during pregnancy (Bann et al., 2017; Zhang et al., 2019). The PSS has an internal consistency of 0.88–0.89 and intraclass correlation of 0.60 across pregnancy (Bann et al., 2017). Higher PSS scores have been related to more perinatal depressive symptoms and poorer perinatal health (Zhang et al., 2019).
2.3.4. Racial discrimination
Exposures to racial discrimination was quantified using the Experiences of Discrimination Scale (EOD) and used as a covariate (Krieger, 1990; Krieger & Sidney, 1996). The EOD asks participants to indicate if, over their lifetime, they have ever experienced discrimination based on their race, ethnicity, or color across nine assessed situations (e.g., at work, getting medical care). If a situation is endorsed, participants are asked to indicate whether the racial discrimination has occurred once, 2–3 times, or 4 or more times. Frequency scores can be calculated, with higher scores indicating greater discrimination exposure. The EOD has an internal consistency of 0.86 and test-retest reliability of 0.70 (Krieger, Smith, Naishadham, Hartman, & Barbeau, 2005). Higher EOD scores have been related to more depressive symptoms among Black American women of childbearing age (Millender et al., 2021).
2.3.5. Systemic inflammation during pregnancy
During the early third trimester study visit, venous whole blood was collected into heparinized vacutainers, placed on ice, and immediately transported to the laboratory for processing. Samples were centrifuged at 1200g at 15°C for 10 minutes. Heparinized plasma was aspirated and stored in aliquots at −80°C until thawed in batches. All plasma cytokine values for this report were determined following first thaw. Plasma levels of the pro-inflammatory cytokines interleukin (IL)-6, IL-8, IL-1β, and tumor necrosis factor (TNF)-α were determined in duplicate as pg/mL using a multi-spot MSD 4-plex immunoassay and the Sector Imager 2400 per manufacturer instructions (Meso Scale Discovery, Gaithersburg, MD). Briefly, this assay uses electrochemiluminescence to capture and quantify levels of multiple proteins simultaneously. Calculated intra-assay coefficients of variation were 7.1%, 4.0%, 15.5%, and 3.9% for IL-6, IL-8, IL-1β, and TNF-α, respectively. Calculated inter-assay coefficients of variation averaged across low and mid-calibration curve values, which most closely approximated sample values, were 5.1%, 4.8%, 2.7%, and 9.2% for IL-6, IL-8, IL-1β, and TNF-α, respectively. Since all plasma cytokine distributions were positively skewed, log transformations were applied to improve the distribution of error terms when plasma cytokines served as dependent variables.
2.3.6. Preterm birth
Cases of preterm birth were identified by manual abstraction of data derived from a detailed review of prenatal and labor and delivery records after the birth of the baby, including all healthcare provider notes. Preterm birth was defined as a birth before 37 weeks 0 days of pregnancy based on the obstetric estimate of due date and actual date of birth. All participants completed a dating ultrasound before 15 weeks of pregnancy, which confirmed or set the estimated due date.
2.4. Data analysis
Participants’ demographic, behavioral, and clinical characteristics were described according to mean and standard deviation or count and frequency as appropriate. Descriptive statistics were generated for the primary variables of interest, and distributions and Spearman rank-order associations were examined.
Next, multivariate multivariable linear regression models were built to examine associations among lifetime stressor exposure, lifetime total and average perceived stressor severity, and log-transformed plasma levels of the key cytokines IL-6, IL-8, IL-1β, and TNF-α. Subscale analyses were then conducted by repeating the above series of analyses with stressor exposure and perceived stressor severity partitioned into chronic versus acute. To address issues of multiple comparisons when testing these planned, a priori hypotheses, we conducted joint significance testing that investigated associations with all four cytokines simultaneously. To examine whether associations between perceived severity of stressors encountered over the lifetime and levels of systemic inflammatory markers were driven by perceived stress levels at the time of assessment or exposures to racial discrimination, significant models were repeated while adjusting for participants’ PSS-14 and EOD scores.
Based on the associations observed between lifetime stressor exposure, perceived stressor severity, and plasma cytokine levels in the above-described models, potential mediational pathways linking lifetime stressor exposure to plasma cytokine levels through lifetime perceived stressor severity were tested using the PROCESS macro developed by Hayes (2013). Briefly, this macro estimates the effect of a predictor on an outcome without considering a proposed mediator (i.e., total effect), controlling for a proposed mediator (i.e., direct effect), and through a proposed mediator (i.e., indirect effect). Statistical inference for testing of the indirect effect involves constructing 10,000 bias-corrected bootstrap confidence intervals, with replacement. Finally, multivariable linear regression models were built to examine if log-transformed plasma IL-6, IL-8, IL-1β, or TNF-α levels differed for women who did versus did not go on to give birth preterm.
STATA 15.0 (College Station, TX) was used for the primary analyses. SPSS 27.0 (New York, NY) was used for testing mediation. Significance was set to α = 0.05 for all tests. Post-estimation diagnostics were performed to review assumption satisfaction. Based on data from our laboratory and published studies (Gillespie, Mitchell, Kowalsky, & Christian, 2018; Lam, Chiang, Chen, & Miller, 2021; Nilsonne et al., 2016), we also considered maternal age, socioeconomic status (operationalized as insurance status), smoking status, pre-pregnancy BMI, parity, and sampling time of day as potential confounders and held these covariates constant in all models.
3. Results
3.1. Descriptive statistics and bivariate associations
Spearman rank-order associations among lifetime stressor exposures, lifetime total perceived stressor severity, lifetime average perceived stressor severity, and plasma pro-inflammatory cytokine levels during the early third trimester of pregnancy are shown in Table 2. Median and interquartile ranges for each variable are also presented. Median values and interquartile ranges for plasma cytokine levels among women falling in the low, mid-, and high tertiles for average perceived stressor severity are also shown in Supplemental Table 1.
Table 2.
Associations among lifetime stressor exposure, perceived stressor severity, and systemic inflammatory markers (N = 92)
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Overall stressor exposure | 1.00 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 2. Overall total perceived stressor severity | 0.88* | 1.00 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 3. Overall average perceived stressor severity | 0.18 | 0.59* | 1.00 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 4. Chronic stressor exposure | 0.83* | 0.82* | 0.31* | 1.00 | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 5. Chronic total perceived stressor severity | 0.82* | 0.94* | 0.57* | 0.89* | 1.00 | -- | -- | -- | -- | -- | -- | -- | -- |
| 6. Chronic average perceived stressor severity | 0.40* | 0.63* | 0.71* | 0.30* | 0.66* | 1.00 | -- | -- | -- | -- | -- | -- | -- |
| 7. Acute stressor exposure | 0.88* | 0.72* | 0.05 | 0.51* | 0.58* | 0.39* | 1.00 | -- | -- | -- | -- | -- | -- |
| 8. Acute total perceived stressor severity | 0.79* | 0.88* | 0.49* | 0.57* | 0.68* | 0.47* | 0.79* | 1.00 | -- | -- | -- | -- | |
| 9. Acute average perceived stressor severity | −0.15 | 0.21* | 0.76* | 0.12 | 0.16 | 0.17 | −0.31* | 0.25* | 1.00 | -- | -- | -- | -- |
| 10. Plasma interleukin-6 | 0.08 | 0.06 | 0.02 | 0.01 | 0.05 | 0.12 | 0.13 | 0.06 | −0.08 | 1.00 | -- | -- | -- |
| 11. Plasma interleukin-8 | −0.03 | 0.07 | 0.20 | −0.03 | 0.01 | 0.09 | −0.06 | 0.10 | 0.25* | 0.02 | 1.00 | -- | -- |
| 12. Plasma interleukin-1β | 0.01 | 0.10 | 0.26* | 0.10 | 0.06 | 0.02 | −0.10 | 0.09 | 0.33* | 0.08 | 0.20 | 1.00 | -- |
| 13. Plasma tumor necrosis factor-α | −0.05 | −0.03 | 0.02 | −0.10 | −0.07 | 0.10 | 0.01 | 0.01 | 0.02 | 0.27* | 0.23* | 0.18 | 1.00 |
| Median | 21.0 | 47.5 | 2.3 | 9.0 | 21.0 | 2.5 | 11.0 | 24.0 | 2.1 | 0.72 | 2.93 | 0.07 | 1.56 |
| 25th Percentile | 13.0 | 27.5 | 1.9 | 5.0 | 9.0 | 1.7 | 7.5 | 15.0 | 1.6 | 0.51 | 2.25 | 0.06 | 1.43 |
| 75th Percentile | 26.0 | 71.0 | 2.8 | 13.5 | 38.0 | 3.2 | 15.0 | 34.0 | 2.9 | 1.02 | 3.09 | 0.10 | 1.78 |
Notes: Results are presented as Spearman’s ρ for rank-order associations. Log-transformed plasma cytokine levels were used in analyses but raw values (in pg/mL) are presented here for descriptive purposes.
p < 0.05
3.2. Lifetime stressor exposure and lifetime perceived stressor severity
As would be expected, the covariate-adjusted regression models revealed that experiencing more overall, chronic, and acute stressors was associated with higher total overall, chronic, and acute perceived stressor severity (ps ≤ 0.001). Interestingly, the covariate-adjusted regression model did not reveal an association between experiencing more overall stressors and participants’ average levels of overall perceived stressor severity [Coef. = 0.008, SE = 0.006, β = 0.135, t(84) = 1.24, p = 0.219]. However, greater chronic stressor exposure was related to experiencing greater average levels of overall perceived stressor severity [Coef. = 0.047, SE = 0.018, β = 0.267, t(84) = 2.65, p = 0.010] and more acute stressor exposure was related to experiencing lower average levels of acute perceived stressor severity [Coef. = −0.033, SE = 0.012, β = −0.292, t(84) = −2.67, p = 0.009].
3.3. Lifetime stressor exposure, lifetime perceived stressor severity, and systemic inflammation during pregnancy
Covariate-adjusted multivariate multivariable regression models did not reveal evidence of associations among overall stressor exposure nor total overall perceived stressor severity and plasma levels of IL-6, IL-8, IL-1β, or TNF-α during pregnancy (ps ≥ 0.207). Likewise, associations were not significant when the effects of chronic and acute stressors were analyzed separately (ps ≥ 0.133).
In contrast, joint tests of significance for covariate-adjusted multivariate regression models revealed that average levels of overall and acute perceived stressor severity were related to plasma cytokine levels [F(4,83) = 2.5, p = 0.049 and F(4, 83) = 3.28, p = 0.015, respectively]. Specifically, there was a significant association between greater average levels of overall perceived stressor severity and higher log-transformed plasma IL-1β levels [Coef. = 0.168, SE = 0.071, β = 0.238, t(84) = 2.34, p = 0.022]. Subscale analyses revealed that this association was significant for average levels of acute perceived stressor severity [Coef. = 0.139, SE = 0.061, β = 0.230, t(84) = 2.26, p = 0.026] but not average levels of chronic perceived stressor severity [Coef. = 0.050, SE = 0.052, β = 0.103, t(84) = 0.97, p = 0.336]. Subscale analyses also revealed an association between greater average levels of acute perceived stressor severity and higher log-transformed plasma IL-8 levels [Coef. = 0.117, SE = 0.056, β = 0.218, t(84) = 2.09, p = 0.040].
Given that average levels of perceived stressor severity (but not stressor count nor total perceived stressor severity) were associated with plasma IL-1β and IL-8 levels, it is possible that higher average levels of perceived stressor severity may simply be a reflection of experiencing more perceived stress at the time of assessment. Therefore, we repeated these analyses while controlling for participants’ PSS-14 scores. However, this did not affect the results: higher average levels of overall perceived stressor severity continued to be related to higher plasma IL-1β levels [Coef. = 0.159, SE = 0.075, β = 0.226, t(83) = 2.14, p = 0.036]; likewise, higher average levels of acute perceived stressor severity continued to predict higher plasma IL-1β [Coef. = 0.141, SE = 0.061, β = 0.234, t(83) = 2.30, p = 0.024] and IL-8 [Coef. = 0.118, SE = 0.056, β = 0.221, t(83) = 2.11, p = 0.038] levels. Moreover, for each of these models, unlike scores from the Adult STRAIN, PSS-14 scores were not significantly related to participants’ plasma pro-inflammatory cytokine levels (ps ≥ 0.109).
In addition, we investigated if average levels of perceived stressor severity over the lifetime were associated with plasma IL-1β and IL-8 levels over and above associations that may be explained by experiencing racial discrimination. To accomplish this, we repeated the above-described analyses while controlling for participants’ EOD scores. However, this did not affect the results: higher average levels of overall perceived stressor severity continued to be related to higher plasma IL-1β levels [Coef. = 0.180, SE = 0.072, β = 0.254, t(83) = 2.50, p = 0.014]; likewise, higher average levels of acute perceived stressor severity continued to be related to higher plasma IL-1β [Coef. = 0.136, SE = 0.062, β = 0.225, t(83) = 2.20, p = 0.030] and IL-8 [Coef. = 0.121, SE = 0.056, β = 0.227, t(83) = 2.17, p = 0.033] levels. Similar to the PSS-14, unlike the Adult STRAIN, EOD scores were not significantly related to participants’ plasma pro-inflammatory cytokine levels in each of these models (ps ≥ 0.193).
3.4. Indirect effects of lifetime stressor exposure on systemic inflammation during pregnancy as mediated by average levels of lifetime perceived stressor severity
The above analyses revealed associations among experiencing more chronic stressors and higher average levels of overall perceived stressor severity and, in turn, higher average levels of overall perceived stressor severity and greater plasma IL-1β levels. Because of these findings, we examined the indirect effect of chronic stressor exposure on plasma IL-1β levels as mediated by average levels of overall perceived stressor severity. As shown in Figure 2, covariate-adjusted tests of mediation revealed an indirect effect of experiencing more chronic stressors on greater plasma IL-1β levels as mediated by higher average levels of overall perceived stressor severity (indirect effect: ab = 0.002, 95% CI 0.002, 0.005). Covariate-adjusted tests of mediation did not reveal an association between chronic stressors and plasma IL-1β levels without considering the proposed mediator [total effect: c = 0.005, t(85) = 1.24, p = 0.217] and controlling for the proposed mediator [direct effect: c’ = 0.003, t(84) = 0.76, p = 0.449].
Figure 2. Indirect effect of lifetime chronic stressor exposure on plasma IL-1β levels as mediated by average levels of lifetime overall perceived stressor severity.
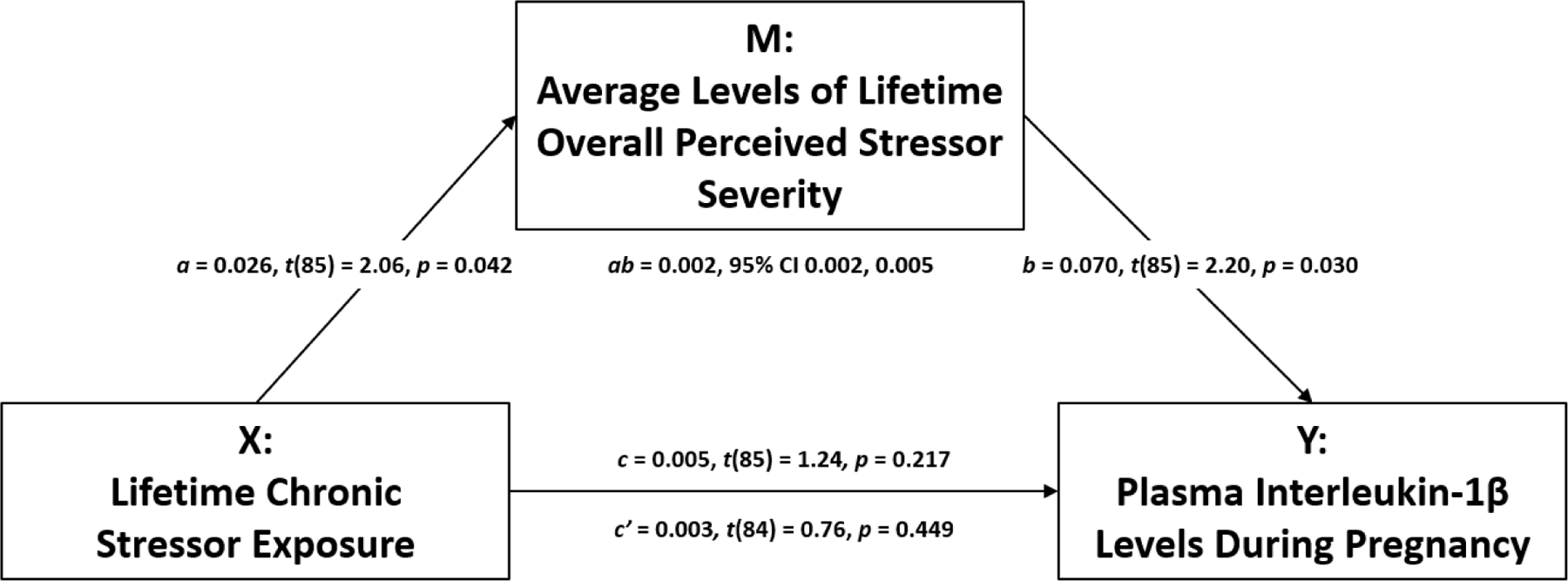
Experiencing more chronic stressors was related to higher average levels of overall perceived stressor severity (a). In addition, higher average levels of overall perceived stressor severity were associated with higher plasma IL-1β levels (b). Finally, exposure to more chronic stressors was related to higher plasma IL-1β levels, as mediated by higher average levels of overall perceived stressor severity (ab), controlling for relevant covariates.
The above analyses also revealed associations between experiencing fewer acute stressors and higher average levels of acute perceived stressor severity and, in turn, higher average levels of acute perceived stressor severity and greater plasma IL-1β and IL-8 levels. Therefore, we examined the indirect effects of acute stressor exposure on plasma IL-1β and IL-8 levels as mediated by average levels of acute perceived stressor severity. As shown in Figure 3, covariate-adjusted tests of mediation revealed an indirect effect of experiencing fewer acute stressors on greater plasma IL-1β levels as mediated by higher average levels of acute perceived stressor severity (indirect effect: ab = −0.002, 95% CI −0.006, −0.0004). Covariate-adjusted tests of mediation did not reveal an association between acute stressor exposure and plasma IL-1β levels without considering the proposed mediator [total effect: c = 0.0002, t(85) = 0.05, p = 0.958] and controlling for the proposed mediator [direct effect: c’ = 0.003, t(84) = 0.75, p = 0.454]. Covariate-adjusted tests of mediation did not yield evidence of a total, direct, or indirect effect (as mediated by average levels of acute perceived stressor severity) of acute stressor exposure on plasma IL-8 levels.
Figure 3. Indirect effect of lifetime acute stressor exposure on plasma IL-1β levels as mediated by average levels of lifetime acute perceived stressor severity.
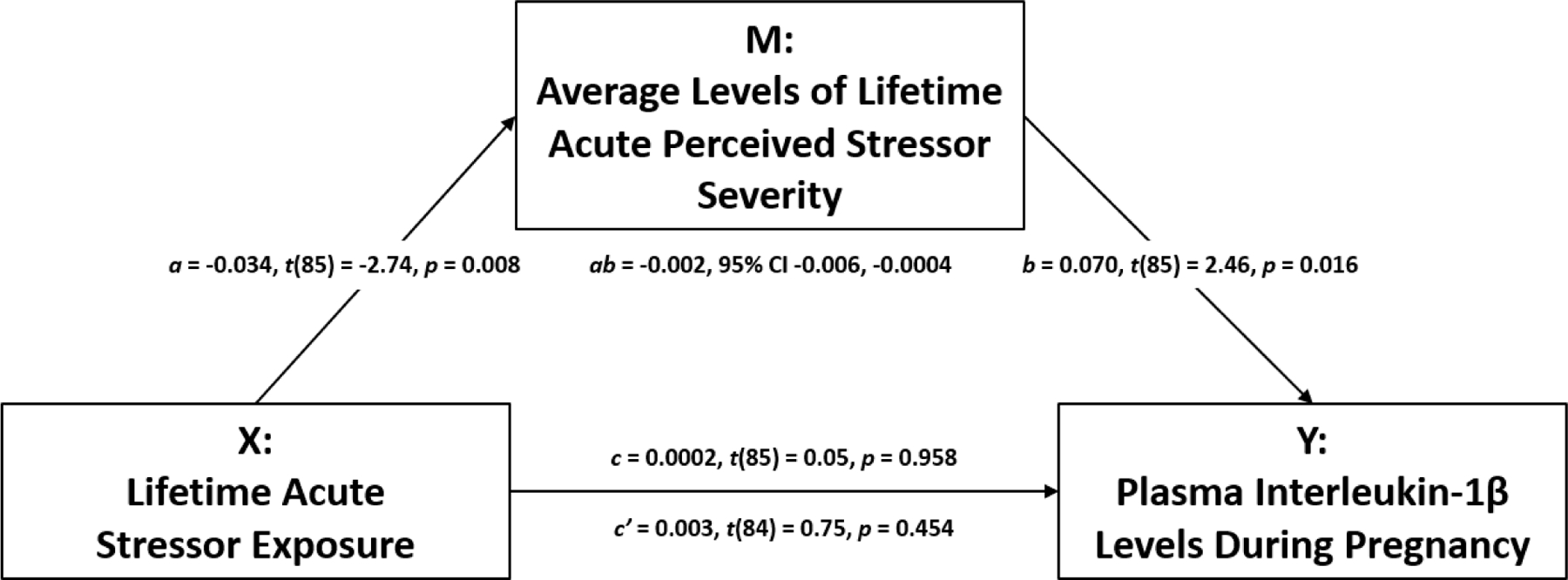
Experiencing fewer acute stressors was related to higher average levels of acute perceived stressor severity (a). In addition, higher average levels of acute perceived stressor severity were associated with higher plasma IL-1β levels (b). Finally, exposure to fewer acute stressors was related to higher plasma IL-1β levels, as mediated by higher average levels of acute perceived stressor severity (ab), controlling for relevant covariates.
In sum, therefore, exposure to more chronic stressors was related to greater plasma interleukin-1β levels, as mediated by higher average levels of overall perceived stressor severity. Experiencing fewer acute stressors was related to greater plasma interleukin-1β levels, as mediated by higher average levels of acute perceived stressor severity.
3.5. Preterm birth and systemic inflammation during pregnancy
Seven of the ninety-two participants went on to give birth preterm, with two presenting in preterm labor (i.e., with contractions), three with preterm premature rupture of membranes (i.e., with breakage of water), and two for a medically-indicated induction or cesarean section. Of these women, the earliest preterm birth occurred at 34 weeks 1 day, and the latest preterm birth occurred at 36 weeks 5 days (M = 35 weeks 5.9 days, SD = 0 weeks 6.1 days). Covariate-adjusted regression revealed that women who went on to give birth preterm exhibited greater log-transformed plasma IL-6 levels during the early third trimester assessment than women who went on to give birth at term [Coef. = 0.694, SE = 0.221, β = 0.304, t(84) = 3.15, p = 0.002]. Median plasma IL-6 levels were 1.381 [interquartile (IQ) range = 0.552–1.832] versus 0.701 (IQ range = 0.513–0.984) for the two groups, respectively. Women who did versus did not go on to give birth preterm did not show significantly different log-transformed plasma IL-8, IL-1β, or TNF-α levels (ps ≥ 0.349), suggesting a possible specific role of IL-6 in indexing this risk.
4. Discussion
To our knowledge, this study provides the first data linking lifetime stressor exposure to plasma IL-1β and IL-8 levels during pregnancy, as mediated by average levels of overall and acute lifetime perceived stressor severity among Black American women. These effects remained significant while controlling for both perceived stress at the time of assessment (i.e., PSS-14 scores) and participants’ exposure to racial discrimination (i.e., EOD scores), indicating that experiencing lifetime stressors is predictive over and above the effects attributable to these variables. Moreover, plasma IL-6 levels were higher during the early third trimester among women who did versus did not go on to give birth preterm. These findings thus suggest that assessing lifetime perceived stressor severity may be important for understanding the effects that major life stressors have on health-relevant biology. Although perceived stress, depressive symptoms, and symptoms of anxiety in adulthood are well-established correlates of lifetime stressor exposure (McLoughlin, Fletcher, Slavich, Arnold, & Moore, 2021; Toussaint, Shields, Dorn, & Slavich, 2016), such snapshots of mental health during clinical care fail to provide a comprehensive picture of individuals’ experiences.
We found that Black American women exposed to more chronic stressors experienced higher average levels of overall perceived stressor severity. Higher average levels of overall perceived stressor severity mediated the association between experiencing more chronic stressors and greater plasma IL-1β levels during pregnancy. These findings are consistent with prior research suggesting that chronic stressor exposure may be particularly deleterious, including for immune-related health outcomes (Dhabhar, 2014). For example, individuals managing chronic health conditions often report a progressive toll on their coping capacity, including their ability to manage other stressors (Bryl et al., 2021; Tracy et al., 2021). Individuals in the midst of ongoing crises have reported feelings of demoralization and hopelessness that contributed to their growing emotional distress (Braun-Lewensohn, Abu-Kaf, & Kalagy, 2021; Elnakib et al., 2021). Some psychoeducational interventions are now focusing on empowering participants that are facing chronic stressors, including racial discrimination (Brown et al., 2017; Saban et al., 2021). Preliminary results from these studies suggest potential for health benefits (Brown et al., 2017; Saban et al., 2021), including for immune-related health (Shields, Spahr, & Slavich, 2020).
We also found that Black American women exposed to fewer acute stressors experienced higher average levels of acute perceived stressor severity. Higher average levels of acute perceived stressor severity mediated the association between experiencing fewer acute stressors and having greater plasma IL-1β levels during pregnancy. These findings are interesting and may be consistent with prior research suggesting the potential for a resilience phenotype in which certain stressors build psychological strength and provide biological protection – but only when experienced in limited doses and under certain circumstances (Dooley et al., 2017; Seery, Leo, Lupien, Kondrak, & Almonte, 2013; Shields et al., 2017). For example, undergraduate students exposed to some (versus few or many) lifetime stressors report lower pain intensity during a cold pressor test (Seery et al., 2013). Cancer survivors exposed to some (versus few or many) lifetime acute (but not chronic) stressors report less frequent cancer-related intrusive thoughts and more positive affect (Dooley et al., 2017). Such findings highlight the complexity of the stressor exposure-health link across the life course.
In addition, we found that average levels of perceived stressor severity but not stressor exposure, total perceived stressor severity, or perceived stress at the time of assessment, was associated with plasma IL-1β and IL-8 levels for the pregnant Black American women assessed here. These findings may suggest that levels of inflammatory markers are dynamically regulated by current perceptions of lifetime stressor severity. If that is the case, systemic levels of some inflammatory markers may be particularly susceptible to interventions such as cognitive behavioral therapy, which aims to reframe how individuals think to improve health (Moraes, Miranda, Loures, Mainieri, & Marmora, 2018; Shields et al., 2020). Indeed, studies describing the beneficial effects of such therapies is growing and improved immune profiles may be a potential mechanism of action (Lau, Cheng, Wong, Yen, & Cheng, 2021; Pearlstein, Staudenmaier, West, Geraghty, & Cosgrove, 2020). What should also be noted is that this report provides evidence that lifetime stressor exposure appears to shape average levels of perceived stressor severity at recall. Such effects, over time, cannot simply be erased, and personal resilience cannot be expected to replace efforts to address root causes. Just as racism cuts across structures, systems, and individual experiences, efforts to eliminate its pervasive effects must do the same.
Finally, it is worth noting that although average levels of perceived stressor severity were associated with plasma IL-1β and IL-8 levels in our sample, women who did versus did not go on to give birth preterm differed only according to plasma IL-6 levels. Although systemic inflammatory markers are often interrelated (as was largely the case here), it remains to be determined whether distinct cytokines are active in the pathophysiology of specific preterm birth subtypes among specific populations. For example, Menon and colleagues published a series of articles that included stratified analyses among Black versus White women. In these analyses, maternal plasma IL-6R, IL-1β, and TNF-α levels and amniotic fluid IL-1β and TNF-α levels were elevated among spontaneous preterm birth cases versus controls in the Black American strata, whereas maternal plasma IL1RA levels and amniotic fluid IL-6 and IL-8 levels were elevated among spontaneous preterm birth cases versus controls in the White American strata (Brou et al., 2012; Menon, Williams, & Fortunato, 2007; Menon, Camargo, Thorsen, Lombardi, & Fortunato, 2008; Menon et al., 2008). Other studies have reported that pregnant Black versus White Americans show higher plasma IL-6 levels in response to stress and impaired vasodilation in response to pregnancy, putting them at higher risk for gestational hypertensive disorders and therefore medically indicated preterm birth (Christian, Glaser, Porter, & Iams, 2013; Christian, Koenig, Williams, Kapuku, & Thayer, 2021). More research combining multi-omics data is certainly needed, and the importance of considering the social determinants of health cannot be understated (Hong et al., 2017).
This study has several strengths, including the use of the Adult STRAIN to assess lifetime stressor exposure and perceived stressor severity in a sizeable cohort of pregnant Black American women (Slavich & Shields, 2018). The Adult STRAIN has excellent validity and reliability and is a National Institute of Mental Health/Research Domain Criteria-recommended instrument. Even in well-designed cohort studies of Black Americans that are paving the way toward health equity (e.g., Jackson Heart Study), only very limited snapshots of life stressor exposure are available [e.g., over the past year (Payne et al., 2005)]. The Adult STRAIN also provides a comprehensive assessment of lifetime stressor exposure, extending assessments that take a life course approach but focus only on trauma (Myers et al., 2015). In addition, we sampled venous blood in the absence of covert infection, fever, antibiotic use, or vaccination, and collection was standardized to time of day. We also controlled for several potential factors that could have confounded the associations observed and introduced systematic bias in the data. Finally, systemic inflammation is a biologically plausible mediator linking stress and health, including major complications of pregnancy such as preeclampsia and preterm birth, which is of great clinical relevance (Black & Horowitz, 2018; Furman et al., 2019; Gomes et al., 2019).
Several limitations should also be noted. First, the Adult STRAIN is designed to systematically query a large set of stressors that might be experienced by heterogeneous populations. More methodological work is needed to advance our understanding of, specifically, the lived experience of racism across structures, systems, and individuals. Both quantitative and qualitative work is needed to realize this goal. Second, the present data are cross-sectional; therefore, directionality and causality cannot be assumed. Moreover, although several well-known correlates of stress and systemic inflammatory markers (e.g., smoking status, BMI) were included as covariates, some data suggest that health-related behaviors partially mediate associations between stress and health outcomes (e.g., Woods-Giscombe et al., 2021). Focused investigations on such pathways are thus important. Third, although the Adult STRAIN partitions lifetime stressors into chronic difficulties versus acute life events, acute life events can have lasting effects. Indeed, rumination and hyperarousal have been observed following discrete yet traumatic events but were not assessed here (Szabo, Warnecke, Newton, & Valentine, 2017). Fourth, only seven women fit the case definition of preterm birth in this sample. As such, the exploratory nature of these analyses should be acknowledged and replication is required. Finally, this report focused on pregnant Black American women, who reported greater lifetime perceived stressor severity than prior studies of pregnant women completing the STRAIN (Smith et al., 2020). This is consistent with prior research showing that pregnant Black Americans report more lifetime stressors than pregnant White Americans (Malat et al., 2020). However, the difference must also be considered in terms of generalizability.
Conclusion
In conclusion, this study provides new data showing that greater lifetime stressor exposure is related to higher levels of the key inflammatory cytokines IL-1β and IL-8 during pregnancy among Black American women and that this association is mediated by average levels of lifetime perceived stressor severity. These findings may point to factors driving the substantial health disparities evident in this population. Furthermore, we found that plasma IL-6 levels were higher among women who did versus did not go on to give birth preterm. These findings are consistent with frameworks such as allostatic load and Social Safety Theory (McEwen, 1998; McEwen & Seeman, 1999; McEwen, 2007; McEwen & Gianaros, 2010; Slavich, 2020), which posit that an individual’s perceptions of stressor severity over the lifetime is an important process linking stressor exposures and health. These data thus highlight the potential importance of assessing and addressing lifetime stressor exposure among the mother during the provision of prenatal care as well as the societal and institutional inequities that place a disproportionate burden of stress on Black American women.
Supplementary Material
Highlights.
We examined lifetime stressors and inflammation in Black pregnant women
Greater lifetime stressor severity was related to higher prenatal IL-1β levels
Stressor severity mediated the association between stressors and IL-1β levels
Prenatal IL-6 levels were higher preceding preterm versus full-term birth
Therefore, assessing and reducing maternal stress is potentially important
Acknowledgements
We would like to thank our undergraduate research assistants, Amy Kole and Patricia Do, for helping collect these data. We would also like Grant Shields and Chandler Spahr for assisting with the STRAIN data and, most importantly, our study participants and the staff at our partnering clinical sites.
Funding
This work was supported by the National Institute of Nursing Research of the National Institutes of Health (F31NR01460; SLG); Association of Women’s Health, Obstetric and Neonatal Nurses (SLG); Midwest Nursing Research Society (SLG); Sigma Theta Tau International, Epsilon (SLG); Cola-Cola Critical Difference for Women and Department of Women’s, Gender and Sexuality Studies, Graduate School, and Office of Diversity and Inclusion of The Ohio State University (SLG). Resources supported by the National Center for Advancing Translational Sciences (UL1TR001070) of the National Institutes of Health were utilized. Preparation of this report was supported by the National Institute of Nursing Research of the National Institutes of Health (K23NR017902; SLG) and the National Institute of Mental Health (K08 MH103443; GMS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Moreover, the funding agencies had no role in study design; in the collection, analysis, or interpretation of these data; in the writing of this report; or in the decision to submit this article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors report no conflicts of interest.
References
- American College of Obstetricians and Gynecologists. (2018). ACOG committee opinion no. 757 summary: Screening for perinatal depression. Obstetrics and Gynecology, 132(5), 1314–1316. doi: 10.1097/AOG.0000000000002928 [doi] [DOI] [PubMed] [Google Scholar]
- Assari S (2020). Social epidemiology of perceived discrimination in the united states: Role of race, educational attainment, and income. International Journal of Epidemiologic Research, 7(3), 136–141. doi: 10.34172/ijer.2020.24 [doi] [DOI] [PubMed] [Google Scholar]
- Bann CM, Parker CB, Grobman WA, Willinger M, Simhan HN, Wing DA, . . . NuMoM2b study. (2017). Psychometric properties of stress and anxiety measures among nulliparous women. Journal of Psychosomatic Obstetrics and Gynaecology, 38(1), 53–62. doi: 10.1080/0167482X.2016.1252910 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black KD, & Horowitz JA (2018). Inflammatory markers and preeclampsia: A systematic review. Nursing Research, 67(3), 242–251. doi: 10.1097/NNR.0000000000000285 [doi] [DOI] [PubMed] [Google Scholar]
- Boyd RW, Lindo EG, Weeks LD, & McLemore MR (2020, July 2, 2020). On racism: A new standard for publishing on racial health inequities Retrieved from 10.1377/hblog20200630.939347/full/ [DOI]
- Braun-Lewensohn O, Abu-Kaf S, & Kalagy T (2021). Hope and resilience during a pandemic among three cultural groups in israel: The second wave of covid-19. Frontiers in Psychology, 12, 637349. doi: 10.3389/fpsyg.2021.637349 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brou L, Almli LM, Pearce BD, Bhat G, Drobek CO, Fortunato S, & Menon R (2012). Dysregulated biomarkers induce distinct pathways in preterm birth. BJOG : An International Journal of Obstetrics and Gynaecology, 119(4), 458–473. doi: 10.1111/j.1471-0528.2011.03266.x [DOI] [PubMed] [Google Scholar]
- Brown AG, Hudson LB, Chui K, Metayer N, Lebron-Torres N, Seguin RA, & Folta SC (2017). Improving heart health among black/african american women using civic engagement: A pilot study. BMC Public Health, 17(1), 112–016–3964–2. doi: 10.1186/s12889-016-3964-2 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryl K, Wenger S, Banz D, Terry G, Ballester D, Bailey C, & Bradt J (2021). Power over pain - an interprofessional approach to chronic pain: Program feedback from a medically underserved community. Journal of Evaluation in Clinical Practice, doi: 10.1111/jep.13552 [doi] Online ahead of print [DOI] [PubMed]
- Cazassa MJ, Oliveira MDS, Spahr CM, Shields GS, & Slavich GM (2020). The stress and adversity inventory for adults (adult STRAIN) in brazilian portuguese: Initial validation and links with executive function, sleep, and mental and physical health. Frontiers in Psychology, 10, 3083. doi: 10.3389/fpsyg.2019.03083 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM (2020). At the forefront of psychoneuroimmunology in pregnancy: Implications for racial disparities in birth outcomes: PART 2: Biological mechanisms. Neuroscience and Biobehavioral Reviews, 117, 327–333. doi:S0149–7634(18)30551–7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Glaser R, Porter K, & Iams JD (2013). Stress-induced inflammatory responses in women: Effects of race and pregnancy. Psychosomatic Medicine, 75(7), 658–669. doi: 10.1097/PSY.0b013e31829bbc89 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Koenig J, Williams DP, Kapuku G, & Thayer JF (2021). Impaired vasodilation in pregnant african americans: Preliminary evidence of potential antecedents and consequences. Psychophysiology, 58(1), e13699. doi: 10.1111/psyp.13699 [doi] [DOI] [PubMed] [Google Scholar]
- Cohen S, Kessler R, & Underwood Gordon L (1995). Measuring stress: A guide for health and social scientists New York, NY: Oxford University Press. [Google Scholar]
- Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L, . . . Cole S (2012). The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain, Behavior, and Immunity, 26(4), 650–659. doi: 10.1016/j.bbi.2012.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS (2014). Effects of stress on immune function: The good, the bad, and the beautiful. Immunologic Research, 58(2–3), 193–210. doi: 10.1007/s12026-014-8517-0 [doi] [DOI] [PubMed] [Google Scholar]
- Dooley LN, Slavich GM, Moreno PI, & Bower JE (2017). Strength through adversity: Moderate lifetime stress exposure is associated with psychological resilience in breast cancer survivors. Stress and Health : Journal of the International Society for the Investigation of Stress, 33(5), 549–557. doi: 10.1002/smi.2739 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnakib S, Elaraby S, Othman F, BaSaleem H, Abdulghani AlShawafi NA, Saleh Al-Gawfi IA, . . . Tappis H (2021). Providing care under extreme adversity: The impact of the yemen conflict on the personal and professional lives of health workers. Social Science & Medicine (1982), 272, 113751. doi:S0277–9536(21)00083–6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely DM, & Driscoll AK (2020). Infant mortality in the united states, 2018: Data from the period linked birth/infant death file. National Vital Statistics Reports : From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 69(7), 1–18. [PubMed] [Google Scholar]
- Finy MS, & Christian LM (2018). Pathways linking childhood abuse history and current socioeconomic status to inflammation during pregnancy. Brain, Behavior, and Immunity, 74, 231–240. doi:S0889–1591(18)30579–8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, . . . Slavich GM (2019). Chronic inflammation in the etiology of disease across the life span. Nature Medicine, 25(12), 1822–1832. doi: 10.1038/s41591-019-0675-0 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie SL, Mitchell AM, Kowalsky JM, & Christian LM (2018). Maternal parity and perinatal cortisol adaptation: The role of pregnancy-specific distress and implications for postpartum mood. Psychoneuroendocrinology, 97, 86–93. doi:S0306–4530(18)30093–3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes J, Au F, Basak A, Cakmak S, Vincent R, & Kumarathasan P (2019). Maternal blood biomarkers and adverse pregnancy outcomes: A systematic review and meta-analysis. Critical Reviews in Toxicology, 49(6), 461–478. doi: 10.1080/10408444.2019.1629873 [doi] [DOI] [PubMed] [Google Scholar]
- Gordon NP, Banegas MP, & Tucker-Seeley RD (2020). Racial-ethnic differences in prevalence of social determinants of health and social risks among middle-aged and older adults in a northern california health plan. PloS One, 15(11), e0240822. doi: 10.1371/journal.pone.0240822 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, White LK, Waller R, Barzilay R, Moore TM, Kornfield S, . . . Elovitz MA (2020). The disproportionate burden of the COVID-19 pandemic among pregnant black women. Psychiatry Research, 293, 113475. doi:S0165–1781(20)33136-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). In Kenny DA, Little TD (Eds.), Introduction to mediation, moderation, and conditional process analysis: A regression-based approach New York, NY: The Guilford Press. [Google Scholar]
- Hong X, Sherwood B, Ladd-Acosta C, Peng S, Ji H, Hao K, . . . Wang X (2017). Genome-wide DNA methylation associations with spontaneous preterm birth in US blacks: Findings in maternal and cord blood samples. Epigenetics,, 0. doi: 10.1080/15592294.2017.1287654 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph KS, Boutin A, Lisonkova S, Muraca GM, Razaz N, John S, . . . Schisterman E (2021). Maternal mortality in the united states: Recent trends, current status, and future considerations. Obstetrics and Gynecology, 137(5), 763–771. doi: 10.1097/AOG.0000000000004361 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Im EO, Liu J, & Ulrich C (2020). Maternal age patterns of preterm birth: Exploring the moderating roles of chronic stress and race/ethnicity. Annals of Behavioral Medicine : A Publication of the Society of Behavioral Medicine, 54(9), 653–664. doi: 10.1093/abm/kaaa008 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N (1990). Racial and gender discrimination: Risk factors for high blood pressure? Social Science & Medicine (1982), 30(12), 1273–1281. [DOI] [PubMed] [Google Scholar]
- Krieger N, & Sidney S (1996). Racial discrimination and blood pressure: The CARDIA study of young black and white adults. American Journal of Public Health, 86(10), 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, & Barbeau EM (2005). Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Social Science & Medicine (1982), 61(7), 1576–1596. doi: 10.1016/j.socscimed.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Lam PH, Chiang JJ, Chen E, & Miller GE (2021). Race, socioeconomic status, and low-grade inflammatory biomarkers across the lifecourse: A pooled analysis of seven studies. Psychoneuroendocrinology, 123, 104917. doi:S0306–4530(20)30340–1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y, Cheng J, Wong S, Yen K, & Cheng L (2021). Effectiveness of digital psychotherapeutic intervention among perinatal women: A systematic review and meta-analysis of randomized controlled trials. World Journal of Psychiatry, 11(4), 133–152. doi: 10.5498/wjp.v11.i4.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malat J, Johns-Wolfe E, Smith T, Shields GS, Jacquez F, & Slavich GM (2020). Associations between lifetime stress exposure, race, and first-birth intendedness in the united states. Journal of Health Psychology,, 1359105320963210. doi: 10.1177/1359105320963210 [doi] [DOI] [PMC free article] [PubMed]
- McCormack C, Lauriola V, Feng T, Lee S, Spann M, Mitchell A, . . . Monk C (2021). Maternal childhood adversity and inflammation during pregnancy: Interactions with diet quality and depressive symptoms. Brain, Behavior, and Immunity, 91, 172–180. doi:S0889–1591(20)31116–8 [pii] [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease. allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87(3), 873–904. doi: 10.1152/physrev.00041.2006 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2010). Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences, 1186, 190–222. doi: 10.1111/j.1749-6632.2009.05331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Seeman T (1999). Protective and damaging effects of mediators of stress. elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences, 896, 30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x [doi] [DOI] [PubMed] [Google Scholar]
- McLoughlin E, Fletcher D, Slavich GM, Arnold R, & Moore LJ (2021). Cumulative lifetime stress exposure, depression, anxiety, and well-being in elite athletes: A mixed-method study. Psychology of Sport and Exercise, 52, 101823. doi: 10.1016/j.psychsport.2020.101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, Camargo MC, Thorsen P, Lombardi SJ, & Fortunato SJ (2008). Amniotic fluid interleukin-6 increase is an indicator of spontaneous preterm birth in white but not black americans. American Journal of Obstetrics and Gynecology, 198(1), 77.e1–77.e7. doi: 10.1016/j.ajog.2007.06.071 [DOI] [PubMed] [Google Scholar]
- Menon R, Thorsen P, Vogel I, Jacobsson B, Morgan N, Jiang L, . . . Fortunato SJ (2008). Racial disparity in amniotic fluid concentrations of tumor necrosis factor (TNF)-alpha and soluble TNF receptors in spontaneous preterm birth. American Journal of Obstetrics and Gynecology, 198(5), 533.e1–533.10. doi: 10.1016/j.ajog.2007.11.025 [DOI] [PubMed] [Google Scholar]
- Menon R, Williams SM, & Fortunato SJ (2007). Amniotic fluid interleukin-1beta and interleukin-8 concentrations: Racial disparity in preterm birth. Reproductive Sciences (Thousand Oaks, Calif.), 14(3), 253–259. doi: 10.1177/1933719107301336 [DOI] [PubMed] [Google Scholar]
- Millender E, Barile JP, R Bagneris J, Harris RM, De Faria L, Wong FY, . . . Taylor JY (2021). Associations between social determinants of health, perceived discrimination, and body mass index on symptoms of depression among young african american mothers. Archives of Psychiatric Nursing, 35(1), 94–101. doi:S0883–9417(20)30609–9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes LJ, Miranda MB, Loures LF, Mainieri AG, & Marmora CHC (2018). A systematic review of psychoneuroimmunology-based interventions. Psychology, Health & Medicine, 23(6), 635–652. doi: 10.1080/13548506.2017.1417607 [doi] [DOI] [PubMed] [Google Scholar]
- Myers HF, Wyatt GE, Ullman JB, Loeb TB, Chin D, Prause N, . . . Liu H (2015). Cumulative burden of lifetime adversities: Trauma and mental health in low-SES african americans and latino/as. Psychological Trauma : Theory, Research, Practice and Policy, 7(3), 243–251. doi: 10.1037/a0039077 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsonne G, Lekander M, Akerstedt T, Axelsson J, & Ingre M (2016). Diurnal variation of circulating interleukin-6 in humans: A meta-analysis. PloS One, 11(11), e0165799. doi: 10.1371/journal.pone.0165799 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne TJ, Wyatt SB, Mosley TH, Dubbert PM, Guiterrez-Mohammed ML, Calvin RL, . . . Williams DR (2005). Sociocultural methods in the jackson heart study: Conceptual and descriptive overview. Ethnicity & Disease, 15(4 Suppl 6), S6–38-48. [PubMed] [Google Scholar]
- Pearlstein JG, Staudenmaier PJ, West AE, Geraghty S, & Cosgrove VE (2020). Immune response to stress induction as a predictor of cognitive-behavioral therapy outcomes in adolescent mood disorders: A pilot study. Journal of Psychiatric Research, 120, 56–63. doi:S0022–3956(19)30436–4 [pii] [DOI] [PubMed] [Google Scholar]
- Saban KL, Motley D, Shawahin L, Mathews HL, Tell D, De La Pena P, & Janusek LW (2021). Preliminary evidence for a race-based stress reduction intervention for black women at risk for cardiovascular disease. Complementary Therapies in Medicine, 58, 102710. doi:S0965–2299(21)00051–0 [pii] [DOI] [PubMed] [Google Scholar]
- Seery MD, Leo RJ, Lupien SP, Kondrak CL, & Almonte JL (2013). An upside to adversity?: Moderate cumulative lifetime adversity is associated with resilient responses in the face of controlled stressors. Psychological Science, 24(7), 1181–1189. doi: 10.1177/0956797612469210 [doi] [DOI] [PubMed] [Google Scholar]
- Shields GS, Moons WG, & Slavich GM (2017). Better executive function under stress mitigates the effects of recent life stress exposure on health in young adults. Stress (Amsterdam, Netherlands), 20(1), 75–85. doi: 10.1080/10253890.2017.1286322 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Spahr CM, & Slavich GM (2020). Psychosocial interventions and immune system function: A systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry, 77(10), 1031–1043. doi: 10.1001/jamapsychiatry.2020.0431 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JM, Winsky L, Zehr JL, & Gordon JA (2021). Priorities in stress research: A view from the U.S. national institute of mental health. Stress (Amsterdam, Netherlands), 24(2), 123–129. doi: 10.1080/10253890.2020.1781084 [doi] [DOI] [PubMed] [Google Scholar]
- Slavich GM (2019). Stressnology: The primitive (and problematic) study of life stress exposure and pressing need for better measurement. Brain, Behavior, and Immunity, 75, 3–5. doi:S0889–1591(18)30393–3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM (2020). Social safety theory: A biologically based evolutionary perspective on life stress, health, and behavior. Annual Review of Clinical Psychology, 16, 265–295. doi: 10.1146/annurev-clinpsy-032816-045159 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Shields GS (2018). Assessing lifetime stress exposure using the stress and adversity inventory for adults (adult STRAIN): An overview and initial validation. Psychosomatic Medicine, 80(1), 17–27. doi: 10.1097/PSY.0000000000000534 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedley A, & Smedley BD (2005). Race as biology is fiction, racism as a social problem is real: Anthropological and historical perspectives on the social construction of race. The American Psychologist, 60(1), 16–26. doi:2005–00117-003 [pii] [DOI] [PubMed] [Google Scholar]
- Smith T, Johns-Wolfe E, Shields GS, Malat J, Jacquez F, & Slavich GM (2020). Associations between lifetime stress exposure and prenatal health behaviors. Stress and Health : Journal of the International Society for the Investigation of Stress, 36(3), 384–395. doi: 10.1002/smi.2933 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper LA, Hart KL, Venkatesh KK, Kaimal AJ, & Perlis RH (2018). Cohort study of the relationship between individual psychotherapy and pregnancy outcomes. Journal of Affective Disorders, 239, 253–257. doi:S0165–0327(17)31171–0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo YZ, Warnecke AJ, Newton TL, & Valentine JC (2017). Rumination and posttraumatic stress symptoms in trauma-exposed adults: A systematic review and meta-analysis. Anxiety, Stress, and Coping, 30(4), 396–414. doi: 10.1080/10615806.2017.1313835 [doi] [DOI] [PubMed] [Google Scholar]
- Thompson JA, & Suter MA (2020). Estimating racial health disparities among adverse birth outcomes as deviations from the population rates. BMC Pregnancy and Childbirth, 20(1), 155–020-2847–9. doi: 10.1186/s12884-020-2847-9 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint L, Shields GS, Dorn G, & Slavich GM (2016). Effects of lifetime stress exposure on mental and physical health in young adulthood: How stress degrades and forgiveness protects health. Journal of Health Psychology, 21(6), 1004–1014. doi: 10.1177/1359105314544132 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy EL, Berg CA, Kelly CS, Kent de Grey RG, Litchman ML, Allen NA, & Helgeson VS (2021). Daily stress spillover and crossover in couples coping with type 1 diabetes. Journal of Family Psychology : JFP : Journal of the Division of Family Psychology of the American Psychological Association (Division 43), 35(5), 618–627. doi: 10.1037/fam0000819 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh KK, Ferguson KK, Smith NA, Cantonwine DE, & McElrath TF (2019). Association of antenatal depression with clinical subtypes of preterm birth. American Journal of Perinatology, 36(6), 567–573. doi: 10.1055/s-0038-1675646 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Bell BA, Huang SJ, & Williams DR (2020). Perceived discrimination trajectories and depressive symptoms among middle-aged and older black adults. Innovation in Aging, 4(5), igaa041. doi: 10.1093/geroni/igaa041 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods-Giscombe CL, Lobel M, Zimmer C, Brooks J, Sheffield-Abdullah K, Bey G, . . . Muhirwa A (2021). Use of food to cope with culturally relevant stressful life events is associated with body mass index in african american women. Nursing Research, 70(5S Suppl 1), S53–S62. doi: 10.1097/NNR.0000000000000532 [doi] [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang Q, Gao T, Kong Y, Qin Z, Hu Y, . . . Mei S (2019). Relations between stress and quality of life among women in late pregnancy: The parallel mediating role of depressive symptoms and sleep quality. Psychiatry Investigation, 16(5), 363–369. doi: 10.30773/pi.2019.02.14 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


