Abstract
Background:
Precision medicine approaches attempt to reduce variability in alcohol use disorder (AUD) outcomes by identifying patient characteristics that predict response to a particular treatment. Recent work has examined the extent to which individuals with AUD may seek alcohol to enhance positive experiences (reward drinking) or relieve negative states (relief drinking) and shown that a high reward/low relief phenotype predicts naltrexone treatment response. Yet, limitations of reward/relief drinking measures may hamper efforts to translate findings to clinical practice. We sought to refine a brief measure of reward/relief drinking and develop cutoff scores to identify reward/relief subgroups that predict pharmacotherapy response.
Methods:
The Inventory of Drinking Situations (IDS), used in previous studies to measure reward/relief drinking, was administered to 426 participants (77% male; average age=45.3) in a clinical trial examining naltrexone and acamprosate.
Results:
Item response theory and tests of differential item functioning across sex, age, and alcohol dependence severity were used to create a 10-item measure, titled the Reward and Relief IDS (RR-IDS). Cutoff scores on the RR-IDS for the reward/relief drinking subgroups were identified using latent profile and area under the curve analyses. The cutoff scores demonstrated good construct validity. Individuals in the high reward/low relief subgroup who received naltrexone or acamprosate had a decreased likelihood of heavy drinking (large effect sizes) versus those who received placebo.
Conclusions:
The RR-IDS is a practical measure for identifying reward/relief subgroups and predicting pharmacotherapy response. Pending replication of these findings, the RR-IDS could be a critical precision medicine tool for prescribing AUD medications.
Keywords: alcohol use disorder, precision medicine, naltrexone, acamprosate, reward drinking, relief drinking
1. Introduction
Despite decades of treatment development, pharmacological and psychological treatments for alcohol use disorder (AUD) are only moderately efficacious (Witkiewitz et al., 2019a). The high degree of heterogeneity among individuals with AUD (Lane and Sher, 2015; Witkiewitz et al., 2020) could explain the modest efficacy of treatments. A better understanding of variability among AUD patients could improve outcomes by identifying subgroups that respond better to certain treatments (Litten et al., 2015).
One approach to classifying variability within AUD is based on the extent to which individuals seek alcohol to enhance positive experiences and social interaction (reward drinking) or to relieve negative emotional and somatic states (relief drinking). Processes underlying reward/relief drinking were initially proposed in the three-pathway psychobiological model of craving for alcohol (Verheul et al., 1999) and may reflect, to a certain extent, the 3-stage cycle of addiction (Koob and Volkow, 2016). Reward drinking is hypothesized to be present in the initial stage of alcohol use (binge-intoxication). This stage is elicited by alcohol’s action as a positive reinforcer and mediated by dopaminergic and opioidergic dysfunction within the ventral striatum. Relief drinking is hypothesized to correspond to the withdrawal-negative affect stage, where individuals increasingly drink to reduce negative emotional and somatic states, which is mediated by the upregulation of corticotropin-releasing factor and norepinephrine in the extended amygdala, as well as glutamate and GABA dysfunction. Whether obsessive drinking corresponds to the preoccupation-anticipation stage needs to be elucidated.
Recent research has translated these hypothesized neurobiological processes into the identification of reward/relief drinking phenotypes using latent variable mixture models to identify four latent profiles: high reward/high relief, high reward/low relief, low reward/high relief, low reward/low relief (Glöckner-Rist et al., 2013; Mann et al., 2018; Roos et al., 2017; Witkiewitz et al., 2019b). Most individuals in these studies reported both reward and relief drinking, indicating that these drinking profiles are not orthogonal or binary. Although obsessive drinking subtypes have not been widely examined, some results indicate that obsessive drinkers show similar alcohol use and craving behavior as relief drinkers (Grodin et al., 2019).
Given the proposed neurobiological underpinnings of reward and relief drinking, primary reward drinkers may respond best to naltrexone, an opioid antagonist. Conversely, primary relief drinkers may respond best to acamprosate, which targets glutamatergic neurotransmission (Mann et al., 2009). There is some evidence that high reward/low relief drinkers respond better to naltrexone than placebo (Mann et al., 2018; Roos et al., 2021; Witkiewitz et al., 2019b), though one study (Roos et al., 2017) did not identify a benefit of naltrexone over placebo among reward drinkers. Although that study showed that low reward/high relief drinkers respond better to acamprosate than placebo (Roos et al., 2017), Mann and colleagues (2018) found no benefit of acamprosate in this subgroup. Despite conflicting findings, which may be partly explained by different abstinent periods prior to randomization, previous studies support the potential utility of reward/relief drinking phenotypes in matching patients to AUD pharmacotherapies.
The use of different measures of reward/relief drinking across studies might also contribute to inconsistent findings. Previous research on reward/relief phenotypes utilized a subset of items from the Inventory of Drinking Situations (IDS) (Mann et al., 2018; Witkiewitz et al., 2019b), the Alcohol Abstinence Self-Efficacy Scale (AASE) (Roos et al., 2017), the Drinking Motives Questionnaire (DMQ) (Roos et al., 2021), and the Reward, Relief, Habit Drinking Scale (RRHDS) (Grodin et al., 2019). Of these, the IDS is the only measure that has identified reward/relief phenotypes and predicted AUD medication response across two independent samples (Mann et al., 2018; Witkiewitz et al., 2019b), supporting the potential clinical utility of this instrument. A limitation of the IDS is length, which may impede efforts to translate findings to clinical practice. The full IDS comprises 100 items (Annis et al., 1987), though prior studies examining reward/relief drinking have used subsets of 30 items (Mann et al., 2018) or 27 items (Witkiewitz et al., 2019b). Additionally, the psychometric properties and potential bias of the IDS have not been thoroughly assessed. Some prior studies have offered observed cutoff scores for identifying reward/relief phenotypes (Mann et al., 2018; Roos et al., 2021; Witkiewitz et al., 2019b), but only a few potential cutoff scores were tested, and statistical techniques for selecting cutoffs were not utilized (Rovner et al., 2019).
Translating findings on reward/relief drinking and precision medicine to clinical practice requires a measure with predictive utility that is easy to administer and score. Using secondary data analysis from a prior study that examined reward/relief subgroups and the response to AUD pharmacotherapy (Mann et al., 2018), we aimed to establish a brief version of the IDS and a scoring algorithm to identify reward/relief profiles that predict pharmacotherapy response. First, we identified a brief version of the IDS that assesses reward and relief drinking and is free of bias across sex, age, and alcohol dependence severity, which we titled the Reward and Relief IDS (RR-IDS). Second, we established observed cutoff scores for the four reward/relief profiles. Third, we examined the concurrent validity of the RR-IDS with alcohol use severity, psychological distress, and personality measures. Lastly, we examined the clinical utility of the RR-IDS in predicting pharmacotherapy response. Consistent with findings reported by Mann and colleagues (2018), we hypothesized that participants in the high reward/low relief subgroup would respond better to naltrexone than placebo, with no other significant effects of naltrexone or acamprosate in the other reward/relief subgroups.
2. Materials and Methods
2.1. Participants and Procedures
This is a secondary analysis of data from the PREDICT study (Mann et al., 2018, 2013, 2009), a randomized clinical trial examining response to naltrexone and acamprosate versus placebo. Participants (N=426) were recruited from inpatient AUD treatment programs in Germany and enrolled after detoxification. Eligible participants received 13 weeks of medical management (Pettinati et al., 2004) and were randomly assigned (in a 2:2:1 ratio) to receive naltrexone (50 mg/day), acamprosate (2 g/day), or placebo over 12 weeks. The present analysis utilized data from baseline and the 12-week medication period.
Inclusion criteria included age 18–65, a DSM-IV diagnosis of alcohol dependence (American Psychiatric Association, 1994), 4–28 days of abstinence before study entry (mean days abstinent =22.1, SD=4.4), and consumption of 14+/21+ drinks per week for women/men during a consecutive 30-day period during the 90 days before detoxification. Exclusion criteria included a lifetime history of other substance dependence (excluding nicotine/cannabis), a DSM-IV diagnosis of a major psychiatric disorder, current receipt of psychiatric treatment, current use of psychoactive drugs, an unstable medical condition, or current pregnancy. Participants were predominately male (77%), had an average age of 45.3 (SD=8.7), with 37% of participants married, 48% employed, and 51% with 12+ years of education. All participants were of European ancestry.
2.2. Measures
2.2.1. Reward and Relief Drinking
The 100-item version of the Inventory of Drinking Situations (IDS; Annis et al., 1987), which assesses the frequency of heavy drinking (1=never, 4=almost always) in different situations (e.g., physical discomfort, pleasant emotions), was administered at baseline, of which the 30 items (Table 1) that identified latent reward/relief drinking profiles in a previous analysis of the PREDICT trial (Mann et al., 2018) were used.
Table 1.
Descriptive statistics for the Inventory of Drinking Situations (IDS)-30 Reward and Relief Drinking Items. Items retained for the RR-IDS are bolded and italicized.
| Item | Reward Drinking Items | M | SD |
|---|---|---|---|
| 1 | When I wanted to celebrate with a friend | 2.14 | 0.92 |
| 2 | When I met with a friend and they suggested we have a drink | 2.09 | 0.91 |
| 3 | When I was out with friends “on the town” and wanted to increase my enjoyment | 2.11 | 0.99 |
| 4 | When I was out with others and they stopped by a bar for a drink | 2.05 | 1.00 |
| 5 | When I was enjoying myself at a party and wanted to feel even better | 2.10 | 0.94 |
| 6 | When I was at a party and others were drinking | 2.29 | 1.01 |
| 7 | When something good happened and I felt like celebrating | 2.33 | 0.87 |
| 8 | When I was relaxed with a good friend and wanted to have a good time | 2.16 | 0.92 |
| 9 | When I was invited to someone’s home and they offered me a drink | 2.09 | 0.94 |
| 10 | When I was enjoying myself | 2.36 | 0.88 |
| 11 | When good friends dropped by and I was full of good feelings | 2.18 | 0.95 |
| 12 | When I was in a restaurant and people with me ordered drinks | 1.84 | 0.90 |
| 13 | When I wanted to celebrate special occasions like Christmas or a birthday | 2.26 | 0.99 |
| 14 | When I was having fun with friends and wanted to increase my enjoyment | 2.07 | 0.93 |
| 15 | When I was enjoying a meal with friends and felt that a drink would make it even more 15 enjoyable | 1.99 | 0.92 |
| Cronbach’s α=0.963 (n=402) | |||
|
| |||
| Item | Relief Drinking Items | M | SD |
|
| |||
| 1 | When I was afraid that things weren’t going to work out | 2.13 | 0.88 |
| 2 | When someone criticized me | 1.97 | 0.86 |
| 3 | When other people treated me unfairly | 1.99 | 0.88 |
| 4 | When I was sad at the memory of something that had happened | 2.40 | 0.92 |
| 5 | When I felt there was nowhere left to turn | 2.41 | 1.01 |
| 6 | When I felt under a lot of pressure | 2.47 | 0.94 |
| 7 | When I felt unsure that I could measure up to other people’s expectations | 1.88 | 0.83 |
| 8 | When nothing I did seems right | 2.04 | 0.93 |
| 9 | When everything was going badly for me | 2.56 | 0.98 |
| 10 | When I began to feel fed up with life | 2.13 | 1.03 |
| 11 | When I felt no one really cared what happened to me | 2.11 | 0.98 |
| 12 | When I felt guilty about something | 2.19 | 0.97 |
| 13 | When I was depressed about things in general | 2.26 | 0.99 |
| 14 | When I thought about the chances I had missed in life | 2.04 | 0.92 |
| 15 | When I felt rejected by friends | 1.67 | 0.80 |
| Cronbach’s α=0.957 (n=405) | |||
Note. IDS=Inventory of Drinking Situations, M=mean, SD=standard deviation.
2.2.2. Alcohol Consumption
Alcohol consumption was measured with a calendar-based method (Miller, 1996; Sobell and Sobell, 1992). Baseline data comprised percent drinking days (PDD) and percent heavy drinking days (PHDD) in the 30 days before the initiation of abstinence. During the 90-day medication phase, the primary drinking outcomes included any heavy drinking (4+/5+ standard drinks for women/men) and time to first heavy drinking episode.
2.2.3. Construct Validity Measures
Baseline measures also included alcohol use severity, psychological distress, and personality traits associated with reward/relief profiles in a prior analysis of the PREDICT data (Mann et al., 2018). These measures included the Alcohol Dependence Scale (ADS; Skinner and Horn, 1984); self-reported age of alcohol dependence onset; Alcohol Use Disorders Identification Test (AUDIT; Bohn et al., 1995); Obsessive-Compulsive Drinking Scale (OCDS; Anton, 2000); Beck Depression Inventory (BDI; Beck et al., 1988); Perceived Stress Scale (PSS; Cohen et al., 1983); trait subscale of the State-Trait Anxiety Inventory (STAI; Spielberger and Sydeman, 1994); and the harm avoidance and novelty seeking subscales of the Temperament and Character Inventory (TCI; Cloninger et al., 1993).
2.3. Data Analyses
2.3.1. Identifying a Brief Version of the Inventory of Drinking Situations
Information on statistical packages and missing data is available in Supplementary Materials. We utilized item response theory (IRT) with a 2-parameter graded response model (2PL; Samejima, 1997) and tests of differential item functioning (DIF) to identify a brief IDS. IRT and DIF analyses were conducted separately for the reward and relief factors, given that IRT assumes unidimensionality (Kline, 2015). In the 2PL models, the discrimination parameter represents the strength of the association between the item and the latent construct. The difficulty parameter represents the level of the latent construct at which 50% of participants endorse a particular response. Given that the IDS has four categorical response options, three difficulty parameters were estimated per item. We retained items for the RR-IDS that demonstrated strong associations with the latent construct and good latent construct coverage.
To assess DIF, or bias, in items across sex, age, and alcohol dependence severity (scores on the ADS), we utilized multiple indicator multiple cause (MIMIC) models (Supplementary Figure 1). MIMIC models can be used to assess item DIF by examining whether individuals from different groups have equivalent scores on a given item after controlling for the level of the latent construct (Stark et al., 2006; Woods, 2009). We separately regressed the reward and relief latent factors on covariates (sex, age, ADS) and then examined modification indices to determine the degree to which the χ2 value decreased (i.e., model fit improves) when a direct effect of a covariate in predicting items was freely estimated. Consistent with prior analyses (Kwako et al., 2019), we re-estimated models to include direct effects for items based on modification indices over 10. Items that demonstrated DIF were excluded from consideration for the RR-IDS.
Using results from the IRT and MIMIC models, we identified ten items for the RR-IDS (bolded/italicized items in Table 1). We then utilized a 2-factor confirmatory factor analysis (CFA; Supplementary Figure 2) and Cronbach’s a to assess the psychometric properties of the RR-IDS. CFA model fit is assessed by a non-significant χ2 test, the Comparative Fit Index (CFI) >.95, and Root Mean Square Error of Approximation (RMSEA) <.06 (Hu and Bentler, 1999).
2.3.2. Developing Cutoff Scores for Reward and Relief Drinking Profiles
We utilized latent profile analyses (LPA) to identify reward/relief drinking profiles with the RR-IDS items as indicators in the LPA. We anticipated a four-profile solution based on prior work (Mann et al., 2018; Witkiewitz et al., 2019b). Appropriateness of the four-profile LPA was evaluated on model interpretability; the Lo-Mendell-Rubin Likelihood Ratio Test (LRT), which provides a statistical significance test that compares a model with k-profiles to a k-1 profile model; and entropy, which provides an estimate of classification precision.
To identify potential cutoff scores, we saved participants’ most likely latent profile membership and plotted summed reward and relief subscale scores from the RR-IDS on a matrix (Rovner et al., 2019). We then tested several iterations of different cutoff scores with Receiver Operating Characteristic (ROC) curves and area Under the ROC Curve (AUC) analysis. The ROC curve plots sensitivity (the true positive rate on the y-axis) against 1 – specificity (the true negative rate on the x-axis). The AUC is an overall summary of correspondence between latent profiles and observed cutoff scores, where >0.80 is considered excellent classification (Mandrekar, 2010).
2.3.3. Examining Construct Validity
Analyses examining the construct validity and clinical utility of the RR-IDS sought to replicate a prior examination of the construct validity of latent reward/relief drinking subgroups in the PREDICT study (Mann et al., 2018). We utilized one-way ANOVA with Tukey’s HSD post hoc tests to assess the concurrent validity of the reward/relief profiles identified using the RR-IDS cutoff scores by examining differences among the profiles in age, sex, baseline PDD and PHDD, and measures described in section 2.2.3.
2.3.4. Evaluating Clinical Utility for Naltrexone Response
We then tested whether reward/relief profiles interacted with medication assignment (i.e., naltrexone, acamprosate, or placebo) to predict any heavy drinking (using logistic regression models) and the time to first heavy drinking episode (using Cox proportional hazard regression models) during the medication period. All models included the main effects of the reward/relief profiles, medication assignment, sex, age, number of days abstinent before baseline, ADS scores, and smoking status. We also conducted sensitivity analyses in a subsample of individuals whose medication adherence rate was > 80% and by including additional covariates (i.e., depression symptoms, trait anxiety, perceived stress, age of AD onset, harm avoidance, novelty seeking). These methods and results, which were essentially unchanged, are presented in Supplementary Materials.
3. Results
3.1. Identifying a Brief Version of the IDS
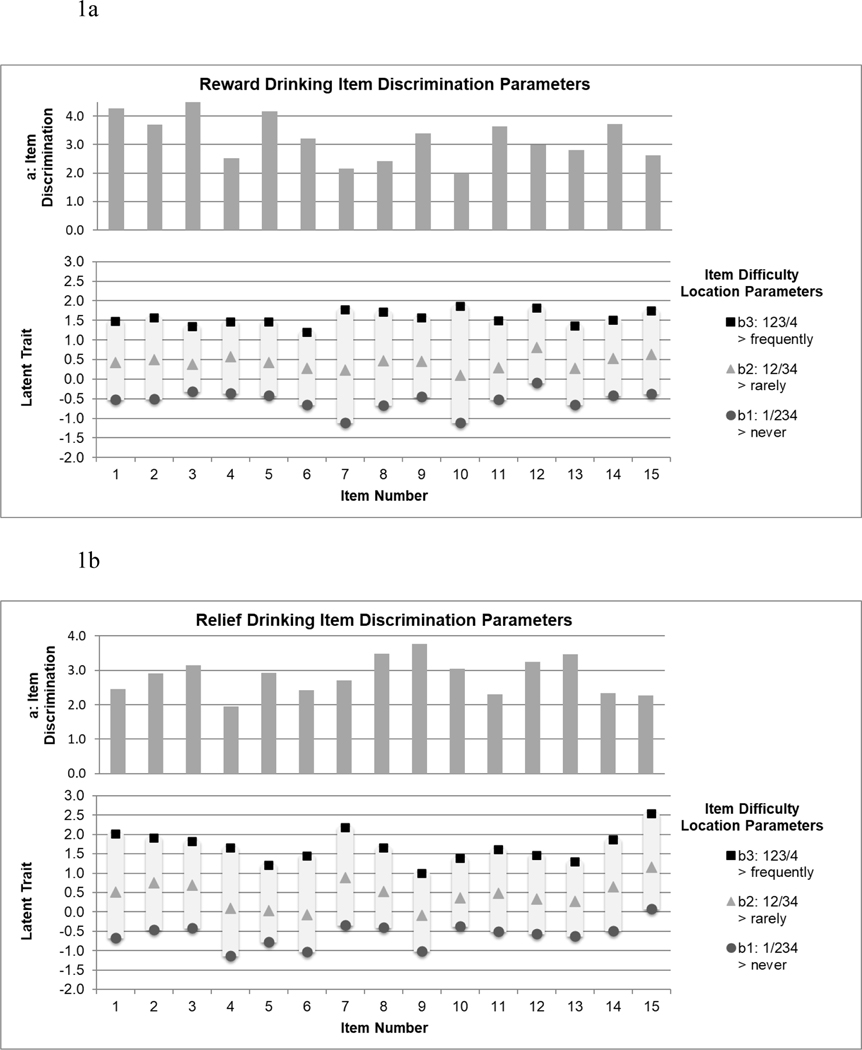
Item discrimination and difficulty parameters are presented in Figure 1a (reward items) and Figure 1b (relief items). Items were strongly related to the latent reward and relief factors (item discriminations: 1.995–4.482 for reward items, 1.954–3.773 for relief items) and were in the moderate range of the latent construct (item difficulties: −1.112–1.861 for reward items, −1.124–2.532 for relief items). The MIMIC models (Supplementary Figure 1) indicated reward and relief items were mostly free of bias across sex, age, and alcohol dependence severity. One item (reward item #15) showed bias across age, with older individuals reporting higher scores on the item at average levels of reward drinking (b(SE)=0.03 (0.01), p<0.001). This item was excluded from further consideration.
Figure 1.
Item discrimination (a) and difficulty (b) parameters for reward drinking items (Figure 1a; n=425) and relief drinking items (Figure 1b; n=426). Note. Descriptions of each item are provided in Table 1.
We then focused on items that were strongly related to the latent reward and relief factors, covered a range of the latent constructs, and had face validity for unique aspects of reward and relief drinking. We retained five reward and five relief items (Table 1 and Table 2) for the RR-IDS. A two-factor CFA, representing reward and relief subscales, demonstrated good fit to the data (n=426; χ2(34)=61.223, p=0.003; CFI=0.997; RMSEA (90% CI)=0.043 (0.025, 0.061), Supplementary Figure 2). The reward drinking (α=0.908) and relief drinking (α=0.905) subscales demonstrated good internal consistency and were positively correlated (r=0.381, p<.001).
Table 2.
Reward and Relief Inventory of Drinking Situations (RR-IDS) (Annis et al., 1987) Listed below are situations in which some people drink heavily. Read each item carefully, and answer in terms of your own heavy drinking in the past year.
| Never drank heavily | Rarely drank heavily | Often drank heavily | Always drank heavily | ||
|---|---|---|---|---|---|
| 1. | When I was at a party and others were drinking | ◻1 | ◻2 | ◻3 | ◻4 |
| 2. | When I felt unsure that I could measure up to other people’s expectations | ◻1 | ◻2 | ◻3 | ◻4 |
| 3. | When I was out with friends “on the town” and wanted to increase my enjoyment | ◻1 | ◻2 | ◻3 | ◻4 |
| 4. | When nothing I did seems right | ◻1 | ◻2 | ◻3 | ◻4 |
| 5. | When everything was going badly for me | ◻1 | ◻2 | ◻3 | ◻4 |
| 6. | When something good happened and I felt like celebrating | ◻1 | ◻2 | ◻3 | ◻4 |
| 7. | When I met with a friend and they suggested we have a drink | ◻1 | ◻2 | ◻3 | ◻4 |
| 8. | When I felt guilty about something | ◻1 | ◻2 | ◻3 | ◻4 |
| 9. | When other people treatment me unfairly | ◻1 | ◻2 | ◻3 | ◻4 |
| 10. | When I was enjoying myself at a party and wanted to feel even better | ◻1 | ◻2 | ◻3 | ◻4 |
Instructions for RR-IDS to identify reward and relief drinkers:
Calculate reward drinking score by summing items 1, 3, 6, 7, and 10: _____
Calculate relief drinking score by summing items 2, 4, 5, 8, and 9: _____
Identify subgroup:
A client is categorized in the low reward/low relief profile if their reward drinking subscale score is between 5–11 and their relief drinking subscale score is between 5–10.
A client is categorized in the high reward/low relief profile if their reward drinking subscale score is between 12–20 and their relief drinking subscale score is between 5–11.
A client is categorized in the low reward/high relief profile if their reward drinking subscale score is between 5–11 and their relief drinking subscale score is between 11–20.
A client is categorized in the high reward/high relief profile if their reward drinking subscale score is between 12–20 and their relief drinking subscale score is between 12–20.
3.2. Developing Cutoff Scores for Reward and Relief Drinking Profiles
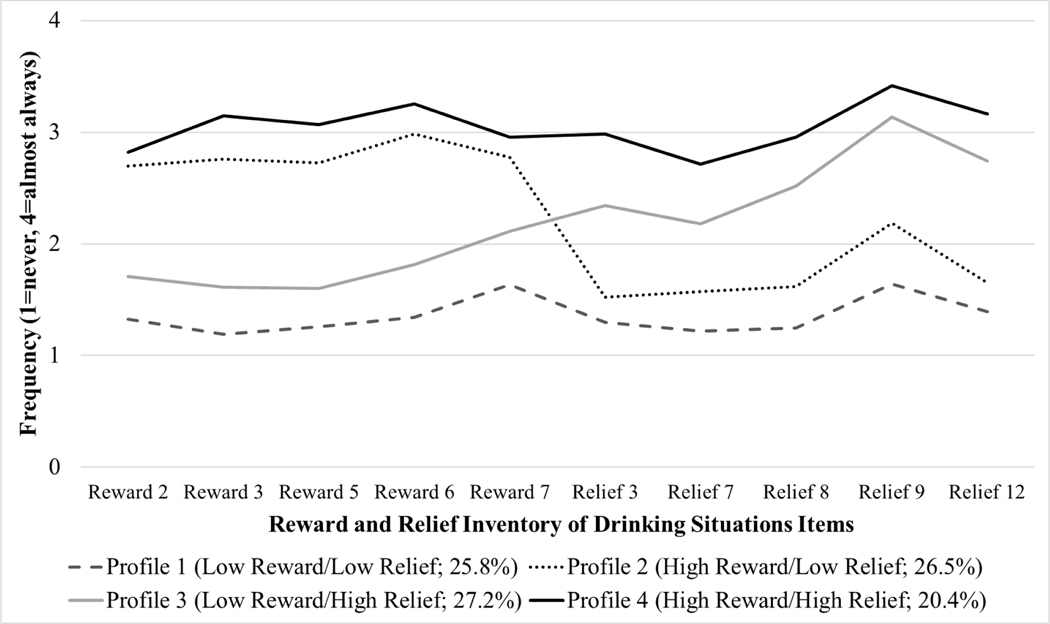
Latent profile analysis (n=426) supported a four-profile solution with excellent classification precision (entropy=0.888) and a significant LRT versus a 3-profile model (p<.001). The four-profile solution (Figure 2) was also similar to the latent profile solutions reported in prior studies (Mann et al., 2018; Witkiewitz et al., 2019b) with profiles that could be characterized as low reward/low relief (n=110; 25.8%), high reward/low relief (n=113; 26.5%), low reward/high relief (n=116; 27.2%), and high reward/high relief (n=87; 20.4%).
Figure 2.
Conditional means on the Reward and Relief Inventory of Drinking Situations (RR-IDS) items by latent profile membership. Note. Descriptions of each item are provided in Table 1.
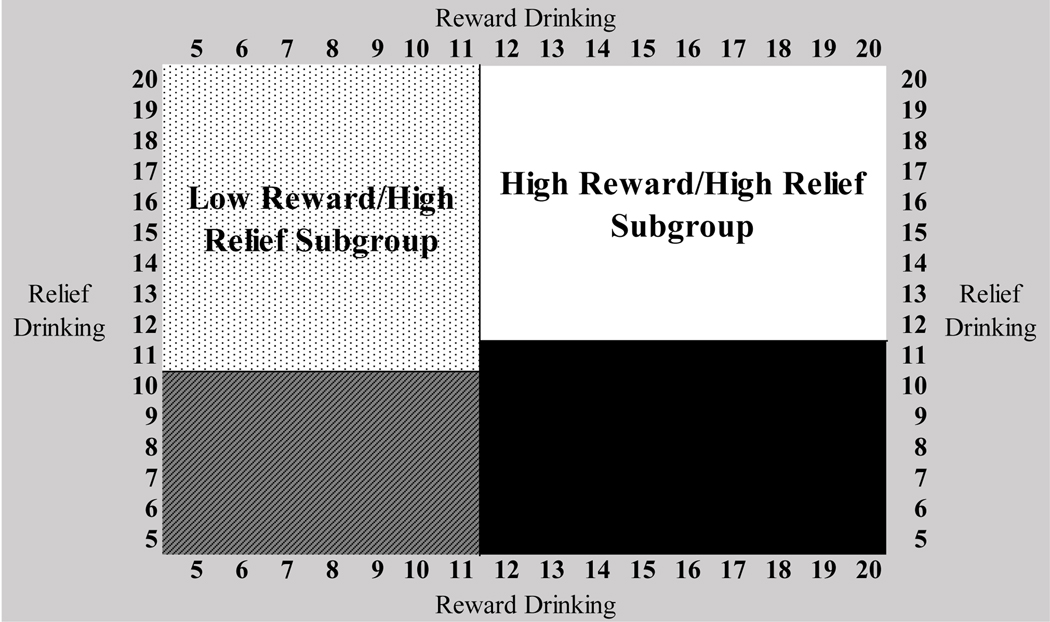
Supplementary Figure 3 depicts the matrix of reward and relief subscale scores from the RR-IDS by latent profile membership. Using this matrix, we evaluated four observed cutoff scores to identify reward/relief drinking subgroups via AUC (Supplementary Table 1). We selected observed cutoff scores (iteration 4, Supplementary Table 1) with AUC values >0.80 for all reward/relief subgroups. These cutoff scores (Figure 3) best represented the subgroups theoretically, had easily interpretable scoring guidelines, and demonstrated good sensitivity (0.798–1.000) and specificity (0.926–1.000). The RR-IDS items and scoring guidelines are presented in Table 2.
Figure 3.
The observed cutoff scores for the Reward and Relief Inventory of Drinking Situations (RR-IDS) to identify reward drinking and relief drinking subgroups. Note: Reward drinking and relief drinking subscale scores are identified by summing the reward drinking and relief drinking items from the RR-IDS, as presented in Table 1 and Table 2.
3.3. Examining Construct Validity
The ANOVAs supported the construct validity of the reward/relief drinking subgroups, as identified with the RR-IDS cutoff scores (Table 3). In comparison to the two subgroups characterized by low levels of reward drinking, the high reward/low relief subgroup had a greater proportion of men, and those in the high reward/high relief subgroup were significantly younger and had greater PDD and PHDD at baseline. Subgroups characterized by high relief drinking had greater alcohol use severity and psychological distress than subgroups characterized by low relief drinking. Subgroups characterized by high reward drinking had greater novelty-seeking scores than those in the low reward/high relief subgroup.
Table 3.
Comparisons among reward/relief subgroups (identified using the Reward and Relief Inventory of Drinking Situations and newly developed cutoff scores) on construct validity measures.
| Mean (SD) or % | Class comparisons | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| n | Profile 1: low reward/low relief (n=129; 31.2%) | Profile 2: high reward/low relief (n=87; 21.1%%) | Profile 3: low reward/high relief (n=100; 24.2%) | Profile 4: high reward/high relief (n=97; 23.5%) | Over all test | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |
| Observed IDS | ||||||||||||
| Subscales | ||||||||||||
| Reward Subscale | 41 3 | 7.41 (2.14) | 14.54 (2.03) | 8.55 (2.10) | 15.03 (2.26) | ** | ** | ** | ** | ** | NS | ** |
| Relief Subscale | 41 3 | 7.20 (1.90) | 8.33 (2.15) | 13.34 (2.15) | 14.87 (2.41) | ** | ** | ** | ** | ** | ** | ** |
| Construct Validity Variables | ||||||||||||
| Male sex | 41 3 | 71.32% | 88.51% | 70.00% | 81.44% | ** | * | NS | NS | * | NS | NS |
| Age | 41 3 | 47.64 (8.44) | 43.39 (10.01) | 45.89 (7.94) | 43.06 (7.84) | ** | ** | NS | ** | NS | NS | NS |
| ADS | 40 6 | 12.00 (5.94) | 14.14 (6.13) | 16.62 (6.53) | 17.88 (6.77) | ** | NS | ** | ** | * | ** | NS |
| Baseline PDD | 41 3 | 60.67 (25.70) | 67.87 (19.46) | 63.30 (21.86) | 71.75 (17.84) | ** | NS | NS | ** | NS | NS | * |
| Baseline PHDD | 41 3 | 57.44 (26.75) | 65.02 (21.39) | 62.06 (22.44) | 70.52 (18.39) | ** | NS | NS | ** | NS | NS | * |
| Age of onset of AD | 40 0 | 32.59 (10.85) | 29.31 (9.40) | 32.92 (10.39) | 27.77 (8.73) | ** | NS | NS | ** | NS | NS | ** |
| AUDIT | 40 9 | 23.98 (6.92) | 25.02 (6.31) | 29.34 (5.61) | 30.18 (4.87) | ** | NS | ** | ** | ** | ** | NS |
| OCDS | 41 3 | 10.99 (5.15) | 12.61 (5.25) | 15.17 (5.69) | 16.53 (6.85) | ** | NS | ** | ** | * | ** | NS |
| BDI | 40 6 | 4.77 (5.02) | 4.99 (4.81) | 7.33 (5.57) | 9.49 (6.87) | ** | NS | ** | ** | * | ** | * |
| PSS | 41 1 | 12.73 (6.04) | 13.22 (5.72) | 17.91 (7.07) | 19.74 (6.92) | ** | NS | ** | ** | ** | ** | NS |
| STAI-Trait | 40 9 | 34.06 (9.85) | 34.45 (8.43) | 41.14 (9.36) | 44.61 (11.29) | ** | NS | ** | ** | ** | ** | NS |
| TCI-harm avoidance | 40 5 | 14.91 (5.55) | 15.09 (5.54) | 18.37 (6.03) | 19.88 (7.13) | ** | NS | ** | ** | ** | ** | NS |
| TCI-novelty seeking | 40 5 | 19.18 (5.17) | 21.10 (5.02) | 18.84 (5.64) | 20.84 (5.42) | ** | NS | NS | NS | * | NS | * |
Note:
p<0.05,
p<0.01.
IDS=Inventory of Drinking Situations, M=mean, SD=standard deviation, ADS=Alcohol Dependence Scale, PDD=percent drinking days, PHDD=percent heavy drinking days, AD=alcohol dependence, AUDIT=Alcohol Use Disorder Identification Test, OCDS=Obsessive-Compulsive Drinking Scale, BDI=Beck Inventory of Drinking Situations, PSS=Perceived Stress Scale, STAI=State-Trait Anxiety Inventory, TCI=Temperament and Character Inventory.
3.4. Evaluating Clinical Utility in Predicting Naltrexone Treatment Response
Results from the logistic regression model predicting any heavy drinking (Supplementary Table 2) showed a significant interaction between the high reward/low relief subgroup (low reward/low relief subgroup as the reference) and naltrexone (placebo as the reference) in predicting any heavy drinking (b(SE)=−1.79 (0.87); p=0.039). There was also an interaction between the high reward/low relief subgroup and acamprosate (b(SE)=−1.74 (0.87); p=0.044). Simple slopes analyses indicated that among the high reward/low relief subgroup (Table 4), active medications were associated with lower odds of heavy drinking than placebo, with a slightly stronger effect for naltrexone (b(SE)=−1.67 (0.76); p=0.029) than acamprosate (b(SE)=−1.53 (0.75); p=0.041).
Table 4.
Summary of medication effects within each reward/relief drinking subgroup (n=405 for regression models, n=413 for descriptive statistics).
| Any heavy drinking during treatment as outcome | |||
|---|---|---|---|
|
| |||
| Subgroup | Naltrexone effect b (SE) OR (95% CI) |
Acamprosate effect b (SE) OR (95% CI) |
n (%) with any heavy drinking |
| Low Reward/Low Relief | 0.10 (0.52) 1.11 (0.40, 3.07) |
0.20 (0.54) 1.23 (0.43, 3.53) |
Placebo: 10/28 (35.7%) Naltrexone: 24/56 (42.9%) Acamprosate: 20/45 (44.4%) |
| High Reward/Low Relief | −1.67 (0.76)* 0.19 (0.04, 0.85) |
−1.53 (0.75)* 0.22 (0.05, 0.94) |
Placebo: 10/14 (71.4%) Naltrexone: 13/34 (38.2%) Acamprosate: 15/39 (38.5%) |
| Low Reward/High Relief | 1.06 (0.60) 2.89 (0.90, 9.26) |
0.91 (0.58) 2.48 (0.81, 7.66) |
Placebo: 8/19 (42.1%) Naltrexone: 25/38 (65.8%) Acamprosate: 27/43 (62.8%) |
| High Reward/High Relief | 0.20 (0.63) 1.22 (0.36, 4.17) |
0.84 (0.66) 2.32 (0.64, 8.43) |
Placebo: 11/22 (50.0%) Naltrexone: 22/39 (56.4%) Acamprosate: 22/36 (61.1%) |
| Time (in days) to first heavy drinking during treatment as outcome | |||
|
| |||
| Subgroup | Naltrexone effect b (SE) OR (95% CI) |
Acamprosate effect b (SE) OR (95% CI) |
Mean (SD) |
|
| |||
| Low Reward/Low Relief | 0.04 (0.41) 1.04 (0.47, 2.31) |
0.15 (0.42) 1.16 (0.51, 2.65) |
Placebo: 69.29 (32.52) Naltrexone: 67.27 (30.26) Acamprosate: 63.13 (33.94) |
| High Reward/Low Relief | −0.97 (0.45)* 0.38 (0.16, 0.92) |
−0.83 (0.42)* 0.44 (0.19, 0.99) |
Placebo: 48.79 (37.60) Naltrexone: 68.29 (30.94) Acamprosate: 66.33 (32.31) |
| Low Reward/High Relief | 0.51 (0.41) 1.67 (0.74, 3.77) |
0.63 (0.42) 1.87 (0.74, 3.77) |
Placebo: 61.89 (37.82) Naltrexone: 58.13 (31.11) Acamprosate: 50.53 (36.58) |
| High Reward/High Relief | −0.10 (0.40) 0.91 (0.41, 1.98) |
0.38 (0.40) 1.46 (0.67, 3.18) |
Placebo: 59.36 (37.22) Naltrexone: 59.00 (32.67) Acamprosate: 54.47 (34.34) |
Note:
p<0.05,
p<0.01.
b=unstandardized beta estimates, SE=standard error, OR=odds ratio, 95% CI=95% confidence interval, SD=standard deviation. Logistic regression was used for models predicting any heavy drinking and Cox proportional hazard regression for models predicting time to first heavy drinking episode; models controlled for sex, age, days abstinent prior to randomization, Alcohol Dependence Scale scores, and smoking status.
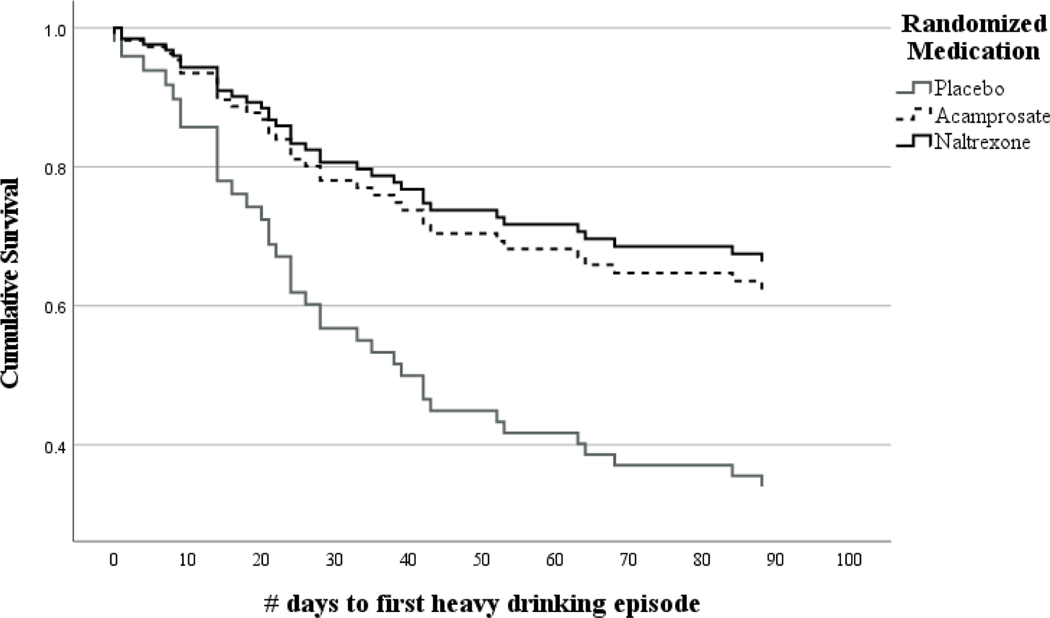
Results from Cox proportional hazard models indicated a significant interaction between the high reward/low relief subgroup and naltrexone (b(SE)=−1.16 (0.59); p=0.048) and the high reward/low relief subgroup and acamprosate (b(SE)=−1.19 (0.58); p=0.040) in predicting the time to first heavy drinking episode (Supplementary Table 2). Simple slopes analyses (Table 4) indicated naltrexone and acamprosate were associated with a longer time to first heavy drinking episode in the high reward/low relief subgroup (Figure 4), with the effect slightly stronger for naltrexone (b(SE)=−0.97 (0.45); p=0.031) than acamprosate (b(SE)=−0.83 (0.42); p=0.047).
Figure 4.
Cox proportional hazard model predicting time to first heavy drinking episode by randomized medication group among the high reward/low relief subgroup (n=86)
4. Discussion
We aimed to identify a brief, psychometrically-valid version of the IDS and empirically-derived, practical cutoff scoring guidelines for identifying reward/relief phenotypes and predicting AUD medication response. Consistent with the reward drinker-naltrexone response hypothesis and prior studies (Mann et al., 2018; Roos et al., 2021; Witkiewitz et al., 2019b), there was an 81% decreased likelihood of heavy drinking–a large effect size–during treatment for the high reward/low relief subgroup that received naltrexone versus placebo. This finding is notable, given there was no benefit of naltrexone or acamprosate over placebo in the overall sample (Mann et al., 2013), and adds to the growing body of literature indicating that naltrexone (in combination with psychosocial treatment) could be a front-line treatment for individuals whose alcohol use is primarily maintained through positive reinforcement processes. Interestingly, the high reward/low relief subgroup also had a 78% decreased likelihood of heavy drinking with acamprosate versus placebo treatment. The RR-IDS could have greater clinical utility in personalizing AUD treatment, given that it is briefer than other available measures of reward/relief drinking.
The RR-IDS differs from other reward/relief drinking measures that assess the strength of drinking temptation in various contexts (i.e., AASE) and individuals’ perceptions of the extent to which their drinking is motivated by various reasons (i.e., DMQ, RRHDS). We believe that the IDS’ focus on actual behavior, rather than the perceived motivation for behavior, is a strength of this measure because individuals’ self-reported motivations might not reflect actual behaviors. Perceived coping motives do not consistently predict drinking on days with higher than average negative affect (Votaw and Witkiewitz, 2021), and perceptions of drinking to obtain a pleasant feeling might not predict sensitivity to the stimulatory effects of alcohol (Grodin et al., 2019). A potential weakness of the RR-IDS is that the reward drinking items primarily capture instrumental positive reinforcement processes (e.g., drinking in social situations) rather than other aspects of reward drinking, including cue-elicited craving and drinking to obtain positive pharmacological effects of alcohol (Verheul et al., 1999). Of note, prior studies have shown that drinking to enhance social situations and obtain positive pharmacological effects of alcohol demonstrate moderate to high correlations with one another (Kuntsche et al., 2005).
Several questions remain regarding the measurement of reward drinking, including whether facets of reward drinking (e.g., social drinking, cue reactivity) cluster together, how they can be measured via self-report questionnaires, and which facets best predict the response to naltrexone. In addition, other reward/relief drinking measures may be better suited to identifying primary relief drinkers. A study using the AASE found that individuals in the low reward/high relief subgroup responded better to acamprosate versus placebo (Roos et al., 2017). Directly comparing reward/relief drinking measures using multimethod designs (e.g., ecological momentary assessment, alcohol administration) might determine which reward/relief drinking measures best reflect the phenotypes and predict response to AUD pharmacotherapy.
Nevertheless, the observed reward/relief profiles derived from the RR-IDS demonstrated good construct validity in the present analysis. Consistent with prior findings using the IDS-30 (Mann et al., 2018), the observed profiles characterized by high relief reported higher psychological distress and alcohol use severity, and profiles characterized by high reward reported higher novelty seeking. Notably, Mann and colleagues (2018) did not identify meaningful age differences between the groups. In contrast, we found that the two groups characterized by high reward drinking were younger than the low reward/low relief group. The high reward/high relief profile also had a significantly lower age of alcohol dependence onset than the profiles characterized by low reward drinking. These findings further support the construct validity of the RR-IDS and observed cutoff scores, given that the 3-stage cycle of addiction implicates reward drinking in earlier stages of addiction and relief drinking in later stages of addiction (Koob and Volkow, 2016).
Unexpectedly, the high reward/low relief subgroup had better drinking outcomes when assigned to acamprosate than placebo. This finding is difficult to explain, given that low reward/high relief drinking in the COMBINE study sample moderated the response to acamprosate, consistent with the glutamatergic mechanisms of action of acamprosate (Roos et al., 2017). Detoxication before medication treatment, which characterized the present sample but not the COMBINE study sample, may be a critical factor that accounts for the different findings on relief/reward drinking as a moderator of acamprosate treatment response (Maisel et al., 2013). In supplementary analyses, the acamprosate effects were no longer significant in the high reward/low relief subgroup among participants with high medication adherence. Still, acamprosate continued to predict a greater time to first heavy drinking episode in this subgroup when additional covariates were included. Further research is needed to understand whether individuals in the high reward/low relief subgroup could benefit from naltrexone or acamprosate treatment and under what circumstances (e.g., medication adherence and detoxification considerations). Those in the other subgroups might not benefit from either medication examined. Psychosocial treatment may be indicated for individuals who are not in the high reward/low relief subgroup, except that those in the low reward/high relief subgroup with short periods of abstinence may benefit from acamprosate (Roos et al., 2017).
4.1. Limitations and Future Directions
The present study has several limitations. First, although we aimed to develop a brief version of the IDS free of systematic bias across sex, age, and alcohol dependence severity, the interpretation of IDS items may also be influenced by cultural factors, which we did not examine here. Future studies should examine reward/relief drinking measures in diverse samples to ensure that the precision medicine implications of the findings are applicable across racial and ethnic groups. Given the small sample, we refined and validated the RR-IDS and observed cutoff scores in the same sample. Future research should do so in independent samples.
Additionally, we excluded individuals with major psychiatric disorders, and thus the sample had relatively low psychological distress. The sample was also predominantly male, and prior research indicates that women are more likely to use substances to cope with negative emotions than men (McHugh et al., 2017). Thus, future studies should examine reward/relief drinking in samples with greater sex and gender diversity who exhibit the full range of psychological distress. The effects of drinking goals and abstinence during treatment, which were not measured in the current study, should be considered in future research on the RR-IDS in studies of naltrexone and acamprosate. Abstinence and moderation goals predict different drinking outcomes in pharmacotherapy clinical trials (Dunn and Strain, 2013). In addition, naltrexone may be more beneficial in reducing heavy drinking, while acamprosate may promote abstinence (Maisel et al., 2013).
4.2. Conclusions
We validated a brief measure, the RR-IDS, showing that it has good psychometric properties and observed cutoff scores to identify four reward/relief phenotypes and predict AUD pharmacotherapy response. Prospective studies are needed to test the reward drinker-naltrexone response hypothesis prospectively. We believe that the RR-IDS will aid in such trials by making it possible to stratify randomization to naltrexone or placebo by observed reward/relief drinking subgroups. Additional studies should also explore the possibility that high reward/low relief drinkers respond better to acamprosate than placebo. Pending replication, the RR-IDS could be a precision medicine tool for prescribing AUD medications.
Supplementary Material
Highlights.
Research has found reward/relief drinking subgroups predict medication response.
Current reward/relief drinking measures might limit translation of findings.
We refined a 10-item measure and cutoff scores to assess reward/relief drinking.
This measure had good construct validity and predicted pharmacotherapy response.
Those with high reward/low relief drinking responded better to naltrexone and acamprosate.
Acknowledgements
We thank the members of the Predict Study Group (Mann et al., 2013) for their support and also for the Co-PIs of the different centers: Michael Smolka, Falk Kiefer, Stefan Wellek, Rosemarie Krämer, Silke Merkel, and Iris Reinhard (Mannheim); Michael Berner, Katrin Frick, and Horst Gann (Freiburg); Johannes Schröder and Johannes Pantel (Heidelberg); Anil Batra (Tübingen); Norbert Wodarz and Monika Johann (Regensburg); Hans Hagenbuch (Emmendingen); and Barbara Richter and Matthias Kluge (Wiesloch). We are also grateful to Drs. Matthew Pearson and Elizabeth Yeater for feedback on the conceptualization of this project.
Author Disclosures
This research was supported by the Federal Government of Germany (01EB0110), the National Institutes of Health, award numbers F31AA029266 (PI: VRV), K23AT011342 (PI: CRR), and R01AA025539 (PI: KW), and the APA Division 50 Student Research Grant (PI: VRV). Medication was donated by Bristol-Myers Squibb and MERCK Serono. These funding sources had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
Dr. Kranzler is a member of advisory boards for Dicerna Pharmaceuticals and Sophrosyne Pharmaceuticals; a consultant to Sobrera Pharmaceuticals; and is named as an inventor on PCT patent application #15/878,640 entitled: “Genotype-guided dosing of opioid agonists,” filed January 24, 2018. Drs. Kranzler, Mann, and Witkiewitz are members of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was supported in the last three years by AbbVie, Alkermes, Dicerna, Ethypharm, Indivior, Lilly, Lundbeck, Otsuka, Pfizer, Arbor, and Amygdala Neurosciences.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association, 1994. Diagnostic and statistical manual of mental disorders (4th ed.; DSM-IV), 4th ed. ed. Author, Washington. [Google Scholar]
- Annis HM, Graham JM, Davis CS, 1987. Inventory of drinking situations: User’s guide. Addiction Research Foundation, Toronto. [Google Scholar]
- Anton RF, 2000. Obsessive-compulsive aspects of craving: development of the Obsessive Compulsive Drinking Scale. Addict. Abingdon Engl. 95 Suppl 2, S211–217. 10.1080/09652140050111771 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG, 1988. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin. Psychol. Rev. 8, 77–100. 10.1016/0272-7358(88)90050-5 [DOI] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler HR, 1995. The Alcohol Use Disorders Identification Test (AUDIT): Validation of a screening instrument for use in medical settings. J. Stud. Alcohol 56, 423–432. 10.15288/jsa.1995.56.423 [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR, 1993. A Psychobiological Model of Temperament and Character. Arch. Gen. Psychiatry 50, 975–990. 10.1001/archpsyc.1993.01820240059008 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396. [PubMed] [Google Scholar]
- Dunn KE, Strain EC, 2013. Pretreatment Alcohol Drinking Goals are Associated with Treatment Outcomes. Alcohol. Clin. Exp. Res. 37, 1745–1752. 10.1111/acer.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glöckner-Rist A, Lémenager T, Mann K, PREDICT Study Research Group, 2013. Reward and relief craving tendencies in patients with alcohol use disorders: results from the PREDICT study. Addict. Behav. 38, 1532–40. 10.1016/j.addbeh.2012.06.018 [DOI] [PubMed] [Google Scholar]
- Grodin EN, Bujarski S, Venegas A, Baskerville W-A, Nieto SJ, David Jentsch J, Ray LA, 2019. Reward, Relief and Habit Drinking: Initial Validation of a Brief Assessment Tool. Alcohol Alcohol 54, 574–583. 10.1093/alcalc/agz075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, Bentler PM, 1999. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. 6, 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Kline RB, 2015. Principles and Practice of Structural Equation Modeling, Fourth Edition. Guilford Publications. [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, Engels R, 2005. Why do young people drink? A review of drinking motives. Clin. Psychol. Rev. 25, 841–61. 10.1016/j.cpr.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Kwako LE, Schwandt ML, Ramchandani VA, Diazgranados N, Koob GF, Volkow ND, Blanco C, Goldman D, 2019. Neurofunctional Domains Derived From Deep Behavioral Phenotyping in Alcohol Use Disorder. Am. J. Psychiatry appiajp201818030357–appiajp201818030357. 10.1176/appi.ajp.2018.18030357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SP, Sher KJ, 2015. Limits of current approaches to diagnosis severity based on criterion counts: An example with DSM-5 alcohol use disorder. Clin. Psychol. Sci. 3, 819–835. 10.1177/2167702614553026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF, 2015. Heterogeneity of Alcohol Use Disorder: Understanding Mechanisms to Advance Personalized Treatment. Alcohol. Clin. Exp. Res. 39, 579–584. 10.1111/acer.12669 [DOI] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW, 2013. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction 108, 275–293. 10.1111/j.1360-0443.2012.04054.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar JN, 2010. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 5, 1315–1316. 10.1097/JTO.0b013e3181ec173d [DOI] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Smolka M, Gann H, Wellek S, Heinz A, 2009. Searching for Responders to Acamprosate and Naltrexone in Alcoholism Treatment: Rationale and Design of the Predict Study. Alcohol. Clin. Exp. Res. 33, 674–683. 10.1111/j.1530-0277.2008.00884.x [DOI] [PubMed] [Google Scholar]
- Mann K, Lemenager T, Hoffmann S, Reinhard I, Hermann D, Batra A, Berner M, Wodarz N, Heinz A, Smolka MN, Zimmermann US, Wellek S, Kiefer F, Anton RF, 2013. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict. Biol. 18, 937–946. 10.1111/adb.12012 [DOI] [PubMed] [Google Scholar]
- Mann K, Roos CR, Hoffmann S, Nakovics H, Leménager T, Heinz A, Witkiewitz K, 2018. Precision Medicine in Alcohol Dependence: A Controlled Trial Testing Pharmacotherapy Response Among Reward and Relief Drinking Phenotypes. Neuropsychopharmacology 43, 891–899. 10.1038/npp.2017.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Votaw VR, Sugarman DE, Greenfield SF, 2017. Sex and gender differences in substance use disorders. Clin. Psychol. Rev. 10.1016/j.cpr.2017.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, 1996. Form 90: A structured assessment interview for drinking and related behaviors, Project MA. ed. National Institute on Alcohol Abuse and Alcoholism, Bethesda. [Google Scholar]
- Pettinati HM, Weiss RD, Miller WR, Donovan DM, Ernst D, Rounsaville BJ, 2004. COMBINE Monograph Series, Volume 2. Medical Management Treatment Manual: A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence, DHHS Publi. ed. National Institute on Alcohol Abuse and Alcoholism, Bethesda. [Google Scholar]
- Roos CR, Bold KW, Witkiewitz K, Leeman RF, DeMartini KS, Fucito LM, Corbin WR, Mann K, Kranzler HR, O’Malley SS, 2021. Reward drinking and naltrexone treatment response among young adult heavy drinkers. Addiction 116, 2360–2371. 10.1111/add.15453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CR, Mann K, Witkiewitz K, 2017. Reward and relief dimensions of temptation to drink: construct validity and role in predicting differential benefit from acamprosate and naltrexone. Addict. Biol. 22, 1528–1539. 10.1111/adb.12427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovner G, Johansson F, Gillanders D, 2019. Cutoff scores for the 8-item version of the Chronic Pain Acceptance Questionnaire (CPAQ-8) to identify different profiles of pain acceptance patterns, levels of function and behavioral flexibility. J. Context. Behav. Sci. 14, 146–156. 10.1016/j.jcbs.2019.07.006 [DOI] [Google Scholar]
- Samejima F, 1997. Graded Response Model, in: Handbook of Modern Item Response Theory. Springer; New York, pp. 85–100. 10.1007/978-1-4757-2691-6_5 [DOI] [Google Scholar]
- Skinner HA, Horn JL, 1984. Alcohol Dependence Scale (ADS) user’s guide. Addiction Research Foundation, Toronto. [Google Scholar]
- Sobell LC, Sobell MB, 1992. Timeline Follow-Back, in: Measuring Alcohol Consumption. Humana Press, pp. 41–72. 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- Spielberger CD, Sydeman SJ, 1994. State-trait anxiety inventory and state-trait anger expression inventory, in: The Use of Psychological Testing for Treatment Planning and Outcome Assessment. [Google Scholar]
- Stark S, Chernyshenko OS, Drasgow F, 2006. Detecting differential item functioning with confirmatory factor analysis and item response theory: Toward a unified strategy. J. Appl. Psychol. 91, 1292–1306. 10.1037/0021-9010.91.6.1292 [DOI] [PubMed] [Google Scholar]
- Verheul R, van den Brink W, Geerlings P, 1999. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol. Oxf. Oxfs. 34, 197–222. 10.1093/alcalc/34.2.197 [DOI] [PubMed] [Google Scholar]
- Votaw VR, Witkiewitz K, 2021. Motives for Substance Use in Daily Life: A Systematic Review of Studies Using Ecological Momentary Assessment. Clin. Psychol. Sci. 9, 535–562. 10.1177/2167702620978614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Litten RZ, Leggio L, 2019a. Advances in the science and treatment of alcohol use disorder. Sci. Adv. 5. 10.1126/sciadv.aax4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Montes KS, Schwebel FJ, Tucker JA, 2020. What Is Recovery? Alcohol Res. Curr. Rev. 40, 01. 10.35946/arcr.v40.3.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Roos CR, Mann K, Kranzler HR, 2019b. Advancing Precision Medicine for Alcohol Use Disorder: Replication and Extension of Reward Drinking as a Predictor of Naltrexone Response. Alcohol. Clin. Exp. Res. 43, 2395–2405. 10.1111/acer.14183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods CM, 2009. Evaluation of MIMIC-Model Methods for DIF Testing With Comparison to Two-Group Analysis. Multivar. Behav. Res. 44, 1–27. 10.1080/00273170802620121 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.