Abstract
Opioid addiction remains a severe health problem. While substantial insights underlying opioid addiction have been yielded from neuron-centric studies, the contribution of non-neuronal mechanisms to opioid-related behavioral adaptations has begun to be recognized. Toll-like receptor 4 (TLR4), a pattern recognition receptor, has been widely suggested in opioid-related behaviors. Interleukin-1 receptor-associated kinase 4 (IRAK4) is a kinase essential for TLR4 responses, However, the potential role of IRAK4 in opioid-related responses has not been examined. Here, we explored the role of IRAK4 in cue-induced opioid-seeking behavior in male rats. We found that morphine self-administration increased the phosphorylation level of IRAK4 in the nucleus accumbens (NAc) in rats; the IRAK4 signaling remained activated after morphine extinction and cue-induced reinstatement test. Both systemic and local inhibition of IRAK4 in the NAc core attenuated cue-induced morphine-seeking behavior without affecting the locomotor activity and cue-induced sucrose-seeking. In addition, inhibition of IRAK4 also reduced the cue-induced reinstatement of fentanyl-seeking. Our findings suggest an important role of IRAK4 in opioid relapse-like behaviors and provide novel evidence in the association between innate immunity and drug addiction.
Keywords: IRAK4, opioid addiction, neuroimmune response, cue-induced opioid-seeking, nucleus accumbens, relapse
1. Introduction
Opioid addiction is a chronic and relapsing disease characterized by compulsive opioid-seeking, continued use of opioids despite the knowledge of adverse consequences and unsuccessful efforts to control the drug use (Cami and Farre, 2003; Dennis et al., 2020; Strang et al., 2020). Dopaminergic and glutamatergic projections that send signals from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) (i.e. mesolimbic system) are crucial to opioid-associated behaviors (Sesack and Grace, 2010). Opioids increase dopaminergic signals in the NAc via activation of dopaminergic neurons, resulting in increased dopamine release (Browne et al., 2020; Fields and Margolis, 2015; Volkow and Morales, 2015). Long-term exposure to opioids also involves the glutamatergic input to the NAc, which is associated with synaptic changes underlying opioid-related behavioral adaptations (Strang et al., 2020). Though these neuron-centric studies have yielded substantial insights into mechanisms underlying opioid addiction, the contribution of non-neuronal mechanisms to behavioral adaptations has been largely underestimated.
Recent studies have begun to illustrate the potential role of innate immunity in opioid-related responses (Lewitus et al., 2016; Sekine et al., 2008). Toll-like receptor 4 (TLR4) is a pattern recognition receptor (Dunne et al., 2011) that recognizes pathogen-associated molecular patterns (PAMPs) and activates innate immunity (Liu et al., 2010). Morphine is shown to dock at the LPS-binding site of MD-2, the co-receptor of TLR4 and induces TLR4 oligomerization. This results in production of pro-inflammatory cytokines and chemokines, which are associated with opioid-related behaviors (Hutchinson et al., 2012; Hutchinson et al., 2010; Wang et al., 2012). Importantly, blockade of TLR4 by (+)-naloxone or (+)-naltrexone, the selective TLR4 antagonists which are μ-opioid receptors (MOR) inactive isomers, reduced opioid-related analgesic tolerance, withdrawal symptoms, heroin incubation, morphine conditioned place preference (CPP) and remifentanil self-administration (Hutchinson et al., 2012; Hutchinson et al., 2011; Hutchinson et al., 2010; Theberge et al., 2013). These studies implicate TLR4 in opioid-related behaviors. However, TLR4 mutant and null mice were reported to maintain opioid-induced tolerance, hyperalgesia and physical dependence (Mattioli et al., 2014), which challenges the role of TLR4 in opioid-related responses. Since studies focusing on TLR4 in opioid-related actions have yielded conflicting results, it is worth exploring whether the downstream effector of TLR4, like interleukin-1 receptor-associated kinase 4 (IRAK4), could be a more specific and reliable target for modulating opioid-related responses.
IRAK4 is a serine/threonine kinase expressed in glia cells in the brain, primarily microglia and astrocytes (Cameron et al., 2012; McCarthy et al., 2017; Yuan et al., 2015). It is the first kinase activated by TLR4 and is essential for the transduction of TLR4 response (De Nardo et al., 2018; Kim et al., 2007; Suzuki et al., 2002a). Upon TLR4 activation, a signaling platform termed “myddosome” is formed. The myddosome is comprised of myeloid differentiation primary response 88 (MyD88) and members of the IRAK family, including IRAK4 (Lin et al., 2010; Motshwene et al., 2009). Myddosome formation promotes IRAK4 auto-activation, which in turn activates down-stream transcription factors and results in production of pro-inflammatory cytokines and chemokines (De Nardo et al., 2018; Ferrao et al., 2014; Kawagoe et al., 2008). Given the indispensable role of IRAK4 in TLR4-mediated immune responses and its potential role in opioid-related responses, we wanted to the first to examine how IRAK4 is involved in opioid addiction.
The tendency to relapse is a core behavioral pathology of opioid addiction, even after long-term abstinence. Thus, strategies that can prevent drug relapse will contribute substantially to cessation of drug use (Sinha, 2011). A major contributor to opioid relapse is the exposure to cues that have previously been associated with opioid self-administration (Lee et al., 2006). Such drug-associated cues can provoke memories of drug effect and induce drug-seeking behaviors (Bossert et al., 2013). Therefore, prolonged vulnerability to cue-induced relapse remains a major problem to the treatment of opioid addiction. While IRAK4 is indispensable for TLR4-mediated immune responses which have been associated with opioid-related behaviors, it remains unclear whether IRAK4 could modulate cue-induced opioid relapse. To answer this question, we first determined whether morphine exposure could activate IRAK4 in the NAc, and then examined the activation of IRAK4 signaling following extinction and cue-reinstatement of morphine-seeking behavior. We also tested whether manipulation of IRAK4 could affect cue-induced morphine-seeking behavior using drug reward behavioral assay and pharmacological approaches. Moreover, we examined the effect of IRAK4 in cue-induced reinstatement of fentanyl-seeking behavior to confirm its role in opioid addiction.
2. Materials and Methods
2.1. Animals
Adult male Sprague–Dawley rats (initial weight 250–280 g; Envigo, Indianapolis, IN) were housed individually on a 12/12-h light/dark cycle with free access to water and food except during some experimental sessions. Rats were allowed to acclimate for at least 1 week before surgery or experiment. All experimental procedures were approved by the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York, and were in accordance with the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington DC).
2.2. Drugs
Morphine sulfate was provided by the National Institute of Drug Abuse (Research Technology Branch, NIH, Rockville, MD), while fentanyl was purchased from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in 0.9% saline. IRAK4 inhibitor PF06650833 (Cat. No. 6373) was purchased from Tocris (R&D System, Bristol, UK) and dissolved in vehicle comprised of 10% DMSO, 10% Emulphor-620 (Rhodia), and 80% physiologic saline. The doses of morphine (0.3mg/kg/infusion) and fentanyl (0.0032mg/kg/infusion) for self-administration were determined according to our previous studies and others (Ezeomah et al., 2020; Liu et al., 2020; Ucha et al., 2019). The doses of PF 06650833 (0.3, 1 and 3 mg/kg, i.p. and 0.3, 1ug/side, intra NAc) were selected according to previous report (Pletinckx et al., 2020).
2.3. Surgery
Rats (weighing 280–300 g at the beginning of the experiments) were anesthetized with ketamine and xylazine (75 and 5 mg/kg, respectively, i.p.) and were injected with Carprofen (5 mg/kg, s.c.) immediately after surgery and for the following 2 days to relieve pain. Rats were allowed to recover from surgery for 1 week.
2.3.1. Catheterization
Rats were implanted with chronic indwelling jugular catheters as previously described (Liu et al., 2018). Catheters were flushed daily with 0.2 ml solution of enrofloxacin (4 mg/ml) mixed in a heparinized saline solution (50 IU/ml in 0.9% sterile saline) for 1 week after surgery to preserve catheter patency and prevent infection (Liu et al., 2018; Thorn et al., 2014).
2.3.2. Stereotaxic Surgery
In experiment 4, we performed the stereotaxic surgery after the morphine self-administration training as previously described (Chai et al., 2014; Liu et al., 2015; Thorn et al., 2016). Guide cannula (23 gauge; Plastics One, Roanoke, VA, USA) were bilaterally implanted 1 mm above the NAc. Coordinate for cannula: angle, 10°; anterior/posterior (AP), +1.7 mm; medial/lateral (ML), ±2.2 mm; dorsal/ventral (DV), −5.7 mm from the bregma (G. and C., 2014). The cannula was anchored to the skull with 3–4 stainless-steel screws and dental cement. A stainless-steel stylet blocker was inserted into each cannula to keep it patent and to prevent infection.
2.4. Intracranial microinjections
PF06650833 (0.3 or 1 μg/side) or vehicle (1 ul/side) were freshly prepared and infused bilaterally over 2 min via microinjection needles, which are 1 mm longer than the cannula. Following the microinjections, the needles were kept in place for an additional 1 min to allow for full drug diffusion. The cannula placements were confirmed in 30-μm-thick sections using Nissl staining under light microscopy after the behavioral tests.
2.5. Tissue collection and western Blotting
Fresh brains were sliced by a rat brain matrix. Punches of the NAc core were taken and stored individually in 1 ml Eppendorf tubes in −80 C for western blotting. The Western blotting was based on our previous studies with some modifications (Liu et al., 2015; Wu et al., 2020; Wu et al., 2017). Brain tissue punches from each rat were homogenized in 25 mmol/L Tris (pH 8.0) and 0.25 mol/L sucrose buffer. The total protein concentrations of all of the samples were determined using the BCA assay kit (Bio-Rad Laboratories, Inc. Hercules, California). 3 X loading buffer (CST, Boston, MA) was added to each sample (2:1, sample: loading buffer) before being boiled for 5 min. The samples were cooled and subjected to SDS-PAGE for ~40 min at 80 V in stacking gel and ~1 h at 120 V in resolving gel. The proteins were electrophoretically transferred to Immobilon-P transfer membranes (Millipore) at 250 mA for 1.5 h. The membranes were washed with phosphate-buffered saline plus 0.05% Tween 20, pH 7.4, (PBST) and then dipped in blocking buffer (5% BSA in PBST) for blocking at room temperature for 1 h. The membranes were incubated for overnight at 4°C on a shaker with primary antibodies in PBST plus 5% BSA. After three 5 min washes in PBST buffer, the blots were incubated for 90 min at room temperature on a shaker with horseradish peroxidase-conjugated secondary antibody diluted 1:5000 in blocking buffer. The blots were then washed three times for 5 min each in TBST and incubated with a layer of Super Signal West Femto maximum sensitivity substrate (Thermo scientific, Rockford, IL). Band intensities were quantified using Image J (National Institutes of Health, Bethesda, MD).
The following primary antibodies were used: anti-phospho-IRAK1 (1:500; cat# SAB4504246; Rabbit; MilliporeSigma, Burlington, MA), anti-tIRAK1 (1:1000; cat# SAB4501559; Rabbit; MilliporeSigma), anti-phospho-IRAK4 (1:500; cat# 11927S; Rabbit; CST, Boston, MA), anti-tIRAK4 (1:1000; cat# 4363S; Rabbit; CST), anti-Phospho-IKKα/β (1:500; cat# 2697S; Rabbit; CST), anti-tIKKβ (1:500; cat# 8943S, Rabbit; CST), anti-TNF-α (1:500; cat# 6945S; Rabbit; CST), β-Actin (1:2000, cat# 8457P; Rabbit; CST), anti-GAPDH (1:2000; cat# ab9485, Rabbit; Abcam, Cambridge, MA). The isolation of soluble-TNF-α and membrane-TNF-α was conducted according to the difference in their molecular weight (soluble-TNF-α: 18 kDa, membrane-TNF-α: 25 kDa). All image acquisition and analyses were conducted by investigators blind to the experimental conditions.
2.6. Operant chambers
Eighteen standard operant chambers (Med Associates, St Albans, Vermont) were used for saline, morphine, fentanyl and sucrose self-administration studies (Liu et al., 2017). Chambers contained two response levers; responses on the active (right) lever were associated with saline, morphine, fentanyl infusions or sucrose deliveries, while responses on the inactive (left) lever were recorded and had no programmed consequence. Stimulus presentations, saline, morphine and fentanyl infusions or sucrose deliveries, and data recording were controlled by Med-PC IV software (Med Associates, St Albans, Vermont).
2.7. Procedures
2.7.1. Drug self-administration
After recovery from surgery (1 week), rats were trained to lever press for saline, morphine (0.3 mg/kg/infusion; 2 hours/session/day) or fentanyl (0.0032mg/kg/infusion; 2 hours/session/day), during which responses to the active lever resulted in intravenous injections of saline, morphine or fentanyl under a fixed ratio (FR) schedule of reinforcement followed by a 30-s time-out period. Drug infusions were accompanied by a 5-s illumination of the stimulus light above the active lever (right cue), and the house light was extinguished for the duration of the time-out period. During this training phase, the response requirement was gradually increased from FR 1 to FR 5. Rats that self-administered more than ten infusions of drug (2 hours) on any day during the training were moved up to the next FR (FRs: 1, 2, 3, 5).
2.7.2. Sucrose self-administration
Sucrose self-administration (2 h/session/day) was performed similar to the drug self-administration, except that a sucrose pellet (45 mg dustless precision pellets; Sucrose; Product # F0023; Bio-Serv) rather than an injection of drug was delivered following responding in the active lever. Rats that trained for sucrose self-administration did not receive catheterization surgery. Initially, a single response produced a sucrose pellet; as performance improved, the response requirement was progressively increased across days to a final FR of 5. During the 2-h sessions, rats could get a maximum of 100 sucrose pellets. Rats have free access to food after the sucrose-administration training.
2.7.3. Extinction
One day after the maintenance of drug or sucrose self-administration, rats underwent daily extinction sessions (2-h session), during which lever presses had no consequence (no drug or cue). At the end of extinction, all rats in the study reached the extinction criteria (no more than 10 active lever responses per 2 h over two consecutive sessions).
2.7.4. Cue-induced reinstatement of drug or sucrose-seeking
One day after the last extinction, rats were tested for cue-induced reinstatement (2 h) of drug- (morphine or fentanyl) or sucrose-seeking during which responding to the active lever (FR5) resulted in the presentation of lights with no drug or sucrose delivery.
2.7.5. Locomotion test
Locomotor activity was recorded by an infrared motion-sensor system (AccuScan Instruments) fitted outside plastic cages (40 × 40 × 30 cm) (Liu et al., 2018). The plastic cages contained a thin layer of corn cob bedding and were cleaned between each test session. The Fusion activity-monitoring system software monitors infrared beam breaks at a frequency of 0.01 s. The interruption of any beam not interrupted during the previous sample was interpreted as an activity score. The Versa Max animal activity monitoring software monitors the distance the rats traveled in 45 min.
2.8. Specific Experiments
2.8.1. Experiment 1
Effect of morphine self-administration on the activation of IRAK4 in the NAc core
In experiment 1, we determined whether morphine self-administration would affect the activation of IRAK4 in the NAc core. We used two groups of rats in an experiment design that included the between-subject factor of drug treatment (saline, n=6; morphine, n=7). We first trained rats to self-administer morphine or saline in 2 weeks (from FR 1 to 5) and then maintained (FR5) for another 2 weeks. Then, these two groups of rats were decapitated immediately after the last training session and brain tissue were collected.
2.8.2. Experiment 2
Effect of morphine extinction and cue-induced reinstatement test on the activation of IRAK4 signaling in the NAc core
In experiment 1, we found selective activation of IRAK4, but not IRAK1, in the NAc core after the morphine self-administration training. Based on these results, we determined whether IRAK4 and its signaling was activated after the extinction and cue-induced reinstatement of morphine-seeking in experiment 2. We used four groups of rats (Morphine-Extinction: n=7; Morphine-Cue: n=7; Saline-Extinction: n=6; Saline-Cue: n=6) in an experimental design that included the between-subject factors of drug treatment (Morphine, saline) and within-subject factors of test condition (extinction, cue-induced reinstatement). The experiment consisted of the following three phases: training phase, extinction phase and cue-induced reinstatement test phase.
Training:
This experiment conditions were similar to those described in experiment 1. Rats were then assigned to different groups according to the responses in the maintenance of morphine self-administration such that groups of the same treatment (i.e. Morphine-Extinction vs. Morphine-Cue; saline-Extinction vs. saline-Cue) had similar levels of morphine or saline self-administration. Repeated-measures of ANOVA was conducted to analyze the morphine or saline self-administration to ensure the groups were not statistically different.
Extinction:
All groups of rats underwent extinction training (7 days) and reached the extinction criteria. Then, Morphine-Extinction group and saline-Extinction group were decapitated 1 day after the last extinction session.
Cue-induced reinstatement test:
One day after the last extinction session, Morphine-Cue group and saline-Cue group were tested for cue-induced drug-seeking and brain tissues were collected immediately after the test.
2.8.3. Experiment 3
Effect of systemic inhibition of IRAK4 in the cue-induced reinstatement of morphine-seeking
In this experiment, we determined whether systemic inhibition of IRAK4 could affect the cue-induced morphine-seeking using PF06650833, a highly potent and selective inhibitor of IRAK4. This compound is already in phase II clinical trials for the treatment of COVID-19 respiratory infection (NCT04575610) and rheumatoid arthritis (NCT02996500) with promising outcomes (Danto S, 2019). We used six groups of rats in an experimental design that included the between-subject factors of PF06650833 dose (0, 0.3, 1 and 3 mg/kg) and drug treatment (saline and morphine), and the within-subject factor of time (last extinction and cue test). For saline self-administration, we included 12 rats for two groups (saline-vehicle and saline-PF 1mg/kg, n = 6). For morphine self-administration, we included 28 rats for four groups (Morphine-vehicle, Morphine-PF 0.3mg/kg, Morphine-PF 1mg/kg, Morphine-PF 3mg/kg, n=7). The experiment consisted of the following three phases: training phase, extinction phase and cue-induced reinstatement test phase.
Training:
Training conditions were similar to those of experiment 1 and 2.
Extinction:
All rats underwent extinction training (7 days) and reached the extinction criteria. Then, rats from saline and morphine self-administration group were assigned to different sub-groups with no difference in the maintenance and extinction phase, i.e. there was no difference in the maintenance and extinction of morphine self-administration among Morphine-vehicle, Morphine-PF 0.3mg/kg, Morphine-PF 1mg/kg and Morphine-PF 3mg/kg group (Table S1). Similarly, the saline-vehicle group had similar responses as the saline-PF 1mg/kg group (Table S1).
Cue-induced reinstatement test:
One day after the last extinction session, rats were tested for cue-induced reinstatement (2 h) of morphine-seeking. PF 06650833 (0.3, 1 and 3 mg/kg, i.p.) or vehicle (1 ml/kg, i.p.) was given 10 min before the test. The pretreatment time was selected based on previous studies (Lee et al., 2017; Seganish, 2016). Rats were decapitated immediately after the test and the dose of 1mg/kg PF06650833 was chosen to further measure the IRAK4 phosphorylation level because it significantly reduced the active lever presses compared to the Morphine-vehicle group.
A separate cohort of rats (n=7) was tested for the effect of IRAK4 inhibition on the locomotion in a design that included the between-subject factor of PF06650833 dose (0, 1 and 3 mg/kg). The locomotor activity of the rats was measured 10 minutes after PF 06650833 (1 or 3 mg/kg, i.p.) or vehicle (1 ml/kg, i.p.) was injected.
To determine whether the effect of PF06650833 on cue-induced morphine-seeking behavior was drug specific, we tested the effect of PF 06650833 using the sucrose self-administration model at the dose of 1mg/kg. This experiment was similar to the morphine self-administration, with two groups of rats (n=8) were included (vehicle and PF06650833 1mg/kg). Rats underwent sucrose training and extinction, and there was no difference in sucrose self-administration and extinction responses between these two groups. One day after the last extinction session, rats were tested for cue-induced sucrose-seeking. PF06650833 (1 mg/kg, i.p.) or vehicle (1 ml/kg, i.p.) was administered 10 min before the test.
2.8.4. Experiment 4
Effect of intra-NAc inhibition of IRAK4 in the cue-induced reinstatement of morphine-seeking
In this experiment, we determined whether the NAc is a key brain region for IRAK4 in mediating cue-induced morphine-seeking behavior. We used four groups of rats (saline-vehicle, n=6; morphine-vehicle, n=8; Morphine-PF 0.3ug/side, n=8; Morphine-PF 1ug/side, n=8) in an experimental design that included the between-subject factors of drug (saline and morphine), PF06650833 dose (0, 0.3 and 1ug/side) and the within-group subject factors of time. This experiment consisted of the following three phases: training phase, extinction phase and cue-induced reinstatement test phase.
Training:
Training conditions were similar to those of experiments 1–3. After the training, rats had stereotaxic surgery and were allowed to recover for 1 week.
Extinction:
One week after cannula implantation, all rats underwent extinction training (7 days) and reached the extinction criteria. Then, rats from morphine self-administration were assigned to different groups with no difference in the maintenance and extinction phase (Table S2).
Cue-induced reinstatement test:
One day after the last extinction session, rats were tested for cue-induced reinstatement of morphine-seeking. PF06650833 (0.3 and 1 ug/side) or vehicle (1ul/side) was microinjected into the NAc 5 min before the test.
2.8.5. Experiment 5
Effect of systemic inhibition of IRAK4 in the cue-induced reinstatement of fentanyl-seeking
In this experiment, we determined whether the effect of IRAK4 inhibition could be generalized to other opioids, like fentanyl. We used four groups of rats (fentanyl-vehicle, n=9; fentanyl-PF, n=10; saline-vehicle, n=6, saline-PF, n=6) in an experimental design that included the between-subject factors of PF06650833 dose (0 and 1 mg/kg) and drug treatment (saline and fentanyl), and the within-subject factors of time. The experiment consisted of the following three phases: training phase, extinction phase and cue-induced reinstatement test phase.
Training:
Training conditions were similar to those of experiments 1–3.
Extinction:
All rats underwent extinction training (7 days) and reached the extinction criteria.
Cue-induced reinstatement test:
One day after the last extinction session, rats were tested for cue-induced reinstatement (2 h) of fentanyl-seeking. PF 06650833 (1 mg/kg, i.p.) or vehicle (1 ml/kg, i.p.) was injected 10 min before the test.
2.9. Data analysis
All results are presented as mean ± SEM and analyzed by IBM SPSS statistics (IBM, New York, NY) software and plotted by the Graphpad Prism 8 software (GraphPad Software, San Diego, CA). Two-tailed unpaired t-tests were conducted for the IRAK4 and IRAK1 activation in experiment 1 (Figure 1C and 1D) as well as the activation of IRAK4 signaling in experiment 2 (Figure 2F, 2G, 2H and 2I). Repeated-measures of ANOVA was conducted for the all other behavioral data with assumed sphericity (within-subjects and between-subject factors were specifically stated in the specific experiment section) followed by post hoc Bonferroni’s multiple comparisons test. Post hoc tests were conducted only if F in ANOVA achieved the necessary level of statistical significance (p < 0.05) and there was no significant variance in homogeneity (which precludes use of parametric statistics).
Figure 1. Rats with morphine self-administration history showed significantly increased phosphorylation level of IRAK4 in the NAc.
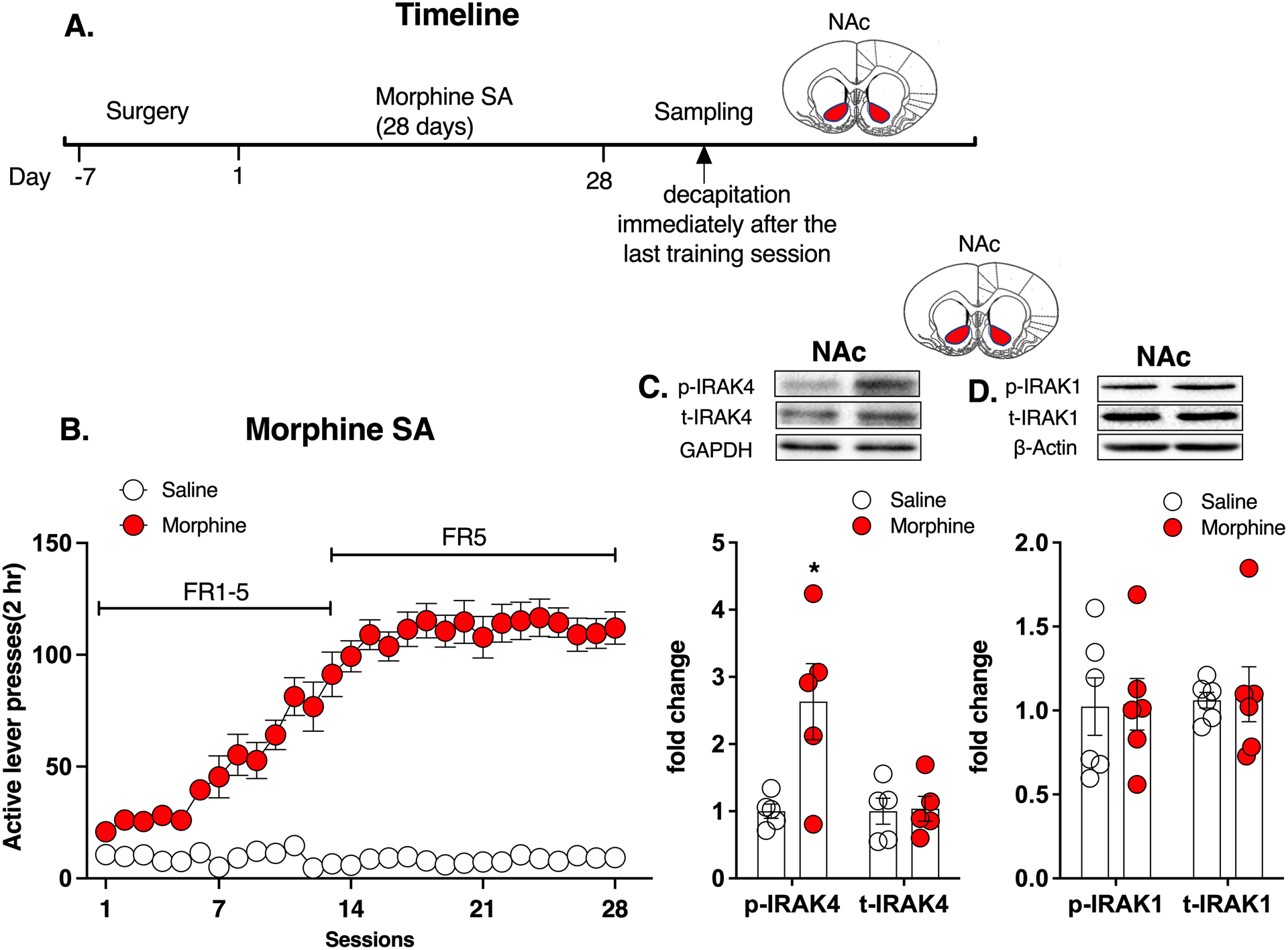
A. Experimental timeline. B. Morphine self-administration. C. Morphine group showed a significantly increased phosphorylation level of IRAK4 in the NAc compared with saline group, while total IRAK4 expression remained unchanged. D. Morphine group showed no difference either in the phosphorylation level or total expression of IRAK1 in the NAc compared with saline group. Data are expressed as mean ± SEM; *p < 0.05, compared with saline group.
Figure 2. IRAK4 was activated after the extinction and cue-induced reinstatement of morphine-seeking behavior.
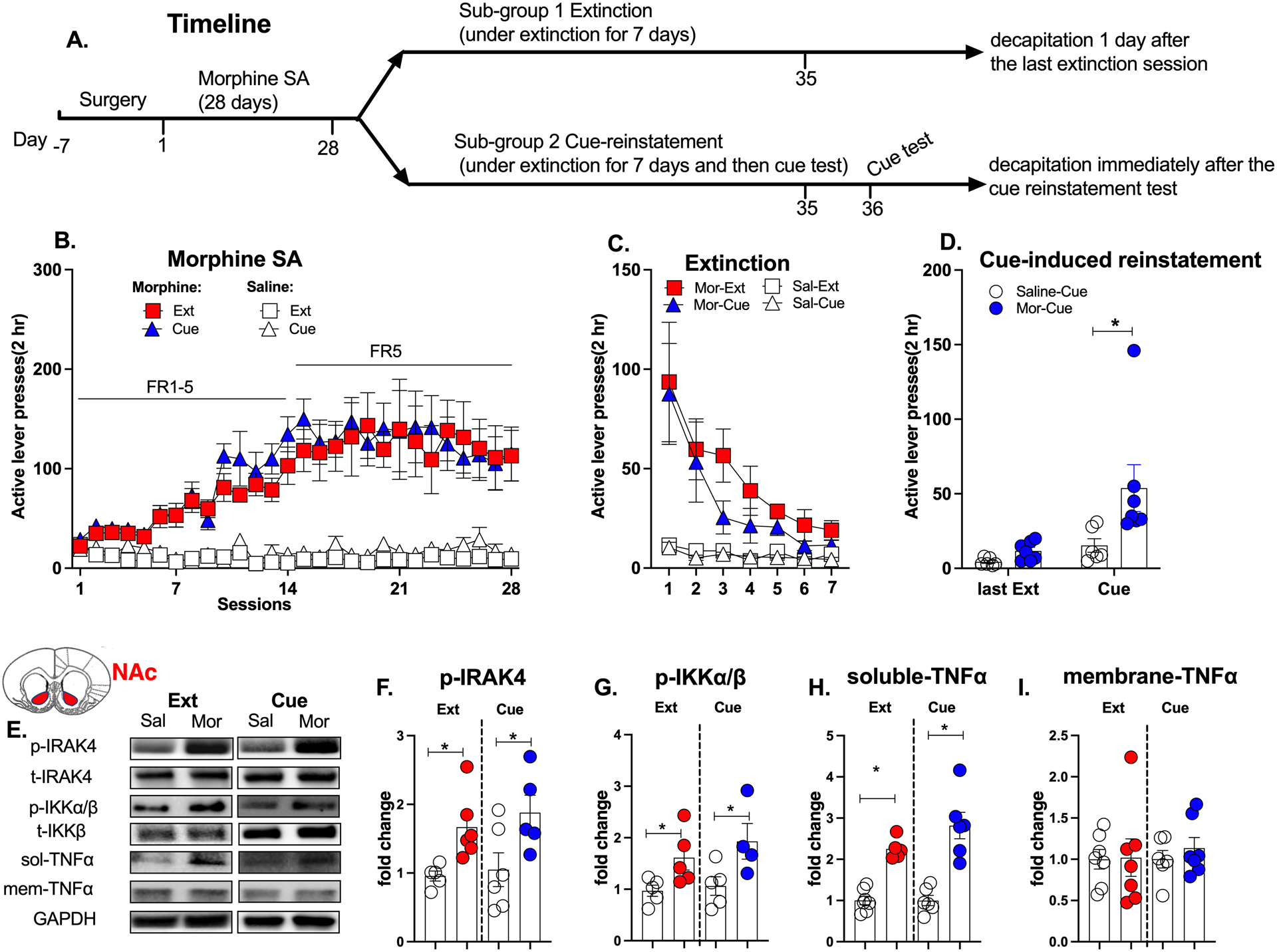
A. Experimental timeline. B. Morphine self-administration. C. Morphine extinction. D. Morphine group showed increased active lever presses at the cue-induced reinstatement test. E. Representative western blot images in the NAc. F. The phosphorylation level of IRAK4 was increased in morphine groups after the extinction and cue-induced reinstatement test. G. The phosphorylation level of IKKα/β was increased in morphine groups after the extinction and cue-induced reinstatement test. H. The expression of soluble-TNFα was increased in morphine groups after the extinction and cue-induced reinstatement test. I. The membrane-TNFα had no changes in these time points. The phosphorylation levels of IRAK4 or IKKα/β were calculated as the ratio of phosphorylated IRAK4 or IKKα/β to total IRAK4 or IKKα/β respectively. Data are expressed as mean ± SEM; *p < 0.05, compared with saline group.
3. Results
3.1. Experiment 1
Effect of morphine self-administration on the activation of IRAK4 in the NAc core
The timeline of experiment 1 is shown in Figure 1A. Rats achieved stable lever presses for morphine than saline after training (Repeated measures of ANOVA, drug: F (1,11) = 172.0, p < 0.05; time: F (4,48) = 34.1, p < 0.05; interaction: F (27,297) = 36.0, p < 0.05; Figure 1B,). Morphine group showed a significantly increased phosphorylation level of IRAK4 in the NAc compared with saline group (unpaired t-test, t15 = 2.825, p < 0.05, Figure 1C), while total IRAK4 expression remained unchanged (t15 = 0.138, p > 0.05, Figure 1C). We also tested the effect of morphine on the phosphorylation of IRAK1, another important kinase in TLR4-mediated innate immunity. However, we found that the morphine group showed no difference either in the phosphorylation level (t15 = 0.063, p > 0.05, Figure 1D) or total expression (t15 = 0.731, p > 0.05, Figure 1D) of IRAK1 in the NAc compared with the saline group. These results indicate that morphine self-administration may specifically activate IRAK4 in the NAc.
3.2. Experiment 2
Effect of morphine extinction and cue-induced reinstatement test on the activation of IRAK4 signaling in the NAc core
The timeline of experiment 2 is shown in Figure 2A. There was no difference in the morphine self-administration training between Morphine-Extinction group and Morphine-Cue group (Repeated measures of ANOVA, F (1,10) = 0.775, p > 0.05, Figure 2B). Then all rats underwent extinction (Figure 2C). At the cue-induced reinstatement test, the morphine-Cue group showed significantly increased active lever presses compared with the saline-Cue group, indicating a relapse-like behavior (Repeated measures of ANOVA, drug: F (1,11) = 5.427, p < 0.05; time: F (1,11) = 11.20, p < 0.05; interaction: F (1,11) = 3.845, p < 0.05; post hoc: Mor-Cue vs Sal-Cue: t22 = 3.045, p < 0.05, Figure 2D).
The activation of IRAK4 and its downstream signaling in the NAc was tested after the extinction and the cue-reinstatement test, respectively (Figure 2E). The phosphorylation level of IRAK4 was increased in the Morphine-Extinction group (unpaired t-test, t10 = 3.147, p < 0.05) and the Morphine-Cue group (t10 = 2.330, p < 0.05, Figure 2F). Consistently, as a downstream effector of IRAK4, the phosphorylation level of IKKα/β was also increased in morphine-Extinction group (t10 = 2.445, p < 0.05) and Morphine-Cue group (t10 = 2.372, p < 0.05, Figure 2G). Interestingly, only the expression of soluble-TNFα was elevated after the extinction (t10 = 8.161, p < 0.05) and cue-test (t10 = 5.312, p < 0.05, Figure 2H), while the membrane-TNFα had no changes at these time points (Figure 2I). This finding is consistent with a previous study that showed that soluble-TNFα was specifically responsible for morphine-induced neuroinflammation and tolerance mediated by TLR4 (Eidson et al., 2017). These results indicate that IRAK4 and its signaling remained activated after the extinction and cue-induced reinstatement test.
3.3. Experiment 3
Effect of systemic inhibition of IRAK4 on the cue-induced reinstatement of morphine-seeking
The timeline of experiment 3 is shown in Figure 3A. Rats underwent morphine self-administration training, until they achieved stable active lever presses for morphine (Repeated measures of ANOVA, drug: F (5,78) = 29.81, p < 0.05; time: F (2,146) = 0.86, p > 0.05; interaction: F (20,312) = 0.63, p > 0.05; post hoc: morphine vs saline: p < 0.05; Figure 3B). Then rats underwent extinction (Figure 3C). For the cue-induced morphine-seeking test, significant main effects for the drug (Repeated measures of ANOVA, F (1,33) = 22.54, p < 0.05), treatment (F (3,33) = 2.97, p < 0.05), time (F (1,33) = 37.67, p < 0.05) and the interaction (F (1,33) = 5.33, p < 0.05) were observed. Bonferroni post hoc test showed that the morphine-vehicle group had a significant increase in the active presses compared with the saline-vehicle group (t67 = 5.853, p < 0.05, Figure 3D). Importantly, 1 and 3 mg/kg of PF06650833 significantly attenuated the cue-induced morphine-seeking behavior (1mg/kg: t67 = 4.798, p < 0.05; 3mg/kg: t67 = 4.057, p < 0.05, Figure 3D) while 0.3mg/kg of PF06650833 didn’t change this abnormal behavior. Consistently, PF06650833 (1mg/kg) significantly abolished the increased phosphorylation level of IRAK4 in the NAc after the cue-induced morphine-seeking test (Repeated measures of ANOVA, drug: F (1,19) = 7.33, p < 0.05; treatment: F (1,19) = 5.34, p < 0.05; interaction: F (1,19) = 9.43, p < 0.05; post hoc: Sal-Veh vs Mor-Veh: t18 = 3.384, p < 0.05; Mor-Veh vs Mor-PF: t18 = 3.087, p < 0.05, Figure 3E). Meanwhile, the observation that PF 06650833 didn’t alter the phosphorylation level of IRAK4 in the saline group may be due to a “basement effect” since the phosphorylation level of IRAK4 in the saline group was relatively low. Moreover, PF 06650833 (1 and 3mg/kg) did not affect locomotor activity (Figure 3F). These results indicate that systemic inhibition of IRAK4 suppresses cue-induced morphine-seeking behavior at a dose-dependent manner.
Figure 3. Systemic inhibition of IRAK4 attenuated cue-induced morphine-seeking.
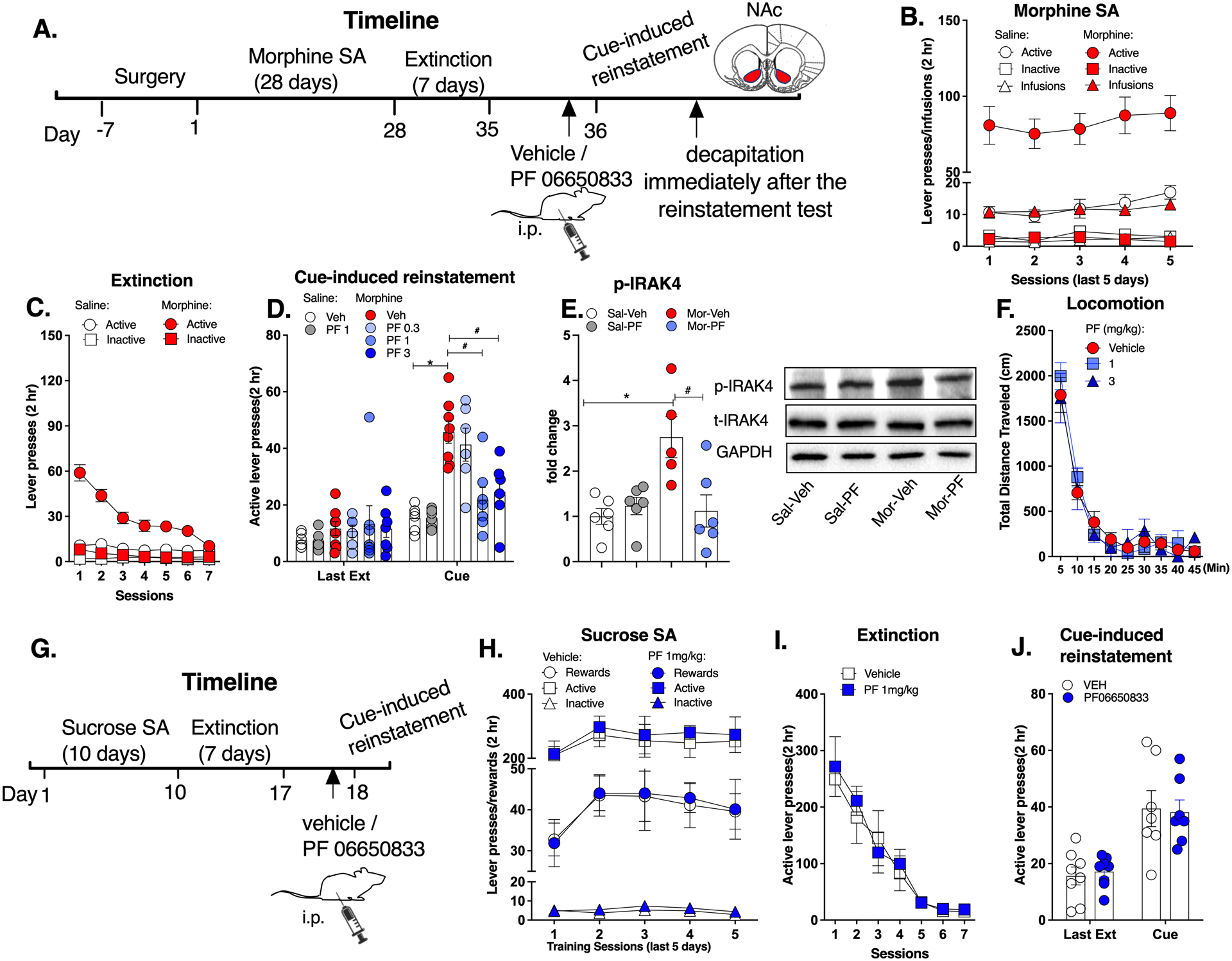
A. Experimental timeline. B. Morphine self-administration. C. Morphine extinction. D. PF06650833 (1 and 3mg/kg) significantly attenuated the cue-induced morphine-seeking behavior. E. PF06650833 (1mg/kg) significantly abolished the increased phosphorylation level of IRAK4 in the NAc after the cue-induced morphine-seeking test. F. PF 06650833 did not affect the locomotor activity. G. Timeline for sucrose self-administration. H. Sucrose self-administration. I. Sucrose extinction. J. PF 06650833 (1mg/kg) did not affect the cue-induced sucrose-seeking. Data are expressed as mean ± SEM; *p < 0.05, compared with saline-vehicle, # p < 0.05, compared with morphine-vehicle.
The timeline of sucrose self-administration experiment is shown in Figure 3G. No difference was observed during the sucrose self-administration training and extinction between vehicle and PF06650833 group (Figure 3H & I). At the cue-induced reinstatement test, there was a significant effect for time (Repeated measures of ANOVA, F (1,12) = 31.16, p < 0.05) with no significant effect for the treatment (F (1,12) = 0.04, p > 0.05) or interaction (F (1,12) = 0.01, p > 0.05, Figure 3J). Both groups demonstrated higher active lever presses in the cue-induced reinstatement test compared with the last extinction (post-hoc test: vehicle: t26 = 4.110, p < 0.05; PF 06650833: t26 = 3.629, p < 0.05, Figure 3J). However, PF 06650833 did not affect the cue-induced sucrose-seeking behavior. These results indicate that inhibition of IRAK4 did not affect the reward behaviors maintained by sucrose.
3.4. Experiment 4
Effect of intra-NAc inhibition of IRAK4 on the cue-induced reinstatement of morphine-seeking
The timeline of experiment 4 is shown in Figure 4A. Rats underwent morphine self-administration training and extinction (Figure 4B & C). No difference was found between the PF06650833 group and the vehicle group at the last extinction session (Figure 4D). At the cue-induced reinstatement of morphine-seeking test, significant main effects for the drug (Repeated measures of ANOVA, F (1,25) = 30.38, p < 0.05), treatment (F (2,25) = 16.32, p < 0.05), time (F (1,25) = 35.89, p < 0.05) and their interactions (F (2,25) = 20.36, p < 0.05, Figure 4D) were observed. Post hoc analysis showed that both doses of PF 06650833 significantly reduced the increased active lever presses in the morphine group (morphine-vehicle vs saline-vehicle: t50 = 8.313, p < 0.05; PF 0.3 ug/side: t50 = 6.834, p < 0.05; 1ug/side: t50 = 7.616, p < 0.05, Figure 4D). These results indicate that IRAK4 inhibition in the NAc attenuates the cue-induced morphine-seeking behavior.
Figure 4. Local inhibition of IRAK4 in the NAc core decreased cue-induced morphine-seeking.
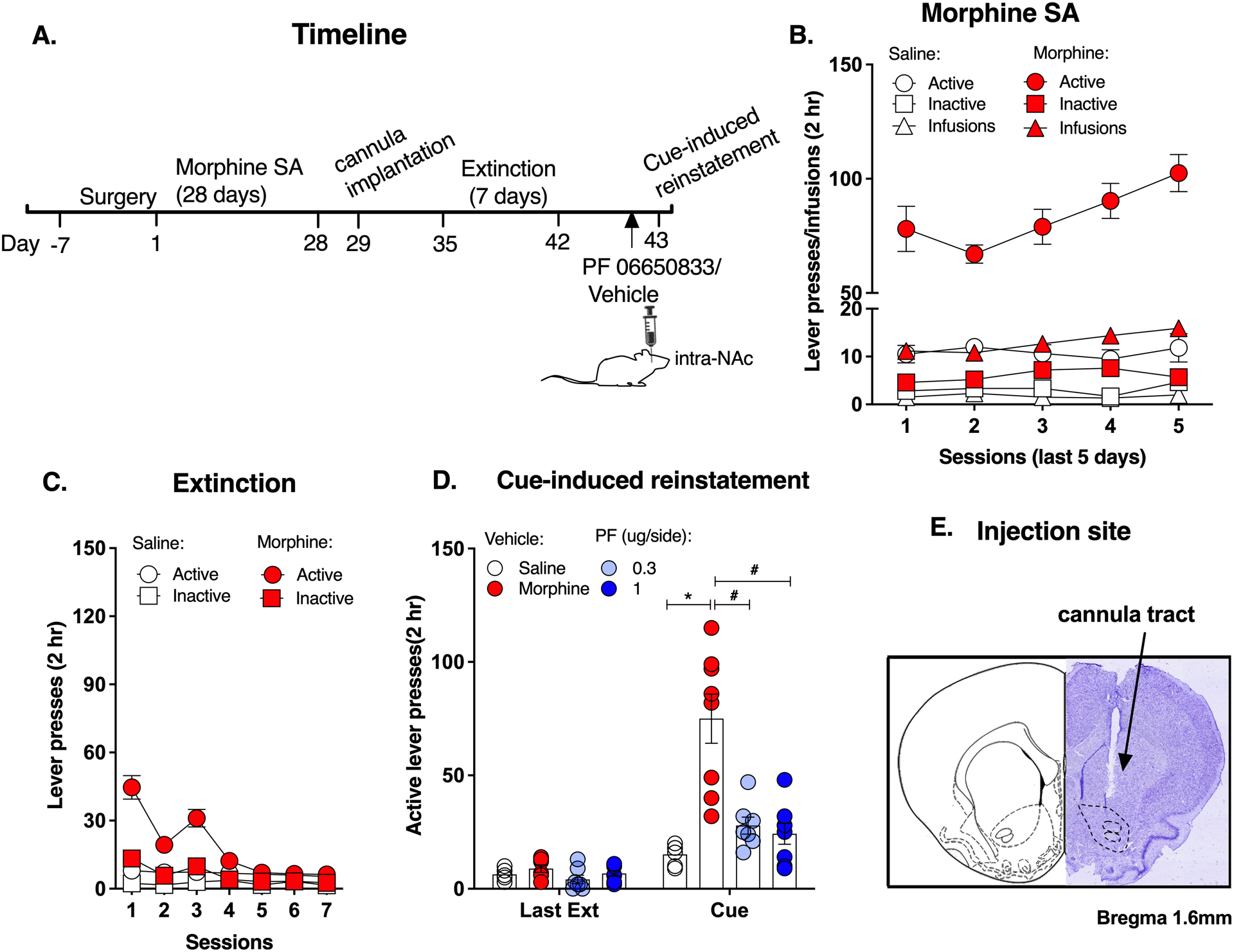
A. Experimental timeline. B. Morphine self-administration. C. Morphine extinction. D. Both doses of PF 06650833 (0.3ug and 1ug/side) significantly reduced the active lever presses. E. Injection site of NAc core. Data are expressed as mean ± SEM; *p < 0.05, compared with saline-vehicle, # p < 0.05, compared with morphine-vehicle.
3.5. Experiment 5
Effect of systemic inhibition of IRAK4 in the cue-induced reinstatement of fentanyl-seeking
The timeline of experiment 5 is shown in Figure 5A. There was a significant effect for the drug (Repeated measures of ANOVA, drug: F (1,25) = 159.71, p < 0.05) while there was no significant effect for PF06650833 treatment (F (1,25) = 0.80, p > 0.05) or time (F (3,63) = 0.73, p > 0.05) or interaction among drug, PF 06650833 treatment and time (F (3,63) = 0.063, p > 0.05, Figure 5B). Post hoc analysis showed that the fentanyl groups had higher infusions than the saline groups during the training sessions (p < 0.05) and there was no difference between the fentanyl-vehicle group and the fentanyl-PF group. Rats then underwent extinction (Figure 5C). At the cue-induced reinstatement of fentanyl-seeking test, there was a significant effect for drug (Repeated measures of ANOVA, F (1,23) = 44.89, p < 0.05), PF06650833 (F (1,23) = 3.90, p < 0.05), time (F (1,23) = 28.02, p < 0.05), and an almost significant effect for their interactions (F (1,23) = 3.820, p = 0.06, Figure 5D). Post hoc analysis showed that PF06650833 remarkably reduced the active lever presses for fentanyl (Sal-Veh vs fen-Veh: t48 = 7.177, p < 0.05; fen-Veh vs fen-PF: t22 = 5.158, p < 0.05, Figure 5D). These results indicate that inhibition of IRAK4 decreased the cue-induced reinstatement of fentanyl-seeking, suggesting a generalized suppressing role of IRAK4 in opioid-related relapse-like behaviors.
Figure 5. Inhibition of IRAK4 disrupted the cue-induced fentanyl-seeking behavior.
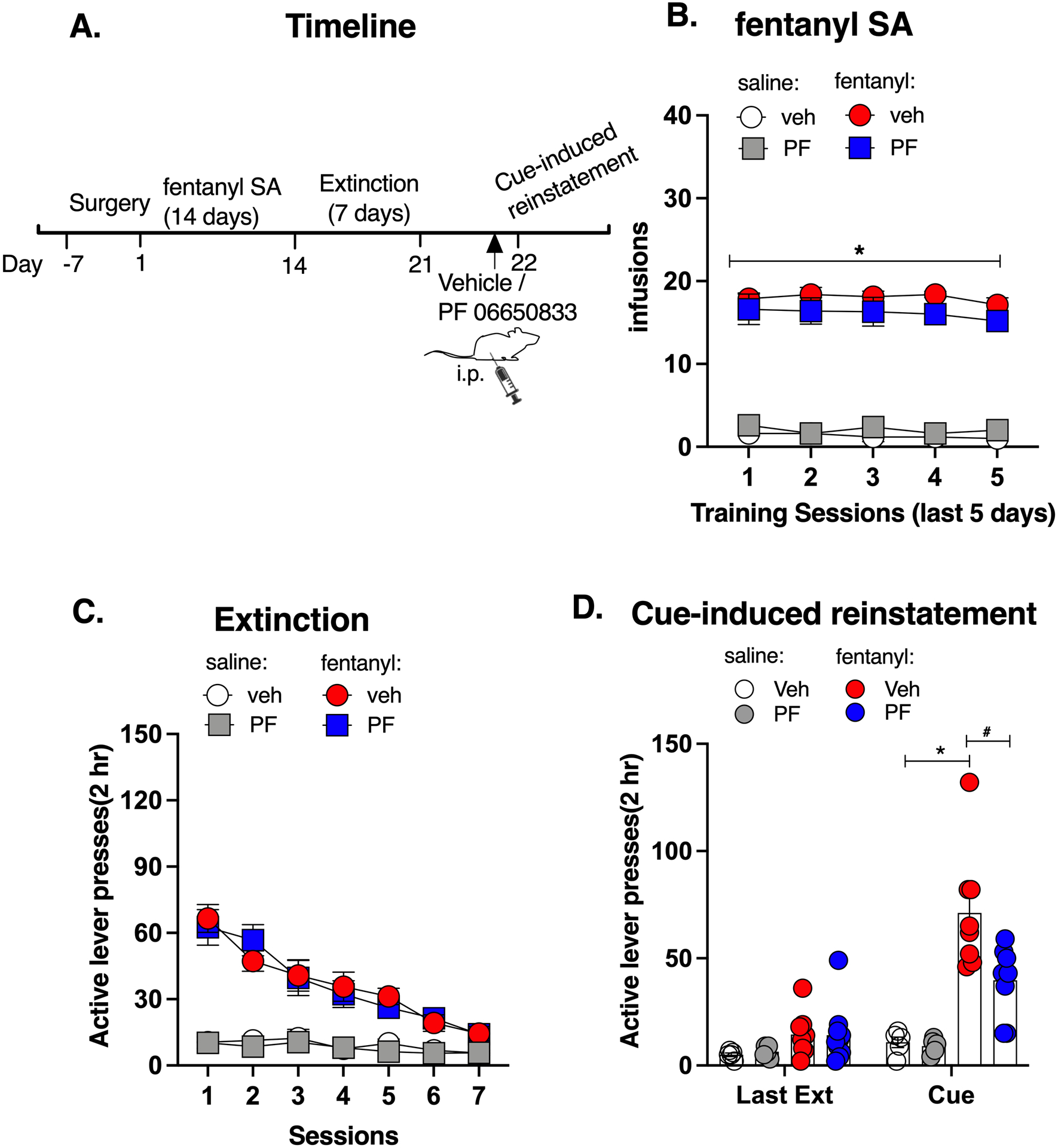
A. Experimental timeline. B. Fentanyl self-administration. C. Fentanyl extinction. D. PF 06650833 reduced the active lever presses for fentanyl. Data are expressed as mean ± SEM; *p < 0.05, compared with saline-vehicle, # p < 0.05, compared with fentanyl-vehicle.
4. Discussion
The present study shows that morphine activated IRAK4 in the NAc while both systemic and intra-NAc inhibition of IRAK4 attenuated cue-induced morphine-seeking behaviors. In addition, inhibition of IRAK4 also reduced the cue-induced reinstatement of fentanyl-seeking. Our study is the first to demonstrate a critical role of IRAK4 in cue-induced reinstatement of opioid-seeking behavior and provide valuable information about the link between immune response and drug addiction.
We showed an increase in IRAK4 related neuro-immune responses due to chronic opioid exposure, which is consistent with previous studies that show a pro-inflammatory role of opioids via the modulation of TLR4 (Narita et al., 2006; Yang et al., 2010). IRAK4 is the first kinase activated by TLR4 ligation and serves as a gatekeeper of TLR4 signaling (Suzuki et al., 2002b). Once activated, TLR4 initiates two main pathways that are dependent on MyD88 and TIR domain-containing adapter-inducing interferon-β (TRIF) (Wu and Li, 2020). IRAK4 is believed to be crucial for MyD88-dependent signaling and activation. IRAK4 auto-activation activates IRAK1 and IRAK2, leading to the activation of TNF receptor-associated receptor 6 (TRAF6). TRAF6 activation triggers the IKK complex and induces the activation of down-stream transcription factors like NF-κB, which results in production of pro-inflammatory cytokines and chemokines that are responsible for inflammatory responses (Latty et al., 2018; Suzuki et al., 2002b). We found that morphine exposure activated IRAK4 and IKKα/β and increased the expression of soluble-TNFα, suggesting a consistent activation of neuro-immune responses. Meanwhile, we found no alteration in IRAK1 by morphine exposure, possibly due to the compensatory role of IRAK1 in TLR4 signaling, as mice lacking IRAK1 show a partial defect in TLR4 signaling while depletion of IRAK4 severely abolished this response (Suzuki et al., 2002a). However, studies have also suggested that IRAK4 is dispensable for TLR4-mediated NF-κB or MAPK signaling and might be dispensable for myddosome assembly (De Nardo et al., 2018), which question the essential role of IRAK4 in TLR4 signaling. Nevertheless, studies agree on the critical role of IRAK4 in MyD88-dependent production of inflammatory cytokines, which may be involved for opioid-associated hyperalgesia, tolerance and dependence (De Nardo et al., 2018; Kim et al., 2007).
Whether opioids are immunosuppressive or immunoactive is a subject of debate (Eisenstein, 2019). A previous study showed a clear link between pain and up-regulation of pro-inflammatory responses (Zhang and An, 2007), and it seems reasonable to conclude that opioids alleviate pain by blocking these inflammatory cytokines (Stein, 2013). However, we found that morphine exposure increased pro-inflammatory responses, which aligns with studies that have shown that morphine induces glial activation (Yang et al., 2010). One possible explanation is the differences in duration of drug exposure. It has been shown that chronic, but not acute, morphine administration induces glial activation (Raghavendra et al., 2003; Tawfik et al., 2005). Chronic morphine exposure results in sepsis, which induces leakage of Gram negative organisms and LPS from gastrointestinal tract (Tawfik et al., 2005). This leaked LPS binds to TLR4 and increases production of pro-inflammatory cytokines. Similarly, the morphine self-administration behavioral assay used in the present study lasted for several weeks, so the finding that chronic exposure to morphine increased IRAK4-related neuro-immune response is unsurprising.
It is noteworthy that IRAK4 signaling remained activated after the extinction and cue-induced morphine-seeking test, which implies that IRAK4 activation may not be specific to the cue-induced morphine-seeking behavior. The observation that IRAK4 remained activated when the drug-seeking behavior was extinguished is hard to reconcile. However, it is possible that chronic morphine exposure leads to a persistent and enduring increase in IRAK4 phosphorylation even when active lever pressing for morphine was low. The relationship between IRAK4 and extinction merits more extensive investigation. Nevertheless, it is likely that morphine-induced persistent abnormal IRAK4 activation form the pathological basis for the cue-induced relapsing-like behavior. Indeed, our findings that both systemic and local inhibition of IRAK4 remarkably reduced the active lever presses at the cue-induced reinstatement test and simultaneously, decreased the phosphorylation level of IRAK4 in the NAc at the dose of 1 mg/kg suggests that the activation of IRAK4 and its signaling may partially underlie the mechanisms of the relapse-like behaviors of opioids. However, because the phosphorylation levels of IRAK4 remained elevated after the extinction training, the levels of p-IRAK4 did not necessarily correspond to the reduced active lever presses. Thus, it remains to be determined whether PF06650833 at the dose of 0.3 mg/kg could reduce the phosphorylation level of IRAK4, although it did not decrease the active lever presses in the cue-induced reinstatement test.
Our result that IRAK4 inhibition attenuates opioid-seeking behavior is in alignment with previous studies which implicate TLR4 and its signaling in opioid addiction. For example, Hutchinson and colleagues found that genetic knock-outs of TLR4-MyD88 signaling reduces opioid-induced CPP in mice (Hutchinson et al., 2012). Moreover, pharmacological inhibition of TLR4 decreased opioid self-administration in rats (Hutchinson et al., 2012). These results suggest that TLR4 and MyD88 signaling are important contributors to acute opioid reward (Jacobsen et al., 2014). Meanwhile, studies also show that TLR4 is involved in the development of opioid incubation. Theberge and colleagues found that chronic delivery of (+)-naltrexone during the withdrawal phase decreases incubated cue-induced heroin-seeking, which suggests that TLR4 is associated with opioid relapse (Theberge et al., 2013). The results of our study are consistent in that IRAK4, the downstream effector of TLR4 might also participate in opioid relapse, further demonstrating the importance of this signaling.
While we found that inhibition of IRAK4 prior to the cue-induced reinstatement test was able to attenuate morphine- and fentanyl-seeking behavior, Theberge et al showed that acute blockade of TLR4 before withdrawal day 13 had no effect on incubated heroin-seeking (Theberge et al., 2013). Two possible explanations for the differences in these studies are as follows. First, rats in the study of Theberge et al were trained under a long-access condition for heroin (9h/d) whereas our rats in the present study had limited-access to morphine or fentanyl (2h/d). It is well known that different drug access conditions can lead to different brain neuroadaptations and different behavioral responses to pharmacological manipulations (Ferrario et al., 2005; Greenwell et al., 2009a; Greenwell et al., 2009b). Second, Theberge and colleagues used a procedure where rats underwent no extinction training except on days 1 and 13, while our rats underwent extinction training for 7 days to extinguish the drug-seeking behavior. Both of these procedures have been used to study drug relapse, and it is likely that different neural substrate mediate drug-seeking after extinction versus withdrawal (Fuchs et al., 2006; Kelamangalath and Wagner, 2009).
We found that PF 06650833 at the dose of 1mg/kg did not change the behavioral responding maintained by sucrose, which is consistent with studies that show unaltered food responding following TLR4 antagonism (Bachtell et al., 2015; Northcutt et al., 2015; Theberge et al., 2013). These studies suggested a drug-specific involvement of TLR4 and IRAK4 in mediating opioid actions. However, Tanda and colleagues found that (+)-naltrexone reduced the rate of food responding, demonstrating a lack of specificity of TLR4 signaling in opioid addiction (Tanda et al., 2016; Yue et al., 2020). One possible explanation is the difference in doses of (+)-naltrexone that were tested in different studies. While lower doses (7.0, 22.4 and 30.0 mg/kg) of (+)-naltrexone (Northcutt et al., 2015; Theberge et al., 2013) were not found to affect food-maintained responding, another study found that higher doses of (+)-naltrexone (32, 56 mg/kg) decreased food-maintained responding (Tanda et al., 2016). Therefore, it is likely that this pharmacological effect is dose-dependent as food responding is suppressed by higher, but not lower doses of (+)-naltrexone.
The inhibition of IRAK4 by a highly selective antagonist PF 06650833 was shown to reduce the cue-induced morphine- and fentanyl-seeking behavior, suggesting a generalized role of IRAK4 in modulating opioid relapse. These findings suggest the broad clinical potential of IRAK4 for the treatment of opioid addiction. The effect of IRAK4 is not due to a potential sedative effect since PF 06650833 did not affect locomotor activity in rats. These findings strongly support IRAK4 as a novel and highly-selective therapeutic target for the treatment of opioid relapse.
The mechanisms underlying IRAK4’s effect in opioid relapse-like behaviors are not clear. It is known that opioid receptor antagonism may result in withdrawal symptoms, which may affect drug-taking or -seeking behaviors. However, it is not likely that inhibition of IRAK4 could induce withdrawal-like effects through opioid receptor modulation since antagonism of TLR4 by (+)-naloxone attenuated remifentanil self-administration (Hutchinson et al., 2012). Nonetheless, the neuro-excitatory and neurotoxic effects of pro-inflammatory cytokines may provide some insights (Watkins et al., 2009). Opioid-induced IRAK4 activation promotes pro-inflammatory responses which subsequently affect neuronal transmission and plasticity associated with opioid-related behaviors (Shah and Choi, 2017). As a downstream effector, TNFα is reported to regulate synaptic transmission by affecting the activity of GABAA receptors, AMPA receptors, and presynaptic metabotropic glutamate receptors (Domercq et al., 2006; Lewitus et al., 2014; Pascual et al., 2012). TNFα decreases the surface expression of GABAA receptors on postsynaptic neurons and reduces the inhibitory tone of GABA on dopamine activity (Pribiag and Stellwagen, 2013), eventually leading to an increase of dopamine release in the NAc. Thus, activation of TNFα in IRAK4 signaling may have a substantial impact on the mesolimbic dopamine system, which contributes to opioid-related behavioral adaptations. These studies emphasize a possible mediating effect of immune responses on synaptic functions and suggests a feasible mechanism underlying IRAK4’s effect in opioids actions. However, it is noteworthy that immune responses do not act independently in the process of opioid addiction. They either cooperate or compensate with the characterized neuronal circuits (Jacobsen et al., 2014) to contribute to drug reward as alone they are not sufficient to produce reward behaviors (Narita et al., 2006).
A limitation in our study is that only male rats were tested in the present study, thus it remains unknown whether inhibition of IRAK4 also attenuates cue-induced opioid-seeking behaviors in female rats. It is well recognized that females show less sensitivity to the analgesic effect of opioid (Fillingim et al., 2009; Sarton et al., 2000). A clinical study showed that women require 30% more morphine to achieve the same level of analgesia as men (Aubrun et al., 2005). Preclinical studies also showed obvious sex differences in morphine action in different pain models (Holtman and Wala, 2005; Murphy et al., 2009), as the 50% effective dose (ED50) for females is almost twice as much as the ED50 for males (Morgan et al., 2008). As morphine action on TLR4 induces pro-inflammatory responses that antagonizes the analgesic effects of morphine (Grace et al., 2016), the percentage of activated microglia in the periaqueductal gray (PAG) of females was increased more significantly than that of males following the administration of TLR4 agonist LPS (Doyle and Murphy, 2018). This effect was linked to the reduced analgesic effects of morphine and the TLR4 antagonist (+)-naloxone significantly restored morphine analgesia in females but not males (Doyle and Murphy, 2018). These studies suggest substantial sex differences in opioid action on production of pro-inflammatory responses, and thus females may differ in the effects of IRAK4 inhibitor in regulation of opioid-related effects, including opioid relapse-like behavior. Studies that include both sex are needed to clarify this question. Another limitation is that we only focused on the NAc core while investigating the role of IRAK4 on cue-induced morphine-seeking behavior. Other brain regions that are also involved in cue-induced reinstatement, such as the NAc shell or prelimbic cortex (Floresco et al., 2008; Rubio et al., 2019). It remains to be examined to further clarify the specificity of neuroadaptations induced by IRAK4 inhibition. Finally, another caveat is that in Experiment 2, brains from the extinction group were taken when rats were in their homecages (one day after the last extinction session), while rats from the reinstatement group were decapitated immediately after the cue-induced reinstatement test. It is unclear whether the difference in experiences (homecage vs. operant box) would affect IRAK4 signaling expression in this study. Nevertheless, the results should be interpreted with caution.
In summary, we show that morphine exposure activates IRAK4 and its signaling in the NAc, while inhibition of IRAK4 attenuates cue-induced opioid-seeking behaviors. These results suggest IRAK4 as a novel and potential therapeutic target for the treatment of opioid addiction. Given the promising role of IRAK4 in modulating opioid relapse, future studies to examine its effect in opioid analgesia and tolerance are warranted.
Supplementary Material
Acknowledgement
We thank Kristen Woodhouse for proofreading the manuscript.
Funding sources
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R21DA040777 and R01DA047967 to J-X.L.]. The authors declare no competing financial interests
Footnotes
Declarations of interest
None.
References
- Aubrun F, Salvi N, Coriat P, Riou B, 2005. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology 103, 156–160. [DOI] [PubMed] [Google Scholar]
- Bachtell R, Hutchinson MR, Wang X, Rice KC, Maier SF, Watkins LR, 2015. Targeting the Toll of Drug Abuse: The Translational Potential of Toll-Like Receptor 4. CNS Neurol Disord Drug Targets 14, 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y, 2013. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 229, 453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CJ, Godino A, Salery M, Nestler EJ, 2020. Epigenetic Mechanisms of Opioid Addiction. Biol Psychiatry 87, 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron B, Tse W, Lamb R, Li X, Lamb BT, Landreth GE, 2012. Loss of interleukin receptor-associated kinase 4 signaling suppresses amyloid pathology and alters microglial phenotype in a mouse model of Alzheimer’s disease. J Neurosci 32, 15112–15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cami J, Farre M, 2003. Drug addiction. N Engl J Med 349, 975–986. [DOI] [PubMed] [Google Scholar]
- Chai N, Liu JF, Xue YX, Yang C, Yan W, Wang HM, Luo YX, Shi HS, Wang JS, Bao YP, Meng SQ, Ding ZB, Wang XY, Lu L, 2014. Delayed noradrenergic activation in the dorsal hippocampus promotes the long-term persistence of extinguished fear. Neuropsychopharmacology 39, 1933–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danto S, S. N, Singh R, Manukyan Z, Mancuso J, Peeva E, Vincent M, Beebe J, 2019. Efficacy and Safety of the Selective Interleukin-1 Receptor Associated Kinase 4 Inhibitor, PF-06650833, in Patients with Active Rheumatoid Arthritis and Inadequate Response to Methotrexate [abstract]. Arthritis Rheumatol. 71 (suppl 10). [Google Scholar]
- De Nardo D, Balka KR, Cardona Gloria Y, Rao VR, Latz E, Masters SL, 2018. Interleukin-1 receptor-associated kinase 4 (IRAK4) plays a dual role in myddosome formation and Toll-like receptor signaling. J Biol Chem 293, 15195–15207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis BB, Sanger N, Bawor M, Naji L, Plater C, Worster A, Woo J, Bhalerao A, Baptist-Mohseni N, Hillmer A, Rice D, Corace K, Hutton B, Tugwell P, Thabane L, Samaan Z, 2020. A call for consensus in defining efficacy in clinical trials for opioid addiction: combined results from a systematic review and qualitative study in patients receiving pharmacological assisted therapy for opioid use disorder. Trials 21, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Brambilla L, Pilati E, Marchaland J, Volterra A, Bezzi P, 2006. P2Y1 receptor-evoked glutamate exocytosis from astrocytes: control by tumor necrosis factor-alpha and prostaglandins. J Biol Chem 281, 30684–30696. [DOI] [PubMed] [Google Scholar]
- Doyle HH, Murphy AZ, 2018. Sex-dependent influences of morphine and its metabolites on pain sensitivity in the rat. Physiol Behav 187, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne A, Marshall NA, Mills KH, 2011. TLR based therapeutics. Curr Opin Pharmacol 11, 404–411. [DOI] [PubMed] [Google Scholar]
- Eidson LN, Inoue K, Young LJ, Tansey MG, Murphy AZ, 2017. Toll-like Receptor 4 Mediates Morphine-Induced Neuroinflammation and Tolerance via Soluble Tumor Necrosis Factor Signaling. Neuropsychopharmacology 42, 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein TK, 2019. The Role of Opioid Receptors in Immune System Function. Front Immunol 10, 2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeomah C, Cunningham KA, Stutz SJ, Fox RG, Bukreyeva N, Dineley KT, Paessler S, Cisneros IE, 2020. Fentanyl self-administration impacts brain immune responses in male Sprague-Dawley rats. Brain Behav Immun 87, 725–738. [DOI] [PubMed] [Google Scholar]
- Ferrao R, Zhou H, Shan Y, Liu Q, Li Q, Shaw DE, Li X, Wu H, 2014. IRAK4 dimerization and trans-autophosphorylation are induced by Myddosome assembly. Mol Cell 55, 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE, 2005. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry 58, 751–759. [DOI] [PubMed] [Google Scholar]
- Fields HL, Margolis EB, 2015. Understanding opioid reward. Trends Neurosci 38, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL 3rd, 2009. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 10, 447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, Haluk DM, 2008. Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience 154, 877–884. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE, 2006. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci 26, 3584–3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- G., P., C., W., 2014. The rat brain in stereotaxic coordinates (Elsevier Academic, Amsterdam: ), 7th Edition. [Google Scholar]
- Grace PM, Strand KA, Galer EL, Urban DJ, Wang X, Baratta MV, Fabisiak TJ, Anderson ND, Cheng K, Greene LI, Berkelhammer D, Zhang Y, Ellis AL, Yin HH, Campeau S, Rice KC, Roth BL, Maier SF, Watkins LR, 2016. Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc Natl Acad Sci U S A 113, E3441–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF, 2009a. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol 14, 130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwell TN, Walker BM, Cottone P, Zorrilla EP, Koob GF, 2009b. The alpha1 adrenergic receptor antagonist prazosin reduces heroin self-administration in rats with extended access to heroin administration. Pharmacol Biochem Behav 91, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman JR Jr., Wala EP, 2005. Characterization of morphine-induced hyperalgesia in male and female rats. Pain 114, 62–70. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR, 2012. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci 32, 11187–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR, 2011. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev 63, 772–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR, 2010. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun 24, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JH, Watkins LR, Hutchinson MR, 2014. Discovery of a novel site of opioid action at the innate immune pattern-recognition receptor TLR4 and its role in addiction. Int Rev Neurobiol 118, 129–163. [DOI] [PubMed] [Google Scholar]
- Kawagoe T, Sato S, Matsushita K, Kato H, Matsui K, Kumagai Y, Saitoh T, Kawai T, Takeuchi O, Akira S, 2008. Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol 9, 684–691. [DOI] [PubMed] [Google Scholar]
- Kelamangalath L, Wagner JJ, 2009. Effects of abstinence or extinction on cocaine seeking as a function of withdrawal duration. Behav Pharmacol 20, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Staschke K, Bulek K, Yao J, Peters K, Oh KH, Vandenburg Y, Xiao H, Qian W, Hamilton T, Min B, Sen G, Gilmour R, Li X, 2007. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J Exp Med 204, 1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latty SL, Sakai J, Hopkins L, Verstak B, Paramo T, Berglund NA, Cammarota E, Cicuta P, Gay NJ, Bond PJ, Klenerman D, Bryant CE, 2018. Activation of Toll-like receptors nucleates assembly of the MyDDosome signaling hub. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ, 2006. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci 26, 5881–5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Ambler CM, Anderson DR, Boscoe BP, Bree AG, Brodfuehrer JI, Chang JS, Choi C, Chung S, Curran KJ, Day JE, Dehnhardt CM, Dower K, Drozda SE, Frisbie RK, Gavrin LK, Goldberg JA, Han S, Hegen M, Hepworth D, Hope HR, Kamtekar S, Kilty IC, Lee A, Lin LL, Lovering FE, Lowe MD, Mathias JP, Morgan HM, Murphy EA, Papaioannou N, Patny A, Pierce BS, Rao VR, Saiah E, Samardjiev IJ, Samas BM, Shen MWH, Shin JH, Soutter HH, Strohbach JW, Symanowicz PT, Thomason JR, Trzupek JD, Vargas R, Vincent F, Yan J, Zapf CW, Wright SW, 2017. Discovery of Clinical Candidate 1-{[(2S,3S,4S)-3-Ethyl-4-fluoro-5-oxopyrrolidin-2-yl]methoxy}−7-methoxyisoquinoli ne-6-carboxamide (PF-06650833), a Potent, Selective Inhibitor of Interleukin-1 Receptor Associated Kinase 4 (IRAK4), by Fragment-Based Drug Design. J Med Chem 60, 5521–5542. [DOI] [PubMed] [Google Scholar]
- Lewitus GM, Konefal SC, Greenhalgh AD, Pribiag H, Augereau K, Stellwagen D, 2016. Microglial TNF-alpha Suppresses Cocaine-Induced Plasticity and Behavioral Sensitization. Neuron 90, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Pribiag H, Duseja R, St-Hilaire M, Stellwagen D, 2014. An adaptive role of TNFalpha in the regulation of striatal synapses. J Neurosci 34, 6146–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Lo YC, Wu H, 2010. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465, 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zhang L, Zhao Y, 2010. Modulation of immune responses through direct activation of Toll-like receptors to T cells. Clin Exp Immunol 160, 168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Johnson B, Wu R, Seaman R Jr., Vu J, Zhu Q, Zhang Y, Li JX, 2020. TAAR1 agonists attenuate extended-access cocaine self-administration and yohimbine-induced reinstatement of cocaine-seeking. Br J Pharmacol 177, 3403–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JF, Seaman R Jr., Siemian JN, Bhimani R, Johnson B, Zhang Y, Zhu Q, Hoener MC, Park J, Dietz DM, Li JX, 2018. Role of trace amine-associated receptor 1 in nicotine’s behavioral and neurochemical effects. Neuropsychopharmacology 43, 2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JF, Siemian JN, Seaman R Jr., Zhang Y, Li JX, 2017. Role of TAAR1 within the Subregions of the Mesocorticolimbic Dopaminergic System in Cocaine-Seeking Behavior. J Neurosci 37, 882–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JF, Yang C, Deng JH, Yan W, Wang HM, Luo YX, Shi HS, Meng SQ, Chai BS, Fang Q, Chai N, Xue YX, Sun J, Chen C, Wang XY, Wang JS, Lu L, 2015. Role of hippocampal beta-adrenergic and glucocorticoid receptors in the novelty-induced enhancement of fear extinction. J Neurosci 35, 8308–8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli TA, Leduc-Pessah H, Skelhorne-Gross G, Nicol CJ, Milne B, Trang T, Cahill CM, 2014. Toll-like receptor 4 mutant and null mice retain morphine-induced tolerance, hyperalgesia, and physical dependence. PLoS One 9, e97361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy GM, Bridges CR, Blednov YA, Harris RA, 2017. CNS cell-type localization and LPS response of TLR signaling pathways. F1000Res 6, 1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Whittier KL, Hegarty DM, Aicher SA, 2008. Periaqueductal gray neurons project to spinally projecting GABAergic neurons in the rostral ventromedial medulla. Pain 140, 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motshwene PG, Moncrieffe MC, Grossmann JG, Kao C, Ayaluru M, Sandercock AM, Robinson CV, Latz E, Gay NJ, 2009. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J Biol Chem 284, 25404–25411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AZ, Suckow SK, Johns M, Traub RJ, 2009. Sex differences in the activation of the spinoparabrachial circuit by visceral pain. Physiol Behav 97, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Miyatake M, Narita M, Shibasaki M, Shindo K, Nakamura A, Kuzumaki N, Nagumo Y, Suzuki T, 2006. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology 31, 2476–2488. [DOI] [PubMed] [Google Scholar]
- Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA, Pomrenze MB, Galer EL, Kopajtic TA, Li CM, Amat J, Larson G, Cooper DC, Huang Y, O’Neill CE, Yin H, Zahniser NR, Katz JL, Rice KC, Maier SF, Bachtell RK, Watkins LR, 2015. DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry 20, 1525–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A, 2012. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A 109, E197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletinckx K, Krings D, Welbers A, Rider DA, Dunkern TR, 2020. Central IRAK-4 kinase inhibition for the treatment of pain following nerve injury in rats. Brain Behav Immun 88, 781–790. [DOI] [PubMed] [Google Scholar]
- Pribiag H, Stellwagen D, 2013. TNF-alpha downregulates inhibitory neurotransmission through protein phosphatase 1-dependent trafficking of GABA(A) receptors. J Neurosci 33, 15879–15893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga F, Rutkowski MD, DeLeo JA, 2003. Anti-hyperalgesic and morphine-sparing actions of propentofylline following peripheral nerve injury in rats: mechanistic implications of spinal glia and proinflammatory cytokines. Pain 104, 655–664. [DOI] [PubMed] [Google Scholar]
- Rubio FJ, Quintana-Feliciano R, Warren BL, Li X, Witonsky KFR, Valle FSD, Selvam PV, Caprioli D, Venniro M, Bossert JM, Shaham Y, Hope BT, 2019. Prelimbic cortex is a common brain area activated during cue-induced reinstatement of cocaine and heroin seeking in a polydrug self-administration rat model. Eur J Neurosci 49, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Teppema L, Dahan A, 2000. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology 93, 1245–1254; discussion 1246A. [DOI] [PubMed] [Google Scholar]
- Seganish WM, 2016. Inhibitors of interleukin-1 receptor-associated kinase 4 (IRAK4): a patent review (2012–2015). Expert Opin Ther Pat 26, 917–932. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL, 2008. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci 28, 5756–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA, 2010. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology 35, 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Choi S, 2017. Toll-like Receptor-Dependent Negative Effects of Opioids: A Battle between Analgesia and Hyperalgesia. Front Immunol 8, 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, 2011. New findings on biological factors predicting addiction relapse vulnerability. Curr Psychiatry Rep 13, 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, 2013. Targeting pain and inflammation by peripherally acting opioids. Front Pharmacol 4, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, Marshall BDL, Tyndall M, Walsh SL, 2020. Opioid use disorder. Nat Rev Dis Primers 6, 3. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, Mirtsos C, Takada H, Wakeham A, Itie A, Li S, Penninger JM, Wesche H, Ohashi PS, Mak TW, Yeh WC, 2002a. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature 416, 750–756. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Suzuki S, Yeh WC, 2002b. IRAK-4 as the central TIR signaling mediator in innate immunity. Trends Immunol 23, 503–506. [DOI] [PubMed] [Google Scholar]
- Tanda G, Mereu M, Hiranita T, Quarterman JC, Coggiano M, Katz JL, 2016. Lack of Specific Involvement of (+)-Naloxone and (+)-Naltrexone on the Reinforcing and Neurochemical Effects of Cocaine and Opioids. Neuropsychopharmacology 41, 2772–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik VL, LaCroix-Fralish ML, Nutile-McMenemy N, DeLeo JA, 2005. Transcriptional and translational regulation of glial activation by morphine in a rodent model of neuropathic pain. J Pharmacol Exp Ther 313, 1239–1247. [DOI] [PubMed] [Google Scholar]
- Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, Baumann MH, Hutchinson MR, Rice KC, Watkins LR, Shaham Y, 2013. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry 73, 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Jing L, Qiu Y, Gancarz-Kausch AM, Galuska CM, Dietz DM, Zhang Y, Li JX, 2014. Effects of the trace amine-associated receptor 1 agonist RO5263397 on abuse-related effects of cocaine in rats. Neuropsychopharmacology 39, 2309–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Qiu Y, Jia S, Zhang Y, Li JX, 2016. Antinociceptive effects of imidazoline I2 receptor agonists in the formalin test in rats. Behav Pharmacol 27, 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucha M, Coria SM, Nunez AE, Santos-Toscano R, Roura-Martinez D, Fernandez-Ruiz J, Higuera-Matas A, Ambrosio E, 2019. Morphine self-administration alters the expression of translational machinery genes in the amygdala of male Lewis rats. J Psychopharmacol 33, 882–893. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M, 2015. The Brain on Drugs: From Reward to Addiction. Cell 162, 712–725. [DOI] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H, 2012. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc Natl Acad Sci U S A 109, 6325–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF, 2009. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci 30, 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Li JX, 2020. Toll-Like Receptor 4 Signaling and Drug Addiction. Front Pharmacol 11, 603445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Xiao D, Shan X, Dong Y, Tao WW, 2020. Rapid and Prolonged Antidepressant-like Effect of Crocin Is Associated with GHSR-Mediated Hippocampal Plasticity-related Proteins in Mice Exposed to Prenatal Stress. ACS Chem Neurosci 11, 1159–1170. [DOI] [PubMed] [Google Scholar]
- Wu R, Zhang H, Xue W, Zou Z, Lu C, Xia B, Wang W, Chen G, 2017. Transgenerational impairment of hippocampal Akt-mTOR signaling and behavioral deficits in the offspring of mice that experience postpartum depression-like illness. Prog Neuropsychopharmacol Biol Psychiatry 73, 11–18. [DOI] [PubMed] [Google Scholar]
- Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ, 2010. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A 107, 11942–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Shen H, Lin L, Su T, Zhong L, Yang Z, 2015. MicroRNA367 negatively regulates the inflammatory response of microglia by targeting IRAK4 in intracerebral hemorrhage. J Neuroinflammation 12, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue K, Tanda G, Katz JL, Zanettini C, 2020. A further assessment of a role for Toll-like receptor 4 in the reinforcing and reinstating effects of opioids. Behav Pharmacol 31, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, An J, 2007. Cytokines, inflammation, and pain. Int Anesthesiol Clin 45, 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


