Abstract
Whereas human spinal cord injury (SCI) is more common in men, the prevalence is growing in women. However, little is known about the effect of biological sex on brain dysfunction and injury mechanisms. To model the highest per capita rate of injury (ages between 16–30 years old) in humans, in the present study, young adult or a young/middle-aged male and female C57BL/6 mice were subjected to moderate contusion SCI. When mice were injured at 10–12-week-old, transcriptomic analysis of inflammation-related genes and flow cytometry revealed a more aggressive neuroinflammatory profile in male than females following 3 d SCI, ostensibly driven by sex-specific changes myeloid cell function rather than cell number. Female mice were generally more active at baseline, as evidenced by greater distance traveled in the open field. After SCI, female mice had more favorable locomotor function than male animals. At 13 weeks post-injury, male mice showed poor performance in cognitive and depressive-like behavioral tests, while injured female mice showed fewer deficits in these tasks. However, when injured at 6 months old followed by 8 months post-injury, male mice had considerably less inflammatory activation compared with female animals despite having similar or worse outcomes in affective, cognitive, and motor tasks. Collectively, these findings indicate that sex differences in functional outcome after SCI are associated with the age at onset of injury, as well as disrupted neuroinflammation not only at the site of injury but also in remote brain regions. Thus, biological sex should be considered when designing new therapeutic agents.
Keywords: Spinal cord injury, sex differences, functional recovery, neuroinflammation
1. Introduction
Traumatic spinal cord injury (SCI) is a catastrophic event for the body that results in individuals facing lifelong disability. Although epidemiological data shows that men are predominantly more likely to suffer from spinal cord and head injury, with over 80% of SCI occurring in males between 25 and 45 years of age, there has been a growing trend of increased case numbers in women (Chen et al., 2016). Biological sex is a key factor in multiple disorders of the central nervous system (CNS), including but not limited to stroke, multiple sclerosis, Alzheimer’s disease (AD), and neuropathic pain (Bartley and Fillingim, 2013; Bushnell et al., 2018; Comfort and Re, 2017; Dubal, 2020; Falk et al., 2013; Giatti et al., 2020). In the neurotrauma field, sex differences in acute neurological outcome have now been widely established in experimental models of traumatic brain injury (TBI) and SCI, however, few studies have investigated the role of sex in the chronic stages of SCI or as it pertains to brain function.
Clinical data show the influence of sex in survivors of traumatic SCI. For example, women are more susceptible to mood disorders and neuropathic pain after SCI but have an overall better recovery process in terms of locomotor function and independence (Wilson et al., 2018; Wilson et al., 2012). However, other clinical studies on functional recovery and rehabilitation after SCI show little to no difference when factoring in sex as a variable (Greenwald et al., 2001; Scivoletto et al., 2004). In the experimental setting, rodent models of contusion SCI show faster functional recovery in adult female rats compared to their male counterparts (Datto et al., 2015; Hauben et al., 2002). However, the majority of these studies have been limited to descriptive observations of motor function recovery or complications following SCI. Other studies examine the possibility of sex hormones as a viable treatment option. This is likely based on the traditional view that sex dimorphism is determined by downstream regulatory pathways that underlie sexual development of the gonads, brain and other organs, along with fluctuations of sex hormones within the test subject’s lifespan (Hochberg et al., 2011). However, this traditional line of thinking has been called into question as several studies in the early 2000’s displayed examples of sex differences in response to either prenatal or early postnatal environmental exposures, a time period that should be attributed to genetic properties instead of fluctuating hormone levels (Gabory et al., 2009; Gallou-Kabani et al., 2007a; Gallou-Kabani et al., 2007b; Sugden and Holness, 2002; Wilcoxon et al., 2003). The present study attempts to identify the genetic factors that drive sexually dimorphic changes in neuroinflammatory responses at acute and chronic time points after SCI.
Despite varying conclusions and experimental designs, it seems likely that the majority of sex differences are mediated by secondary injury mechanisms, of which, inflammatory responses play a major role in delayed cell death and the formation of glial scars. As reported by our lab and others (Li et al., 2020a; Li et al., 2020b; Maldonado-Bouchard et al., 2016), this process of ongoing neuroinflammation not only affects the spinal cord but also the brain of injured mice, especially at chronic time points. Based on preclinical and clinical studies in AD and mood disorders, the onset and severity of these diseases also relies heavily on sex (Lai et al., 2020; Todorovic et al., 2020). Thus, the question of whether biological sex modifies the development of cognitive decline after SCI is also highly relevant and one in which the present study attempts to address. It is also unclear if sex differences in SCI become more or less pronounced when older females enter perimenopause. In the present study, we separately controlled for matching age at 10–12 week-old and 6 month-old when injured to model the highest per capita rate of injury (ages between 16–30 years old) in humans. Using molecular and cellular experimental methods, we assessed the functional and pathological outcomes of each sex at three different time points after SCI. Importantly, we demonstrate a novel role for sex as a risk factor for the development of depressive-like behavior and neuroinflammation in the brain after chronic SCI.
2. Materials and Methods
2.1. Animals and surgery
Young adult (10–12-week-old) or young/middle aged (6-month-old) male and female C57BL/6 mice were obtained from Jackson Laboratories to model the highest per capita rate of injury (ages between 16–30 years old) in humans according to Spinal Cord Injury Facts & Statistics from the National Spinal Cord Injury Statistical Center (NSCISC, 2019). Male and female mice were housed in the same room of the animal care facility at the University of Maryland School of Medicine under a 12 h light/dark cycle, with ad libitum access to food and water. After inducing anesthesia with isoflurane, a laminectomy was performed at the thoracic T9 and T11 region. The spinal column was then stabilized with lateral clamps. The mice underwent contusion injury of the spinal cord at the T10 level using the Infinite Horizon Spinal Cord Impactor (Precision Systems and Instrumentation) at 65 or 70 kilodynes of force, which is considered to be moderate/severe (Li et al., 2021; Li et al., 2020b; Scheff et al., 2003; Wu et al., 2016). For the first two weeks of injury, manual bladder expression was performed on SCI mice for at least three times per day, until reflex bladder emptying could be reestablished. For mice being used as control animals, only laminectomy was performed after anesthesia. Individuals who performed functional assessment and were involved in data analysis were blinded to group designations throughout all stages of the experiment. We did not assess estrous cycle, thus, female mice were blindly assigned to surgical condition with respect to hormonal status. The number of mice at various time points and used for each experiment is indicated in the figure legends. All procedures were performed under protocols approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee (IACUC).
2.2. Experimental design
Study 1:
To examine sex differences on transcriptomic levels of neuroinflammation-related genes in the acute phase of SCI, age-matched male and female C57BL/6 mice at 10–12 weeks old were subjected to moderate/severe (65 kdyn) SCI. At 3 days post-injury, mice were euthanized and the ~0.5 cm of spinal cord tissue surrounding the epicenter of the lesion site was dissected and processed for NanoString neuroinflammation panel analysis.
Study 2:
To better understand our transcriptomic findings in the context of cellular inflammation, age-matched male and female C57BL/6 mice at 10–12 weeks old were subjected to moderate/severe (65 kdyn) SCI. At 3 days post-injury, mice were euthanized and the fresh ~1cm of spinal cord tissue surrounding the epicenter of the lesion site was dissected and the cell suspension was prepared for flow cytometry assays.
Study 3:
To investigate whether there were sex differences in neurological function recovery after SCI, C57BL/6 male and female mice at 10–12 weeks old were subjected to moderate/severe (65 kdyn) contusion injury or sham surgery. All animals underwent locomotor function testing (BMS) on 1 day and 3 days and weekly thereafter for up to 10 weeks post-injury. Beginning 12 weeks post-injury, all mice underwent a battery of neurobehavioral tasks [Motor function: open field (OF); Cognitive function: Y maze (YM), novel object recognition (NOR); Depressive-like behavior: tail suspension (TS), forced swim (FS), novelty suppressed feeding (NSF)].
Study 4:
To examine the inflammatory response when injury is elicited at middle-age, age-matched male and female C57BL/6 mice at 6 months old were subjected to severe (70 kdyn) SCI. Our unpublished data indicates that total body weight does affect displacement of the cord during injury. To obtain similar displacement in different cohorts of mice in the present study, injury force was adjusted to 70 kdyn in 6-month-old mice. At 3 days post-injury, mice were euthanized and the ~0.5 cm of spinal cord tissue surrounding the epicenter of the lesion site was dissected and processed for total RNA and analyzed by qRT-PCR.
Study 5:
To determine whether there were sex differences in pathological changes and functional recovery after chronic SCI, age-matched male and female C57BL/6 mice at 6 months old were subjected to severe (70 kdyn) SCI followed by 8.5 months post-injury. All animals underwent locomotor function testing (BMS) and neurological behavioral tests. By 34 weeks post-injury, injured spinal cord (~0.5 cm of spinal cord tissue surrounding the epicenter of the lesion site) and brain tissue (the somatosensory cortex) were collected and processed for NanoString neuroinflammation panel analysis. Table 1 summarizes body weight and injury biomechanics for each experimental cohort.
Table 1.
Body weight and injury biomechanics for different experimental cohorts.
| Experimental cohorts | Body weight (g) | Force (kdyne) | Displacement (μm) |
|---|---|---|---|
| Study 1: d3 SCI for NanoString | |||
| Male (n=4) | 21.78±0.39 | 66.50±0.65 | 719.5±19.3 |
| Female (n=4) | 20.18±0.56* | 66.25±0.75 | 683.0±56.6 |
| 1 mouse was euthanized due to surgical complications | |||
| Study 2: d3 Flow cytometry | |||
| Male (n=10) | 25.50±0.67 | 67.00±0.8 | 632.7±30.2 |
| Female (n=10) | 18.00±0.30*** | 666.70±0.76 | 623.6±28.1 |
| Study 3: long-term behavior | |||
| Male (n=10) | 23.03±0.31 | 69.00+2.17 | 767.5±55.9 |
| Female (n=10) | 21.05±0.34*** | 67.70±0.79 | 715.8±75.3 |
| 3 mice were excluded due to dural tears at the time of surgery | |||
| Study 4: older mice-d3 SCI qPCR | |||
| Male (n=8) | 31.61±0.99 | 71.75±0.56 | 739.8±39.0 |
| Female (n=8) | 25.41±1.34** | 71.63±0.82 | 727.1±32.4 |
| Study 5: older mice-behavior | |||
| Male (n=11) | 32.27±0.60 | 81.55±7.89 | 729.1±50.9 |
| Female (n=11) | 25.09±0.74*** | 76.82±3.56 | 709.3±40.0 |
p<0.05,
p<0.01,
p<0.001 vs Male group, Unpaired t test.
2.3. NanoString neuroinflammation panel analysis
RNA samples were obtained after sample processing with the Qiagen RNA extraction kit from 0.5 cm of spinal cord tissue at 3 days and 8 months post-injury, tissue puncta of the somatosensory cortex were also taken from mice euthanized at the 8-month timepoint. Total RNA (20 ng/ul) was run on a NanoString nCounter® system for Mouse Neuroinflammation v1.0 panel (NanoString Technologies, Seattle, WA) to profile RNA transcript counts for 757 genes and 13 housekeeping genes (Li et al., 2021; Li et al., 2020b).
Sample gene transcript counts were normalized prior to downstream analysis and pairwise differential expression analysis was performed with NanoString’s nSolver software Version 4.0. All statistical analysis of NanoString data was performed in the R language using RStudio Version 1.2.5033. Multidimensional Scaling (MDS) was performed with the Log2 distance measurement method. The four pairwise comparisons were as follows and described in this manuscript as: (1) Sham/Female vs. Sham/Male – Set 1; (2) SCI/Male vs. Sham/Male – Set 2; (3) SCI/Female vs. Sham/Female — Set 3; and (4) SCI/Female vs. SCI/Male — Set 4. All comparisons “Group 1 vs. Group 2” were interpreted as “Group 1 relative to Group 2” in the text and figures. A p-value of less than 0.05 was used to identify differentially expressed (DE) genes in each comparison. Subsets of DE genes displayed as heatmaps were normalized across samples as z-scores and then averaged to a single value per group before plotting using GraphPad Prism Version 8.4.2. Pathway enrichment analysis was performed through the online Enrichr website with KEGG 2019 mouse database as our main gene set library (Kuleshov et al., 2016). Follow-up subnetwork enrichment analysis was performed with the R package PathfindR (Ulgen et al., 2019).
2.4. Flow cytometry assays
Mice were sacrificed as described above at three days post-SCI and transcardially-perfused with 40 ml of cold saline. The ~1 cm of spinal cord tissue surrounding the epicenter of the lesion site was dissected out, washed again with saline to remove any residual or contaminant blood cells, and mechanically digested through a 70 μm filter followed by enzymatic digestion precisely as described (Li et al., 2021). Spinal cord tissue weights (mg) were recorded for each mouse immediately after harvest. Total cell counts were obtained after collecting all events for each spinal cord sample and normalizing to mg tissue weight. All cells were blocked using TruStain FcX antibody (Clone: 93, Cat# 101320, Biolegend) prior to cell surface antibody staining. CNS leukocytes were identified after first gating for leukocyte scatter properties, singlets, and exclusion of the fixable viability dye Zombie Aqua (Cat# 423102, Biolegend). Living microglia were identified as CD45intCD11b+, whereas infiltrating myeloid cells (CD45hiCD11b+) were divided into Ly6Chi inflammatory monocyte and Ly6G+ neutrophil subsets. Intracellular staining for CD68-PerCP/Cy5.5 (Clone: FA-11, Cat# 137010, Biolegend), Ki67-PE (Clone: 16A8, Cat# 652404, Biolegend), NOX2/GP91PHOX-AF647 (Cat# BS-3889R-A647, Bioss), and phospho(Ser139)-H2A.X-PE/Cy7 (Clone: 2F3, Cat# 613420, Biolegend) was performed according to the manufacturer’s suggestions (Doran et al., 2019; Ritzel et al., 2019). Intracellular staining for IL1β-PerCP/eF710 (Clone: NJTEN3, Cat# 46-7114-82, Thermofisher), IL6-PE (Clone: MP5–20F3, Cat# 504504, Biolegend), TNF-PE/Cy7 (Clone: MP6-XT22, Cat# 506324, Biolegend), and TGFβ-APC (Clone: TW7–16B4, Cat# 141406, Biolegend) cytokine production was performed using 1X Brefeldin A (Cat# 420601, Biolegend) in Roswell Park Memorial Institute (RPMI)-1640 media (Lonza) and incubating for 3h prior at 37°C to fixation/permeabilization. Data were acquired on a BD LSRFortessa cytometer using FACSDiva 6.0 (BD Biosciences) and analyzed using FlowJo (Treestar Inc.). Cell type-matched fluorescence minus one (FMO) controls were used to determine the positivity of each antibody. Prior to assessment on the cytometer, isolated cells were briefly probed to determine phagocytosis activity, oxidative stress level, and mitochondrial function as described below.
Assessment of reactive oxygen species production was achieved by staining with the free radical sensor H2DCF-DA (DCF) (Cat# D399, ThermoFisher) as described (Ritzel et al., 2021). Bead (Cat# L4655, Sigma) engulfment assay was performed exactly as described (Ritzel et al., 2018; Ritzel et al., 2020). Mitochondrial mass and membrane potential were measured using MitoSpy Green FM (Cat# 424806, Biolegend) and MitoSpy Red CMXRos (Cat# 424802, Biolegend) according to manufacturer’s instructions.
2.5. Neurological behavioral tests
Basso mouse scale (BMS) for locomotion:
For assessment of locomotor function with the BMS (Basso et al., 2006), mice were placed on a flat, enclosed surface with a diameter of 100 cm and continuously observed for a minimum of 4 min by two trained researchers blinded to the sex of each mouse. Animals were rated on a scale of 0–9: 0 being complete paralysis of the hind limbs and 9 being normal locomotor activity. The scoring parameters are based on hind limb joint movement, weight support, plantar stepping, and coordination. Mice were tested for BMS scores on day 1, day 3 and weekly time points after injury.
Spontaneous motor activity:
The open field (OF) test was used to measure locomotor activity at 13 or 32 weeks post-injury (Wu et al., 2016). Each mouse was individually placed in the same corner facing the wall of the open-field chamber (22.5 cm × 22.5 cm) and allowed to freely explore the chamber for 5min. The distance travelled, average speed, time immobile vs mobile were recorded by Any-Maze software (Stoelting Co).
Y-maze test:
The Y-maze was performed at 13 or 32 weeks after injury to test spatial working memory in mice (Li et al., 2020b; Ritzel et al., 2021). The Y-maze (Stoelting Co.) consisted of three identical arms (A, B, C). During testing, one arm was randomly selected as the “start” arm, and the mouse was placed in the maze freely for 5 min. Arm entries were recorded, and one alternation was designated as when the mouse entered three different arms consecutively. The percentage of spontaneous alternations was calculated as follows: total alternations × 100/(total arm entries − 2). If a mouse scored significantly >50% alternations (the chance level for choosing the unfamiliar arm), this was indicative of spatial working memory.
Novel object recognition (NOR):
NOR testing was performed at 13 or 32 weeks to assess non-spatial hippocampal-mediated memory, as previously described (Li et al., 2020b; Ritzel et al., 2020). On day 1, mice were individually placed in an open field (22.5 cm × 22.5 cm) for 5min free moving for habituation. Next day, mice were placed in an open field where two identical objects were placed near the left and right corners of the chamber for training (sample phase). After 24 h, object recognition was tested by substituting a novel object for a familiar training object (the novel object location was counterbalanced across mice). Time spent with two identical objects was recorded; because mice inherently prefer to explore novel objects, a preference for the novel object (more time than chance (15 s) spent with the novel object) indicates intact memory for the familiar object.
Novelty Suppressed Feeding (NSF):
The NSF test was performed at 13 or 33 weeks after SCI (Stedenfeld et al., 2011). This is a conflict-based test in which the subject mouse that has undergone food deprivation for a full 24 h faces the choice of approaching and consuming a piece of chow pellet in the center of a brightly lit, novel open field arena or staying to the side. The latency time for each mouse to reach the food pellet in the novel arena is recorded within a maximum time of 10 min. For animals that did not eat the food pellet in the testing period, a latency of 600 s was assigned to them. Upon returning to their home cage, the latency time to eat was also recorded to test whether feeding differences in the novel arena are due to differences in hunger or motivation.
Tail-suspension (TS) test:
The TS test assesses depression-like behavior in mice and is based on the observation that mice develop an immobile posture when placed in an inescapable hemodynamic stress of being hung by their tail. The TS was performed at 13 weeks as described previously (Li et al., 2020b; Wu et al., 2016). Each mouse was suspended at a height of 28 cm using 3M adhesive tape. The tip of the mouse tail was not wrapped around the rod while being suspended. The duration of immobility was recorded throughout the 5-minute test period. The definition of immobility is passive hanging and complete motionlessness. Foam padding (3” deep) was placed under the beam in case animals fall from the beam during the experiment.
Forced swim (FS) test:
FS testing is one of the most commonly used assays for the study of depressive-like behavior in rodents. At 13 or 33 weeks after SCI, mice were placed in transparent plastic cylinder (45 cm high × 20 cm diameter) filled with water (23 ± 2 °C; 28 cm in depth) for 6 min (Ritzel et al., 2021; Ritzel et al., 2020). The duration of immobility was recorded.
2.6. RNA extraction and qPCR
Total RNA was extracted from flash frozen tissue samples with the miRNeasy Mini Kit (Qiagen, Cat# 74104). Complementary DNA (cDNA) was synthesized with the Verso cDNA RT kit (Thermo Scientific, Cat# AB1453B). Both kits were used according to the manufacturer’s instructions included in the kit box. Real-time PCR for target mRNAs was performed using TaqMan gene expression assays for Il1b (IL-1β), Mm00434228_m1; IL-6, Mm00446190_m1; Cd74, Mm00658576_m1; Psmb8, Mm00440207_m1; chil3 (Ym1), Mm00657889_mH; C1qa, Mm00432142_m1; C1qb, Mm00437836_m1; C1qc, Mm00776126_m1; C4a, Mm01132415_g1; ITGAM (CD11b), Mm00434455_m1; Nlrp3, Mm00840904_m1; Hcar2, Mm01199527_s1; Igf1, Mm00439560_m1; TGFβ, Mm01178820_m1; SOCS3, Mm01342740_g1; TNFα, Mm00443258_m1; Cd68, Mm03047343_m1; Cxcl10, Mm00445235_m1; Cd44, Mm01277161_m1; Serping1, Mm00437835_m1; Pmp22, Mm01333393_m1; Flt1, Mm00438980_m1; Egfr, Mm01187858_m1; C3, Mm00437838_m1; and GAPDH, Mm99999915_g1 (Applied Biosystems, Carlsbad, CA] on an QuantStudio™ 5 Real-Time PCR System (Applied Biosystems). Samples were assayed in duplicate in 1 run (40 cycles), which was composed of 3 stages, 50 °C for 2 min, 95 °C for 10 s for each cycle (denaturation), and finally, the transcription step at 60 °C for1 min. Gene expression was normalized by GAPDH and compared to the control sample to determine relative expression values by the 2–ΔΔCt method.
2.7. Statistical analysis
Data presented as individual data points with mean ± S.E.M. Animal numbers in each experiment were derived from the power calculation based on effect sizes defined by Cohen (Cohen, 1992) and variability estimated from published data. Conventional definitions of effect sizes of difference have been offered as follows: 0.10 (small), 0.25 (medium), and 0.40 (large). This design achieved 90% power to detect 0.3 effect size difference of each of main effects or two-way interactions at a 5% significance level. For specific experiments, one mouse in study 1 was euthanized due to surgical complications and three mice in study 3 were excluded due to dural tears at the time of surgery. No exclusion criteria were pre-established. All statistical analyses were conducted by using the GraphPad Prism Program, Version 3.02 for Windows (GraphPad Software; RRID:SCR_002798). BMS scores were analyzed using two-way ANOVA with repeated measures followed by Sidak’s multiple followed by Tukey’s multiple comparisons post hoc test for parametric (normality and equal variance passed) data. Statistical analysis in each assay was detailed in figure legends. A p value of < 0.05 was considered statistically significant.
3. Results
3.1. Acute SCI increases a transcriptional inflammatory response in the injured spinal cord with distinct sex effects
To model the highest per capita rate of injury (ages between 16–30 years old) in humans and to assess sex differences in acute phase of SCI, young adult (10–12-week-old) age-matched male and female C57BL/6 mice were subjected to moderate contusion SCI. Injury biomechanics indicated that though body weight of female mice was significantly less than male animals before injury, there were no differences in injury forces and displacement between SCI/Male and SCI/Female groups (Table 1). At 3 d post-injury, the injury site was evaluated using Nanostring’s nCounter technology. The Neuroinflammation panel tested a total of 757 genes within three themes of Immunity & Inflammation, Neurobiology & Neuropathology and Metabolism & Stress. MDS of all normalized gene counts revealed a distinct separation of samples into individual groups across the first two principal coordinates (Fig. 1A). The first component accounted for the majority of the variation (90.2%) across samples and separated the groups by injury, while the second principal coordinate (3.4%) separated the groups by sex. Interestingly, the two SCI groups clustered closer together on the y-axis compared to the two Sham groups, which is probably due to the vast majority of inflammatory genes being increased at three days post-SCI.
Figure 1. SCI significantly alters the transcriptome at the injury site in acute phase.
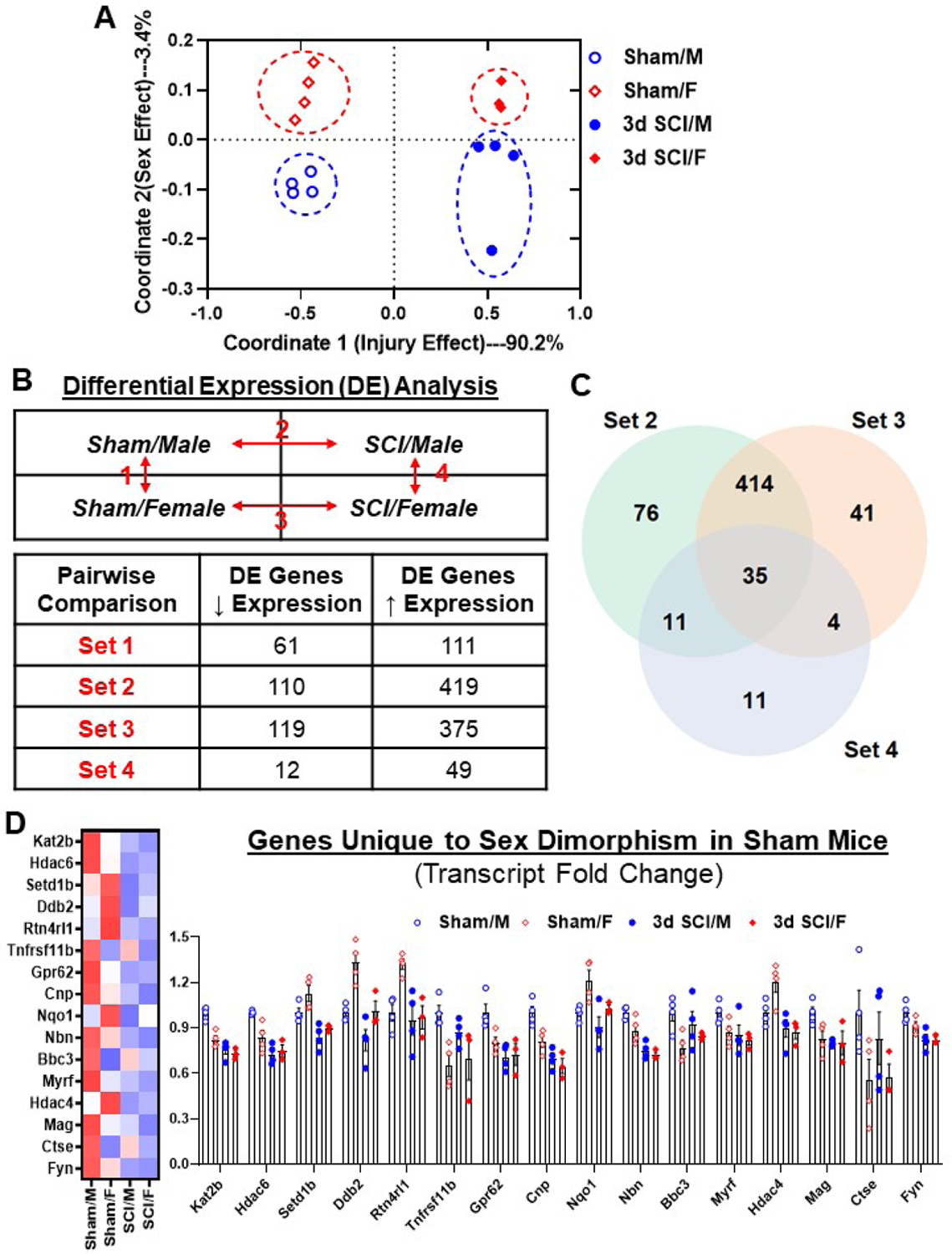
Age-matched male and female C57BL/6 mice at 10–12 weeks old were subjected to moderate (65 kdyn) SCI. A NanoString nCounter® Neuroinflammation panel was used to assess transcriptional changes at the injured site at 3 d post-injury. (A) Multi-dimensional Scaling (MDS) was performed using all normalized gene counts from the NanoString panel. The four sample groups were Sham/Male (open circle), Sham/Female (open square), 3d SCI/Male (closed circle), and 3d SCI/Female (closed square). PCA revealed distinct clustering (dashed ellipses) of the four sample groups across the first two principal coordinates, which accounted for 90.2% and 3.4%, respectively, of the total variation across samples. Injury-related effects were captured on Coordinate 1, separating the SCI groups on the right from the left. (B) Differential expression (DE) analysis was performed on pairwise group comparisons using nSolver (p-value < 0.05). Four pairwise comparisons were performed: (1) Sham/Female vs. Sham/Male; (2) SCI/Male vs. Sham/Male; (3) SCI/Female vs. Sham/Female; and (4) SCI/Female vs. SCI/Male. (C) A Venn diagram demonstrates the separation of total injury genes (i.e., those genes DE in SCI/Male vs. Sham/Male (Set 2) and SCI/Female vs. Sham/Female (Set 3) into those showing sex dimorphism and those that do not, based on based on membership in the gene list of Set 4 (SCI/Female vs. SCI/Male). Although the vast majority showed upregulation after injury, only a small percentage of genes showed significant changes between SCI/Female and SCI/Male. (D) Plot of transcript fold changes normalized by Sham/Male for genes unique to sex dimorphism between sham mice (Set 1, p-value<0.05). N=3–4 mice/group.
Four pairwise comparisons of spinal cord tissues were performed and outlined in Fig. 1B: (1) Sham/Female vs. Sham/Male – (Set 1); (2) SCI/Male vs. Sham/Male – (Set 2); (3) SCI/Female vs. Sham/Female — (Set 3); and (4) SCI/Female vs. SCI/Male — (Set 4). SCI resulted in increased expression of genes in both male and female mice (Set 2 and 3), while sex differences were seen in both baseline (Set 1) and SCI conditions (Set 4). At baseline, 172 genes (22.7%) were differentially expressed between male and female, indicating that sex affected the homeostatic regulation of genes related to the inflammatory and immune process in a fundamental way. A Venn diagram illustrates the number of DE genes that overlap between Sets 2–4 (Fig. 1C). With further analysis we were able to identify a unique subset of 16 differentially expressed genes that only showed significant changes between Sham/Male and Sham/Female (Fig. 1D). Several genes in this subset showed as much as a 40% increase or decrease in linear fold changes. For example, Sham/Female mice showed significantly higher transcription levels of Ddb2, which encodes Damage Specific DNA binding Protein 2, a protein that participates in DNA repair after injury (Stoyanova et al., 2009). The gene Rtn4rl1 encodes cell surface receptor Reticulon 4 Receptor-Like 1, which plays a role in regulating axon regeneration in the CNS and promotes neuroprotection for motoneurons against apoptosis. On the other hand, Sham/Female mice showed significantly lower transcription levels of Ctse, which encodes the lysosomal enzyme Cathepsin E, a protein thought to be involved in age-related neuronal death and excitotoxicity in transient cerebral ischemia (Qin et al., 2008). The higher transcription levels of neuroprotective genes and lower pro-inflammatory genes may potentially set the stage for vastly different reactions to SCI between the sexes. These differences in expression level may also coincide with faster functional recovery following SCI.
To further investigate the effects of sex on transcriptional activation after SCI, we compared the percentage of genes modified by injury for each pathway annotation (Fig. 2A). All pathways were affected by injury at this acute stage, but genes that facilitate Microglia Function, Astrocyte Function, Cellular Stress, and Cytokine Signaling were far more likely to be activated in SCI/Male mice compared to SCI/Females. Subnetwork enrichment analysis of injury genes for both males and females using PathfindR indicate that each pathway was differentially activated in response to injury (Fig. 2B). The most notable difference was seen in the number of upregulated pro-apoptotic genes with higher ranking p-values in SCI/Males (Set 2, 78 genes) than SCI/Females (Set 3, 52 genes).
Figure 2. Acute SCI alters different neuroinflammatory pathways between male and female.
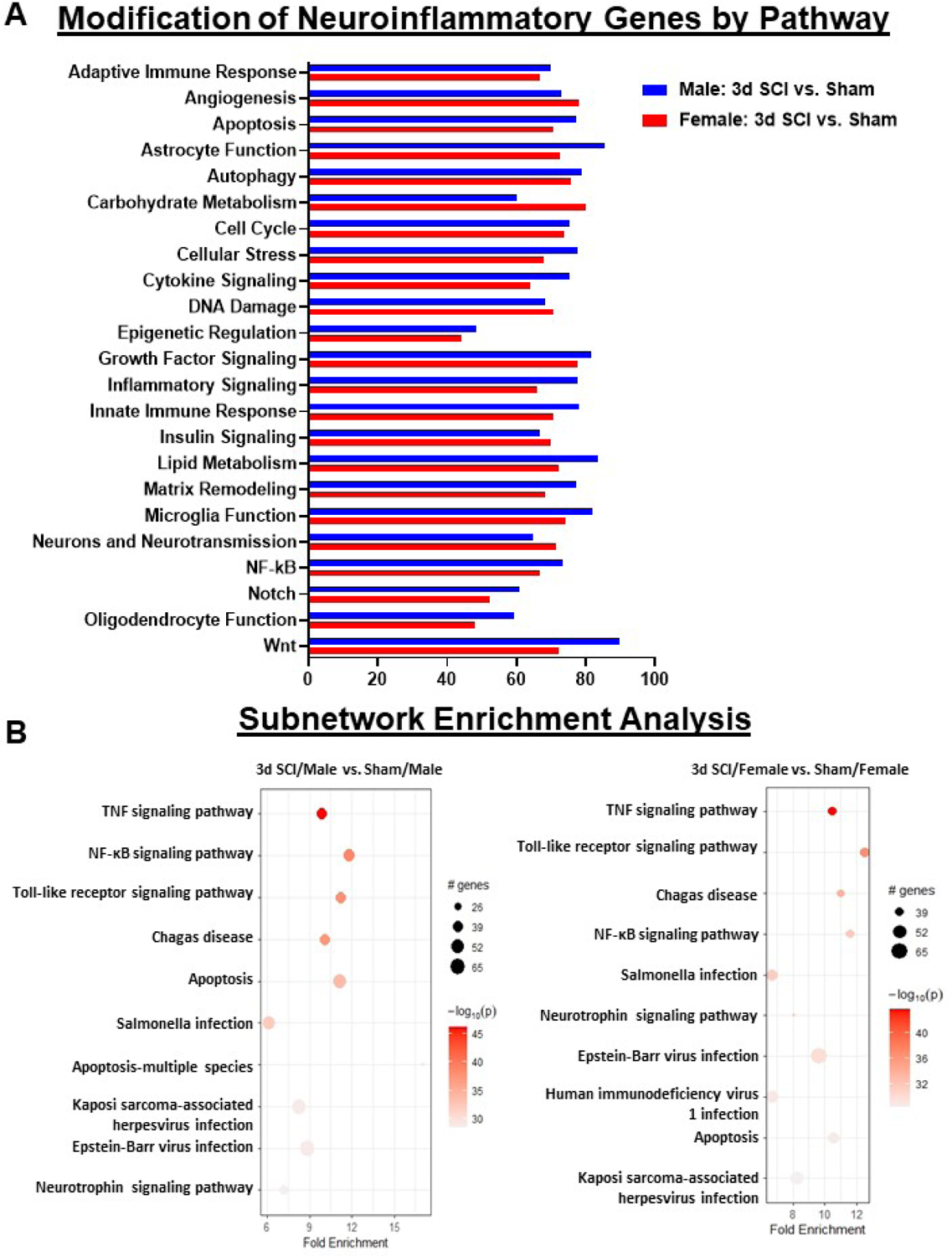
(A) Graph illustrating the neuroinflammatory genes altered by spinal cord injury in different sexes. Injury genes in male mice indicated higher alteration in Astrocyte Function, Cellular Stress, Cytokine Signaling, Inflammatory Signaling, Lipid Metabolism and Wnt Signaling pathway. Female mice indicated higher levels of alteration in Angiogenesis, Carbohydrate Metabolism, DNA Damage and Neurotransmission. (B) Sub-network enrichment analysis with Rstudio’s PathfindR package shows more significant enrichment of genes related to NF-kappaB and Apoptosis Signaling pathway in male mice after injury, while female mice showed higher alterations to Toll-like Receptor and Neurotrophin Signaling pathways. N=3–4 mice/group.
To further analyze the role of sex on the neuroinflammatory response to acute SCI, we isolated three subsets of genes that were unique to Set 2, Set 3 and Set 4, i.e., genes that were only significantly altered in one comparison set and not observed in any other sets. These three subsets allow us to understand which genes may be driving the observed sexual dimorphism at the impact site during acute SCI. We identified 53 genes uniquely altered by injury in male mice, 31 genes uniquely altered by injury in female mice, and 7 differentially expressed genes between SCI/Female and SCI/Male groups (Fig. 3A–C). Pathway enrichment analysis of the unique injury genes in male mice show an abundance of genes that facilitate Cytokine-Cytokine Receptor Signaling (9 genes, Il1rn, Il1rl2, Il1b, Clcf1, Il3ra, Ccl4, Il21R, Tnfrsf10b, Ngf) and Apoptosis (5 genes, Il3ra, Tnfrsf10b, Traf1, Ngf, Bid), whereas the unique injury genes in female mice show a high ranking for genes involved with Non-homologous End-joining (2 genes, Xrcc6, Rad50) and cAMP Signaling Pathway (5 genes, Bdnf, Ptger3, Pik3cb, Rapgef3) (Fig. S1A–C).
Figure 3. Determination of neuroinflammatory genes unique to SCI/Male, SCI/Female and sex dimorphism after injury.
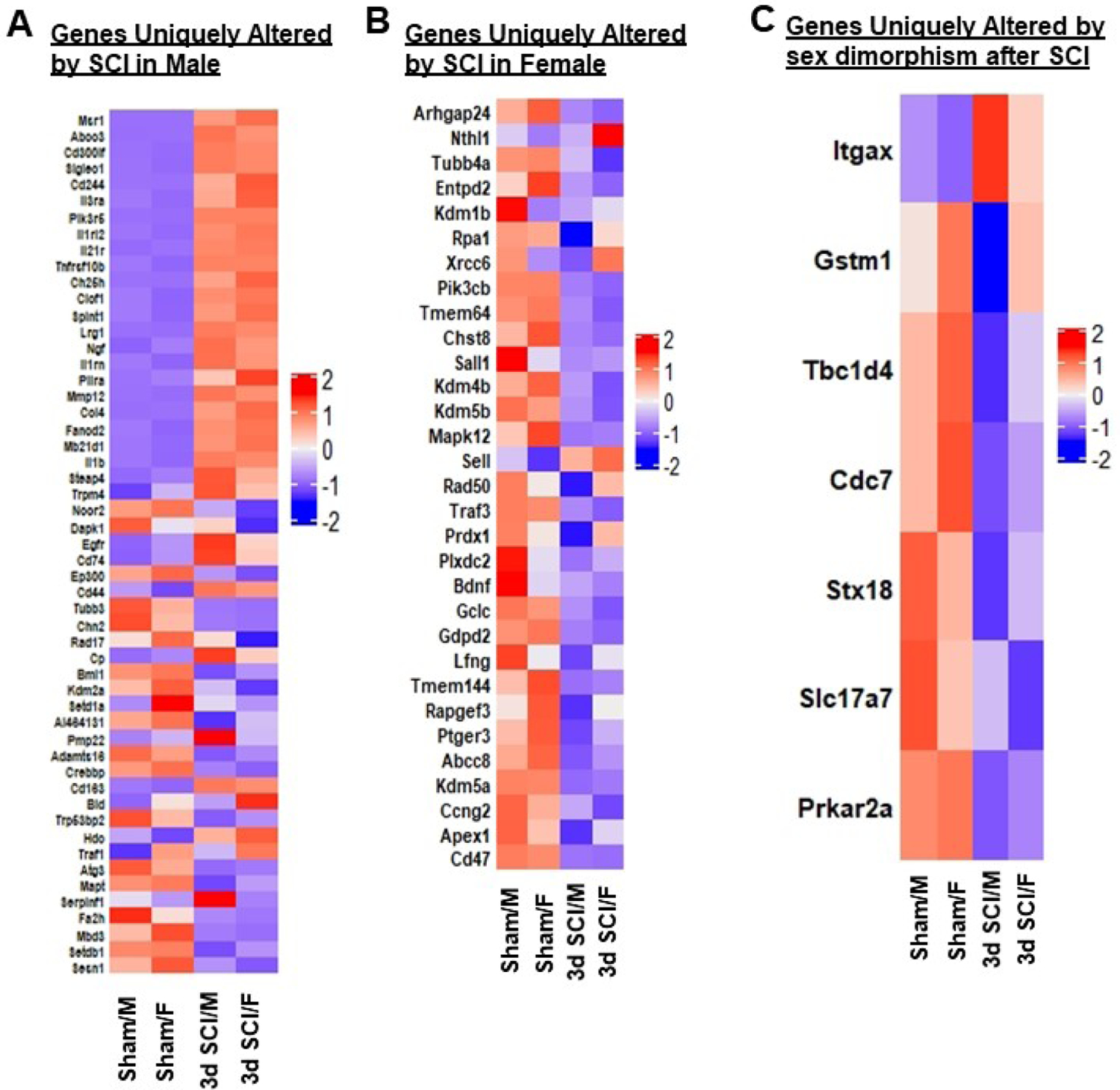
(A) Heatmap of genes that are uniquely altered in DE between SCI/Male and Sham/Male, i.e., genes only altered in Set 2. Color coding was based on z-score scaling. (B) Heatmap of genes that are uniquely altered between SCI/Female and Sham/Female, i.e., genes only altered in Set 3. Color coding is based on z-scores of normalized transcription counts. (C) Heatmap of genes unique to sex dimorphism after injury, i.e., genes only significantly altered in Set 4. N=3–4 mice/group.
Because the genes related to Astrocyte and Microglia Function, Cytokine Signaling, and Cellular Stress showed a high degree of difference between sexes, we teased out the injury genes that were only altered in either male (Set 2) or female mice (Set 3) in these pathways to further elucidate the effects of sex. A high number of genes associated with Astrocyte Function, such as the cell cycle regulatory genes S100b and S100a10, the iron-trafficking gene Lcn2, and the cytokine Csf1, were only altered by SCI in male mice (Fig. S2A–E). For the SCI/Female group, we identified the genes Entpd2 and Gdpd2, both of which are involved with hydrolase activity. Analysis of injury genes involved with Cytokine Signaling showed an increase in multiple cytokine-encoding genes, such as Ccl2, Ccl4 and Ccl7 in Set 2, while the genes Kit (KIT Proto-Oncogene Receptor Tyrosine Kinase), Nefl (Neurofilament Light), Pik3cb (Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Beta) and Traf3 (TNF Receptor Associated Factor 3) were specifically down-regulated in Set 3. Within the Cellular Stress pathway, the pro-apoptotic gene Bad (BCL2 Associated Agonist Of Cell Death) and the proinflammatory cytokine Il1b (Interleukin-1β) were significantly increased in SCI/Males. For Set 3, we see the upregulation of two genes that regulate DNA repair after damage, Rpa1 (Gene Replication Protein A1) and Rad50 (Rad50 Double Strand Break Repair Protein), and the anti-oxidant enzyme Prdx1 (Peroxiredoxin 1). Taken together, our results show a sexually dimorphic transcriptional response to acute SCI highlighted by increased inflammatory and pro-apoptotic pathways in males, and DNA repair pathways in females.
3.2. Sex differences in microglial function, but not number, during acute SCI
To better understand our gene expression data in the context of cellular inflammation, we performed flow cytometry on sham and injured spinal cord tissue at 10–12-week-old age. Injury biomechanics indicated that although body weight of female mice was significantly less than males before injury, there were no differences in injury forces or displacement between SCI/Male and SCI/Female groups (Table 1). Male and female mice showed an equivalent number of newly proliferated microglia in the contusion region of the spinal cord at three days post-injury (Fig. 4A, n=6–10/group). We detected no significant main effect of sex (F(1, 28)=1.590, p=0.2177; Fig. 4A). Intracellular staining for the nuclear protein Ki67 also demonstrated similar rates of ongoing microglial proliferation between sexes at this acute timepoint (F(1, 28)=0.03990, p=0.8431; Fig. 4B). Interestingly, microglia in SCI/Males exhibited a markedly more robust increase in both mitochondrial mass and membrane potential compared SCI/Females, suggesting increased mitochondrial biogenesis and/or activity after injury (Fig. 4C–D). While there was no significant main effect of sex (F(1, 28)=2.063, p=0.1620; Fig. 4C) in the MFI of MitoSpyGreen+ microglia, we detected a significant main effect of sex in the MFI of MitoSpyRed+ microglia (F(1, 28)=8.523, p=0.0069; Fig. 4D). Surprisingly, no sex differences were seen in microglial expression of NOX2 (F(1, 27)=2.015, p=0.1672), reactive oxygen species (ROS, F(1, 27)=0.2148, p=0.6467) production, DNA damage (i.e., p-H2AX, F(1, 27)=1.528, p=0.2271) or cytokine production [i.e., TNF (F(1, 28)=0.7215, p=0.4029), IL1β (F(1, 28)=0.8351, p=0.3686), IL6 (F(1, 28)=0.07602, p=0.7848), and TGFβ (F(1, 28)=0.003623, p=0.9524)] (Fig. S3A–G). To determine whether changes in metabolic function and/or energy production may have affected phagocytic behavior we probed for the expression of CD68, a type-1 transmembrane protein found in the endosomal/lysosomal compartments of phagocytes. Indeed, male microglia showed significantly higher expression of CD68 engulfment of fluorescent latex beads after injury were found (F(1, 28)=0.2901, p=0.5944; Fig. 4F), indicating that pre-existing differences in SCI-induced phagocytosis in vivo became normalized ex vivo. These findings show a strong association between mitochondrial activity and phagocytic activity which was further augmented in males during the acute phase of SCI.
Figure 4. Effect of sex on cellular inflammation during the acute phase of spinal cord injury.
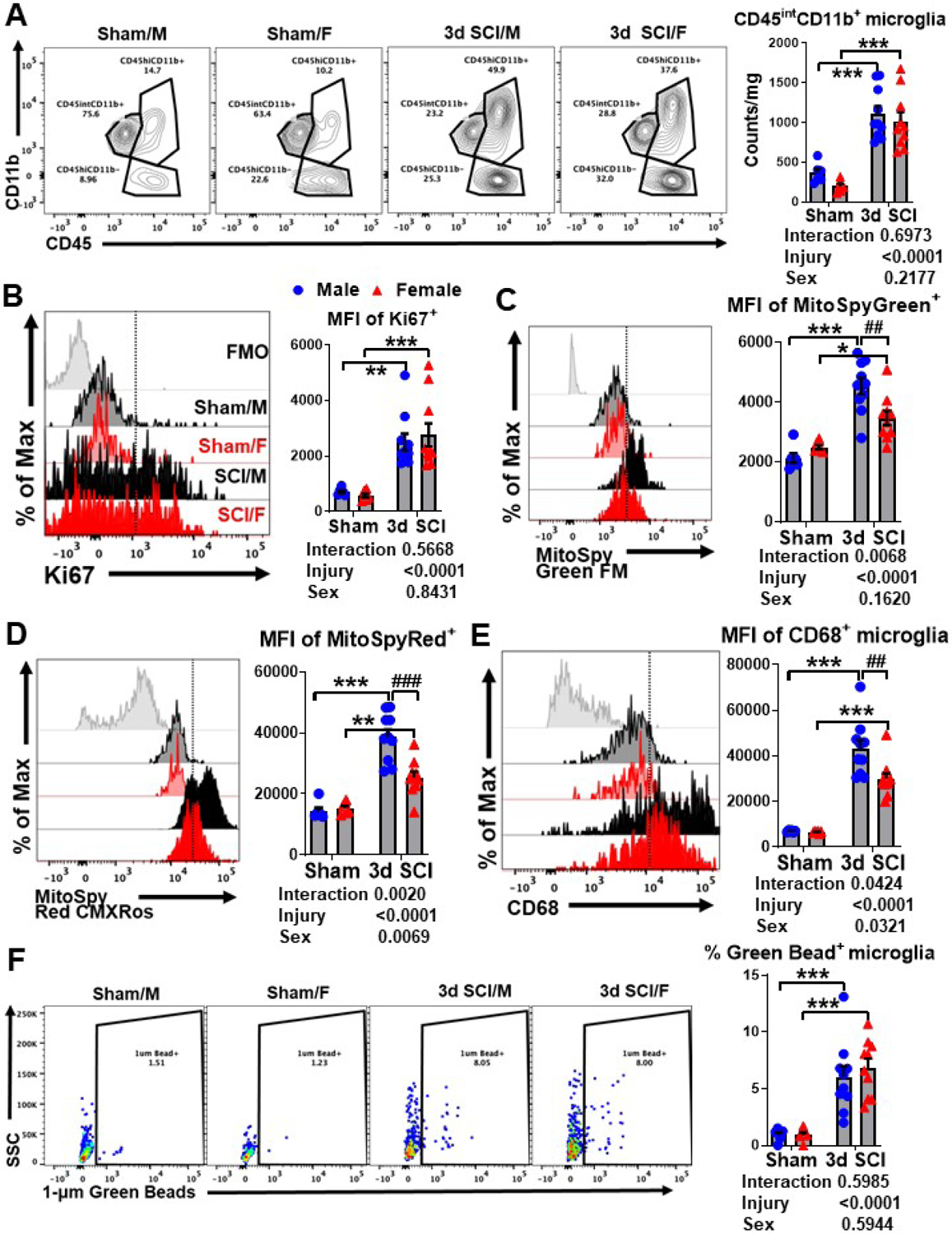
(A) A representative panel of contour plots depicts the leukocyte composition in the lesion area of the spinal cord at 3 d post-injury. Quantification of CD45intCD11b+ microglia cell counts in male and female mice after sham or SCI surgery is shown. (B) A representative histogram shows the relative expression level of Ki67 in microglia at 3d post-injury [FMO = gray, Sham/Male (M) = light black, Sham/Female (F) = light red, SCI/M = dark black, and SCI/F = dark red]. The MFI of Ki67-positive microglia was quantified. Representative Histograms of (C) MitoSpy Green FM and (D) MitoSpy Red CMXRos show similar trends in MFI levels in male and female microglia after injury. (E) A representative histogram shows the relative intracellular expression of CD68 in microglia after injury. MFI quantification of CD68-positive microglia is shown. (F) Representative dot plots show the percentage of microglia that engulfed fluorescent latex beads. The frequency of microglia that actively phagocytosed green beads was quantified. For each sex, N=6 mice per sham group, and N=10 mice per SCI group. **p<0.01, ***p<0.001 vs. Sham group; ##p<0.01, ###p<0.001 vs. SCI group. Two-way ANOVA following Tukey’s multiple comparisons test. Abbreviations: d (day), FMO (fluorescence minus one), int (intermediate), Max (maximum), mg (milligram), MFI (mean fluorescence intensity), SCI (spinal cord injury), SSC (side scatter), μm (micrometer).
Next, we examined the potential for sex differences in peripheral leukocyte extravasation. Injury-related increases in the total number of CD45hiCD11b+ cells and Ly6Chi inflammatory monocyte and Ly6G+ neutrophil subsets were not affected by sex (Fig. 5A). There was also no effect of sex in Ly6Clo monocyte counts after d3 SCI. Additionally, the activation phenotype of Ly6Clo monocytes as indicated by ROS production (Fig. S4A–B) and the expression of NOX2 (Fig. S4C) and CD68 (Fig. S4D) showed no sex differences except for mitochondrial membrane potential which was significantly lower in females (Fig. S4E). Consistent with these findings, we observed higher mitochondrial mass and activity in spinal cord-infiltrating myeloid cells from SCI/Male mice relative to SCI/Female mice (Fig. 5B–C). As with microglia, ex vivo bead engulfment in infiltrating myeloid cells showed no effect of sex (Fig. 5D), however, ROS production in Ly6Chi inflammatory monocytes was significantly higher in SCI/Females (Fig. 5E). These findings highlight a potential role for mitochondria in mediating sex-specific neuroinflammatory profiles in the immediate aftermath of SCI. In sum, these data imply that sex differences in the acute inflammatory response to SCI arise from complex changes in cellular function rather than changes in absolute number or immune composition of resident and infiltrating leukocyte populations.
Figure 5. Sex differences in infiltrating myeloid function during the acute phase of spinal cord injury.
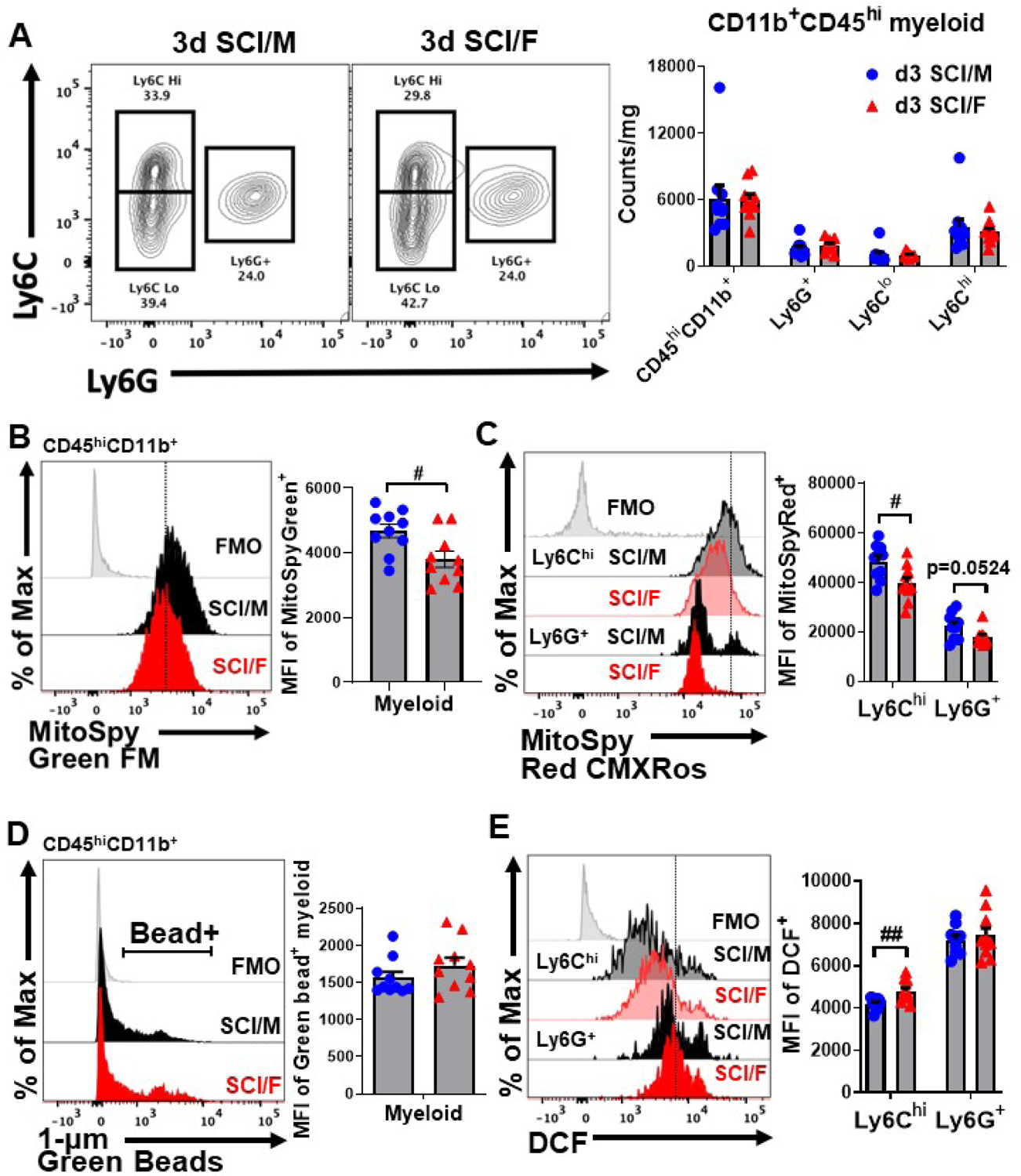
(A) Identification of spinal cord-infiltrating CD45hiCD11b+Ly6Chi inflammatory monocytes, CD45hiCD11b+Ly6G+ neutrophils, and CD45hiCD11b+Ly6Clo monocytes in sham and SCI mice at 3d is shown and cell counts were quantified. (B) A representative histogram depicts the relative level of mitochondrial mass in the CD45hiCD11b+ population (FMO = gray, SCI/M = dark black, SCI/F = dark red) and the MFI was quantified. (C) A representative shows the relative level of mitochondrial activity based on membrane potential in CD45hiCD11b+ subsets (FMO = gray, Sham/M = light black, Sham/F = light red, SCI/M = dark black, SCI/F = dark red). MFI quantification of MitoSpy Red CMXRos-positive microglia is shown. (D) No sex differences were seen between the percentage or MFI of bead-positive microglia in CD45hiCD11b+ populations. (E) A representative histogram shows the relative level of reactive oxygen species production in CD45hiCD11b+ subsets quantified. For each sex, N=6 mice per sham group, and N=10 mice per SCI group. #p<0.05, ##p<0.01 vs. SCI group. Two-way ANOVA following Tukey’s multiple comparisons test. Abbreviations: d (day), hi (high), FMO (fluorescence minus one), Max (maximum), MFI (mean fluorescence intensity), mg (milligram), SCI (spinal cord injury), μm (micrometer).
3.3. Sex differences in motor, cognitive, and depressive-like behavior after SCI
Next, we attempted to address whether there were sex differences in functional recovery after SCI. Age-matched mice of both sexes at 10–12 weeks old were subjected to moderate/severe contusion injury with equal force and displacement (Table 1) after SCI. Gradual recovery of hindlimb locomotor activity was quantified using the BMS for up to ten weeks before putting the mice through a battery of behavior tests designed to evaluate their spontaneous motor activity, cognitive ability, and depressive-like behaviors (Fig. 6A).
Figure 6. Female mice exhibit better locomotor functional recovery after moderate/severe SCI.
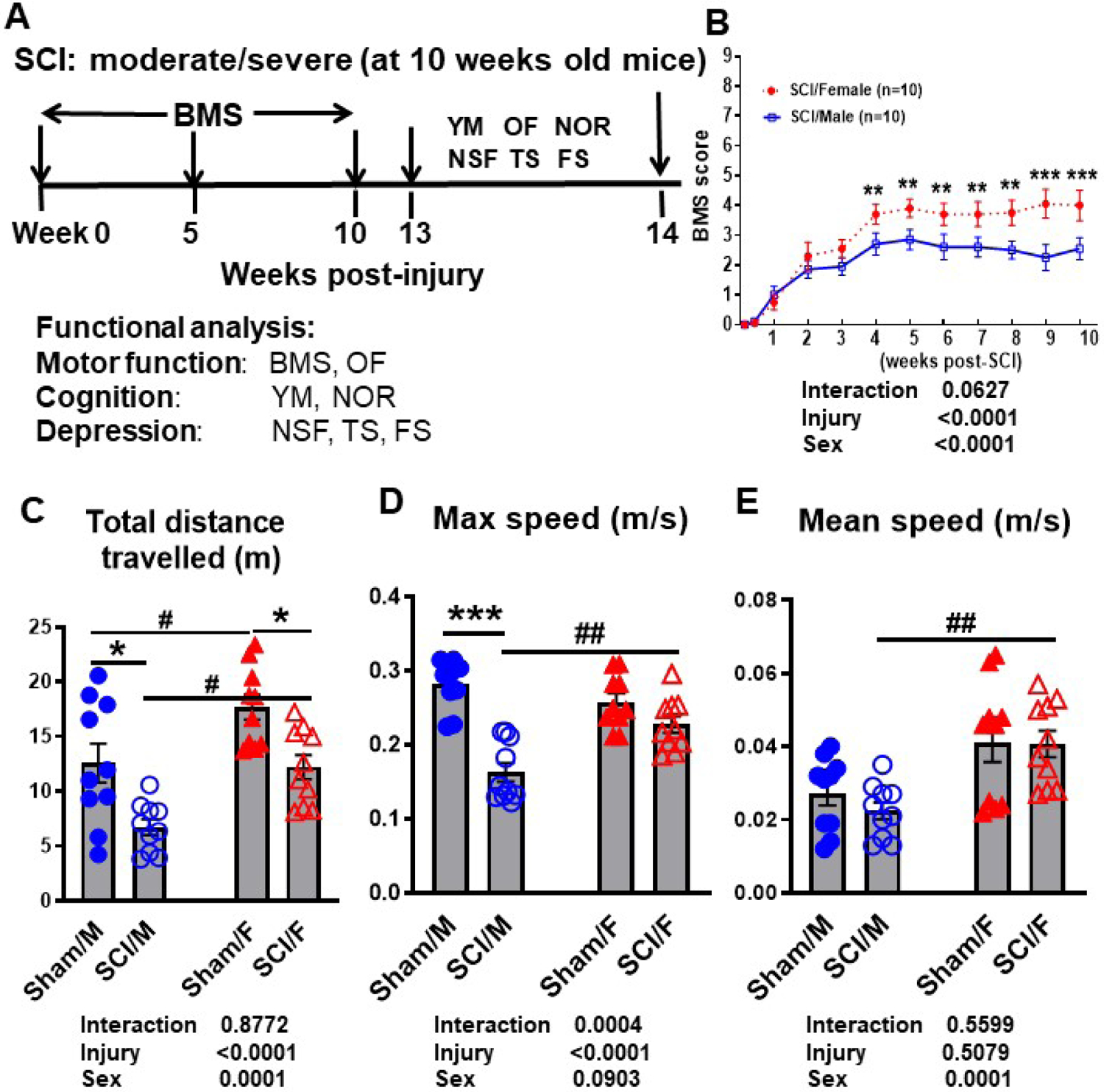
Age-matched male and female C57BL/6 mice at 10–12 weeks old were subjected to moderate/severe (65 kdyn) SCI followed by 14 weeks post-injury. (A) Diagram illustrating timepoints by weeks post-injury and behavior experiments conducted after the initial moderate injury. All behavior tests are categorized by the functional outcome that was examined. (B) Weekly BMS scores were recorded to quantify hindlimb locomotor recovery after SCI. Starting from week 4, SCI/Female mice showed significantly higher BMS scores compared to SCI/Male mice (N=10/group, p=0.007). This robust and dramatic difference in recovery rate was persistent until the end of the testing period at week 10. (C-E) Spontaneous activity in an OF apparatus was recorded and analyzed with use of the AnyMaze animal behavior system. Injury effects could be observed in two parameters: total distance travelled and mobility. Sex effects were seen (C) at baseline, (D) after injury, and (C-E) between injury groups. For all tests, N=10/group. **p<0.01, ***p<0.001 vs. Sham group; #p<0.05, ##p<0.01, ###p<0.001 vs. SCI/M group. Two-way ANOVA following Tukey’s multiple comparisons test. Abbreviations: BMS (Basso motor scale), cm (centimeter), FS (forced swim), m (meters), m/s (meters/second), Max (maximum), NSF (novelty suppressed feeding), NOR (novel object recognition), OF (open field), TS (tail suspension), YM (Y-maze).
Weekly assessment of BMS scores showed SCI/Females were recovering at a faster rate than their male counterparts (Fig. 6B). At four weeks post-injury, the average score of SCI/Female mice was 3.7 + 0.3348, indicating that the majority of female mice within the group were able to place their hind paws in a plantar position with or without weight support. Some mice were able to have occasional plantar stepping. At the same timepoint, SCI/Male mice only had an average score of 2.7 + 0.3710, which is indicative of extensive ankle movement and plantar placement of the paw. This dramatic difference in BMS scores at four weeks post-injury (N=10/group, p=0.007) was the start of a persistent trend and ever widening gap that would continue for six weeks (p<0.001) at which point motor function recovery appeared to have reached a plateau point for both sexes. We detected a significant main effect of sex (F(1, 216)=32.09, p<0.0001; Fig. 6B). Following ten weeks of BMS scoring, we examined motor function based on spontaneous activity in the OF test. The total distance travelled was significantly decreased after injury in both male (p<0.05) and female (p<0.05) mice (Fig. 6C, N=10/group). We detected a significant main effect of sex (F(1, 36)=18.27, p=0.0001). Incidentally, SCI/Female mice travelled more than SCI/Male within the same time frame (p<0.05). However, it is important to note the significant baseline difference between Sham/Female and Sham/Male groups (p<0.05). The maximum speed of male mice was also significantly decreased after injury (Fig. 6D, p<0.001). This deficit in movement speed due to SCI was not observed in female mice, and a significantly higher level of both maximum speed (p<0.01) and mean speed (Fig. 6E, p<0.01) could be seen in the SCI/Female group when compared to SCI/Male mice. While there was no significant main effect of sex (F(1, 36)=3.030, p=0.0903) in the maximum speed, we detected a significant main effect of sex in the mean speed (F(1, 36)=18.55, p=0.0001).
We then assessed the effects of SCI on cognitive function. Y-maze results showed a significant decrease in spontaneous alternations in SCI/Male compared to Sham/Male mice (Fig. 7A, p<0.05, N=10/group), but the same deficit was not apparent in female mice after injury. There was no significant main effect of sex (F(1, 36)=0.4231, p=0.5195). Both male and female injury groups entered a lower number of arms within the Y-maze apparatus compared to their respective sham control groups (Fig. 7B). We detected a significant main effect of sex (F(1, 36)=8.419, p=0.0063). Learning and memory deficits were further assessed via the NOR test, wherein both sex groups showed significantly less time spent exploring the novel object versus the familiar object during the choice phase (Fig. 7C–D, N=10/group). However, compared to SCI/Male, SCI/Females showed a significantly higher ratio of time spent with novel/familiar objects (p<0.05).
Figure 7. Sex differences in cognitive function and depression after SCI.
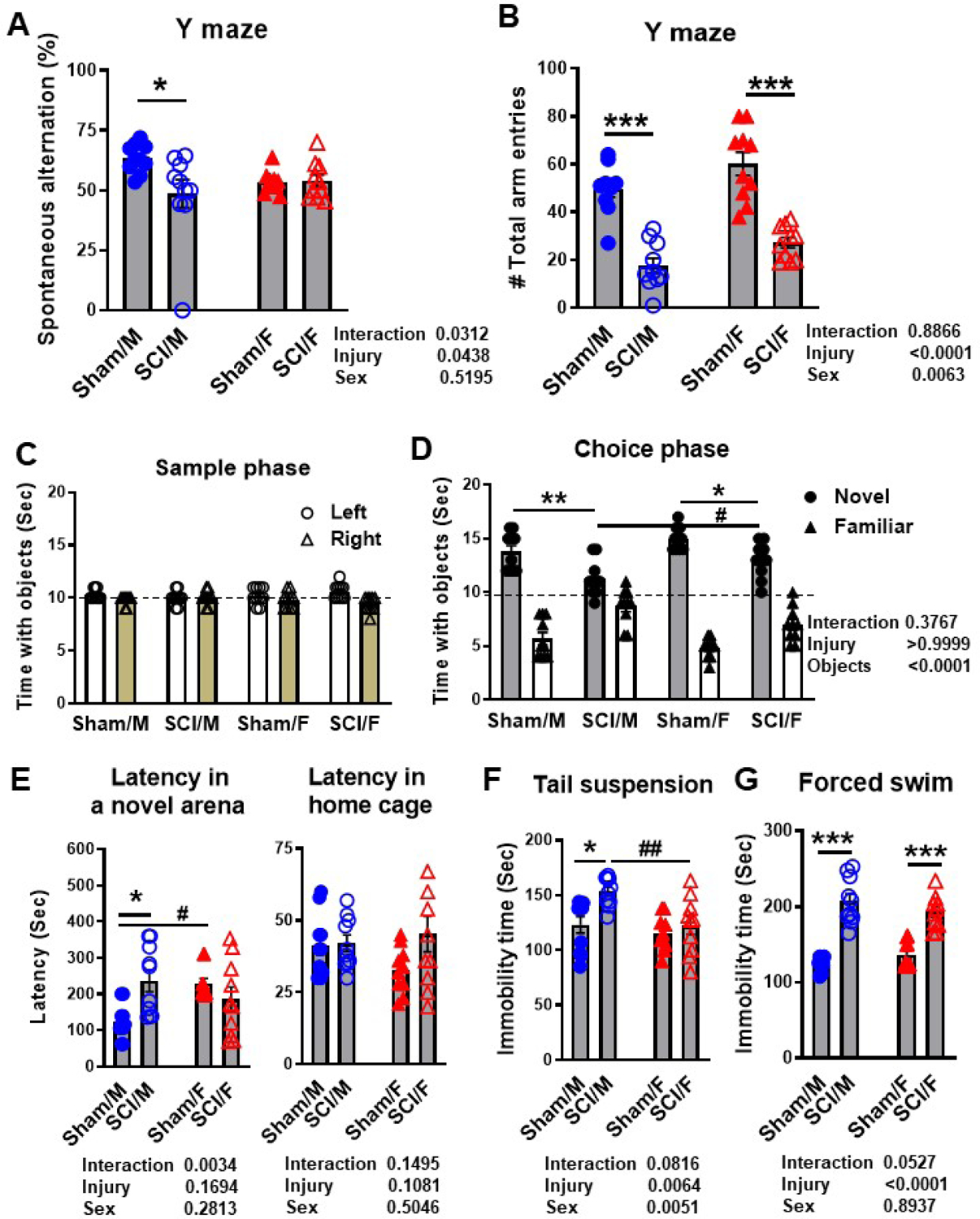
Age-matched male and female C57BL/6 mice at 10–12 weeks old were subjected to moderate/severe (65 kdyn) SCI. At 13 weeks post-surgery male and female mice were subjected to Y-maze, NOR, NSF, TS, and FS testing to evaluate cognition and depression-like phenotypes. (A) The percentage of spontaneous alternation in the Y-maze showed sex differences at baseline and injury effects in SCI/Males. (B) The total number of arm entries was similarly decreased in both sexes late after SCI. (C) No differences were seen in exploration time between left and right-side objects during the sample phase of the NOR task. (D) Significant effects of injury and sex were seen in time spent exploring the novel versus familiar object late after SCI. (E) The latency to reach food in the center of a novel arena was recorded for the NSF test. No difference was found in latency time in the home cage, confirming that the novel environment was a source of anxiety in SCI/Males. (F) SCI/Males displayed greater immobility during TS testing. (G) Both sexes displayed increased immobility after SCI in the FS test. N=10/group. *p<0.05, **p<0.01, ***p<0.001 vs. Sham group; #p<0.05, ##p<0.01, ###p<0.001 vs. SCI/M group. Two-way ANOVA following Tukey’s multiple comparisons test.
To further examine whether SCI-induced depression-like behavior is affected by sex we implemented the NSF, TS, and FS testing. Latency to food in a novel environment showed a dramatic increase after injury in male, but not female mice (Fig. 7E, N=10/group, p<0.05), indicating a higher level of anxiety or depressive-like behavior. To exclude the possibility of confounding error due to mobility issues or lack of desire to ingest food, we also recorded the latency time within their respective home cages for each mouse. No statistical differences in latency to food in home cage were observed between groups (Fig. 7E). There was no significant main effect of sex in the NSF test (F(1, 36)=1.196, p=0.2813 in a novel area and F(1, 36)=0.4543, p=0.5046 in home cage). Depression-like behavior was subsequently confirmed through two learned helplessness tests, the TS and FS test. In TS, SCI/Male mice displayed higher immobility time compared to Sham/Males (Fig. 7F, N=10/group, p<0.05). Similar trends were not observed between SCI/Female and Sham/Female mice. In fact, the average immobility time of SCI/Females was significantly lower than that of the SCI/Male group (p<0.01). We detected a significant main effect of sex (F(1, 36)=8.917, p=0.0051). FS assessment revealed greater immobility times in both SCI/Males (p<0.001) and SCI/Females (p<0.001) when compared to their respective sham control group (Fig. 7G). However, no significance difference was observed between SCI/Male and SCI/Female groups (F(1, 36)=0.01811, p=0.8937). Taken together, these findings indicate that young adult age-matched female mice generally display greater restoration of neurological function compared to males during the first 13 weeks of injury.
3.4. Age alters the inflammatory response in the injured spinal cord with distinct sex effects
To examine whether sex differences would affect the acute inflammatory response to SCI in an age-dependent manner, age-matched male and female C57BL/6 mice were subjected to SCI at 6 months of age. At 3 days post-surgery, injured spinal cord tissue was processed for total RNA and qPCR analysis.
Revisiting the NanoString results for young adult mice at 3d post-injury, we isolated a total number of 23 genes presented as volcano plot (Fig. 8A) for further validation with qPCR in the middle-aged groups. In order to obtain an overview of their inflammatory profile during acute stage SCI, the list was intentionally spread out between genes that showed significant changes between SCI/F vs. SCI/M and those that showed only minimal regulation by sex difference. In addition, 3 genes (Cd44, Cd74, Egfr) were selected from the list of genes uniquely altered by SCI in male mice. For better effects in side-by-side comparison between young adults and middle-aged mice, the relative linear fold change obtained through qPCR were transformed into Log2(Fold Change) and a volcano graph was plotted in the same format as that obtained through NanoString (Fig. 8B).
Figure 8. Mice display distinct sex differences in inflammatory profile at 3d post-injury.
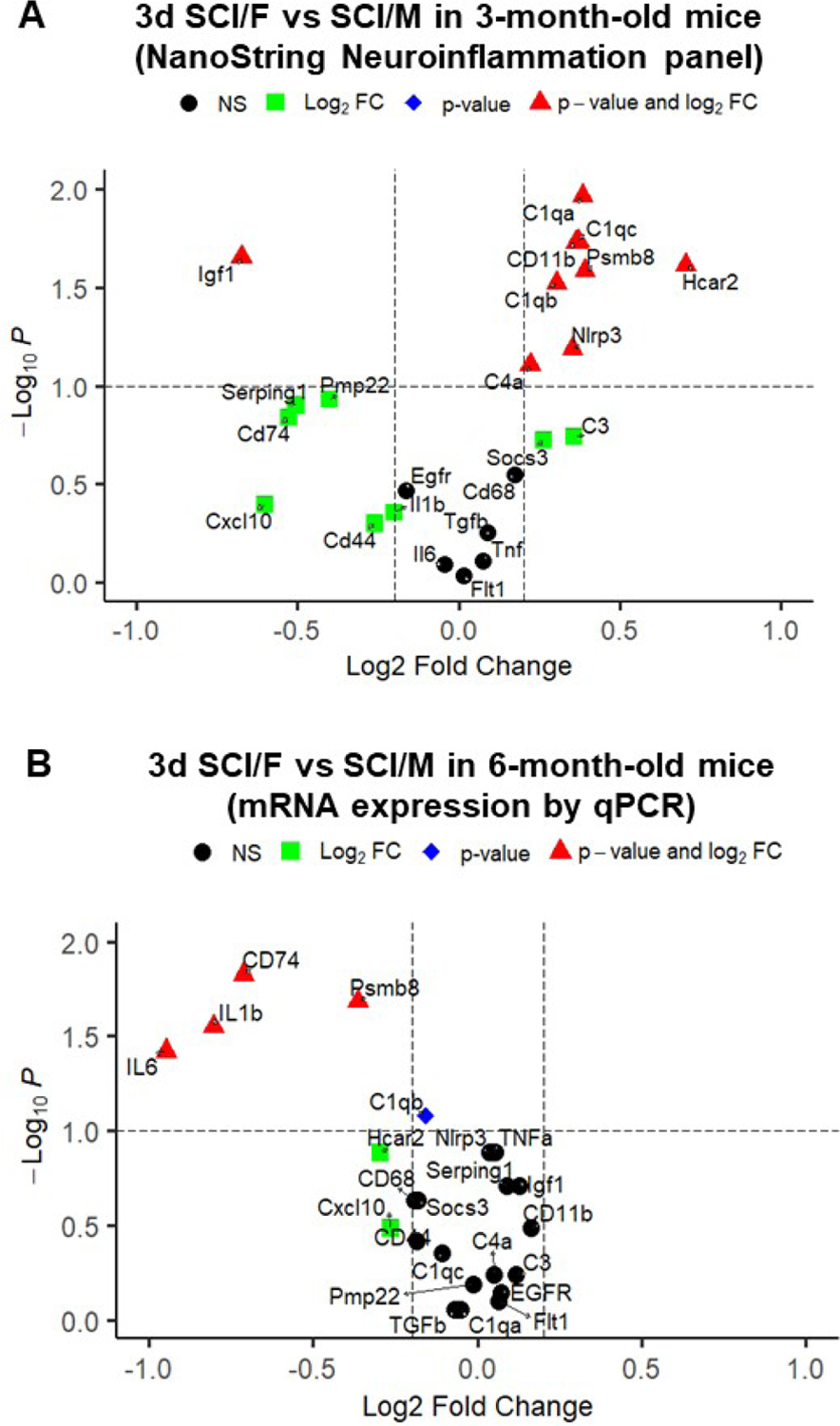
(A) Volcano plot of select differential expressed genes derived from SCI/Female vs. SCI/Male pairwise comparisons in NanoString data of young adult mice (3-month-old). n=3–4/group. (B) Six month-old male and female mice were subjected to SCI and injured spinal cord tissue was collected at 3 d post-injury and processed for RNA extraction and a list of inflammatory genes was examined by qPCR analysis. Linear fold change (FC) and p-values derived from t-test were transformed into Log2FC and Log10(P-value), respectively and presented as Volcano plot. Black circle=not significant (NS); Green square=Log2(Fold change)>0.25; Blue diamond=Log10(P-value)>1; Red triangle=Log2(Fold change)>0.25 and Log10(P-value)>1. n=8/group.
Quantitative RT-PCR analysis of gene expression showed 4 genes related to neuroinflammation were robustly downregulated in SCI/F group when comparing between middle-aged mice (Fig. 8B & S5, n=8 mice/group). The pro-inflammatory genes IL1β (p=0.0281), IL6 (p=0.0379), CD74 (p=0.0148), and Psmb8 (p=0.0207) showed significantly lower levels of expression in SCI/F mice (Fig. S5A). The gene Chil3, which encodes the molecule Ym1 and has been reported to be an anti-inflammatory marker, underwent significant upregulation in the SCI/F group (Fig. S5B, p=0.0499). Of the 23 genes that were examined, a total of 10 genes in young adult mice showed relatively higher expression in SCI/F, while another 10 genes showed relatively lower expression (Fig. 8A). However, many of these robust changes derived from young adult animals were not observed in middle-aged mice at 3d post-injury (Fig. S5C–D). Of note, the gene Igf1, which encodes a potent neurotrophic factor, had been downregulated in SCI/F mice in young adults, but not in the middle-aged group (Fig. S5E). Furthermore, the anti-inflammatory genes TGFβ and Socs3 did not show significant sex differences at either age (Fig. S5F). In addition, assessment of well-known pro-inflammatory genes in middle-aged groups failed to demonstrate sex differences (Fig. S5G). Finally, there were no differences between SCI/F and SCI/M in several genes pertaining to astrocyte (Serping1 and Egfr) and microglial (Pmp22, Flt1 and C3) function (Fig. S5H). Taken together, these findings indicate that the inflammatory profile is altered by the age when the insult occurs.
3.5. Middle age-matched male and female mice display differential changes of neurological function after SCI
Next, we used age-matched male and female mice at 6 months old to further elucidate the sexual dimorphism in neurological function after SCI. Gradual recovery of hindlimb locomotor activity was quantified using the BMS for up to 17 weeks before putting the mice through a battery of behavior tests designed to evaluate their spontaneous motor activity, cognitive ability, and depressive-like behaviors (Fig. S6). Injury biomechanics indicated that body weight of female mice was less than males before injury, and no differences in injury forces and displacement between SCI/Male and SCI/Female groups (Table 1). We performed weekly recording of BMS scores to evaluate their endogenous recovery process in motor function. Surprisingly, the clear advantage of SCI/Female compared to SCI/Male mice in the rate and degree of locomotor functional recovery that we saw in the first study was no longer apparent for this second cohort (Fig. 9A). There was no significant main effect of sex (F(1, 198)=3.014, p=0.0841). At 8 months after SCI, the final BMS score for SCI/Male and SCI/Female showed no significant difference between the two groups of injured mice (Fig. 9B). When examining spontaneous activity in the OF test, a clear decrease in activity levels for SCI/Female could be observed when compared to Sham/Females, as measured by several parameters including total distance travelled, maximum speed, and time immobility (Fig. 9C). Similar trends could be observed for SCI/Male mice in comparison to Sham/Male mice, but only max speed showed statistical significance. However, no difference was observed between sexes with or without injury except for the percentage of distance travelled within the inside zone. We detected a significant main effect of sex in total distance travelled (F(1, 29)=9.683, p=0.0042), time immobility (F(1, 29)=4.357, p=0.0458), and % inside zone distance (F(1, 29)=5.659, p=0.0242), but not in max speed (F(1, 29)=1.126, p=0.2974). Next, we assessed spatial learning and memory with the Y-maze test. Results showed a significant decline in cognitive function for SCI/Male mice compared to Sham/Male groups (Fig. 8D). Interestingly, females showed no change after injury, however, the percentage of spontaneous alterations was comparable to SCI/Male mice. There was no significant main effect of sex (F(1, 29)=1.323, p=0.2595). In the NOR test, exploration of the novel object decreased significantly for male mice at 8 months after SCI (Fig. 9E). However, female mice showed no such detriment when comparing the SCI group to their same sex sham control group. In fact, SCI/Female mice were observed to have a higher ratio of novel/familiar exploration time compared to SCI/Male. Finally, depression-like behavior was observed through NSF and immobility time in the FS test. No statistical differences in latency to food in both novel area and home cage were observed between groups (Fig. 9F). Both sexes displayed injury-related increases in forced swim immobility compared to their respective sham controls (Fig. 9G). There was no significant main effect of sex in NSF (F(1, 29)=1.188, p=0.2847) and FS (F(1, 29)=0.1657, p=0.6870).
Figure 9. Middle age-matched male and female mice display differential changes in neurological function late after SCI.
C57BL/6 mice at 6 months old were subjected to severe (70 kdyn) SCI followed by 8 months post-injury. (A) Weekly BMS scores were recorded to quantify hindlimb locomotor recovery after SCI, which showed no sexual dimorphism between injury groups for up to 17 weeks after injury. (B) BMS score of SCI/Male and SCI/Female at 8 months after injury. (C) Graphs depicting the parameters measured in the OF test at 8 months post-injury. Sex differences were seen at baseline in time spent traveling in the inside zone, after injury in total distanced travel and immobility time. (D) The percentage of spontaneous alternations in the Y-maze test is significantly decreased in SCI/Males, but not SCI/Females. The total number of arm entries was recorded for each mouse. (E) No preference was seen in exploration time between left and right objects during the sample phase of the NOR task. Significant effects of injury and sex were seen in time spent exploring the novel versus familiar object at 32 weeks after SCI during the choice phase. (F) The latency to reach food in the center of a novel arena was recorded for the NSF test. No difference was found in latency time in both novel area and the home cage. (G) Both sexes displayed increased immobility after SCI in the FS test. N=4–5 mice/group (Shams) and 10–14 mice/group (SCI groups). *p<0.05, **p<0.01, ***p<0.001 vs. Sham group; #p<0.05, ##p<0.01, vs. SCI/M group. Two-way ANOVA following Tukey’s multiple comparisons test.
3.6. Neuroinflammation genes exhibit persistent increase at the injury site during chronic SCI
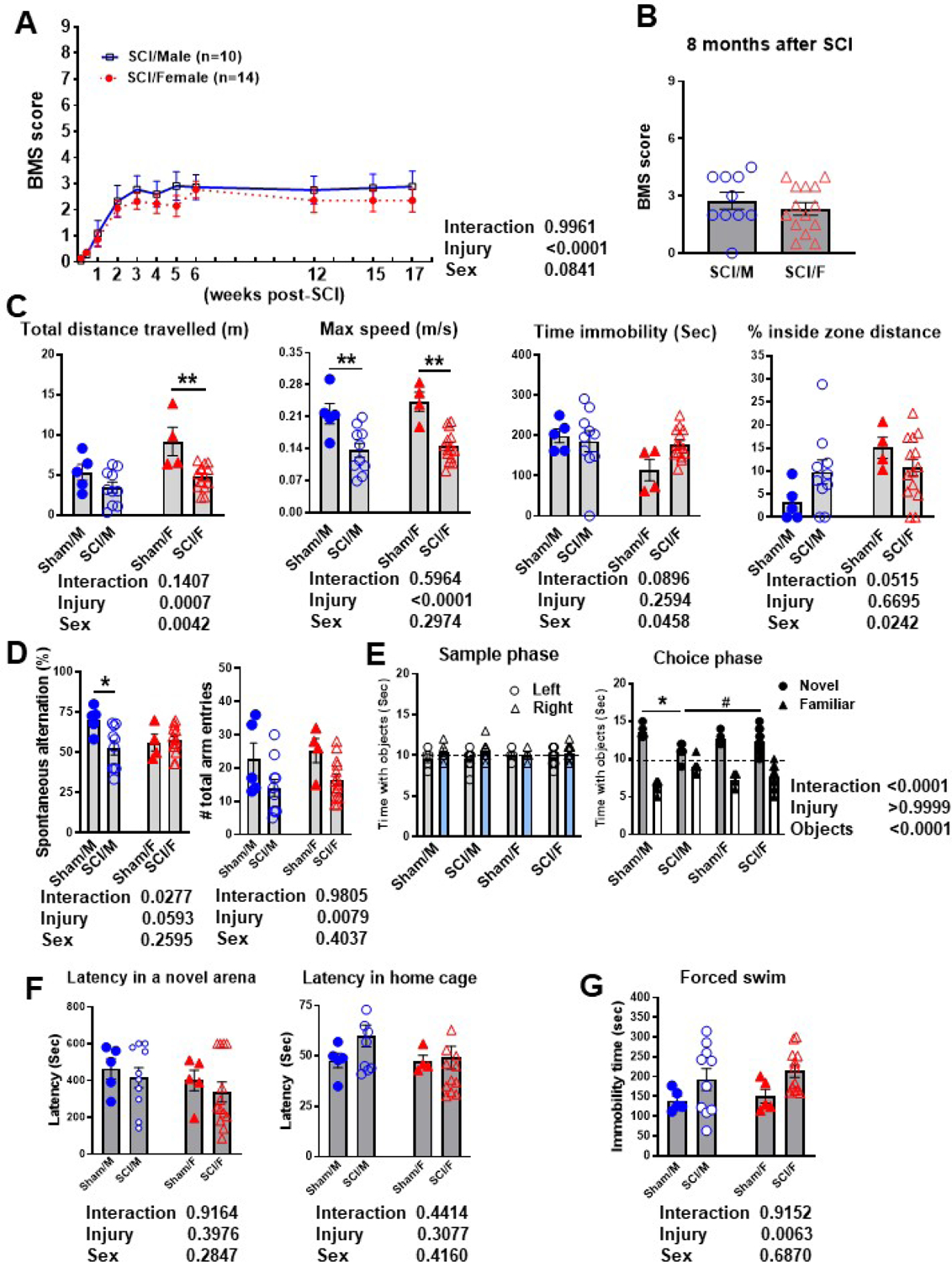
To evaluate the role of sex in the injury site during chronic SCI, we extracted spinal cord tissue from around the epicenter at 8 months post-injury and assessed the inflammatory profile using the Nanostring nCounter Neuroinflammation panel. MDS of all normalized gene counts revealed a distinct separation of samples into individual groups across the first two principal coordinates (Fig. 10A). The first principal coordinate accounted for the majority of the variation (66.3%) across samples and separated the groups by injury, while the second principal coordinate (7%) separated the groups by sex. Four pairwise comparisons were performed and outlined in Fig. 10B–C: (1) Sham/Female vs. Sham/Male–(Set 1); (2) SCI/Male vs. Sham/Male–(Set 2); (3) SCI/Female vs. Sham/Female—(Set 3); and (4) SCI/Female vs. SCI/Male—(Set 4). SCI primarily resulted in increased expression of genes in both male and female mice (Set 2 and 3), while sex differences were seen in both baseline (Set 1) and SCI conditions (Set 4). Although by this time endogenous recovery had attenuated the expression levels of inflammatory genes in both sexes after SCI, we could still observe as many as 171 (20.7%) differentially expressed genes in SCI/Males and 400 (52%) differentially expressed genes for SCI/Females. However, only 21 genes were differentially expressed when directly comparing SCI/Female to SCI/Male. Through further analysis of the DE genes, we were able to identify a subset of injury genes that are significantly altered in male but not female mice and vice versa (Fig. 10D). Due to the large quantity of genes, the gene sets were differentiated into up/downregulated genes for SCI/Male (Fig. 10E) and SCI/Female mice (Fig. 10F). As depicted in Fig. 11A, pathway analysis of the DE gene sets showed a higher number of genes modulating the carbohydrate and lipid metabolism processes for SCI/Male mice. SCI/Female mice appeared to be undergoing changes in a more widespread range of pathways, with a significantly higher number of genes involved in Angiogenesis, Apoptosis, Autophagy, Cellular Stress, Cytokine signaling and Wnt signaling pathways being altered by injury. With further analysis of the DE genes between the two injury groups (Set 4) we were able to identify 10 genes that were different at baseline in Sham mice and 11 genes that were only DE under injury conditions (Fig. 11B). We identified two genes that were only modified by sex after SCI, Uty and Xiap (Fig. 11C). Uty (Ubiquitously-transcribed TPR Protein On The Y Chromosome) is a minor histocompatibility antigen which may induce graft rejection of male stem cell grafts and is also involved in pathways for chromatin organization (Wang et al., 2018). The gene Xiap (X-Linked Inhibitor of Apoptosis) is a multifunctional regulator of apoptosis, inflammation, and immunity (Estornes and Bertrand, 2015).
Figure 10. SCI significantly alters the transcriptome at the injury site in chronic phase.
A NanoString nCounter® Neuroinflammation panel was used to assess transcriptional changes at the spinal cord injury site at 8 months) post-injury. (A) MDS (multi-dimensional scaling) was performed using all normalized gene counts from the NanoString panel. The four sample groups were Sham/Male (open circle), Sham/Female (open square), 8m SCI/Male (closed circle), and 8m SCI/Female (closed square). PCA revealed distinct clustering (dashed ellipses) of the four sample groups across the first two principal coordinates, which accounted for 66.3% and 7%, respectively, of the total variation across samples. Injury-related effects were captured on Coordinate 1, separating the SCI groups on the right from the left. (B) DE analysis was performed on pairwise group comparisons using nSolver (p-value < 0.05). Four pairwise comparisons were performed: (1) Sham/Female vs. Sham/Male; (2) SCI/Male vs. Sham/Male; (3) SCI/Female vs. Sham/Female; and (4) SCI/Female vs. SCI/Male. (C) A Venn diagram demonstrates the separation of total injury genes (i.e., those genes DE in SCI/Male vs. Sham/Male (Set 2) and SCI/Female vs. Sham/Female (Set 3) into those showing sex dimorphism and those that do not, based on based on membership in the gene list of Set 4 (SCI/Female vs. SCI/Male). Although the vast majority showed upregulation after injury, only a small percentage of genes showed significant changes between SCI/Female and SCI/Male. (D) Plot of transcript fold changes normalized by Sham/Male for genes unique to sex dimorphism between SCI/Male mice (Set 2, p-value<0.05). (E) Plot of transcript fold changes normalized by Sham/Female for genes unique to sex dimorphism between SCI/Female mice (Set 3, p-value<0.05). N=3–4 mice/group.
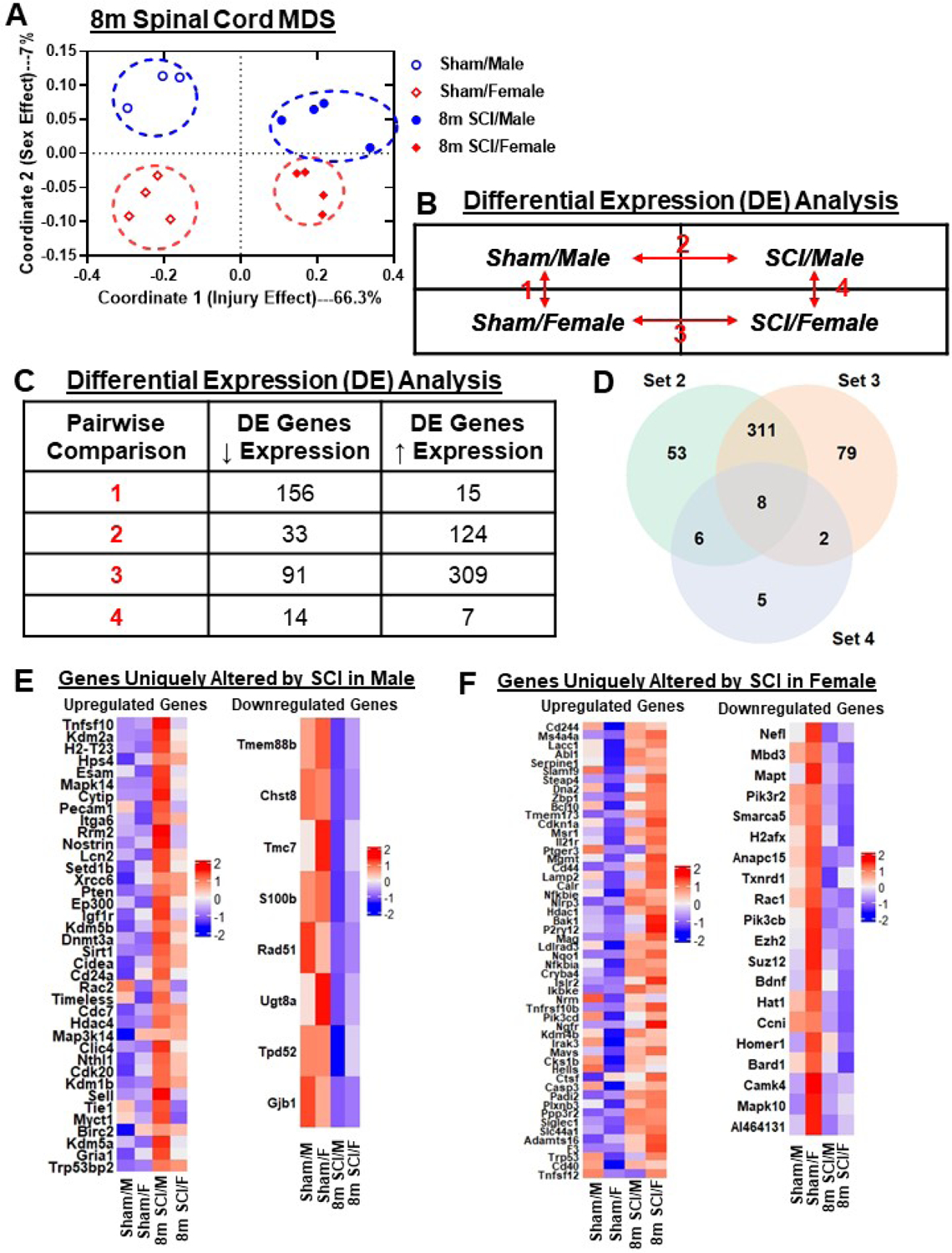
Figure 11. Chronic SCI alters different neuroinflammatory pathways in the injury site of male and female mice.
(A) Graph illustrating the neuroinflammatory genes altered by spinal cord injury in different sexes. Injury genes in male mice indicated higher alteration in Carbohydrate Metabolism and Lipid Metabolism. Female mice indicated higher levels of alteration in Angiogenesis, Apoptosis, Autophagy, Cellular Stress, Cytokine Signaling and Wnt Signaling pathways. (B) Heatmap of genes that are altered in DE between SCI/Female and SCI/Male, 10 of the genes that were different at baseline in Sham mice and 11 genes that were only DE under injury conditions. Color coding was based on z-score scaling. (C) Heatmap of genes that are uniquely altered in DE by sex late after SCI, genes only altered in Set 4. Color coding based on z-scores of normalized transcription counts.
3.7. Chronic SCI alters transcription patterns in the cerebral cortex in a sex-dependent manner
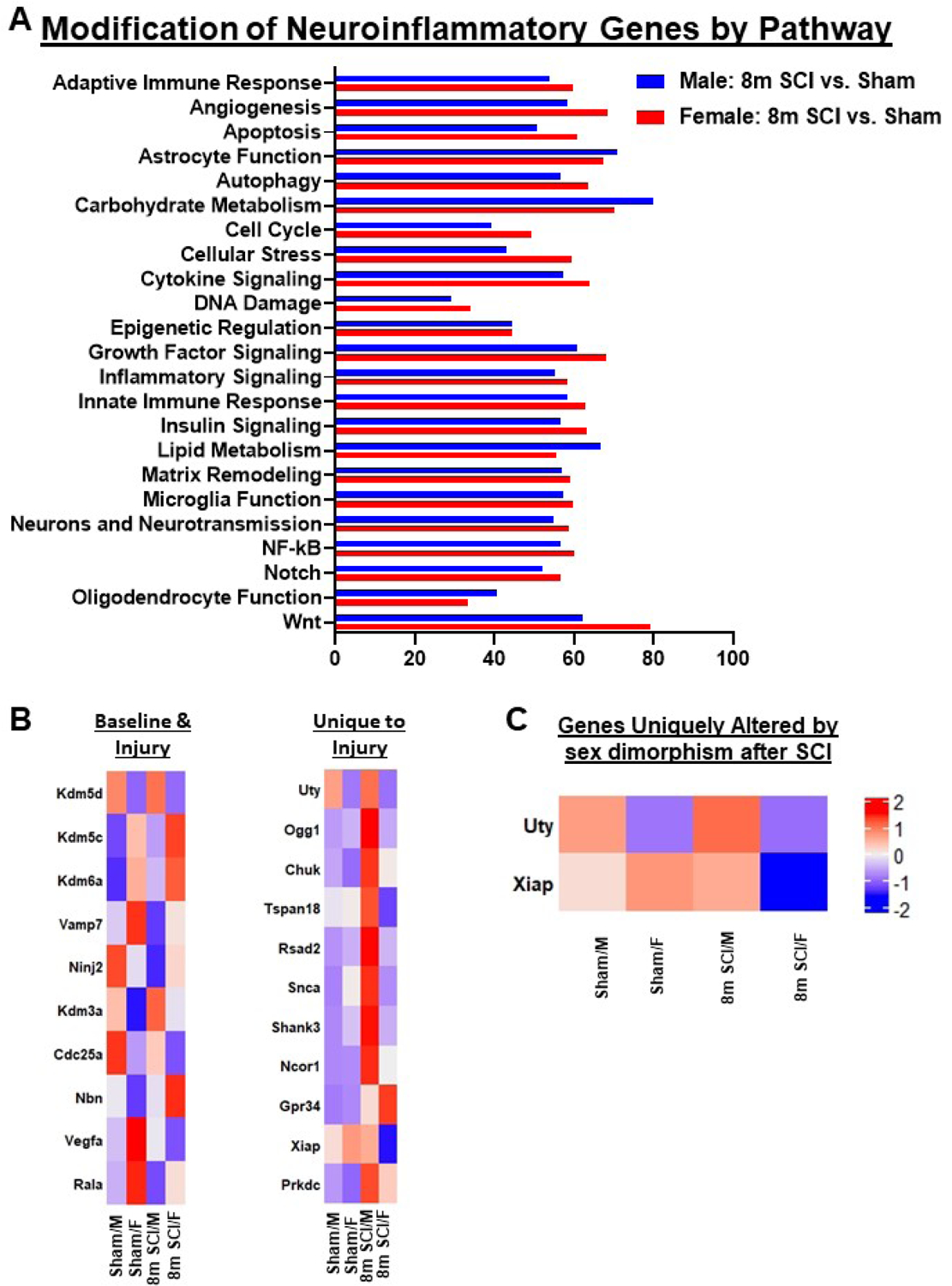
Our behavioral data on long-term cognitive outcome and depression phenotype indicate that SCI/Female mice are generally less inclined to exhibit depressive-like symptoms and cognitive decline at chronic timepoints following SCI. Although earlier studies by our lab have uncovered the cellular and molecular mechanisms of this phenomenon, this is the first time that we examined post-SCI brain inflammation in the context of sex. Initial analysis with MDS showed a clear distinction between SCI and Sham mice, with Sham/Female and SCI/Female groups clustered closer together along the y-axis, indicating the injury effects were not as significant (Fig. 12A). Differential expression between the four comparison sets is outlined in Fig. 12B–C and shows a discrete group of inflammatory genes altered by SCI in both sexes. We sorted out the genes uniquely altered in either male or female mice and differentiated them into upregulated and downregulated groups (Fig. 12D–E). Several genes of interest in male mice include the upregulation of Vegfa (Vascular Endothelial Growth Factor A), Mmp14 (Matrix Metallopeptidase 14), Ulk1 (Unc-51 Like Autophagy Activating Kinase 1), Sumo1 (Small Ubiquitin Like Modifier 1) and Atg14 (Autophagy Related 14), all of which have been reported to be upregulated in models of AD, and coincide with deficiencies in learning and memory in this study (Hamano et al., 2018; Marcelli et al., 2017; Martins et al., 2017). Pathway analysis of injury genes indicate that those regulating Matrix Remodeling, NF-kappa B and Oligodendrocyte Function are highly altered in the cerebral cortex of male mice after long-term SCI (Fig. 13A). Interestingly, female mice showed higher alteration of genes involved with Angiogenesis, Autophagy, Carbohydrate Metabolism, Insulin Signaling and Wnt Signaling pathways. Analysis of genes exhibiting differential expression between the two sex groups after injury show a total of 18 genes, most of which are upregulated in females relative to males (Fig. 13B). Pathway enrichment analysis showed a high abundance of genes related to Cytosolic DNA-sensing (Ikbkb, Ripk1, Ikbkg) and RIG-I-like receptor signaling pathways (Ikbkb, Ripk1, Ikbkg) (Fig. 13C). The former is a part of the innate immune system that functions to detect the presence of cytosolic DNA, triggering the expression of inflammatory genes that can result in chronic activation (Yang et al., 2017). The latter pathway is also part of the innate immune response and may be a specific marker for early AD pathogenesis (de Rivero Vaccari et al., 2014).
Figure 12. Neuroinflammatory genes in the cerebral cortex are altered by chronic SCI.
(A) MDS was performed using all normalized gene counts from the NanoString panel. The four sample groups were Sham/Male (open circle), Sham/Female (open square), 8m SCI/Male (closed circle), and 8m SCI/Female (closed square). MDS revealed distinct clustering (dashed ellipses) of the four sample groups across the first two principal components, PC1 and PC2, which accounted for 14.6% and 20.8%, respectively, of the total variation across samples. Sex effects were captured on Coordinate 1, separating the Male groups on the right from the Female mice in left. Injury effects were captured on Coordinate 2, separating Sham from SCI mice. (B) DE analysis was performed on pairwise group comparisons using nSolver (p-value < 0.05). Four pairwise comparisons were performed: (1) Sham/Female vs. Sham/Male; (2) SCI/Male vs. Sham/Male; (3) SCI/Female vs. Sham/Female; and (4) SCI/Female vs. SCI/Male. (C) Venn diagram demonstrates the separation of total injury genes (i.e., those genes DE in SCI/Male vs. Sham/Male (Set 2) and SCI/Female vs. Sham/Female (Set 3) into those showing sex dimorphism and those that do not, based on based on membership in the gene list of SCI/Female vs. SCI/Male (Set 4). (D) Heatmap of genes that are uniquely altered in DE between SCI/Male and Sham/Male. Color coding was based on z-score scaling. (E) Heatmap of genes that are uniquely altered in DE between SCI/Female and Sham/Female. Color coding based on z-scores of normalized transcription counts. N=3–4 mice/group.
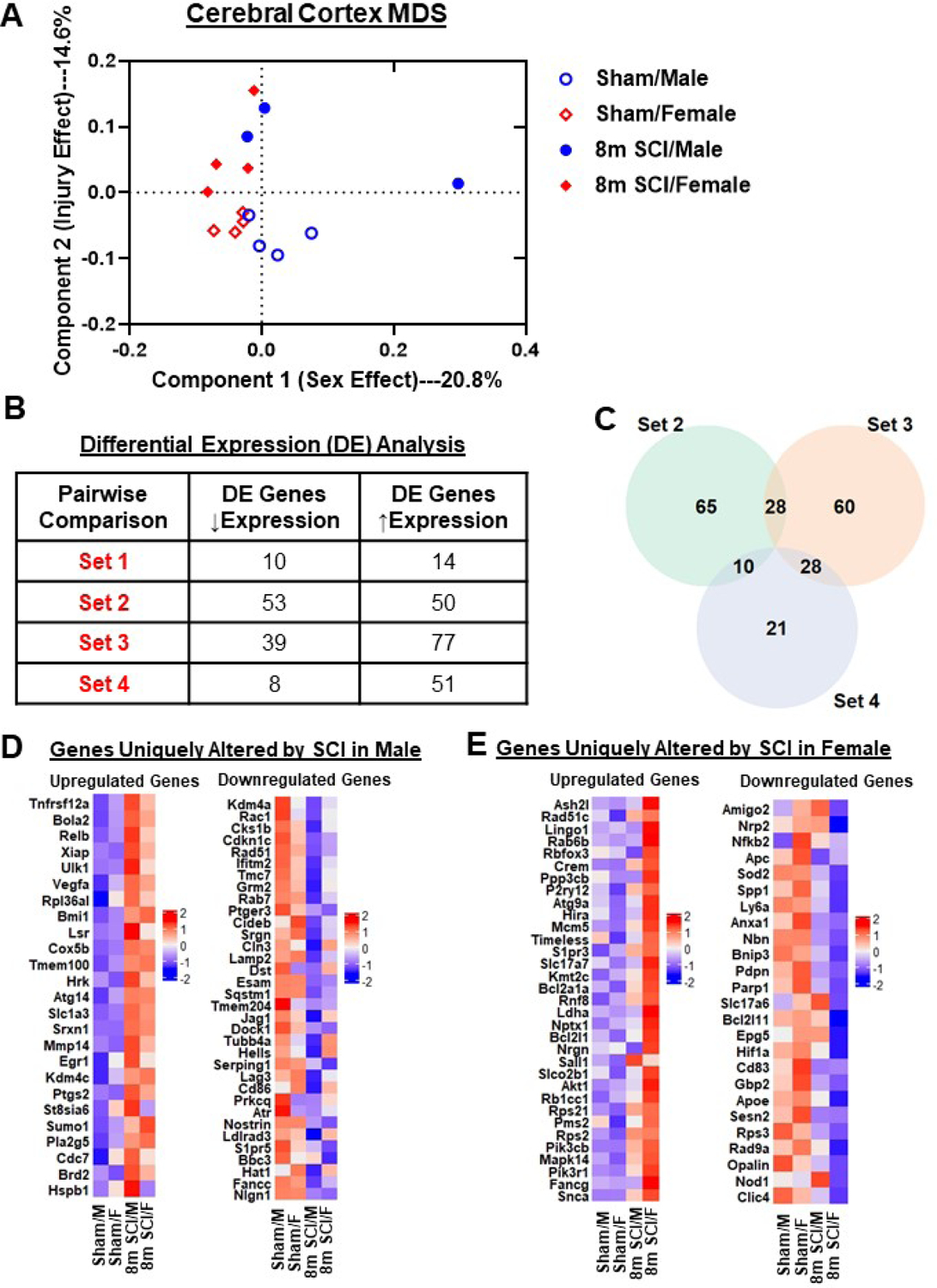
Figure 13. Chronic SCI alters different neuroinflammatory pathways in the cerebral cortex of male and female mice.
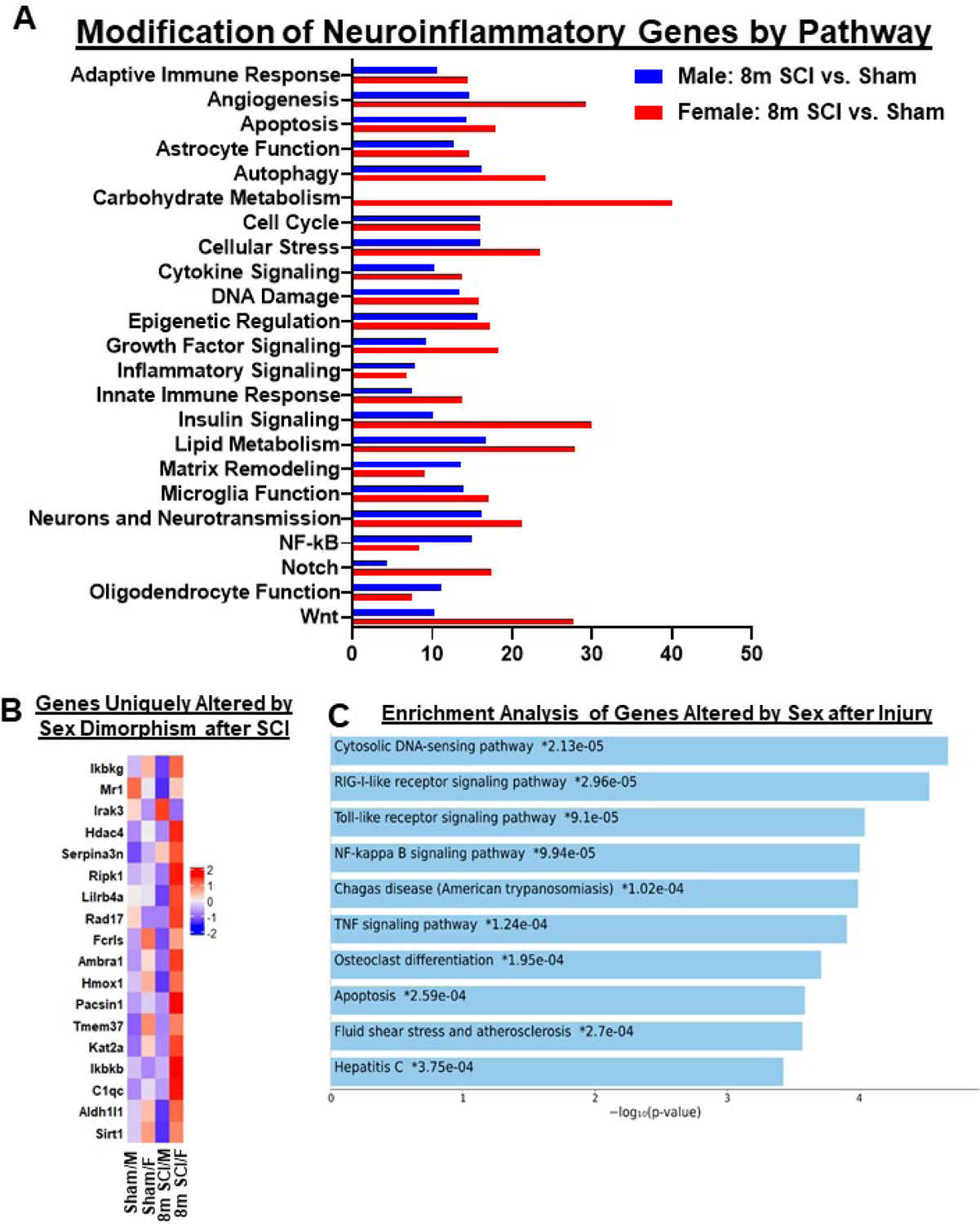
(A) Graph illustrating the neuroinflammatory genes altered by spinal cord injury in different sexes. Injury genes in male mice indicated higher alteration in Carbohydrate Metabolism and Lipid Metabolism. Female mice indicated higher levels of alteration in Angiogenesis, Apoptosis, Autophagy, Cellular Stress, Cytokine Signaling and Wnt Signaling pathways. (B) Heatmap of genes that are altered in differential expression between SCI/Female and SCI/Male, 10 of the genes overlap with those of. Color coding was based on z-score scaling. (C) Pathway analysis of genes unique to sex dimorphism after injury.
4. Discussion (2383 words)
Factors governing sex-dependent outcomes following CNS injury remain an ongoing issue of contention in the neurotrauma field. This applies to SCI, where incidence rates disproportionately affect males at younger ages and females at older ages (Stewart et al., 2020). Using age-matched sexually mature male and female mice subjected to injury at 10–12 week-old, we demonstrated that SCI in female mice is characterized by an attenuated acute neuroinflammatory response, independent of immune cell composition or number, followed by enhanced functional recovery in most, but not all motor, cognitive, and affective behavioral tests by 13 weeks post-injury. In contrast to females which showed increased transcription of DNA damage repair genes, male mice showed higher expression of genes associated with microglial function, cytokine signaling, and apoptosis at the injury site, and later developed more severe depressive-like behaviors by 13 weeks. When mice were subjected to injury at 6 month-old followed by 8 months post-injury, SCI/Females showed considerably more transcriptional activation of neuroinflammatory genes at both the local injury site and in distal regions of the brain, which was no longer associated with a sex advantage in functional recovery. To summarize, we found that gonadally intact, young adult female C57BL/6 mice exhibit temporary neuroprotection during the acute and post-acute periods following SCI, which gradually normalizes to male levels with both increased age at injury and increased time post-injury. Importantly, these sex-related differences were temporally associated with acute decreases and chronic increases in distinct inflammation-related genes in SCI/Female mice relative to SCI/Males.
The effect of biological sex on the recovery process from SCI is controversial in the clinical setting, with several studies claiming different outcomes using different outcome measures (Chan et al., 2013; Farooque et al., 2006; Luchetti et al., 2010; Stewart et al., 2020). One reason for this is that women are underrepresented in clinical studies because of their lower incidence rates, and as such, are purposely excluded to make the study population more homogenous (Raguindin et al., 2021). Moreover, the fact that SCI is more common in older females suggests that sample sizes for younger SCI patients are too small. Experimentally, data are also mixed. Some studies have reported protective effects of endogenous sex hormones and hormone therapy, while others show subtle but significant enhancements in functional recovery of SCI/Females independent of estrogen (Swartz et al., 2007; Zendedel et al., 2018). SCI in rodents historically show similar or less tissue loss in females compared to their male counterparts (Farooque et al., 2006; Hauben et al., 2002). We demonstrate that the acute neuroprotection reported for female SCI mice is fleeting, implying a gradual loss of protective and/or restorative mechanisms. This finding may explain the disparity between studies (i.e., experimental design) and highlights the important role of sex-dependent secondary injury mechanisms in shaping the trajectory of long-term outcomes. Most notably, we found that neuroinflammation was acutely attenuated and chronically elevated at the injury site of SCI/Females relative to males. We also discovered higher levels of neuroinflammation in distal regions of the CNS such as the cerebral cortex. What accounts for this reversal of fortune is unclear. Previous studies have shown that SCI can alter the estrous cycle and cause a chronic reduction in circulating estradiol concentrations (Stewart et al., 2020). However, it is not unreasonable to assume that male mice might have a delayed enhancement of neurorestorative pathways. Thus, the answer may lie with either sex or both. The effects of normal age-related processes might also interact in a sex-specific manner to affect SCI outcome. What is clear is that the chronicity of events that lead to progressive neurodegeneration after SCI have a sexually dimorphic neuroinflammatory signature that corresponds with recovery status.
Recent findings show that resident microglia in the CNS adapt to changing demands in a sex and age specific fashion. This work showed that females had higher numbers of monocyte-derived macrophages relative to microglia after acute SCI, whereas males had more total microglia within the lesion area (Stewart et al., 2021). We did not perform histology in our study so the relationship between gene expression profiling and the spatial distribution of activated immune cells in the spinal cord remains unclear. Examination of immune-deficient nude mice revealed no effect of sex, suggesting that the relatively milder level of injury-induced neurodegeneration in wildtype female mice may be driven by immune cells, namely mature T cells (Luchetti et al., 2010). Our study did not examine lymphocyte function for the primary reason that myeloid cells far outnumber lymphocytes within the injury site at very acute timepoints. Interestingly, we found no interaction between sex and injury in the number of any leukocyte population at d3. With the confounding variables of cell number and composition held constant, it is likely that any differences in the activation of immune-related genes is driven by the local impact of sex on cell function and neuroinflammation.
To our knowledge, the present study is the first to provide a comprehensive analysis of sex differences after SCI in the context of neuroinflammation at both the gene and cellular level. In the acute 3d timepoint, our results clearly showed a sexually dimorphic inflammatory response to SCI. In addition to gene expression differences seen between injury groups, we were also able to observe a distinct difference in pathways activated by injury. For example, male mice showed greater DE in genes related to Astrocyte and Microglia Function, while female mice showed a higher percentage in genes involved with Carbohydrate Metabolism. This was in line with our flow cytometry data showing male-specific increases in mitochondrial activity/biogenesis and upregulation of CD68, a marker of phagocytosis. In the subnetwork enrichment analysis, the top three represented pathways for male mice were TNF, NF-kB, and Toll-like Receptor signaling pathways. Interestingly, our flow cytometry data did not detect a difference in the protein production of TNF or several other inflammatory cytokines, which could be explained by changes in posttranscriptional regulation. Although few labs if any have delved into these sex-dependent dynamics using this approach, transcriptional and bioinformatic studies aimed at genetic profiling after SCI appear to coincide with our findings (Gong et al., 2020; Zhu et al., 2019). Gong and others found that TNF signaling, Cytokine-Cytokine Receptor interaction, and lysosome/phagosome pathways were highly DE during the first week of SCI. Recent efforts to characterize the role of sex in SCI-induced inflammation have utilized NanoString technology to analyze 27 genes of interest (Stewart et al., 2021). Of these, only one, C1qa, was differentially affected by sex and shown to be higher in male tissue-infiltrating myeloid cells at 3d. Our study adds to the growing list of neuroinflammatory genes uniquely altered by sex and its interaction with SCI. Included in this list was itgax, or CD11c, which encodes a protein that combines with the beta 2 chain (ITGB2) to form a leukocyte-specific integrin referred to as inactivated-C3b (iC3b) receptor 4 (CR4). This protein is involved in the complement pathway, is highly expressed on the surface of dendritic cells, and upregulated in males acutely after SCI. Sex-specific activation of the alternative complement pathway after SCI is consistent with the acute increases in microglial phagocytosis seen in males and might indicate greater neuronal apoptosis. Indeed, we found predominantly higher representation of Apoptosis pathways in male mice at 3d. Although the mechanisms are currently unclear, estrogens have been known to exert anti-apoptotic effects in SCI by inhibiting inflammasome and calpain activity (Sribnick et al., 2006; Yune et al., 2008; Zendedel et al., 2018). Future studies are required to determine the up-stream drivers of SCI pathophysiology to understand whether these sex-dependent effects are primarily neuroprotective or immunoprotective.
One of the most interesting findings from our study was the lack of sexual dimorphism at 8 months after SCI when the mice were at 6 months of age at the time of injury. Altered acute inflammatory response after SCI in middle-aged mice suggests that the same ‘protective’ sex-specific transcriptional response to acute SCI seen in young mice was largely absent in older, middle-aged mice. This may account for the worsened functional outcome of older SCI females and suggests protection in young females is mediated by sex hormones. Another possible reason for this phenomenon is that mice usually enter perimenopause by nine months of age and reach reproductive senescence by twelve months of age (Diaz Brinton, 2012). Because these mice were already at six months of age at the time of injury, the terminal endpoint behavior and all consecutive NanoString data for this cohort was carried out at 14 months of age, a time when female mice would have already entered reproductive senescence. In multiple sclerosis, a neurodegenerative disease cause by inflammation affecting the spinal cord, sex differences have been extensively reported (Ghaffarinia et al., 2016; Li et al., 2017; Shibasaki and Kuroiwa, 1976). However, Bove and group reported that multiple sclerosis patients with onset age of 50 or older have similar disease progressions between men and women, suggesting that menopause may affect sexual dimorphism in disease severity and progression (Bove et al., 2013; Bove et al., 2012). Similarly, our NanoString data on spinal cord and cerebral cortex tissue taken at eight months show fewer DE genes between SCI/Male and SCI/Female (a total of 21 genes, with 14 upregulated and 7 downregulated) compared to the younger adult mice that were in the first part of our study (a total of 61 genes, with 12 upregulated and 49 downregulated genes). This would implicate female sex hormones as a hidden driver of neuroinflammation in our study. Though it is important to note that BMS scores for six-month old male and female mice were statistically similar prior to reaching menopause age.
Unlike many other brain injury models, the majority of SCI studies prefer to utilize female mice because males exhibit more severe post-operative complications. Implicit in this empirical design is that sex differences in SCI outcome are robust, seen across labs, and likely underreported. The exclusion of males in most SCI studies means we lack deeper understanding and discovery of male-specific disease mechanisms. For example, Hauben et al. reported that testosterone may interfere with recovery from SCI after demonstrating that the endogenous recovery of females treated with dihydrotestosterone was nullified (Hauben et al., 2002). Yet others argue a protective role for testosterone on motoneuron and muscle morphology after SCI (Byers et al., 2012). Our study did not evaluate sex hormone levels in either sex which may be seen as a major limitation, particularly because we used mice of different ages at the time of injury and different endpoints. It is often argued that inherent differences in body mass and sex hormone levels predispose outcome. Accordingly, differences in body mass may extend to spinal cord size. Larger spinal cords could affect displacement and increase the cellular density and total area within a percentage volume relative to smaller spinal cords. The importance of these variables is reviewed elsewhere (Stewart et al., 2020). Earlier work has shown that female rats have better spontaneous recovery than males after a severe contusive SCI when they are matched by age and body weight (Hauben et al., 2002). Not only did the authors report better recovery in locomotor function, but histopathology also showed a severe loss of neural tissue in males after injury, while female rats had better preservation of neural tissue, more organized tissue structure, and greater continuity of myelinated fibers. The results of that study and many that have followed bare resemblance to our own reporting of BMS scores and CNS inflammation.
Chronic pain is an important problem following SCI and a major impediment to rehabilitation. Mechanical allodynia and heat hyperalgesia after SCI develops similarly in both sexes, however, mechanisms of SCI-induced pain may be sex-specific (Gensel et al., 2019; McFarlane et al., 2020). We did not assess pain in our study for this reason, choosing instead to focus on the development of anxiety/depression and associated brain inflammatory mechanisms which have been less studied. Previously we found that SCI could induce depressive-like behavior in male mice and rats at chronic timepoints (Li et al., 2020b; Wu et al., 2014a; Wu et al., 2014b). Clinical studies have reported higher prevalence of cognitive deficits and mood disorders after SCI (Dowler et al., 1997; Huang et al., 2016; Mahmoudi et al., 2021; Roth et al., 1989; VanDerwerker et al., 2020). The results were further confirmed in 2016 by a clinical study of veterans with SCI which showed women had a higher rates of diagnosis in lifetime depression and exhibited more depressive symptoms compared to men, which indicate evidence for sex-dependency in the development of SCI-induced mood disorders (Wilson et al., 2018). Female mice have also been shown to have increased anxiousness after SCI compared to males, independent of sensorimotor function and lesion size (Fukutoku et al., 2020). In general, we found that females had less depressive-like phenotypes compared to males at 13 weeks post-injury. While we did not directly assess mood behavior at 8 months after SCI, one can infer based on open field behavior (i.e., immobility time, inner/outer zone preference) and cognitive performance that anxiety may be worsened in females. However, we cannot be sure if this is due to age at injury or time post-injury. Regardless, SCI/Females showed a dramatic increase in DE genes in the brain late after SCI. These pathways include autophagy, cellular stress, cytokine and growth factor signaling, adaptive and innate immune responses, and microglia function. This suggests that SCI-induced neuroinflammation interacts with normal aging in a sex-specific manner to influence recovery.
In conclusion, we demonstrated that age at the time of SCI and time post-injury can differentially affect long-term outcome in a sex-dependent manner. Young males showed increased expression of genes associated with glial function, cytokine signaling, and apoptosis during acute SCI, including greater functional activation of microglia. Females exhibited acute attenuation and chronic exacerbation of neuroinflammation which correlated with the temporal course of functional recovery. We observed deficits in learning and memory and mood behavior in both sexes at very late stages of injury which were associated with sex-specific neuroinflammatory processes in the cerebral cortex. Taken together, our findings support a growing literature indicating female sex confers a modest, but significant, if not temporary, level of protection in SCI. The distinct neuroinflammatory gene clusters activated in male and female mice suggest that preclinical therapies that interfere with inflammatory pathways should be tested in both sexes before efficacy can be proven.
Supplementary Material
Figure S1. Enrichment analysis of genes unique to sex after injury. (A) Enrichment analysis of injury genes unique to male mice show a high content of genes related to Cytokine-Cytokine Receptor Interaction and Apoptosis. The length of the bar represents the significance of that specific gene-set or term. Greater statistical significance is denoted by the increased brightness of the bar color. (B) Enrichment analysis of injury genes unique to female mice show a high content of genes related to Non-Homologous End-Joining and cAMP Signaling pathway. (C) Pathway analysis of genes unique to sex dimorphism after injury. N=3–4 mice/group.
Figure S2. Sex dimorphism differentially modifies genes related to astrocyte function, cytokine signaling, cellular stress and microglia function in acute stages of SCI. (A) Heatmap of genes related to Astrocyte Function that are DE by injury in male mice but not in females and vice versa. Color coding was based on z-score scaling. (B) Heatmap of genes related to Cytokine Signaling that are DE by injury and male specific (left) or female specific (right). (C) Heatmap of genes related to Apoptosis that are DE by injury and male specific (left) or female specific (right). (D) Heatmap of genes related to Cellular Stress that are DE by injury and male specific (left) or female specific (right). (E) Heatmap of genes related to Microglia Function that are DE by injury and male specific (left) or female specific (right). N=3–4 mice/group.
Figure S3. Microglial production of reactive oxygen species and inflammatory cytokines during acute spinal cord injury. Microglial activity in male and female mice at 3d post-SCI was measured using flow cytometry. Representative histograms and subsequent MFI quantification of (A) NOX2 protein expression, (B) reactive oxygen species production, (C) DNA damage, and production of (D) TNF, (E) IL-1β, (F) IL-6 and (G) TFGβ cytokines in CD45intCD11b+ microglia are shown. Significant differences were seen after injury, but not between sexes. For each sex, N=6 mice per sham group, and N=10 mice per SCI group. ***p<0.001 vs. Sham group; Two-way ANOVA following Tukey’s multiple comparisons test. Abbreviations: d (day), FMO (fluorescence minus one), Max (maximum), MFI (mean fluorescence intensity), SCI (spinal cord injury), μm (micrometer).
Figure S4. Sex differences in infiltrating CD45hiCD11b+Ly6Clo monocytes at 3 days post-injury. (A-B) No sex differences were seen in ROS production as evidenced by both MFI of DHR+ and DCF+ Ly6Clo monocytes. (C) MFI quantification of NOX2 protein expression. (D) MFI quantification of CD68-positive Ly6Clo monocytes. (E) MFI of mitochondrial membrane potential in Ly6Clo monocytes using MitoSpyRed+ was significantly lower in females vs. males after SCI. N=10 mice per SCI group. **p<0.01 vs. SCI/M with unpaired t test.
Figure S5. Middle-age alters inflammatory genes in response to acute SCI. Six month-old male and female mice were subjected to SCI and spinal cord samples (~5-mm segments) were collected at 3 d post-injury and processed for RNA extraction and a list of inflammatory genes qPCR analysis. (A) Pro-inflammatory genes (IL1β, IL6, CD74, Psmb8) were downregulated in SCI/F mice compared to SCI/M animals. (B) Anti-inflammatory gene Chil3 (Ym1) expression was increased in SCI/F group vs SCI/M mice. (C-D) Complement pathway genes (C1qa, C1qb, C1qc, C4a), and Nlrp3, Hcar2 (hydroxycarboxylic acid receptor 2), and CD11b genes that were elevated in young SCI/F mice showed no differential changes between SCI/M and SCI/F in middle-age mice. (E) Igf1 (insulin-like growth factor I) mRNA expression that was significantly downregulated in young SCI/F mice showed no alteration between SCI/M and SCI/F in middle-age mice. (F-H) There were no differential changes between SCI/F and SCI/M groups in anti-inflammatory genes (TGFβ, Socs3), pro-inflammatory genes (TNFα, CD68, Cxcl10, CD44, Pmp22, C3, Flt1), and astrocyte function (Serping1, Egfr). Gene expression was normalized by GAPDH and expressed as a fold-change relative to sham vehicle control. n=8 mice/group with Mann Whitney test, *p < 0.05.
Figure S6. Diagram illustrating timepoints by weeks post-injury and behavior experiments conducted after the initial injury. All behavior tests are categorized by the functional outcome that was examined.
Highlights.
Inflammation-related genes were attenuated in young females after acute SCI
Sex differences were seen in myeloid cell function, but not count or composition
Young adult female mice showed modestly enhanced neurological recovery
Functional recovery was differentially affected by sex in mice injured at 6 months.
Spinal cord and brain inflammation is higher in older females late after SCI
Acknowledgements
The work was supported by the National Institutes of Health Grants R01 NS094527 (JW), R01 NS110635 (AIF/JW), RF1 NS110637 (JW), R01 NS110825 (JW), K99 NS116032 (RMR), and the Craig H. Neilsen Foundation Research (340442) (AIF). We would like to thank Lulu Liu, Niaz Khan, and Hui Li for assistance with the tissue preparation and Flow cytometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental information
Supplemental Information includes Supplemental six figures and figure legends.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Data availability
The data that support the findings of this study are available from the corresponding authors upon request.
References
- Bartley EJ, Fillingim RB, 2013. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 111, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG, 2006. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23, 635–659. [DOI] [PubMed] [Google Scholar]
- Bove R, Musallam A, Healy BC, Houtchens M, Glanz BI, Khoury S, Guttmann CR, De Jager PL, Chitnis T, 2013. No sex-specific difference in disease trajectory in multiple sclerosis patients before and after age 50. BMC Neurol 13, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove RM, Healy B, Augustine A, Musallam A, Gholipour T, Chitnis T, 2012. Effect of gender on late-onset multiple sclerosis. Mult Scler 18, 1472–1479. [DOI] [PubMed] [Google Scholar]
- Bushnell CD, Chaturvedi S, Gage KR, Herson PS, Hurn PD, Jimenez MC, Kittner SJ, Madsen TE, McCullough LD, McDermott M, Reeves MJ, Rundek T, 2018. Sex differences in stroke: Challenges and opportunities. J Cereb Blood Flow Metab 38, 2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers JS, Huguenard AL, Kuruppu D, Liu NK, Xu XM, Sengelaub DR, 2012. Neuroprotective effects of testosterone on motoneuron and muscle morphology following spinal cord injury. The Journal of comparative neurology 520, 2683–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WM, Mohammed Y, Lee I, Pearse DD, 2013. Effect of gender on recovery after spinal cord injury. Translational stroke research 4, 447–461. [DOI] [PubMed] [Google Scholar]
- Chen Y, He Y, DeVivo MJ, 2016. Changing Demographics and Injury Profile of New Traumatic Spinal Cord Injuries in the United States, 1972–2014. Archives of physical medicine and rehabilitation 97, 1610–1619. [DOI] [PubMed] [Google Scholar]
- Cohen J, 1992. A power primer. Psychological bulletin 112, 155–159. [DOI] [PubMed] [Google Scholar]
- Comfort N, Re DB, 2017. Sex-Specific Neurotoxic Effects of Organophosphate Pesticides Across the Life Course. Curr Environ Health Rep 4, 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datto JP, Bastidas JC, Miller NL, Shah AK, Arheart KL, Marcillo AE, Dietrich WD, Pearse DD, 2015. Female Rats Demonstrate Improved Locomotor Recovery and Greater Preservation of White and Gray Matter after Traumatic Spinal Cord Injury Compared to Males. J Neurotrauma 32, 1146–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Brand FJ 3rd, Sedaghat C, Mash DC, Dietrich WD, Keane RW, 2014. RIG-1 receptor expression in the pathology of Alzheimer’s disease. J Neuroinflammation 11, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Brinton R, 2012. Minireview: translational animal models of human menopause: challenges and emerging opportunities. Endocrinology 153, 3571–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowler RN, Harrington DL, Haaland KY, Swanda RM, Fee F, Fiedler K, 1997. Profiles of cognitive functioning in chronic spinal cord injury and the role of moderating variables. Journal of the International Neuropsychological Society : JINS 3, 464–472. [PubMed] [Google Scholar]
- Dubal DB, 2020. Sex difference in Alzheimer’s disease: An updated, balanced and emerging perspective on differing vulnerabilities. Handbook of clinical neurology 175, 261–273. [DOI] [PubMed] [Google Scholar]
- Estornes Y, Bertrand MJ, 2015. IAPs, regulators of innate immunity and inflammation. Semin Cell Dev Biol 39, 106–114. [DOI] [PubMed] [Google Scholar]
- Falk S, Uldall M, Appel C, Ding M, Heegaard AM, 2013. Influence of sex differences on the progression of cancer-induced bone pain. Anticancer Res 33, 1963–1969. [PubMed] [Google Scholar]
- Farooque M, Suo Z, Arnold PM, Wulser MJ, Chou CT, Vancura RW, Fowler S, Festoff BW, 2006. Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal cord 44, 182–187. [DOI] [PubMed] [Google Scholar]
- Fukutoku T, Kumagai G, Fujita T, Sasaki A, Wada K, Liu X, Tanaka T, Kudo H, Asari T, Nikaido Y, Ueno S, Ishibashi Y, 2020. Sex-Related Differences in Anxiety and Functional Recovery after Spinal Cord Injury in Mice. J Neurotrauma 37, 2235–2243. [DOI] [PubMed] [Google Scholar]
- Gabory A, Attig L, Junien C, 2009. Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol 304, 8–18. [DOI] [PubMed] [Google Scholar]
- Gallou-Kabani C, Vige A, Gross MS, Boileau C, Rabes JP, Fruchart-Najib J, Jais JP, Junien C, 2007a. Resistance to high-fat diet in the female progeny of obese mice fed a control diet during the periconceptual, gestation, and lactation periods. Am J Physiol Endocrinol Metab 292, E1095–1100. [DOI] [PubMed] [Google Scholar]
- Gallou-Kabani C, Vige A, Junien C, 2007b. Lifelong circadian and epigenetic drifts in metabolic syndrome. Epigenetics 2, 137–146. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Donahue RR, Bailey WM, Taylor BK, 2019. Sexual Dimorphism of Pain Control: Analgesic Effects of Pioglitazone and Azithromycin in Chronic Spinal Cord Injury. J Neurotrauma 36, 2372–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffarinia A, Parvaneh S, Jalili C, Riazi-Rad F, Yaslianifard S, Pakravan N, 2016. Immunomodulatory Effect of Chymotrypsin in CNS Is Sex-independent: Evidence of Anti-inflammatory Role for IL-17 in EAE. Iran J Allergy Asthma Immunol 15, 145–155. [PubMed] [Google Scholar]
- Giatti S, Rigolio R, Diviccaro S, Falvo E, Caruso D, Garcia-Segura LM, Cavaletti G, Melcangi RC, 2020. Sex dimorphism in an animal model of multiple sclerosis: Focus on pregnenolone synthesis. J Steroid Biochem Mol Biol 199, 105596. [DOI] [PubMed] [Google Scholar]
- Gong L, Lv Y, Li S, Feng T, Zhou Y, Sun Y, Mi D, 2020. Changes in transcriptome profiling during the acute/subacute phases of contusional spinal cord injury in rats. Ann Transl Med 8, 1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald BD, Seel RT, Cifu DX, Shah AN, 2001. Gender-related differences in acute rehabilitation lengths of stay, charges, and functional outcomes for a matched sample with spinal cord injury: a multicenter investigation. Archives of physical medicine and rehabilitation 82, 1181–1187. [DOI] [PubMed] [Google Scholar]
- Hamano T, Hayashi K, Shirafuji N, Nakamoto Y, 2018. The Implications of Autophagy in Alzheimer’s Disease. Curr Alzheimer Res 15, 1283–1296. [DOI] [PubMed] [Google Scholar]
- Hauben E, Mizrahi T, Agranov E, Schwartz M, 2002. Sexual dimorphism in the spontaneous recovery from spinal cord injury: a gender gap in beneficial autoimmunity? Eur J Neurosci 16, 1731–1740. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Feil R, Constancia M, Fraga M, Junien C, Carel JC, Boileau P, Le Bouc Y, Deal CL, Lillycrop K, Scharfmann R, Sheppard A, Skinner M, Szyf M, Waterland RA, Waxman DJ, Whitelaw E, Ong K, Albertsson-Wikland K, 2011. Child health, developmental plasticity, and epigenetic programming. Endocr Rev 32, 159–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SW, Wang WT, Chou LC, Liou TH, Lin HW, 2016. Risk of Dementia in Patients with Spinal Cord Injury: A Nationwide Population-Based Cohort Study. J Neurotrauma. [DOI] [PubMed] [Google Scholar]
- Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, Koplev S, Jenkins SL, Jagodnik KM, Lachmann A, McDermott MG, Monteiro CD, Gundersen GW, Ma’ayan A, 2016. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44, W90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Mhatre PG, Yang Y, Wang MC, Schupf N, Rosas HD, 2020. Sex differences in risk of Alzheimer’s disease in adults with Down syndrome. Alzheimers Dement (Amst) 12, e12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Sun X, Shu Y, Mao Z, Xiao L, Qiu W, Lu Z, Hu X, 2017. Sex differences in outcomes of disease-modifying treatments for multiple sclerosis: A systematic review. Mult Scler Relat Disord 12, 23–28. [DOI] [PubMed] [Google Scholar]
- Li Y, Cao T, Ritzel RM, He J, Faden AI, Wu J, 2020a. Dementia, Depression, and Associated Brain Inflammatory Mechanisms after Spinal Cord Injury. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ritzel RM, He J, Cao T, Sabirzhanov B, Li H, Liu S, Wu LJ, Wu J, 2021. The voltage-gated proton channel Hv1 plays a detrimental role in contusion spinal cord injury via extracellular acidosis-mediated neuroinflammation. Brain Behav Immun 91, 267–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ritzel RM, Khan N, Cao T, He J, Lei Z, Matyas JJ, Sabirzhanov B, Liu S, Li H, Stoica BA, Loane DJ, Faden AI, Wu J, 2020b. Delayed microglial depletion after spinal cord injury reduces chronic inflammation and neurodegeneration in the brain and improves neurological recovery in male mice. Theranostics 10, 11376–11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchetti S, Beck KD, Galvan MD, Silva R, Cummings BJ, Anderson AJ, 2010. Comparison of immunopathology and locomotor recovery in C57BL/6, BUB/BnJ, and NOD-SCID mice after contusion spinal cord injury. J Neurotrauma 27, 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi E, Lin P, Peterson MD, Meade MA, Tate DG, Kamdar N, 2021. Traumatic Spinal Cord Injury and Risk of Early and Late Onset Alzheimer’s Disease and Related Dementia: Large Longitudinal Study. Archives of physical medicine and rehabilitation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Bouchard S, Peters K, Woller SA, Madahian B, Faghihi U, Patel S, Bake S, Hook MA, 2016. Inflammation is increased with anxiety- and depression-like signs in a rat model of spinal cord injury. Brain Behav Immun 51, 176–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelli S, Ficulle E, Iannuzzi F, Kovari E, Nistico R, Feligioni M, 2017. Targeting SUMO-1ylation Contrasts Synaptic Dysfunction in a Mouse Model of Alzheimer’s Disease. Molecular neurobiology 54, 6609–6623. [DOI] [PubMed] [Google Scholar]
- Martins D, Moreira J, Goncalves NP, Saraiva MJ, 2017. MMP-14 overexpression correlates with the neurodegenerative process in familial amyloidotic polyneuropathy. Dis Model Mech 10, 1253–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane K, Otto TE, Bailey WM, Veldhorst AK, Donahue RR, Taylor BK, Gensel JC, 2020. Effect of Sex on Motor Function, Lesion Size, and Neuropathic Pain after Contusion Spinal Cord Injury in Mice. J Neurotrauma 37, 1983–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin AP, Zhang HL, Qin ZH, 2008. Mechanisms of lysosomal proteases participating in cerebral ischemia-induced neuronal death. Neurosci Bull 24, 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguindin PF, Muka T, Glisic M, 2021. Sex and gender gap in spinal cord injury research: Focus on cardiometabolic diseases. A mini review. Maturitas 147, 14–18. [DOI] [PubMed] [Google Scholar]
- Ritzel RM, Doran SJ, Barrett JP, Henry RJ, Ma EL, Faden AI, Loane DJ, 2018. Chronic Alterations in Systemic Immune Function after Traumatic Brain Injury. J Neurotrauma 35, 1419–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, He J, Li Y, Cao T, Khan N, Shim B, Sabirzhanov B, Aubrecht T, Stoica BA, Faden AI, Wu LJ, Wu J, 2021. Proton extrusion during oxidative burst in microglia exacerbates pathological acidosis following traumatic brain injury. Glia 69, 746–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Li Y, He J, Khan N, Doran SJ, Faden AI, Wu J, 2020. Sustained neuronal and microglial alterations are associated with diverse neurobehavioral dysfunction long after experimental brain injury. Neurobiol Dis 136, 104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth E, Davidoff G, Thomas P, Doljanac R, Dijkers M, Berent S, Morris J, Yarkony G, 1989. A controlled study of neuropsychological deficits in acute spinal cord injury patients. Paraplegia 27, 480–489. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE Jr., 2003. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma 20, 179–193. [DOI] [PubMed] [Google Scholar]
- Scivoletto G, Morganti B, Molinari M, 2004. Sex-related differences of rehabilitation outcomes of spinal cord lesion patients. Clin Rehabil 18, 709–713. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Kuroiwa Y, 1976. Sex difference of multiple sclerosis in Japan. Neurology 26, 821–824. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL, 2006. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosci Res 84, 1064–1075. [DOI] [PubMed] [Google Scholar]
- Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF, 2011. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiology & behavior 103, 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AN, Lowe JL, Glaser EP, Mott CA, Shahidehpour RK, McFarlane KE, Bailey WM, Zhang B, Gensel JC, 2021. Acute inflammatory profiles differ with sex and age after spinal cord injury. J Neuroinflammation 18, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AN, MacLean SM, Stromberg AJ, Whelan JP, Bailey WM, Gensel JC, Wilson ME, 2020. Considerations for Studying Sex as a Biological Variable in Spinal Cord Injury. Frontiers in neurology 11, 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanova T, Roy N, Kopanja D, Raychaudhuri P, Bagchi S, 2009. DDB2 (damaged-DNA binding protein 2) in nucleotide excision repair and DNA damage response. Cell Cycle 8, 4067–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden MC, Holness MJ, 2002. Gender-specific programming of insulin secretion and action. J Endocrinol 175, 757–767. [DOI] [PubMed] [Google Scholar]
- Swartz KR, Fee DB, Joy KM, Roberts KN, Sun S, Scheff NN, Wilson ME, Scheff SW, 2007. Gender differences in spinal cord injury are not estrogen-dependent. J Neurotrauma 24, 473–480. [DOI] [PubMed] [Google Scholar]
- Todorovic S, Loncarevic-Vasiljkovic N, Jovic M, Sokanovic S, Kanazir S, Mladenovic Djordjevic A, 2020. Frailty index and phenotype frailty score: Sex- and age-related differences in 5XFAD transgenic mouse model of Alzheimer’s disease. Mech Ageing Dev 185, 111195. [DOI] [PubMed] [Google Scholar]
- Ulgen E, Ozisik O, Sezerman OU, 2019. pathfindR: An R Package for Comprehensive Identification of Enriched Pathways in Omics Data Through Active Subnetworks. Frontiers in genetics 10, 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDerwerker CJ, Gregory CM, Simpson KN, 2020. Using Inferred Mobility Status to Estimate the Time to Major Depressive Disorder Diagnosis Post-Spinal Cord Injury. Archives of physical medicine and rehabilitation 101, 658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Huang H, Halagan M, Vierra-Green C, Heuer M, Brelsford JE, Haagenson M, Scheuermann RH, Telenti A, Biggs W, Pearson NM, Udell J, Spellman S, Maiers M, Kennedy CJ, 2018. Chromosome Y-encoded antigens associate with acute graft-versus-host disease in sex-mismatched stem cell transplant. Blood Adv 2, 2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcoxon JS, Schwartz J, Aird F, Redei EE, 2003. Sexually dimorphic effects of maternal alcohol intake and adrenalectomy on left ventricular hypertrophy in rat offspring. Am J Physiol Endocrinol Metab 285, E31–39. [DOI] [PubMed] [Google Scholar]
- Wilson CS, Nassar SL, Ottomanelli L, Barnett SD, Njoh E, 2018. Gender differences in depression among veterans with spinal cord injury. Rehabilitation psychology 63, 221–229. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Cadotte DW, Fehlings MG, 2012. Clinical predictors of neurological outcome, functional status, and survival after traumatic spinal cord injury: a systematic review. J Neurosurg Spine 17, 11–26. [DOI] [PubMed] [Google Scholar]
- Wu J, Stoica BA, Luo T, Sabirzhanov B, Zhao Z, Guanciale K, Nayar SK, Foss CA, Pomper MG, Faden AI, 2014a. Isolated spinal cord contusion in rats induces chronic brain neuroinflammation, neurodegeneration, and cognitive impairment: Involvement of cell cycle activation. Cell Cycle 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhao Z, Kumar A, Lipinski MM, Loane DJ, Stoica BA, Faden AI, 2016. Endoplasmic Reticulum Stress and Disrupted Neurogenesis in the Brain Are Associated with Cognitive Impairment and Depressive-Like Behavior after Spinal Cord Injury. J Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhao Z, Sabirzhanov B, Stoica BA, Kumar A, Luo T, Skovira J, Faden AI, 2014b. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J Neurosci 34, 10989–11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Ren J, Chen Q, Chen ZJ, 2017. cGAS is essential for cellular senescence. Proc Natl Acad Sci U S A 114, E4612–E4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yune TY, Park HG, Lee JY, Oh TH, 2008. Estrogen-induced Bcl-2 expression after spinal cord injury is mediated through phosphoinositide-3-kinase/Akt-dependent CREB activation. J Neurotrauma 25, 1121–1131. [DOI] [PubMed] [Google Scholar]
- Zendedel A, Monnink F, Hassanzadeh G, Zaminy A, Ansar MM, Habib P, Slowik A, Kipp M, Beyer C, 2018. Estrogen Attenuates Local Inflammasome Expression and Activation after Spinal Cord Injury. Molecular neurobiology 55, 1364–1375. [DOI] [PubMed] [Google Scholar]
- Zhu W, Chen X, Ning L, Jin K, 2019. Network Analysis Reveals TNF as a Major Hub of Reactive Inflammation Following Spinal Cord Injury. Scientific reports 9, 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Enrichment analysis of genes unique to sex after injury. (A) Enrichment analysis of injury genes unique to male mice show a high content of genes related to Cytokine-Cytokine Receptor Interaction and Apoptosis. The length of the bar represents the significance of that specific gene-set or term. Greater statistical significance is denoted by the increased brightness of the bar color. (B) Enrichment analysis of injury genes unique to female mice show a high content of genes related to Non-Homologous End-Joining and cAMP Signaling pathway. (C) Pathway analysis of genes unique to sex dimorphism after injury. N=3–4 mice/group.
Figure S2. Sex dimorphism differentially modifies genes related to astrocyte function, cytokine signaling, cellular stress and microglia function in acute stages of SCI. (A) Heatmap of genes related to Astrocyte Function that are DE by injury in male mice but not in females and vice versa. Color coding was based on z-score scaling. (B) Heatmap of genes related to Cytokine Signaling that are DE by injury and male specific (left) or female specific (right). (C) Heatmap of genes related to Apoptosis that are DE by injury and male specific (left) or female specific (right). (D) Heatmap of genes related to Cellular Stress that are DE by injury and male specific (left) or female specific (right). (E) Heatmap of genes related to Microglia Function that are DE by injury and male specific (left) or female specific (right). N=3–4 mice/group.
Figure S3. Microglial production of reactive oxygen species and inflammatory cytokines during acute spinal cord injury. Microglial activity in male and female mice at 3d post-SCI was measured using flow cytometry. Representative histograms and subsequent MFI quantification of (A) NOX2 protein expression, (B) reactive oxygen species production, (C) DNA damage, and production of (D) TNF, (E) IL-1β, (F) IL-6 and (G) TFGβ cytokines in CD45intCD11b+ microglia are shown. Significant differences were seen after injury, but not between sexes. For each sex, N=6 mice per sham group, and N=10 mice per SCI group. ***p<0.001 vs. Sham group; Two-way ANOVA following Tukey’s multiple comparisons test. Abbreviations: d (day), FMO (fluorescence minus one), Max (maximum), MFI (mean fluorescence intensity), SCI (spinal cord injury), μm (micrometer).
Figure S4. Sex differences in infiltrating CD45hiCD11b+Ly6Clo monocytes at 3 days post-injury. (A-B) No sex differences were seen in ROS production as evidenced by both MFI of DHR+ and DCF+ Ly6Clo monocytes. (C) MFI quantification of NOX2 protein expression. (D) MFI quantification of CD68-positive Ly6Clo monocytes. (E) MFI of mitochondrial membrane potential in Ly6Clo monocytes using MitoSpyRed+ was significantly lower in females vs. males after SCI. N=10 mice per SCI group. **p<0.01 vs. SCI/M with unpaired t test.
Figure S5. Middle-age alters inflammatory genes in response to acute SCI. Six month-old male and female mice were subjected to SCI and spinal cord samples (~5-mm segments) were collected at 3 d post-injury and processed for RNA extraction and a list of inflammatory genes qPCR analysis. (A) Pro-inflammatory genes (IL1β, IL6, CD74, Psmb8) were downregulated in SCI/F mice compared to SCI/M animals. (B) Anti-inflammatory gene Chil3 (Ym1) expression was increased in SCI/F group vs SCI/M mice. (C-D) Complement pathway genes (C1qa, C1qb, C1qc, C4a), and Nlrp3, Hcar2 (hydroxycarboxylic acid receptor 2), and CD11b genes that were elevated in young SCI/F mice showed no differential changes between SCI/M and SCI/F in middle-age mice. (E) Igf1 (insulin-like growth factor I) mRNA expression that was significantly downregulated in young SCI/F mice showed no alteration between SCI/M and SCI/F in middle-age mice. (F-H) There were no differential changes between SCI/F and SCI/M groups in anti-inflammatory genes (TGFβ, Socs3), pro-inflammatory genes (TNFα, CD68, Cxcl10, CD44, Pmp22, C3, Flt1), and astrocyte function (Serping1, Egfr). Gene expression was normalized by GAPDH and expressed as a fold-change relative to sham vehicle control. n=8 mice/group with Mann Whitney test, *p < 0.05.
Figure S6. Diagram illustrating timepoints by weeks post-injury and behavior experiments conducted after the initial injury. All behavior tests are categorized by the functional outcome that was examined.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon request.


