Abstract
Background:
An emerging body of literature has indicated that broad, transdiagnostic dimensions of psychopathology are associated with alterations in brain structure across the lifespan. The current study aimed to investigate the relationship between brain structure and broad dimensions of psychopathology in the critical preadolescent period when psychopathology is emerging.
Methods:
This study included baseline data from the Adolescent Brain and Cognitive Development (ABCD) Study® (n = 11,721; age range = 9–10 years; male = 52.2%). General psychopathology, externalizing, internalizing, and thought disorder dimensions were based on a higher-order model of psychopathology and estimated using Bayesian plausible values. Outcome variables included global and regional cortical volume, thickness, and surface area.
Results:
Higher levels of psychopathology across all dimensions were associated with lower volume and surface area globally, as well as widespread and pervasive alterations across the majority of cortical and subcortical regions studied, after adjusting for sex, race/ethnicity, parental education, income and maternal psychopathology. The relationships between general psychopathology and brain structure were attenuated when adjusting for cognitive functioning. There were no statistically significant relationships between psychopathology and cortical thickness in this sample of preadolescents.
Conclusions:
The current study identified lower cortical volume and surface area as transdiagnostic biomarkers for general psychopathology in preadolescence. Future research may focus on whether the widespread and pervasive relationships between general psychopathology and brain structure reflect cognitive dysfunction that is a feature across a range of mental illnesses.
Keywords: generalized psychopathology, externalizing, internalizing, brain structure, preadolescence
Introduction
Recent meta-analyses of case-control studies indicate that the neural substrates underpinning mental disorders appear to be largely shared (Goodkind et al., 2015; McTeague et al., 2017; McTeague et al., 2020; Sha, Wager, Mechelli, & He, 2019). Given this evidence, recent studies have focused on uncovering the neurobiological underpinnings of broad dimensional spectra that represent latent liabilities towards psychopathology across a range mental disorders. These dimensional spectra have been organized into a hierarchy, with an overarching liability towards general psychopathology (or the ‘p factor’) at the top (Caspi et al., 2014; Kotov et al., 2017; Lahey et al., 2012). General psychopathology reflects a broad liability towards the whole range of psychopathology, which can then be subdivided into lower-order sub-spectra, such as externalizing (e.g., antisocial behavior, hyperactivity), internalizing (e.g., depression, anxiety), and thought disorder (i.e., disorganized thoughts, delusional beliefs, hallucinations, obsessions, compulsions) dimensions (Caspi et al., 2014; Caspi & Moffitt, 2018). An emerging body of literature has demonstrated the utility of working within this latent variable framework to uncover structural brain alterations associated with dimensional psychopathology across the lifespan (Latzman & DeYoung, 2020; Zald & Lahey, 2017).
Working within this framework of latent dimensions, increased levels of general psychopathology have been associated with less gray matter in prefrontal (Snyder, Hankin, Sandman, Head, & Davis, 2017), cerebellar (Moberget et al., 2019; Romer et al., 2018; Romer et al., 2019), occipital (Romer et al., 2018; Romer et al., 2019), and striatal (Gong et al., 2019) regions, suggesting a distributed effect across brain structural networks. Consistent with this distributed effect, two recent studies have identified global structural brain alterations as a pervasive feature of general psychopathology. In a cross-sectional study of the Philadelphia Neurodevelopmental Cohort (PNC; mean age 15 years), higher levels of general psychopathology were associated with smaller gray matter volumes across structural networks globally (Kaczkurkin et al., 2019). Meanwhile, in the Dunedin cohort study, higher levels of general psychopathology (measured longitudinally from age 18 to age 45) were associated with a pervasively thinner neocortex measured at age 45 (Romer et al., 2020). Previous studies therefore converge to indicate that general psychopathology is associated with global alterations in brain structure.
The majority of mental disorders have their onset in adolescence and young adulthood. This peak period of risk coincides with increased myelination and synaptic pruning that extend from preadolescence into the mid-twenties (Tamnes et al., 2017). Despite late childhood and early adolescence representing a period of intense neural development, coinciding with the emergence of many mental disorders, few studies have focused on the neural correlates of general psychopathology in preadolescents. To address this gap, the current study aimed to investigate structural brain alterations associated with general psychopathology in preadolescents using baseline data from the Adolescent Brain and Cognitive Development (ABCD) Study® (n = 11,875; age range = 9–10 years) (Barch et al., 2018). Structural brain alterations associated with the lower-order externalizing, internalizing and thought disorder dimensions that comprise general psychopathology were also investigated. The study aims and the multilevel modelling analyses were preregistered (https://osf.io/qxegb). Consistent with prior research in older samples (Kaczkurkin et al., 2019; Romer et al., 2020), we hypothesized that general psychopathology would be associated with non-specific and pervasive alterations in brain structure.
Materials and Methods
Study Population
Baseline cross-sectional data from the ABCD Study consisted of 11,875 participants, born between 2005 and 2008. A probability sample was recruited through schools at 21 sites across the US, and selected based on sex, race/ethnicity, socioeconomic status, and urbanicity (Garavan et al., 2018). Baseline data collection (included in the 2.0.1 data release) occurred between September 1, 2016 and October 15, 2018. Table 1 provides the baseline demographic characteristics of the ABCD sample. All parents provided written informed consent and all children provided assent to the research protocol approved by the institutional review board at each of the 21 data collection sites. Of the 11,875 participants enrolled, 154 were removed from the factor analysis stage because of missing data for all indicators (n=11,721). A further 863 participants who did not pass the ABCD quality control measures were removed from the structural MRI analyses (n=10,858). Those excluded were comparable to those included in terms of many clinical characteristics, although they were more likely to be diagnosed with ADHD, had lower total cognition scores, lower levels of parental education and were less likely to be Caucasian (see Table S1).
Table 1.
Baseline demographic and clinical characteristics of the participants in the ABCD study (n=11,721)
| Mean | SD | |
|---|---|---|
| Age (years) | 9.91 | 0.62 |
| Total cognition score | 47.71 | 11.21 |
| Parental education (years) | 16.6 | 2.8 |
| N | % | |
| Male | 6,118 | 52.2 |
| Race/ethnicity | ||
| Caucasian | 6,108 | 52.1 |
| Black | 1,753 | 15.0 |
| Hispanic | 2,360 | 20.1 |
| Asian | 250 | 2.1 |
| Other | 1,230 | 10.5 |
| Lifetime indicators of psychopathology | ||
| Major Depressive Disorder | 318 | 2.7 |
| Generalized Anxiety Disorder | 510 | 4.3 |
| Panic Disorder | 32 | 0.3 |
| Separation Anxiety | 1,049 | 8.8 |
| Social Anxiety Disorder | 547 | 4.6 |
| Hallucinations | 55 | 0.5 |
| Delusions | 216 | 1.8 |
| Attention Deficit Hyperactivity | 2,429 | 20.5 |
| Oppositional Defiant Disorder | 1,667 | 14.0 |
| Conduct Disorder | 374 | 3.1 |
| Obsessive-Compulsive Disorder | 1,099 | 9.3 |
| Bipolar Disorder (Unspecified) | 429 | 3.6 |
| Post-Traumatic Stress Disorder | 231 | 1.9 |
| Specific Phobia | 3 133 | 26 4 |
Note: Cognition scores ascertained from fully corrected total cognition t-score from the NIH toolbox
Indicators of psychopathology
Previous studies using ABCD data have delineated the structure of psychopathology using the parent-reported Child Behavior Checklist (CBCL) (Clark et al., 2020; Karcher, Michelini, Kotov, & Barch, 2021; Michelini et al., 2019). The lower order dimensions derived from the CBCL, however, do not provide coverage of the full spectrum of psychopathology, especially with respect to the thought disorder dimension. While the CBCL does contain a thought problems sub-scale, a thought disorder dimension has not emerged in previous studies of the CBCL using the ABCD data (Clark et al., 2020; Karcher et al., 2021; Michelini et al., 2019). The CBCL dimensions also do not align with the lower order dimensions examined in previous studies examining the neural correlates of general psychopathology (Kaczkurkin et al., 2019; Romer et al., 2020). As such, the current study is based on categorical indicators derived from the parent-reported Kiddie Schedule for Affective Disorders and Schizophrenia for DSM-5 (KSADS-5) (Kobak, Kratochvil, Stanger, & Kaufman, 2013), which allowed the delineation of the structure of psychopathology including thought disorder pathology. This approach is also consistent with our previous study examining neural connectivity and activation patterns associated with psychopathology using functional magnetic resonance imaging (MRI) data and KSADS diagnoses from the ABCD Study (Lees et al., 2020).
Consistent with this previous study (Lees et al., 2020), 14 categorical indicators (present/absent) of lifetime mental disorders were examined (see Table 1). All disorders assessed as part of the baseline ABCD Study were included, except those not assessed in the whole sample (agoraphobia, autism spectrum disorders, disruptive mood dysregulation disorder) and the eating disorders which had low base prevalence rates. To adequately model the thought disorder dimension, the hallucinations and delusions criteria from the schizophrenia module were included as separate indicators, rather than the full DSM-5 schizophrenia diagnosis. In total, 5,831 (49.7%) youth had at least one lifetime indicator present, consistent with previous US community samples (Merikangas et al., 2010). Comorbidity was common, with less youth meeting criteria for a single indicator (n=2,856) than multiple indicators (n=2,975). Among those meeting criteria for more than one indicator, the mean number of indicators present was 3.11 (SD=1.39) and the maximum number was 12.
MRI Data Acquisition.
MRI acquisition and scanning parameters are described elsewhere (Casey et al., 2018) (see the Supporting Information Appendix S1 for details). Brain data were collected on 3T scanners (Siemens Prisma and Prisma Fit, General Electric MR 750, Philips Achieva dStream and Ingenia). The T1 images were corrected for gradient nonlinearity distortions using scanner-specific, nonlinear transformations. Cortical reconstruction and volumètric segmentation were performed by the Data Analysis, Informatics, and Resources Centre (DAIRC) of ABCD using FreeSurfer v5.3.0 (Dale, Fischl, & Sereno, 1999). The present study used post-processed structural (i.e., cortical volume, thickness, and surface area) data mapped to 34 cortical parcellations per hemisphere (68 total regions of interest) based on the Desikan-Killiany brain registration atlas (Desikan et al., 2006). For cortical volume, 8 subcortical segmentations per hemisphere were also investigated (16 regions in total) (Fischl et al., 2002). DAIRC used a combination of automated and manual methods to review the datasets for quality control prior to sharing data via the NDA database.
Statistical analysis
Confirmatory factor analysis (CFA) model comparisons indicated that the higher-order model was the best fit for the 14 psychopathology indicators when compared with both a unidimensional model and a bifactor model (Tables S2 and S3). These methods have been detailed previously (Lees et al., 2021) (also see the Supporting Information Appendix S2 for detailed CFA methods). Briefly, all three models fit the data well, with both the higher-order model and the bifactor model demonstrating excellent fit and outperforming the unidimensional model. However, the reliability [estimated using the H coefficient; (Rodriguez, Reise, & Haviland, 2016)] of the specific factors in the modified bifactor model was low (H coefficients: externalizing = 0.50; internalizing = 0.53). Whereas reliability was higher and within the acceptable range for the lower order factors in the higher-order model (H coefficients: externalizing = 0.84; internalizing = 0.91, thought disorder = 0.74). The path diagram for the higher-order model and standardized factor loadings are provided in Figure 1. The confirmatory approach to factor analysis, and the assignment of indicators to respective factors, were based on an extensive literature examining the structure of psychopathology across the lifespan (Carragher et al., 2016; Caspi et al., 2020; Caspi et al., 2014; Caspi & Moffitt, 2018; Kotov et al., 2017).
Figure 1:
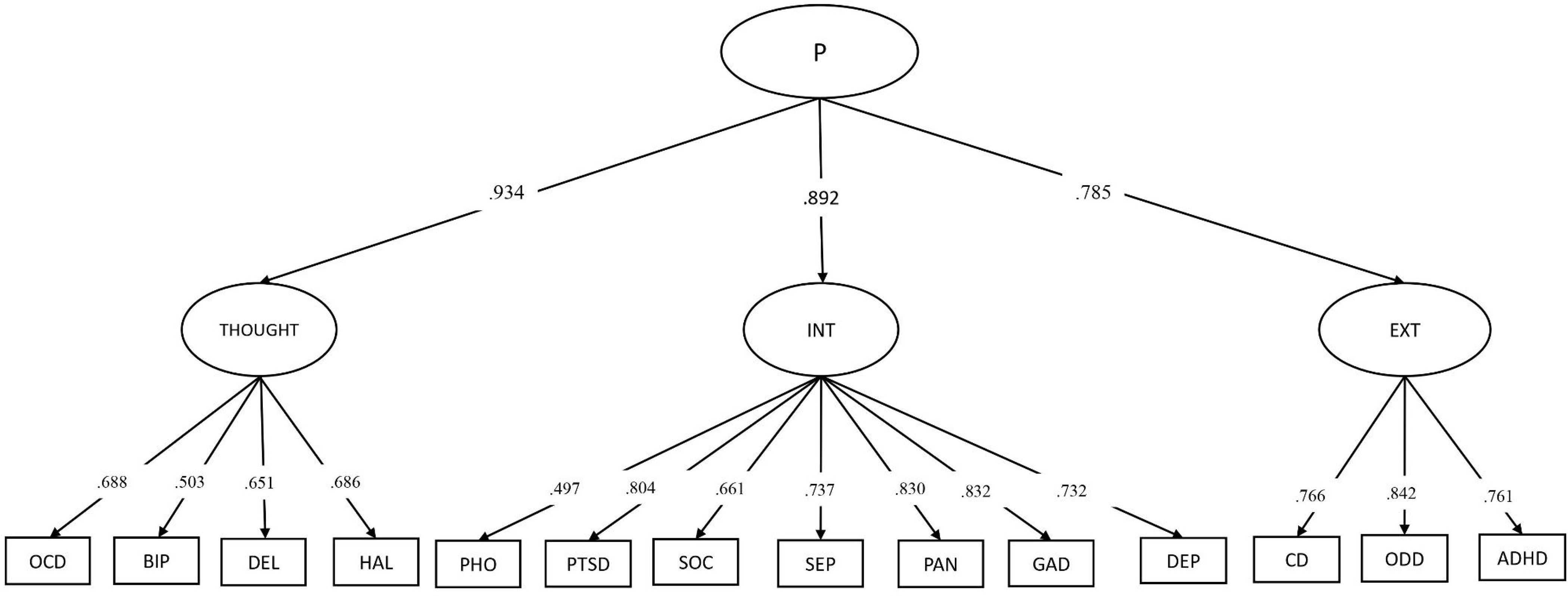
Hierarchical structure of psychopathology in the ABCD Study (n = 11,721)
Note: ADHD=attention deficit hyperactivity disorder, ODD=oppositional defiant disorder, CD=conduct disorder, MDD=major depressive disorder, GAD=generalized anxiety disorder, PAN=panic disorder, SEP=separation anxiety disorder, SOC=social anxiety disorder, PTSD=post-traumatic stress disorder, PHO=specific phobia, HAL=hallucinations, DEL=delusions, OCD=obsessive-compulsive disorder, BIP=bipolar disorder.
Bayesian plausible values were estimated for each participant for secondary use in linear mixed model regression analyses. Almost all previous studies focusing on the neural correlates of general psychopathology have been based on secondary analysis of factor scores. Factor scores directly estimate a participants’ underlying psychopathology using a single point estimate based on the pattern of participant responses. The population distribution of factor scores is therefore likely to be highly skewed when derived from categorical and, to a lesser extent, ordinal (i.e., symptom count) indicators. As an alternative to factor scores, plausible values are multiple random draws from the posterior distribution for each participant and represent a range of possible values a participant might reasonably have, given the presence or absence of the 14 model indicators. As random draws from the posterior distribution, plausible values provide an unbiased estimate of the population mean and variance of psychopathology which more accurately approximates its underlying continuous distribution when compared with single point estimates such as factor scores (Wu, 2005). As an illustration, the difference in the population distributions derived from general psychopathology factor scores and plausible values for the present study are presented in Figure S1. As single point estimates, factor scores also potentially contain a significant amount of random error (i.e., factor indeterminacy) which is not taken into account in analyses that treat these estimates as observable outcomes (Wu, 2005). Plausible values provide less biased parameter estimates than factor scores by directly modelling the uncertainty around these estimates through multiple random draws (i.e., imputations) from the posterior distribution.
For each individual, 100 plausible values were estimated for each latent variable (i.e., 100 imputations), using the higher order model as specified in Figure 1, with the Bayes estimator (see the Supporting Information Appendix S3 for more detail on estimation of plausible values as well as Mplus syntax). Factor analysis and plausible value estimation were conducted using Mplus Version 8.4 (Muthén & Muthén, 2018). The 100 datasets, each with different plausible value estimates for each of the latent dimensions, were then analyzed in R version 3.5.3 using multilevel modelling [R package lme4; (Bates, Mächler, Bolker, & Walker, 2015)] within a multiple imputation framework [package mitml; (Grund, Robitzsch, Luedtke, & Grund, 2019)]. Outcome variables included global cortical volume, global surface area, and mean cortical thickness, as well as regional cortical volume (n=68 cortical, 16 subcortical regions), surface area (n=68 regions), and thickness (68 regions). Given the correlated nature of the lower order factors derived from higher order models, analyses included one latent dimension as a predictor at a time (without adjusting for the other factors) and included crossed random intercepts for family (there were 3,724 participants who were siblings) and scanner device (there were 29 scanners across the 21 sites). Baseline models were also adjusted for non-modifiable participant characteristics, including sex (reported as female or male) and race/ethnicity (reported as White, Black, Hispanic, Asian, Other). Consistent with prior research in this area (Kaczkurkin et al., 2019; Romer et al., 2020), the primary analyses did not adjust for total cortical volume, average surface area, or mean cortical thickness because we were interested in examining absolute, rather than relative, regional effects. Sensitivity analyses focusing on relative regional effects were also conducted that adjusted for total cortical volume (mm3), average surface area (mm2), or mean cortical thickness (mm3), depending on the outcome variables of interest. Given there is currently no consensus on which is most informative (Mills et al., 2016), this provides both absolute and relative relationships between psychopathology and brain structure.
A series of analyses were also conducted to determine whether the results were robust to the inclusion of modifiable characteristics which act as confounders that may potentially capture some of the variation between psychopathology and brain structure. The baseline model (adjusted for sex and race/ethnicity) was first also adjusted for parental education and income (as indicators of socio-economic status). In the next series of analyses, maternal psychopathology (maternal reported experiences of either depression, psychosis, anxiety, antisocial behavior or mania) was included as an additional covariate. Finally, total cognition score (as determined by the fully corrected total cognition composite t-score from the NIH toolbox®) was included as an additional covariate.
Within each set of analyses (i.e., cortical volume, thickness, surface area) the false discovery rate (p(FDR) <0.05) was used to correct for multiple comparisons and findings based on the adjusted p-values are reported (Benjamini & Hochberg, 1995). All three indices of brain structure were examined on the basis of prior research in older samples which has indicated that psychopathology may be associated with cortical volume, surface area and thickness in different ways (Kaczkurkin et al., 2019; Romer et al., 2020), although specific hypotheses regarding these relationships were not preregistered due to the different age range of the participants in the current study. Analyses pertaining to each index of brain structure were therefore treated as separate ‘families’, with the error rate controlled for within, rather than across, the sets of analyses focusing on cortical volume, surface area and thickness, respectively. Analyses based on cortical volume corrected for a total of 272 comparisons (68 regions analyzed for the each of the four underlying dimensions), as did the analyses based on cortical thickness and surface area. Analyses based on subcortical volume corrected for 64 comparisons (16 regions analyzed for each of the four underlying dimensions).
Results
Structural alterations associated with general psychopathology
Table 2 presents the associations between psychopathology (in standardized units) and global brain structure (in standardized units). General psychopathology was negatively associated with global cortical volume and surface area, but not mean cortical thickness (Table 2). The relationships between general psychopathology and global brain structure were robust to the inclusion of potential confounders, including parental education, income, maternal psychopathology and total cognition scores, although relationships were attenuated as more variables were progressively entered into the models (Table 2).
Table 2.
Relationship between psychopathology (in standardized units) and global cortical volume, global surface area and mean cortical thickness (n=10,868)
| Global cortical volume | Global surface area | Mean cortical thickness | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | |
| General psychopathology a | |||||||||
| Baseline model | −0.042 | −0.061, −0.025 | <.0001 | −0.048 | −0.061, −0.025 | <.0001 | −0.003 | −0.022, 0.017 | .792 |
| Adjusting education and income | −0.038 | −0.055, −0.020 | <.0001 | −0.039 | −0.056, −0.021 | <.0001 | −0.001 | −0.020, 0.019 | .424 |
| Adjusting maternal psychopathology | −0.037 | −0.055, −0.019 | <.0001 | −0.037 | −0.055, −0.019 | <.0001 | −0.002 | −0.021, 0.018 | .871 |
| Adjusting total cognition b | −0.034 | −0.052, −0.015 | 0.0004 | −0.033 | −0.052, −0.015 | 0.0004 | −0.002 | −0.023, 0.018 | .827 |
| Externalizing psychopathology a | |||||||||
| Baseline model | −0.047 4 | −0.064, −0.029 | <.0001 | −0.048 | −0.065, −0.031 | <.0001 | −0.001 | −0.020, 0.018 | .951 |
| Adjusting education and income | −0.043 | −0.060, −0.025 | <.0001 | −0.045 | −0.062, −0.028 | <.0001 | −0.001 | −0.018, 0.020 | .951 |
| Adjusting maternal psychopathology | −0.042 | −0.060, −0.024 | <.0001 | −0.043 | −0.061, −0.026 | <.0001 | −0.0001 | −0.019, 0.019 | .992 |
| Adjusting total cognition b | −0.038 | −0.056, −0.019 | <.0001 | −0.038 | −0.056, −0.021 | <.0001 | 0.001 | −0.020, 0.021 | .959 |
| Internalizing psychopathology a | |||||||||
| Baseline model | −0.039 | −0.057, −0.021 | <.0001 | −0.039 | −0.056, −0.021 | <.0001 | −0.004 | −0.022, 0.015 | .713 |
| Adjusting education and income | −0.035 | −0.052, −0.017 | 0.0001 | −0.035 | −0.052, −0.017 | 0.0001 | −0.002 | −0.020, 0.017 | .848 |
| Adjusting maternal psychopathology | −0.033 | −0.052, −0.015 | 0.0003 | –0.033 | −0.051, −0.015 | 0.0004 | −0.003 | −0.021, 0.016 | .787 |
| Adjusting total cognition b | −0.031 | −0.050, −0.012 | 0.001 | −0.032 | −0.051, −0.014 | 0.002 | −0.004 | −0.024, 0.016 | .716 |
| Thought disorder psychopathology a | |||||||||
| Baseline model | −0.041 | −0.059, −0.023 | <.0001 | −0.041 | −0.030, −0.077 | <.0001 | −0.003 | −0.023, 0.017 | .780 |
| Adjusting education and income | −0.036 | −0.053, −0.018 | <.0001 | −0.037 | −0.054, −0.019 | <.0001 | −0.001 | −0.021, 0.019 | .919 |
| Adjusting maternal psychopathology | −0.035 | −0.053, −0.017 | 0.0001 | −0.035 | −0.053, −0.017 | 0.0001 | −0.002 | −0.022, 0.018 | .864 |
| Adjusting total cognition b | −0.032 | −0.051, −0.014 | 0.0008 | −0.032 | −0.050, −0.013 | 0.0009 | −0.002 | −0.023. 0.019 | .820 |
All models adjusted for additional variables in a progressive, step-wise manner
Models corrected for total cognition scores based on the NIH Toolbox
In regional analyses, a higher level of general psychopathology was associated with lower volume and surface area that was widespread and pervasive across the majority of brain regions studied (Figure 2, Tables S4 and S5). For cortical volume, 51 of the 68 regions passed FDR correction. For subcortical volume, four of the 16 regions passed FDR correction. For surface area, 57 of the 68 regions passed FDR correction. General psychopathology was not associated with regional cortical thickness for any of the 68 brain regions studied (Table S6). Consistent with the global and pervasive pattern of relationships between general psychopathology and brain structure, none of the regional relationships were related to volume or surface area after accounting for global volume and surface area, respectively.
Figure 2:
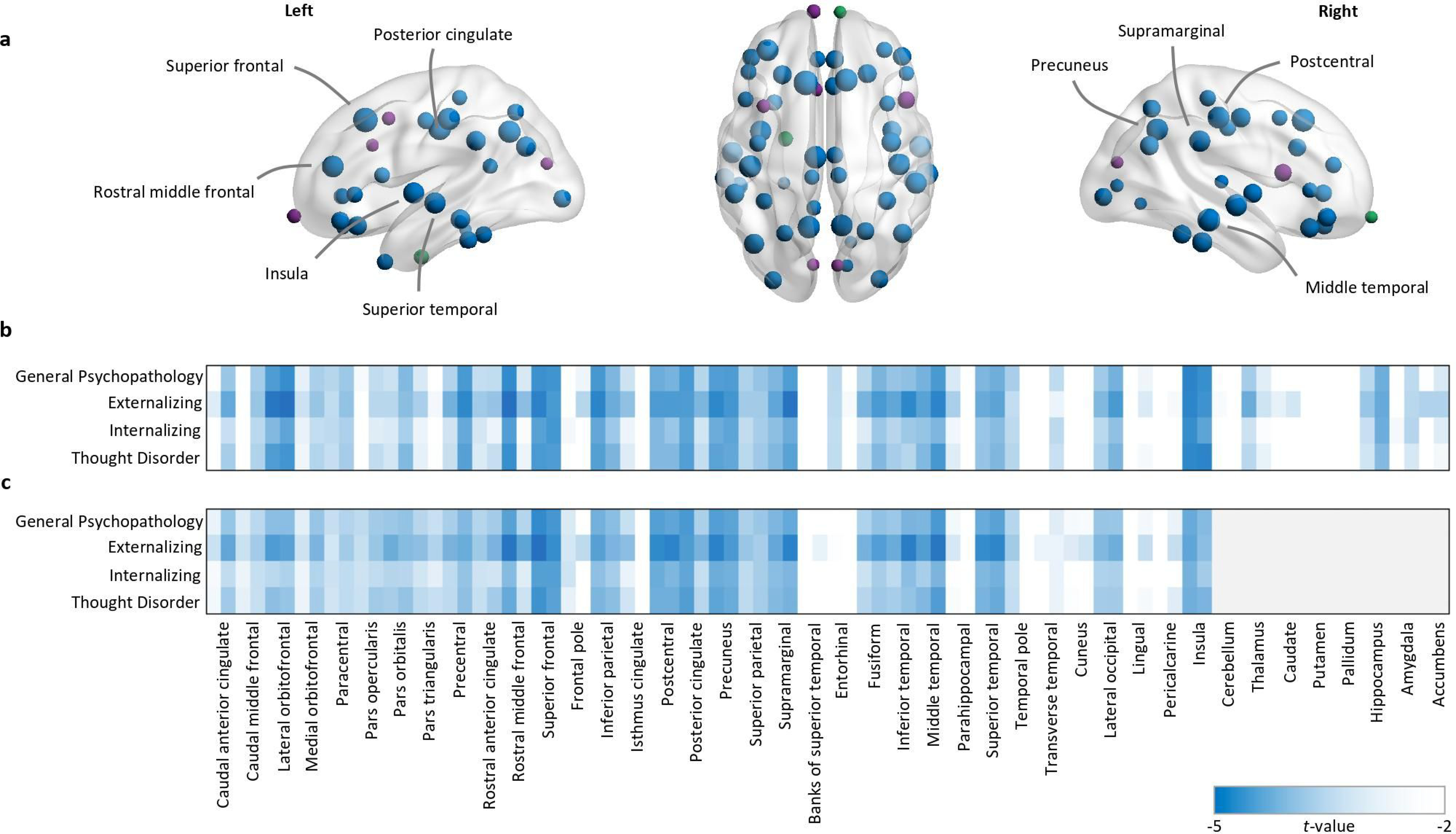
In the baseline model, controlling for sex and race/ethnicity, psychopathology was associated with lower volume and surface area. a Regions exhibiting significantly lower volume and surface area (blue nodes), volume-only (green nodes), or surface area-only (purple nodes), mapped for the general psychopathology dimension. Regions with the strongest loadings are labelled (t<3.5). Node size represents the t values. b-c t values for all psychopathology dimensions and volumetric (b) or surface area (c) associations, ordered left hemisphere-right hemisphere for each cortical (n=68) and subcortical (n=16) region where non-significant findings are coded white.
The majority (~85%) of the relationships between general psychopathology and regional volume and surface area were robust to the inclusion of parental education and income. None of the relationships with the subcortical structures were statistically significant after adjusting for parental education and income (Tables S7 and S8). After additionally adjusting for maternal psychopathology, 37 of the 68 regions passed FDR correction for cortical volume and 47 of the 68 regions passed correction for surface area (Tables S9 and S10). After additionally adjusting for total cognition scores, none of the regional relationships between general psychopathology and cortical volume were statistically significant, whilst 26 of the 68 regions passed FDR correction for surface area (Tables S11 and S12).
The neural correlates of lower order dimensions
A similar pattern of results emerged for each of the lower-order externalizing, internalizing, and thought disorder dimensions when examining global measures of cortical volume, thickness, and surface area. Global cortical volume and surface area were robustly associated with each of the lower-order dimensions, while mean cortical thickness was not (Table 2). None of the relationships between lower-order dimensions of psychopathology and regional cortical thickness were statistically significant (Table S6). In the regional analyses, there were very few relationships with cortical volume and surface area that were specific to one lower-order dimension and not shared with either general psychopathology and/or one or more of the other lower-order dimensions (Figure 2, Tables S4 and S6). The externalizing factor was specifically associated with less volume in the left caudal anterior cingulate cortex and the nucleus accumbens bilaterally, as well as lower surface area in the right frontal pole, the right banks of the superior temporal sulcus, and the left transverse temporal gyrus. Meanwhile, the thought disorder factor was specifically associated with less volume in the left amygdala. Consistent with the global and pervasive pattern of relationships between the lower-order dimensions and brain structure, none of the regional relationships were related to volume or surface area after accounting for global volume and surface area, respectively.
As with the analyses focusing on general psychopathology, the majority of relationships between the lower-order dimensions and regional cortical volume and surface area were robust to the inclusion of parental education and income (Tables S4, S6, S7, S8). Additionally adjusting for maternal psychopathology and total cognition scores attenuated the relationships between brain structure and each of the lower order dimensions (Tables S4, S5, S-S12).
Post hoc analyses
Post hoc analyses were conducted to investígate the relationships between individual indicators of mental disorders and global brain structure. Within a linear mixed model framework, relationships between each of the 14 indicators used in the CFA (as predictors in separate models) and global brain structure (volume, surface area and thickness as outcomes in separate models) were examined. These analyses controlled for sex and race/ethnicity and included random intercepts for family membership and scanner. There were statistically significant relationships between global cortical volume and each of the indicators of psychopathology, except for hallucinations. There were statistically significant relationships between global surface area and each of the indicators, except for the hallucinations and delusions indicators. Meanwhile, none of the relationships between mean cortical thickness and the 14 disorder-level indicators were statistically significant. These findings are largely consistent with those reported with respect to the main analysis focusing on dimensional psychopathology.
The relationships with brain structure were very similar across the different factors, indicating that their shared, rather than unique, variance (or the general psychopathology factor) may be driving the univariable results. Post hoc analyses were therefore also conducted to further investigate whether there were any unique associations between the lower-order factors and gray matter volume and surface area. Models were run with externalizing, internalizing, and thought disorder entered simultaneously in a multiple regression analysis predicting each of the brain regions. There were no unique associations between the lower order dimensions of psychopathology and volume or surface area when considered in the context of the other lower order dimensions (see Tables S13 and S14).
Discussion
The current study extends findings from adolescents, young adults, and midlife adults to suggest that general psychopathology is associated with broad, nonspecific alterations in brain structure in preadolescents (Kaczkurkin et al., 2019; Romer et al., 2020). The relationship between psychopathology and brain structure appears to be driven by general psychopathology, with few unique relationships between the lower-order dimensions and brain structure. General psychopathology has similarly been associated with non-specific aberrations in white matter microstructure (Alnæs et al., 2018; Neumann et al., 2020) and task-based functional activation (Lees et al., 2021; Shanmugan et al., 2016). Neurobiological findings converge across a variety of methods and are consistent with the genetics literature, which suggests there is little specificity in the brain structures related to general psychopathology (Lahey, Krueger, Rathouz, Waldman, & Zald, 2017; Lahey, Van Hulle, Singh, Waldman, & Rathouz, 2011; Zald & Lahey, 2017). The current study adds to the growing body of literature suggesting that broad alterations in brain structure relate non-specifically to general psychopathology and extends these previous findings to include brain structure in the critical preadolescent period when psychopathology is emerging.
In the current study, there were very few unique relationships between the lower-order dimensions of psychopathology and brain structure. This is in contrast to our previous study using the same dataset and factor analytic approach but focusing on functional connectivity and task-evoked activation (Lees et al., 2021). In this prior study, common patterns of aberrant functional connectivity and task-evoked activation throughout neurocognitive networks were identified across all dimensions of psychopathology. However, unique patterns of neurocircuitry were also observed for all lower-order dimensions, particularly with respect to externalizing pathology. Taken together, these findings indicate that during the preadolescent period functional connectivity and activation may more clearly differentiate dimensions of psychopathology when compared with indices of brain structure.
Understanding the link between brain structure and general psychopathology
These findings need to be interpreted within the context of normal cortical development, where gray matter volume and surface area decrease from late childhood, throughout adolescence into young adulthood (Tamnes et al., 2017). The lower volume and surface area found in the current study may indicate decreased cortical development throughout childhood and/or acceleration of the gray matter loss that is characteristic of normal adolescent development. While the current study suggests that brain structure is related to general psychopathology relatively early in development, the cross-sectional nature of the data precludes any comment on the causal nature of this relationship. Longitudinal data spanning early development and adolescence are needed to model the links between trajectories of psychopathology and neurobiology. Towards this end, a recent prospective study in the Dunedin cohort found that general psychopathology was associated with indices of poorer brain health and cognitive function from age 3 through to age 45 (Caspi et al., 2020). Prior evidence therefore suggests that compromised brain health is an antecedent for psychopathology, whether that be through genetics, prenatal exposures, early life stressors, or some other mechanism.
General psychopathology, cortical thickness and surface area
The current study indicates that the lower cortical volume associated with general psychopathology appears to be related to alterations in surface area, not cortical thickness. This is largely consistent with the previous study of PNC youth, where general psychopathology was associated with cortical thickness in only one of the 18 networks studied (Kaczkurkin et al., 2019). The present findings are also consistent with other large-scale studies of youth, which have consistently shown that psychopathology, operationalized in various ways, is associated with reduced cortical volume, but largely unrelated to cortical thickness (Jalbrzikowski et al., 2019; Parkes et al., 2020; Schmaal et al., 2017). In contrast, a thinner neocortex, but not surface area, was pervasively associated with psychopathology in the older Dunedin cohort (Romer et al., 2020). Discrepancies across studies may be due to the relatively lower reliability of cortical thickness when compared with measures of cortical volume and surface area (Drobinin et al., 2020). It is also possible that cortical thickness may become more closely associated with psychopathology across adolescent (and adult) development. Cortical thickness and surface area both decrease across adolescence, but the functional form of these decreases appear to differ. While surface area is marked by an increase across childhood, followed by a subtle decrease in adolescence, cortical thickness appears to decrease monotonically across childhood and adolescence (Tamnes et al., 2017). Surface area may therefore be associated with childhood psychopathology, as captured by using lifetime diagnoses in the current study, resulting in a lower peak surface area during the preadolescent period. Cortical thickness, on the other hand, shows a sharper decrease during adolescence and may be more related to interruptions from psychopathology during the later adolescent period. Analyses of future waves of the ABCD data can test whether cortical thickness becomes a more important index of psychopathology across adolescent development.
General psychopathology and cognitive functioning
The relationships between psychopathology and brain structure were robust to potentially confounding variables, including indicators of socioeconomic status (parental education and income), maternal psychopathology and cognitive function. Whilst adjusting for socioeconomic status and maternal psychopathology attenuated the relationships between psychopathology and brain structure, many of the relationships remained statistically significant. The relationships between psychopathology and brain structure were less robust to adjustment for cognitive function, particularly with respect to the relationships between psychopathology and regional cortical volume. Poorer cognitive function, particularly executive dysfunction, has been proposed as a mechanism underpinning the relationship between general psychopathology and altered brain structure (Caspi & Moffitt, 2018; Goodkind et al., 2015; Romer et al., 2018; Romer et al., 2019; Snyder et al., 2017). The current findings indicate that cognitive function may confound, or possibly mediate, the relationship between psychopathology and aberrations in gray matter structure, a hypothesis that may be investigated in future waves of ABCD data when longitudinal data will allow the modelling of causal relationships between these variables. Future research could also focus on whether the current findings with respect to cognitive function replicate in other samples and across the lifespan.
Lower order dimensions of psychopathology
In the current study, very few unique relationships between the lower-order dimensions of psychopathology and brain structure were identified. This is consistent with the previous study of the Dunedin cohort, but inconsistent with the previous study of PNC youth, where dissociable links were identified between dimensions of psychopathology and brain structure. This may be due to differences in analyses based on a higher-order model of psychopathology (in the Dunedin cohort and the current study) and the bifactor model of psychopathology (in the PNC study). In the higher-order model, the first-order factors capture the shared variance among each set of indicators. In the bifactor model, the specific factors capture what the indicators share with each other, but not with the other factors in the model. Given these differences, it is difficult to compare first-order and specific factors, and their correlates, across studies. Whether psychopathology should be modelled in a higher order or bifactor framework is a current source of debate (Greene et al., 2019; Watts, Poore, & Waldman, 2019). In the current study, the higher reliability (H coefficient) of the lower order factors (from the higher order model) when compared with the reliability of the specific factors (from the bifactor model), ultimately informed our decisión to adopt a higher order modelling framework.
Strengths and Limitations
The current study has several strengths. The ABCD study is a multisite, nationally-representative, population-based study of 9–10 year olds that is the largest of its kind to investigate brain development. This is a well-characterized cohort, with neuroimaging data and comprehensive neuropsychiatric and behavioral assessment, all of which was incorporated into the current study. The large sample size allowed the inclusion of low base rate indicators of psychopathology that are rare in the preadolescent period. The narrow age range included in this study allowed the exploration of developmentally specific relationships between detailed neurobiological indices and psychopathology during a critical developmental period when many mental disorders are emerging. Rather than factor scores, we used Bayesian plausible values to robustly estimate the full population distribution of psychopathology and model factor indeterminacy. Reliability of the externalizing, internalizing, and thought disorder lower-order dimensions was also assessed in the current study and found to be acceptable.
However, these findings need to be interpreted within the context of some limitations. ABCD data are cross-sectional at present and cannot determine whether general psychopathology is the cause or effect of lower volume and surface area. While the current study analysed cortical parcellations and subcortical segmentations using standard ABCD pipelines, future studies could explore voxel-wise analytical approaches which may be more suited to the dimensional analysis of regional differences. Those excluded from the analysis based on poor quality scans were different to those who were included in terms of total cognition scores, parental education, race/ethnicity and a diagnosis of ADHD. These systematic differences between those participants with and without structural imaging data may limit the broader applicability of these findings to certain sub-groups of the population. As an issue that affects all analyses based on ABCD structural imaging data, coordinated efforts should be undertaken to incorporate methods to increase sample representativeness, such as inverse probability or post-stratification weighting, that will allow future research to harness the full power of this rich dataset. It is also noted that the validity of Freesurfer’s automatic segmentation of subcortical structures has been questioned, especially with respect to the segmentation of the amygdala and hippocampus (Hanson et al., 2012; Morey et al., 2009). The KSADS stem questions and skip structure were preserved in the ABCD Study which precluded the use of more comprehensive dimensional measures of psychopathology based on individual symptoms or symptom counts. Unlike other measures, such as the GOASSESS used in the PNC, the KSADS was not designed for dimensional analysis in the general population. Future work may therefore focus on whether the current findings with respect to general psychopathology replicate across different instruments (i.e., the CBCL) in the ABCD Study. Finally, the baseline data from the ABCD Study only include measures of parent-reported psychopathology. Prior research comparing youth- and parent-reported psychopathology indicates that parents may over-report problem behavior when compared with youth (Salbach-Andrae, Klinkowski, Lenz, & Lehmkuhl, 2009). As future waves of the ABCD Study incorporate measures of youth-reported psychopathology, research may focus on the concordance of youth and parent reports of symptomatology and the extent to which the current findings replicate when extended to youth-reported psychopathology.
Conclusion
The current study identified lower global cortical volume and surface area as transdiagnostic biomarkers for general psychopathology in preadolescence. Studies from preadolescence to middle age converge to suggest that psychopathology is associated with broad neural features that may not be dissociable on the basis of traditional diagnostic boundaries. Future research may focus on the extent to which cognitive dysfunction may contribute to the relationship between psychopathology and brain structure, as well as longitudinal follow-up of these relationships across adolescence using the ABCD data.
Supplementary Material
Key points.
This study examined the relationships between brain structure and psychopathology during the critical preadolescent period when mental illness is emerging.
Lower global cortical volume and surface area, but not cortical thickness, were identified as transdiagnostic biomarkers of general psychopathology.
Some relationships between psychopathology and regional brain structure were attenuated when controlling for cognition, indicating that psychopathology may index cognitive dysfunction.
Acknowledgements
B.L. is supported by a National Health and Medical Research Council Postgraduate Scholarship (GNT1169377). L.M.S. is supported by the National Institutes of Health (U01 DA041093; K23 AA025399; R01 AA027399). M.K.F. is supported by a Macquarie University Research Fellowship, R.K. is supported in part by the U.S. National Institute on Aging grants R01AG053217 and U19AG051426.
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners “under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147”. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from 10.15154/1504431 (DOI). DOIs can be found at https://nda.nih.gov/study.html?id=796.
Footnotes
The authors have declared that they have no competing or potential conflicts of interest.
References
- Alnæs D, Kaufmann T, Doan NT, Córdova-Palomera A, Wang Y, Bettella F, . . . Westlye LT (2018). Association of heritable cognitive ability and psychopathology with white matter properties in children and adolescents. JAMA psychiatry, 75(3), 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, . . . Yurgelun-Todd D (2018). Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: rationale and description. Developmental cognitive neuroscience, 32, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1). [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Carragher N, Teesson M, Sunderland M, Newton N, Krueger R, Conrod P, . . . Slade T (2016). The structure of adolescent psychopathology: a symptom-level analysis. PsychologicalMedicine, 46(5), 981–994. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, . . . Workgroup, A. I. A. (2018). The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci, 32, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Ambler A, Danese A, Elliott ML, Hariri A, . . . Ramrakha S (2020). Longitudinal Assessment of Mental Health Disorders and Comorbidities Across 4 Decades Among Participants in the Dunedin Birth Cohort Study. JAMA Network Open, 3(4), e203221–e203221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, . . . Poulton R (2014). The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science, 2(2), 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, & Moffitt TE (2018). All for one and one for all: Mental disorders in one dimension. American Journal of Psychiatry, 175(9), 831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Hicks BM, Angstadt M, Rutherford S, Taxali A, Hyde LW, . . . Sripada C (2021). The General Factor of Psychopathology in the Adolescent Brain Cognitive Development (ABCD) Study: A Comparison of Alternative Modeling Approaches. Clinical Psychological Science 9 (2), 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–194. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, . . . Hyman BT (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- Drobinin V, Van Gestel H, Helmick CA, Schmidt MH, Bowen CV, & Uher R (2020). Reliability of multimodal MRI brain measures in youth at risk for mental illness. Brain and behavior, 10(6), e01609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, . . . Klaveness S (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, . . . Zahs D (2018). Recruiting the ABCD sample: Design considerations and procedures. Dev Cogn Neurosci, 32, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Scarpazza C, Dai J, He M, Xu X, Shi Y, . . . Ai Y (2019). A transdiagnostic neuroanatomical signature of psychiatric illness. Neuropsychopharmacology, 44(5), 869–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, . . . Korgaonkar MS (2015). Identification of a common neurobiological substrate for mental illness. JAMA psychiatry, 72(4), 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene AL, Eaton NR, Li K, Forbes MK, Krueger RF, Markon KE, . . . Docherty AR (2019). Are fit indices used to test psychopathology structure biased? A simulation study. Journal of abnormal psychology, 128(7), 740. [DOI] [PubMed] [Google Scholar]
- Grund S, Robitzsch A, Luedtke O, & Grund MS (2019). Package ‘mitml’.
- Hanson J, Suh J, Nacewicz B, Sutterer M, Cayo A, Stodola D, . . . Yushkevich P (2012). Robust automated amygdala segmentation via multi-atlas diffeomorphic registration. Frontiers in neuroscience, 6, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Freedman D, Hegarty CE, Mennigen E, Karlsgodt KH, Loohuis LMO, . . . Bearden CE (2019). Structural brain alterations in youth with psychosis and bipolar spectrum symptoms. Journal of the American Academy of Child & Adolescent Psychiatry, 58(11), 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Park SS, Sotiras A, Moore TM, Calkins ME, Cieslak M, . . . Cui Z (2019). Evidence for dissociable linkage of dimensions of psychopathology to brain structure in youths. American Journal of Psychiatry, 176(12), 1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher NR, Michelini G, Kotov R, & Barch DM (2021). Associations Between Resting-State Functional Connectivity and a Hierarchical Dimensional Structure of Psychopathology in Middle Childhood. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 6(5), 508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobak KA, Kratochvil C, Stanger C, & Kaufman J (2013). Computerized screening of comorbidity in adolescents with substance or psychiatric disorders. Anxiety Disorders and Depression.(La Jolaa, CA). [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, . . . Clark LA (2017). The Hierarchical Taxonomy of Psychopathology (HiTOP): a dimensional alternative to traditional nosologies. Journal of abnormal psychology, 126(4), 454. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, & Rathouz PJ (2012). Is there a general factor of prevalent psychopathology during adulthood? Journal of abnormal psychology, 121(4), 971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, & Zald DH (2017). A hierarchical causal taxonomy of psychopathology across the life span. Psychological bulletin, 143(2), 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Van Hulle CA, Singh AL, Waldman ID, & Rathouz PJ (2011). Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Archives of general psychiatry, 68(2), 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latzman RD, & DeYoung CG (2020). Using empirically-derived dimensional phenotypes to accelerate clinical neuroscience: the Hierarchical Taxonomy of Psychopathology (HiTOP) framework. Neuropsychopharmacology 45, 1083–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees B, Squeglia LM, McTeague LM, Forbes MK, Krueger RF, Sunderland M, . . . Mewton L (2021). Altered Neurocognitive Functional Connectivity and Activation Patterns Underlie Psychopathology in Preadolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 6(4), 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, & Etkin A (2017). Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. American Journal of Psychiatry, 174(7), 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Rosenberg BM, Lopez JW, Carreon DM, Huemer J, Jiang Y, . . . Etkin A (2020). Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. American Journal of Psychiatry, 177(5), 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, . . ., & Swendsen J (2010). Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication – Adolescent Supplement (NCSA). Journal of the American Academy of Child and Adolescent Psychiatry, 49(10), 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini G, Barch DM, Tian Y, Watson D, Klein DN, & Kotov R (2019). Delineating and validating higher-order dimensions of psychopathology in the Adolescent Brain Cognitive Development (ABCD) study. Translational psychiatry, 9(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings A-L, Herting MM, Meuwese R, Blakemore S-J, Crone EA, . . . Sowell ER (2016). Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. Neuroimage, 141, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberget T, Alnaes D, Kaufmann T, Doan NT, Cordova-Palomera A, Norbom LB,. . . Westlye LT (2019). Cerebellar gray matter volume is associated with cognitive function and psychopathology in adolescence. Biological psychiatry, 86(1), 65–75. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR II, Lewis DV, . . . McCarthy G (2009). A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage, 45(3), 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L, & Muthén B (2018). Mplus. The comprehensive modelling program for applied researchers: User’s guide, 5. [Google Scholar]
- Neumann A, Muetzel RL, Lahey BB, Bakermans-Kranenburg MJ, van IJzendoorn MH, Jaddoe VW, . . . Tiemeier H (2020). White Matter Microstructure and the General Psychopathology Factor in Children. Journal of the American Academy of Child & Adolescent Psychiatry, 59(11), 1285–1296. [DOI] [PubMed] [Google Scholar]
- Parkes L, Moore TM, Calkins ME, Cook PA, Cieslack M, Roalf DR, . . . Satterthwaite TD (2020). Transdiagnostic dimensions of psychopathology explain individuals’ unique deviations from normative neurodevelopment in brain structure. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Reise SP, & Haviland MG (2016). Evaluating bifactor models: calculating and interpreting statistical indices. Psychological methods, 21(2), 137. [DOI] [PubMed] [Google Scholar]
- Romer AL, Elliott ML, Knodt AR, Sison ML, Ireland D, Houts R, . . . Melzer TR (2020). Pervasively thinner neocortex as a transdiagnostic feature of general psychopathology. American Journal of Psychiatry, appi. ajp. 2020.19090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer AL, Knodt AR, Houts R, Brigidi BD, Moffitt TE, Caspi A, & Hariri AR (2018). Structural alterations within cerebellar circuitry are associated with general liability for common mental disorders. Molecular psychiatry, 23(4), 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer AL, Knodt AR, Sison ML, Ireland D, Houts R, Ramrakha S, . . . Moffitt TE (2019). Replicability of structural brain alterations associated with general psychopathology: evidence from a population-representative birth cohort. Molecular psychiatry, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salbach-Andrae H, Klinkowski N, Lenz K, & Lehmkuhl U (2009). Agreement between youth-reported and parent-reported psychopathology in a referred sample. European child & adolescent psychiatry, 18(3), 136–143. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Hibar D, Sämann P, Hall G, Baune B, Jahanshad N, . . . Ikram M (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Molecular psychiatry, 22(6), 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z, Wager TD, Mechelli A, & He Y (2019). Common dysfunction of large-scale neurocognitive networks across psychiatric disorders. Biological psychiatry, 85(5), 379–388. [DOI] [PubMed] [Google Scholar]
- Shanmugan S, Wolf DH, Calkins ME, Moore TM, Ruparel K, Hopson RD, . . . Jackson C (2016). Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. American Journal of Psychiatry, 173(5), 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Hankin BL, Sandman CA, Head K, & Davis EP (2017). Distinct patterns of reduced prefrontal and limbic gray matter volume in childhood general and internalizing psychopathology. Clinical Psychological Science, 5(6), 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Herting MM, Goddings A-L, Meuwese R, Blakemore S-J, Dahl RE, . . . Crone EA (2017). Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. Journal of Neuroscience, 37(12), 3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AL, Poore HE, & Waldman ID (2019). Riskier tests of the validity of the bifactor model of psychopathology. Clinical Psychological Science, 7(6), 1285–1303. [Google Scholar]
- Wu M (2005). The role of plausible values in large-scale surveys. Studies in Educational Evaluation, 31(2–3), 114–128. [Google Scholar]
- Zald DH, & Lahey BB (2017). Implications of the hierarchical structure of psychopathology for psychiatric neuroimaging. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(4), 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


