Abstract
Among the key metabolites produced by probiotic lactic acid bacteria (LAB), the use of gamma-aminobutyric acid (GABA), which alleviates hypertension, depression, and sleepiness in humans, is gaining popularity. Thus, GABA-producing LAB are sought after. GABA-producing LAB were preliminarily screened in acidified-MRS broth and quantified via GABase assays. The one-factor-at-a-time strategy was applied to determine the optimal conditions for GABA production. GABA production in reconstituted skim milk medium (RSM) and antibiotic susceptibility testing were performed to evaluate the potential of the strain as a yogurt starter. L. plantarum Y7 produced 4,856.86 ± 82.47 μg/mL of GABA at optimal culture conditions. Co-cultivation of Y7 and commercial Lactobacillus bulgaricus affected the amount of GABA production (6.85 ± 0.20 μg/mL) in RSM. Y7 was susceptible to ampicillin, erythromycin, and tetracycline. Therefore, L. plantarum Y7 represents a promising strain for GABA production in the food industry.
Keywords: Gamma-aminobutyric acid, Fructophilic bacterium, Lactiplantibacillus plantarum, Optimization, Yogurt starter
Introduction
Gamma-aminobutyric acid (GABA) plays a major role as an inhibitory neurotransmitter in mammalian nervous systems (Dhakal et al., 2012). It is widely used as a bioactive natural product in foods and pharmaceuticals owing to its antihypertension, diuretic, anti-depression, and sleep-inducing effects (Tsai et al., 2006). Therefore, the interest in using pure GABA and GABA-containing functional foods has steadily increased (Kim et al., 2009).
GABA production is mainly performed via fermentation using fungi, yeast, and bacteria (Dhakal et al., 2012). Among these, lactic acid bacteria (LAB), which are generally recognized as safe organisms, have been reported to synthesize a large amount of GABA compared to that produced by other microorganisms (Hwanhlem et al., 2010). In particular, Lactiplantibacillus plantarum and Levilactobacillus brevis have been investigated as the key species for GABA production (Li and Cao, 2010). Although, GABA-producing LAB have been isolated from various foods, additional functional microorganisms are sought after based on desirable strain characteristics and optimal fermentation conditions (Seo et al., 2013).
The amount of GABA synthesized by microorganisms depends on several factors including pH, temperature, concentration of glutamate and pyridoxal 5′-phosphate (PLP), and medium composition (Dhakal et al., 2012). These environmental and nutritional factors have been optimized via a one-factor-at-a-time (OFAT) strategy for improving commercial GABA production (Binh et al., 2014; Sun et al., 2010). According to several studies, Lactobacillus spp. isolated from plants lack genes encoding extracellular or cell wall-anchored proteinases (Ji et al., 2021; Wu et al., 2015). These findings suggest that plant-derived Lactobacillus spp. may not grow in milk-containing environments owing to their non-proteolytic nature (Li et al., 2019). Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus represent important starter microorganisms and are required for the production of fermented dairy foods such as yogurt (Sozzi and Smiley, 1980). Therefore, it was hypothesized that GABA-producing LAB isolated from plants can survive and produce high concentrations of GABA in co-cultures with these starters.
In this study, GABA-producing L. plantarum Y7 was isolated from kimchi. The optimal fermentation conditions, including temperature, initial pH, carbon source, nitrogen source, supplementation of L-monosodium glutamate (MSG) and PLP, were determined via the OFAT strategy. Thereafter, the potential of L. plantarum Y7 in production of GABA-containing fermented milk was evaluated via co-culturing with dairy starters in reconstituted skim milk medium (RSM).
Materials and methods
Isolation of GABA-producing LAB
Twenty varieties of Korean kimchi were collected for the isolation of wild LAB. Each sample was diluted with 0.85% (w/v) NaCl (saline). The diluted solution was filtered through filter paper (Toyo Roshi Kaisha, Ltd., Tokyo, Japan). Screening of GABA-producing LAB was performed as described previously (Wu and Shah, 2015), with slight modifications. Briefly, the diluted samples were centrifuged at 6,000 × g for 10 min. The bacterial pellet was resuspended in MRS broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) containing 50 mM MSG (pH 6.5; Sigma-Aldrich, St. Louis, MO, USA) and incubated at 37 °C for 3 h. Thereafter, the pH of the culture medium was adjusted to 4.0, and the medium was incubated at 37 °C for 3 h. Bromocresol purple (BCP)-MRS agar plates containing 0.02% (w/v) sodium azide (NaN3; Sigma-Aldrich) were inoculated with each diluted solution and incubated at 37 °C for 24 ± 3 h. Colonies showing yellowish circles were picked and incubated in MRS containing 50 mM MSG (pH 6.5) at 37 °C for 24 h.
GABase assay
Putative GABA-producing LAB isolates were quantified via a spectrophotometry assay using GABase (GABA‐aminotransferase + succinic semialdehyde dehydrogenase; Sigma-Aldrich). Culture supernatants were treated with Carrez reagents (BioVision Inc., Milpitas, CA, USA) for 30 min. The test mixture comprised 2.3 mL of 100 mM potassium pyrophosphate buffer, 0.1 mL of 100 mM 2-mercaptoethanol solution, 0.15 mL of 25 mM β-NADP and 100 mM α-ketoglutarate solution, and 0.3 mL of sample solution. Subsequently, the enzyme solution was added to each cuvette. The cuvettes were incubated at 25 °C for 1 h. GABA concentration was determined at 340 nm via spectrophotometry (Multiskan Sky; Thermo Fisher Scientific, Waltham, MA, USA).
Bacterial strains and culture conditions
Lactiplantibacillus plantarum Y7, an LAB producing a high concentration of GABA isolated from kimchi, S. thermophilus KCCM 40430, and L. bulgaricus isolated from a commercial dairy product (Binggrae, Namyangju-si, Gyeonggi-do, Republic of Korea) were used in this study. The Lactobacillus strains and S. thermophilus were cultivated in MRS broth and M17 broth, respectively. Each culture (approximately 107–108 CFU/mL) of LAB was inoculated into MRS broth or RSM for determination of GABA production.
16S rRNA gene sequencing
DNA sequencing was performed by Cosmogentech (Seoul, Republic of Korea). Sequencing reactions were performed using a DNA analyzer (model 3730xl; Applied Biosystems, Waltham, MA, USA). Sequence comparisons were performed using Basic Local Alignment Search Tool (BLAST) available at the National Center for Biotechnology Information (NCBI; National Institutes of Health, Bethesda, MD, USA). Similarity of the type strain was confirmed via analysis using the Ezbiocloud database (Yoon et al., 2017). Sequence alignments and editing were performed using BioEdit (Ibis Biosciences, Carlsbad, CA, USA).
HPLC
GABA production was analyzed using the automated pre-column ortho-phthalaldehyde-derivatization method (Winspear and Oaks, 1983). The automated injection program for GABA analysis was performed as described by Agilent Technologies (Santa Clara, CA, USA) with slight modifications. Briefly, mobile phase A contained 10 mM Na2HPO4, 10 mM Na2B4O7, and 5 mM NaN3, and mobile phase B contained acetonitrile:methanol:water (45:45:10, v:v:v). The solutions were filtered through a 0.45 μm membrane via vacuum filtration. The bubble decay process was performed using an ultra-sonicator (JAC-1505, 40 kHz; Kodo, Hwaseong-si, Republic of Korea) for 1 h (Bartolomeo and Maisano, 2006). The gradient conditions were: 2% mobile phase B from 0 min to 0.35 min, 100% mobile phase B from 13.4 min to 15.7 min, and 2% mobile phase B from 15.8 min to 18 min. GABA analysis was performed using Agilent 1260 Infinity HPLC. A Poroshell120 HPH-C18 (4.6 mm × 150 mm × 4 μm; Agilent technologies) was used during the analysis.
OFAT-based culture optimization for GABA production
The OFAT strategy was applied to determine the optimal carbon and nitrogen source. The carbon source present in the defined MRS medium (2% (w/v) glucose) was replaced with 2% of other carbon sources (glucose, mannitol, maltose, lactose, lactulose, galactose, fructose, xylose, and sucrose). In the same manner, nitrogen sources (1% proteose peptone No. 3 and 1% beef extract) present in the defined MRS medium were replaced with 2% of various nitrogen sources (control, peptone, soytone, malt extract, beef extract, proteose peptone No. 3, and tryptone). Other parameters were individually optimized by altering their ranges, such as MSG concentration (25–200 mM), PLP concentration (0–100 μM), initial pH (3.5–9.5), and co-culturing with bacterial starters for dairy fermentation.
Antibiotic susceptibility testing
Antibiotic susceptibility was determined using a modification of the agar overlay diffusion method described by the National Committee for Clinical Laboratory Standards (Hecht et al., 2007). MRS plates containing 1.5% (w/v) agar were overlaid with 4 mL of soft agar inoculated with 200 μL of an active culture. The plates were solidified at room temperature (25 °C) for 10 min prior to dispensing antibiotic-containing discs (Becton, Dickinson and Company). Subsequently, the plates were anaerobically incubated at 37 °C for 24 h. Inhibition zone diameters were measured using sliding calipers, and the results are presented in terms of resistance, moderate susceptibility, or susceptibility, according to interpretative standards. The precision and accuracy of the antimicrobial susceptibility test were monitored using Escherichia coli ATCC 25922 as a control (Lorian, 2005).
Sequence accession number
The 16S rRNA gene sequence of L. plantarum Y7 (accession no. OL587486) is available on GenBank (NCBI).
Statistical analysis
Data are presented as the mean ± standard deviation in bar charts. A significant difference (p < 0.01) among the group was evaluated via one-way analysis of variance and Duncan’s multiple range tests or independent sample t-test using IBM SPSS Statistics 25 software (IBM Corp., Armonk, NY, USA).
Results and discussion
Isolation and 16S rRNA gene identification of GABA-producing LAB
LAB strains were isolated from 20 varieties of kimchi as potential GABA producers. The isolates exhibited different colony morphologies on BCP-MRS agar plates. In total, 500 colonies were obtained. The glutamate-GABA system is known to function as an acid-resistance system that supports cell survival and metabolic activity in LAB during acidic conditions (De Biase and Pennacchietti, 2012; Teixeira et al., 2014) This system protects bacterial cells exposed to a pH ≤ 2.5 for a few hours (Lin et al., 1996). GABA-producing bacteria may survive after exposure to acidic conditions by reducing proton (H+) and producing CO2. Based on this acid-maintenance, the 500 colonies that showed growth in acidified-MRS broth were considered as GABA-producing LAB. Thereafter, we performed a spectrophotometry assay using GABase enzyme to screen for the isolate that produced the largest amount of GABA. Although thin-layer chromatography is generally used to screen GABA-producing LAB (Kanklai et al., 2021), the GABase assay is a useful method for determining GABA content because of its ease and speed (Saito et al., 2008).
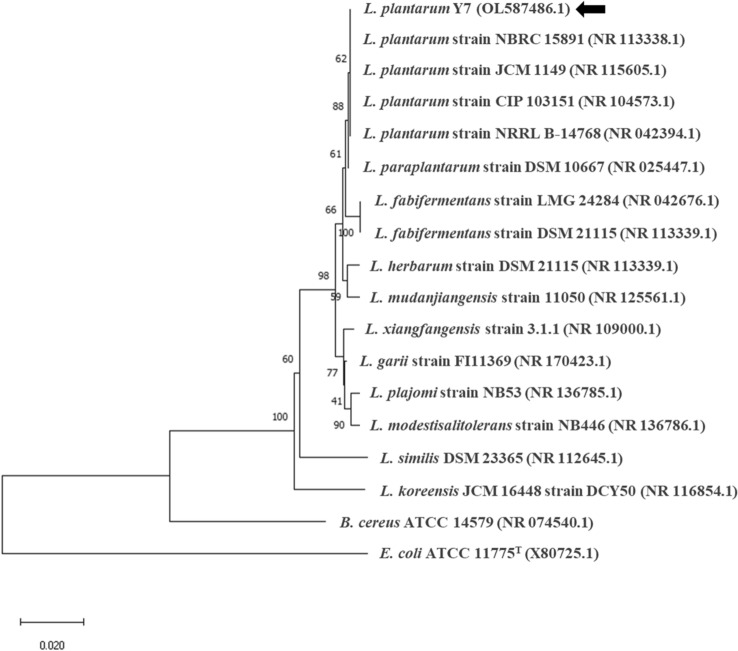
Among the 500 colonies, 8 isolates produced the highest GABA content (Fig. 1). GABA produced by the selected 8 isolates was within in the range of 11.84 ± 1.15 to 401.49 ± 8.98 μg/mL. Among the isolates, isolate Y7 produced the highest GABA content at 401.49 ± 8.98 μg/mL and was selected for further experiments. Isolate Y7 was identified via 16S rRNA gene sequencing analysis using the BLAST search program and universal primers 27F and 1492R (Weisburg et al., 1991). A phylogenetic tree containing Y7 and 17 other standard strains of the Lactobacillaceae family is shown in Fig. 2. Bacillus cereus ATCC 14579 and E. coli ATCC 11775 served as the outgroups. Isolate Y7 was identified as L. plantarum.
Fig. 1.
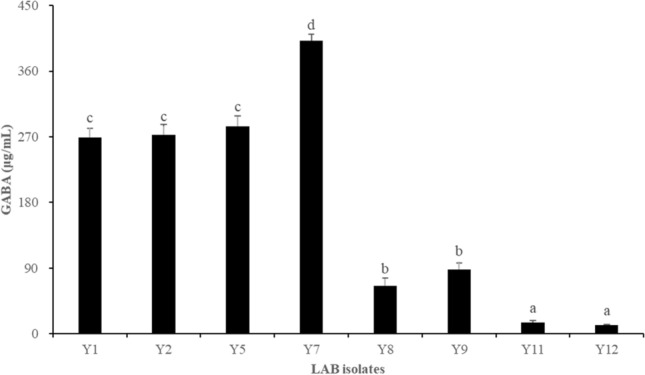
Concentration of GABA produced by LAB isolates in modified-MRS broth supplemented with 50 mM MSG at 37 °C for 48 h. GABA concentration was determined using the GABase assay. GABA, gamma-aminobutyric acid; LAB, lactic acid bacteria; MSG, monosodium glutamate
Fig. 2.
Phylogenetic tree of Lactiplantibacillus plantarum Y7 and other related strains based on 16S rRNA gene sequences. The tree was generated using the Neighbor-joining method with bootstrap trials of 1,000. The arrow indicates the position of L. plantarum Y7 in the tree. The bar represents 0.02 substitutions per nucleotide position
GABA production in modified-MRS medium
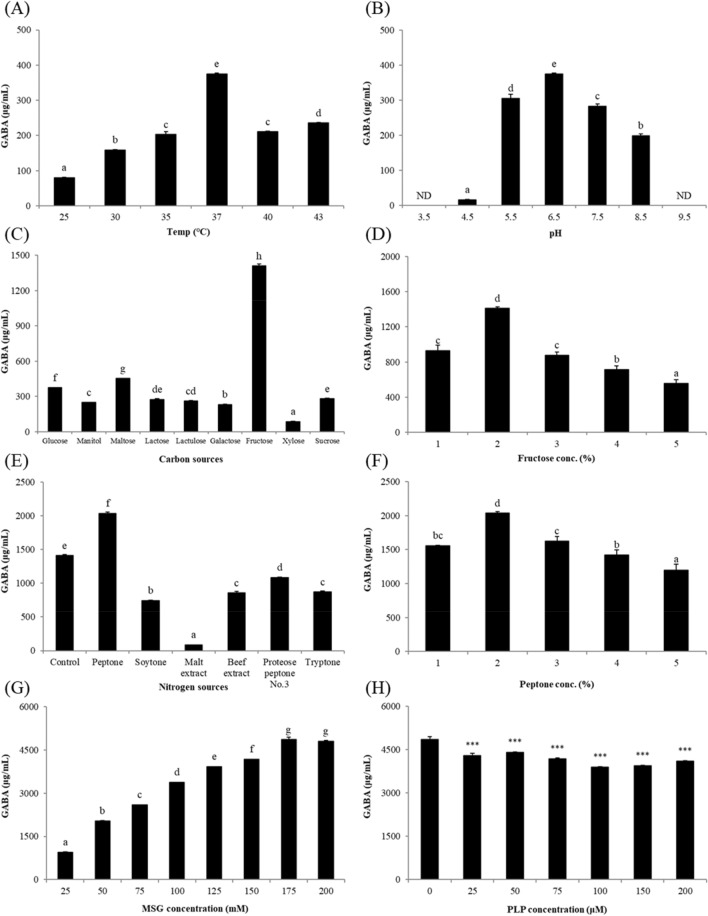
The OFAT strategy was applied to determine the optimal culture conditions for GABA production. The effect of temperature on GABA production was investigated in modified-MRS medium containing 50 mM MSG at 25 °C, 30 °C, 35 °C, 40 °C, and 43 °C. The optimal temperature for GABA production was determined at 37 °C (375.29 ± 2.60 μg/mL, Fig. 3A). GABA production considerably decreased at temperatures lower than 30 °C (79.86 ± 1.43 μg/mL). L. plantarum is a mesophilic bacterium and has an optimal growth temperature of approximately 37 °C (Matejcekova et al., 2019). Microbial biosynthesis of GABA is regulated by acidity (Li et al., 2010). Therefore, the influence of initial pH on GABA production was investigated using the aforementioned medium at pH 3.5, 4.5, 5.5, 6.5, 7.5, 8.5, and 9.5. Investigation of the initial pH revealed that GABA was produced within a pH range between 4.5–8.5, suggesting that the optimal initial pH for GABA production was 6.5 (Fig. 3B).
Fig. 3.
Concentration of GABA produced by Lactiplantibacillus plantarum Y7 in modified-MRS broth. (A) The optimal temperature was determined at various temperatures (25 °C, 30 °C, 35 °C, 37 °C, 40 °C, and 43 °C) supplemented with 50 mM MSG. (B) The optimal initial pH was investigated at different pH conditions (pH 3.5–9.5) supplemented with 50 mM MSG at 37 °C. (C) The optimal carbon
source was determined using 9 different carbon sources (glucose, mannitol, maltose, lactose, lactulose, galactose, fructose, xylose, and sucrose) supplemented with 50 mM MSG at 37 °C and pH 6.5. (D) Optimal fructose concentration was determined at the concentration range of 1–5% supplemented with 50 mM MSG at 37 °C and pH 6.5. (E) The optimal nitrogen source was investigated using 7 different nitrogen sources (control, peptone, soytone, malt extract, beef extract, proteose peptone No. 3, and tryptone) supplemented with 50 mM MSG and 2% fructose at 37 °C and pH 6.5. (F) Optimum peptone concentration was investigated within the range of 1–5% supplemented with 50 mM MSG and 2% fructose at 37 °C and pH 6.5. (G) Effect of MSG supplementation was evaluated under various concentrations (25 mM, 50 mM, 75 mM, 100 mM, 125 mM, 150 mM, 175 mM, and 200 mM) substituted with 2% fructose and 2% peptone at 37 °C and pH 6.5. (H) Effect of PLP supplementation was performed at various concentrations (0 μM, 25 μM, 50 μM, 75 μM, 100 μM, 150 μM, and 200 μM) supplemented with 175 mM MSG, 2% fructose, and 2% peptone at 37 °C and pH 6.5. MSG, monosodium glutamate; PLP, pyridoxal 5′-phosphate
To optimize carbon and nitrogen sources, the effect of carbon source on GABA production by L. plantarum Y7 was first evaluated in modified-MRS medium containing 2% of each carbon source and 50 mM MSG at 37 °C (pH 6.5). Fructose was determined as the best carbon source for GABA production (1,409.62 ± 17.54 μg/mL), and GABA production was slightly increased in maltose-containing medium (452.80 ± 2.48 μg/mL) compared to that in glucose-containing medium (375.29 ± 2.60 μg/mL), which represents the commercial MRS medium. In contrast, mannitol, lactose, lactulose, galactose, xylose, and sucrose were associated with lower GABA production than that obtained using glucose (Fig. 3C). The optimal fructose concentration for GABA production was determined as 2% (Fig. 3D). The metabolism of glucose affects rapid bacterial growth compared to that obtained via metabolism of other simple carbohydrates; the accumulated active bacterial cells contribute to increased secretion of GAD, which converts glutamate into GABA (Hussin et al., 2021). However, GABA production by L. plantarum Y7 was considerably higher in fructose-containing medium than that in glucose-based medium. Previous studies have reported that certain LAB strains prefer other carbohydrates over glucose as a carbon source: 4% sucrose for Lactobacillus sakei B2-16 (Kook et al., 2010), 3% sucrose for L. brevis 340G (Seo et al., 2013), and 2% maltose for L. brevis HYE1 (Lim et al., 2017). In addition, because the growth of L. plantarum FPL was the highest in fructose-based medium, it was considered to have fructophilic properties (Gustaw et al., 2018). Similarly, L. plantarum Y7 can be regarded as a fructophilic bacterium.
The effect of nitrogen source on GABA production was evaluated in modified-MRS medium containing 2% fructose as a carbon source, 50 mM MSG, and 2% of each nitrogen compound. Among the 7 nitrogen sources tested (including control), peptone was determined as the best nitrogen source for GABA production (2,036.44 ± 20.71 μg/mL), whereas soytone, malt extract, beef extract, proteose peptone No. 3, and tryptone were associated with lower GABA content than that obtained using the control mixture of the commercial MRS medium (Fig. 3E). The optimal peptone concentration for GABA production was determined as 2% (Fig. 3F). Contrary to our findings, previous studies have reported yeast extract as an optimal nitrogen source (Binh et al., 2014; Kook et al., 2010; Seo et al., 2013). Therefore, the optimum carbon and nitrogen sources for GABA production by L. plantarum Y7 were determined as 2% fructose and 2% peptone, respectively.
The effect of MSG and PLP, which is considered a co-factor of glutamate decarboxylase (GAD) enzyme in GABA production, was evaluated in the optimized culture medium (modified-MRS medium containing 2% fructose and 2% peptone) under identical culture conditions (37 °C, pH 6.5, and 48 h). Maximum GABA production was obtained on addition of 175 mM MSG (4,856.86 ± 82.47 μg/mL, Fig. 3G). Therefore, optimizing the culture conditions via the OFAT strategy increased GABA production by 10 times. In contrast, GABA production slightly decreased upon addition of PLP (Fig. 3H). In a recent study, the addition of PLP did not increase GABA synthesis by L. brevis 15f (Yunes et al., 2016). Although the stable binding of the apoenzyme to its co-factor might influence the synthesis, further studies are needed.
GABA production in RSM
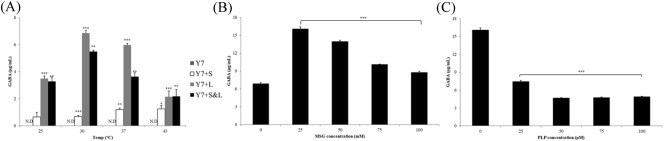
To investigate the potential of L. plantarum Y7 as a starter for producing GABA-containing fermented milk, Y7 was cultivated in RSM at 37 °C for 48 h. However, GABA production was not detected at any temperature. L. plantarum is a well-known GABA producer, which may not be able to ferment milk due to its poor proteolytic nature (Daeschel et al., 1987). Therefore, L. plantarum Y7 was co-cultured with conventional dairy starters, S. thermophilus and L. bulgaricus, which show proteolysis activity, to improve cell viability and GABA production. The commercial L. bulgaricus promoted GABA production by L. plantarum Y7 (6.85 ± 0.20 μg/mL) at 30 °C (Fig. 4). In contrast, the co-cultures of L. plantarum Y7 with S. thermophilus KCCM 40430 or both dairy starters showed low GABA production.
Fig. 4.
Concentration of GABA produced by L. plantarum Y7 co-cultured with dairy starters in RSM. S, Streptococcus thermophilus KCCM 40430; L, commercial Lactobacillus bulgaricus. (A) The optimal temperature and co-cultivation conditions were determined under various conditions (25 °C, 30 °C, 37 °C, and 43 °C). (B) Effect of MSG concentration was investigated at various concentrations (0 mM (control), 25 mM, 50 mM, 75 mM, and 100 mM). (C) Effect of PLP concentration was determined under various concentrations (0 μM (control), 25 μM, 50 μM, 75 μM, and 100 μM). RSM, reconstituted skim milk; MSG, monosodium glutamate; PLP, pyridoxal 5′-phosphate
To determine the effect of additives related to enhancing GABA production, MSG and PLP were added to RSM. GABA production by L. plantarum Y7 in co-culture with L. bulgaricus isolate was increased in RSM containing 25 mM MSG (16.06 ± 0.34 μg/mL). However, the GABA production in RSM was slightly decreased upon addition of PLP. Although, co-culturing dairy starters can improve GABA production by L. plantarum Y7, the concentration of GABA was still low. A previous study reported that chloride ions influence GABA production by Lactococcus lactis NCDO 2118 exposed to acidic stress (Laroute et al., 2021). Hence, further studies are needed on the optimization of culture conditions and co-culturing with various strains of dairy starters.
A previous study reported that yeast produce amino acids that enable the survival of LAB (Ponomarova et al., 2017). Therefore, Y7 was co-cultured with yeast in RSM to investigate the symbiotic relation of yeast and LAB in a variety of naturally fermented foods such as kefir. However, GABA was not detected in any culture containing yeast strains in RSM (data not shown).
Antibiotic susceptibility
Antibiotic susceptibility is a key factor associated with the safety of using probiotic bacteria. Therefore, the antibiotic susceptibility of L. plantarum Y7 was evaluated based on the diameter of the zone of inhibition in the disc diffusion test (Table 1). L. plantarum Y7 was susceptible to 3 of the 5 different antibiotics tested with the inhibition zone ranging from 17.0 ± 0.5 to 20.7 ± 0.3 mm; Y7 was resistant to streptomycin and colistin. The diameters of inhibition zones of ampicillin, erythromycin, and tetracycline were 20.3 ± 4.0 mm, 20.7 ± 0.3 mm, and 17.0 ± 0.5 mm, respectively.
Table 1.
Antibiotic resistance of Lactiplantibacillus plantarum Y7
| Antibiotics | Interpretative zone diameter (mm) | Results | |||
|---|---|---|---|---|---|
| R | MS | S | |||
| Streptomycin | ≤ 11 | 12–14 | ≥ 15 | – | R |
| Erythromycin | ≤ 13 | 14–17 | ≥ 18 | 20.7 ± 0.3 | S |
| Colistin | ≤ 8 | 9–10 | ≥ 11 | – | R |
| Tetracyclin | ≤ 14 | 15–18 | ≥ 19 | 17.0 ± 0.5 | MS |
| Ampicillin | ≤ 12 | 13–15 | ≥ 16 | 20.3 ± 4.0 | S |
The National Committee for Clinical Laboratory Standards guidelines was applied
*Susceptibility is expressed as R (resistant), MS (moderately susceptible), or S (susceptible). Results are presented as mean ± standard deviation (n = 3)
In this study, L. plantarum Y7 was susceptible to ampicillin, erythromycin, and tetracycline, but was resistant to streptomycin and colistin. These results are consistent with previous findings, where Lactobacilli have been found to be most sensitive to ampicillin, erythromycin, and tetracycline (Kaktcham et al., 2012). Regarding the resistance of L. plantarum Y7 to streptomycin and colistin, intrinsic resistance to aminoglycosides such as streptomycin has been reported as a general feature of Lactobacilli owing to aminoglycoside-modifying enzymes (Danielsen and Wind, 2003). Moreover, gram-positive bacteria are intrinsically resistant to polymyxins owing to the absence of an outer membrane in which lipopolysaccharide binds to colistin (Xiong et al., 2005).
In summary, we isolated the GABA-producing LAB strain L. plantarum Y7 from kimchi and optimized GABA production under various conditions in this study. For enabling efficient and practical use, further studies are required which investigate the increase in GABA production in RSM or investigate the in vivo physiological effects of GABA produced by L. plantarum Y7. Such follow-up studies on L. plantarum Y7 may facilitate the industrial production of GABA-containing foods.
Acknowledgements
This work was supported by a grant from the Commercializations Promotion Agency for R&D Outcomes (COMPA) funded by the Ministry of Science and ICT, Republic of Korea (Project Number: 1711150496)
Declarations
Conflict of interest
The authors declare no conflict of interest.
Human and animal rights
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jaegon Kim, Email: kjgyap1022@yonsei.ac.kr.
Yong-Won Yoon, Email: y2036591@naver.com.
Min-Sun Kim, Email: alstjs98@yonsei.ac.kr.
Myung-Hyun Lee, Email: ynh1999@yonsei.ac.kr.
Geun-Ah Kim, Email: kkiisskga@naver.com.
Kiho Bae, Email: kbae@yonsei.ac.kr.
Sung-Sik Yoon, Email: sungsik@yonsei.ac.kr.
References
- Bartolomeo MP, Maisano F. Validation of a reversed-phase HPLC method for quantitative amino acid analysis. Journal of Biomolecular Techniques. 2006;17:131–137. [PMC free article] [PubMed] [Google Scholar]
- Binh TT, Ju WT, Jung WJ, Park RD. Optimization of gamma-amino butyric acid production in a newly isolated Lactobacillus brevis. Biotechnology Letters. 2014;36:93–98. doi: 10.1007/s10529-013-1326-z. [DOI] [PubMed] [Google Scholar]
- Daeschel M, Andersson RE, Fleming H. Microbial ecology of fermenting plant materials. FEMS Microbiology Reviews. 1987;3:357–367. doi: 10.1111/j.1574-6968.1987.tb02472.x. [DOI] [Google Scholar]
- Danielsen M, Wind A. Susceptibility of Lactobacillus spp. to antimicrobial agents. International Journal of Food Microbiology. 2003;82:1–11. doi: 10.1016/S0168-1605(02)00254-4. [DOI] [PubMed] [Google Scholar]
- De Biase D, Pennacchietti E. Glutamate decarboxylase-dependent acid resistance in orally acquired bacteria: Function, distribution and biomedical implications of the gadBC operon. Molecular Microbiology. 2012;86:770–786. doi: 10.1111/mmi.12020. [DOI] [PubMed] [Google Scholar]
- Dhakal R, Bajpai VK, Baek KH. Production of GABA (gamma-Aminobutyric acid) by microorganisms: A review. Brazilian Journal of Microbiology. 2012;43:1230–1241. doi: 10.1590/S1517-83822012000400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustaw K, Michalak M, Polak-Berecka M, Wasko A. Isolation and characterization of a new fructophilic Lactobacillus plantarum FPL strain from honeydew. Annals of Microbiology. 2018;68:459–470. doi: 10.1007/s13213-018-1350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht DW, Citron DM, Cox M, Jacobus N, Jenkins S, Onderdonk A, Wexler H. Methods for antimicrobial susceptibility testing of anaerobic bacteria: Approved Standard. Clinical and Laboratory Standards Institute, Wayne, PA, USA (2007)
- Hussin FS, Chay SY, Hussin ASM, Ibadullah WZW, Muhialdin BJ, Abd Ghani MS, Saari N. GABA enhancement by simple carbohydrates in yoghurt fermented using novel, self-cloned Lactobacillus plantarum Taj-Apis362 and metabolomics profiling. Scientific Reports. 2021;11:1–12. doi: 10.1038/s41598-021-88436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwanhlem N, Watthanasakphuban N, Riebroy S, Benjakul S, Kittikun AH, Maneerat S. Probiotic lactic acid bacteria from Kung-Som: Isolation, screening, inhibition of pathogenic bacteria. International Journal of Food Science and Technology. 2010;45:594–601. doi: 10.1111/j.1365-2621.2010.02172.x. [DOI] [Google Scholar]
- Ji D, Ma J, Xu M, Agyei D. Cell-envelope proteinases from lactic acid bacteria: Biochemical features and biotechnological applications. Comprehensive Reviews in Food Science and Food Safety. 2021;20:369–400. doi: 10.1111/1541-4337.12676. [DOI] [PubMed] [Google Scholar]
- Kaktcham PM, Zambou NF, Tchouanguep FM, El-Soda M, Choudhary MI. Antimicrobial and safety properties of lactobacilli isolated from two Cameroonian traditional fermented foods. Scientia Pharmaceutica. 2012;80:189–204. doi: 10.3797/scipharm.1107-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanklai J, Somwong TC, Rungsirivanich P, Thongwai N. Screening of GABA-producing lactic acid bacteria from Thai fermented foods and probiotic potential of Levilactobacillus brevis F064A for GABA-fermented mulberry juice production. Microorganisms. 2021;9:33. doi: 10.3390/microorganisms9010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Lee MY, Ji GE, Lee YS, Hwang KT. Production of gamma-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. International Journal of Food Microbiology. 2009;130:12–16. doi: 10.1016/j.ijfoodmicro.2008.12.028. [DOI] [PubMed] [Google Scholar]
- Kook M-C, Seo M-J, Cheigh C-I, Pyun Y-R, Cho S-C, Park H. Enhanced production of gamma-aminobutyric acid using rice bran extracts by Lactobacillus sakei B2–16. Journal of Microbiology and Biotechnology. 2010;20:763–766. [PubMed] [Google Scholar]
- Laroute V, Mazzoli R, Loubiere P, Pessione E, Cocaign-Bousquet M. Environmental Conditions Affecting GABA Production in Lactococcus lactis NCDO 2118. Microorganisms. 9 (2021) [DOI] [PMC free article] [PubMed]
- Li H, Cao Y. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids. 2010;39:1107–1116. doi: 10.1007/s00726-010-0582-7. [DOI] [PubMed] [Google Scholar]
- Li HX, Qiu T, Huang GD, Cao YS. Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microbial Cell Factories. 9 (2010) [DOI] [PMC free article] [PubMed]
- Li S, Tang S, He Q, Hu J, Zheng J. Changes in proteolysis in fermented milk produced by Streptococcus thermophilus in Co-culture with Lactobacillus plantarum or Bifidobacterium animalis subsp. lactis during refrigerated storage. Molecules. 2019;24:3699. doi: 10.3390/molecules24203699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HS, Cha IT, Roh SW, Shin HH, Seo MJ. Enhanced production of gamma-aminobutyric acid by optimizing culture conditions of Lactobacillus brevis HYE1 isolated from Kimchi, a Korean fermented food. Journal of Microbiology and Biotechnology. 2017;27:450–459. doi: 10.4014/jmb.1610.10008. [DOI] [PubMed] [Google Scholar]
- Lin J, Smith MP, Chapin KC, Baik HS, Bennett GN, Foster JW. Mechanisms of acid resistance in enterohemorrhagic Escherichia coli. Applied and Environmental Microbiology. 1996;62:3094–3100. doi: 10.1128/aem.62.9.3094-3100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorian V. Antibiotics in laboratory medicine. Lippincott Williams & Wilkins (2005)
- Matejcekova Z, Spodniakova S, Dujmic E, Liptakova D, Valik L. Modelling growth of Lactobacillus plantarum as a function of temperature: Effects of media. Journal of Food and Nutrition Research. 2019;58:125–134. [Google Scholar]
- Ponomarova O, Gabrielli N, Sevin DC, Mulleder M, Zirngibl K, Bulyha K, Andrejev S, Kafkia E, Typas A, Sauer U, Ralser M, Patil KR. Yeast creates a niche for symbiotic lactic acid bacteria through nitrogen overflow. Cell Systems. 2017;5:345–357 e6. doi: 10.1016/j.cels.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Matsukura C, Sugiyama M, Watahiki A, Ohshima I, Iijima Y, Konishi C, Fujii T, Inai S, Fukuda N. Screening for γ-aminobutyric acid (GABA)-rich tomato varieties. Journal of the Japanese Society for Horticultural Science. 2008;77:242–250. doi: 10.2503/jjshs1.77.242. [DOI] [Google Scholar]
- Seo M-J, Lee J-Y, Nam Y-D, Lee S-Y, Park S-L, Yi S-H, Lee M-H, Roh SW, Choi H-J, Lim S-I. Production of γ-aminobutyric acid by Lactobacillus brevis 340G isolated from kimchi and its application to skim milk. Korean Society for Food Engineering. 2013;17:418–423. [Google Scholar]
- Sozzi T, Smiley MB. Antibiotic resistances of yogurt starter cultures Streptococcus thermophilus and Lactobacillus bulgaricus. Applied and Environmental Microbiology. 1980;40:862–865. doi: 10.1128/aem.40.5.862-865.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li T, Yan J, Liu J. Technology optimization for polysaccharides (POP) extraction from the fruiting bodies of Pleurotus ostreatus by Box-Behnken statistical design. Carbohydrate Polymers. 2010;80:242–247. doi: 10.1016/j.carbpol.2009.11.018. [DOI] [Google Scholar]
- Teixeira JS, Seeras A, Sanchez-Maldonado AF, Zhang C, Su MS, Ganzle MG. Glutamine, glutamate, and arginine-based acid resistance in Lactobacillus reuteri. Food Microbiology. 2014;42:172–180. doi: 10.1016/j.fm.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Tsai J-S, Lin Y, Pan B, Chen T. Antihypertensive peptides and γ-aminobutyric acid from prozyme 6 facilitated lactic acid bacteria fermentation of soymilk. Process Biochemistry. 2006;41:1282–1288. doi: 10.1016/j.procbio.2005.12.026. [DOI] [Google Scholar]
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winspear MJ, Oaks A. Automated pre-column amino acid analyses by reversed-phase high-performance liquid chromatography. Journal of Chromatography a. 1983;270:378–382. doi: 10.1016/S0021-9673(01)96389-7. [DOI] [Google Scholar]
- Wu Q, Law YS, Shah NP. Dairy Streptococcus thermophilus improves cell viability of Lactobacillus brevis NPS-QW-145 and its gamma-aminobutyric acid biosynthesis ability in milk. Scientific Reports. 2015;5:12885. doi: 10.1038/srep12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu QL, Shah NP. Gas release-based prescreening combined with reversed-phase HPLC quantitation for efficient selection of high-gamma-aminobutyric acid (GABA)-producing lactic acid bacteria. Journal of Dairy Science. 2015;98:790–797. doi: 10.3168/jds.2014-8808. [DOI] [PubMed] [Google Scholar]
- Xiong YQ, Mukhopadhyay K, Yeaman MR, Adler-Moore J, Bayer AS. Functional interrelationships between cell membrane and cell wall in antimicrobial peptide-mediated killing of Staphylococcus aureus. Antimicrobial Agents and Chemotherapy. 2005;49:3114–3121. doi: 10.1128/AAC.49.8.3114-3121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. International Journal of Systematic and Evolutionary Microbiology. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunes RA, Poluektova EU, Dyachkova MS, Klimina KM, Kovtun AS, Averina OV, Orlova VS, Danilenko VN. GABA production and structure of gadB/gadC genes in Lactobacillus and Bifidobacterium strains from human microbiota. Anaerobe. 2016;42:197–204. doi: 10.1016/j.anaerobe.2016.10.011. [DOI] [PubMed] [Google Scholar]