Abstract
Abstract
Nectarine fruit is highly perishable due to its high moisture content (89%) and susceptibility to decay. Continuous degradation in quality attributes due to physiological responses and ripening result ultimately in post-harvest losses. Drying of fruit offers the possibility to minimize losses and add value to fresh produce. Thus, this study evaluated the impacts of sodium metabisulphite (SMB; 10 g/kg) and characterized the influence of hot air (50 °C) drying on the kinetics, fruit tissue microstructure, and the physicochemical properties of dried 'Sunectwentyone' nectarines (Super star®). Out of the nine mathematical models, Logarithmic and Henderson, and Pabis models were the most suitable to predict the drying behaviour of sliced nectarines (R2 = 0.94). Based on the microstructural analysis, prolonged drying led to higher tissue displacement/disruption in dehydrated nectarine slices. Results showed that SMB treatment was more effective in maintaining both the freshness and the color of 'Sunectwentyone' nectarine than the untreated.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-022-01039-6.
Keywords: Fruit tissue deformation, Moisture content, Convective drying, Non-enzymatic browning, Prunus persica L
Introduction
Nectarines (Prunus persica L. Batsch) belong to the Prunus genus and the Rosaceae family (Wills et al., 1983). It is rich in vitamins, phenols, flavonoids and anthocyanins (Durst and Weaver, 2013; Zhao et al., 2018). Nectarine is a climacteric fruit due to its ethylene production and cellular respiration, which increase during the ripening stage, affecting the biochemical compounds and limiting their storage life (Cano-Salazar et al., 2013; Mathooko et al., 2001; Zhang et al., 2017). Post-harvest techniques such as dehydration and drying have been used to extend the shelf life and availability of the fruit during off-season. Drying is a low‐cost method in fruit processing to generate new products, minimize packaging requirements, reduce shipping weights, prevent post-harvest losses and extend produce shelf life by removing water content and reducing water activity (Le and Konsue, 2021) and inhibits microbial growth in fruit (Macedo et al., 2021).
Several drying methods have been proposed for the preservation of nectarine fruit quality. This includes sun drying, which depends on the climate and requires an average of 23 h for nectarines to dry (İsmail et al., 2016). Near infrared-vacuum drying systems present undesirable effects as it causes rapid and direct heat concentration on the nectarines slices (Alaei et al., 2015). Another drying technology reported for nectarines includes convective drying, also called hot air drying (Hiranvarachat et al., 2011). This conventional (hot air) method is the most widely used in the food industry since it is inexpensive and easy to operate due to its simplicity (Hiranvarachat et al., 2011). Hot air drying influences physical properties such as texture, color, nutritional content, and shape of the fruit (Hiranvarachat et al., 2011; Macedo et al., 2021). During the drying process, the air temperature is one of the principal parameters that influences the dried product's physicochemical and drying kinetic properties (Lee et al., 2013). Consequently, the physicochemical changes can lead to undesired characteristics in the products, such as a decrease in the of nutrients in the fruit and changing their organoleptic properties. Nevertheless, adapting suitable drying temperature conditions could lead to high-quality products with better nutritional value and extended shelf-life (Treybal, 1995).
During the drying process, removing water from the fruit tissue leads to a disturbed balance of forces and microstructural deformation. The tissue structure of the dried fruit is changed due to micro-contractions, leading to an increase in wrinkling, warping and porosity (Aral and Beşe, 2016), as well as modified surface topology (Ashtiani et al., 2018). An approach to describe the macroscopic and microscopic mechanisms of heat and mass transfer and to predict the behaviour of food material during the drying process is to analyze the drying kinetics (Lee and Zuo, 2013). A key advantage of drying kinetics is to mathematically model and optimize the fruit drying process to provide the most suitable working conditions during the drying phase (Aral and Beşe, 2016). Drying rate is one of the most important factors to be measured to predict the fruit's drying time (Lee and Zuo, 2013). During the drying process of fruit, water leaves the cell by creating a pressure on the cell, which leads to a decrease in the tension exerted on the cell wall, hence the shrinkage of the sample (Araujo et al., 2004). The quantification of this phenomenon enables the analysis of the drying kinetics and the drying process.
Furthermore, the color quality of dried fruit is an important attribute and this qualitative factor influences customers (Sturm et al., 2012). Hence, it is crucial to maintain the freshness of color attributes to attract customers and ensure marketability (Jafari et al., 2016). Sodium metabisulphite is used at a varying concentrations as an anti-browning agent, to maintain dried fruit color attributes. It has a strong inhibitory effect on polyphenol oxidase activity (Noorafshan et al., 2013). Studies on drying of nectarine have focused on: effects of drying treatments on volatile compounds (Sunthonvit et al., 2007), modelling of dried nectarine under near infrared-vacuum (Alaei and Chayjan, 2015), effects of hot-air and hybrid hot air-microwave drying on drying kinetics and textural quality of sliced nectarine (Ashtiani et al. 2018). Despite the various studies reported on drying nectarines, no specific study investigated the drying of nectarines with sodium metabisulphite as pre-treatment before drying nor elucidate microstructural changes in the fruit tissue. Therefore, the main objectives of this study are: (a) to compare the effect of sodium metabisulphite as pre-treatment on the qualitative parameters of dried nectarine; (b) to evaluate the drying kinetics and select the best mathematical models from various hot air drying models, and (c) to elucidate the phenomenal microstructural changes during the drying of 'Sunectwentyone' nectarines slices.
Materials and methods
Plant material
Fresh nectarine fruit (Prunus persica var. nucipersica cv. Sunectwentyone) were obtained at commercial maturity stage fully ripe from Blue Jay’s farm (33º92′36.852′′S, 18º87′44.714′′E) in Western Cape, South Africa. Fruit were transported to the Agricultural Research Council (ARC) Infruitec-Nietvoorbij, Agro-Processing Pilot Plant, Stellenbosch, South Africa. Nectarine fruit was sorted carefully to ensure uniformity in size, shape, and color and to eliminate damaged or decayed fruit. Before the experiments, the nectarine fruit was washed with tap water, air-dried for 2–3 h and the water droplets removed with sterile tissue paper at room temperature. Fruit samples were peeled using a sharp, sterile stainless steel knife and were cut into 6.5 mm-thick round slices with a sterilized slicer (Rheninghaus, Tecmal, Torino, Italy).
Drying and experimental procedure
Drying experiments were conducted in an in-house designed dehydrator tunnel (assembled in Western Cape, Stellenbosch, South Africa) at the ARC, Agro-Processing Pilot Plant. Fresh-cut nectarine slices (6.5 mm thick) were treated with 1% (10 g/kg) sodium metabisulphite (SMB) for 2 min, and non-treated fruit was used for the control. Processed fruit treated and non-treated were arranged in a single layer on trays, and the drying experiments were performed at a constant air temperature of 50 °C and airflow rate of 49.50 Hz, at 35% RH. The moisture content and weight of the nectarine slices were monitored hourly during the drying process for 6 h using a moisture meter (Mettler Toledo HR73 Halogen, Switzerland). The drying process was stopped when the moisture of the dried mango slice reached 13.53%. All experiments were conducted in triplicate for each treatment and the mean value was calculated.
The following physical characteristics of the fresh samples were measured before drying experiments using a digital calliper with ± 0.01 mm accuracy (Mitutoyo, Japan): average major diameter (70.53 mm), intermediate diameter (68 mm), and minor diameter (65.59 mm). After drying, the samples were cooled to room temperature (Zhang et al., 2019) and packed in airtight glass containers and stored in a dark cupboard at room temperature. Samples required for analysis were transferred to a vacuum dryer (Heraeus GMBH, HANAU, Germany) for 16 h until the samples were dried entirely and were milled to a fine powder using a grinder (Model Micro Universal; 0.05 μm, 14 000 rpm; Interscience Sdn. Bhd, Retsch, Germany). The powder obtained was kept at room temperature (20 ± 1 °C) in zip-lock polyethene bags (Zhang et al., 2019) and kept in hermetically sealed airtight glass for further qualitative analysis.
Mathematical modelling of drying kinetics
The initial moisture content of the fresh ‘Sunectwentyone’ nectarine slices was obtained at 71.23% (wet basis, w.b.), and the size of the selected sample were 9.35 g. Moisture ratio (MR) was recorded for five separate occasions at an hourly interval. The moisture ratio as a function of drying time was determined using nine different semi-theoretical thin-layer drying models mainly used in modelling drying curves of agricultural products and using non-linear regression analysis to find the most suitable model. The experimental data were fitted to the following hot air drying mathematical model:
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
where MR is the moisture ratio, k, k1, k2, a, b, c, n, g are model drying constants, t is the drying time. Correlation coefficient (R2) and the root mean square error (RMSE) was used to evaluate the best fit (Erenturk et al., 2004).
In order to determine the moisture ratio as a function of drying time, the experimental drying data were used using the following equations:
| 10 |
where t is the time in hours, Mt is the dry basis moisture content at any time t, Mo is the initial dry basis moisture content of the sample, Me is the equilibrium moisture content (Mercer, 2012).
Moisture ratio was investigated by directly comparing the drying of nectarine samples with different moisture contents at any time, t, and divided by the initial dry basis moisture (Doymaz, 2008)].
| 11 |
where Mt is the dry basis moisture content at any time t and M0 is the sample's initial dry basis moisture content. MR is further simplified to Mt/M0, for long drying hours as Me becomes small compared to Mt or M0. Moisture ratio at time t = 0 = 1.00.
The experimental drying data described from Eq. 10, was used for the drying rate (DR) calculation:
| 12 |
where M0+t is the moisture content at intervals (kg water/kg dry mass). The Eq. 12 was used to describe the drying rate as a function drying time.
Scanning electron microscope (SEM) analysis
The SEM (ZEISS Gemini SEM 300, Carl Zeiss Microscopy GmbH, Oberkochen, Germany) was used to capture the microstructure images on fresh and dried nectarine (after 2.5 h and 5 h of drying). The sample preparation and SEM image analysis with some modifications were performed according to the method described by Ashtiani et al. (2018) and Bao et al. (2021). Small pieces of nectarine fruit (≈ 3 × 3 × 2 mm) were taken from both the fresh and the dried fruit with a razor blade. The first step involved the fixation of the samples overnight at 4 ºC with 2.5% glutaraldehyde for 15 min in phosphate buffer (0.1 M, pH 7.0) to preserve the samples morphology. Furthermore, the samples were gradually dehydrated using a mixture of ethanol–water, with increasing ratios of ethanol concentrations from 30, 50, 70, 90, to 100% at regular interval (15 min). Thereafter, the dehydrated samples were dried for 15 min using 2 × 100% Hexamethyldisilazane. Samples were mounted on a glass slide with double-sided carbon tape. The samples were then coated with a thin (~ 10 nm thick) layer of gold, using a Leica EM ACE200 Gold Sputter Coater. Samples were loaded and observed with SEM and were viewed using a voltage of 3 kV, 100 IProbe at a magnification of 50 X, 100 X and 300 X to identify the surface characteristics.
Biochemical analysis of fresh nectarine fruit
The juice was extracted from fresh ‘Sunectwentyone’ nectarines using a laboratory juice blender (De Longhi Braun Household GmbH, China) for titratable acidity (TA), total soluble solids (TSS) and pH measurement. Titratable acidity (TA) was determined using 60 mL of fresh juice and titrating against standardized 0.33 N NaOH solution to the endpoint of pH 8.2 using a CRISON titrosampler (Crison Instruments, S.A. E-08328 ALELLA-Barcelona, made in Spain) (Nsumpi et al., 2020). The results were expressed as percentage citric acid (% CA). The pH was measured using a digital pH meter (Crison Model 00,924 basic 20 + , South Africa). Total soluble solids (TSS) were measured using a digital handheld refractometer (Atago N1, Tokyo, Japan) and expressed as ºBrix. For the dried nectarines, the grounded samples previously described in Sect. 2.2 was used. Powder samples (6 g) was mixed with 60 mL distilled water and used for TA, TSS and pH according to the method reported by Bao et al. (2021).
Color
A calibrated Minolta Chroma Meter CR-400 (Minolta Corp., Osaka, Japan) was used to measure the color on opposite sides of each fruit slice before and after drying at pre-specified time intervals during hot air. For each drying strategy, a total of 5 slices were evaluated in triplicate. The color was expressed as lightness (L*), redness (a*) and yellowness (b*) according to the CIELAB color coordinates (Ashtiani et al., 2018). Color intensity or chroma (C*), which describes the quantitative attribute of color intensity, hue angle (h°), which denotes the qualitative attribute of color shades, and the total color difference between the fresh and the dried nectarine slices were calculated using the Eqs. (13), (14) and (15) (Maskan, 2006):
| 13 |
| 14 |
| 15 |
where L*0, a*0 and b*0 are the color parameters of the fresh-cut nectarines fruit, while L*, a* and b* are the color values of the dried fruit slices.
Statistical analysis
Factorial analysis of variance (ANOVA) was performed in order to understand the effects of treatments. Mean values were tested using Fisher’s LSD test at a level of significance of 95% (Statistica13.5.0.17 TIBCO, StatSoft Inc., Tulsa, OK, USA). The moisture ratio curves obtained from experimental data were fitted with nine mathematical models to describe dehydrated nectarine slices' drying characteristics. The experimental data were evaluated with the coefficient of determination (R2), and the root means square error (RMSE). The higher the R2 values, and the lower the RMSE values, the better is the model goodness of fit (Ertekin and Yaldiz, 2004; Mercer et al., 2012; Wang et al., 2007).
Results and discussion
Moisture content and ratio
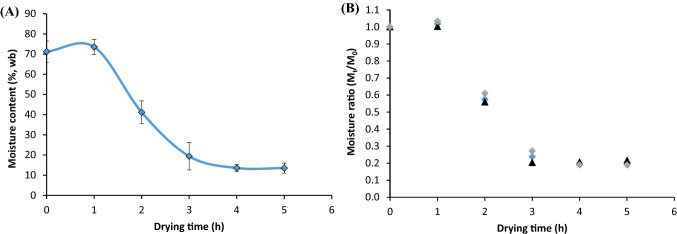
Effect of hot air drying on the changes of the moisture content and ratio (MR) versus drying time for ‘Sunectwentyone’ nectarines slices at the drying temperature of 50 °C is shown in Fig. 1. Within the first hour of drying, the moisture content of nectarines increased slightly from 71.2 to 73.5%, and thereafter, decreased continuously to 13.53% after 6 h. Sunjka and Raghavan (2004) reported that minimal processing such as cutting fruit results in a substantial increase in moisture ratio loss because the surface area for mass transfer is greater in fresh-cut slices compared to the whole fruit. In the current study, the higher moisture ratio at the beginning of the experiment could be due to the initial increase in moisture content. This implies that the temperature gradient is from the surface to the centre, the heat transfer is much slower, causing a reduction in the mass migration and transfer (Talens et al., 2017).
Fig. 1.
Changes of the moisture content (A) and ratio (MR) (B) versus drying time for ‘Sunectwentyone’ nectarines slices at the drying temperature of 50 °C. Errors bar represent standard deviation from MR mean values (n = 3)
As drying time increased from 2 to 6 h, there was a continuous decrease in the moisture ratio of dehydrated nectarines slices. This could be attributed to heat transferred from the fruit surface to the nectarine slices inner part, that increase the vapour pressure. This facilitated the migration of water molecules towards the surface of the samples, causing a faster drying rate and a decline in the moisture ratio (Swain et al., 2012), as observed in Fig. 1. Similarly, the drying of ‘Kalocsa 622’ paprika berries was investigated at 60ºC in a flow dryer and a laboratory-scale cross-flow dryer by Ramesh et al. (2001). The authors reported that during the drying process, some amount of heat is transferred to the product's surface, another portion propagates to the interior, and the remaining is utilized in the evaporation of moisture from the surface. Their study further highlighted the diffusing of moisture from the interior to replace the evaporated moisture on the product surface hence the decrease in the moisture ratio of dried samples. This is consistent with this study, where the moisture ratio of the dehydrated nectarine slices decreased from the second hour of drying.
The mechanism of moisture movement during thin-layer drying of biological materials is governed by diffusion, as described by Fick's second law of diffusion (Karathanos et al., 1995). For instance, Swain et al. (2012) demonstrated the effects of microwaved treatments at 30, 45 and 60 ºC and osmotic dehydration after pre-treatment of sweet pepper using salt and sucrose. The results indicated that increasing the drying time leads to a continuous decrease in the moisture content over time and too little energy absorbed. Furthermore, Ashtiani et al. (2018) concluded from their studies on drying of ‘Moghan’ nectarines slices using hot-air and hybrid hot air-microwave drying methods at 50 ºC, that the drying rate of nectarine slices using both methods had no constant drying rate period. However, the falling-rate period occurred in the process due to diffusion-controlled mechanisms inside the nectarine. This finding is similar to the results in this study, where no constant drying rate period was observed throughout the drying process. Moisture ratio in dehydrated nectarine slices at 50 ºC showed increase at the beginning of drying, due to the surface area for mass transfer, but declined after 2 h. This change was attributed to the evaporation of moisture from the surface of the sample. However, the dehydration method used in the current study could be responsible for the moisture ratio change.
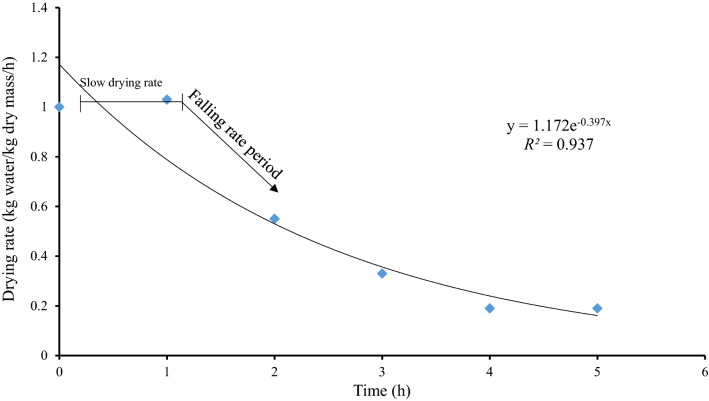
Drying rate
Figure 2 shows the drying rate based on moisture content as a function of time for ‘Sunectwentyone’ nectarine. In the early stages of nectarine drying, a slow drying rate was observed. This period can be considered as a warming up a step where the temperature rises quickly without significant mass losses. This was explained by Andrés et al. (2004) in their study on the drying kinetics of ‘Granny Smith’ apple under combined hot air microwave dehydration at 25, 30, 40 and 50 ºC. Drying rates for dehydrated nectarine slices followed the pattern of falling rate period considered a phenomenon of diffusion-efficiency and could probably be influenced by the physical properties of nectarines (Ashtiani et al., 2018).
Fig. 2.
Drying rate of 'Sunectwentyone' nectarines as a function of drying time with annotated description of the slow drying rate, falling rate to the constant drying time (drying rate was maximized after 1 h to 4 h)
Internal diffusion was assumed to be the mechanism responsible for the loss of water during the dehydration process. This is consistent with reports by Andrés et al. (2004) and Schössler et al. (2012). Similarly, Okos et al. (1992) suggested that the falling rate period indicates that an internal mass transfer resistance controls moisture removal. Moreover, moisture diffusion depends on the product, pore structure, and moisture interactions with the respective food product (Schössler et al., 2012). Lemus-Mondaca et al. (2013) reported that the drying rate of dried ‘Chilean’ papaya slices continuously decreases as the period progresses, representing falling rate periods. Schössler et al. (2012) reported that the drying of hygroscopic products, including fruits, generally has two falling rate periods. The first falling rate period is described as the ‘‘zone of unsaturated surface drying’’, and during that period, the drying rate decreases with an increasing proportion of surface areas being no longer saturated with moisture. Furthermore, they reported that internal moisture movement controls the drying rate at the beginning of the second falling rate period (Perry et al., 1984).
Ashtiani et al. (2018) explained that higher moisture content at the initial phase of drying nectarine slices using hot air and hybrid hot air-microwave at 50 ºC leads to higher absorption of a more significant amount of microwave energy producing a high drying rate. Similarly, the current study observed a high drying rate, which could be linked to the microstructural properties of the nectarines, especially the porosity and shrinkage of the fruit mesocarp during the drying process, which led to wide spaces between neighbouring cells (Andrés et al., 2004). This phenomenon can also be observed in this study as shown in Figs. 3 and 4. The slight initial increase in the moisture content of nectarine slices at the early drying stages could be attributed to initial water vapour condensation on the fruit surface. Moreover, the water inside the fruit slice, primarily present as a liquid, was transferred by capillary diffusion leading to a progressive decrease in the drying rate.
Fig. 3.
Mathematical model of dried 'Sunectwentyone' nectarines slices at 50 °C for 5 h with A representing the Henderson and Pabis model, B Lewis, C Midilli-Kucuk, d Page, E Verma et al. F Logarithmic, G Weibull, H Wang and Singh and I Aghbashlo et al.
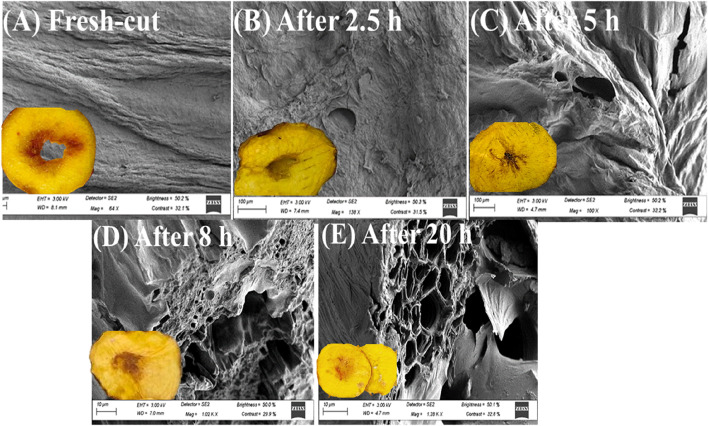
Fig. 4.
SEM micrographs and pictures of 'Sunectwentyone' nectarine slices as fresh-cut slices prior to drying (A), and during the drying process at 50 °C after 2.5 h (B), 5 h (C), 8 h (D) and 20 h (E)
Fitting of drying curve
The coefficient of determination (R2) and root mean square error (RMSE) was used to determine the goodness of the fit model, as shown in Table 1. The RMSE values obtained in our experiment varied between 35.30 and 6.57, while R2 varied between 0.94 and 0.10. The highest R2 values were observed in the Logarithmic model (0.94) (Fig. 3f) as well as the Henderson and Pabis (0.94) models (Fig. 3a). The higher the R2 values, the lower the RMSE values and, consequently, the better the fitting of the model (Wang et al., 2007). Henceforth, they were the best mathematical models among the nine fitted explaining the drying process and describing the drying curves of nectarine slices. The others models included Lewis (Fig. 3b), Midilli-Kucuk (Fig. 3c), Page (Fig. 3d), Verma et al. (Fig. 3e), Weibull (Fig. 3g), Wang and Singh (Fig. 3h) and Aghbashlo et al. (Fig. 3i) and had the lowest R2. On the contrary to this study, Ashtiani et al. (2018) revealed that the Midilli-Kucuk model had the highest accuracy in predicting the drying process of nectarine slices after drying at 50 ºC using hot-air hybrid hot air-microwave methods. However, the study conducted by Bao et al. (2021) for ‘Junzao’ jujube slices using a hot air dryer at 50, 60 and 70 ºC, showed the highest accuracy was achieved by the Logarithmic and Henderson & Pabis models (R2 > 0.97). These models adequately described the drying curves for jujube slices during the hot air drying process. A similar outcome was obtained in this study for sliced nectarine hot air-dried at 50 °C. This suggests that these models can be used for further optimization and upscaling processes.
Table 1.
Curve fitting criteria for nine mathematical models and parameters for 'Sunectwentyone' nectarines
| Parameters | Mathematical models | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lewis | Page | Verma et al | Midilli-Kucuk | Henderson & Pabis | Logarithmic | Wang and Singh | Weibull | Aghbashlo et al | |
| K | 0 | 0 | 0.5461 | 0.7810 | 0.3485 | 0.3485 | NA | NA | NA |
| K1 | NA | NA | NA | NA | NA | NA | NA | NA | 1.0780 |
| K2 | NA | NA | NA | NA | NA | NA | NA | NA | 1.6873 |
| A | NA | NA | 124.6925 | 71.2399 | 79.8103 | 79.8105 | 1 | 1 | NA |
| B | NA | NA | NA | 0 | NA | NA | 2 | NA | NA |
| C | NA | NA | NA | NA | NA | 0 | NA | NA | NA |
| N | NA | 0.6876 | NA | 75.9711 | NA | NA | NA | 1 | NA |
| g | NA | NA | 11.8690 | NA | NA | NA | NA | NA | NA |
| R2 | 0.8878 | 0.8878 | 0.1008 | 0.4286 | 0.9415 | 0.9415 | 0.8878 | 0.8878 | 0.8511 |
| RMSE | 35.3060 | 35.3060 | 22.2999 | 15.9116 | 6.5722 | 6.5722 | 35.3060 | NA | 34.2174 |
Microstructural analysis
Micrographs showed that dehydrated ‘Sunectwentyone’ nectarine slices presented higher surface morphological damages as the drying time progressed compared to the fresh-cut samples, which showed fewer pores and no intracellular spaces (Fig. 4). The cellular deformation and shrinkages observed at the microstructural level in the dried nectarine mesocarp could be associated with the internal vapour condensation on the fresh-cut samples fruit surface, which led to an enlargement of the dried nectarine slices tissue (Fig. 4B-E). Similarly, Bde Bruijn and Borquez (2014) reported that internal vapour condensation leads to a more open structure due to vapour expansion within strawberries fruit dried at 50 ºC by vacuum microwave. In another study conducted by Bilbao et al. (2000) and Andrés et al. (2004), microwaved air-dried apple slices at 40 ºC present a porous structure, and the cell walls were vastly shrunken, leaving wide spaces between the neighbouring fruit cells.
Generally, convective drying of fruit tissues is characterized by wrinkling and significant microstructural changes. Ashtiani et al. (2018) reported that nectarine slices dried with hot-air at 50 ºC exhibit severe wrinkling and failure of the mesocarp. Tissue shrinkage and cell wall collapse were observed for selected horticultural commodities such as apples dehydrated at 50 ºC, strawberries at 40 ºC, tomatoes at 50 ºC and mushrooms at 50 ºC (Askari et al., 2009). Similar microstructural changes were observed in our study when the drying time were prolonged up to 20 h (Fig. 4). Longer drying time and higher vapour pressure would lead to larger pores and deeper grooves in the fruit mesocarp. Hence, it is essential to establish optimal drying time in order to minimize adverse microstructural changes.
Biochemical properties of nectarines
Titratable acidity, Total soluble solids and pH
Titratable acidity (TA) and total soluble solids (TSS) significantly decreased (p < 0.05) in all untreated nectarines and sodium metabisulphite pretreated samples dried for 8 and 20 h compared to the fresh-cut samples used as control (Table 2). For 8 h dried samples, the TA value decreased from the initial value of 0.52 g/L for fresh-cut samples to 0.27 g/L and 0.35 g/L for untreated and pre-treated dried nectarines, respectively. For 20 h dried samples, the TA value decreased from the initial value of 0.52 g/L for fresh-cut samples to 0.33 g/L and 0.32 g/L, respectively for untreated and pretreated nectarines with sodium metabisulphite. The observed reduction could be related to the decomposition of acids into sugars (Wei et al, 2014). These results are in agreement with those reported by Piga et al. (2004), where a decrease was observed in TA after hot-air drying at 55 °C of figs (Ficus carica L.). Similar results were observed in studies that reported a significant decrease in TA value after drying ‘Amoroso’, ‘Berlinto’ and ‘Messina’ tomatoes at 55 °C, 65 °C and 75 °C (Ashebir et al., 2009).
Table 2.
Effects of pre-treatment and hot air drying on the quality index of dried 'Sunectwentyone' nectarine slices
| Parameters | TA (g/L) | pH | TSS | C* | h ° | ΔE |
|---|---|---|---|---|---|---|
| Control fresh cut | 0.52 a ± 0.05 | 4.15 d ± 0.06 | 11.70 a ± 0.06 | 38.73 b ± 3.62 | 42.07 c ± 15.07 | – |
| Untreated dried 8 h | 0.27 c ± 0.00 | 4.28 c ± 0.03 | 5.07 c ± 0.31 | 49.18 a ± 5.67 | 82.24 ab ± 5.96 | 32.42 a ± 7.56 |
| Treated SMB dried 8 h | 0.35 b ± 0.05 | 4.53 a ± 0.02 | 7.70 b ± 0.12 | 47.00 ab ± 7.01 | 87.90 ab ± 5.04 | 38.87 a ± 6.27 |
| Untreated dried 20 h | 0.33 b ± 0.00 | 4.42 b ± 0.02 | 7.97 b ± 0.12 | 41.07 ab ± 9.06 | 75.64 b ± 10.03 | 25.40 b ± 9.93 |
| Treated SMB dried 20 h | 0.32 b ± 0.01 | 4.48 a ± 0.01 | 7.83 b ± 0.45 | 52.79 a ± 6.66 | 90.19 a ± 2.14 | 45.74 a ± 5.27 |
SMB – sodium metabisulphite; Values represent means ± standard deviation. Different letters in the same column indicate a significant difference
The TSS value decreased from the initial value of 11.70 g/L for fresh-cut samples to 5.07 g/L and 7.70 g/L for untreated and pretreated dried nectarines with sodium metabisulphite, respectively after 8 h of drying (Table 2). Similarly, the TSS value decreased from the initial value of 11.70 g/L for fresh-cut samples to 7.97 g/L and 7.83 g/L, respectively for untreated and pretreated nectarines with sodium metabisulphite in dried samples after 20 h. The changes observed could be attributed to drying under high temperatures. These results agree with the findings of Piga et al. (2004), who observed a decrease in TSS after applying hot-air drying at 55 °C to figs. Furthermore, the pH value in all the treated and untreated nectarines samples dried for 8 and 20 h show a slight but significant increase compared to the fresh-cut control (Table 2). The observed changes in pH values found in the studies could be explained by factors such as the water loss of the samples during drying (Misra et al., 2014). Overall, the results obtained in this study could be linked to the pre-treatment applied and to the drying temperature used.
Color change
Nectarine fruit were dried for a total duration of 20 h, and S-Fig. 1 (Supplementary S-Fig. 1) shows a pictographic representation of fresh untreated (control samples) and freshly treated nectarine with metabisulphite before and after drying. Nectarine slices samples pretreated with metabisulphite appeared brighter than the untreated ones (S-Fig. 1). The untreated samples dried for 8 and 20 h showed a brown color, which could result from the occurrence of the Maillard reactions compared to the samples treated with metabisulphite and dried for 8 and 20 h appeared pale (S-Fig. 1).
Chroma indicates the degree of color in the sample, which varied from low Chroma values (dull or low colors) to high Chroma values (bright colors) (Alagbe et al., 2020). Table 2 shows that fresh-cut control fruit had the lowest color intensity (C* = 38.7), while untreated fruit dried for 8 h and 20 h had a C* of 49.2 and 41.1, respectively, and the treated fruit had a C* of 47.0 and 52.8, respectively. Generally, high C* values represent a more excellent retention of color in samples (Bilbao et al., 2000). Drying nectarine fruit for 8 h affected the hue angle, which generally increased from the initial value of 42.1 for fresh-cut control samples to 82.2 and 87.9 for untreated and pre-treated samples, respectively. Similarly, after 20 h, the h° value increased from the initial value of 42.1 for fresh-cut control samples to 75.6 and 90.2 for untreated and pre-treated nectarines, respectively. Lower h° values were associated with less colored fruit samples, while higher h° values were correlated with bright colored mango slices (Askari et al 2009).
The total color difference (TCD, ΔE) indicates the color disparity between the untreated and treated dried fruit, and this was influenced by the pre-treatment applied (sodium metabisulphite). After 8 h of drying, ΔE for untreated and treated nectarines were 32.4 and 38.9, respectively (Table 2). Likewise, after 20 h, ΔE were 25.4 and 45.7 for untreated and treated nectarines, respectively. Alagbe et al. (2020) reported that the initial purpose of pre-treatment is to retain color by inactivating the enzymes responsible for color changes. Maskan (2006) reported that during thermal processes of agricultural products, the various changes observed in redness is associated with the decrease of anthocyanin and carotenoids concentrations, whereas authors such as Sturm et al. (2012) associated the changes with enzymatic and non-enzymatic browning.
Changes observed in the nectarine yellowness could be linked to its tissue breakdown and other mechanisms such as the degradation of pigments and the occurrence of browning and non-enzymatic browning, which occurred during the drying process (Ashtiani et al., 2018). The color change could be related to the sodium metabisulphite pre-treatment applied, which maintained the color of the dried slices. The anti-browning effect of sodium metabisulphite was reported by Noorafshan et al. (2013). In summary, browning is one of the main factors, which can occur during drying and is highly correlated with the visual appearance of untreated fresh-cut nectarines. Furthermore, it strongly affects the shelf life of fresh-cut fruit as well as consumer purchase decisions. The variations of color can be associated with a series of chemical reactions during the drying process, including the degradation of pigments and the browning and non-enzymatic browning. Hot air drying with a dehydrator at 50 ºC had a significant effect on the color of nectarine slices. Moreover, pre-treating nectarine fruit with sodium metabisulphite decreases the browning in the fruit sample.
In conclusion, Henderson and Pabis and Logarithmic were the most suitable models for describing the drying kinetics of nectarine slices dehydrated at 50 ºC, and had the highest R2 (0.94) compared to other models. Pre-treating minimally processed nectarines with 1% sodium metabisulphite before drying improved the color properties and decreased browning. The results of this study can be used to simulate the hot air drying process of nectarine slices and improve the drying process's efficiency. Based on the outcomes from this study, future studies could investigate the effects of hot air drying using a dehydrator in combination with other non-thermal post-harvest pre-treatments such as a cold plasma or electric pulse field on the drying efficiency and quality index of nectarines.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgement
The authors are grateful to the Agriculture Research Council (ARC) Infruitec-Nietvorbij, Stellenbosch, South Africa for providing the platform and facility to conduct this study. The research support provided by Mr. George Dico, from the Plant Bioactives Group is gratefully acknowledged.
Funding
This work is based upon research supported wholly/in part by the Agriculture Research Council (ARC) and National Research Foundation (NRF), South Africa (Grant Nos.: 116272 and 119,192). The award of a PhD Fellowship by the Organisation for Women in Science for the Developing World (OWSD) is gratefully acknowledged.
Declarations
Conflict of interest
The authors declare that they have no known competing/conflicting interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Loriane A. Yanclo, Email: YancloL@arc.agric.za
Gunnar Sigge, Email: GOS@sun.ac.za.
Zinash A. Belay, Email: BelayZ@arc.agric.za
Feroza October, Email: PohplonkerF@arc.agric.za.
Oluwafemi J. Caleb, Email: oluwafemi@sun.ac.za
References
- Aghbashlo M, Samimi-Akhijahani H. Influence of drying conditions on the effective moisture diffusivity, energy of activation and energy consumption during the thin-layer drying of berberis fruit (Berberidaceae) Energy Conversion and Management. 2008;49(10):2865–2871. [Google Scholar]
- Alaei B, Chayjan RA. Modelling of nectarine drying under near infrared? Vacuum conditions. Acta Scientiarum Polonorum Technologia Alimentaria. 2015;14:15–27. doi: 10.17306/J.AFS.2015.1.2. [DOI] [PubMed] [Google Scholar]
- Alagbe EE, Amlabu YS, Daniel EO, Ojewumi ME. Effect of varying drying temperature on the soluble sugar and nutritional content of banana. The Open Chemical Engineering Journal. 14 (2020)
- Andrés A, Bilbao C, Fito P. Drying kinetics of apple cylinders under combined hot air–microwave dehydration. Journal of Food Engineering. 2004;63(1):71–78. [Google Scholar]
- Aral S, Beşe AV. Convective drying of hawthorn fruit (Crataegus spp.): Effect of experimental parameters on drying kinetics, color, shrinkage, and rehydration capacity. Food Chemistry. 2016;210:577–584. doi: 10.1016/j.foodchem.2016.04.128. [DOI] [PubMed] [Google Scholar]
- Araujo EA, Ribeiro S, Azoubel PM, Murr FE. Drying kinetics of nectarine (Prunus persica) with and without shrinkage. InEmbrapa Semiárido-Artigo em anais de congresso (ALICE) 2004. In: INTERNATIONAL DRYING SYMPOSIUM, 14, 2004, São Paulo, SP. Proceedings Campinas: State University of Campinas, Chemical Engineering School, 2004
- Ashebir D, Jezik K, Weingartemann H, Gretzmacher R. Change in color and other fruit quality characteristics of tomato cultivars after hot-air drying at low final-moisture content. International Journal of Food Sciences and Nutrition. 2009;60(7):308–315. doi: 10.1080/09637480903114128. [DOI] [PubMed] [Google Scholar]
- Ashtiani SH, Sturm B, Nasirahmadi A. Effects of hot-air and hybrid hot air-microwave drying on drying kinetics and textural quality of nectarine slices. Heat and Mass Transfer. 2018;54(4):915–927. [Google Scholar]
- Askari GR, Emam-Djomeh Z, Mousavi SM. An investigation of the effects of drying methods and conditions on drying characteristics and quality attributes of agricultural products during hot air and hot air/microwave-assisted dehydration. Drying Technology. 2009;27(7–8):831–841. [Google Scholar]
- Bao T, Hao X, Shishir MR, Karim N, Chen W. Cold plasma: An emerging pre-treatment technology for the drying of jujube slices. Food Chemistry. 2021;337:127783. doi: 10.1016/j.foodchem.2020.127783. [DOI] [PubMed] [Google Scholar]
- Bilbao C, Albors A, Gras M, Andrés A, Fito P. Shrinkage during apple tissue air-drying: Macro and microstructural changes. In Proceedings of the 12th International Drying Symposium. IDS2000. Paper 2000 (No. 419) (2000)
- Cano-Salazar J, López ML, Crisosto CH, Echeverría G. Volatile compound emissions and sensory attributes of ‘Big Top’ nectarine and ‘Early Rich’ peach fruit in response to a pre-storage treatment before cold storage and subsequent shelf-life. Postharvest Biology and Technology. 2013;76:152–162. [Google Scholar]
- de Bruijn J, Bórquez R. Quality retention in strawberries dried by emerging dehydration methods. Food Research International. 2014;63:42–48. [Google Scholar]
- Doymaz I. Convective drying kinetics of strawberry. Chemical Engineering and Processing: Process Intensification. 2008;47(5):914–919. [Google Scholar]
- Durst RW, Weaver GW. Nutritional content of fresh and canned peaches. Journal of the Science of Food and Agriculture. 2013;93(3):593–603. doi: 10.1002/jsfa.5849. [DOI] [PubMed] [Google Scholar]
- Erenturk S, Gulaboglu MS, Gultekin S. The thin-layer drying characteristics of rosehip. Biosystems Engineering. 2004;89(2):159–166. [Google Scholar]
- Ertekin C, Yaldiz O. Drying of eggplant and selection of a suitable thin layer drying model. Journal of Food Engineering. 2004;63(3):349–359. [Google Scholar]
- Henderson SM. Grain drying theory (I) temperature effect on drying coefficient. Journal of Agricultural Engineering Research. 1961;6(3):169–174. [Google Scholar]
- Hiranvarachat B, Devahastin S, Chiewchan N. Effects of acid pre-treatments on some physicochemical properties of carrot undergoing hot air drying. Food and Bioproducts Processing. 2011;89(2):116–127. [Google Scholar]
- İsmail O, Beyribey B, Doymaz İ. Effect of drying methods on drying characteristic, energy consumption and color of nectarine. Journal of Thermal Engineering. 2016;2(2):801–806. [Google Scholar]
- Jafari SM, Azizi D, Mirzaei H, Dehnad D. Comparing quality characteristics of oven-dried and refractance window-dried kiwifruits. Journal of Food Processing and Preservation. 2016;40(3):362–372. [Google Scholar]
- Karathanos VT, Kostaropoulos AE, Saravacos GD. Air-drying kinetics of osmotically dehydrated fruits. Drying Technology. 1995;13(5–7):1503–1521. [Google Scholar]
- Le D, Konsue N. Mass transfer behavior during osmotic dehydration and vacuum impregnation of “Phulae” pineapple and the effects on dried fruit quality. Current Research in Nutrition and Food Science Journal. 2021;9(1):308–319. [Google Scholar]
- Lee JH, Zuo L. Mathematical modeling on vacuum drying of Zizyphus jujuba Miller slices. Journal of Food Science and Technology. 2013;50(1):115–121. doi: 10.1007/s13197-011-0312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemus-Mondaca RA, Zambra CE, Vega-Gálvez A, Moraga NO. Coupled 3D heat and mass transfer model for numerical analysis of drying process in papaya slices. Journal of Food Engineering. 2013;116(1):109–117. [Google Scholar]
- Macedo LL, Corrêa JL, Araújo CD, Vimercati WC, Pio LA. Process optimization and ethanol use for obtaining white and red dragon fruit powder by foam mat drying. Journal of Food Science. 2021;86(2):426–433. doi: 10.1111/1750-3841.15585. [DOI] [PubMed] [Google Scholar]
- Maskan M. Production of pomegranate (Punica granatum L.) juice concentrate by various heating methods: color degradation and kinetics. Journal of Food Engineering. 2006;72(3):218–224. [Google Scholar]
- Mathooko FM, Tsunashima Y, Owino WZ, Kubo Y, Inaba A. Regulation of genes encoding ethylene biosynthetic enzymes in peach (Prunus persica L.) fruit by carbon dioxide and 1-methylcyclopropene. Postharvest Biology and Technology. 2001;21(3):265–281. [Google Scholar]
- Mercer DG. A comparison of the kinetics of mango drying in open-air, solar, and forced-air dryers. African Journal of Food, Agriculture, Nutrition and Development. 12(7) (2012)
- Midilli AD, Kucuk HA, Yapar Zİ. A new model for single-layer drying. Drying Technology. 2002;20(7):1503–1513. [Google Scholar]
- Misra NN, Keener KM, Bourke P, Mosnier JP, Cullen PJ. In-package atmospheric pressure cold plasma treatment of cherry tomatoes. Journal of Bioscience and Bioengineering. 2014;118(2):177–1782. doi: 10.1016/j.jbiosc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Motevali A, Minaei S, Khoshtaghaza MH, Amirnejat H. Comparison of energy consumption and specific energy requirements of different methods for drying mushroom slices. Energy. 2011;36(11):6433–6441. [Google Scholar]
- Noorafshan A, Asadi-Golshan R, Karbalay-Doust S, Abdollahifar MA, Rashidiani-Rashidabadi A. Curcumin, the main part of turmeric, prevents learning and memory changes induced by sodium metabisulfite, a preservative agent, in rats. Experimental Neurobiology. 2013;22(1):23. doi: 10.5607/en.2013.22.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsumpi AN, Belay ZA, Caleb OJ. Good intentions, bad outcomes: Impact of mixed-fruit loading on banana fruit protein expression, physiological responses and quality. Food Packaging and Shelf Life. 2020;26:100594. [Google Scholar]
- Okos MR, Narsimhan G, Singh RK, Witnauer AC, In Food dehydration, R. Heldman, D.B. Lund (Eds.). Handbook of food engineering. Marcel Dekker. New York (1992).
- Perry RH, Green DW, Maloney JO. Prediction and correlation of physical and transport properties. Perry’s Chemical Engineers’ Handbook (ed. RH Perry), 6th edition. McGraw-Hill. New York. London. 3–213 (1984)
- Piga A, Pinna I, Özer KB, Agabbio M, Aksoy U. Hot air dehydration of figs (Ficus carica L): drying kinetics and quality loss. International Journal of Food Science & Technology. 2004;39(7):793–799. [Google Scholar]
- Ramesh MN, Wolf W, Tevini D, Jung G. Influence of processing parameters on the drying of spice paprika. Journal of Food Engineering. 2001;49(1):63–72. [Google Scholar]
- Roberts JS, Kidd DR, Padilla-Zakour O. Drying kinetics of grape seeds. Journal of Food Engineering. 2008;89(4):460–465. [Google Scholar]
- Schössler K, Jäger H, Knorr D. Effect of continuous and intermittent ultrasound on drying time and effective diffusivity during convective drying of apple and red bell pepper. Journal of Food Engineering. 2012;108(1):103–110. [Google Scholar]
- Sturm B, Hofacker WC, Hensel O. Optimizing the drying parameters for hot-air–dried apples. Drying Technology. 2012;30(14):1570–1582. [Google Scholar]
- Sunjka PS, Raghavan GS. Assessment of pre-treatment methods and osmotic dehydration for cranberries. Canadian Biosystems Engineering. 2004;46(1):45–48. [Google Scholar]
- Sunthonvit N, Srzednicki G, Craske J. Effects of drying treatments on the composition of volatile compounds in dried nectarines. Drying Technology. 2007;25(5):877–881. [Google Scholar]
- Swain S, Samuel DV, Bal LM, Kar A, Sahoo GP. Modeling of microwave assisted drying of osmotically pretreated red sweet pepper (Capsicum annum L.) Food Science and Biotechnology. 2012;21(4):969–978. [Google Scholar]
- Talens C, Arboleya JC, Castro-Giraldez M, Fito PJ. Effect of microwave power coupled with hot air drying on process efficiency and physico-chemical properties of a new dietary fibre ingredient obtained from orange peel. LWT – Food Science and Technology. 77: 110–118 (2017)
- Treybal RE. Operaciones de transferencia de masa, Cap. 12 Secado. Universidad de Rhode Island, México, 2da ed.: McGraw Hill, (1995)
- Verma LR, Bucklin RA, Endan JB, Wratten FT. Effects of drying air parameters on rice drying models. Transactions of the American Society of Agricultural Engineers. 1985;28(1):296–301. [Google Scholar]
- Wang Z, Sun J, Liao X, Chen F, Zhao G, Wu J, Hu X. Mathematical modeling on hot air drying of thin layer apple pomace. Food Research International. 2007;40(1):39–46. [Google Scholar]
- Wang CY, Singh RP. A single layer drying equation for rough rice. St. Joseph, MI, USA: American Society of Agricultural Engineers, (1978).
- Wei M, Zhou L, Song H, Yi J, Wu B, Li Y, Zhang L, Che F, Wang Z, Gao M, Li S. Electron beam irradiation of sun-dried apricots for quality maintenance. Radiation Physics and Chemistry. 2014;97:126–133. [Google Scholar]
- Wills RB, Scriven FM, Greenfield H. Nutrient composition of stone fruit (Prunus spp.) cultivars: apricot, cherry, nectarine, peach and plum. Journal of the Science of Food and Agriculture. 1983;34(12):1383–1389. doi: 10.1002/jsfa.2740341211. [DOI] [PubMed] [Google Scholar]
- Zhang J, Cheng D, Wang B, Khan I, Ni Y. Ethylene control technologies in extending post-harvest shelf life of climacteric fruit. Journal of Agricultural and Food Chemistry. 2017;34:7308–7319. doi: 10.1021/acs.jafc.7b02616. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Zhong CS, Mujumdar AS, Yang XH, Deng LZ, Wang J, Xiao HW. Cold plasma pre-treatment enhances drying kinetics and quality attributes of chili pepper (Capsicum annuum L.) Journal of Food Engineering. 2019;241:51–57. [Google Scholar]
- Zhao H, Shu C, Fan X, Cao J, Jiang W. Near-freezing temperature storage prolongs storage period and improves quality and antioxidant capacity of nectarines. Scientia Horticulturae. 2018;228:196–203. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.