Abstract
In the last years we have witnessed tremendous advancements in the treatment landscape of metastatic breast cancer (MBC), leading to a progressive prolongation of progression-free survival and, in some cases, also of overall survival. This led to a substantial increase of advanced disease treatability. In the present review we comprehensively and critically describe the most significant progresses in the therapeutic scenario of MBC according to BC subtype. In particular, we reviewed studies reporting practice-changing data in hormone receptor-positive/human epidermal growth factor receptor 2 (HER2)-negative, HER2-positive and triple-negative BC, with also a hint to BRCA-related tumors and the emerging HER2-low-positive category.
Key words: metastatic breast cancer, patient selection, endocrine therapy, chemotherapy, targeted therapy
Highlights
-
•
The treatment landscape of MBC has progressively widened in the last years.
-
•
Several therapeutic strategies proved to be capable of prolonging survival of patients with MBC.
-
•
We comprehensively reviewed practice-changing data on MBC treatment according to BC subtype.
Introduction
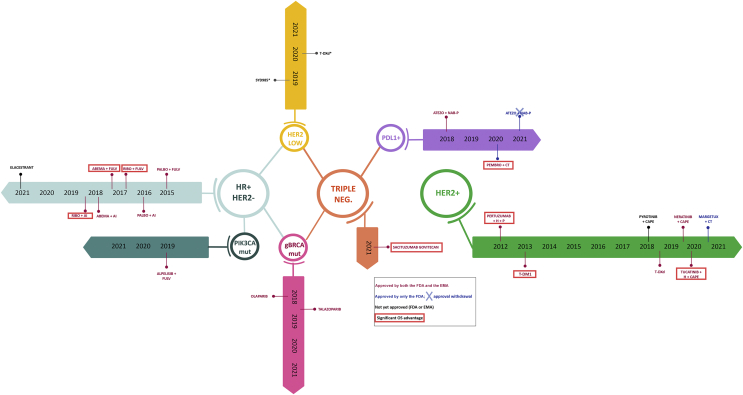
Although breast cancer (BC)-associated survival rates have dramatically improved over the past decades, once metastatic, BC still represents an incurable condition. Fortunately, the landscape of metastatic BC (MBC) has undergone profound advancements in all BC subtypes, leading to a progressive prolongation of progression-free survival (PFS) and, in some cases, also of overall survival (OS).1 In the present review we comprehensively describe the most significant progresses in the therapeutic scenario of MBC, with a particular focus on practice-changing data. Data will be reviewed by considering separately hormone receptor-positive (HR+)/human epidermal growth factor receptor 2 (HER2)-negative (HER2–), HER2+ and triple-negative (TN) BC, with also a mention to BRCA-related MBC and the emerging category of HER2-low-positive BC. Figure 1 and Table 1 summarize timeline and value according to ESMO-Magnitude of Clinical Benefit Scale, respectively, of main breakthrough therapies across BC subtypes in the advanced setting.2
Figure 1.
Timeline of main breakthrough therapies across breast cancer (BC) subtypes.
HR, hormone receptor; PALBO, palbociclib; FULV, fulvestrant; AI, aromatase inhibitor; RIBO, ribociclib; ABEMA, abemaciclib; mut, mutated; gBRCA, BRCA germline mutation; neg, negative; PDL1, Programmed death-ligand 1; ATEZO, atezolizumab; NAB-P, nab-paclitaxel; PEMBRO, pembrolizumab, CT, chemotherapy; H, trastuzumab; P, docetaxel; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan; CAPE, capecitabine; MARGETUX, margetuximab; FDA, Food and Drug Administration; EMA, European Medicines Agency; OS, overall survival.
∗phase I trials.
Table 1.
Main breakthrough therapies across BC subtypes in the advanced setting and their value according to ESMO-Magnitude of Clinical Benefit Scale
| Treatment class | Subtype | Treatment strategy | Phase III Clinical Trials | Significant results (a if primary endpoint) |
Practice changing | Current clinical positioning | ESMO MCBS |
||
|---|---|---|---|---|---|---|---|---|---|
| PFS | OS | ||||||||
| CDK 4/6 inhibitors | HR+/HER2– | CDK 4/6 inhibitors + AI | Palbociclib + letrozole | PALOMA-23 | Yesa | N.A. | Yes | First-line treatment for endocrine-sensitive HR+/HER2– MBC with postmenopausal status. | 3 |
| Ribociclib + letrozole | MONALEESA-24, 5 | Yesa | Yes | Yes | First-line treatment for endocrine-sensitive HR+/HER2– MBC with postmenopausal status. | 3 | |||
| Abemaciclib + NSAI | MONARCH-36 | Yesa | N.A. | Yes | First-line treatment for endocrine-sensitive HR+/HER2– MBC with postmenopausal status. | 3 | |||
| HR+/HER2– | CDK 4/6 inhibitors + endocrine therapy | Ribociclib + AI/Tam | MONALEESA-77, 8 | Yesa | Yes | Yes | First-line treatment for endocrine-sensitive HR+/HER2– MBC with premenopausal status. | 5 | |
| HR+/HER2– | CDK 4/6 inhibitors + fulvestrant | Palbociclib + fulvestrant | PALOMA-39 | Yesa | No | Yes | Second- or first-line treatment (DFI ≤12 months from ET for EBC) for endocrine-resistant HR+/HER2– MBC | 4 | |
| Ribociclib + fulvestrant | MONALEESA-310, 11 | Yesa | Yes | Yes | Second- or first-line treatment (DFI ≤12 months from ET for EBC) for endocrine-resistant HR+/HER2– MBC and first-line treatment for endocrine-sensitive HR+/HER2– MBC | 4 | |||
| Abemaciclib + fulvestrant | MONARCH-212, 13 | Yesa | Yes | Yes | Second- or first-line treatment (DFI ≤12 months from ET for EBC) for endocrine-resistant HR+/HER2– MBC | 4 | |||
| PI3K inhibitors | HR+/HER2– | Alpelisib + fulvestrant | SOLAR-114 | Yesa (in PIK3CA-mutant cohort) | No | Yes | Treatment for endocrine-resistant HR+/HER2– MBC (EMA: no prior CDK 4/6) | 3 | |
| Oral SERDs | HR+/HER2– | Elacestrant | EMERALD15 | Yesa (in ITT and ESR1-mutant cohort) | No (immature) | Potentially | Treatment for endocrine-resistant HR+/HER2– MBC | N.A. | |
| mTOR inhibitors | HR+/HER2– | Everolimus + exemestane | BOLERO-216, 17 | Yesa | No | Yes | Treatment for HR+/HER2– MBC progressing to AI | 2 | |
| Dual HER2 blockade with anti-HER2 MABS | HER2+ | Pertuzumab + trastuzumab + CT | CLEOPATRA18, 19 | Yesa | Yes | Yes | First-line treatment for HER2+ MBC | 4 | |
| Anti-HER2 ADCS | HER2+ | T-DM1 | EMILIA20, 21 | Yesa | Yesa | Yes | Second-line treatment for HER2+ MBC or first line if DFI ≤6 months after completion of adjuvant trastuzumab | 4 | |
| HER2+ | T-DXd | DESTINY-Breast0322 | Yesa | No (immature) | Yes | Upcoming second-line treatment for HER2+ MBC with ≥1 prior anti-HER2-based lines. | 2 (based on DESTINY-Breast01) | ||
| HER2+ | Trastuzumab duocarmazine | TULIP23 | Yesa | No | Uncertain | Treatment as third-line or higher setting | NA | ||
| Anti-HER2 TKIs | HER2+ | Tucatinib + trastuzumab + capecitabine | HER2CLIMB24, 25 | Yesa | Yes | Yes | Treatment for HER2+ MBC HER2+ MBC with two or more prior anti-HER2-based lines (second line or higher for the FDA; third line or higher for the EMA) | 3 | |
| Novel anti-HER2 MAB | HER2+ | Margetuximab + trastuzumab + CT | SOPHIA26 | Yesa | Noa | Yes | Treatment for HER2+ MBC HER2+ MBC with two or more prior anti-HER2-based lines (at least one for MBC) | 2 | |
| PAN-HER TKIs | HER2+ | Neratinib + capecitabine | NALA27 | Yesa | Noa | Yes | Treatment for HER2+ MBC HER2+ MBC with two or more prior anti-HER2-based lines | 1 | |
| HER2+ | Pyrotinib + capecitabine | PHOEBE28 | Yesa | No | Not outside China | Treatment in patients with one or more prior line | N.A. | ||
| Anti-PD-1/PD-L1 agents | TN—PD-L1+ | Atezolizumab + nab-paclitaxel | IMPASSION13029, 30 | Yesa (in ITT) | Noa (in ITT not significant → PD-L1+ not tested) | Yes | First-line treatment for TN and PD-L1+ MBC (atezolizumab marketing authorization withdrawn in the United States) | 3 | |
| TN—PD-L1+ | Pembrolizumab + CT | KEYNOTE35531 | Yesa (in PD-L1+ CPS ≥10 → PD-L1+ CPS ≥1 → ITT) | Yesa (in PD-L1+ CPS ≥10 → PD-L1+ CPS ≥1 not significant → ITT not tested) | Yes | First-line treatment for TN and PD-L1+ MBC | 3 | ||
| Anti-TROP2 ADC | TN | Sacituzumab govitecan | ASCENT32 | Yesa (in patients without brain metastases) | Yes | Yes | Treatment for TN MBC with two or more prior lines (at least one for MBC) | 4 | |
| PARP inhibitors | gBRCA+ HER2– | Olaparib | OlympiAD33, 34 | Yesa | No | Yes | Treatment for gBRCA+ TN MBC with prior chemotherapy | 4 | |
| gBRCA+ HER2– | Talazoparib | EMBRACA35, 36 | Yesa | No | Yes | Treatment for gBRCA+ TN MBC with prior chemotherapy | 4 | ||
MCBS, Magnitude of Clinical Benefit Scale; AI, aromatase inhibitor; NSAI, non-steroidal aromatase inhibitor; BC, breast cancer; DFI, disease-free interval; EBC, early breast cancer; CPS, combined positive score; CT, chemotherapy; EMA, European Medicines Agency; ET, endocrine therapy; FDA, Food and Drug Administration; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; ITT, intention to treat; MBC, metastatic breast cancer; mTOR, mammalian target of rapamycin; OS, overall survival; PARP, poly(adenosine diphosphate ribose) polymerase; PFS, progression-free survival; PI3K, phosphatidylinositol 3-kinase; SERD, selective estrogen downregulator; Tam, tamoxifen; T-DXd, trastuzumab deruxtecan; TN, triple negative.
Study primary endpoint.
Hormone receptor-positive/HER2-negative breast cancer
HR+/HER2– BC accounts for approximately two-thirds of all BCs and endocrine-based therapy represents the preferred frontline treatment strategy for advanced disease, with the exception of those cases where the presence (or high menace of) visceral crisis demands to give priority to chemotherapy to minimize the risk of organ failure. In this context, type and duration of first-line chemotherapy have not been well established; however, depending on timing/magnitude of response as well as tolerability, switch to maintenance endocrine therapy (ET) is generally considered with the aim of delaying disease progression.37,38
Furthermore, the establishment of a state of endocrine resistance and the exhaustion of endocrine-based strategies represent clinical scenarios where chemotherapy is still commonly used.39, 40, 41
CDK 4/6 inhibitors
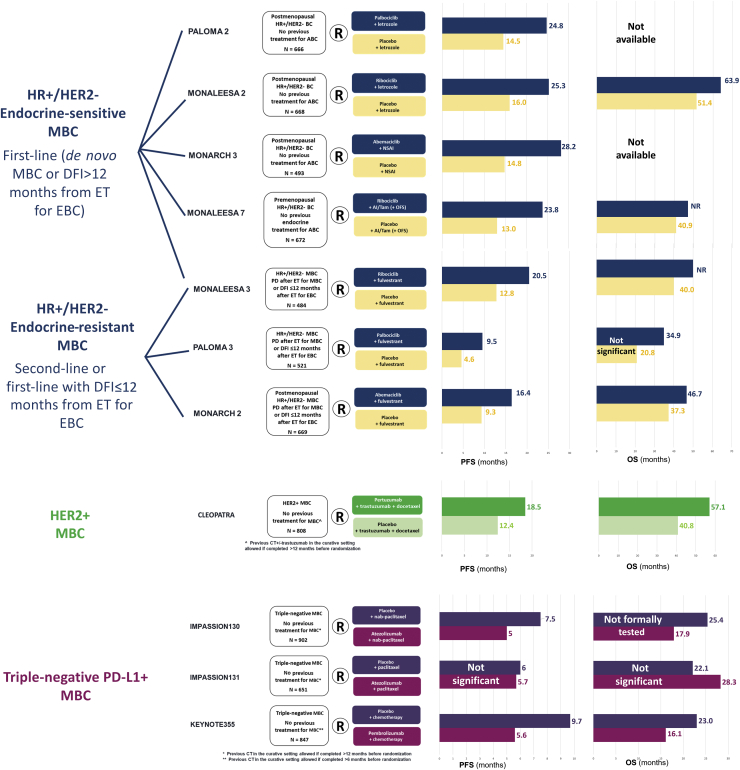
The incorporation of CDK4/6 inhibitors in the treatment armamentarium of HR+/HER2– MBC represents one of the major advancements we have witnessed, and currently it is the preferred first-line approach for the vast majority of newly diagnosed HR+/HER2– MBC patients, as highlighted in Figure 2.
Figure 2.
Main trials investigating first-line strategies across breast cancer (BC) subtypes with progression-free survival (PFS) and overall survival (OS) results.
Trials investigating CDK 4/6 inhibitors in endocrine-resistant HR+/HER2– metastatic breast cancer (MBC) have been included because they also covered the first-line setting in case of early relapse from endocrine therapy for early-stage disease.
HR, hormone receptor; HER2, human epidermal growth factor receptor 2; MBC, metastatic breast cancer; DFI, disease-free interval; ET, endocrine therapy; EBC, early breast cancer; R, randomization; PD, progressive disease; NSAI, non-steroidal aromatase inhibitor; OFS, ovarian function suppression; Tam, tamoxifene; PFS, progression-free survival; OS, overall survival; CT, chemotherapy; NR, not reached.
CDK4/6 inhibitors act by inhibiting the transition from G0/G1 to S phase of the cell cycle, which is mediated, among others, by the interaction of cyclin-D and CDK4/6 resulting in Rb phosphorylation, and ultimately, in the release of the transcription factor E2F. The rationale for testing CDK4/6 inhibitors in HR+/HER2– BC relies on the notion that cyclin D/CDK 4/6 pathway is frequently overexpressed in HR+/HER2– BC via ER signalling.42 Currently, three CDK4/6 inhibitors are available in clinical practice based on results from phase III trials leading to Food and Drug Administration (FDA)/European Medicines Agency (EMA) approval: palbociclib, ribociclib, and abemaciclib. Although comparable clinical activity has been reported, major pharmacological differences may be outlined across the three agents in terms of potency in CDK4/6 inhibition, target activity, drug administration schedule, and metabolism. In particular, abemaciclib is associated, as compared with ribociclib and palbociclib, with higher potency in the inhibition of CDK4/CDK6, as well as wider target activity, also including CDK9, thus possibly accounting for its increased activity when administered as a single agent, as well as increased number of off-target interactions, as compared with the other 2 CDK4/6 inhibitors.43, 44, 45 In addition, while ribociclib and palbociclib are administered with a 3 weeks on/1 week off schedule, abemaciclib is given continuously. As far as the metabolism is concerned, all the three drugs are metabolized at liver through CYP3A4 activity, with also a role for SULT2A1 enzyme in palbociclib metabolism.46 CDK4/6 inhibitors show differences also in terms of toxicity profile. In particular, although bone marrow toxicity may be considered a class effect, it is more pronounced with palbociclib and ribociclib as compared with abemaciclib. By contrast, abemaciclib shows a more pronounced gastrointestinal toxicity as compared with the other two agents, mainly in terms of diarrhea. Finally, an increased risk of QTc prolongation and liver enzyme abnormalities has been mostly associated with ribociclib.47
The combination of either one of the three CDK4/6 agents + aromatase inhibitor (AI) has received FDA/EMA approval as first-line treatment for endocrine-sensitive HR+/HER2– MBC patients, namely, those presenting with de novo stage IV disease or experiencing relapse >12 months after the completion of adjuvant ET. This regulatory scenario builds on results of four trials: PALOMA-2,3 MONARCH-3,6 MONALEESA-2,4,5 and MONALEESA-7.7,8 This latter trial specifically focused on premenopausal patients, as summarized in Figure 2. All these trials met their primary endpoint (investigator-assessed PFS), demonstrating a significant PFS improvement associated with the CDK4/6 inhibitor arm as compared with the placebo arm. In particular, in postmenopausal patients, the addition of palbociclib, abemaciclib, and ribociclib to an AI resulted, respectively, in 42%, 56%, and 54% relative reduction in the risk of PFS events as compared with ET alone. In the PALOMA-2 and MONALEESA-2 trials, leuko-neutropenia was the most common adverse event (AE) significantly associated with the CDK4/6 inhibitor, while diarrhea, mostly grade 1, represented the most frequent AE associated with abemaciclib. Finally, 3.3% of patients receiving ribociclib experienced a QTc prolongation >480 ms, which has proved to be fatal in one patient.
OS results from the MONALEESA-2 trial have been recently reported, revealing that ribociclib + letrozole was associated with a significant reduction in the risk of death as compared with letrozole + placebo.5 In particular, ribociclib + letrozole determined a 12.5-month prolongation of OS as compared with letrozole + placebo with an increasing OS delta over time (OS delta at 4 and 6 years: 5.7% and 12.2%, respectively).
The MONALEESA-7 trial was designed to assess ribociclib efficacy and safety as first-line treatment in 672 premenopausal patients, who were randomized to receive ribociclib/placebo in association with either tamoxifen or AI, all with goserelin. The primary endpoint analysis (investigator-assessed PFS) revealed a significant improvement of PFS with the CDK4/6 inhibitor as compared with placebo, and a significant improvement of OS was also reported, consistently across all major subgroups, including that of patients receiving an AI as endocrine partner for ribociclib. Consistently with the MONALEESA-2 trial, neutropenia was confirmed as the most common AE, and QTc prolongation >480 ms occurred in 7% of ribociclib-treated patients. Importantly, the mean QTcF increase from baseline was >10 ms higher with tamoxifen representing the endocrine partner for ribociclib over AI, thus compelling drug regulatory agencies to exclude tamoxifen from ribociclib-based combinations which were given approval in the premenopausal population.
Besides survival impact, the incorporation of CDK4/6 inhibitors to AIs as first-line treatment has also been demonstrated to delay the start of subsequent chemotherapy, thus strengthening the clinical value of this endocrine-based strategy.5,48,49 In addition, first-line administration of CDK4/6 inhibitors has been reported to yield remarkable antitumor activity, with objective response rate (ORR) ranging from 51% to 59.2% across trials. The combination or AI + palbociclib/abemaciclib has been associated with median time to response of 3.4 and 3.6 months, respectively; similarly, the proportion of patients with an ORR at 2 months was 16% in a pooled analysis of the MONALEESA 2 and MONALEESA 7 trials.50 Indeed, although international guidelines recommended to consider upfront chemotherapy only in patients with visceral crisis, even in the pre-CDK4/6 inhibitor era, a not negligible proportion of HR+/HER– MBC patients with no evidence of high risk of imminent organ failure were still offered cytotoxic treatment as frontline treatment.51, 52, 53 In this context, data regarding magnitude and promptness of CDK4/6-driven antitumor response overall reassure on the value of these endocrine-based strategies even in clinical situations where a high burden of disease compels to achieve a rapid response.
The combination of CDK4/6 inhibitors + fulvestrant has been FDA/EMA approved in patients presenting with endocrine-resistant MBC, based on results from the PALOMA-3,9 MONARCH-2,12 and MONALEESA-310 trials, as shown in Figure 2.9, 10, 12 In addition, based on results from the MONALEESA-3 trial, ribociclib + fulvestrant approval has been expanded as initial endocrine-based therapy in both the United States and Europe. The PALOMA-3, MONARCH-2, and MONALEESA-3 trials showed a significant investigator-assessed PFS improvement with the CDK4/6 inhibitor over placebo, in combination with fulvestrant, thus all meeting their primary endpoint. In particular, the addition of palbociclib, abemaciclib, and ribociclib to fulvestrant led to, respectively, a 58%, 45%, and 41% relative reduction in the risk of progression and death as compared with placebo + fulvestrant. Interestingly, in the MONALEESA-3 trial, the PFS benefit derived from the addition of ribociclib to fulvestrant was consistent irrespective of the endocrine sensitiveness setting. Importantly, in both MONALEESA-3 and MONARCH-2 trials a significant improvement on OS was also reported with the CDK4/6 inhibitor-containing arm as compared with placebo.11,13 In particular, ribociclib + fulvestrant was associated with a 28% difference in the relative risk of death as compared with fulvestrant + placebo in the MONALEESA-3 trial.11 In the MONARCH-2 trial, abemaciclib + fulvestrant was associated with a 24% relative reduction in the risk of death as compared with abemaciclib + placebo, with a median OS delta of 9.4 months.13
Importantly, the combination of CDK4/6 inhibitor + ET has been reported to yield comparable clinical efficacy as compared with chemotherapy in AI-resistant MBC, while being associated with a less toxic profile and a lower/delayed impact on QoL, as recently suggested in the context of the PEARL trial. In particular, although it failed to formally demonstrate the noninferiority of palbociclib + ET over capecitabine, it reported superimposable PFS rates, with lower rates of nonhematological toxicity, serious AEs, and treatment discontinuation due to AEs, as well as longer time to deterioration in patient-reported global health status.54
In the absence of head-to-head comparisons and by acknowledging the questionability of direct cross-trial comparisons, in the everyday treatment decision process it is reasonable to base the choice across the three CDK4/6 inhibitors on country-specific availability and regulatory/reimbursement policies, agent-specific toxicity spectrum falling outside the overlapping class-effect AEs as well as on efficacy information progressively maturing and getting released, especially in terms of OS impact, while cautioning on the confounding role of treatment crossover and postprogression therapies, which may mitigate the peremptoriness of not statistically significant OS results.
Beyond CDK 4/6 inhibitors
As comprehensively outlined in the preceding text, undeniable and dramatic enhancement of patients’ prognosis has been obtained with the incorporation of CDK4/6 inhibitors to ET; however, acquired resistance to ET remains a challenge and, in this context, further efforts have been directed toward obtaining a further extension of PFS/OS among patients with HR+/HER2– MBC. Within this framework, phosphatidylinositol 3-kinase (PI3K) inhibitors stood out as a promising strategy in patients experiencing relapse or progression to AI-based therapy, with, however, a controversial regulatory positioning, as comprehensively outlined in the following text. The value of PI3K inhibitors seems to be restricted to patients harboring PIK3CA activating mutations, mediating the hyperactivation of PI3K. This represents an acknowledged mechanism of endocrine resistance, which may potentially be reverted by negatively interfering with PI3K itself.55,56 Several trials investigated pan-PI3K inhibitors, which inhibit the kinase activity of all four PI3K isoforms, thus affecting multiple downstream pathways.57,58 This inevitably results in increased risk of both on-target and off-target toxicities, which has ultimately limited the clinical implementation of such agents.59,60 For this reason, the experimental scenario veered into the investigation of PI3K isoform-specific inhibitors, which are expected to retain a wider therapeutic index, with fewer off-target toxicities. In this context, the most promising results have been obtained with the α-specific PI3K inhibitor alpelisib in combination with fulvestrant. The SOLAR-1 trial enrolled 572 HR+/HER2– MBC patients, who had received prior ET, in two cohorts based on PIK3CA status assessed on tumor tissue (mutated and wild-type), with investigator-assessed PFS in the PIK3CA-mutated cohort representing the primary endpoint. Patients were randomized to receive either placebo or the PI3K inhibitor alpelisib in combination with fulvestrant. The trial succeeded in demonstrating a significant PFS improvement with alpelisib as compared with placebo in the PIK3CA-mutated cohort, with median PFS of 11.0 months versus 5.7 months, respectively. No significant PFS improvement was instead observed in the PIK3CA wild-type cohort. Hyperglycemia represented the most relevant grade 3/4 AE associated with the PI3K inhibitor, followed by rash and diarrhea. Regarding hyperglycemia, it represents an on-target effect of alpelisib, linked with α-specific PI3K inhibition, leading, in the SOLAR-1 trial, to permanent discontinuation of alpelisib in >6% of the patients, thus outlining that rigorous monitoring is required to both minimize treatment discontinuation and maximize alpelisib benefit.14 Based on these results, the combination of alpelisib + fulvestrant has been approved by the FDA for patients with HR+/HER2– MBC and mutation of PIK3CA following progression on or after ET. Subsequently, this combination also received approval by the EMA, which however restricted alpelisib indication to HR+/HER2– MBC patients previously treated with ET as monotherapy, thereby precluding this strategy to patients progressing to CDK4/6 inhibitor. This regulatory limitation is based on the ascertainment that only 6% of patients enrolled in the SOLAR-1 trial had received prior CDK4/6 inhibitor, thus precluding the possibility to solidly establishing the efficacy of alpelisib in this specific cohort of patients.
The role of PI3K inhibitor in a post-CDK4/6 inhibitor setting has subsequently been addressed in the context of the BYLieve phase II open-label noncomparative study, which investigated the combination of alpelisib + ET in HR+/HER2– MBC patients with PIK3CA mutation, following progression on or after previous therapy, including CDK4/6 inhibitors, allocating them into three cohorts based on the most recently received ET (cohort A: alpelisib + fulvestrant in patients treated with previous CDK4/6 inhibitor + AI; cohort B: alpelisib + letrozole in patients treated with CDK4/6 inhibitor + fulvestrant; cohort C: alpelisib + fulvestrant in patients treated with previous systemic chemotherapy or ET). In cohort A, alpelisib + fulvestrant was associated with a 6-month PFS of 50.4% and median PFS of 7.3 months, thus suggesting this combination as an active therapeutic option with manageable safety profile in HR+/HER2– PIK3CA-mutated MBC patients also in a ‘pure’ post-CDK4/6 inhibitor + AI setting.61 Interestingly, interim findings from BYLieve cohort B suggested promising activity also with the combination of alpelisib + letrozole, thus further supporting the role of alpelisib in patients priorly treated with CDK4/6 inhibitors.62 Although these results overall solidify the role of alpelisib in a more contemporary clinical scenario, the EMA considered the BYLieve trial results of limited value from a regulatory point of view, given the small sample size, the highly selected population, and the limited number of matching variables used, thus demanding a prospective randomized controlled clinical trial to consider expanding the current indication for alpelisib in Europe also to patients pretreated with CDK4/6 inhibitor.
Besides the subgroup of patients harboring a PIK3CA mutation, the use of sequential ET is endorsed by major international guidelines when there is no evidence of visceral crisis, until all endocrine-based options have been exhausted, despite the well-acknowledged uncertainty regarding the optimal endocrine-based sequence and the ideal timing for considering switching to chemotherapy (besides the emergence of visceral crisis).
The occurrence of therapeutic resistance to ET has been linked to several mechanisms, among which the acquisition of ESR1 mutations making ER activation independent from estrogen ligand stands up as one of the most well-established and common drivers,63,64 especially after exposure to an AI, with a reported prevalence up to 20%-30%. In this setting, selective estrogen downregulators (SERDs) may be capable of circumventing such resistance mechanism by inhibiting both estrogen-dependent and estrogen-independent ER signaling. However, although fulvestrant has been proved to retain promising activity in HR+/HER2– MBC patients with ESR1 mutations, it is still associated with poor median PFS in endocrine-resistant patients, including those pretreated with CDK 4/6 inhibitors,65,66 thus outlining a challenging clinical scenario. In this context, orally bioavailable SERDs with antiestrogenic and ER degrading capability have emerged as a promising strategy, showing to retain antitumor activity in endocrine-resistant HR+/HER2– MBC patients, including those previously treated with CDK 4/6 inhibitors and fulvestrant, as well as those harboring ESR1 mutations.67,68 In this context, potentially practice-changing data have been reported in the context of the EMERALD study, the first phase III trial comparing the oral SERD elacestrant with treatment of physician’s choice (TPC) (fulvestrant or AIs) in 477 HR+/HER2– MBC patients progressed or relapsed on or after one to two endocrine-based lines for advanced disease, including CDK 4/6 inhibitor.15 The trial met its dual primary endpoints, by showing a ≈30% and 45% relative reduction in the risk of PFS events with elacestrant over TPC in the intention-to-treat (ITT) population and in the subgroup of patients harboring ESR1 mutation (by ctDNA analysis), respectively, with also a signal of OS improvement at the interim analysis. Elacestrant was associated with a manageable and predictable safety profile. These results are expected to impact future clinical practice, with a growing interest on this novel drug class, mirrored by the fact that several trials are currently ongoing, investigating other single-agent SERDs as well as diverse SERD-based combination strategies, with those involving CDK 4/6 inhibitors being awaited with particular interest.
In the context of pre-treated MBC, it is also worth mentioning the combination of exemestane with the mTOR inhibitor everolimus, the clinical value of which has been established in the context of the BOLERO-2 phase III trial, where the addition of everolimus to exemestane provided PFS benefit over exemestane + placebo, while failing to provide an OS advantage.16,17 Based on these results, since 2012 everolimus has been formally incorporated in the treatment armamentarium of HR+/HER2– MBC in both Europe and the United States, with a contemporary clinical positioning in a pretreated MBC population.
Although the increasing availability of effective endocrine-based strategies has progressively moved forward the timing of chemotherapy being considered, during the course of HR+/HER2– MBC natural history it still represents a viable treatment, preferably as a single agent, in patients deemed endocrine resistant and/or ruling out main endocrine-based strategies.
HER2+ breast cancer
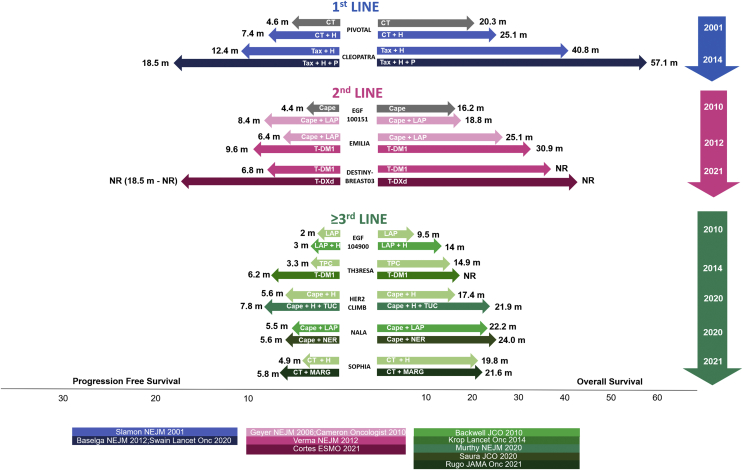
HER2+ BC accounts for ∼15%-20% of all BCs and represents one of the most aggressive BC subtypes.69 However, since 1998, which marked the beginning of the anti-HER2 targeted therapy era, survival rates of patients with metastatic disease have dramatically and progressively improved, as shown in Figure 3.
Figure 3.
Progression-free survival (PFS) and overall survival (OS) results from major randomized clinical trials testing anti-HER2 strategies according to treatment line.18, 19, 20,22,25, 26, 27,70, 71, 72, 73, 74
HER2, human epidermal growth factor receptor 2; CT, chemotherapy; H, trastuzumab; P, pertuzumab; Tax, taxane; Cape, capecitabine; LAP, lapatinib; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan; TPC, treatment of physician choice; TUC, tucatinib; NER, neratinib; MARG, margetuximab; NR, not reached; m, months.
First-line treatment
The current standard of care as first-line treatment has been established in the context of the CLEOPATRA phase III trial, which randomized 808 patients not previously treated in the advanced setting (>50% were presented with de novo stage IV MBC) to receive trastuzumab + docetaxel + pertuzumab/placebo, as shown in Figure 2. The trial demonstrated a highly clinically significant improvement of PFS with docetaxel + dual-HER2 blockade.18 Importantly, in the end-of-study analysis, pertuzumab + trastuzumab + docetaxel was associated with a median OS of 57.1 months, significantly superior to that observed in the placebo arm (40.8 months), thus crystallizing this treatment strategy as the standard first-line treatment up to the present time.19 A consideration worth raising is that, in the CLEOPATRA trial, maintenance ET in patients with ER coexpression was not allowed, thus precluding the possibility of formally establishing its value in this setting. However, the strategy of switching to maintenance dual HER2-blockade (trastuzumab + pertuzumab) + ET in ER+ patients after completing at least six cycles of upfront concomitant taxane-based chemotherapy is currently enshrined in major international guidelines.39,40,75
Second-line treatment
Treatment with trastuzumab emtansine (T-DM1) has represented the long-established standard in the second-line setting based on results from the EMILIA trial.20 In particular, T-DM1 is an anti-HER2 antibody–drug conjugate (ADC) consisting of the anti-HER2 antibody Trastuzumab to which a cytotoxic payload (maytansine) is linked through a not cleavable linker. The antibody’s primary function is to selectively bind to the target antigen (HER2) and deliver the cytotoxic drug payload directly to the site of tumor, thus minimizing the risk of off-target effects. Once the antibody–antigen complex is internalized, the cytotoxic material is released in its active form, thus exerting its action selectively inside the target cells.76 The EMILIA phase III trial randomized 991 HER2+ MBC patients (progressing to taxane + trastuzumab in the metastatic setting or within 6 months after completing the anti-HER2 adjuvant treatment) to either capecitabine + lapatinib or T-DM1. The trial demonstrated a significant improvement in both PFS and OS with T-DM1 over the control arm.20,21 The value of T-DM1 has been also strengthened in a third-line setting scenario by the TH3RESA phase III trial, which randomized 602 HER2+ MBC patients previously treated with two anti-HER2 lines to receive T-DM1 versus TPC. The TH3RESA trial demonstrated a significant PFS and OS improvement with T-DM1 over the control arm.70,77 Based on these results, on 2013, T-DM1 was granted approval by the FDA/EMA for HER2+ MBC patients experiencing either disease progression on taxane+anti-HER2-based treatment in the metastatic setting or disease relapse within 6 months after the completion of adjuvant treatment.
The role of T-DM1 as standard second-line treatment has been recently challenged by the results of the DESTINY-Breast03 phase III trial, which demonstrated a highly statistically significant and clinically meaningful superiority of the novel anti-HER2 ADC trastuzumab deruxtecan (T-DXd) over T-DM1 in the second-line setting.22 The key structural components of T-DXd are the anti-HER2 monoclonal antibody trastuzumab, a cleavable linker, and a topoisomerase I inhibitor as payload. Some major differences between these two ADCs may account for the higher potency of T-DXd over T-DM1. In detail, T-DXd is characterized by a higher drug-to-antibody ratio, the linker is cleavable, and the payload has a high membrane solubility. Both the linker cleavability and the payload permeability contribute to the so-called bystander effect, which allows the payload to carry out its cytotoxic properties also on tumor cells located in the neighborhood of the one the ADC specifically binds to, and, in the case of T-DXd, this occurs mostly after the internalization thanks to the payload transmembrane permeabilization and diffusion.78
The DESTINY-Breast03 trial builds on the impressive preliminary results regarding T-DXd antitumor activity observed in the DESTINY-Breast01 phase II trial, where 184 HER2+ MBC patients, treated with at least two prior lines of anti-HER2 therapy, including T-DM1 (median: 6 previous treatments), were treated with T-DXd. In the ITT analysis, ORR was 60.9%, median duration of response was 14.8 months, median PFS was 16.4 months, and OS at 6 and 12 months was 93.9% and 86.2%, respectively. Activity results were consistent across all major subgroups, including that of pertuzumab pretreated patients and those defined based on HR status, thus suggesting a high level of clinical activity in heavily pretreated HER2+ MBC patients. The most common grade ≥3 AEs were hematological and gastrointestinal. Importantly, 13.6% of patients experienced interstitial lung disease, proving to be fatal in 2.2% of the total patients.79 Based on these results, in 2019 the FDA granted accelerated approval to T-DXd for patients with HER2+ MBC who have received two or more prior anti-HER2-based regimens in the metastatic setting. Contextually, the FDA cautioned about T-DXd-associated interstitial lung disease by inserting a warning box in T-DXd label, endorsing the monitor and the prompt investigation of signs and symptoms of respiratory nature. Subsequently, the EMA echoed the FDA decision by recommending T-DXd for conditional marketing authorization with the same indication. On this groundwork laid the DESTINY-Breast03 phase III trial, comparing head-to-head T-DXd versus T-DM1 in 524 HER2+ MBC patients previously treated with taxane + trastuzumab in the advanced setting or experiencing disease relapse within 6 months after completing adjuvant treatment. More than 60% of patients had also received prior pertuzumab. The trial met its primary endpoint by showing a 72% relative reduction in the risk of PFS events with T-DXd as compared with T-DM1, with median PFS not reached in the experimental arm versus 6.8 months in the control arm with an impressive HR of 0.28 (95% confidence interval (CI) 0.22-0.37, P < 0.001). The benefit appeared consistent across all major subgroups, including those defined by HR status, prior pertuzumab, prior treatment lines, visceral disease, and the presence of brain metastases. Interestingly, T-DXd was associated with an impressive ORR (79.7%), which was significantly superior to T-DM1 (34.2%). T-DXd and T-DM1 were associated with similar rates of drug-related treatment emergent adverse event (TEAE), with interstitial lung disease/pneumonitis being the most commonly reported drug-related TEAE leading to treatment discontinuation with T-DXd (any grade 8.2%, mostly grade 1-2, no grade 4-5). Finally, early and immature OS data also showed an initial trend for survival benefit.22 Based on these compelling results, the FDA granted T-DXd a breakthrough therapy designation for the treatment of HER2+ MBC patients who have received one or more prior anti-HER2-based regimens. This evolving regulatory landscape will shift T-DM1 positioning in subsequent lines. In this context, it is currently largely unknown whether T-DM1 administration in a T-DXd-resistant setting would be biologically rational, also considering that an expected increasing proportion of HER2+ BC patients will have been already treated with T-DM1 in the post-neoadjuvant setting.80 Of course, a better understanding of mechanisms driving resistance to T-DXd will provide crucial insights in this—so far—unexplored issue and, in this regard, translational analyses of the DESTINY-Breast03 trial are highly awaited.
Third-line treatment
Treatment of HER2+ MBC in subsequent lines represents a challenging scenario, with several agents with demonstrated efficacy in pretreated patients, without, however, an established univocal optimal sequence.39,40,75 Currently, from the third-line setting onward, the treatment decision process is typically conditional on the uncertainty of results obtained in the context of clinical trials enrolling a patient population no longer representative of the contemporary clinical scenario. In this context, the driving principle should be continuing HER2 blockade beyond progression, by tailoring the treatment sequence based on—all other things being equal—the demonstrated efficacy in the context of clinical trials in terms of both PFS and OS, the agent-specific toxicity spectrum, and the country-specific regulatory scenario.
The HER2CLIMB trial investigated safety and efficacy of tucatinib, an oral tyrosine kinase inhibitor (TKI) that shows high selectiveness for the kinase domain of HER2 with minimal inhibition of HER1, combined with trastuzumab + capecitabine in patients with HER2+ MBC previously treated with trastuzumab, pertuzumab, and T-DM1. Interestingly, patients with brain metastases, including those with active lesions could be eligible, based on the efficient brain penetration demonstrated by tucatinib in an early-phase clinical trial.24 A total of 480 patients were randomized to receive either tucatinib + trastuzumab + capecitabine or placebo + tucatinib + capecitabine. The tucatinib-containing arm was associated with significantly improved PFS as compared with placebo arm, with also a significant improvement of OS. The most prevalent grade 3/4 AEs with tucatinib were palmar–plantar erythrodysesthesia, diarrhea, increased liver enzymes, and fatigue.25 Interestingly, tucatinib was demonstrated to yield an impressive efficacy—both intracranially and extracranially—in the subgroup of patients with brain metastases, including those with active brain metastases, which represents a subgroup typically excluded from clinical trials (or at least severely underrepresented), thus traditionally presenting a high level of unmet need.81 Based on these data, in 2020 the FDA granted regular approval to the combination of tucatinib + trastuzumab + capecitabine for patients with HER2+ MBC, including those with brain metastases, who have received one or more prior anti-HER2-based regimens in the advanced setting. The FDA also granted tucatinib breakthrough therapy designation. The same year, the EMA also recommended marketing authorization for tucatinib + trastuzumab + capecitabine in HER2+ MBC patients already treated with at least two prior anti-HER2-based lines.
In the changing landscape of HER2+ MBC, with T-DXd becoming the standard second-line treatment in a near future, the expected positioning of tucatinib + trastuzumab + capecitabine may be as third-line treatment, following progression to T-DXd, based on the assumption that having the efficacy of tucatinib-based therapy been demonstrated in a T-DM1-resistant setting, it would be conceivable to indirectly transfer this observation to a T-DXd-resistant scenario. A separate consideration may be done for patients with active brain metastases, included in the HER2CLIMB trial, but excluded from the DESTINY-Breast03 trial. Indeed, in this patients’ subgroup, the tucatinib-based combination yielded highly significant and clinically meaningful benefit, both intracranially and extracranially, thus being suggested as a potential preferred second-line option over T-DXd in patients with active brain metastases progressing to first-line dual-HER2 blockade.82 However, this near-future scenario may be an object of intense debate pending the results from the phase II—Simon’s two stage—TUXEDO-1 trial, assessing T-DXd in HER2+ MBC patients, priorly exposed to trastuzumab and pertuzumab, with newly diagnosed brain metastases or brain lesions radiologically progressing after prior local therapy. The preliminary report suggests a 83.3% rate of intracranial response with T-DXd, thus meeting the criteria for moving to the second stage, as well as generating the hypothesis that T-DXd might be highly effective also in this subgroup of patients.83
Beyond third-line treatment
Beyond the third line, there are various combinations of anti-HER2 agents with chemotherapy which may represent valid options.
In particular, in the SOPHIA phase III trial, safety and efficacy of the novel anti-HER2 antibody margetuximab as compared with trastuzumab—all in combination with chemotherapy—have been evaluated in 536 HER2+ MBC patients progressing to at least two prior lines of anti-HER2-based therapy.26 In detail, margetuximab represents an anti-HER2 chimeric antibody, which, while sharing with trastuzumab the epitope specificity and the Fc-independent antiproliferative effect, has been engineered in its IgG1 Fc portion to increase the affinity for activating Fc γ CD16A receptor and simultaneously to decrease the affinity for inhibitory Fc γ CD32B receptor. These features result in the increased ability of margetuximab, as compared with trastuzumab, to enhance both the innate and adaptive antitumor immune response. The SOPHIA trial showed a PFS improvement with margetuximab over trastuzumab added to chemotherapy, with a 24% relative reduction in the risk of progression or death. No solid conclusion may instead be drawn on OS—the sequential primary endpoint—given the immaturity of data. Interestingly, in the SOPHIA trial the hypothesis that CD16A genotype may drive clinical benefit has been tested, with appealing results. In particular, this exploratory analysis generated the hypothesis that the PFS benefit captured with margetuximab over trastuzumab may be mainly driven by the subgroup of patients harboring low-affinity CD16A-158F genotype, which, conversely, may account for a diminished clinical response to trastuzumab. To validate this observation, a clinical trial comparing margetuximab versus trastuzumab in low-affinity CD16A genotype carriers is currently ongoing (MARGOT: NCT04425018). Based on SOPHIA trial results, in 2020, the FDA granted margetuximab (+ chemotherapy) regular approval for HER2+ MBC patients who have already received two or more prior anti-HER2 regimens, at least one of which for metastatic disease. The EMA decision on margetuximab’s possible marketing authorization is still pending.
Another class of anti-HER2 agents exhibiting promising activity and efficacy in pretreated patients with HER2+ MBC is represented by pan-HER TKI. In particular, neratinib and pyrotinib have been compared with lapatinib, in association with capecitabine, in the context of two phase III trials, NALA and PHOEBE, respectively.27,28 Both trials showed modest PFS improvements with novel anti-HER2 TKI as compared with lapatinib, while both failed to capture a significant benefit in terms of OS. Interestingly, secondary endpoint analysis of the NALA trial revealed that neratinib significantly delayed the time to intervention for symptomatic disease on central nervous system. The major safety concern associated with these novel anti-HER2 TKIs was diarrhea, with grade ≥3 events occurring in ∼25% and 31% of patients receiving neratinib and pyrotinib, respectively. One of the major limitations of both trials is that patients pretreated with the current standard of care were highly underrepresented, with only one-third of patients in the NALA trial and none of the patients in the PHOEBE study having received prior pertuzumab and T-DM1; in addition, the appropriateness of the control arms—lapatinib + capecitabine—has been questioned given that trastuzumab + capecitabine has been previously shown to provide increased OS benefit as compared with lapatinib + capecitabine.84 Both these limitations contribute to complicating the contextualization of NALA and PHOEBE trial results in a contemporary scenario. In addition, it should be mentioned that the PHOEBE trial was entirely conducted in China, thus precluding the possibility to draw conclusions on pyrotinib efficacy in a White population. Currently, the combination of neratinib + capecitabine is FDA approved for HER2+ MBC patients who have already received two or more prior anti-HER2 therapies for MBC, while pyrotinib (+ capecitabine) was granted regular approval by the China National Medical Products Administration as second-line standard-of-care treatment for HER2+ MBC.
Finally, the role of the anti-HER2 ADC trastuzumab duocarmazine is worth a mention. In particular, this novel ADC, based on trastuzumab linked through a cleavable linker to the cytotoxic payload duocarmycin, has been shown to retain a promising antitumor activity in 50 heavily pretreated HER2+ BC patients in the dose-expansion cohort of a phase I trial.85 In particular, ORR was 33%, and median PFS was 7.6 months. Importantly, 80% of the HER2+ cohort had received prior T-DM1, thus strengthening the activity of novel anti-HER2 ADCs in a T-DM1-refractory or T-DM1-resistant setting and leading to FDA fast-track designation in 2018 for pretreated HER2+ MBC patients (≥2 prior lines). The subsequent phase III TULIP trial randomized 437 HER2+ MBC patients receiving two or more lines of T-DM1 for MBC to receive either trastuzumab duocarmazine or TPC.23 Although the study met its primary endpoint by showing a significant improvement of PFS, the 2.1-month PFS delta between the experimental arm and the control arm (median PFS 7.0 months versus 4.9 months) may not reflect a clinically meaningful result. For this reason, and in the light of compelling results from the DESTINY-Breast03 trial, the future positioning of trastuzumab duocarmazine is uncertain. Indeed, the integration of the TULIP results within the rapidly evolving landscape of HER2+ MBC imposes to consider the biological rationale of administering trastuzumab duocarmazine after progression to T-DXd. In this context, the type of resistance mechanisms driving progression to T-DXd has been put forward as a possible key determinant in affecting the subsequent response to trastuzumab duocarmazine. In this context, loss of HER2 membrane expression and defects in ADC internalization or lysosomal proteolytic activity have been suggested as possible resistance mechanisms shared also with other ADCs, while mechanisms involving specifically T-DXd payload may represent non-cross-resistant drivers, thus possibly leaving space for the subsequent administration of trastuzumab duocarmazine.86, 87, 88 Of course, these are just speculations that need to be confirmed in the context of clinical trials.
‘Triple-positive’ breast cancer (hr+/her2+)
A further layer of complexity in HER2+ BC heterogeneity may be added by the simultaneous expression of HER2 and HR, which accounts for ∼50% of all HER2+ BC. Interestingly, the complex bidirectional crosstalk between HER2 and ER has been put forward as a possible key determinant of treatment resistance to both endocrine and anti-HER2 targeted therapies, thus providing a strong biological rationale for the evaluation of combination strategies.89 In this context, based on pivotal trials suggesting that the addition of anti-HER2 targeted therapy to ET may prolong PFS in patients with HER2+/HR+ MBC, subsequent studies have been conducted to investigate dual HER2 blockade in association with ET. In particular, the addition of pertuzumab or lapatinib to trastuzumab + AI has been demonstrated to be more effective than single HER2 blockade + ET in the context of the randomized PERTAIN90 and ALTERNATIVE91 trials, respectively, both meeting their primary endpoint. Recently, preliminary results from the Sysucc-002 trial further broadened the base on which the rationale for sparing chemotherapy in HR+/HER2+ MBC builds. Interestingly, the Sysucc-002 trial, which randomized 392 MBC patients simultaneously expressing HER and HR to receive first-line trastuzumab in association with either ET or chemotherapy, met its primary endpoint by formally demonstrating the noninferiority of the chemotherapy-free regimen over trastuzumab + chemotherapy, while being associated with a more manageable toxicity profile.92
Based on these reassuring data, international guidelines currently provide for the possibility of considering such chemotherapy-free, de-escalated regimens in selected cases of ‘triple-positive’ MBC patients not suitable for chemotherapy (based on clinical contraindications or patient’s preferences). However, before these considerations can be widely extended to all MBC patients with HR+/HER2+ tumors, more solid data are needed.
Triple-negative breast cancer
First-line treatment
TNBC, clinically defined by the absence of both HR expression and HER2 protein overexpression or gene amplification, is typically associated with poor outcome as compared with other BC subtypes.93,94 In particular, when diagnosed at an early stage, it exhibits a strong inclination to precocious metastatic dissemination, with high visceral tropism and, once metastatic, it is associated with particularly dismal prognosis, with estimated median OS of ∼18 months. Focusing on MBC, chemotherapy is still the mainstay of treatment, with taxane and anthracycline-based chemotherapy representing the preferred options in earlier lines, with also a role for platinum salts.39,40 In this scenario, the most remarkable advancement has been represented by the incorporation of immune checkpoint inhibitors (ICIs) in the first-line therapeutic armamentarium of TN MBC patients with PD-L1+ tumors, as shown in Figure 2. In detail, ICIs with a current regulatory positioning in this setting are the anti PD-L1 inhibitor atezolizumab and the PD-1 inhibitor pembrolizumab, based on the results from the Impassion130 and Keynote355 phase III trials, respectively.29,31 In particular, the Impassion130 trial randomized 900 TN MBC patients who were treatment naive in the metastatic setting to receive nab-paclitaxel with either placebo or atezolizumab. In the ITT population, patients receiving immunotherapy experienced a statistically significant absolute improvement of PFS by 1.7 months as compared with those receiving placebo. However, this benefit appeared to be driven by the subgroup of patients showing a PD-L1+ status on tumor tissue, as defined by at least 1% of tumor-infiltrating immune cells staining for PD-L1 by Ventana SP142 assay, where atezolizumab led to a 38% relative reduction in the risk of PFS events as compared with placebo with a 1.5-month absolute PFS improvement. At the final OS analysis, the association of atezolizumab + nab-paclitaxel determined a trend toward improved OS as compared with placebo in the ITT population, which however failed to cross the statistical significance boundary, thus precluding the possibility to formally test the OS endpoint in the PD-L1+ subgroup, given the hierarchical statistic design. However, an exploratory OS analysis in this patient subgroup was still reported, revealing a numerical absolute improvement in OS of 7.5 months in PD-L1+ patients receiving atezolizumab as compared with those allocated to placebo.29,30 Based on these results, atezolizumab was the first ICI to receive FDA accelerated approval and EMA approval as first-line treatment in combination with nab-paclitaxel for TN MBC patients harboring PD-L1+ tumor.
Subsequently, the Impassion131 trial, representing an Impassion130 twin study, investigated whether the addition of atezolizumab to conventional paclitaxel could improve outcome in TN MBC patients as first-line treatment. In particular, 651 TN MBC patients not selected for PD-L1 status were randomized to receive either atezolizumab + paclitaxel or placebo + paclitaxel, with PFS set as the primary endpoint. Surprisingly, the Impassion131 trial failed to demonstrate a significant PFS improvement with the addition of chemotherapy to conventional paclitaxel in the ITT population and in the PD-L1+ subgroup. Similarly, no OS improvement was observed neither in the ITT nor in the PD-L1+ populations.95
Several assumptions have been made to provide an explanation for such inconsistency of results between Impassion130 and Impassion 131 trials; however, none of them has been considered exhaustive enough in this regard.96 In particular, among others, the larger use of steroid premedication required with paclitaxel as compared with nab-paclitaxel and the possible exceptional performance of the control arm in the Impassion131 trial have been put forward as potential contributing factors. It should also be noted that a possible role of differences in population composition and features is not plausible, because sample size, metastatic burden, prior systemic treatment exposure in the early setting, and the percentage of PD-L1+ patients were similar across the two trials.
Following Impassion131 trial results, the EMA released a warning to physicians, reminding them to use atezolizumab only in combination with nab-paclitaxel and not with conventional paclitaxel when treating TN MBC patients with PD-L1+ tumor in the first-line setting. Conversely, in the United States, where atezolizumab was granted accelerated approval, the negative results of the Impassion131 trial, upon which the continued approval of atezolizumab was contingent, led to a withdrawal of its indication by the manufacturer following a consultation with the FDA, without however affecting the European Commission’s approval in this regard. Consequently, since August 2021, the combination of atezolizumab + taxane is no longer available in the United States as frontline treatment of TN MBC patients with PD-L1 positivity.
The efficacy of pembrolizumab as first-line treatment of TN MBC was evaluated in the context of the Keynote355 trial which randomized 847 patients not previously treated in the metastatic setting to receive pembrolizumab or placebo in association with chemotherapy per physician’s choice among paclitaxel, nab-paclitaxel, or carboplatin-gemcitabine. Dual-primary endpoints were PFS and OS in patients with PD-L1+ tumors [as defined by combined positive score (CPS) ≥10 and CPS ≥1 by 22C3 pharmDx] and in the ITT population, according to a hierarchical statistical design. The trial met its primary endpoint by showing a statistically significant improvement of both PFS and OS in patients with CPS ≥10. Hierarchically subsequent analyses in the CPS ≥1 and in the ITT populations did not show significant differences in terms of PFS and OS. Interestingly, pembrolizumab benefit was consistent across all major subgroups, including that defined by the choice of the chemotherapy backbone.31 Following these results, pembrolizumab has received accelerated approval from the FDA and approval from the EMA in combination with chemotherapy in patients with TN MBC whose tumors are PD-L1+ (CPS ≥10).
Although, as outlined earlier, the incorporation of immunotherapy represents one of the major achievements for TN MBC, it is unfortunately restricted to the 20%-40% of TN MBC patients harboring PD-L1+ status. In this context, the expansion of the pool of TN MBC patients who may benefit from immunotherapy represents an appealing research area deserving to be delved into. The technical and biological heterogeneity affecting PD-L1 testing is well acknowledged,97 with possible substantial implications in terms of treatment accessibility. Indeed, although some insights have already been put forward,98 specific guidelines defining the ideal timing, tumor site, and tumor source to assess PD-L1 for therapeutic purposes are lacking. In this context, given that the access to immunotherapy is currently contingent to PD-L1 positivity, and TN MBC presents an high level of unmet clinical need, it is mandatory to enhance the likelihood of detecting a PD-L1+ status to minimize the risk of false-negative results. By contrast, it might be worth exploring immune-induction strategies capable of priming TN tumors for response to ICI. In this context, the TONIC trial recently provided the proof of principle that a short course of induction chemotherapy, especially with anthracyclines or platinum salts, may be capable of turning cold tumors into hot ones, thus resulting in the enhancement of response to ICI.99 Of course, these data need to be confirmed in larger and late-in-stage clinical trials.
Second-line treatment and beyond
Beyond first-line, single-agent chemotherapy still represents the main treatment option for TN MBC patients, with, however, unsatisfactory response and survival rates,100, 101, 102, 103, 104 thus outlining an unmet clinical need.
Under this scenario, the ASCENT phase III trial was conducted, comparing sacituzumab govitecan with single-agent chemotherapy of physician’s choice.32 Sacituzumab govitecan represents a novel ADC composed of the anti-TROP2 IgG1 antibody, linked through a hydrolyzable linker to SN-38 (irinotecan active metabolite). The chemical and structural properties of this ADC, in terms of both hydrolizability of the linker and membrane permeability of SN-38, allow it to elicit a bystander effect. The target of sacituzumab govitecan is TROP2, which represents a transmembrane transducer of the calcium signal which has been found to be overexpressed in >90% of TN tumors.105
The ASCENT trial randomized 468 TN MBC patients, including patients with stable brain metastases, relapsed or refractory to two or more prior standard chemotherapy regimens to receive either sacituzumab govitecan or single-agent chemotherapy per physician’s choice among eribulin, vinorelbine, capecitabine, or gemcitabine. Primary endpoint analysis of PFS in patients without brain metastases revealed a statistically significant and clinically meaningful benefit in favor of sacituzumab govitecan as compared with single-agent chemotherapy, with a 59% relative reduction in the risk of progression or death, with also a significant impact in terms of OS. The benefit associated with sacituzumab govitecan over chemotherapy was consistent across all major subgroups and was also confirmed in the ITT population (which also included patients with stable brain metastases). In addition, when focusing on the subgroup of 61 patients with brain metastases, sacituzumab govitecan appeared to be numerically superior to chemotherapy, although the small sample size precluded the possibility to formally establish a statistically defined superiority of sacituzumab govitecan in this subgroup. The key treatment-related AEs were hematological toxicities and diarrhea. In addition, 5% and 9% of patients receiving sacituzumab govitecan experienced ocular toxicity and rash of any grade, respectively. Based on these compelling results, both the FDA and EMA granted approval to sacituzumab govitecan for patients with TN MBC receiving at least two prior lines of systemic treatment, at least one of them in the metastatic setting. Some interesting hints regarding the predictive value of TROP-2 expression may also be captured in the context of ASCENT translational analyses. In particular, the benefit provided by sacituzumab govitecan appeared to be greater in patients with high/medium TROP-2 levels than those with low levels of TROP-2. However, the small number of patients exhibiting low levels of TROP-2 and the lack of a formal treatment-sensitivity testing preclude the possibility to draw solid conclusions and additional biomarker data are needed to better elucidate the actual predictive role of TROP-2.106
gBRCA-associated breast cancer
Poly(adenosine diphosphate ribose) polymerase inhibitors
BRCA-associated BC accounts for ∼5% of all—unselected—breast tumors.107,108 The presence of a deleterious mutation in BRCA1/2 genes, which are involved in homologous recombination-driven DNA repairing mechanisms, makes the affected cells dependent on single-strand breaks repairing pathways, regulated by the enzyme poly(adenosine diphosphate ribose) polymerase (PARP). In this context, there is a strong biological rationale supporting the pharmacological inhibition of PARP as an effective antitumor strategy in BRCA-associated BC, leading to the accumulation of irreparable DNA damages, ultimately resulting in tumor cell death through the so-called synthetic lethality.109, 110, 111 Currently, two PARP inhibitors, olaparib and talazoparib, find a regulatory clinical positioning in HER2-negative, BRCA-associated MBC, based on the results from the OlympiAD and EMBRACA phase III trials, respectively.
In detail, the OlympiAD trial randomized 302 HER2-negative MBC patients with germline BRCA1/2 mutations, pretreated with no more than two prior chemotherapy lines in the metastatic setting (including taxane and anthracycline and not showing disease progression to platinum salts), to receive either olaparib or standard therapy with single-agent chemotherapy per physician’s choice among capecitabine, eribulin, or vinorelbine.33 The EMBRACA trial enrolled 431 HER2-negative MBC patients with germline BRCA1/2 mutations previously treated with no more than three chemotherapy regimens for MBC, to receive talazoparib or chemotherapy per physician’s choice (capecitabine, eribulin, gemcitabine, or vinorelbine).35 Both OlympiAD and EMBRACA studies met their primary endpoint, by showing a PFS improvement with the PARP inhibitor as compared with single-agent chemotherapy, reporting a significant 42% and 46% relative reduction of the risk of progression or death, respectively. However, both trials failed to capture a significant OS improvement with PARP inhibitor over chemotherapy.34,36 The major safety warning associated with both these PARP inhibitors is hematological toxicity. Based on the OlympiAD and EMBRACA trials, both the FDA and EMA granted approval to olaparib and talazoparib for HER2-negative MBC patients harboring a deleterious germline mutation in BRCA 1 or BRCA 2 genes, previously exposed to chemotherapy either in the early (neoadjuvant and/or adjuvant) or metastatic setting.
gBRCA/PD-L1-positive breast cancer
The subgroup of TN MBC patients exhibiting both a PD-L1+ status and gBRCA 1/2 mutation deserves a separate discussion because, in this specific subset of patients, the treatment armamentarium encompasses the combination of immunotherapy and chemotherapy as well as PARP inhibitors. In this context, no head-to-head comparison between these two treatment strategies is currently available, thus precluding to draw definitive and formal conclusions regarding their mutual positioning in terms of treatment sequence. Interestingly, the translational analysis of the Impassion130 trial revealed that the relative benefit conferred by the incorporation of atezolizumab to first-line nab-paclitaxel over nab-paclitaxel alone was consistent irrespective of gBRCA status, thus supporting the use of immunotherapy also in patients simultaneously harboring germline BRCA mutation and somatic PD-L1+ status.112 In addition, it should be noted that the use of immunotherapy has been tested in a pure first-line setting while olaparib and talazoparib efficacy has been demonstrated in patients already treated with up to three or four previous lines, respectively. Overall, these observations indirectly support giving priority to immunotherapy + chemotherapy as preferred initial treatment for MBC in PD-L1+ TN MBC patients even in the presence of gBRCA mutations.
HER2-low-positive breast cancer
As already reviewed, in the past decades, several anti-HER2 strategies have been developed, with compelling advancements in terms of antitumor activity and efficacy in MBC. However, so far, access to anti-HER2 agents has been restricted to patients classified as HER2+, based on IHC and ISH assessment.113 Nevertheless, the dichotomization between HER2+ versus HER2– actually relies on the predictive value in terms of trastuzumab benefit established in the context of pivotal trials,71,114 thus not necessarily fitting for novel anti-HER2 drugs. Indeed, HER2 expression should be considered a continuum. In fact, it has been shown that HER2 mRNA expression levels progressively increase from tumors scored as 0 to those scored as 3+ at IHC and this gradient may not be fully recapitulated by the mere distinction in HER2 positive versus negative.115 Indeed, this notion finds further support from results of early-phase clinical trials testing novel anti-HER2 ADCs in MBC patients traditionally classified as HER2 negative, though harboring HER2-low-expressing tumors. In detail, promising antitumor activity has been recently reported with the novel ADCs T-DXd116 and trastuzumab duocarmazine85 investigated in, respectively, 54 and 49 heavily pretreated HER2-low-positive MBC patients, defined as those exhibiting HER2 IHC score of 1+ or 2+ in the absence of HER2 gene amplification by IHC, as shown in Table 2. In particular, T-DXd led to 37.0% of ORR, with 10.4 months of median duration of response; in addition, median PFS and median OS were 11.1 and 29.4 months, respectively. Similarly, trastuzumab duocarmazine has been reported to provide 28% and 4.1 months of median PFS in the HR+/HER2-low-positive cohort and 40% of ORR and 4.9 months in the HR–/HER2-low-positive cohort. The major safety concerns are represented by interstitial lung disease (occurring in 5% of patients, in one patient resulting to be fatal) for T-DXd and ocular toxicity (at least one ocular event in 71% of patients) for trastuzumab duocarmazine.
Table 2.
Phase I trials assessing novel ADCs in HER2-low-positive MBC
| Treatments | Subgroups | Number of patients | ORR (95% CI) | Median treatment duration, months (95% CI) | Median PFS, months (95% CI) | Median OS, months, (95% CI) | |
|---|---|---|---|---|---|---|---|
| NCT025649035 | Trastuzumab deruxtecan | — | 54 | 37.0% (24.3%-51.3%) | 6.1 (0.7-29.2) | 11.1 (7.6-NE) | 29.4 (12.9-29.4) |
| NCT0227771725 | Trastuzumab duocarmazine | ER+/HER2 low-positive ER–/HER2 low-positive |
32 15 |
28% (13.8%-46.8%) 40% (16.3%-67.6%) |
3.7 3.3 |
4.1 (2.4-5.4) 4.9 (1.2-NE) |
— |
ADC, antibody–drug conjugate; CI, confidence interval; HER2, human epidermal growth factor receptor 2; MBC, metastatic breast cancer; ORR, objective response rate.
Based on these results, a large number of late-phase clinical trials, including two phase III randomized trials (NCT03734029/Destiny-Breast04 and NCT04494425/ Destiny-Breast06), have been initiated to deepen the activity and efficacy of anti-HER2 treatments in HER2-low-positive BC patients. For these reasons, although anti-HER2 ADCs are not yet available in the context of the regulatory scenario worldwide, results from aforementioned pivotal trials forced us to reconsider our current approach for determining anti-HER2 drug access. Indeed, they have already triggered an historical change, which is expected to radically affect the diagnostic and therapeutic landscape of MBC.
Ongoing research, future perspectives and conclusions
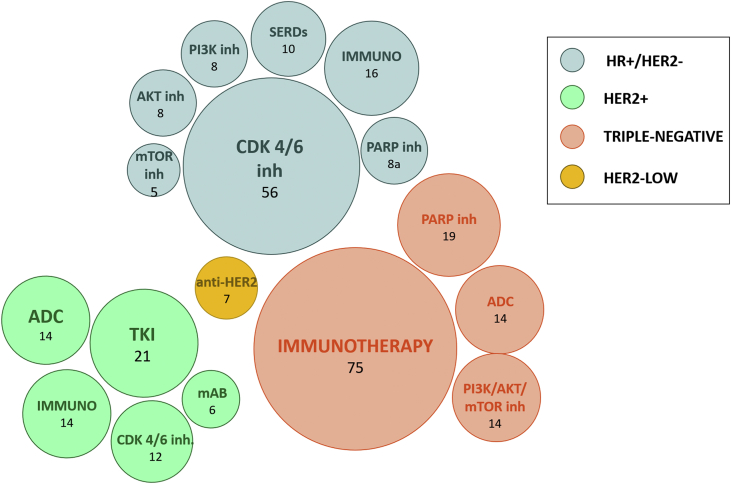
To conclude, in the last few years, several effective therapeutic strategies have been made available for MBC given the compelling results outlined in their clinical development. Importantly, this breakthrough has transversally concerned all BC subtypes, with the major achievement in terms of survival improvement observed within HR+/HER2– and HER2+ BC. In addition, a prolific research activity is currently ongoing, striving to bring to light novel treatment strategies capable of further enhancing MBC patients’ prognosis, as highlighted in Figure 4. Great expectations have been placed on combination strategies, namely, among others, CDK 4/6 inhibitor + immunotherapy, CDK 4/6 inhibitor + oral SERDs, CDK 4/6 inhibitor + anti-HER2 treatment, immunotherapy + anti-HER2 therapy, anti-HER2 ADC + anti-HER2 monoclonal antibodies. In addition, on the wave of the enthusiasm generated by T-DXd in HER2+ and sacituzumab govitecan in TN MBC, several other novel ADCs are currently under development and investigation. Disease chronicization no longer represents an unrealistic goal and the symbolic wall of 5 year of median OS in the metastatic setting has already been broken down in HR+/HER2– BC5 (and we are getting close in HER2+ MBC). In this context, it is expected that future advancements will go through a rethinking of BC subtype classification, implementing more flexible boundaries at the service of drug access, with HER2-low-positive BC serving as an example and standard bearer of this (r)evolution.117
Figure 4.
Currently active phase II and III trials for metastatic breast cancer (MBC) divided according to BC subtypes and drug categories (source:ClinicalTrials.gov; at 25 November 2021).
inh, inhibitor; mTOR, mammalian target of rapamycin; SERDs, selective estrogen receptor degraders, IMMUNO; immunotherapy; ADC, antibody drug conjugate; mAB, monoclonal antibody; TKI, tyrosine kinase inhibitor; PARP, poly-ADP-ribose polymerase; HR, hormone receptor.
Acknowledgments
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
GG reports personal fees from Eli Lilly and Novartis, outside the submitted work. MVD reports personal fees from Eli Lilly, Exact Sciences, Novartis, Pfizer, Seagen, outside the submitted work. VG reports personal fees from Novartis, Roche, Eli Lilly, MSD, outside the submitted work. The remaining authors declare no competing interests.
References
- 1.Guarneri V., Conte P. Metastatic breast cancer: therapeutic options according to molecular subtypes and prior adjuvant therapy. Oncologist. 2009;14:645–656. doi: 10.1634/theoncologist.2009-0078. [DOI] [PubMed] [Google Scholar]
- 2.Cherny N.I., Dafni U., Bogaerts J., et al. ESMO-magnitude of clinical benefit scale version 1.1. Ann Oncol. 2017;28:2340–2366. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 3.Finn R.S., Martin M., Rugo H.S., et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 4.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 5.Hortobagyi G.N., Stemmer S.M., Burris H.A., et al. LBA17 Overall survival (OS) results from the phase III MONALEESA-2 (ML-2) trial of postmenopausal patients (pts) with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocri. Ann Oncol. 2021;32:S1290–S1291. [Google Scholar]
- 6.Goetz M.P., Toi M., Campone M., et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 7.Tripathy D., Im S.A., Colleoni M., et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19:904–915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 8.Im S.-A., Lu Y.-S., Bardia A., et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 9.Turner N.C., Ro J., André F., et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 10.Slamon D.J., Neven P., Chia S., et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 11.Slamon J.S., Chia S., Fasching P.A., et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med. 2020;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 12.Sledge G.W., Toi M., Neven P., et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 13.Sledge G.W.J., Toi M., Neven P., et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6:116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.André F., Ciruelos E., Rubovszky G., et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. N Engl J Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 15.Bardia A, Neven P, Streich G, et al. Elacestrant, an oral selective estrogen receptor degrader (SERD), vs investigator’s choice of endocrine monotherapy for ER+/HER2- advanced/metastatic breast cancer (mBC) following progression on prior endocrine and CDK4/6 inhibitor therapy: results of the EMERALD phase 3 trial. Presented at SABCS 2021. December 7-10, 2021; San Antonio, TX. Abstract GS2-02.
- 16.Baselga J., Campone M., Piccart M., et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2011;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piccart M., Hortobagyi G.N., Campone M., et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2. Ann Oncol. 2014;25:2357–2362. doi: 10.1093/annonc/mdu456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baselga J., Cortés J., Kim S.-B., et al. CLEOPATRA Study Group Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swain S.M., Miles D., Kim S.-B., et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 20.Verma S., Miles D., Gianni L., et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diéras V., Miles D., Verma S., et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer ( EMILIA ): a descriptive analysis of final overall survival results from a randomised, open-label , phase 3 trial. Lancet Oncol. 2017;18:732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortés J., Kim S.-B., Chung W.-P., et al. LBA1 trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): results of the randomized phase III DESTINY-Breast03 study. Ann Oncol. 2021;32:S1287–S1288. [Google Scholar]
- 23.Saura Manich C., O’Shaughnessy J., Aftimos P.G., et al. Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol. 2021;32:S1288. [Google Scholar]
- 24.Murthy R., Borges V.F., Conlin A., et al. Tucatinib with capecitabine and trastuzumab in advanced HER2-positive metastatic breast cancer with and without brain metastases: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:880–888. doi: 10.1016/S1470-2045(18)30256-0. [DOI] [PubMed] [Google Scholar]
- 25.Murthy R.K., Loi S., Okines A., et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 26.Rugo H.S., Im S.-A., Cardoso F., et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7:573–584. doi: 10.1001/jamaoncol.2020.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saura C., Oliveira M., Feng Y., Dai M., Chen S. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38:3138–3149. doi: 10.1200/JCO.20.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu B., Yan M., Ma F., et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:351–360. doi: 10.1016/S1470-2045(20)30702-6. [DOI] [PubMed] [Google Scholar]
- 29.Schmid P., Adams S., Rugo H.S., et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 30.Emens L.A., Adams S., Barrios C.H., et al. First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann Oncol. 2021;32:983–993. doi: 10.1016/j.annonc.2021.05.355. [DOI] [PubMed] [Google Scholar]
- 31.Cortes J., Cescon D.W., Rugo H.S., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet (London, England) 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 32.Bardia A., Hurvitz S.A., Tolaney S.M., et al. ASCENT Clinical Trial Investigators Sacituzumab govitecan in metastatic triple-negative breast cancer. N Eng J Med. 2021;384:1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong A., Wu W., Goessl C., et al. Olaparib for metastatic breast cancer in patients with a germline. N Eng J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 34.Robson M.E., Tung N., Conte P., et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;2:558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Litton J.K., Rugo H.S., Ettl J., et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Litton J.K., Hurvitz S.A., Mina L.A., et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. 2020;31(11):1526–1535. doi: 10.1016/j.annonc.2020.08.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutherland S., Miles D., Makris A. Use of maintenance endocrine therapy after chemotherapy in metastatic breast cancer. Eur J Cancer. 2016;69:216–222. doi: 10.1016/j.ejca.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Guarneri V., Giorgi C.A., Cinieri S., et al. Everolimus plus aromatase inhibitors as maintenance therapy after first-line chemotherapy: final results of the phase III randomised MAIN-A (MAINtenance Afinitor) trial. Eur J Cancer. 2021;154:21–29. doi: 10.1016/j.ejca.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Gennari A., André F., Barrios C.H., et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32:1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 40.NCCN Clinical Practice Guidelines in Oncology - Breast Cancer. Version 12022 2022. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf Available at.
- 41.Burstein H.J., Somerfield M.R., Barton D.L., et al. Endocrine treatment and targeted therapy for hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: ASCO guideline update. J Clin Oncol. 2021;39:3959–3977. doi: 10.1200/JCO.21.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finn R.S., Aleshin A., Slamon D.J. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016;18:17. doi: 10.1186/s13058-015-0661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gelbert L.M., Cai S., Lin X., et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32:825–837. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torres-Guzmán R., Calsina B., Hermoso A., et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget. 2017;8:69493–69507. doi: 10.18632/oncotarget.17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickler M.N., Tolaney S.M., Rugo H.S., et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin Cancer Res. 2017;23:5218–5224. doi: 10.1158/1078-0432.CCR-17-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu Y., Loi C.-M., Hoffman J., Wang D. Physiologically based pharmacokinetic modeling of palbociclib. J Clin Pharmacol. 2017;57:173–184. doi: 10.1002/jcph.792. [DOI] [PubMed] [Google Scholar]
- 47.Thill M., Schmidt M. Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Ther Adv Med Oncol. 2018;10 doi: 10.1177/1758835918793326. 1758835918793326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rugo H.S., Finn R.S., Diéras V., et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat. 2019;174:719–729. doi: 10.1007/s10549-018-05125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martín M., Johnston S., Huober J., et al. MONARCH 3: Updated time to chemotherapy and disease progression following abemaciclib plus aromatase inhibitor (AI) in HR+, HER2- advanced breast cancer (ABC) Ann Oncol. 2019;30:v113–v114. [Google Scholar]
- 50.Tripathy D., Hortobagyi G., Chan A., et al. 155PPooled efficacy analysis of first-line ribociclib (RIB) plus endocrine therapy (ET) in HR+/HER2: advanced breast cancer (ABC) Ann Oncol. 2019;30 [Google Scholar]
- 51.Lobbezoo D.J.A., van Kampen R.J.W., Voogd A.C., et al. In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: a study of the Southeast Netherlands breast cancer consortium. Ann Oncol. 2016;27:256–262. doi: 10.1093/annonc/mdv544. [DOI] [PubMed] [Google Scholar]
- 52.Jacquet E., Lardy-Cléaud A., Pistilli B., et al. Endocrine therapy or chemotherapy as first-line therapy in hormone receptor-positive HER2-negative metastatic breast cancer patients. Eur J Cancer. 2018;95:93–101. doi: 10.1016/j.ejca.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 53.André F., Neven P., Marinsek N., et al. Disease management patterns for postmenopausal women in Europe with hormone-receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer. Curr Med Res Opin. 2014;30:1007–1016. doi: 10.1185/03007995.2014.887002. [DOI] [PubMed] [Google Scholar]
- 54.Martin M., Zielinski C., Ruiz-Borrego M., et al. Palbociclib in combination with endocrine therapy versus capecitabine in hormonal receptor-positive, human epidermal growth factor 2-negative, aromatase inhibitor-resistant metastatic breast cancer: a phase III randomised controlled trial—PEARL. Ann Oncol. 2021;32:488–499. doi: 10.1016/j.annonc.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Goncalves M.D., Hopkins B.D., Cantley L.C. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N Engl J Med. 2018;379:205–262. doi: 10.1056/NEJMra1704560. [DOI] [PubMed] [Google Scholar]
- 56.Mollon L.E., Anderson E.J., Dean J.L., et al. A systematic literature review of the prognostic and predictive value of PIK3CA mutations in HR(+)/HER2(-) metastatic breast cancer. Clin Breast Cancer. 2020;20:e232–e243. doi: 10.1016/j.clbc.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Bosch A., Li Z., Bergamaschi A., et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med. 2015;7:283ra51. doi: 10.1126/scitranslmed.aaa4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller T.W., Balko J.M., Fox E.M., et al. ERα-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1:338–351. doi: 10.1158/2159-8290.CD-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baselga J., Im S.-A., Iwata H., et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:904–916. doi: 10.1016/S1470-2045(17)30376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Leo A., Johnston S., Lee K.S., et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:87–100. doi: 10.1016/S1470-2045(17)30688-5. [DOI] [PubMed] [Google Scholar]
- 61.Rugo H.S., Lerebours F., Ciruelos E., et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489–498. doi: 10.1016/S1470-2045(21)00034-6. [DOI] [PubMed] [Google Scholar]
- 62.Rugo H.S., Lerebours F., Ciruelos E., et al. Alpelisib (ALP) + fulvestrant (FUL) in patients (pts) with PIK3CA-mutated (mut) hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (ABC) previously treated with cyclin-dependent kinase 4/6 inh. J Clin Oncol. 2020;38:1006. [Google Scholar]
- 63.Oesterreich S., Davidson N.E. The search for ESR1 mutations in breast cancer. Nat Genet. 2013;45(12):1415–1416. doi: 10.1038/ng.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O’Leary B., Cutts R.J., Huang X., et al. Circulating tumor DNA markers for early progression on fulvestrant with or without palbociclib in ER+ advanced breast cancer. J Natl Cancer Inst. 2021;113(3):309–317. doi: 10.1093/jnci/djaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liendema G.J., Bowen R., Jerzak K.J., et al. Results from VERONICA: a randomized, phase II study of second-/third-line venetoclax (VEN) + fulvestrant (F) versus F alone in estrogen receptor (ER)-positive, HER2-negative, locally advanced, or metastatic breast cancer (LA/MBC) J Clin Oncol. 2021;39(15_suppl) (15_suppl):1004-1004. [Google Scholar]
- 66.Turner N.C., Kingston B., Kilburn L.S., et al. Circulating tumour DNA analysis to direct therapy in advanced breast cancer (plasmaMATCH): a multicentre, multicohort, phase 2a, platform trial. Lancet Oncol. 2020;21(10):1296–1308. doi: 10.1016/S1470-2045(20)30444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bihani T., Patel H.K., Arlt H., et al. Elacestrant (RAD1901), a selective estrogen receptor degrader (SERD), has antitumor activity in multiple ER+ breast cancer patient-derived xenograft models. Clin Cancer Res. 2017;23(16):4793–4804. doi: 10.1158/1078-0432.CCR-16-2561. [DOI] [PubMed] [Google Scholar]
- 68.Bardia A., Kaklamani V., Wilks S., et al. Phase I study of elacestrant (RAD1901), a novel selective estrogen receptor degrader, in ER-positive, HER2-negative advanced breast cancer. J Clin Oncol. 2021;39(12):1360–1370. doi: 10.1200/JCO.20.02272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ross J.S., Slodkowska E.A., Symmans W.F., Pusztai L., Ravdin P.M., Hortobagyi G.N. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 70.Krop I.E., Kim S.-B., González-Martín A., et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:689–699. doi: 10.1016/S1470-2045(14)70178-0. [DOI] [PubMed] [Google Scholar]
- 71.Slamon D.J., Leyland-Jones B., Shak S., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 72.Blackwell K.L., Burstein H.J., Storniolo A.M., et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 73.Geyer C.E., Forster J., Lindquist D., et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 74.Cameron D., Casey M., Oliva C., et al. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase III randomized trial. Oncologist. 2010;15:924–934. doi: 10.1634/theoncologist.2009-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giordano S.H., Temin S., Chandarlapaty S., et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36:2736–2740. doi: 10.1200/JCO.2018.79.2697. [DOI] [PubMed] [Google Scholar]
- 76.Lewis Phillips G.D., Li G., Dugger D.L., et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 77.Krop I.E., Kim S.B., Martin A.G., et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18:743–754. doi: 10.1016/S1470-2045(17)30313-3. [DOI] [PubMed] [Google Scholar]
- 78.Ogitani Y., Hagihara K., Oitate M., Naito H., Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107:1039–1046. doi: 10.1111/cas.12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Modi S., Saura C., Yamashita T., et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.von Minckwitz G., Huang C.-S., Mano M.S., et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 81.Lin N.U., Borges V., Anders C., et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38:2610–2619. doi: 10.1200/JCO.20.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shanu M. Plenary discussion of the phase 3 randomized trial of T dxd versus T DM1 (destiny breast 03 trial) Invit Discussant LBA1. 2021 [Google Scholar]
- 83.Bartsch R., Berghoff A.S., Furtner J., et al. 280P Intracranial activity of trastuzumab-deruxtecan (T-DXd) in HER2-positive breast cancer patients with active brain metastases: results from the first stage of the phase II TUXEDO-1 trial. Ann Oncol. 2021;32:S486. [Google Scholar]
- 84.Pivot X., Manikhas A., Żurawski B., et al. CEREBEL (EGF111438): a phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2015;33:1564–1573. doi: 10.1200/JCO.2014.57.1794. [DOI] [PubMed] [Google Scholar]
- 85.Banerji U., van Herpen C.M.L., Saura C., et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20:1124–1135. doi: 10.1016/S1470-2045(19)30328-6. [DOI] [PubMed] [Google Scholar]
- 86.Ogitani Y., Aida T., Hagihara K., et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22:5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]
- 87.Dokter W., Ubink R., van der Lee M., et al. Preclinical profile of the HER2-targeting ADC SYD983/SYD985: introduction of a new duocarmycin-based linker-drug platform. Mol Cancer Ther. 2014;13:2618–2629. doi: 10.1158/1535-7163.MCT-14-0040-T. [DOI] [PubMed] [Google Scholar]
- 88.Linehan A.S., Fitzpatrick O.M., Morris P.G. Profile of trastuzumab deruxtecan in the management of patients with HER2-positive unresectable or metastatic breast cancer: an evidence-based review. Breast Cancer (Dove Med Press) 2021;13:151–159. doi: 10.2147/BCTT.S245024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thanopoulou E., Khader L., Caira M., et al. Therapeutic strategies for the management of hormone receptor-positive, human epidermal growth factor receptor 2-positive (HR+/HER2+) breast cancer: a review of the current literature. Cancers (Basel) 2020;12:3317. doi: 10.3390/cancers12113317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rimawi M., Ferrero J.-M., de la Haba-Rodriguez J., et al. First-line trastuzumab plus an aromatase inhibitor, with or without pertuzumab, in human epidermal growth factor receptor 2-positive and hormone receptor-positive metastatic or locally advanced breast cancer (PERTAIN): a randomized, open-label phase II T. J Clin Oncol. 2018;36(28):2826–2835. doi: 10.1200/JCO.2017.76.7863. [DOI] [PubMed] [Google Scholar]
- 91.Johnston S.R.D., Hegg R., Im S.-A., et al. Phase III, randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-positive, hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2018;36:741–748. doi: 10.1200/JCO.2017.74.7824. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Yuan Z., Huang J.-J., Hua X., et al. Trastuzumab plus endocrine therapy or chemotherapy as first-line treatment for metastatic breast cancer with hormone receptor-positive and HER2-positive: the sysucc-002 randomized clinical trial. J Clin Oncol. 2021;39:1003. doi: 10.1158/1078-0432.CCR-21-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.den Brok W.D., Speers C.H., Gondara L., Baxter E., Tyldesley S.K., Lohrisch C.A. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res Treat. 2017;161:549–556. doi: 10.1007/s10549-016-4080-9. [DOI] [PubMed] [Google Scholar]
- 94.Bonotto M., Gerratana L., Poletto E., et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist. 2014;19:608–615. doi: 10.1634/theoncologist.2014-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miles D., Gligorov J., André F., et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32:994–1004. doi: 10.1016/j.annonc.2021.05.801. [DOI] [PubMed] [Google Scholar]
- 96.Franzoi M.A., de Azambuja E. Atezolizumab in metastatic triple-negative breast cancer: IMpassion130 and 131 trials - how to explain different results? ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miglietta F., Griguolo G., Guarneri V., Dieci M.V. Programmed cell death ligand 1 in breast cancer: technical aspects, prognostic implications, and predictive value. Oncologist. 2019;24:e1055–e1069. doi: 10.1634/theoncologist.2019-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rugo H.S., Loi S., Adams S., et al. LBA20 - performance of PD-L1 immunohistochemistry (IHC) assays in unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC): post-hoc analysis of IMpassion130. Ann Oncol. 2019;30:v858–v859. [Google Scholar]
- 99.Voorwerk L., Slagter M., Horlings H.M., et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25:920–928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 100.Perez E.A., Patel T., Moreno-Aspitia A. Efficacy of ixabepilone in ER/PR/HER2-negative (triple-negative) breast cancer. Breast Cancer Res Treat. 2010;121:261–271. doi: 10.1007/s10549-010-0824-0. [DOI] [PubMed] [Google Scholar]
- 101.Brufsky A., Valero V., Tiangco B., et al. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: subgroup analysis of the RIBBON-2 trial. Breast Cancer Res Treat. 2012;133:1067–1075. doi: 10.1007/s10549-012-2008-6. [DOI] [PubMed] [Google Scholar]
- 102.Pivot X., Marmé F., Koenigsberg R., Guo M., Berrak E., Wolfer A. Pooled analyses of eribulin in metastatic breast cancer patients with at least one prior chemotherapy. Ann Oncol. 2016;27:1525–1531. doi: 10.1093/annonc/mdw203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park I.H., Im S.-A., Jung K.H., et al. Randomized open label phase III trial of irinotecan plus capecitabine versus capecitabine monotherapy in patients with metastatic breast cancer previously treated with anthracycline and taxane: PROCEED trial (KCSG BR 11-01) Cancer Res Treat. 2019;51:43–52. doi: 10.4143/crt.2017.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cortés J., Lipatov O., Im S.-A., et al. LBA21 - KEYNOTE-119: Phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC) Ann Oncol. 2019;30:v859–v860. [Google Scholar]
- 105.Goldenberg D.M., Cardillo T.M., Govindan S.V., Rossi E.A., Sharkey R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC) Oncotarget. 2015;6(26):22496–22512. doi: 10.18632/oncotarget.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bardia A., Tolaney S.M., Punie K., et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021;32:1148–1156. doi: 10.1016/j.annonc.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 107.Malone K.E., Daling J.R., Doody D.R., et al. Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years. Cancer Res. 2006;66:8297–8308. doi: 10.1158/0008-5472.CAN-06-0503. [DOI] [PubMed] [Google Scholar]
- 108.Kurian A.W., Gong G.D., John E.M., et al. Performance of prediction models for BRCA mutation carriage in three racial/ethnic groups: findings from the Northern California Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2009;18:1084–1091. doi: 10.1158/1055-9965.EPI-08-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5:387–393. doi: 10.1016/j.molonc.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Javle M., Curtin N.J. The potential for poly (ADP-ribose) polymerase inhibitors in cancer therapy. Ther Adv Med Oncol. 2011;3:257–267. doi: 10.1177/1758834011417039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lord C.J., Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–1158. doi: 10.1126/science.aam7344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Emens L.A., Molinero L., Loi S., et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer: biomarker evaluation of the IMpassion130 study. J Natl Cancer Inst. 2021;113(8):1005–1016. doi: 10.1093/jnci/djab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wolff A.C., Elizabeth Hale Hammond M., Allison K.H., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36:2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 114.Balduzzi S., Mantarro S., Guarneri V., et al. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014;2014:CD006242. doi: 10.1002/14651858.CD006242.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Griguolo G., Brasó-Maristany F., González-Farré B., et al. ERBB2 mRNA expression and response to ado-trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Cancers (Basel) 2020;12:1902. doi: 10.3390/cancers12071902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Modi S., Park H., Murthy R.K., et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38:1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dieci M.V., Miglietta F. HER2: a never ending story. Lancet Oncol. 2021;22:1051–1052. doi: 10.1016/S1470-2045(21)00349-1. [DOI] [PubMed] [Google Scholar]