Highlights
-
•
Since tuberculosis still become an important health problem in the world, especially in developing countries, CKD patients also become a high-risk population to TBC infection.
-
•
Due to immunity impairment in CKD patients, particularly who are routinely hemodialyzed, tuberculosis is not always clinically manifested (latent).
-
•
However, tuberculosis among CKD patients contribute to greater morbidity, quality of life and morbidity. Hence, we investigated the factors that associated with latent tuberculosis among CKD on haemodialysis patients.
-
•
By understanding it, management of CKD patients could be more comprehensive, and the morbidity and mortality could be decreased while quality of life could be increased.
-
•
We also provide the first documentation study of tuberculosis among CKD on haemodialysis patients in Indonesia, one of the tuberculosis endemic country.
-
•
According our study, smoking status and HD adequacy based on URR < 73% are associated factors that contribute to LTB among CKD on HD patients.
Keywords: Chronic kidney disease, Haemodialysis, Latent tuberculosis, Risk factors
Abstract
Introduction
Since immune system alteration occurs, chronic kidney disease (CKD) on routine haemodialysis (HD) patients have a greater risk for latent tuberculosis (LTB). LTB needs special attention so that it does not develop into an active form, because infection in CKD patients increases the mortality. This study aims to determine the risk factors that associated with LTB among CKD on routine HD patients.
Methods
This was a cross-sectional study conducted in Haemodialysis Unit, Hasan Sadikin General Hospital, Bandung. The subjects were recruited from March–May 2020. Subjects aged > 18 years at least have undergoing HD in 3 months and twice a week HD were included in this study. Patients with active tuberculosis (TB) suspected, malignancy, or immunocompromised were excluded. LTB was diagnosed using interferon-γ release assays (IGRA). All data including age, sex, CKD etiologies, smoking status, HD adequacy that assessed using KT/V and urea reduction ratio (URR), and contact status with TB patients were obtained and recorded in case report form.
Results
A total of 120 subjects were involved. LTB based on IGRA was occurred in 39.2% subjects, while 56.7% and 4.1% subjects had negative and indeterminate IGRA, respectively. Adequacy of HD based on KT/V value was not significantly different between positive and negative IGRA subjects. Positive IGRA subjects had lower URR (p = 0.042). Smoking status had significant association with LTB (OR = 2.5[95%CI 1.2–5.4, p = 0.017). Furthermore, URR < 73% also had significant association with LTB (OR = 2.6[1.2–5.6, p = 0.013).
Conclusion
Smoking status and HD adequacy based on URR < 73% are associated factors that contribute to LTB among CKD on HD patients.
1. Introduction
Tuberculosis (TB) risk among chronic kidney disease (CKD) patients is greater than normal kidney function subjects [1]. Coexistence of systemic inflammation and immunocompromised are the common implication of uremic state. These two mechanisms contribute to the morbidity and mortality of CKD patients [1], [2]. Uremic state disrupts the immune response through several pathways including decreased phagocytosis activity of granulocytes and monocytes/macrophages, impaired antigen presentation capacity of antigen presenting cells (APC), decreased in amount of presented antigen on dendritic cells surface, decreased capacity of B cells production, increased T cell apoptosis, and disrupted cell-mediated immunity (CMI) [2].
LTB among CKD patients are prevalent. Based on tuberculin subcutaneous test (TST), the prevalence of LTB among CKD patients in Brazil was 10.3%, with average age of 53 years old and predominantly in male individuals [3]. Another study that conducting screening for LTB in dialysis unit, revealed 9 of 41 patients had LTB [4]. An Indian 10-years cohort study yielded 10.5% LTB diagnosis after HD initiation. The mid-point duration after HD initiation were 24 months. The study conducted in Taiwan yielded 21.3% LTB according on IGRA [5].
Various factors are purposed to be predictors of LTB. Inadequate nutrition intake such as carbohydrates, vitamin, and minerals increase the probability of LTB 2–4 times. Inadequate nutrition and dense population settlement, in combine, also increase 2-times probability LTB. In-house contact with TB patients propagates the probability of LTB up to 14-times [6]. Among CKD patients, the risk is increased and it is contributed by increased age, previous history of TB, and smoking habits, both still active smokers and the quitters [7].
CKD patients are the high-risk population to develop LTB. Moreover, TB reactivation risk is increased by 10–25 times among CKD on dialysis [8]. Unfortunately, LTB is often not documented adequately. To date, diagnosing LTB is still to be challenging. Two-most modalities, IGRA and TST are used to detect LTB infection among CKD patients [9]. Compared to immunocompetent individuals, immunocompromised patients might have decreased IGRA response. However, IGRA is more superior than TST [10].
LTB is the one of crucial steps in mortality of CKD patients that accompanied by active TB. Infection contributes to mortality and morbidity among CKD patients, even though HD has been started [11]. Active TB is an infection which worsens the prognosis of CKD patients and increases the mortality [12]. Hence, LTB needs serious attention so it could not be progressed to active form. Due to the magnitude and its urgencies of LTB among CKD on HD patients, the investigation about risk factors that associated with LTB is needed. Hence, the management of CKD on HD patients could be more comprehensive, and as its implications, the morbidity and mortality could be decreased while quality of life could be increased. The aim of the study was to identify the risk factors that associated with LTB among CKD on HD patients.
2. Methods
2.1. Study design and subjects
This was an observational-analytic study with cross sectional approach. The study was conducted on CKD on HD patients in Haemodialysis Unit, Hasan Sadikin General Hospital, Bandung, with the subject recruitment period of March–May 2020. The subjects were recruited consecutively. Subjects aged more 18 years with minimum routine HD period of 3 months at least twice a week were included in this study. Patients with malignancy, infected by human immunodeficiency virus/ acquired immune deficiency syndrome (HIV/AIDS), or being treated using immunosuppressive regiment were excluded. Patients with TB history, being treated using anti-tuberculosis drugs, or radiologically suspected for TB were also not involved in this study.
2.2. Data collection
Subjects that met the inclusion criteria but not met the exclusion criteria were examined. The examinations were consisted of history taking, physical examination, as well as laboratory investigations to exclude active TB. Symptoms and signs of TB were fever, dyspneu, decreased body weight, chronic diarrhea, and lymph node enlargement. All subjects were examined for thoracic x-ray. Those were expertized by independent radiologist (not involved in this study). Subjects were also assessed for sputum acid-fast bacilli, culture, abdominal ultrasound, and lymph node examination according to the presence of sign and/or symptoms. IGRA was examined to each patient without signs and symptoms of TB, before HD procedure.
Some data were obtained to accommodate this study. The age of subjects was calculated and noted according to birthdate in identity card and the date of the examination. Adequacy of HD was based on Kt/V value and urea reduction ratio (URR). Etiologies of CKD was defined as underlying disease that causing CKD, that could be diabetes mellitus, hypertension, glomerulonephropathy, chronic glomerulonephropathy, pyelonephritis, and polycystic kidney disease. Smoking status was categorized as smokers and non-smokers, while smoker was defined as an adults who has smoked 100 cigarettes in his/her lifetime, both everyday and somedays smokers [13]. History of TB patients contact was defined as a presence of in-house contact with patient that has been diagnosed as TB patients or proven by positivity of acid-fast bacilli in sputum. LTB was diagnosed according to the IGRA result, which have done during 24–48 h and could be interpreted qualitatively based on the amount of yielded interferon gamma. The criteria of IGRA interpretation is presented in on Table 1[14]. Each indetermined IGRA results were re-examined to confirming the results. Study data were collected and managed using REDCap electronic data capture tools hosted at Universitas Padjadjaran [15], [16].
Table 1.
Interpretation of QuantiFERON-TB IGRA.
| Interpretation | Nill | TB response | Mitogen Response |
|---|---|---|---|
| Positive | ≤8.0 | ≥0.35 IU/ml and ≥ 25% of Nill | Any |
| Negative | ≤8.0 | <0.35 IU/ml and < 25% of Nill | ≥0.5 |
| Indeterminate | ≤8.0 | <0.35 IU/ml and < 25% of Nill | <0.5 |
| ≥8.0 | Any | Any |
2.3. Statistic analysis
All date were analysed using SPSS (IBM corporation. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp). Two-groups numerical comparisons were analyzed using unpaired-T test with Mann-Whitney as the alternative test. Categorical bivariate analyses were performed by chi-square test, or Fisher’s exact as the alternative test. Multivariate logistic regression analysis was conducted to produce adjusted odds ratio. Each p value < 0.05 was considered as statistically significant. Results have been reported according to the STROBE guidelines on the reporting of observational studies [17], [18].
2.4. Ethical approval
This study was approved by Ethical Committee of Hasan Sadikin General Hospital Bandung. This study was a part of “Tuberculosis among Chronic Kidney Disease on Haemodialysis in Hasan Sadikin General Hospital and Habibie Kidney Hospital Bandung, with registered number LB.02.01/X6.5/302/2019.
3. Results
3.1. Subject characteristics
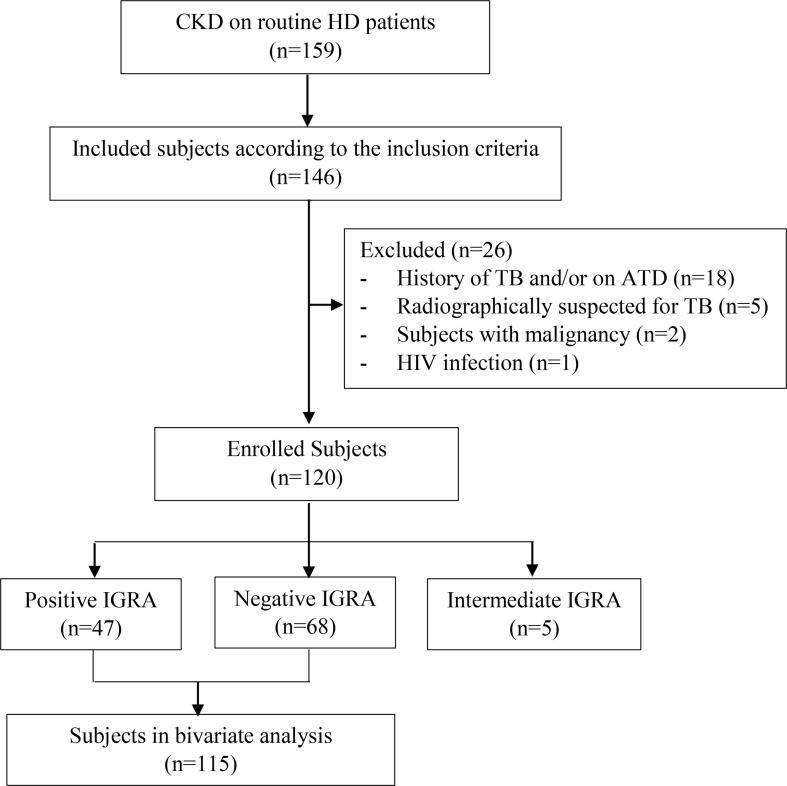
CKD on HD patients in Haemodialysis Unit Hasan Sadikin General Hospital Bandung were 159. The detailed process of subject recruitment was depicted on Fig. 1. A total of 120 patients (53.0% females and 47% males) fulfilled the inclusion criteria and did not met the exclusion criteria. According to IGRA, forty-seven (39,17%) subjects were positive, meanwhile 68 (56.7%) subjects were negative. Indeterminate results of IGRA were found in 5 subjects (4.2%). Subject characteristics are presented in Table 2. Mean age of the subjects was 47 ± 13 years. Median of the HD period was 50 months. Hypertension (52.2%) was the dominant CKD etiology, followed by diabetes mellitus, primary glomerulonephropathy, chronic pyelonephritis, uric acid nephropathy, lupus nephropathy, obstructive nephropathy, and polycystic kidney disease. There was no significant difference in subject characteristics between positive and negative IGRA subjects (p value > 0.05).
Fig. 1.
Flowchart of Subjects Recruitment. ATD: antituberculosis drugs; CKD: chronic kidney disease; HD: haemodialysis; HIV: human immunodeficiency virus; IGRA: interferon-γ release assay; TB: tuberculosis.
Table 2.
Subject characteristics.
| Characteristics | Total n = 115 |
IGRA (+) n = 47 |
IGRA (–) n = 68 |
P value |
|---|---|---|---|---|
| Age (years) | ||||
| Mean ± SD | 47 ± 13 | 48 ± 12 | 47 ± 14 | 0.669a |
| Sex | ||||
| Male | 54 (47.0) | 26 (55.3) | 28 (41.2) | 0.135c |
| Female | 61 (53.0) | 21 (44.7) | 40 (58.8) | |
| Period of haemodialysis (month) | ||||
| Median (Interquartile range) | 50 (27–83) | 45 (26–80) | 52 (28–87) | 0.527b |
| CKD Etiologies | ||||
| Hypertensive kidney disease | 60 (52.2) | 26 (55.3) | 34 (50.0) | 0.619c |
| Diabetes kidney disease | 18 (15.7) | 9 (19.1) | 9 (13.2) | |
| Uric acid nephropathy | 4 (3.5) | 2 (4.3) | 2 (2.9) | |
| Lupus nephropathy | 2 (1.7) | 1 (2.1) | 1 (1.5) | |
| Obstructive nephropathy | 1 (0.9) | 1 (2.1) | 0 (0.0) | |
| Glomerulonephropathy | 23 (20.0) | 6 (12.8) | 17 (25.0) | |
| Chronic pyelonephritis | 6 (5.2) | 2 (4.3) | 4 (5.9) | |
| Polycystic kidney | 1 (1.5) | 0 (0.0) | 1 (1.5) |
Notes: Used tests: aUnpaired t-test bMann Whitney, cChi Square, dFisher Exact.
3.2. Bivariate analysis of HD Adequacy, smoking Status, and TB contact with IGRA
Bivariate analysis of HD adequacy, smoking status, and TB contact with IGRA results are presented in Table 3. However, indeterminate IGRA subjects were not involved on bivariate analysis due to small number of subjects. Adequacy of HD according to KT/V value, positive IGRA subjects had lower value, but not statistically significant, compared to negative IGRA subjects (1.6 [8] vs 1.7[1.5–1.8], p value = 0.24). Subjects with positive IGRA had significant lower URR than subjects with negative IGRA (70.4[65.7–76.6] vs. 74.2[70.7–77.3], p value = 0.04). Positive IGRA results were significantly more prevalent in smokers than non-smokers (63.8% vs 41.2%, p value = 0.017). There was no significant difference in the frequencies of contact with TB patients between positive and negative IGRA subjects (4.3% vs 11.8%, p value = 0.16).
Table 3.
Bivariate analysis between study variables with latent tuberculosis.
| Variables | IGRA (+) n = 47 |
IGRA (–) n = 68 |
P value |
|---|---|---|---|
| Haemodialysis adequacy | |||
| KT/V | |||
| Median (Interquartile range) | 1.58 (1.42–1.88) | 1.71 (1.52–1.82) | 0.238a |
| Urea reduction ratio | |||
| Median (Interquartile range) | 70.45 (65.70–76.61) | 74.15 (70.71–77.33) | 0.042a* |
| Smoking status | |||
| Smokers | 30 (63.8) | 28 (41.2) | 0.017b* |
| Non-smokers | 17 (36.2) | 40 (58.8) | |
| Tuberculosis contact history | 2 (4.3) | 8 (11.8) | 0.160c |
Notes: aMann Whitney, bChi Square, cFisher Exact, *statistically significant.
3.3. Categorical bivariate analysis of URR and smoking status with IGRA
Table 4 shows the results of categorical bivariate analysis of URR and smoking status with IGRA results. Smokers were significantly more frequent in subjects with positive IGRA results (OR = 2.5[1.2–5.4], p value = 0.017]. Subjects with URR < 73% significantly more frequent among positive IGRA subjects than negative IGRA subjects (OR = 2.6[1.2–5.6], p value = 0.33).
Table 4.
Odds ratio of smoking status and urea reduction ratio of < 73% with latent tuberculosis.
| Variables | IGRA (+) n = 47 |
IGRA (–) n = 68 |
P value | OR (95% CI) |
|---|---|---|---|---|
| Smoking status | ||||
| Smokers | 30 (63.8) | 28 (41.2) | 0.017* | 2.52 (1.17–5.42) |
| Non-smokers | 17 (36.2) | 40 (58.8) | ||
| Urea reduction ratio | ||||
| <73% | 29 (61.7) | 26 (38.2) | 0.013* | 1.58 (0.62–4.03) |
| ≥73% | 18 (38.3) | 42 (61.8) | ||
4. Discussion and conclusion
In our study, the prevalence of LTB among CKD on HD patients based on IGRA was 39.2%. It is slightly greater than in a study conducted by Shu et al [19]. in Taiwan (21.3%), that also used IGRA as diagnostic tools. In such study, age, albumin serum, dialysis requirement, and previous history of TB were identified as independent predictors of LTB. Grant et al [20]. has studied the presence of LTB among CKD on HD patients, which revealed the prevalence of 27–29%% according to IGRA. Kim et al. also reported the IGRA positivity of 42.1% patients whose undergoing to kidney transplantation [21]. A study by Agarwal et al. [22] in India yielded similar results with our study, which was 36% LTB among CKD on HD patients.
Our study also revealed no significant difference between positive and negative IGRA, in age and sex distributions. Rao et al. also reported the similar results with our study [5]. On the other hand, younger average of subject ages and male-dominant distribution were reported in Agarwal et al. [22] study. Shu et al reported the older average of ages according to IGRA positivity [19].
Although no difference in sex distribution, our results indicated that the positivity of IGRA is greater in male subjects, meanwhile negativity of IGRA is more common in female. These might be explained by smoking habits. Agarwal et al. [22] and Rao et al [5] also showed the accordant results to our study. Moreover, in general population, the incidence of TB is 2-times more common in male [5].
In CKD patients, haemodialysis could alter immune response through pro-apoptotic effect due to direct contact to dialysis membrane which affects the CMI [23]. In our study, period length of HD in positive IGRA subjects was shorter than in negative IGRA subjects. This similar fact has documented by Shu et al. in previous study [19]. Period of HD in negative IGRA subjects was significantly longer than in positive IGRA subjects. Nevertheless, our study revealed non-significant difference.
In our study, DM in positive IGRA subjects, is more prevalent than in the negative one. DM has known as risk factor for TB [24]. Patients with DM also have greater risk for TB reactivation [24], [25]. Moreover, In Indonesia, the prevalence of LTB among DM patients is quietly high (38.9%) [26]. Weakening of immune response increases the risk of TB infection development, from latent to active form. In fact, besides TB, DM is also an independent risk for lower respiratory tract infections and predisposing factor to have higher risk for severe complications [27]. Increased pro-inflammatory cytokines is correlated with increased blood glucose. Delayed adaptive immune response including T cell-produced IFN-γ, mycobacteria lung-to-lymph dissemination, and leukocytes aggregation have observed in DM patients [28].
Haemodialysis adequacy is an important factor that represents urea clearance during HD session. Uremic retention implies disruption of immune response in various mechanisms [2]. Our study assessed HD adequacy through KT/V and URR value. Positive IGRA subjects had non-significant lower KT/V value. On the other hand, they had markedly lower URR value. In categorical analysis with 73% cut-off, the association of URR value with LTB was significant. These discordance results between KT/V and URR value is probably due to KT/V is more sensitive than URR because involving the urea volume that distribute based on body weight and dialysis duration.
According to our results, IGRA positivity is more frequent in smokers significantly. Cigarettes smoke and its components alter respiratory tract structure. These alterations consist of peribronchial inflammation and fibrosis, increased mucosal permeability, disrupted mucocilliar clearance, altered pathogen environment, and disrupted respiratory epithelia. As consequences, development of respiratory tract infection and its inflammation are enhanced. Both humoral and cell-mediated immunity are also disturbed. These abnormal processes cause decreased circulating immunoglobulin levels, depressed antibody response to certain antigen, decreased amount of CD4+ lymphocytes, increased CD8+ lymphocytes, supressed phagocytes activities, and decreased proinflammatory cytokines release. Nicotine could stimulate catecholamines and corticosteroids which also supressed host immunity. These biological defects put the smokers as vulnerable individuals to TB progression [29]. Moreover, smoking habits is also an independent risk factor for LTB [19], [30]. In general population, the prevalence of LTB is higher in still active smokers than in who had previous smoking history and non-smokers, respectively [7].
In our study, no significant difference of TB contact history between positive and negative IGRA subjects. Using contact with TB patient history and suggestive thoracic x-ray to define high-risk individuals. In general population, the occurrence of LTB also was not associated to in-house contact with TB patients. On the other hand, Fox et alconcluded that in-house contact with TB patients increased the probability to develop LTB [6]. Suggestively, the discordance results are caused by the fact that in our study the contact with TB patients was occurred before diagnosis of CKD and treat using HD.
As a limitation, since we conducted the cross-sectional study, it merely described one-point of time frame conditions. Hence, it does not yet represent a stronger causal relationship. However, our study is first documentation in Indonesia that investigate important risk factors associated with LTB among CKD on HD patients. Further longitudinal study is needed to determine and evaluate the strength of these causal inferences. Moreover, the outcome of LTB therapy might be recommended. High proportion of people receiving chronic haemodialysis can complete LTB therapy [31]. LTB therapy in chronic haemodialysis may be safe but requires close evaluation and monitoring. Further study for this issue is also needed.
In conclusion, LTB is prevalent among CKD on HD patients. In such population, smoking habit increases the probability to develop LTB. Adequacy HD according to URR < 73% is also significantly associated with the occurrence of LTB. There is no significant difference in age, sex, CKD etiologies, and contact status with diagnosed TB patient. Further study to investigate the outcome and treatment matters of LTB on HD patients is needed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement & Funding Source
This study was funded by Research Grant from Ministry of Science and Technology of the Republic of Indonesia.
References
- 1.Ruzangi J., Iwagami M., Smeeth L., Mangtani P., Nitsch D. The association between chronic kidney disease and tuberculosis; a comparative cohort study in England. BMC Nephrol. 2020;21(1):1–9. doi: 10.1186/s12882-020-02065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen G., Hörl W.H. Immune dysfunction in uremia—an update. Toxins. 2012;4(11):962–990. doi: 10.3390/toxins4110962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaziri N.D., Pahl M.V., Crum A., Norris K. Effect of uremia on structure and function of immune system. J Renal Nutrit. 2012;22(1):149–156. doi: 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chagas A.C.F., Hans Filho G., Oliveira S.M.d.V.L.d., Ivo M.L., Corrêa Filho R.A.C., Donatti M.I. Prevalence of latent tuberculosis and treatment adherence among patients with chronic kidney disease in Campo Grande, State of Mato Grosso do Sul. Rev Soc Bras Med Trop. 2014;47(2):204–211. doi: 10.1590/0037-8682-0035-2014. [DOI] [PubMed] [Google Scholar]
- 5.Brij S.O., Beck S.C., Kleemann F., Jack A.L., Wilkinson C., Enoch D.A. Tuberculosis screening in a dialysis unit: detecting latent tuberculosis infection is only half the problem. J Hosp Infect. 2014;87(4):241–244. doi: 10.1016/j.jhin.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Rao T.M., Ram R., Swarnalatha G., Pai B.H.S., Ramesh V., Rao C.S.S., et al. Tuberculosis in haemodialysis patients: A single centre experience. Indian J Nephrol. 2013;23(5):340. doi: 10.4103/0971-4065.116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox G.J., Lee R.S., Lucas M., Khan F.A., Proulx J.-F., Hornby K., et al. Inadequate diet is associated with acquiring Mycobacterium tuberculosis infection in an inuit community. A case–control study. Ann Am Thoracic Soc. 2015;12(8):1153–1162. doi: 10.1513/AnnalsATS.201503-156OC. [DOI] [PubMed] [Google Scholar]
- 8.Horne D.J., Campo M., Ortiz J.R., Oren E., Arentz M., Crothers K., et al. Association between smoking and latent tuberculosis in the US population: an analysis of the National Health and Nutrition Examination Survey. PLoS ONE. 2012;7(11):e49050. doi: 10.1371/journal.pone.0049050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zumla A., George A., Sharma V., Herbert R.H.N., Oxley A., Oliver M. The WHO 2014 global tuberculosis report—further to go. Lancet Global Health. 2015;3(1):e10–e12. doi: 10.1016/S2214-109X(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 10.Myall K., Milburn H.J. An update on the management of latent tuberculosis infection and active disease in patients with chronic kidney disease. Pol Arch Intern Med. 2017;127(10):681–686. doi: 10.20452/pamw.4093. [DOI] [PubMed] [Google Scholar]
- 11.Seyhan E.C., Gunluoglu G., Gunluoglu M.Z., Tural S., Sökücü S. Predictive value of the tuberculin skin test and QuantiFERON-tuberculosis Gold In-Tube test for development of active tuberculosis in hemodialysis patients. Ann Thorac Med. 2016;11(2):114. doi: 10.4103/1817-1737.180023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang C.-H., Fan P.-C., Kuo G., Lin Y.-S., Tsai T.-Y., Chang S.-W., et al. Infection in advanced chronic kidney disease and subsequent adverse outcomes after dialysis initiation: a nationwide cohort study. Sci Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-59794-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura H., Tateyama M., Tasato D., Teruya H., Chibana K., Tamaki Y., et al. Active tuberculosis in patients undergoing hemodialysis for end-stage renal disease: a 9-year retrospective analysis in a single center. Intern Med. 2009;48(24):2061–2067. doi: 10.2169/internalmedicine.48.2660. [DOI] [PubMed] [Google Scholar]
- 14.Levy D.T., Zavala-Arciniega L., Reynales-Shigematsu L.M., Fleischer N.L., Yuan Z., Li Y., et al. Measuring smoking prevalence in a middle income nation: An examination of the 100 cigarettes lifetime screen. Global Epidemiol. 2019;1:100016. doi: 10.1016/j.gloepi.2019.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ringshausen F.C., Schablon A., Nienhaus A. Interferon-gamma release assays for the tuberculosis serial testing of health care workers: a systematic review. J Occup Med Toxicol. 2012;7(1):1–9. doi: 10.1186/1745-6673-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31–S34. doi: 10.4103/sja.SJA_543_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandenbroucke J.P., von Elm E., Altman D.G., Gøtzsche P.C., Mulrow C.D., Pocock S.J., et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10) doi: 10.1371/journal.pmed.0040297. e297-e. e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu C-C, Wu V-C, Yang F-J, Pan S-C, Lai T-S, Wang J-Y, et al. Predictors and prevalence of latent tuberculosis infection in patients receiving long-term hemodialysis and peritoneal dialysis. 2012. [DOI] [PMC free article] [PubMed]
- 21.Grant J., Jastrzebski J., Johnston J., Stefanovic A., Jastrabesky J., Elwood K., et al. Interferon-gamma release assays are a better tuberculosis screening test for hemodialysis patients: A study and review of the literature. Can J Infect Dis Med Microbiol. 2012;23(3):114–116. doi: 10.1155/2012/287181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S.Y., Jung G.S., Kim S.K., Chang J., Kim M.S., Kim Y.S., et al. Comparison of the tuberculin skin test and interferon-γ release assay for the diagnosis of latent tuberculosis infection before kidney transplantation. Infection. 2013;41(1):103–110. doi: 10.1007/s15010-012-0291-0. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal S.K., Singh U.B., Zaidi S.H., Gupta S., Pandey R.M. Comparison of interferon gamma release assay & tuberculin skin tests for diagnosis of latent tuberculosis in patients on maintenance haemodialysis. Indian J Med Res. 2015;141(4):463. doi: 10.4103/0971-5916.159297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martín-Malo A., Carracedo J., Ramírez R., Rodriguez-Benot A., Soriano S., Rodriguez M., et al. Effect of uremia and dialysis modality on mononuclear cell apoptosis. J Am Soc Nephrol. 2000;11(5):936–942. doi: 10.1681/ASN.V115936. [DOI] [PubMed] [Google Scholar]
- 25.Ai J.-W., Ruan Q.-L., Liu Q.-H., Zhang W.-H. Updates on the risk factors for latent tuberculosis reactivation and their managements. Emerg Microbes Infect. 2016;5(1):1–8. doi: 10.1038/emi.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar N.P., Banurekha V.V., Nair D., Dolla C., Kumaran P., Babu S. Modulation of iron status biomarkers in tuberculosis-diabetes co-morbidity. Tuberculosis. 2018;108:127–135. doi: 10.1016/j.tube.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koesoemadinata R.C., McAllister S.M., Soetedjo N.N.M., Febni Ratnaningsih D., Ruslami R., Kerry S., et al. Latent TB infection and pulmonary TB disease among patients with diabetes mellitus in Bandung, Indonesia. Trans R Soc Trop Med Hyg. 2017;111(2):81–89. doi: 10.1093/trstmh/trx015. [DOI] [PubMed] [Google Scholar]
- 28.Remy WL. The association between latent tuberculosis infection and diabetes mellitus control in the United States. 2016.
- 29.Baker M.A., Lin H.-H., Chang H.-Y., Murray M.B. The risk of tuberculosis disease among persons with diabetes mellitus: a prospective cohort study. Clin Infect Dis. 2012;54(6):818–825. doi: 10.1093/cid/cir939. [DOI] [PubMed] [Google Scholar]
- 30.Alavi-Naini R., Sharifi-Mood B., Metanat M. Association between tuberculosis and smoking. Int J High Risk Behav Addict. 2012;1(2):71–74. doi: 10.5812/ijhrba.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang L.Y., Baumann B., Romanowski K., Kumar D., Campbell J.R., Djurdjev O., et al. Latent tuberculosis therapy outcomes in dialysis patients: A retrospective cohort. Am J Kidney Dis. 2021;77(5):696–703. doi: 10.1053/j.ajkd.2020.06.017. [DOI] [PubMed] [Google Scholar]