Abstract
Eltrombopag has been used in ITP and found its use in AA armamentarium recently.
We retrospectively analyzed 61 patients at a tertiary care center in Pakistan from January 2015 to January 2021. They included patients with severe AA who were refractory to at least one course of immunosuppressive therapy and persistent/chronic ITP who have received at least one previous treatment for ITP. Responses to Eltrombopag in our population were comparable to real-world experiences while tolerable hepatotoxicity and GI issues were notable.
We found Eltrombopag to be a safe and efficacious agent for treating patients with ITP and AA.
Keywords: Eltrombopag, Immune thrombocytopenia, Severe aplastic anemia, Platelet response, Complete resposne
Abbreviations
- AA:
Aplastic anemia
- allo-HSCT:
Allogeneic hematopoietic stem cell transplantation
- ATG:
Anti-thymocyte globulin
- CTCAE:
Criteria for Adverse Events
- CYA:
cyclosporine A
- ELT:
Eltrombopag
- GI:
Gastrointestinal disturbance
- HSCs:
Hematopoietic stem cells
- ITP:
Immune thrombocytopenia
- SAA:
Severe aplastic anemia
1. Introduction
Aplastic anemia (AA) is a form of bone marrow failure caused by T-cell mediated destruction of hematopoietic stem cells (HSCs) and is life-threatening if untreated [1]. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is potentially curative, however, for those not eligible, the standard therapy consists of immunosuppression with horse anti-thymocyte globulin (ATG) and cyclosporine A (CYA) [2]. About 70% of patients do respond, but survival with good marrow function without relapse is in the order of only 30–40% [3]. Treatment options are unsatisfactory for patient's refractory to or relapsing after first-line treatment especially when they are transplant ineligible. Furthermore, the intensive immunosuppressive ATG-CYA combination cannot be applied in a proportion of AA patients due to severe infections [4].
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by an abnormally low number of circulating platelets, <100 × 103/µl which promotes bleeding tendency. Intracranial hemorrhage is a life-threatening complication and occurs especially when the platelet count drops below 10 × 103/µl [5]. The incidence of primary ITP in adults is 3.3/100,000/year. ITP incidence is multimodal and exhibits three peaks in children, young adults, women aged 30–40 years, and the elderly. The prevalence in adults is 9.5/100,000. The exact mechanism that leads to ITP is poorly understood, although it is well known that antiplatelet autoantibodies play a key role. These appear because of an altered T-cell response, in which the splenic T follicular helper cells are involved as inducers of the proliferation and differentiation of autoreactive B-cells [6]. These produce antiplatelet autoantibodies, predominantly of the immunoglobulin (Ig) G isotype, which can react with a series of platelet receptors, mainly glycoprotein (GP) IIb/IIIa and GPIb/IX, but also GPV, GPIa/IIa, or GPIV. These auto epitopes [7] induce cell destruction. These platelets experience splenic sequestration and subsequent phagocytosis by mononuclear macrophages, which react with the cell-complexed autoantibodies. Macrophages still play an additional harmful role in ITP since they behave as the main antigen-presenting cell [8]. CD8+T-cells also contribute to thrombocytopenia by increasing platelet apoptosis [9]. Recently, the Ashwell–Morell receptor (AMR), which is an asialoglycoprotein counter receptor predominantly expressed in hepatocytes, has also been shown to play an important role in anti-GPIbα antibody-mediated platelet clearance. Consequently, the circulating half-life of platelets is markedly reduced. In addition, bone marrow megakaryocytes are unable to produce platelets normally, which further increases thrombocytopenia. The likely reason is that on one hand, there are autoimmune responses against megakaryocytes while on the other hand, the circulating thrombopoietin (TPO), which is the main growth factor of megakaryocytes, does not increase to a level high enough to cause sufficient stimulation [10].
Currently, ITP is categorized according to duration, as newly diagnosed (<3 months), persistent (3–12 months), or chronic (> 12 months) [11].
ELT is a synthetic, low molecular weight thrombopoietin receptor agonist (TPO-RA). It has been used in ITP and recently found its use in the AA therapy armamentarium. Unlike native TPO, which binds to the extracellular domain of thrombopoietin receptor (TPO-R), ELT interacts with the transmembrane domain of the latter. Therefore, the Janus kinases (JAKs), signal transducer, and activator of transcription proteins (STAT) i.e., JAK/STAT signaling pathway stimulate megakaryocytopoiesis, while not detecting autoantibody generation [12]. ELT does not influence agonist-induced platelet aggregation or activation. With good oral bioavailability, circulating peaks are found 2–6 h after oral administration. Metabolism takes place in the liver via cytochrome P450 isoenzymes with a half-life of 21–32 h [13].
In contrast to previous failed attempts to stimulate the HSC compartment by diverse growth factors such as G-CSF, GM-CSF, or EPO, ELT induced trilinear hematopoietic responses in refractory AA patients [14]. Mechanistically, ELT binds to the TPO receptor c-MPL expressed on HSCs and leads to their proliferation and expansion [15, 16]. Additional immunomodulatory effects may be involved. Results of a phase II trial examining ELT monotherapy in refractory AA patients showing a 40% response rate led to the approval of ELT as monotherapy in relapsed-refractory AA in the USA and Europe. Recently, the increased response rate to ELT in combination with standard ATG-CYA immunosuppression as compared to historical controls has been reported by the NIH group [17] and the use of ELT in the first-line setting is currently underway.
Our work is the first such effort in the region, where the role of ELT in both benign hematological disorders is studied retrospectively. We hypothesize that the role of ELT in our population would be reflective of the studies performed in the EXTEND trial [18] for ITP patients and in concordance with previous studies in severe AA [19].
2. Methods
This retrospective chart review was conducted using electronic medical records (EMR) for 60 male and female patients who were treated with ELT for Severe Aplastic anemia and Immune thrombocytopenia from January 2015 to January 2021 at a tertiary care hospital in Karachi, Pakistan.
Severe Aplastic anemia was defined as having a hypocellular bone marrow when two of three blood counts were met (absolute neutrophil count <500/μL, absolute reticulocyte count <60,000/μL, platelet count <20,000/μL). Included patients were refractory to at least one course of immunosuppressive therapy, initiated at least in the last 6 months, and were not candidates for stem cell transplant. Patients were excluded if they had a diagnosis of Fanconi anemia on peripheral chromosomal breakage analysis; MDS (ruled out on morphology and cytogenetics); and/or diagnosed with paroxysmal nocturnal hemoglobinuria based on the absence of CD 55 and CD 59 by gel card method. Women who were nursing or pregnant were also excluded from the study.
Immune thrombocytopenia was defined as having platelets, <100 × 103/µl in the absence of any underlying disorder. Included patients had at least a 3-month history of ITP (Persistent/Chronic); included both primary and secondary (associated with autoimmune diseases, malignancy, infections, and others cause) ITP; received at least one previous treatment for ITP and had a platelet count of less than 30,000 per cubic millimeter at enrollment. Patients who were receiving maintenance immunosuppressive regimens, primarily glucocorticoids, were eligible if the dose had been stable for at least 1 month. The dose had to remain unchanged throughout the study. Patients were excluded if they had hemoglobin levels of less than 9 gs per deciliter; had congestive heart failure; had a history of arrhythmias; had a history of thrombosis or myocardial infarction within 3 months and/or were women who were nursing or pregnant.
Following data was extracted from medical records after departmental and ethical approval: patient age, sex, severe aplastic anemia (SAA), ITP, presenting complete blood counts, history of bleeding, and splenectomy status in ITP patients. Following post-intervention variables were extracted in AA: 1) platelet response; 2) erythroid lineage response; 3) leucocyte response; and 4) treatment failure. While post-intervention variables extracted in ITP were as follows: 1) good response; 2) partial response and 3) no response. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 was used to assess the toxicity profile. It included hepatotoxicity, thromboembolic event, portal vein thrombosis, nasopharyngitis, upper respiratory tract infection, nausea, vomiting, diarrhea, and skin rash. ELT treatment was initiated at a minimum daily dose of 50 mg and a maximum of 75 mg. This was adjusted as per response during a total duration of at least 6 weeks. Lower doses compared to the western population were used based on the population pharmacokinetics of ELT in East Asia [20, 21].
Response to the treatment in each group was assessed according to National Institutes of Health (NIH) criteria, which is as follows.
2.1. Response for aplastic anemia
A platelet response was defined by a platelet count increase of 20 × 109/L above baseline or stable platelet counts with no transfusions for at least 8 weeks. An erythroid lineage response was defined as a hemoglobin increase of >1.5 g/dL or a reduction of >4 units of transfused erythrocytes for 8 consecutive weeks. A leukocyte response was defined as an absolute neutrophil increase of 100% or an absolute neutrophil count increase of >0.5 × 109/L. Not achieving at least one of these criteria during ELT treatment was considered a treatment failure.
2.2. Response for immune thrombocytopenia
Platelet count of more than 50 × 109/L was considered a good response; partial response when the platelet count ranged between 30 and 50 × 109/L with at least a 2-fold increase in the initial platelet count; and no response when the platelet count was 30 × 109/L and less.
No missing data on scale variables were observed. Median and Interquartile range (IQR) was computed for continuous variables like age, length of hospital stays, and blood parameters. Frequencies and percentages were calculated for categorical variables like gender, bleeding, and toxicity. The data was stratified accordingly i.e., SAA and ITP, baseline, clinical characteristics, and treatment response was compared using the Chi-Square test. In addition, a comparative analysis of response between ELT versus non-ELT (standard treatment) group was also performed. One-way ANOVA test was used to compare the pre- and post-treatment quantitative data. A p-value less than or equal to 0.05 was considered significant. The data were analyzed using Stata version-15.
3. Results
From January 2015 to January 2021, 61 patients including 32 Severe Aplastic Anemia (SAA) and 29 Immune Thrombocytopenia (ITP) received ELT. The baseline characteristics of patients are presented in Table 1.
Table 1.
Patients’ baseline characteristics received ELT in immune thrombocytopenia and acquired idiopathic aplastic anemia (n = 61).
| Characteristics | Disease n (%) | p-value | |
|---|---|---|---|
| IAA (n = 32) | ITP (n = 29) | ||
| Median Age (years) | 22.5 (16–34.5) | 49 (38–57) | <0.001 |
|
Gender Male Female |
12 (37.5%) 20 (62.5%) |
19 (65.5%) 10 (34.5%) |
0.029 |
|
WHO bleeding scale None Grade I Grade II Grade III Grade IV |
15 (48.4%) 2 (6.5%) 1 (3.2%) 12 (38.7%) 1 (3.2%) |
13 (44.8%) 0 (0.0%) 13 (44.8%) 2 (6.9%) 1 (3.5%) |
0.033 |
The median (IQR) age of AA patients was 22.5 (16–34.5) years and 49 (38–57) years of ITP patients. Most of the AA patients were female 20 (62.5%), unlike the ITP patients who were mostly males, 19 (65.5%). World Health Organization (W.H.O.) bleeding scale was used to grade bleeding. At presentation 12 (38.7%) AA patients had grade III bleeding and 13 (44.8%) ITP patients had grade II bleeding.
The toxicity profile of Immune Thrombocytopenia and Aplastic Anemia patients are presented in Table 2. Hepatotoxicity and GI issues were notable in both groups.
Table 2.
Patient's toxicity profile received ELT in immune thrombocytopenia and acquired idiopathic aplastic anemia (n = 61).
| Characteristics | Disease n (%) | p-value | |
|---|---|---|---|
| IAA (n = 32) | ITP (n = 29) | ||
| Hepatotoxicity Grade 1/2 Grade 3/4 |
4 (12.9%) 3 (9.7%) |
6 (20.7%) 1 (3.4%) |
0.046 |
| Thromboembolic event Grade 1/2 Grade 3/4 |
0 (0.0%) 1 (3.2%) |
1 (3.4%) 1 (3.4%) |
0.568 |
| UTI Grade 1/2 Grade 3/4 |
0 (0.0%) 7 (21.9%) |
0 (0.0%) 1 (3.4%) |
0.033 |
| Gastrointestinal Grade 1/2 Grade 3/4 |
4 (12.5%) 10 (31.3%) |
0 (0.0%) 1 (3.4%) |
0.008 |
| Rash Grade 1/2 Grade 3/4 |
0 (0.0%) 5 (15.6%) |
0 (0.0%) 0 (0.0%) |
0.260 |
Response to ELT was classified into two groups for both AA and ITP. The response of AA patients was categorized as platelet, erythroid lineage, and leucocyte response respectively, which when all present considered a complete response. In this population, platelet response was seen in 29 (90.6%), erythroid lineage in 27 (84.4%), and leucocyte response in 21 (65.6%) patients respectively (Fig. 1). A complete response to ELT was seen in 17 (53.1%) AA patients (p = 0.001). There was only 1 (3.1%) patient who failed treatment in the AA group (p = 0.692).
Fig. 1.
Treatment response to ELT in acquired idiopathic aplastic anemia.
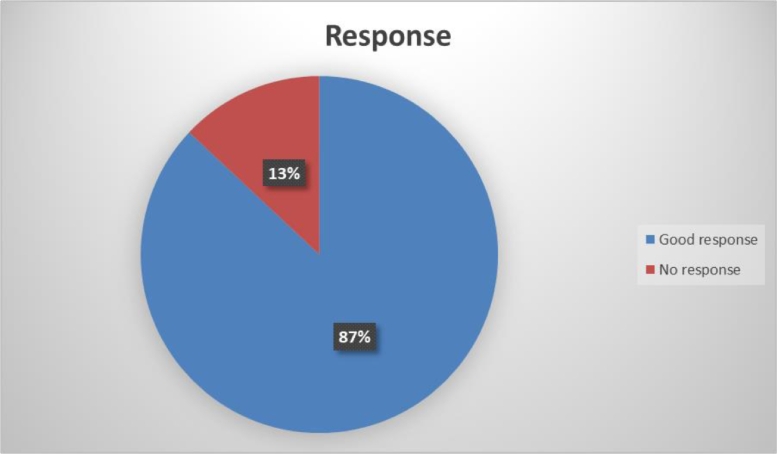
The response of ITP patients to ELT was classified as a ‘no’, ‘partial’, and ‘good response’ respectively. In this group 25 (86.2%, p = 0.033) ITP patients showed good response to the ELT (Fig. 2).
Fig. 2.
Treatment response to ELT in immune thrombocytopenia.
Analysis on the outcomes of AA and ITP patients who received ELT compared with those who received standard (non-ELT) treatment was also performed. Both treatment groups had an equivalent number of patients (61 vs. 60).
ELT group showed significant improvement in clinical characteristics compared with the non-ELT group. Median hemoglobin (g/dl) pre- vs. post-treatment in AA patients who received ELT was 7.6 vs. 11.1 g/dl while in the non-ELT group was 7.5 vs. 9.2 g/dl. Likewise, the median white blood cell count in AA who received ELT pre- vs. post-treatment was (1.4 vs. 6.9) x 109/L, while in the non-ELT group was (3.0 vs. 3.5) x 109/L. Similarly, platelets count also showed an increase in both groups but significantly in the ELT group. The median platelet counts in AA and ITP patients who received ELT pre- vs. post-treatment were (9 vs. 70) x 109/L and (6 vs. 166) x 109/L respectively while (12 vs. 19) x 109/L and (12 vs. 128) x 109/L in the non-ELT group. Similarly 17 (53.1%) AA and 25 (86.2%) ITP patients in ELT group gave complete/good response to treatment, while four (13.3%) AA and 23 (76.7%) ITP patients in non-ELT group gave complete/good response to treatment, which was significant (Table 3).
Table 3.
Response and Outcomes of ELT vs. standard (non-ELT) treatment in immune thrombocytopenia and acquired idiopathic aplastic anemia.
| Characteristic | ELT (n = 61) | Non-ELT (n = 60) | p-value | ||
|---|---|---|---|---|---|
| IAA | ITP | IAA | ITP | ||
| Median Hb pre-treatment (g/dl) | 7.6 | 11.3 | 7.5 | 11.1 | 0.045 |
| Median Hb post-treatment (g/dl) | 11.1 | 12.5 | 9.2 | 12.1 | 0.033 |
| Median WBC pre-treatment (10E9/L) | 1.4 | 3.0 | 3.0 | 7.9 | 0.042 |
| Median WBC post-treatment (10E9/L) | 6.9 | 14.0 | 3.5 | 10.9 | 0.038 |
| Median Platelets pre-treatment (10E9/L) | 9 | 6 | 12 | 12 | 0.028 |
| Median Platelets post-treatment (10E9/L) | 70 | 166 | 19 | 128 | 0.021 |
| Response No Yes |
15 (46.9%) 17 (53.1%) |
4 (13.8%) 25 (86.2%) |
26 (86.7%) 4 (13.3%) |
7 (23.3%) 23 (76.7%) |
0.0006 |
4. Discussion
ELT is approved by both, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as a single agent in severe AA patients who show an inadequate response to initial immunosuppression. FDA has also approved ELT as a component of triple IST in treatment-naïve AA patients.
There has been no consensus yet about the most effective dose of ELT in either AA or ITP. Individuals of East or Southeast Asian ancestry have known population pharmacokinetic dynamics [22] and so are recommended to use lower doses(75 mg) of ELT compared to its normal dose of 150 mg. The usual starting dosage at our center is 50 mg, increased to 75 mg at maximum.
Two important considerations with ELT are the cost and long-term compliance to medications. In Pakistan, the government has taken some initiatives along with pharmaceutical companies to provide ELT at subsidized rates for the indication of ITP. However, financial issues in third-world countries remain a significant hindrance to its use.
Previous medical literature has shown promising results with ELT in different populations spanning different parts of the globe.
A prospective phase 1, 2 study on 92 patients by Danielle et al. found that the addition of ELT to immunosuppressive therapy was associated with markedly higher rates of hematologic response among patients with severe aplastic anemia than in a historical cohort [23].
A phase 2 study by Matthew J et al. involving patients with aplastic anemia that were refractory to immunosuppression was performed to determine the response of ELT on blood counts. Eleven of 25 patients (44%) had a hematologic response in at least one lineage at 12 weeks, with minimal toxic effects [24].
In previous studies, ELT has been considered generally safe, although skin reactions or elevation of hepatic transaminases have been noted [23].
Common adverse events (≥20%) include nausea, fatigue, cough, diarrhea, and headache while mild to moderate increase in indirect bilirubin is a common finding, however without any clinically significant consequence to the patient [25]. It is notable, however, that the effects of long-term use of ELT in AA have not been thoroughly evaluated. This includes the risk of clonal evolution.
In relevance to ITP, the ELT extended Dose study (EXTEND), in which patients were followed for 3 years, the median daily dose was approximately 50 mg in ITP patients [26]. Polyvalent cations such as iron, calcium, aluminum, magnesium, selenium, and zinc are known to reduce the absorption of ELT.
Xiaofan et al. in phase III, randomized, placebo-controlled study assessed the long-term efficacy and safety of ELT use in chronic immune thrombocytopenia (ITP) among the Chinese population. 32% of patients achieved platelet counts ≥50 × 109/L in more than 75% of platelet count assessments overall. The results further established a sustainable long-term efficacy and tolerability of ELT in these patients [27].
Raymond et al. in the open-label EXTEND study evaluated the long-term safety and efficacy of ELT in adults with ITP who had completed a previous ELT study. Among 302 enrolled patients who were followed for a median duration of 2.37 years, 259 patients (85.8%) achieved a response (platelet count ≥50 × 109/L at least once in the absence of rescue), while 133 (52%) of the responders achieved a continuous response of a minimum of 25 weeks. Bleeding symptoms decreased by 41% in 1 year of treatment from baseline. 14% of patients withdrew because of adverse events, which included hepatobiliary adverse events, cataracts, deep vein thrombosis, cerebral infarction, headache, and myelofibrosis. Treatment past one year did not increase rates of thromboembolic or hepatobiliary adverse events. Overall EXTEND trial demonstrated that long-term use of ELT was effective in maintaining a reliable platelet count of 50 × 109/L or more while reducing bleeding in most patients with ITP of more than 6 months. Adverse events were also very infrequent [18].
In a randomized phase 3 trial (RAISE) by Gregory et al. that included 197 patients, 13 percent had adverse events of which the most common were liver enzyme abnormalities and thromboembolic events [28]. They found no significant differences in the development of malignancies or cataracts in the ELT and placebo groups.
In a study by Brynes et al. 2 of 117 patients (1.7%) showed moderate reticulin fibrosis of the bone marrow in patients receiving ELT who were followed for up to 5.5 years [29]. However monitoring of bone marrow is not generally recommended nor part of guidelines by the American Society of Hematology [30] The reason is, the absence of any evidence of progression to myelofibrosis or serial bone marrow testing leading to better outcomes. In addition, bone marrow biopsy is an uncomfortable procedure for patients. Hence, patients in our center did not undergo bone marrow biopsy post-treatment.
Other TPO-RAs include romiplostim and avatrombopag. Romiplostim is a once-weekly subcutaneous injection while ELT and avatrombopag is a once-daily pill. Romiplostim has also shown a good platelet response of around 80% in chronic ITP patients with a relatively acceptable safety profile [31]. Avatrombopag was approved in 2018 however; there is very limited clinical experience with avatrombopag compared with the other TPO-RAs.
The overall response rates are very promising in our population and are comparable to previous experiences with eltrombopag in the European and Chinese populations with a much better safety profile. The resolution of cytopenias especially platelet counts within 6 weeks of initiation of ELT in both AA and ITP makes them less prone to life-threatening bleed.
Our study has several limitations. Being retrospective study causality cannot be determined in terms of side effects, and so prospective trials need to be designed in the future to assess better side effect profiles and related additional factors. We also have a relatively smaller sample size with a shorter follow-up. Further studies that monitor patients for a longer duration would help us ascertain the durability of response and toxicity profile.
5. Conclusion
This retrospective study found very promising results for ELT in both ITP and AA. This makes it a reasonable choice for patients who can afford the treatment and are compliant with long-term medications. ELT showed complete response in 53.1% AA patients while approximately 90% platelet response in both AA and ITP patients. We also found a significantly better response in AA and ITP patients when comparing ELT with other standard treatment options. ELT is a reasonably efficacious and safe drug. We suggest consideration of ELT in this subgroup of patients to avoid early life-threatening infections and bleed.
Ethical approval
Ethical approval was taken from Ethical Review Committee, Aga Khan University Karachi, Pakistan (ERC, AKU) Ref #: 2020–5494–14,246
Financial support and sponsorship
None
Declaration of Competing Interest
The authors do not declare any relevant conflicts of interest.
Acknowledgments
Department of Health Information and Management Systems, Aga Khan University Hospital Karachi, Pakistan
References
- 1.Luzzatto L., Risitano A.M. Advances in understanding the pathogenesis of acquired aplastic anaemia. 2018;182(6):758–776. [DOI] [PubMed]
- 2.Killick S.B., Bown N., Cavenagh J., Dokal I., Foukaneli T., Hill A., et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br. J. Haematol. 2016;172(2):187–207. doi: 10.1111/bjh.13853. [DOI] [PubMed] [Google Scholar]
- 3.Tichelli A., Schrezenmeier H., Socié G., Marsh J., Bacigalupo A., Dührsen U., et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA working party of the European group for blood and marrow transplantation. Blood. 2011;117(17):4434–4441. doi: 10.1182/blood-2010-08-304071. [DOI] [PubMed] [Google Scholar]
- 4.Kao S.Y., Xu W., Brandwein J.M., Lipton J.H., Messner H.A., Minden M.D., et al. Outcomes of older patients (>or = 60 years) with acquired aplastic anaemia treated with immunosuppressive therapy. Br. J. Haematol. 2008;143(5):738–743. doi: 10.1111/j.1365-2141.2008.07389.x. [DOI] [PubMed] [Google Scholar]
- 5.Neunert C., Noroozi N., Norman G., Buchanan G.R., Goy J., Nazi I., et al. Severe bleeding events in adults and children with primary immune thrombocytopenia: a systematic review. J. Thromb. Haemost. 2015;13(3):457–464. doi: 10.1111/jth.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Audia S., Mahévas M., Samson M., Godeau B., Bonnotte B. Pathogenesis of immune thrombocytopenia. Autoimmun. Rev. 2017;16(6):620–632. doi: 10.1016/j.autrev.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Lambert M.P., Gernsheimer T.B. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129(21):2829–2835. doi: 10.1182/blood-2017-03-754119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuwana M., Okazaki Y., Ikeda Y. Splenic macrophages maintain the anti-platelet autoimmune response via uptake of opsonized platelets in patients with immune thrombocytopenic purpura. J. Thromb. Haemost. 2009;7(2):322–329. doi: 10.1111/j.1538-7836.2008.03161.x. [DOI] [PubMed] [Google Scholar]
- 9.Qiu J., Liu X., Li X., Zhang X., Han P., Zhou H., et al. CD8(+) T cells induce platelet clearance in the liver via platelet desialylation in immune thrombocytopenia. Sci. Rep. 2016;6:27445. doi: 10.1038/srep27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kosugi S., Kurata Y., Tomiyama Y., Tahara T., Kato T., Tadokoro S., et al. Circulating thrombopoietin level in chronic immune thrombocytopenic purpura. Br. J. Haematol. 1996;93(3):704–706. doi: 10.1046/j.1365-2141.1996.d01-1702.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodeghiero F., Stasi R., Gernsheimer T., Michel M., Provan D., Arnold D.M., et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 12.Erickson-Miller C.L., DeLorme E., Tian S.S., Hopson C.B., Stark K., Giampa L., et al. Discovery and characterization of a selective, nonpeptidyl thrombopoietin receptor agonist. Exp. Hematol. 2005;33(1):85–93. doi: 10.1016/j.exphem.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Erhardt J., Erickson-Miller C.L., Tapley P. SB 497115-GR, a low molecular weight TPOR agonist, does not induce platelet activation or enhance agonist-induced platelet aggregation in vitro. Blood. 2004;104(11):3888. [Google Scholar]
- 14.Desmond R., Townsley D.M., Dumitriu B., Olnes M.J., Scheinberg P., Bevans M., et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014;123(12):1818–1825. doi: 10.1182/blood-2013-10-534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovtonyuk L.V., Manz M.G., Takizawa H. Enhanced thrombopoietin but not G-CSF receptor stimulation induces self-renewing hematopoietic stem cell divisions in vivo. Blood. 2016;127(25):3175–3179. doi: 10.1182/blood-2015-09-669929. [DOI] [PubMed] [Google Scholar]
- 16.Sun H., Tsai Y., Nowak I., Liesveld J., Chen Y. Eltrombopag, a thrombopoietin receptor agonist, enhances human umbilical cord blood hematopoietic stem/primitive progenitor cell expansion and promotes multi-lineage hematopoiesis. Stem Cell Res. 2012;9(2):77–86. doi: 10.1016/j.scr.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Townsley D.M., Scheinberg P., Winkler T., Desmond R., Dumitriu B., Rios O., et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N. Engl. J. Med. 2017;376(16):1540–1550. doi: 10.1056/NEJMoa1613878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong R.S.M., Saleh M.N., Khelif A., Salama A., Portella M.S.O., Burgess P., et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130(23):2527–2536. doi: 10.1182/blood-2017-04-748707. [DOI] [PubMed] [Google Scholar]
- 19.Drexler B., Passweg J. Current evidence and the emerging role of eltrombopag in severe aplastic anemia. Ther. Adv. Hematol. 2021;12 doi: 10.1177/2040620721998126. 2040620721998126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gibiansky E., Zhang J., Williams D., Wang Z., Ouellet D. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J. Clin. Pharmacol. 2011;51(6):842–856. doi: 10.1177/0091270010375427. [DOI] [PubMed] [Google Scholar]
- 21.Tomiyama Y., Miyakawa Y., Okamoto S., Katsutani S., Kimura A., Okoshi Y., et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J. Thromb. Haemost. 2012;10(5):799–806. doi: 10.1111/j.1538-7836.2012.04695.x. [DOI] [PubMed] [Google Scholar]
- 22.Gibiansky E., Zhang J., Williams D., Wang Z., Ouellet D. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J. Clin. Pharmacol. 2011;51(6):842–856. doi: 10.1177/0091270010375427. [DOI] [PubMed] [Google Scholar]
- 23.Townsley D.M., Scheinberg P., Winkler T., Desmond R., Dumitriu B., Rios O., et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N. Engl. J. Med. 2017;376(16):1540–1550. doi: 10.1056/NEJMoa1613878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olnes M.J., Scheinberg P., Calvo K.R., Desmond R., Tang Y., Dumitriu B., et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N. Engl. J. Med. 2012;367(1):11–19. doi: 10.1056/NEJMoa1200931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheinberg P. Activity of eltrombopag in severe aplastic anemia. Blood Adva. 2018;2(21):3054–3062. doi: 10.1182/bloodadvances.2018020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saleh M.N., Bussel J.B., Cheng G., Meyer O., Bailey C.K., Arning M., et al. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. 2013;121(3):537–545. doi: 10.1182/blood-2012-04-425512. [DOI] [PubMed] [Google Scholar]
- 27.Liu X., Hou M., Li J., Jin J., Huang M., Yu Z., et al. Efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia: stage 2 results from a multicenter phase III study. Platelets. 2020:1–7. doi: 10.1080/09537104.2020.1847267. [DOI] [PubMed] [Google Scholar]
- 28.Cheng G., Saleh M.N., Marcher C., Vasey S., Mayer B., Aivado M., et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377(9763):393–402. doi: 10.1016/S0140-6736(10)60959-2. [DOI] [PubMed] [Google Scholar]
- 29.Brynes R.K., Orazi A., Theodore D., Burgess P., Bailey C.K., Thein M.M., et al. Evaluation of bone marrow reticulin in patients with chronic immune thrombocytopenia treated with eltrombopag: data from the EXTEND study. Am. J. Hematol. 2015;90(7):598–601. doi: 10.1002/ajh.24011. [DOI] [PubMed] [Google Scholar]
- 30.Neunert C., Lim W., Crowther M., Cohen A., Solberg Jr. L., Crowther M.A. The American society of hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–4207. [DOI] [PubMed]
- 31.Kuter D.J., Bussel J.B., Lyons R.M., Pullarkat V., Gernsheimer T.B., Senecal F.M., et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395–403. doi: 10.1016/S0140-6736(08)60203-2. [DOI] [PubMed] [Google Scholar]