Abstract
Objective
Infection with Theiler's murine encephalomyelitis virus (TMEV) in C57Bl/6J mice results in handling‐induced seizures and is useful for evaluating compounds effective against infection‐induced seizures. However, to date only a few compounds have been evaluated in this model, and a comprehensive study of antiseizure medications (ASMs) has not yet been performed. Furthermore, as the TMEV infection produces marked neuroinflammation, an evaluation of prototype anti‐inflammatory compounds is needed as well.
Methods
Male C57Bl/6J mice were inoculated with TMEV (day 0) followed by daily administrations of test compounds (day 3‐7) and subsequent handling sessions (day 3‐7). Doses of ASMs, comprising several mechanistic classes, were selected based on previously published data demonstrating the effect of these compounds in reducing seizures in the 6 Hz model of pharmacoresistant seizures. Doses of anti‐inflammatory compounds, comprising several mechanistic classes, were selected based on published evidence of reduction of inflammation or inflammation‐related endpoints.
Results
Several prototype ASMs reduced acute seizures following TMEV infection: lacosamide, phenytoin, ezogabine, phenobarbital, tiagabine, gabapentin, levetiracetam, topiramate, and sodium valproate. Of these, phenobarbital and sodium valproate had the greatest effect (>95% seizure burden reduction). Prototype anti‐inflammatory drugs celecoxib, dexamethasone, and prednisone also moderately reduced seizure burden.
Significance
The TMEV model is utilized by the Epilepsy Therapy Screening Program (ETSP) as a tool for evaluation of novel compounds. Compounds reducing seizures in the TMEV comprise distinct mechanistic classes, some with mechanisms of action that extend beyond traditional ASMs.
Keywords: animal models, antiseizure medications, infection‐induced seizures, inflammation, pharmacoresistant epilepsy
Key Points.
The Theiler's murine encephalomyelitis virus (TMEV) model is a model of viral‐induced acute seizures and subsequent temporal lobe epilepsy (TLE).
While some studies in this model have evaluated prototype antiseizure medications (ASMs) and anti‐inflammatory drugs, there has not been a comprehensive study encompassing several mechanistic drug classes.
Several ASMs, comprising multiple mechanisms of action, reduced handling‐induced seizure burden during the acute infection period following cortical injections of TMEV.
Celecoxib and steroid compounds (dexamethasone and prednisone), but not diclofenac and ibuprofen, also reduced seizure burden in this model.
The TMEV model is well suited to evaluate both traditional ASMs and anti‐inflammatory compounds that may be effective in reducing seizure burden following central nervous system (CNS) infection.
1. INTRODUCTION
The Theiler's murine encephalomyelitis virus (TMEV) model is recognized as a model of viral‐induced acute seizures and subsequent temporal lobe epilepsy (TLE). 1 , 2 , 3 Following intracerebral injection of the Daniels strain of TMEV, C57Bl/6J mice develop acute spontaneous and handling‐induced seizures. 2 , 4 , 5 , 6 , 7 . TMEV inoculation followed by evaluation of spontaneous and handling‐induced seizures has been proposed as a platform for evaluating compounds for the treatment of CNS infection‐induced seizures. 8 However, a comprehensive evaluation of antiseizure medication (ASM) prototypes has not yet been conducted, though some compounds (eg, valproic acid, carbamazepine, minocycline, wogonin, and cannabidiol) have been evaluated for their ability to prevent seizures in this model. 4 , 8 , 9
Several studies have examined the role of the immune system and inflammation in response to TMEV infection. 9 , 10 , 11 , 12 For example, TGF‐β levels are increased in TMEV‐infected mice that display seizures during the early (eg, day 0‐10) postinfection period. 1 Inflammation in this model is also marked by cytokine expression including interleukin 6 (IL‐6), tumor necrosis factor alpha (TNFα), and IL‐1 beta (IL‐1β). 13 , 14 Further, TNFα‐mediated signaling may be a key inflammatory pathway contributing to acute seizures following TMEV infection. 14 TMEV infection also produces astrogliosis, astrocyte proliferation, microgliosis, microglial proliferation, increased production of reactive oxygen species (ROS), and neuronal cell loss 15 , 16 in the hippocampus. As inflammation and pro‐convulsant cytokines can affect neural circuit excitability and therefore potentially contribute to the development of epilepsy, 17 it is important to identify compounds that can reduce inflammation as well as compounds that can protect against acute seizures.
Traditional drug screening has employed seizure induction by electrical or chemical means in naïve mice or rats. 18 , 19 After verification of antiseizure efficacy, more time‐intensive models can be employed to determine whether selected compounds are effective against kindled seizures or spontaneous seizures. 20 This approach allows for identification and differentiation of novel compounds and comparison to prototype ASMs. The majority of currently available ASMs act via modulation of ion channels, excitatory neurotransmission, or inhibitory neurotransmission. 21 , 22 However, novel treatments for seizures arising from CNS infections, inflammation, and/or immune‐related pathologies may include compounds with mechanisms of action that extend beyond those of traditional ASMs. For example, drugs acting to reduce inflammation or modulate immune system activity 23 , 24 , 25 may be effective in reducing seizures in this model. Therefore, the TMEV model of infection‐induced seizures may aid in the evaluation of novel ASMs with unique mechanisms of action. Herein, we describe the evaluation of several prototype ASMs, comprising several mechanisms of action. Efficacy was assessed during the acute infection period (day 3‐7) following TMEV infection. Further, as inflammation is known to contribute to seizure generation in this model, we also evaluated anti‐inflammatory compounds. This study sets the stage for future investigations within the NIH/NINDS funded Epilepsy Therapy Screening Program (ETSP).
2. METHODS
2.1. Compound preparation
All compounds were prepared in 0.5% methylcellulose (Sigma), except for valproic acid, which was prepared using saline (0.9% NaCl). Carbamazepine, clonazepam, celecoxib, diclofenac, dexamethasone, ethosuximide, ibuprofen, minocycline, phenobarbital, phenytoin, prednisone, ezogabine, and valproic acid were obtained from Sigma (Sigma). Gabapentin, levetiracetam, tiagabine, and topiramate were obtained from TCI America. Lacosamide was obtained from Axon Medchem, and lamotrigine was obtained from AK Scientific. Doses for each compound are shown in Tables 1, 2, 3. All compounds were administered as suspensions, with the exceptions of ethosuximide, ezogabine, gabapentin, levetiracetam, tiagabine, and valproic acid.
TABLE 1.
Effect of prototype antiseizure compounds on seizure burden in the Theiler's murine encephalomyelitis virus (TMEV) model against handling‐induced seizures
| Compound | Mechanism of action |
Mouse 6 Hz (44 mA) ED a 50 |
Dose mg/kg, IP |
% VEH cumulative seizure burden |
Protection (# Protected/# Tested) |
|---|---|---|---|---|---|
| Vehicle | 100.0 ± 18.8 | 5/20 | |||
| Carbamazepine | Na+ channel blocker | 38.2 | 40 | 40.6 ± 13.8 | 10/17 |
| Lacosamide | Na+ channel blocker | 12.9 | 13 | 18.3 ± 4.6** | 6/20 |
| Lamotrigine b | Na+ channel blocker | 43.4 | 20 | 36.5 ± 9.4 | 6/20 |
| Phenytoin | Na+ channel blocker | 44.6 | 20 | 15.6 ± 4.5** | 6/20 |
| Ezogabine | K+ channel opener | 32.5 | 20 | 46.8 ± 8.1** | 5/20 |
| Clonazepam | GABAA receptor modulator | 0.17 | 0.2 | 33.7 ± 10.2 | 8/20 |
| Phenobarbital | GABAA receptor modulator | 35.3 | 35 | 1.7 ± 1.3**** | 7/20 |
| Tiagabine | GABA reuptake inhibition | 1.0 | 1.3 | 19.2 ± 6.0** | 4/20 |
| Ethosuximide | T‐Type Ca++ channel blocker | 271 | 300 | 121 ± 22.3 | 5/20 |
| Gabapentin | α2δ Ca++ chanel modulator | >500 | 350 | 9.8 ± 3.3**** | 4/20 |
| Levetiracetam | SV2A ligand | >1000 | 1000 | 11.3 + 5.4**** | 10/20 |
| Topiramate | Mixed | >300 | 300 | 42.7 ± 9.8* | 3/20 |
| Valproic Acid | Mixed | 239 | 239 | 0.6 ± 0.6**** | 3/18 |
Vehicle: 0.5% methylcellulose. *P < .05, **P < .01, ****P < .0001 compared to vehicle (VEH) seizure burden from the same testing cohort; Mann‐Whitney U test. The vehicle‐treated group was in the same testing cohort as topiramate (300 mg/kg) and ethosuximide (300 mg/kg) and was representative of other vehicle treatment groups.
Metcalf et al, 2017.
Lamotrigine was administered once daily (QD), whereas other compounds noted were administered twice daily (BID).
TABLE 2.
Dose‐response evaluation of selected antiseizure medications (ASM) prototypes in the Theiler's murine encephalomyelitis virus (TMEV) model against handling‐induced seizures
| Compound | Dose mg/kg, IP |
% VEH Cumulative seizure burden |
Protection (# Protected/# Tested) |
|---|---|---|---|
| Carbamazepine | 10 | 141 ± 19 | 3/20 |
| 20 | 72 ± 17 | 7/20 | |
| 40 a | 40.6 ± 13.8 | 10/17 | |
| Ethosuximide | 300 a | 121.4 + 22.3 | 5/20 |
| 400 | 44.2 ± 9.0** | 8/20 | |
| Levetiracetam | 10 | 54.1 ± 8.7 | 3/20 |
| 30 | 33.9 ± 7.5** | 4/20 | |
| 350 | 35.9 ± 7.0** | 3/20 | |
| 700 | 21.9 ± 4.9**** | 3/20 | |
| 1000 a | 11.3 ± 5.4**** | 10/20 | |
| Phenytoin | 2.5 | 112.9 ± 16.9 | 1/20 |
| 5 | 80.5 ± 17.4 | 6/20 | |
| 10 | 84.0 ± 15.0 | 1/20 | |
| 20 a | 15.6 ± 4.5** | 6/20 | |
| Ezogabine | 15 | 47.3 ± 16.2 | 11/17 |
| 20 a | 46.8 ± 8.1** | 5/20 | |
| Valproic Acid | 50 | 77.9 ± 18.4 | 7/20 |
| 100 | 51.3 ± 15.1 | 11/20 | |
| 200 | 52.3 ± 15.9 | 8/20 | |
| 239 a | 0.6 ± 0.6**** | 3/18 | |
| Phenobarbital | 20 | 4.0 + 3.0*** | 7/20 |
| 35 a | 1.7 ± 1.3**** | 4/20 |
**P < .01, ***P < .001, ****P < .0001 compared to vehicle (VEH) seizure burden from the same testing cohort; Mann‐Whitney U test.
Data from Table 1.
TABLE 3.
Effect of prototype anti‐inflammatory compounds on seizure burden in the Theiler's murine encephalomyelitis virus (TMEV) model against handling‐induced seizures
| Compound | Mechanism of action | Dose mg/kg, IP |
% VEH Cumulative seizure burden |
Protection (# Protected/# Tested) |
|---|---|---|---|---|
| Diclofenac | NSAID | 5 | 74.2 ± 17.1 | 8/20 |
| 10 | 74.6 ± 19.8 | 10/20 | ||
| Ibuprofen | NSAID | 10 | 110 ± 14.0 | 2/20 |
| 50 | 60.8 ± 12.8 | 7/20 | ||
| Celecoxib | Cox‐2 inhibitor | 5 | 69.5 ± 9.1* | 2/20 |
| 10 | 73.7 ± 8.6* | 1/20 | ||
| Dexamethasone | Corticosteroid | 20 | 38.3 ± 6.1**** | 4/20 |
| Prednisone | Corticosteroid | 5 | 91.8 ± 15.0 | 4/20 |
| 10 | 108 + 15.5 | 3/20 | ||
| 20 a | 68.3 ± 8.2* | 2/20 | ||
| Minocycline | Unknown | 50 a | 58.0 ± 14.2 | 6/19 |
*P < .05, ****P < .0001 compared to VEH seizure burden from the same testing cohort; Mann‐Whitney U test.
Compound was administered once daily (QD), whereas other compounds noted were administered twice daily (BID).
2.2. Dose selection
Doses of prototype ASMs were selected using efficacy observed in the mouse 6 Hz (44 mA) model. 26 Median effective dose (ED50) values obtained in the 6 Hz assay were used as screening doses in the TMEV model. In some cases, lower doses were used if initial studies suggested that the screening dose was not well tolerated. As anti‐inflammatory drugs do not consistently produce antiseizure efficacy in the mouse 6 Hz model, doses for these compounds were selected based on known dose ranges that reduce signs of inflammation in mice (described below). For selected compounds, the dose‐response relationship in the TMEV model was explored using multiple doses.
2.3. Animals
Male C57Bl/6J mice, 4‐5 weeks old, were obtained from the Jackson Laboratory. Animals were allowed free access to food and water, except during drug treatment and handling sessions. After delivery, animals were allowed at least 4 days to habituate prior to inoculation. All mice were housed in plastic cages, in rooms with controlled humidity, ventilation, and lighting (12 hours on—12 hours off). Animals were maintained in a manner consistent with the recommendations in the “Guide for Care and Use of Laboratory Animals” (National Research Council). Housing, handling, and testing were performed in accordance with Public Health Service policy and an animal protocol that was approved by the Institutional Animal Care and Use Committee at the University of Utah. Animal experiments were conducted in a manner consistent with Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (https://www.nc3rs.org.uk/arrive‐guidelines).
2.4. TMEV infection and postinfection monitoring
The study design for TMEV infection followed by daily injections and testing in shown in Figure 1. On Day 0, mice were briefly anesthetized using a mixture of isofluorane and oxygen (1.5%–3% isofluorane). Each mouse was injected intracortically with 20 µL of the Daniels strain of TMEV (3 x 105 plaque‐forming units). The intracortical injection was performed unilaterally to a depth of 2 mm in the parietal region of the right hemisphere as previously described (posterior and medial to the right eye). 2 , 3 , 8 , 10 , 11 , 15 , 27 , 28 Inoculation occurred at day 0, which was followed by daily handling sessions (including cage shaking, and individual handling of mice, lasting a total of 1‐2 minutes per mouse) starting on day 1 and continuing through day 7. Post‐inoculation days 3‐7 included once or twice‐daily drug injection followed by monitoring sessions, which included cage agitation and individual handling (handling of the tail and rotation of the mouse 3‐5 times). The day 3‐7 observation period was based on previous work demonstrating the occurrence of handling‐induced seizures starting at day 3 and reaching a maximum around day 5. 3 , 4 , 8 , 27 Animals were observed at the time of injection and one hour after injection for the presence of seizures. An experimenter blinded to treatment group observed seizures during the postinjection monitoring session. Seizure burden allows for incorporation of both seizure frequency and severity and is used in both clinical and preclinical settings. 4 , 27 , 29 , 30 , 31 Seizure burden was used as the primary outcome measure in this model. A heat map was generated for each treatment group, showing seizure scores for each observation made, and used for visualization of experimental data and quantification of cumulative seizure burden (Figure 2). Data presented herein include the postinjection monitoring sessions (main body) and time of injection observations (Tables [Link], [Link], [Link]). During this time, mice were observed for behavioral seizures, which were quantified using the Racine scale. 32 Experimenters observing seizures during handling sessions were blinded to experimental treatments.
FIGURE 1.
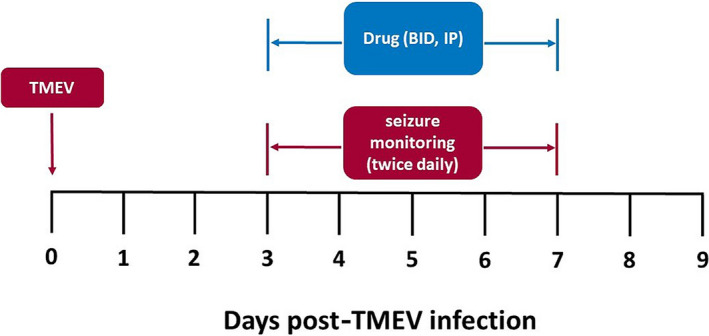
Study design for screening of compounds in the Theiler's murine encephalomyelitis virus (TMEV) model. Treatment groups (N = 20 male C57Bl/6J mice per group) are infected via intracortical injection (Day 0) with TMEV. Each group received twice‐daily intraperitoneal injections starting on Day 3 and continuing through Day 7 (Day 3‐Day 7) intraperitoneal injections. One or two hours following injection, animals were subjected to handling epochs and observed for handling‐induced seizures (twice daily, Day 3‐Day 7)
FIGURE 2.
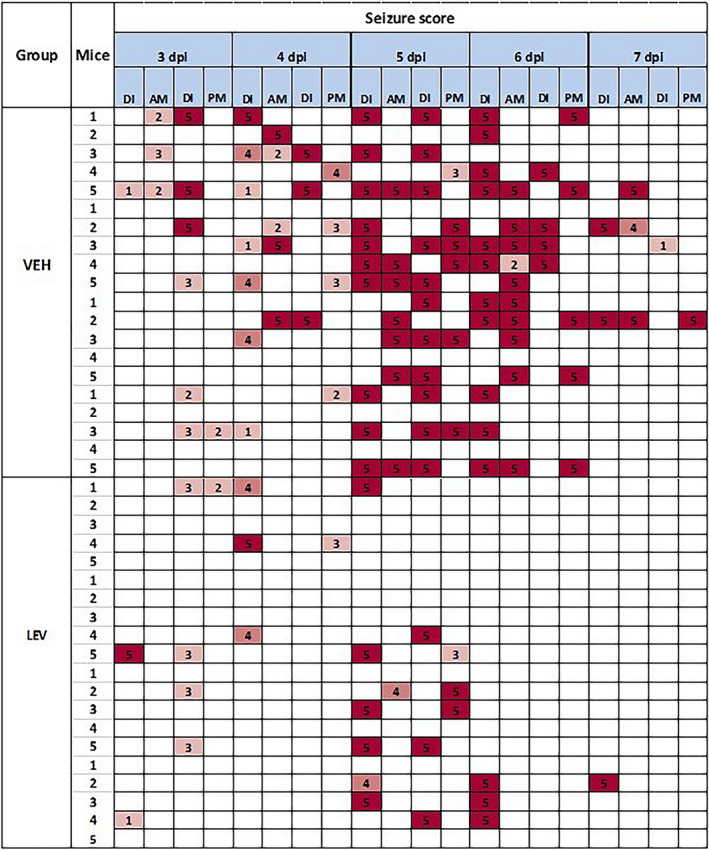
Example heat map for screening of levetiracetam (LEV) in the Theiler's murine encephalomyelitis virus (TMEV) model. LEV or vehicle (VEH) was administered twice daily at a dose of 1000 mg/kg (intraperitoneal administration) starting on Day 3 and continuing through Day 7 (Day 3‐Day 7) following inoculation. One hour following each injection, animals were subjected to handling epochs and observed for handling‐induced seizures (am and pm for each day). If seizures were observed during LEV or VEH administration (DI), seizures scores were noted. Racine scores of 1‐3 are noted in green whereas generalized seizures (score 4‐5) are noted in yellow and orange, respectively
2.5. Compound administration
Test compounds were administered in an optimal fluid‐to‐body volume ratio (0.01 mL/g). All compounds were administered by intraperitoneal (IP) injection either twice daily (BID) or once daily (QD), 1 hour prior to handling sessions, on days 3‐7 postinfection. The only exception was gabapentin, which was administered two hours prior to daily handling sessions. Pretreatment times were determined according to known (eg, 6 Hz model) times of peak effect for each compound. A minimum period of one hour was required for pre‐treatment, to allow for animals to recover from any seizures that may occur during handling for injections. For compounds administered QD, the compound was administered in the morning, followed by a vehicle (VEH) injection for the afternoon treatment and monitoring session. Thus, all animals received two daily handling sessions as well as two injections (9 am and 3 pm injections, 10 am and 4 pm handling sessions, D3‐D7). Each treatment cohort consisted of N = 20 mice for each treatment arm. A power analysis suggests that this group size is sufficient to identify a 50% reduction of seizure burden (standard deviation 50%, alpha 0.05, power 80%). Treatment cohorts consisted of two or three treatment arms and always included a TMEV + VEH‐treated group (eg, TMEV inoculation followed by day 3‐7 VEH administration), as well as one or two treatment groups.
2.6. Statistical analysis
Data are presented as means ± standard error. Daily seizure scores were summed in order to obtain a cumulative seizure burden for the day 3‐7 period. As seizures may occur during the time of injection as a consequence of handling, analysis was performed only using seizures observed following treatment (ie, during posttreatment handling sessions 2 , 4 , 5 , 6 , 7 ). Cumulative seizure burdens were obtained for each cohort of animals. Each animal's cumulative seizure burden was normalized to the group mean seizure burden of the vehicle‐treated TMEV group and presented as a percentage of the seizure burden of the TMEV + VEH treatment group. Statistical comparisons were made between each treatment group and the VEH control group (TMEV + VEH) tested in the same cohort of mice and compared using a Mann‐Whitney U test. Although a drug or dose tested may significantly reduce seizure burden, it is also important to know whether the condition tested eliminated motor seizures in animals tested. Therefore, the number protected value was also used as another measure for assessing drug efficacy, wherein animals were considered protected if they did not display any Racine scale seizures greater than 2. Therefore, data are also presented as number protected/number tested, and these values were compared to vehicle data (number protected/number tested) for animals treated with vehicle during the same cohort as the drug analyzed (Fisher's exact test).
3. RESULTS
3.1. Prototype ASMs can reduce seizures induced by TMEV
Behavioral generally seizures lasted 15‐60 seconds but seizure duration was not quantified for the purposes of this study. Each animal had only one seizure during each observation session (during injection and during postinjection monitoring). Across several cohorts of mice, a majority of VEH‐treated animals consistently demonstrated behavioral seizures. Several prototype ASMs were tested in order to determine whether these compounds could reduce seizure burden for TMEV‐induced seizures during the acute infection period (see Tables 1 and 2). Doses of each compound were selected largely based on the ED50 values, where possible, in the mouse 6 Hz (44 mA) model of psychomotor seizures. 26 The doses described for all compounds evaluated were well tolerated, with the exception of lamotrigine, phenytoin, and phenobarbital, which produced ataxia and sedation at the initial doses tested.
As shown in Table 1, sodium channel blockers were variably effective in this model at reducing handling‐induced seizures. Initial studies with lamotrigine showed that BID treatment (20 mg/kg) reduced seizures, but was not well tolerated (data not shown). Therefore, lamotrigine was administered QD (20 mg/kg), which did not reduce seizures. Similarly, phenytoin was administered at an initial dose of 40 mg/kg, but was not well tolerated (study halted before completion, data not shown), and the dose was reduced to 20 mg/kg. This dose of phenytoin significantly reduced seizure burden (P < .01, Table 1). Similarly, lacosamide (13 mg/kg) also significantly reduced seizure burden (P < .01, Table 1). The GABAA receptor modulators clonazepam and phenobarbital, and the GABA reuptake inhibitor tiagabine, were also evaluated in this model (see Table 1). While clonazepam did not significantly reduce seizures, phenobarbital reduced seizure burden, but only at a poorly tolerated dose (35 mg/kg, P < .0001, Table 1; sedation, lethargy, ataxia were observed). The GABA reuptake inhibitor tiagabine (1.3 mg/kg) also significantly reduced seizure burden (P < .01, Table 1). Table 1 also includes data from compounds acting on Ca++ channels and compounds with mixed mechanisms of action. Gabapentin was not effective in the mouse 6 Hz (44 mA) model 26 at doses up to 500 mg/kg, and therefore, we identified a dose (350 mg/kg) that was approximately 2× greater than doses that are effective in in pentylenetetrazol and maximal electroshock seizure models 33 , 34 Despite lack of efficacy in the mouse 6 Hz model, gabapentin significantly reduced seizure burden at this dose in TMEV‐treated mice (P < .0001, Table 1). Similarly, levetiracetam is not efficacious (up to 1000 mg/kg) in the mouse 6 Hz (44 mA) model, 26 and therefore, a dose of 1000 mg/kg was used in the present experiments and significantly reduced seizure burden and was well tolerated (P < .0001, Table 1). Also, topiramate was evaluated at a maximally tolerated dose (300 mg/kg) and modestly, but significantly, reduced seizure burden (P < .05, Table 1). The potassium channel modulator, ezogabine, also moderately reduced seizure burden at the dose tested (20 mg/kg, P < .01, Table 1). Sodium valproate, at a dose equivalent to the 6 Hz 44 mA ED50 (239 mg/kg) significantly reduced seizure burden (P < .0001, Table 1).
Following initial experiments using prototype ASMs, selected compounds were evaluated at additional doses. Carbamazepine did not significantly reduce seizure burden (Table 2), up to a dose of 40 mg/kg. Additional doses of carbamazepine were not attempted, as the median toxic dose of this compound in mice is ~45 mg/kg. 26 Ethosuximide at a dose of 400 mg/kg (1.3‐fold higher than the initial dose tested) had a modest, but significant effect on seizure burden (Table 2). Additional doses of this compound were not attempted, as the median toxic dose of this compound is ~350 mg/kg. 26 Levetiracetam was evaluated at high doses (350, 700, and 1000 mg/kg) as well as low doses (10 and 30 mg/kg). Interestingly, levetiracetam dose‐dependently reduced seizure burden, with significant seizure burden reduction at doses of 30‐1000 mg/kg (Table 2). Although the initial dose of phenytoin reduced seizure burden, subsequent lower doses (2.5/5/10 mg/kg) had no effect on seizure burden (Table 2). Ezogabine had a modest but significant effect on seizure burden at a dose of 20 mg/kg (Table 2), but with similar efficacy to that observed at a lower dose (15 mg/kg) (Table 2). While valproic acid dramatically reduced seizures at the initial dose tested (239 mg/kg), lower doses (200/100/50 mg/kg) did not similarly reduce seizure burden (Table 2), in contrast to previously published data. 8 Because the screening dose of phenobarbital was effective but with notable side effects, a lower dose of 20 mg/kg was tested. This dose was well tolerated and significantly reduced seizures (P < .001, Table 2).
Finally, for all compounds tested, the number protected was obtained for each condition and it was observed that no dose for any drug tested significantly affected protection from motor seizures. Although some compounds (carbamazepine, levetiracetam) show apparent dose‐related increases in the number of protected animals, this was not consistently seen with other compounds (phenytoin, ezogabine, valproic acid, and phenobarbital). Further, this lack of significant changes in the number protected was independent of whether drugs/doses significantly reduced seizure burden.
3.2. Prototype anti‐inflammatory corticosteroids, but not nonsteroidal anti‐inflammatory drugs or minocycline, reduced seizure burden in the TMEV model
Following evaluation of standard ASMs, we next sought to determine whether anti‐inflammatory compounds can reduce seizures following TMEV infection. The nonsteroidal anti‐inflammatory drugs (NSAIDs) diclofenac (10 mg/kg) and ibuprofen (50 mg/kg) did not reduce seizure burden (Table 3). Further, celecoxib (5 and 10 mg/kg) had a minor but significant effect on seizure burden (Table 3). By contrast, dexamethasone (20 mg/kg) was highly efficacious in reducing seizure burden following TMEV infection (Table 3). Prednisone (20 mg/kg) produced a modest decrease in seizure burden following TMEV infection (Table 3). Minocycline (50 mg/kg) was administered QD, based on previous experience with this compound suggesting BID treatment was not well tolerated (data not shown), and did not significantly reduce seizure burden at the dose tested (Table 3). Finally, as it was observed for prototype ASMs, no drug or dose tested significantly affected the number protected, despite some conditions significantly affecting seizure burden.
4. DISCUSSION
CNS infections can produce acute seizures 35 and contribute to the development of epilepsy in humans. 36 , 37 While infection‐related seizures occur at a lower rate in much of the developed world, it can be a significant problem in underdeveloped countries. 38 Further, seizures arising from CNS infection may be physiologically unique compared to other seizure types, as they may result from infection‐related inflammation, fevers, or unknown causes. 37 While currently available ASMs may demonstrate efficacy in acute seizure screening models or in chronic epilepsy models, until the present studies, it was unknown whether these ASMs could be effective in preventing virally induced seizures. Herein, we have demonstrated that several prototype ASMs are effective in reducing seizure burden in the TMEV model at doses that are also effective in a model of refractory partial seizures. In addition, the TMEV model is utilized by the Epilepsy Therapy Screening Program (ETSP) as a screening tool for evaluation of investigational compounds active against special populations of epilepsy. 20 Given the unique mechanism for seizure generation in this model, test compounds that demonstrate efficacy in this model may also have unique mechanisms of action that go beyond traditional ASM mechanisms and suggest utility in different patient populations.
Several ASMs, with varying mechanisms of action, were effective in this model. The sodium channel blockers lacosamide and phenytoin were effective in reducing seizure burden, whereas carbamazepine and lamotrigine did not significantly reduce seizures. This differs from previously published data, although of note the current study used a higher viral titer during infection. 8 The GABA reuptake inhibitor tiagabine and the GABAA receptor modulator phenobarbital significantly reduced seizures. In contrast, the GABAA receptor modulator clonazepam was ineffective. Gabapentin (calcium channel modulator), levetiracetam (SV2A ligand), topiramate (mixed mechanism), and valproic acid (mixed mechanism) were also efficacious in this model. These data suggest that seizures in this model may be refractory to some of the currently approved ASMs at the doses tested in this study.
In order to determine appropriate doses to test for the TMEV model, the mouse 6 Hz 44 mA model is useful in estimating effective dose(s). However, in cases where compounds are not effective in the mouse 6 Hz 44 mA model, other acute seizure or epilepsy models may be used to inform a dosing strategy. For example, levetiracetam has limited effect in the 6 Hz 44 mA assay 26 , 39 and the maximal electroshock test 39 , 40 but potently blocks seizures in the mouse corneal kindling assay. 40 , 41 Therefore, levetiracetam was evaluated in the TMEV model at higher doses of 350‐1000 mg/kg, corresponding to the dose range previously used to evaluate levetiracetam in the mouse 6 Hz 44 mA assay, 39 , 40 , 42 and doses of 10 and 30 mg/kg, corresponding to doses that are effective in corneal kindling. 40 , 41 Interestingly, levetiracetam reduced seizures in the TMEV model at a dose of 30 mg/kg as well as higher doses of 350, 700, and 1000 mg/kg. It is currently unknown whether the potency of levetiracetam in this model is related to the primary mechanism of action for levetiracetam (eg, SV2A modulation) or other potential mechanisms that reduce inflammation. 43 , 44
TMEV‐mediated infection has been associated with innate immune system‐mediated inflammation which includes a “cytokine storm”. 10 , 13 Therefore, compounds with anti‐inflammatory activity may be effective in this model. To this end, we also evaluated several broad‐spectrum prototype anti‐inflammatory compounds. Doses used for screening of anti‐inflammatory compounds were selected based on known CNS anti‐inflammatory effects. Diclofenac crosses the blood‐brain barrier after peripheral administration, 45 and was administered at a dose that reduces carrageenan‐induced inflammation in mice (10 mg/kg IP). 46 This dose of diclofenac also reduces seizures in the electroshock seizure model 47 in mice and the pentylenetetrazol kindling model in rats. 48 Ibuprofen was administered at a dose (50 mg/kg) that reduces disease progression in an Alzheimer's disease model 49 and reduces zymosan‐induced ear inflammation in mice. 50 Ibuprofen also reduces electroconvulsive seizures, but only at a dose of 100 mg/kg. 51 Celecoxib was administered at doses (5‐10 mg/kg) that have been shown to possess analgesic and anti‐inflammatory effects in rodents. For example, celecoxib at comparable doses reduces inflammation in a model of autoimmune encephalitis 52 and pain behaviors in a model of inflammatory hyperalgesia (ED50 ~8 mg/kg). 53 However, a dose of 10 mg/kg of celecoxib was without effect against electroshock seizures in mice. 47 Dexamethasone was administered at a dose twofold greater than a dose that reduces maximal electroshock‐induced seizures in a model of sepsis 54 and which is fivefold to sixfold greater than a dose that reduces central and peripheral inflammation in mice. 55 , 56 Prednisone was administered in a dose range (5‐20 mg/kg) that has been shown to reduce experimental autoimmune encephalomyelitis in mice (in combination with minocycline), 57 reduces carrageenan‐induced paw edema in rats, 58 and reduces signs of adjuvant‐induced rheumatoid arthritis in rats. 59 Minocycline did not affect seizure burden, as demonstrated previously. 27
Epilepsy involves a number of neuropathological processes, both during the development and maintenance of chronic seizure states. For example, there is increasing evidence that neuroinflammation plays an important role in several types of epilepsy, 17 especially those that involve encephalopathies arising from acute or chronic infections. 37 In this manner, the TMEV model is well suited to identify potential therapies for inflection‐related seizure conditions. Inflammation plays a critical role in driving acute seizures in this model. 9 , 10 , 11 , 13 , 15 , 27 , 60 Furthermore, as seizures following TMEV infection are be driven largely by inflammation, 6 , 10 , 11 , 13 the TMEV model can be used to screen compounds with anti‐inflammatory mechanisms of action for seizure reduction. Of note, however, in these studies several antiseizure and anti‐inflammatory agents were screened using twice‐daily administration. These treatment conditions were not optimized for half‐life and exposure but were rather used as a means for screening of these compounds. Further studies with optimal drug administration paradigms would be needed for further study of specific drugs evaluated herein.
Various forms of epilepsy, such as childhood epileptic encephalopathies and mesial temporal lobe epilepsy, tend to involve greater rates of pharmacoresistance, have a pronounced neuroinflammatory component, and tend to respond to immunomodulatory therapies. 35 , 37 Cytokines, prostaglandins, and other immune signaling molecules can become elevated following seizures in various animal models and have wide‐ranging effects on seizure pathophysiology, including increasing intracellular calcium, 61 , 62 upregulating sodium channels, 63 reducing HCN and TRP channels 64 and activation of microglia and astrocytes. 17 , 65 Therefore, as the TMEV model has a strong inflammatory component, this model is useful for evaluating compounds with anti‐inflammatory mechanisms that may be efficacious in types of epilepsy where inflammation plays a role in seizure susceptibility. We observed that the anti‐inflammatory steroid dexamethasone reduced seizures to an extent similar to that of the ASMs that were effective in this model. Dexamethasone can reduce seizures following lithium‐pilocarpine, with varying efficacy, 66 but has not been extensively evaluated for its potential antiseizure effects. The efficacy of DEX in this model is consistent with the putative pathophysiology behind TMEV‐induced seizures and that reduction of inflammation may be a viable means to prevent seizures. However, it is noteworthy that the NSAIDs DCL and IBU were ineffective in this model when administered at maximally tolerated doses. While CXB significantly reduced seizures, the effect was moderate at the doses tested. Similarly, PDN modestly reduced seizures. Therefore, despite evidence that reduction of inflammation can prevent seizures in the TMEV model, additional work is needed to further explore other anti‐inflammatory mechanisms in this model to better understand which may be viable drug candidates. This work, for example, may include specific targeting of cytokines or prostaglandins.
The number protected value was used as another measure for assessing drug efficacy, allowing for evaluation of whether the drug tested eliminated motor seizures in any animals tested. For all conditions tested in this report, it was observed that there were no significant improvements in number protected, despite several drugs and conditions being effective in reducing seizure burden. This observation is noteworthy, as it suggests a need for compounds that not only improve seizure burden, but also eliminate or dramatically reduce motor seizures in a majority of individuals tested. Therefore, future endeavors with this model should continue to use the number protected as an indicator of compound efficacy.
The endpoint of seizure protection was applied to this data set and demonstrated that none of the drugs evaluated completely blocked behavioral seizures. Further, none of the anti‐inflammatory agents used in this study led to significant protection in a large number of animals. It is therefore likely that cumulative seizure burden is the more reliable endpoint for identifying compounds effective in this model. Furthermore, although inflammation plays a key role in the development of seizures following TMEV infection, future studies are needed with general (eg, steroid and nonsteroid anti‐inflammatories) and specific (eg, cytokine inhibitors or comparable agents) drugs, administered using bioanalytical methods to confirm exposure sufficient to reduce inflammation. The primary outcome measure in the current study was seizure burden. However, other measures of behavioral seizures (seizure frequency) may be similarly useful to evaluate drug efficacy. Combination video and electroencephalography monitoring with subchronic drug administration may provide further information about potential drug efficacy. Finally, in the present study male animals were used for screening and evaluation of prototype compounds. Both male and female C57Bl/6J mice infected with TMEV have behavioral seizures. 1 , 67 , 68 , 69 Female mice may be differentially responsive to ASMs, 70 which may similarly be present in this seizure model. Future studies comparing prototype compounds in this model may suggest potential sex‐dependent differences.
In summary, the TMEV model has been previously shown to be a useful model to study the pathophysiology of infection‐induced seizures and the development of TLE. Herein, we demonstrate that evaluation of ASMs and anti‐inflammatory compounds for their potential to reduce seizures during the acute infection period may help to differentiate compounds with novel mechanisms of action. We observed that some ASMs with varying mechanisms of action reduce seizures in this model at doses that are effective in the 6 Hz 44 mA model, a model of pharmacoresistant epilepsy. We also demonstrated dose dependency of efficacy in this model with several ASMs. Finally, we showed that dexamethasone reduced seizure burden in this model and that other compounds, including prednisone and celecoxib, were mildly effective and that the NSAIDS were ineffective. This pharmacologic profile of the TMEV model may be helpful in designing and identifying novel drugs for various forms of epilepsy.
CONFLICT OF INTEREST
KSW is a consultant for Xenon Pharmaceuticals and is a member of the scientific advisory boards of Mend Neuroscience and Blackfynn, Inc CM is a consultant for SEA Pharmaceuticals. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Table S1
Table S2
Table S3
ACKNOWLEDGMENTS
The authors would also like to thank the ETSP at the National Institutes of Neurological Diseases and Stroke for their review and comments on this manuscript. This project has been funded in whole or in part with Federal funds from the National Institute of Neurological Disorders and Stroke, Epilepsy Therapy Screening Program, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN271201600048C.
Metcalf CS, Vanegas F, Underwood T, et al. Screening of prototype antiseizure and anti‐inflammatory compounds in the Theiler's murine encephalomyelitis virus model of epilepsy. Epilepsia Open.2022;7:e12550. 10.1002/epi4.12550
REFERENCES
- 1. Libbey JE, Kirkman NJ, Smith MC, Tanaka T, Wilcox KS, White HS, et al. Seizures following picornavirus infection. Epilepsia. 2008;49(6):1066–74. [DOI] [PubMed] [Google Scholar]
- 2. Stewart KA, Wilcox KS, Fujinami RS, White HS. Development of postinfection epilepsy after Theiler's virus infection of C57BL/6 mice. J Neuropathol Exp Neurol. 2010;69(12):1210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stewart KA, Wilcox KS, Fujinami RS, White HS. Theiler's virus infection chronically alters seizure susceptibility. Epilepsia. 2010;51(8):1418–28. [DOI] [PubMed] [Google Scholar]
- 4. Patel DC, Wallis G, Fujinami RS, Wilcox KS, Smith MD. Cannabidiol reduces seizures following CNS infection with Theiler's murine encephalomyelitis virus. Epilepsia Open. 2019;4(3):431–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Waltl I, Kaufer C, Gerhauser I, Chhatbar C, Ghita L, Kalinke U, et al. Microglia have a protective role in viral encephalitis‐induced seizure development and hippocampal damage. Brain Behav Immun. 2018;74:186–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Broer S, Kaufer C, Haist V, Li L, Gerhauser I, Anjum M, et al. Brain inflammation, neurodegeneration and seizure development following picornavirus infection markedly differ among virus and mouse strains and substrains. Exp Neurol. 2016;279:57–74. [DOI] [PubMed] [Google Scholar]
- 7. Waltl I, Kaufer C, Broer S, Chhatbar C, Ghita L, Gerhauser I, et al. Macrophage depletion by liposome‐encapsulated clodronate suppresses seizures but not hippocampal damage after acute viral encephalitis. Neurobiol Dis. 2018;110:192–205. [DOI] [PubMed] [Google Scholar]
- 8. Barker‐Haliski ML, Dahle EJ, Heck TD, Pruess TH, Vanegas F, Wilcox KS, et al. Evaluating an etiologically relevant platform for therapy development for temporal lobe epilepsy: effects of carbamazepine and valproic acid on acute seizures and chronic behavioral comorbidities in the Theiler's murine encephalomyelitis virus mouse model. J Pharmacol Exp Ther. 2015;353(2):318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS. Infiltrating macrophages are key to the development of seizures following virus infection. J Virol. 2013;87(3):1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia. 2010;51(3):454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Libbey JE, Kirkman NJ, Wilcox KS, White HS, Fujinami RS. Role for complement in the development of seizures following acute viral infection. J Virol. 2010;84(13):6452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaufer C, Chhatbar C, Broer S, Waltl I, Ghita L, Gerhauser I, et al. Chemokine receptors CCR2 and CX3CR1 regulate viral encephalitis‐induced hippocampal damage but not seizures. Proc Natl Acad Sci USA. 2018;115(38):E8929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS. Lack of correlation of central nervous system inflammation and neuropathology with the development of seizures following acute virus infection. J Virol. 2011;85(16):8149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patel DC, Wallis G, Dahle EJ, McElroy PB, Thomson KE, Tesi RJ, et al. Hippocampal TNFα signaling contributes to seizure generation in an infection‐induced mouse model of limbic epilepsy. eNeuro. 2017;4(2):ENEURO.0105‐17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhuyan P, Patel DC, Wilcox KS, Patel M. Oxidative stress in murine Theiler's virus‐induced temporal lobe epilepsy. Exp Neurol. 2015;271:329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loewen JL, Barker‐Haliski ML, Dahle EJ, White HS, Wilcox KS. Neuronal injury, gliosis, and glial proliferation in two models of temporal lobe epilepsy. J Neuropathol Exp Neurol. 2016;75(4):366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilcox KS, Vezzani A. Does brain inflammation mediate pathological outcomes in epilepsy? Adv Exp Med Biol. 2014;813:169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loscher W. Fit for purpose application of currently existing animal models in the discovery of novel epilepsy therapies. Epilepsy Res. 2016;126:157–84. [DOI] [PubMed] [Google Scholar]
- 19. Loscher W. Animal models of seizures and epilepsy: past, present, and future role for the discovery of antiseizure drugs. Neurochem Res. 2017;42(7):1873–88. [DOI] [PubMed] [Google Scholar]
- 20. Kehne JH, Klein BD, Raeissi S, Sharma S. The National Institute of Neurological Disorders and Stroke (NINDS) Epilepsy Therapy Screening Program (ETSP). Neurochem Res. 2017;42(7):1894–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rogawski MA, Tofighy A, White HS, Matagne A, Wolff C. Current understanding of the mechanism of action of the antiepileptic drug lacosamide. Epilepsy Res. 2015;110:189–205. [DOI] [PubMed] [Google Scholar]
- 22. Porter RJ, Dhir A, Macdonald RL, Rogawski MA. Mechanisms of action of antiseizure drugs. Handb Clin Neurol. 2012;108:663–81. [DOI] [PubMed] [Google Scholar]
- 23. He F, Liu B, Meng Q, Sun Y, Wang W, Wang C. Modulation of miR‐146a/complement factor H‐mediated inflammatory responses in a rat model of temporal lobe epilepsy. Biosci Rep. 2016;36(6):e00433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Hanak TJ, Libbey JE, Doty DJ, Sim JT, DePaula‐Silva AB, Fujinami RS. Positive modulation of mGluR5 attenuates seizures and reduces TNF‐alpha(+) macrophages and microglia in the brain in a murine model of virus‐induced temporal lobe epilepsy. Exp Neurol. 2019;311:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. DeSena AD, Do T, Schulert GS. Systemic autoinflammation with intractable epilepsy managed with interleukin‐1 blockade. J Neuroinflammation. 2018;15(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Metcalf CS, West PJ, Thomson KE, Edwards SF, Smith MD, White HS, et al. Development and pharmacologic characterization of the rat 6 Hz model of partial seizures. Epilepsia. 2017;58(6):1073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barker‐Haliski ML, Heck TD, Dahle EJ, Vanegas F, Pruess TH, Wilcox KS, et al. Acute treatment with minocycline, but not valproic acid, improves long‐term behavioral outcomes in the Theiler's virus model of temporal lobe epilepsy. Epilepsia. 2016;57(12):1958–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Umpierre AD, Remigio GJ, Dahle EJ, Bradford K, Alex AB, Smith MD, et al. Impaired cognitive ability and anxiety‐like behavior following acute seizures in the Theiler's virus model of temporal lobe epilepsy. Neurobiol Dis. 2014;64:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Payne ET, Zhao XY, Frndova H, McBain K, Sharma R, Hutchison JS, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137(Pt 5):1429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kharoshankaya L, Stevenson NJ, Livingstone V, Murray DM, Murphy BP, Ahearne CE, et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic‐ischemic encephalopathy. Dev Med Child Neurol. 2016;58(12):1242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lynch NE, Stevenson NJ, Livingstone V, Murphy BP, Rennie JM, Boylan GB. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia. 2012;53(3):549–57. [DOI] [PubMed] [Google Scholar]
- 32. Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–94. [DOI] [PubMed] [Google Scholar]
- 33. Yuen ES, Troconiz IF. Can pentylenetetrazole and maximal electroshock rodent seizure models quantitatively predict antiepileptic efficacy in humans? Seizure. 2015;24:21–7. [DOI] [PubMed] [Google Scholar]
- 34. Borowicz KK, Swiader M, Luszczki J, Czuczwar SJ. Effect of gabapentin on the anticonvulsant activity of antiepileptic drugs against electroconvulsions in mice: an isobolographic analysis. Epilepsia. 2002;43(9):956–63. [DOI] [PubMed] [Google Scholar]
- 35. Eeg‐Olofsson O. Virological and immunological aspects of seizure disorders. Brain Dev. 2003;25(1):9–13. [DOI] [PubMed] [Google Scholar]
- 36. Ficker DM, So EL, Shen WK, Annegers JF, O'Brien PC, Cascino GD, et al. Population‐based study of the incidence of sudden unexplained death in epilepsy. Neurology. 1998;51(5):1270–4. [DOI] [PubMed] [Google Scholar]
- 37. Pardo CA, Nabbout R, Galanopoulou AS. Mechanisms of epileptogenesis in pediatric epileptic syndromes: Rasmussen encephalitis, infantile spasms, and febrile infection‐related epilepsy syndrome (FIRES). Neurotherapeutics. 2014;11(2):297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Preux PM, Druet‐Cabanac M. Epidemiology and aetiology of epilepsy in sub‐Saharan Africa. Lancet Neurol. 2005;4(1):21–31. [DOI] [PubMed] [Google Scholar]
- 39. Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47(3):217–27. [DOI] [PubMed] [Google Scholar]
- 40. Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353(2–3):191–206. [DOI] [PubMed] [Google Scholar]
- 41. Rowley NM, White HS. Comparative anticonvulsant efficacy in the corneal kindled mouse model of partial epilepsy: correlation with other seizure and epilepsy models. Epilepsy Res. 2010;92(2–3):163–9. [DOI] [PubMed] [Google Scholar]
- 42. Metcalf CS, Klein BD, Smith MD, Pruess T, Ceusters M, Lavreysen H, et al. Efficacy of mGlu2 ‐positive allosteric modulators alone and in combination with levetiracetam in the mouse 6 Hz model of psychomotor seizures. Epilepsia. 2017;58(3):484–93. [DOI] [PubMed] [Google Scholar]
- 43. Stienen MN, Haghikia A, Dambach H, Thone J, Wiemann M, Gold R, et al. Anti‐inflammatory effects of the anticonvulsant drug levetiracetam on electrophysiological properties of astroglia are mediated via TGFbeta1 regulation Br. J Pharmacol. 2011;162(2):491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thone J, Ellrichmann G, Faustmann PM, Gold R, Haghikia A. Anti‐inflammatory effects of levetiracetam in experimental autoimmune encephalomyelitis. Int Immunopharmacol. 2012;14(1):9–12. [DOI] [PubMed] [Google Scholar]
- 45. Fukuda M, Kitaichi K, Abe F, Fujimoto Y, Takagi K, Takagi K, et al. Altered brain penetration of diclofenac and mefenamic acid, but not acetaminophen, Shiga‐like Toxin II‐treated Mice. J Pharmacol Sci. 2005;97(4):525–32. [DOI] [PubMed] [Google Scholar]
- 46. Gupta AK, Parasar D, Sagar A, Choudhary V, Chopra BS, Garg R, et al. Analgesic and anti‐inflammatory properties of gelsolin in acetic acid induced writhing, tail immersion and carrageenan induced paw edema in mice. PLoS One. 2015;10(9):e0135558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suemaru K, Yoshikawa M, Tanaka A, Araki H, Aso H, Watanabe M. Anticonvulsant effects of acetaminophen in mice: comparison with the effects of nonsteroidal anti‐inflammatory drugs. Epilepsy Res. 2018;140:22–8. [DOI] [PubMed] [Google Scholar]
- 48. Vieira V, Glassmann D, Marafon P, Pereira P, Gomez R, Coitinho AS. Effect of diclofenac sodium on seizures and inflammatory profile induced by kindling seizure model. Epilepsy Res. 2016;127:107–13. [DOI] [PubMed] [Google Scholar]
- 49. Van Dam D, Coen K, De Deyn PP. Ibuprofen modifies cognitive disease progression in an Alzheimer's mouse model. J Psychopharmacol. 2010;24(3):383–8. [DOI] [PubMed] [Google Scholar]
- 50. Hougee S, Hartog A, Sanders A, Graus YM, Hoijer MA, Garssen J, et al. Oral administration of the NADPH‐oxidase inhibitor apocynin partially restores diminished cartilage proteoglycan synthesis and reduces inflammation in mice. Eur J Pharmacol. 2006;531(1–3):264–9. [DOI] [PubMed] [Google Scholar]
- 51. Kaminski R, Kozicka M, Parada‐turska J, Dziki M, Kleinrok Z, Turski WA, et al. Effect of non‐steroidal anti‐inflammatory drugs on the anticonvulsive activity of valproate and diphenylhydantoin against maximal electroshock‐induced seizures in mice. Pharmacol Res. 1998;37(5):375–81. [DOI] [PubMed] [Google Scholar]
- 52. Miyamoto K, Miyake S, Mizuno M, Oka N, Kusunoki S, Yamamura T. Selective COX‐2 inhibitor celecoxib prevents experimental autoimmune encephalomyelitis through COX‐2‐independent pathway. Brain. 2006;129(Pt 8):1984–92. [DOI] [PubMed] [Google Scholar]
- 53. Stepanovic‐Petrovic RM, Micov AM, Tomic MA, Kovacevic JM, Boskovic BD. Antihyperalgesic/antinociceptive effects of ceftriaxone and its synergistic interactions with different analgesics in inflammatory pain in rodents. Anesthesiology. 2014;120(3):737–50. [DOI] [PubMed] [Google Scholar]
- 54. Sewal RK, Modi M, Saikia UN, Chakrabarti A, Medhi B. Increase in seizure susceptibility in sepsis like condition explained by spiking cytokines and altered adhesion molecules level with impaired blood brain barrier integrity in experimental model of rats treated with lipopolysaccharides. Epilepsy Res. 2017;135:176–86. [DOI] [PubMed] [Google Scholar]
- 55. Castardo JC, Prudente AS, Ferreira J, Guimaraes CL, Monache FD, Filho VC, et al. Anti‐inflammatory effects of hydroalcoholic extract and two biflavonoids from garcinia gardneriana leaves in mouse paw oedema. J Ethnopharmacol. 2008;118(3):405–11. [DOI] [PubMed] [Google Scholar]
- 56. Gamache DA, Ellis EF. Effect of dexamethasone, indomethacin, ibuprofen, and probenecid on carrageenan‐induced brain inflammation. J Neurosurg. 1986;65(5):686–92. [DOI] [PubMed] [Google Scholar]
- 57. Chen X, Hu X, Zou Y, Pi R, Liu M, Wang T, et al. Combined treatment with minocycline and prednisone attenuates experimental autoimmune encephalomyelitis in C57 BL/6 mice. J Neuroimmunol. 2009;210(1‐2):22–9. [DOI] [PubMed] [Google Scholar]
- 58. Fan H, Qi D, Yang M, Fang H, Liu K, Zhao F. In vitro and in vivo anti‐inflammatory effects of 4‐methoxy‐5‐ hydroxycanthin‐6‐one, a natural alkaloid from Picrasma quassioides. Phytomedicine. 2013;20(3‐4):319–23. [DOI] [PubMed] [Google Scholar]
- 59. Pal R, Chaudhary MJ, Tiwari PC, Babu S, Pant KK. Protective role of theophylline and their interaction with nitric oxide (NO) in adjuvant‐induced rheumatoid arthritis in rats. Int Immunopharmacol. 2015;29(2):854–62. [DOI] [PubMed] [Google Scholar]
- 60. Patel DC, Wallis G, Dahle EJ, McElroy PB, Thomson KE, Tesi RJ, et al. Hippocampal TNFalpha signaling contributes to seizure generation in an infection‐induced mouse model of limbic epilepsy. eNeuro. 2017;4(2):ENEURO.0105‐17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bali A, Gupta S, Singh N, Jaggi AS. Implicating the role of plasma membrane localized calcium channels and exchangers in stress‐induced deleterious effects. Eur J Pharmacol. 2013;714(1–3):229–38. [DOI] [PubMed] [Google Scholar]
- 62. Stebbing MJ, Cottee JM, Rana I. The role of ion channels in microglial activation and proliferation – a complex interplay between ligand‐gated ion channels, K(+) channels, and intracellular Ca(2.). Front Immunol. 2015;6:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gudes S, Barkai O, Caspi Y, Katz B, Lev S, Binshtok AM. The role of slow and persistent TTX‐resistant sodium currents in acute tumor necrosis factor‐alpha‐mediated increase in nociceptors excitability. J Neurophysiol. 2015;113(2):601–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Frigerio F, Flynn C, Han Y, Lyman K, Lugo JN, Ravizza T, et al. Neuroinflammation alters integrative properties of rat hippocampal pyramidal cells. Mol Neurobiol. 2018;55(9):7500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chong SA, Balosso S, Vandenplas C, Szczesny G, Hanon E, Claes K, et al. Intrinsic inflammation is a potential anti‐epileptogenic target in the organotypic hippocampal slice model. Neurotherapeutics. 2018;15(2):470–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Al‐Shorbagy MY, El Sayeh BM, Abdallah DM. Diverse effects of variant doses of dexamethasone in lithium‐pilocarpine induced seizures in rats. Can J Physiol Pharmacol. 2012;90(1):13–21. [DOI] [PubMed] [Google Scholar]
- 67. Tsunoda I, Sato F, Omura S, Fujita M, Sakiyama N, Park AM. Three immune‐mediated disease models induced by Theiler's virus: multiple sclerosis, seizures and myocarditis. Clin Exp Neuroimmunol. 2016;7(4):330–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bijalwan M, Young CR, Tingling J, Zhou XJ, Rimmelin AR, Leibowitz JL, et al. Characterization of plaque‐sized variants of Daniel's (DA) strain in Theiler's virus‐induced epilepsy. Sci Rep. 2019;9(1):3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bell LA, Wallis GJ, Wilcox KS. Reactivity and increased proliferation of NG2 cells following central nervous system infection with Theiler's murine encephalomyelitis virus. J Neuroinflammation. 2020;17(1):369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Reddy DS. The neuroendocrine basis of sex differences in epilepsy. Pharmacol Biochem Behav. 2017;152:97–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3


