Abstract
Aluminum (Al) toxicity and poor phosphorus (P) availability are factors that limit plant growth on many agricultural soils. Previous work reported that expression of a Pseudomonas aeruginosa citrate synthase gene in tobacco (Nicotiana tabacum; CSb lines) resulted in improved Al tolerance (J.M. de la Fuente, V. Ramírez-Rodríguez, J.L. Cabrera-Ponce, L. Herrera-Estrella [1997] Science 276: 1566–1568) and an enhanced ability to acquire P from alkaline soils (J. López-Bucio, O. Martínez de la Vega, A. Guevara-García, L. Herrera-Estrella [2000] Nat Biotechnol 18: 450–453). These effects were attributed to the P. aeruginosa citrate synthase increasing the biosynthesis and efflux of citrate from roots. To verify these findings we: (a) characterized citrate efflux from roots of wild-type tobacco; (b) generated tobacco lines expressing the citrate synthase gene from P. aeruginosa; and (c) analyzed selected CSb lines described above. Al stimulated citrate efflux from intact roots of wild-type tobacco and root apices were found to be responsible for most of the efflux. Despite generating transgenic tobacco lines that expressed the citrate synthase protein at up to a 100-fold greater level than the previously described CSb lines, these lines did not show increased accumulation of citrate in roots or increased Al-activated efflux of citrate from roots. Selected CSb lines, similarly, failed to show differences compared with controls in either citrate accumulation or efflux. We conclude that expression of the P. aeruginosa citrate synthase gene in plants is unlikely to be a robust and easily reproducible strategy for enhancing the Al tolerance and P-nutrition of crop and pasture species.
Organic acids exuded by roots have been shown to have important roles in the mineral nutrition of plants. In particular, organic acid exudation is involved in Al tolerance and is a mechanism by which plants acquire P from soil. A range of species exude organic acids from roots in response to Al, and this response is associated with Al tolerance within species and across different species (Ma, 2000). It is postulated that the organic acids chelate Al external to the root into forms that are not toxic to plants. The relative strength of organic acid:Al complexes correlates well with the observed effectiveness of different organic acids to protect plants from Al-toxicity (Hue et al., 1986). In addition, some plant species exude large quantities of organic acids when P deficient. For example, Lupinus albus forms specialized roots that release large amounts of citrate (Gardner et al., 1983) and, in some cases, the amount of citrate accumulated around roots on calcereous soils precipitate as Ca citrate (Dinklaker et al., 1989). Citrate exuded from roots can liberate phosphate from poorly soluble forms of P present in soils, and this phosphate is available for plant uptake.
Acid soils account for a large proportion of the earth's arable land and on many of these soils Al toxicity and poor availability of P limits plant production (von Uexküll and Mutert, 1995). In addition, on alkaline soils P is poorly available as it forms sparingly soluble compounds with Ca. The application of genetic engineering has the potential to generate crop and pasture plants better adapted to these soils. Toward this end, de la Fuente et al. (1997) reported that tobacco (Nicotiana tabacum) engineered to over-produce citrate had enhanced citrate efflux with a corresponding increase in Al tolerance. Furthermore, López-Bucio et al. (2000) recently reported that these same transgenic tobacco plants were able to acquire P more efficiently than control plants when grown in an alkaline soil. The potential benefits of improved P acquisition by use of this technology are likely to be significant since every year over 30 million tons of P fertilizer as P2O5 equivalents are applied worldwide (www.fertilizer.org/stats.htm). More efficient use of this applied P would reduce farmer costs as well as having environmental benefits. Enhancing the Al tolerance of selected species, similarly, would also result in substantial benefits, particularly in low input agricultural systems where the application of lime to correct soil acidity is uneconomical. In the work of de la Fuente et al. (1997) and López-Bucio et al. (2000), the citrate synthase (CS) gene from Pseudomonas aeruginosa was expressed in tobacco under the control of the 35S cauliflower mosaic virus (35S CaMV) promoter. This resulted in an increased internal citrate concentration of up to 10-fold compared with the control line not expressing the CS gene. The increase in citrate efflux was somewhat less (up to 4-fold), and this was attributed to saturation of the mechanism involved in transporting citrate to the external medium.
In view of the potential benefits that enhanced citrate efflux can confer to plants, we have attempted to verify these observations using both transgenic tobacco plants that we generated as well as using some of the CSb lines described by de la Fuente et al. (1997). However, despite at least a 100-fold greater expression of the P. aeruginosa CS protein in our lines compared with the best CSb lines previously reported, we were unable to show either increased internal citrate concentrations or increased citrate efflux from roots above control plant levels. Characterization of the CSb lines similarly failed to show any increases in either internal citrate concentrations or citrate efflux above control plants.
RESULTS AND DISCUSSION
Characterization of Citrate Efflux from Roots of Wild-Type Tobacco
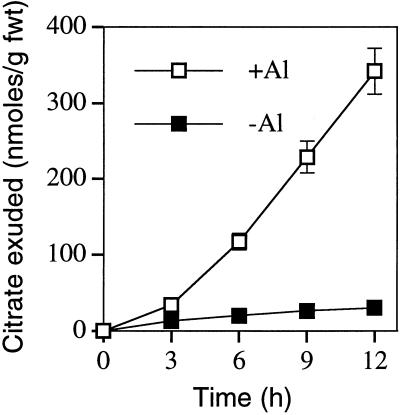
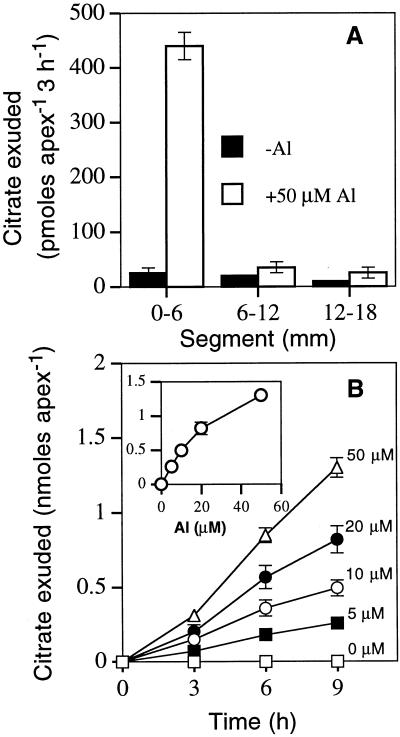
To quantify citrate efflux from wild-type tobacco plants, we immersed whole roots in aerated CaCl2 solution for various times and analyzed the resulting solutions for organic acids. Because Al has been shown to stimulate the efflux of organic acids from a range of other plant species (Ma, 2000), we also assessed the effect that Al had on organic acid efflux. In the absence of Al, low levels of organic acids were exuded from tobacco as determined by either enzymatic or HPLC analysis. By contrast, exposure of whole roots to Al stimulated the efflux of citrate (Fig. 1), and this efflux increased with increasing concentrations of Al (data not shown). The use of excised root segments showed that the majority of the citrate efflux was localized to the terminal 6 mm of roots (Fig. 2A) and, similar to whole roots, the efflux from root apices responded to the concentration of Al (Fig. 2B). Al stimulates citrate efflux from roots of maize (Pellet et al., 1995), triticale (Ma et al., 2000), and Cassia tora (Ma et al., 1997a), and in all of these cases there is a considerable lag phase before maximal efflux is attained. Although whole roots of tobacco showed a lower efflux over the first 3 h of Al exposure compared with later times (Fig. 1), this lag phase was not as evident in excised root tips particularly at the lower Al concentrations (Fig. 2B). The kinetics of efflux resembled those observed for malate efflux from root apices of wheat (Ryan et al., 1995) and oxalate efflux from buckwheat roots (Ma et al., 1997b). The absence of a clear lag-phase in tobacco suggests that, like wheat and buckwheat, Al activates a pre-existing transport system for organic acid release. Because some plant species show enhanced organic acid efflux from roots under P deficiency in the absence of Al (Gardner et al., 1983; Lipton et al., 1987; Hoffland et al., 1992), the effect of P deficiency on efflux from tobacco roots was also examined. However, citrate efflux from whole roots of tobacco was not increased even when plants were grown to be severely P-deficient, yet these same P-deficient plants still showed an Al-activated citrate efflux (data not shown).
Figure 1.
Al-activated efflux of citrate from whole roots of tobacco. Tobacco (cv Wisconsin 38) plants were grown for 11 d in full nutrients, then transferred to either control solution (−Al; 0.2 mm CaCl2, pH 4.3) or Al solution (+Al; 0.2 mm CaCl2 plus 50 μm Al, pH 4.3). Solutions were replaced at 3-h intervals, and the citrate exuded by roots over this time interval was assayed and the cumulative amounts of citrate exuded are shown for each time point. The means of four replicate plants ± se are shown.
Figure 2.
A, Al-activated efflux of citrate from various root segments of tobacco; B, the effect of Al concentration (0–50 μm) on citrate exuded over 9 h by 6-mm root apices. The inset in B shows the relationship of citrate exuded over 9 h to the Al concentration in solution. The root segments were washed in control solution (−Al; 0.2 mm CaCl2, pH 4.3) for 60 min before incubation with shaking in 1 mL of control solution or control solution that contained Al at the concentrations shown. Solutions were replaced at 3-h intervals and citrate exuded by the root segments over this time interval was assayed and the cumulative amounts of citrate exuded are shown for each time point. The means of three replicate plants ± se are shown.
Expression of Bacterial CS Genes in Tobacco
The coding region of the P. aeruginosa CS gene was expressed in tobacco under the control of the 35S CaMV promoter. The integrity of the gene was assessed in a number of ways. First, sequencing confirmed that the P. aeruginosa coding region generated by PCR translated into a predicted protein of identical amino acid sequence to that published in GenBank (accession no. AAG04969). Second, expression of the P. aeruginosa coding region in the Escherichia coli mutant W620, that is defective in the CS gene (Guest, 1981), resulted in a high level of CS activity (1.3 μmol min−1 mg−1; n = 1; activity in the mutant without the CS gene was below the limits of detection). Third, the binary vector containing the CS gene under the control of the 35S CaMV promoter, which has a low level of bacterial promoter activity, resulted in a low but measurable CS activity when introduced into the W620 mutant. Finally the P. aeruginosa gene was amplified from genomic DNA of transgenic line PA12 (see below) by PCR and expression of this PCR product in the W620 mutant yielded approximately the same activity as the initial CS clone used to transform the tobacco (0.83 ± 0.35 μmol min−1 mg−1, n = 2 range shown). When this PCR product was sequenced, it yielded an identical DNA sequence to the CS cDNA that was originally introduced into the plant. Taken together these results indicate that the P. aeruginosa CS gene expressed in line PA12 was not adversely affected by the transformation procedure and encoded a functional protein when expressed in E. coli.
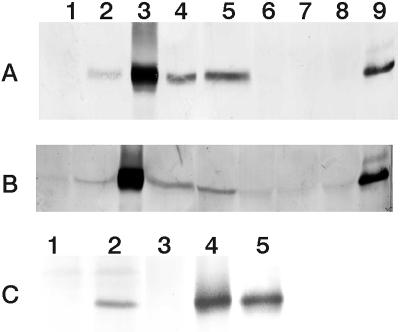
Sixty-three primary (T0) tobacco transformants were analyzed for expression of the P. aeruginosa CS gene (PA lines) by either western- or northern-blot analysis. Of these plants, 36 showed detectable levels of P. aeruginosa CS expression, and four T0 lines encompassing a wide range of expression levels were selected for further analysis (lines P5, P12, P49, and P57; Fig. 3A). Expression of P. aeruginosa CS protein was also detected in the T1 or T2 generations of these PA lines but apart from line PA12, the level of expression was lower than the corresponding T0 lines (Fig. 3B). The levels of expression in all of the PA lines, whether they were T0, T1, or T2, were greater than those apparent in either of the CSb lines (Fig. 3). Western blots showed that the highest expressing PA line (PA12) had over 100-fold greater levels of CS protein than the highest expressing CSb line (CSb47) where the level of antigen was at or below the limits of detection. The presence of the gene in lines CSb18 and CSb47 was confirmed by Southern-blot analysis, and expression at the mRNA level was detected in line CSb47 by northern-blot analysis (data not shown). Line PA12 was exceptional in its level of expression of CS protein and in leaves of plants grown in tissue culture, P. aeruginosa CS accumulated to up to 5% of the total soluble protein. Most of the P. aeruginosa CS protein was localized to the cytosol and in roots it comprised approximately 2% of the total cytosolic protein of line PA12 (Fig. 3C).
Figure 3.
Expression of the P. aeruginosa CS gene in a range of transgenic tobacco lines determined by western-blot analysis. P. aeruginosa CS protein in leaf extracts of T0 plants from four different PA lines (A) and T1 or T2 lines originating from these same four T0 lines (B). Included for comparison are the two CSb lines and control (T2 lines in both A and B). Identity of samples are: 1, P502 (control for PA lines); 2, PA5; 3, PA12; 4, PA49; 5, PA57; 6, CM1522 (control for CSb lines); 7, CSb18; 8, CSb47; and 9, 0.25 μg pure P. aeruginosa CS. For A, 70 μg of protein was loaded per lane except for PA12 where 35 μg was loaded. For B, 240 μg of protein was loaded per lane except for PA12 where 20 μg was loaded. C, P. aeruginosa CS protein in the mitochondrial (1, P502; 2, PA12) and cytosolic (3, P502; 4, PA12) fractions of root extracts from lines PA12 and P502 (30 μg protein loaded per lane). Lane 5 is 0.5 μg of pure P. aeruginosa CS.
Two of the PA lines showed phenotypes that may have resulted from expression of the foreign gene. Line PA49 consistently showed necrosis of the leaf margins and had necrotic patches on leaves, which resulted in misshapen leaves. Occasional plants of line PA12 had similar necrotic patches on leaves but the phenotype was less severe than apparent for line PA49.
Citrate Concentrations in Roots of Transgenic Tobacco
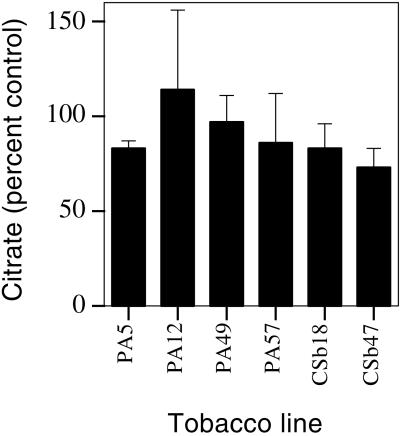
Of the parameters measured in the transgenic CSb lines by de la Fuente et al. (1997), the citrate concentration of roots was reported to be affected the greatest. For example, CSb18 was reported to have approximately a 10-fold greater citrate concentration in roots than the control line and citrate concentrations increased in accordance with increased expression of the P. aeruginosa CS gene. In direct contrast to this finding, we were unable to find any effect of expressing the bacterial CS gene in tobacco on citrate concentrations of roots for any line including the CSb18 and CSb47 lines generated by de la Fuente et al. (1997) (Fig. 4). Even the PA lines, which showed the greatest levels of CS expression, had similar citrate concentrations to the control. Analysis of root citrate concentrations of some of the T0 lines similarly showed no differences to the control line (citrate concentrations of T0 lines as a percent of the P502 control were: PA49, 118 ± 21; PA57, 87 ± 11; and PA12, 115 ± 19; means ± se, n = 6). Root citrate concentrations were also determined in selected lines when grown in a greenhouse (PA12 and control), under low-light conditions (PA12 and control), or with a nutrient solution based on one described by López-Bucio et al. (2000) (CSb lines, high nitrate solution). In all cases, roots had similar citrate concentrations to the control plants (data not shown). Furthermore, de la Fuente et al. (1997) reported very high citrate concentrations in roots that ranged from 0.4 to 4 m. This is in contrast to our values in the sub-millimolar range, which are consistent with values reported by Scheible et al. (1997) for tobacco roots grown with a low nitrate supply. A continuously high concentration of nitrate supplied to tobacco can enhance citrate accumulation by roots with concentrations reaching approximately 3 mm (Scheible et al., 1997).
Figure 4.
Citrate concentrations of roots from various transgenic lines expressed as a percent of control lines. Plants were grown for either 10 (CSb lines) or 11 d (PA lines) in hydroponic culture after transfer from agar plates. Experiments were undertaken on three different occasions and appropriate controls were included with each set of lines. Citrate concentrations for P502 (control for PA lines) ranged from 0.29 ± 0.01 mm to 0.56 ± 0.11 mm and was 0.30 ± 0.04 mm for CM1522 (control for CSb lines). The means of four replicate plants ± se are shown. No statistically significant differences were found for any line when means were compared with their relevant controls as determined by a Student's t test.
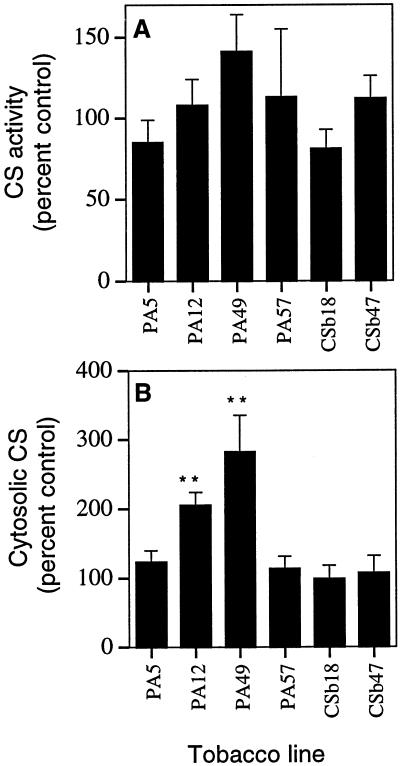
Root CS Activity of Transgenic Tobacco Lines
Total CS activities measured in root extracts of the various transgenic lines were not statistically different to the controls (Fig. 5A). Specific activities reported by de la Fuente et al. (1997) and López-Bucio et al. (2000) ranged from approximately 200 to 700 mg CoA min−1 mg−1 (equivalent to 260–900 μmol CoA min−1 mg−1), which are in excess of 1,000-fold greater than those reported here and greater than activities reported for highly purified CS from a range of organisms (Donald et al., 1989; Unger and Vasconcelos, 1989; Mitchell et al., 1995; Ruijter et al., 2000). In the PA12 line, the majority of the P. aeruginosa CS protein in roots was localized to the cytosolic fraction (Fig. 3C). We reasoned that since most of the endogenous CS activity is present in the mitochondria, an assay of the cytosolic fraction should provide a more sensitive measure of the CS activity resulting from expression of the bacterial gene. Increased CS activity was observed in two of the PA lines (Fig. 5B), but CS activity did not correlate with the level of protein expressed. Line PA49 had the greatest CS activity but based on the western blot (Fig. 3B) had considerably less P. aeruginosa CS protein than line PA12. Consistent with the low level of CS expression in lines CSb18 and CSb47, CS activities in the cytosolic fraction of both of these lines were the same as the CM1522 control.
Figure 5.
Total CS (A) and cytosolic CS (B) activities of roots from various transgenic lines expressed as a percent of control lines. Plants were grown for either 10 (CSb lines) or 11 d (PA lines) in hydroponic culture after transfer from agar plates. Experiments were undertaken on several different occasions, and appropriate controls were included with each set of lines. Total CS activities for P502 (control for PA lines) ranged from 73 ± 11 to 91 ± 8 nmol min−1 mg−1 protein and CM1522 (control for CSb lines) had 77 ± 5 nmol min−1 mg−1 protein. Cytosolic CS activities for P502 ranged from 7.1 ± 1.2 to 8.6 ± 0.5 nmol min−1 mg−1 protein and was 5.7 ± nmol min−1 mg−1 protein for CM1522. The means of four or three replicates ± se are shown for each line and values that are significantly different to the control line at P < 0.01 as determined by a Student's t test are denoted by **.
From the western blot described above, we estimated that the cytosolic fraction of line PA12 contained approximately 2% of total protein as P. aeruginosa CS (Fig. 3C). Pure CS from P. aeruginosa has been reported to have a specific activity of approximately 3 μmol min−1 mg−1 protein when assayed at 20°C (Donald et al., 1989) and, depending on the CS isoform, from 4 to 11 μmol min−1 mg−1 protein when assayed at 25°C (Mitchell et al., 1995). Therefore, we expected that the activity of CS in the cytosolic fraction of line PA12 should be in the order of 60 to 220 nmol min−1 mg−1 protein. However, CS activity for the PA12 cytosolic fraction when measured at 20°C was found to be 11.3 ± 0.7 nmol−1 min−1 mg protein (mean ± se; n = 4), which indicates that only a small proportion of the total P. aeruginosa CS was active in this line. On the basis of these results we suggest that the CS from P. aeruginosa is largely inactive in plants when expressed at a high level and this might result from incorrect folding of the protein or the formation of inactive aggregates in the plant host. It is unlikely that the lower than expected activity was associated with a mutation in the gene introduced into line PA12 since, as discussed above, we were able to rescue a PCR product from line PA12 that could express a functional CS enzyme in E. coli. By contrast, the increase in CS activity over the control found in the root cytosolic fraction of line PA49 was consistent with all of the P. aeruginosa CS protein been active suggesting that at lower levels of expression, most of the protein remains functional in the plant. Similar calculations for the CSb18 line indicate that, at the lower level of expression in this line, the increase in CS activity in the cytosolic fraction would be less than 0.5-fold even if all the P. aeruginosa CS were active. For total CS activity of root extracts (cytosolic plus mitochondrial), the increase would only be approximately 0.05-fold. We conclude that the 3-fold enhancement in total root CS activity of line CSb18 reported previously (de la Fuente et al., 1997) is either not due to expression of the microbial gene or that some environmental factor, not replicated in our work, is required to maximize activity. The gene used to generate the transgenic CSb tobacco lines was a modified version of the wild-type P. aeruginosa CS gene resulting in an N-terminal truncation of the protein and this alteration may have influenced both the expression and activity of the bacterial CS protein (Herrera-Estrella, personal communication).
Citrate Efflux from Roots of Transgenic Tobacco Lines
The most important parameter in terms of enhancing P-nutrition and Al tolerance is the efflux of organic acids from roots. In the absence of Al, efflux was at or below the limits of detection for all lines tested. As shown above (Figs. 1 and 2), Al activated the efflux of citrate but there were no statistically significant differences in Al-activated efflux from either whole roots or root apices between the CS transgenic lines and their respective controls (Table I). The CM1522 control line (tobacco cv Xanthia) had approximately a 3-fold greater Al-activated efflux from intact roots than the P502 control line (tobacco cv Wisconsin 38; Table I). However, this difference was not apparent when citrate efflux from excised root apices was compared in these control lines (Table I). This result suggests that the differences observed in intact roots may be due to differences in root morphology between these lines. By contrast, de la Fuente et al. (1997) reported a 4-fold increase in citrate efflux from whole roots of CSb18 compared with the CM1522 control line. In those experiments roots were incubated in water only and as such it would be expected that only basal efflux, similar to that measured in the absence of Al, would be observed. When we incubated roots of tobacco in distilled water overnight, we found small amounts of citrate exuded (at the limits of detection) that were comparable with those obtained for roots incubated in CaCl2 over the same period (data not shown).
Table I.
Al-activated efflux of citrate from whole roots and root apices of selected transgenic linesa
| Tobacco Line | Citrate
Efflux
|
|
|---|---|---|
| Whole roots | Root apices | |
| nmol h−1 g−1 fresh wt | pmol h−1 apex−1 | |
| P502 | 29 ± 6 | 204 ± 14 |
| PA12 | 72 ± 27 | 249 ± 11 |
| CM1522 | 88 ± 10 | 371 ± 26b; 235 ± 2c |
| CSb18 | 74 ± 12 | 359 ± 21b |
| CSb47 | 93 ± 8 | 169 ± 47c |
Efflux of citrate was measured over the 3- to 6-h interval after exposure of whole roots or root apices to 50 μm Al in 0.2 mm CaCl2, pH 4.3. Citrate efflux in control solution (0.2 mm CaCl2, pH 4.3) over this same interval was at or below the limits of detection. The control line for PA12 is P502, whereas CM1522 is the control line for the CSb lines. No statistically significant differences were found for any line compared with the appropriate control line at P < 0.05 as determined by a student's t test. The mean of four (whole roots) or three (root apices) replicates ± se is shown for each line.
Citrate efflux from apices of line CM1522 was measured on two different occasions, once as a control for line CSb18 and once as a control for line CSb47.
There is evidence that overexpression of a plant gene encoding the mitochondrial form of CS in Arabidopsis plants can enhance citrate efflux with associated benefits in P-acquisition and Al tolerance (Koyama et al., 2000). The highest expressing line had about a 3-fold greater CS activity than controls and this was associated with an increase of 1.6-fold in citrate exuded. Although the increase in citrate efflux was small, it appeared to be sufficient to confer a correspondingly small increase in Al tolerance. Carrot cells grown in culture that overexpress a mitochondrial CS gene from Arabidopsis, similarly, show enhanced citrate exudation (Koyama et al., 1999), whereas mutant carrot cell lines selected for greater citrate exudation had greater mitochondrial CS activity than wild-type cells (Takita et al., 1999). Hoffland et al. (1992) also attributed the enhanced citrate and malate efflux from roots of P-deprived Brassica napus plants to greater activity of enzymes involved in organic acid biosynthesis. These observations support the notion that enhanced activity of enzymes involved in organic acid biosynthesis in plants can alter metabolism and result in increased exudation of organic acids. By contrast, overexpression in Aspergillus niger of its mitochondrial CS gene did not increase internal citrate concentrations or citrate exudation despite an increase CS activity of up to 11-fold (Ruijter et al., 2000).
CS activity in a single 6-mm-long root apex from a wild-type tobacco plant was estimated to be approximately 500 pmol min−1 and citrate efflux from the same tissue exposed to 50 μm Al to be approximately 3 pmol min−1. Therefore, citrate efflux from root apices in the presence of Al uses less than 1% of the endogenous CS capacity. Even if only 10% of the cells of the root apex are involved in supplying citrate for efflux, then this still represents less than 10% of the CS capacity. This suggests that CS activity is unlikely to limit citrate efflux from root apices, a similar finding to that of Ruijter et al. (2000) who concluded that CS activity was not limiting the flow of metabolites toward citrate production in A. niger.
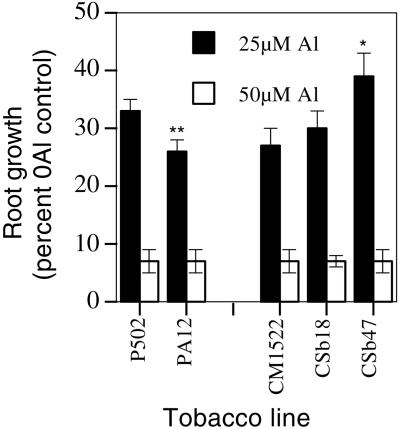
Al Tolerance of Transgenic Tobacco and Alfalfa Lines
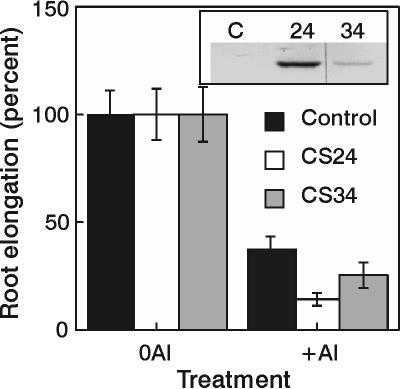
Al tolerances of line PA12 and both CSb lines were assessed. Lines PA12 and CSb18 did not show enhanced Al tolerance, whereas line CSb47 showed a small, yet statistically significant, increase in Al tolerance at 25 μm Al but not at 50 μm Al when compared with the CM1522 control (Fig. 6). The increase in Al tolerance observed in CSb47 could not be attributed to increased citrate concentrations (Fig. 4), resulting in enhanced citrate efflux (Table I). The increased Al tolerance of this line may have resulted from somaclonal variation that can occur during the transformation procedure used to generate transgenic tobacco. These findings contrast with those of de la Fuente et al. (1997) who showed increased Al tolerance of line CSb18 over the control line for a range of Al concentrations. Al tolerance in transgenic alfalfa (Medicago sativum) lines expressing the P. aeruginosa CS protein was also assessed. Since alfalfa is more sensitive of Al than tobacco, it provides a lower background to test the effectiveness of transgenes to enhance Al tolerance. Although expression of the CS protein was clearly apparent in two lines (estimated at approximately 0.02% and 0.1% of total leaf protein for lines CS34 and CS24, respectively), neither line showed enhanced Al tolerance to 6 μm Al (Fig. 7).
Figure 6.
Al tolerance of selected transgenic lines of tobacco. Root elongations during exposure to Al are expressed as a percent of root growth in the zero Al control solutions for the various transgenic lines. The means ± se are shown for 17 to 36 replicate seedlings of each line. Values for the transgenic lines that are significantly different from the appropriate control lines at P < 0.05 and P < 0.01 as determined by a Student's t test are denoted by * and **, respectively. Root elongations for the lines grown in the control solution (−Al) over the same time interval used for Al exposure were 15.6 ± 0.5 mm for P502, 12.8 ± 0.7 mm for PA12, 14.9 ± 0.5 mm for CM1522, 13.6 ± 0.6 mm for CSb18, and 12.6 ± 0.5 mm for CSb47.
Figure 7.
Al tolerance of transgenic lines of alfalfa. Root elongations during exposure to 6 μm Al for 15 d are expressed as a percent of root growth in the zero Al solutions for the various transgenic lines. The means ± se are shown for six to eight replicate seedlings of each line. Root elongations for the lines grown in the zero Al solution over the same time interval used for Al exposure were 70 ± 8 mm for the control line, 59 ± 7 mm for line CS24, and 71 ± 9 mm for line CS34. The inset shows the relative level of P. aeruginosa CS protein in the transgenic lines (C = control line with plasmid only) as determined by a western blot. Each lane contained 100 μg of total leaf protein.
Concluding Remarks
Significant improvements in Al tolerance and P-nutrition were previously reported for transgenic tobacco plants expressing a P. aeruginosa CS gene (de la Fuente et al., 1997; López-Bucio et al., 2000). These effects were attributed to increased production of citrate and its subsequent efflux from roots. We have been unable to replicate the previously reported differences in CS activity, internal citrate, citrate efflux, and Al tolerance in these same CSb lines as well as in newly developed transgenic lines that express the P. aeruginosa CS at up to a 100-fold greater level. This would suggest that the activity of the P. aeruginosa CS in transgenic tobacco is either sensitive to environmental conditions or that the improvements in Al tolerance and P-nutrition observed previously are due to some other variable. In any case, our results indicate that the expression of the P. aeruginosa CS gene is unlikely to be a robust and easily reproducible strategy for enhancing the Al tolerance and P-nutrition of crop and pasture species.
MATERIALS AND METHODS
Plant Material
The transgenic tobacco (Nicotiana tabacum cv Xanthia) lines CM1522 (control) and CSb18, previously described by de la Fuente et al. (1997), used the 35S CaMV promoter to drive the Pseudomonas aeruginosa CS gene. Line CSb47 was produced using the same binary construct and was identified as a line with greater expression than CSb18 (L. Herrera-Estrella, personal communication). The PA lines were generated in the cv Wisconsin 38 and also used the 35S CaMV promoter to drive expression of the coding region of the P. aeruginosa CS gene. Primary transgenic lines were analyzed for expression by western blots to identify lines with high levels of expression. Selected lines were grown in soil and seed collected to generate T1 lines. These lines were either used directly for experiments after selection on Murashige and Skoog (Murashige and Skoog, 1962) agar plates that contained 100 μg mL−1 kanamycin or were grown to produce the T2 generation from which homozygous lines were identified (line PA12). Several lines of alfalfa (CS lines; Medicago sativum cv Aquarius) were also generated using the same binary constructs as was used for the PA tobacco lines. These alfalfa lines were maintained as clonal explants.
Cloning and Preparation of Plasmid Constructs
The coding region of the P. aeruginosa CS gene was amplified from genomic DNA with the primers CGTGGATCCGATGGCTGACAAAAAAGC and GTATCTAGATCAGCCGCGATCCTTG. Inclusion of BamHI and XbaI restriction sites (shown italicized) in the primers allowed the PCR products to be cloned into the BamHI/XbaI site of the pDH51 expression cassette (Pietrzak et al., 1986), which contains the 35S CaMV promoter and terminator. The resulting EcoRI fragment that carried the CS gene was then introduced into the EcoRI site of the binary vector pPLEX502, originally described as pPLEX101 by Surin et al. (1998), and this vector was used to generate lines PA12 and PA5. For other lines (PA49 and PA57) the pART7 expression cassette (Gleave, 1992) containing the 35S CaMV promoter and octopine synthase terminator was used, and the NotI fragment containing the CS gene, cloned into the BamHI/XbaI site, was introduced into the NotI site of pPLEX502. The binary vectors were introduced into tobacco using Agrobacterium-mediated transformation (Horsch et al., 1985). Alfalfa was transformed with the same binary vectors using a procedure described by Tabe et al. (1995).
Plant Growth
Plants grown from seed were first selected for kanamycin resistance on 0.4% (w/v) agar and after 20 to 30 d growth were transferred to hydroponic culture in 2.2-L containers using methods described previously for Arabidopsis (Delhaize and Randall, 1995). The nutrient solution was as described by Delhaize et al. (1993) except that 500 μm Ca(NO3)2 was replaced with 500 μm CaCl2 and the pH adjusted to 4.3. When plants were used for citrate efflux, the K phosphate concentration was reduced to 10 μm on the final day of growth to prevent formation of Al phosphate complexes that may have reduced the concentration of free Al in solution. For the cv Xanthia, nutrient solution was changed after 7 and 9 d of growth and plants harvested on d 10. For the cv Wisconsin 38, nutrient solution was changed after 8 and 10 d of growth and plants were harvested on d 11. Plants were grown in a controlled environment cabinet at constant temperature (25°C) and photon flux density of approximately 500 μmol m−2 s−1 with a 16-h-light/8-h-dark period. Some lines were also grown in (a) a glasshouse under natural daylight where temperature varied between 18°C to 23°C and (b) in a growth room under low intensity fluorescent light (approximately photon flux density of 100 μmol m−2 s−1) at 22°C to 25°C with a 12-h-light/12-h-dark period. The CSb lines were also grown in the nutrient solution (solution J) described by López-Bucio et al. (2000). However, since this solution lacked Ca as described by López-Bucio et al. (2000), 3 mm Ca(NO3)2 was included. Growth of seedlings was initiated on the above low pH nutrient solution for 5 d then the solution was replaced with one-quarter strength solution J and after an additional 2 d of growth, finally replaced with one-half-strength solution J and plants harvested 2 d later. For the analysis of T0 plants, clonal explants were propagated on Murashige and Skoog medium, and six plants from each line were transferred to nutrient solution and grown for 7 d before analysis of root citrate concentrations.
Plant Analysis
For internal citrate concentrations, roots were excised, patted dry between tissue paper, weighed, and then frozen in liquid nitrogen. The frozen tissue was ground to a powder and extracted in 2 volumes of 0.6 m perchloric acid and neutralized with 2 m KOH before assay for citrate using an enzymatic procedure (Dagley, 1974). Some root samples were also extracted using 10 volumes of ethanol at 80°C for 2 h and the resulting ethanol extracts assayed for citrate. Both types of analysis yielded similar values and the perchloric extraction method was used for routine analyses. For total CS assay, the frozen tissues were ground in liquid nitrogen, thawed into 2 volumes of buffer, and assayed for activity using previously described methods (Landschütze et al., 1995). Cytosolic and mitochondrial fractions for CS assay were prepared from fresh tissues as described by Landschütze et al. (1995). CS activities are expressed as nmol acetylCoA-hydrolyzed min−1 mg−1 protein. Citrate efflux from whole roots was determined by immersing the roots of one plant in 50 mL of continuously aerated solution and the resulting solution was measured for citrate by either enzymatic or HPLC methods. Organic acids were analyzed by HPLC using an ion-exclusion column (IC-Pak, 7.8 × 300 mm, Waters, Milford, MA) and 13 mm H2SO4 as the running solvent (1 mL min−1) with UV detection at 214 nm. The solutions for efflux experiments consisted of either 200 μm CaCl2, pH 4.3, or the same solution with Al added as AlCl3. To determine citrate efflux from excised root segments, 20 root segments were incubated with shaking in 1 mL of solution using procedures described previously for wheat (Ryan et al., 1995) and the resulting solution assayed for citrate by the enzymatic procedure. Proteins were extracted with the CS extraction buffer and assayed using the method described by Bradford (1976). An antibody prepared against the P. aeruginosa CS (Donald et al., 1989) was used to detect expression of the protein by western-blot analysis using a second antibody conjugated to alkaline phosphatase (Rerie et al., 1991). Samples were extracted for RNA using a method described by Rerie et al. (1991). Southern blots, northern blots, and sequencing were undertaken using procedures described by Sambrook et al. (1989).
Al Tolerance
Al tolerance of tobacco was determined using a modification of a procedure described by Toda et al. (1999). Sixteen seeds were placed on nylon mesh held up by slide mounts floating on 200 mL of nutrient solution (as described above except K phosphate at 10 μm). After 7 d of growth in the absence of Al, seedlings were thinned to obtain a uniform set and root lengths were measured. Seedlings were then transferred to 2 L of aerated nutrient solution that contained the Al treatment and after a further 7 (cv Xanthia) or 8 (cv Wisconsin 38) d growth, root lengths were measured. For alfalfa, clonally-propagated explants were grown in batches of 42 L of aerated nutrient solution (10 or 11 plants per batch) of the same composition as used for the tobacco experiments except that the pH was maintained at 4.4 to 4.5. Root lengths were measured on the day of transfer to hydroponic culture when treatments were imposed and 15 d later.
ACKNOWLEDGMENTS
We thank Dr. Luis Herrera-Estrella for making available the CSb tobacco lines, CS antibodies, and purified CS protein. We thank Terese Richardson for generating the transgenic alfalfa lines.
Footnotes
This work was supported by the Australian Grains Research and Development Corporation.
LITERATURE CITED
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dagley S. Citrate, UV spectrophotometric determination. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 1585–1589. [Google Scholar]
- de la Fuente JM, Ramírez-Rodríguez V, Cabrera-Ponce JL, Herrera-Estrella L. Aluminum tolerance in transgenic plants by alteration of citrate synthesis. Science. 1997;276:1566–1568. doi: 10.1126/science.276.5318.1566. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ. Aluminum tolerance in wheat (Triticum aestivumL.): I. Uptake and distribution of aluminum in root apices. Plant Physiol. 1993;103:685–693. doi: 10.1104/pp.103.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Randall PJ. Characterization of a phosphate-accumulator mutant of Arabidopsis thaliana. Plant Physiol. 1995;107:207–213. doi: 10.1104/pp.107.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinklaker B, Römheld V, Marschner H. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albusL.) Plant Cell Environ. 1989;12:285–292. [Google Scholar]
- Donald L, Molgat G, Duckworth H. Cloning, sequencing, and expression of the gene for NADH-sensitive citrate synthase of Pseudomonas aeruginosa. J Bacteriol. 1989;171:5542–5550. doi: 10.1128/jb.171.10.5542-5550.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner WK, Barber DA, Parbery DG. The acquisition of phosphorus by Lupinus albusL.: III. The probable mechanism by which phosphorus movement in the soil/root interface is enhanced. Plant Soil. 1983;70:107–124. [Google Scholar]
- Gleave AP. A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol. 1992;20:1203–1207. doi: 10.1007/BF00028910. [DOI] [PubMed] [Google Scholar]
- Guest J. Hybrid plasmids containing the citrate synthase gene (gltA) of Escherichia coliK12. J Gen Microbiol. 1981;124:17–23. doi: 10.1099/00221287-124-1-17. [DOI] [PubMed] [Google Scholar]
- Hoffland E, Van Den Boogaard R, Nelemans J, Findenegg G. Biosynthesis and root exudation of citric and malic acids in phosphate-starved rape plants. New Phytol. 1992;122:675–680. [Google Scholar]
- Horsch RB, Fry JE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227:1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Hue NV, Craddock GR, Adams F. Effect of organic acids on aluminum toxicity in subsoils. Soil Sci Am J. 1986;50:28–34. [Google Scholar]
- Koyama H, Kawamura A, Kihara T, Hara T, Takita E, Shibata D. Overexpression of mitochondrial citrate synthase in Arabidopsis thalianaimproved growth on a phosphorus limited soil. Plant Cell Physiol. 2000;41:1030–1037. doi: 10.1093/pcp/pcd029. [DOI] [PubMed] [Google Scholar]
- Koyama H, Takita E, Kawamura A, Hara T, Shibata D. Over expression of mitochondrial citrate synthase gene improves the growth of carrot cells in Al-phosphate medium. Plant Cell Physiol. 1999;40:482–488. doi: 10.1093/oxfordjournals.pcp.a029568. [DOI] [PubMed] [Google Scholar]
- Landschütze V, Willmitzer L, Müller-Röber B. Inhibition of flower formation by antisense repression of mitochondrial citrate synthase in transgenic potato plants leads to a specific disintegration of the ovary tissues of flowers. EMBO J. 1995;14:660–666. doi: 10.1002/j.1460-2075.1995.tb07044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton DS, Blanchar RW, Blevins DG. Citrate, malate, and succinate concentration in exudates from P-sufficient and P-stressed Medicago sativaL. seedlings. Plant Physiol. 1987;85:315–317. doi: 10.1104/pp.85.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Martínez de la Vega O, Guevara-García A, Herrera-Estrella L. Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nat Biotech. 2000;18:450–453. doi: 10.1038/74531. [DOI] [PubMed] [Google Scholar]
- Ma JF. Role of organic acids in detoxification of aluminum in higher plants. Plant Cell Physiol. 2000;41:383–390. doi: 10.1093/pcp/41.4.383. [DOI] [PubMed] [Google Scholar]
- Ma JF, Taketa S, Yang ZM. Aluminum tolerance genes on the short arm of chromosome 3R are linked to organic acid release in Triticale. Plant Physiol. 2000;122:687–694. doi: 10.1104/pp.122.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H. Specific secretion of citric acid induced by Al stress in Cassia toraL. Plant Cell Physiol. 1997a;38:1019–1025. [Google Scholar]
- Ma JF, Zheng SJ, Matsumoto H, Hiradate S. Detoxifying aluminum with buckwheat. Nature. 1997b;390:569–570. [Google Scholar]
- Mitchell CG, Anderson SCK, El-Mansi EMT. Purification and characterization of citrate synthase isoenzymes from Pseudomonas aeruginosa. Biochem J. 1995;309:507–511. doi: 10.1042/bj3090507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for a rapid growth and bioassay with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Pellet DM, Grunes DL, Kochian LV. Organic acid exudation as an aluminum tolerance mechanism in maize (Zea maysL.) Planta. 1995;196:788–795. [Google Scholar]
- Pietrzak M, Shillito RD, Hohn T, Potrykus I. Expression in plants of two bacterial antibiotic resistance genes after protoplast transformation with a new plant expression vector. Nucleic Acids Res. 1986;14:5857–5868. doi: 10.1093/nar/14.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerie WG, Whitecross M, Higgins TJV. Developmental and environmental regulation of pea legumin genes in transgenic tobacco. Mol Gen Genet. 1991;225:148–157. doi: 10.1007/BF00282653. [DOI] [PubMed] [Google Scholar]
- Ruijter G, Panneman H, Xu D-B, Visser J. Properties of Aspergillus niger citrate synthase and effects of citAoverexpression on citric acid production. FEMS Lett. 2000;184:35–40. doi: 10.1111/j.1574-6968.2000.tb08986.x. [DOI] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Randall PJ. Characterization of Al-stimulated malate efflux from the root apices of Al-tolerant genotypes of wheat. Planta. 1995;196:103–110. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Scheible W-R, González-Fontes A, Lauerer M, Müller-Röber B, Caboche M, Stitt M. Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell. 1997;9:783–798. doi: 10.1105/tpc.9.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surin B, Boevink P, Keese P, Chu P, Larkin P, Llewellyn D, Kahn R, Ellacott G, Waterhouse P. A suite of promoters and terminators for plant biotechnology. In: Larkin PJ, editor. Proceedings of the 4th Asia-Pacific Conference on Agricultural Bio/Technology. Canberra UTC Publishing, Darwin, pp121–122. 1998. [Google Scholar]
- Tabe LM, Wardley-Richardson T, Ceriotti A, Aryan A, McNabb W, Moore A, Higgins TJV. A biotechnological approach to improving the nutritive value of alfalfa. J Anim Sci. 1995;73:2752–2759. doi: 10.2527/1995.7392752x. [DOI] [PubMed] [Google Scholar]
- Takita E, Koyama H, Hara T. Organic acid metabolism in aluminum-phosphate utilizing cells of carrot (Daucus carotaL.) Plant Cell Physiol. 1999;40:489–495. [Google Scholar]
- Toda T, Koyama H, Hara T. A simple hydroponic culture method for the development of a highly viable root system in Arabidopsis thaliana. Biosci Biotechnol Biochem. 1999;63:210–212. doi: 10.1271/bbb.63.210. [DOI] [PubMed] [Google Scholar]
- Unger EA, Vasconcelos AC. Purification and characterization of mitochondrial citrate synthase. Plant Physiol. 1989;89:719–723. doi: 10.1104/pp.89.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Uexküll HR, Mutert E. Global extent, development and economic impact of acid soils. Plant Soil. 1995;171:1–15. [Google Scholar]