Abstract
Objective
To estimate the prevalence of chronic obstructive pulmonary disease (COPD) and chronic bronchitis in eight countries in South Asia through a systematic review and meta-analysis.
Methods
We searched MEDLINE® Complete, Web of Science, Embase®, Scopus, CINAHL and reference lists of screened studies for research on the prevalence of COPD and chronic bronchitis in South Asian countries published between January 1990 and February 2021. We used standardized diagnostic criteria for definitions of COPD and chronic bronchitis. Two reviewers undertook study screening, full-text review, quality appraisal and data extraction.
Findings
Of 1529 studies retrieved, 43 met the inclusion criteria: 32 provided data from India; four from Bangladesh; three from Nepal; two from Pakistan; and two from both India and Sri Lanka. Twenty-six studies used standardized diagnostic definitions and 19 were included in the meta-analysis. The estimated pooled prevalence of COPD was 11.1% (95% confidence interval, CI: 7.4–14.8%), using the Global Initiative for Chronic Obstructive Lung Disease fixed criteria and 8.0% (95% CI: 5.6–10.4%) using the lower limit of normal criteria. The prevalence of COPD was highest in north India (19.4%) and Bangladesh (13.5%) and in men. The estimated pooled prevalence of chronic bronchitis was 5.0% (95% CI: 4.1–6.0%) in India and 3.6% (95% CI: 3.1–4.0%) in Pakistan.
Conclusion
Included countries have a high prevalence of COPD although it varied by geographical area and study characteristics. Future research in South Asia should use standardized diagnostic criteria to examine the contribution of setting-specific risk factors to inform prevention and control strategies.
Résumé
Objectif
Estimer la prévalence de la bronchopneumopathie chronique obstructive (BPCO) et de la bronchite chronique dans huit pays d'Asie du Sud par le biais d'une revue systématique et d'une méta-analyse.
Méthodes
Nous avons exploré les bases de données MEDLINE® Complete, Web of Science, Embase®, Scopus et CINAHL, ainsi que des listes de référence d'études sélectionnées, afin de compiler les recherches sur la prévalence de la BPCO et de la bronchite chronique dans les pays d'Asie du Sud publiées entre janvier 1990 et février 2021. Nous avons défini la BPCO et la bronchite chronique en nous fondant sur des critères de diagnostic normalisés. Enfin, deux réviseurs ont trié les études, examiné le texte intégral, évalué la qualité et extrait les données.
Résultats
Sur 1529 études récupérées, 43 correspondaient aux critères d'inclusion: 32 fournissaient des informations sur l'Inde; quatre sur le Bangladesh; trois sur le Népal; deux sur le Pakistan et deux sur l’Inde et le Sri Lanka. Au total, 26 études reposaient sur une définition standard du diagnostic, et 19 étaient intégrées dans la méta-analyse. La prévalence groupée de la BPCO a été estimée à 11,1% (intervalle de confiance de 95%, IC: 7,4–14,8%) selon les critères fixés par l'Initiative mondiale contre la bronchopneumopathie chronique obstructive, et à 8,0% (IC de 95%: 5.6–10.4%) selon la limite inférieure des critères normaux. C'est dans le nord de l'Inde (19,4%) et au Bangladesh (13,5%) que la prévalence la plus élevée a été observée pour la BPCO, et surtout chez les hommes. De son côté, la prévalence groupée de la bronchite chronique a été estimée à 5,0% (IC de 95%: 4,1–6,0%) en Inde et à 3,6% (IC de 95%: 3,1–4,0%) au Pakistan.
Conclusion
Les pays pris en considération présentent une forte prévalence de la BPCO, bien qu'il existe des variations en fonction des zones géographiques et des caractéristiques des études. Les futures recherches menées en Asie du Sud devraient utiliser des critères de diagnostic normalisés pour examiner le rôle des facteurs de risque propres au contexte, afin d'orienter les stratégies de prévention et de lutte.
Resumen
Objetivo
Estimar la prevalencia de las enfermedades pulmonares obstructivas crónicas (EPOC) y de la bronquitis crónica en ocho países del sur de Asia mediante una revisión sistemática y un metanálisis.
Métodos
Se realizaron búsquedas en MEDLINE® Complete, Web of Science, Embase®, Scopus, CINAHL y en las listas de referencias de los estudios seleccionados en busca de investigaciones sobre la prevalencia de las EPOC y de la bronquitis crónica en los países del sur de Asia publicadas entre enero de 1990 y febrero de 2021. Se utilizaron criterios de diagnóstico estandarizados para las definiciones de EPOC y bronquitis crónica. Dos revisores realizaron la selección de los estudios, la revisión del texto completo, la valoración de la calidad y la extracción de los datos.
Resultados
De los 1529 estudios recuperados, 43 cumplían los criterios de inclusión: 32 proporcionaron datos de la India; cuatro de Bangladesh; tres de Nepal; dos de Pakistán; y dos tanto de la India como de Sri Lanka. Veintiséis estudios utilizaron definiciones diagnósticas estandarizadas y 19 se incluyeron en el metanálisis. La prevalencia conjunta estimada de las EPOC fue del 11,1 % (intervalo de confianza del 95 %, IC: 7,4-14,8 %), utilizando los criterios fijos de la Iniciativa Mundial para las Enfermedades Pulmonares Obstructivas Crónicas y del 8,0 % (IC del 95 %: 5,6-10,4 %) utilizando los criterios del límite inferior de la normalidad. La prevalencia de las EPOC fue mayor en el norte de la India (19,4 %) y en Bangladesh (13,5 %) y en los varones. La prevalencia conjunta estimada de bronquitis crónica fue del 5,0 % (IC del 95 %: 4,1-6,0 %) en la India y del 3,6 % (IC del 95 %: 3,1-4,0 %) en Pakistán.
Conclusión
Los países incluidos tienen una alta prevalencia de EPOC, aunque esta varía según la zona geográfica y las características del estudio. Las investigaciones futuras en el sur de Asia deberían utilizar criterios de diagnóstico estandarizados para analizar la contribución de los factores de riesgo específicos del entorno con el fin de fundamentar las estrategias de prevención y control.
ملخص
الغرض
تقدير مدى انتشار مرض الانسداد الرئوي المزمن (COPD)، والتهاب الشعب الهوائية المزمن في جنوب آسيا من خلال مراجعة منهجية وتحليل تلوي.
الطريقة
قمنا بالبحث في MEDLINE® Complete، وWeb of Science، وEmbase®، وScopus، وCINAHL، وقوائم مرجعية للدراسات التي تم فحصها بغرض الأبحاث عن انتشار مرض الانسداد الرئوي المزمن (COPD)، والتهاب الشعب الهوائية المزمن في دول جنوب آسيا، والتي تم نشرها في الفترة ما بين يناير/كانون ثاني 1990 وفبراير/شباط 2021. كما قمنا باستخدام معايير التشخيص القياسية لتعريفات مرض الانسداد الرئوي المزمن (COPD)، والتهاب الشعب الهوائية المزمن. تولى اثنان من المراجعين فحص الدراسة، ومراجعة النص الكامل، وتقييم الجودة، واستخراج البيانات.
النتائج
من بين الـ 1529 دراسة تم استرجاعها، استوفت 43 منها معايير الاشتمال: قدمت 32 منها بيانات من الهند؛ وأربعة من بنغلاديش؛ وثلاثة من نيبال؛ واثنتان من باكستان؛ واثنتان من كل من الهند وسري لانكا. استخدمت ستة وعشرون دراسة تعريفات تشخيصية قياسية، وتم تضمين 19 منها في التحليل التلوي. كان معدل الانتشار المجمّع التقديري لمرض الانسداد الرئوي المزمن (COPD) هو 11.1% (بفاصل ثقة مقداره: 7.4 إلى 14.8%)، باستخدام المعايير الثابتة للمبادرة العالمية لمرض الانسداد الرئوي المزمن، و8.0% (بفاصل ثقة 95%: 5.6 إلى 10.4%) باستخدام حد أقل للمعايير العادية. كان معدل انتشار مرض الانسداد الرئوي المزمن (COPD) هو الأعلى في شمال الهند (19.4%) وبنغلاديش (13.5%) وفي الرجال. كان معدل الانتشار المجمّع التقديري لمرض التهاب الشعب الهوائية المزمن هو 5.0% (بفاصل ثقة مقداره 95%: 4.1 إلى 6.0%) في الهند و3.6% (بفاصل ثقة مقداره 95%: 3.1 إلى 4.0%) في باكستان.
الاستنتاج
كان لدى البلدان المشمولة نسبة عالية من معدل انتشار مرض الانسداد الرئوي المزمن (COPD) على الرغم من أنها تختلف حسب المنطقة الجغرافية وخصائص الدراسة. يجب أن تستخدم الأبحاث المستقبلية في جنوب آسيا معايير تشخيصية قياسية لفحص مساهمة عوامل الخطر ذات الأوضاع المحددة لزيادة التوعية باستراتيجيات الوقاية والسيطرة.
摘要
目的
旨在通过系统评价和元分析来评估南亚八个国家的慢性阻塞性肺疾病 (COPD) 和慢性支气管炎的患病率。
方法
我们搜索了 MEDLINE® Complete、科学网、Embase®、Scopus、CINAHL 和 1990 年 1 月至 2021 年 2 月期间发布的筛查研究参考文献列表来调查南亚国家的 COPD 和慢性支气管炎的患病率。我们采用规范化的诊断标准来确定 COPD 和慢性支气管炎的定义。由两名评审人员负责研究筛选、全文评审、质量评估和数据提取工作。
结果
在检索到的 1529 项研究中,有 43 项符合纳入标准:其中 32 项数据来自印度;4 项来自孟加拉国;3 项来自尼泊尔;2 项来自巴基斯坦;其余两项分别来自印度和斯里兰卡。二十六项研究采用规范化诊断定义,19 项被纳入元分析中。我们采用《慢性阻塞性肺疾病全球倡议》修订版标准评估的 COPD 汇总患病率是 11.1%(95% 置信区间,CI:7.4–14.8%),采用正常标准的下限评估的患病率是 8.0% (95% CI: 5.6–10.4%)。COPD 在北印度 (19.4%) 和孟加拉国 (13.5%) 以及男性间的患病率最高。印度的 COPD 汇总患病率估计值是 5.0% (95% CI: 4.1–6.0%),巴基斯坦的估计值是 3.6% (95% CI: 3.1–4.0%)。
结论
尽管患病率因地理区域和研究特征而异,但研究所纳入国家的 COPD 患病率较高。南亚地区未来的研究应该采用规范化的诊断标准来检验特定环境的风险因素影响程度,为制定预防和管控策略提供理论依据。
Резюме
Цель
Оценить распространенность хронической обструктивной болезни легких (ХОБЛ) и хронического бронхита в восьми странах Южной Азии посредством систематического обзора и метаанализа.
Методы
Авторы провели поиск по базам данных MEDLINE® Complete, Web of Science, Embase®, Scopus, CINAHL и по спискам литературы отобранных исследований, опубликованных в период с января 1990 года по февраль 2021 года, для определения распространенности ХОБЛ и хронического бронхита в странах Южной Азии. Для определения ХОБЛ и хронического бронхита авторы использовали стандартизированные диагностические критерии. Два рецензента провели скрининг исследования, обзор полного текста, оценку качества и выборку данных.
Результаты
Из 1529 полученных исследований 43 соответствовали критериям включения: 32 исследования предоставили данные из Индии; четыре — из Бангладеш; три — из Непала; два — из Пакистана и два — из Индии и Шри-Ланки вместе.. В 26 исследованиях использовались стандартизированные диагностические определения, а 19 исследований были включены в метаанализ. Показатель объединенной распространенности ХОБЛ составил 11,1% (95%-й доверительный интервал, ДИ: 7,4–14,8%) при использовании фиксированных критериев Глобальной инициативы по хронической обструктивной болезни легких, и 8,0% (95%-й ДИ: 5,6–10,4%) при использовании нижней границы нормы критериев. Распространенность ХОБЛ была самой высокой среди мужчин в северной Индии (19,4%) и Бангладеш (13,5%). Показатель объединенной распространенности хронического бронхита составил 5,0% (95%-й ДИ: 4,1–6,0%) в Индии и 3,6% (95%-й ДИ: 3,1–4,0%) в Пакистане.
Вывод
Включенные страны имеют высокую распространенность ХОБЛ, хотя она варьировалась в зависимости от географического региона и характеристик исследования. Чтобы разработать стратегии по профилактике и борьбе с заболеванием, в будущих исследованиях в Южной Азии следует использовать стандартизированные диагностические критерии для изучения вклада факторов риска, зависимых от условий.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable and treatable disease, with a worldwide prevalence of 10.1% in people aged 40 years or older.1,2 In 2019, COPD was the third leading cause of deaths globally, contributing to 3.23 million deaths, with most deaths (80%) occurring in low-and middle-income countries.3,4 A systematic review on COPD showed that estimates of the number of cases of COPD in countries of the World Health Organization South-East Asia Region had increased from 44.5 million to 75.1 million between 1990 and 2010, a 68.8% increase.5
According to the World Bank, South Asia comprises Afghanistan, Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan and Sri Lanka, and is home to a quarter of the global population.6 The area is currently undergoing a demographic transition, because of ageing and increased life expectancy.7 According to the 2017 Global Burden of Disease study, despite a lower prevalence of COPD in South Asia, the attributable morbidity and premature mortality due to chronic respiratory diseases was highest in South Asia with COPD being the most common cause of premature deaths among chronic respiratory diseases.8 The area is also experiencing a change in the burden of risk factors with ambient air pollution becoming a greater risk due to rapid economic development.8 While systematic reviews on the prevalence of COPD from Latin America9 and sub-Saharan Africa10 have been published previously, literature on current prevalence estimates of COPD and its common risk factors in South Asia is scarce.11 Notably, few published data exist on the rural–urban, sex, and within and between country differences in the prevalence of COPD. Relevant and timely information on the prevalence of COPD in the area is crucial to inform, develop and implement context-appropriate policies and programmes for its prevention and control, in a setting where the burden is rising.8
In COPD, airflow in and out of the lungs is limited due to chronic inflammation and narrowing of airways, which is a result of repeated and long-term exposure of the respiratory tract to noxious stimuli (tobacco smoke, indoor air pollution and repeated respiratory tract infections during childhood).4 COPD presents as chronic bronchitis and emphysema.4 However, variation in the underlying pathology and clinical presentation, and overlap with the symptoms of asthma and other chronic lung diseases often pose a challenge to making an accurate diagnosis of COPD.12 Moreover, the gold standard diagnosis of COPD, which needs evaluation of lung air volumes using a spirometer, requires skilled personnel and quality assurance measures, which makes obtaining accurate data more challenging, especially in low- and middle-income countries where a skilled workforce is limited.1,12
A 2012 systematic review reported limitations in estimates of the prevalence of COPD in India due to a lack of data, concerns about the quality of studies, inconsistencies in study settings and population characteristics.11 The availability of more recently published studies provides an opportunity to conduct a systematic review to obtain up-to-date estimates of the prevalence of COPD. These data can be used to inform policy-makers when planning and implementing population-level risk mitigation strategies to control the rising burden of COPD in South Asia.
Methods
Design
We conducted a systematic review and meta-analysis of peer-reviewed literature to estimate the prevalence of COPD and chronic bronchitis in South Asia, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.13 We used the World Bank’s classification of countries of the South Asia area: Afghanistan, Bhutan, Bangladesh, India, Maldives, Nepal, Pakistan and Sri Lanka.6
We used standardized diagnostic criteria for definitions of COPD and chronic bronchitis. We defined COPD as the presence of persistent airflow limitation according to the Global Initiative for Chronic Obstructive Lung Disease, i.e. a post-bronchodilator ratio of forced expiratory volume in one second (FEV1) to the forced vital capacity (FVC), FEV1/FVC < 0.70 (fixed criteria),1 or post-bronchodilator FEV1/FVC below the lower limit of normal, i.e. the lower fifth centile of values from a reference population.14 We defined chronic bronchitis as the presence of chronic cough and phlegm according to the criteria of the Medical Research Council in the United Kingdom of Great Britain and Northern Ireland, i.e. cough and sputum production on most days continuously for 3 months for more than 2 consecutive years.15
We registered the protocol for this systematic review in the international prospective register of systematic reviews (CRD42020206189).
Data sources and searches
We searched MEDLINE® Complete, Web of Science, Embase®, Scopus and CINAHL databases using keywords: chronic obstructive pulmonary disease; COPD; obstructive airway disease; obstructive lung disease; airflow obstruction; chronic bronchitis; emphysema; prevalence; and other related terms. These keywords were combined with terms for individual countries and demonyms: Afghan*; Bangladesh*; Bhutan*; India*; Maldiv*; Nepal*; Pakistan*; Sri Lanka*; and associated global areas – South Asia*; Central Asia* (see data repository for search terms).16
The study inclusion criteria were (i) community-based studies reporting the prevalence of COPD or chronic bronchitis; (ii) cross-sectional, cohort and case–control design; and (iii) publication date between 1 January 1990 and 28 February 2021. We had no language restrictions. We hand-searched the reference lists of screened studies for additional relevant citations.
Study selection
We used Covidence for management of systematic review citations.17 Two authors independently screened the study titles and abstracts using the inclusion criteria to identify studies for full-text review. These authors undertook an independent full-text review of shortlisted articles, made the final decision to include or exclude studies from the review, independently assessed the methodological quality of included studies using the Joanna Briggs Institute checklist for prevalence studies,18 and extracted data from all included studies into a structured data extraction sheet. The following data were extracted from the studies: authors’ names; year of publication and data collection; study title and area; method of disease ascertainment; sample size; sampling technique; study participants; residence; sex; age group; prevalence of COPD and/or chronic bronchitis with their 95% confidence intervals (CIs); and associations between risk factors for and prevalence of COPD and/or chronic bronchitis.
We resolved inconsistencies between reviewers in screening, inclusion and exclusion of studies, quality appraisal and data extraction decisions through discussion. In cases of disagreement, the third reviewer made the final decision. Where necessary, we also contacted the authors of some publications for further information about the methods and data.
Data analysis
We included studies that used probability sampling techniques and standard definitions for disease ascertainment for the meta-analyses. We did not include studies conducted before 2000 in the meta-analyses. If more than one published study reported the prevalence from the same data set, we included the prevalence from the most recently published study. However, if the older studies provided more details on prevalence data compared with recently published studies, we included the older study data. In the qualitative summary we described characteristics of the studies and charted patterns of the prevalence data according to area, sex and criteria for COPD diagnosis.
We used Stata, version 16.1 (Stata Corp, College Station, United States of America) for all meta-analyses. We used the Stata metaprop command to estimate the pooled prevalence and generate forest plots of COPD (total, men and women) according to fixed criteria and lower limit of normal criteria.19 We assessed statistical heterogeneity using the χ2 test, percentage of variance due to heterogeneity using the I2 test and estimated standard deviation of prevalence using the τ2 test. Due to the considerable heterogeneity across studies, we used random-effects models to calculate the pooled prevalence estimates.
Results
Included studies
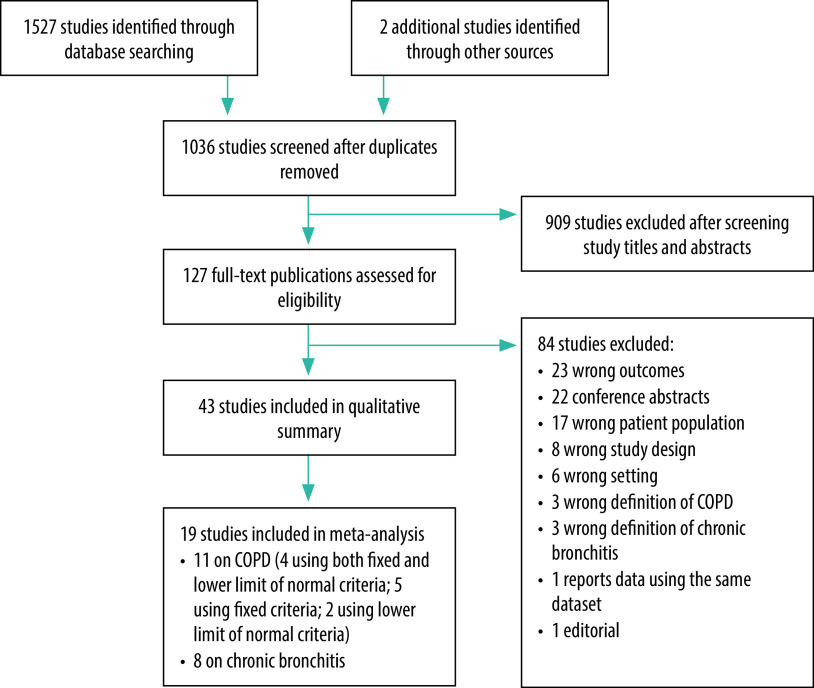
Our search of electronic databases and reference lists yielded a total of 1529 studies. After removing 493 duplicates, we screened 1036 titles and abstracts for inclusion and reviewed the full texts of 127 studies. Of these studies, 43 met the inclusion criteria and were included in the qualitative summary and 19 were included in the meta-analysis: 11 studies on COPD with the most recent or detailed data on prevalence of COPD and eight on chronic bronchitis that reported the overall prevalence (Fig. 1).
Fig. 1.
Study selection for the systematic review of chronic obstructive pulmonary disease, eight countries, 2021
COPD: chronic obstructive pulmonary disease.
Note: We searched for peer-reviewed literature from Afghanistan, Bhutan, Bangladesh, India, Maldives, Nepal, Pakistan and Sri Lanka.
Of the studies included, 32 were undertaken in India, four in Bangladesh, three in Nepal, two in Pakistan and two in both India and Sri Lanka. Five of the studies were part of the multinational Burden of Obstructive Lung Disease study.20–24 No studies from Afghanistan, Bhutan and Maldives met the inclusion criteria. We judged 16 of the included studies had acceptable quality in all appraisal criteria (data repository).16,20–35 Only three studies (two from India and one from Nepal) collected the data on COPD prevalence in the past 5 years (Table 1).
Table 1. Sample characteristics and study outcomes of research reporting the prevalence of chronic obstructive pulmonary disease, Bangladesh, India, Nepal, Sri Lanka, 2021.
| Country and study (year data collected) | Study area | Age, years | Sex | Sample size | COPD criteria |
Prevalence of COPD based on GOLD fixed criteria, % (95% CI) |
Prevalence of COPD based on the lower limit of normal criteria, % |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fixeda | Lower limit of normalb | Overall | Male | Female | Overall | Male | Female | |||||
| COPD confirmed with post-bronchodilation spirometry | ||||||||||||
| Studies included in meta-analysis | ||||||||||||
| Bangladesh | ||||||||||||
| Alam et al., 2015 (2011–2012)25 | Rural Matlab and suburban Kamlapur | ≥ 40 | Male, female | 3660 | Yes | Yes | Total: 13.5 (12.4–14.6), rural: 17.0, urban: 9.9 | Total: 22.0 | Total: 6.4 | Total: 10.3 (9.3–11.3), rural: 12.5, urban: 8.0 | Total:16.2 | Total: 5.3 |
| Biswas et al., 2016 (2010–2011)36 | Rural Chittagong | > 40 | Female | 250 | Yes | No | NA | NA | 20.4 | NA | NA | NA |
| Islam et al., 2013 (2008)26 | Urban Dhaka | ≥ 35 | Male, female | 900 | Yes | No | 11.4 | 11.7 | 10.6 | NA | NA | NA |
| India | ||||||||||||
| Burney et al., 2020 (NR)24c | Mumbai | ≥ 40 | Male, female | 275 males; 165 females | No | Yes | NA | NA | NA | NR | 6.2 | 7.9 |
| Pune | ≥ 40 | Male, female | 501 males; 341 females | No | Yes | NA | NA | NA | NR | 5.8 | 6.7 | |
| Srinagar | ≥ 40 | Male, female | 411 males; 341 females | No | Yes | NA | NA | NA | NR | 17.3 | 15.5 | |
| Mysore | ≥ 40 | Male, female | 256 males; 345 females | No | Yes | NA | NA | NA | NR | 11.3 | 5.5 | |
| Christopher et al., 2020 (2018)28 | Rural Vellore | ≥ 30 | Male, female | 787 | Yes | Yes | 4.1 (2.7–5.5) | 5.7 | 2.9 | 4.6 | 4.2 | 4.9 |
| Johnson et al., 2011 (2007)29 | Rural Tiruvallur | ≥ 30 | Femaled | 900 | Yes | No | NA | NA | 2.4 (1.4–3.5) | NA | NA | NA |
| Koul et al., 2016 (2010–2011)21c | Rural Srinagar | ≥ 40 | Male, female | 757 | Yes | Yes | 19.3 | 23.7 | 14.5 | 16.1 | 17.3 | 14.8 |
| Mukhmohit et al., 2014 (NR)37 | Rural Ambala | ≥ 35 | Female | 1027 | Yes | No | NA | NA | 5.1 | NA | NA | NA |
| Sinha et al., 2017 (2012–2013)38 | Urban Delhi | ≥ 30 | Male, female | 1203 | Yes | No | 10.1 (8.5–11.9) | 12.2 | 7.7 | NA | NA | NA |
| Triest et al., 2019 (NR)22c | Srinagar | ≥ 40 | Male, female | 739 | No | Yes | NA | NA | NA | 16.4 | NR | NR |
| Mumbai | ≥ 40 | Male, female | 440 | No | Yes | NA | NA | NA | 6.8 | NR | NR | |
| Pune | ≥ 40 | Male, female | 843 | No | Yes | NA | NA | NA | 6.2 | NR | NR | |
| Nepal | ||||||||||||
| Adhikari et al., 2020 (2019)30 | Semi-urban Pokhara | ≥ 40 | Male, female | 1508 | Yes | Yes | 8.5 (7.2–10.0) | 10.9 (8.7–13.5) | 6.4 (4.9–8.4) | 5.4 (4.2–6.6) | 7.6 (5.8–9.9) | 3.5 (2.4–5.0) |
| Sri Lanka | ||||||||||||
| Triest et al., 2019 (NR)22c | Colombo | ≥ 40 | Male, female | 1020 | No | Yes | NA | NA | NA | 7.3 | NR | NR |
| Studies not included in meta-analysis | ||||||||||||
| Bangladesh | ||||||||||||
| Grigsby et al., 2016 (2011–2012)27e | Rural Matlab | ≥ 40 | Male, female | 1846 | No | Yes | NA | NA | NA | 15.0 | NR | NR |
| Urban Dhaka | ≥ 40 | Male, female | 1878 | No | Yes | NA | NA | NA | 10.0 | NR | NR | |
| India | ||||||||||||
| Burney et al., 2014c: Mumbai (2006–2008); Pune (2008–2009); Srinagar (2010–2011)20e | Mumbai | ≥ 40 | Male, female | 440 | No | Yes | NA | NA | NA | NR | 6.0 | 7.6 |
| Pune | 843 | No | Yes | NA | NA | NA | NR | 5.7 | 6.8 | |||
| Srinagar | 763 | No | Yes | NA | NA | NA | NR | 17.3 | 14.8 | |||
| Townend et al., 2017 (NR)23c,e | Kashmir | ≥ 40 | Male, female | 738 | No | Yes | NA | NA | NA | 16.0 | NR | NR |
| Mahesh et al., 2018 (2014–2016)39f | Rural Mysuru | > 30 | Male, female | Phase 1: 8457, phase 2: 1085 | Yes | No | 0.92 | 1.0 | 0.6 | NA | NA | NA |
| Sri Lanka | ||||||||||||
| Townend et al., 2017 (NR) 23c,e | NR | ≥ 40 | Male, female | 1035 | No | Yes | NA | NA | NA | 8.0 | NR | NR |
| COPD without confirmation with post-bronchodilation spirometry | ||||||||||||
| India | ||||||||||||
| Arora et al., 2018 (2015)40 | Urban Delhi | 18–59 | Female | 299g | Yes | No | NA | NA | 5.0 | NA | NA | NA |
| Chaturvedi et al., 2015 (2014–2015)41 | Rural Muzaffarnagar | ≥ 30 | Male, female | 908 | Yes | No | 7.8 | NR | NR | NA | NA | NA |
| Mukherjee et al., 2014 (NR)42 | Rural West Bengal | 23–43 | Femaleh,i | 1119 | Yes | No | NA | NA | 2.8 | NA | NA | NA |
| Panigrahi et al., 2018 (NR)43 | Rural Khordha | 18–49 | Femaled,i | 1120 | Yes | No | NA | NA | All: 22.4 Exposed to biomass fuel smoke: 31.0 Exposed to mixed fuel smoke: 22.8 Not exposed: 7.8 |
NA | NA | NA |
| Parasuramalu et al., 2014 (2008)44 | Rural Bengaluru | > 35 | Male, female | 1400 | Yes | No | 4.4 | NR | NR | NA | NA | NA |
| Pathak et al., 2019 (NR)45 | Rural western Uttar Pradesh | > 18 | Female | 310 | Yes | No | NA | NA | 17.42 | NA | NA | NA |
| Shanmugananth et al., 2019 (NR)46 | Chennai, Surendranagar and Hisar | > 30 | Male, female | 1000 | Yes | No | 9.0 | NR | NR | NA | NA | NA |
| Sharma et al., 2016 (2016)47 | Rural Jammu | > 20 | Male, female | 2018 | Peak expiratory flow rate | No | 4.2 | 5.4 | 2.8 | NA | NA | NA |
| Sharma et al., 2019 (2012–2013)48 | Urban Ludhiana | > 20 | Male, female | 8128 | Yes | No | 3.2/1000 | NR | NR | NA | NA | NA |
| Nepal | ||||||||||||
| Dhimal et al., 2019 (2016–2018)49 | Nationwide | ≥ 20 | Male, female | 13 200 | Yes | No | 11.7 (10.5–12.9) | 12.6 (11.2–14.1) | 11.0 (9.6–12.4) | NA | NA | NA |
| Kurmi et al., 2013 (2006–2007)50 | Rural and urban Kathmandu | ≥ 16 | Male, female | 1392 | Yes | Yes | NR | NR | NR | Exposed to biomass fuel smoke: 8.1 Not exposed: 3.6 |
Exposed to biomass fuel smoke: 7.4 Not exposed: 3.3 |
Exposed to biomass fuel smoke: 10.8 Not exposed: 3.8 |
CI: confidence interval; COPD: chronic obstructive pulmonary disease; GOLD: Global Initiative for Chronic Obstructive Lung Disease; NA: not applicable; NR: not reported.
a Fixed criteria defined as a post-bronchodilator ratio of forced expiratory volume in one second (FEV1) to the forced vital capacity (FVC), FEV1/FVC < 0.70.
b The lower limit of normal criteria defined as a post-bronchodilator FEV1/FVC below the lower limit of normal, i.e. the lower fifth centile of values from a reference population.
c Data from the Burden of Obstructive Lung Disease (BOLD) Study.
d Non-smokers.
e Because more recent or detailed data available from another study.
f Only 15% of study participants underwent spirometry.
g 500 women consented and acceptable spirometry data for 299 women were used for analysis.
h Premenopausal women.
i Involved with cooking.
Prevalence of COPD
Overall, 15 studies reported the prevalence of COPD using the standard diagnostic criteria and post-bronchodilation spirometry (Table 1). Four studies reported data from Bangladesh, eight studies reported data from India, two from both India and Sri Lanka and one from Nepal.20–30,36–39 Four studies with data from India20,22–24 and two from Sri Lanka22,23 reported the overall prevalence of COPD using the lower limit of normal criteria, six studies used the fixed criteria26,29,36–39 and four studies (two from India and one each from Bangladesh and Nepal)21,25,28,30 used a combination of both the fixed and lower limit of normal criteria (Table 1). Three studies, two from India29,37 and one from Bangladesh,36 reported the prevalence using the fixed criteria among women only (Table 1).
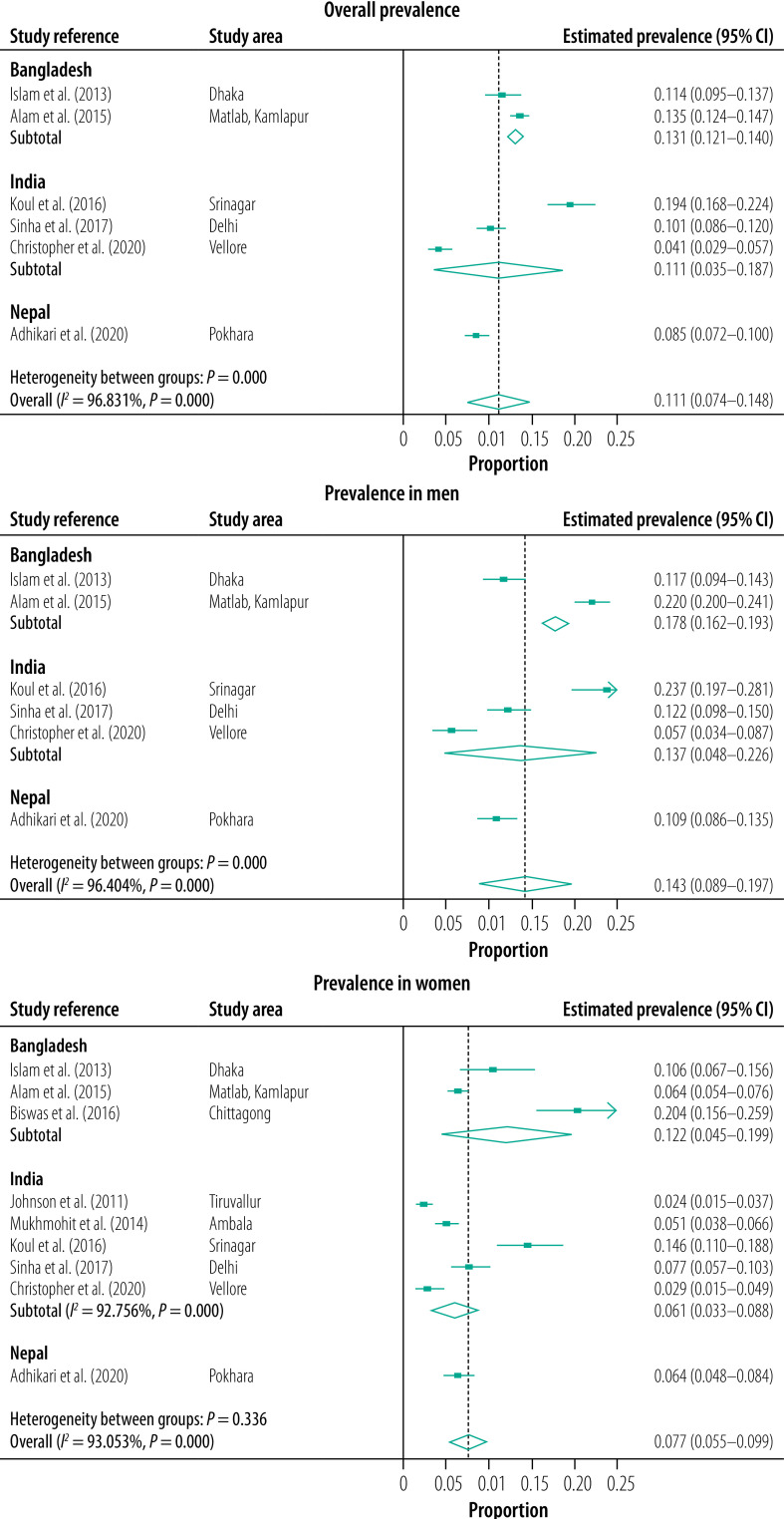
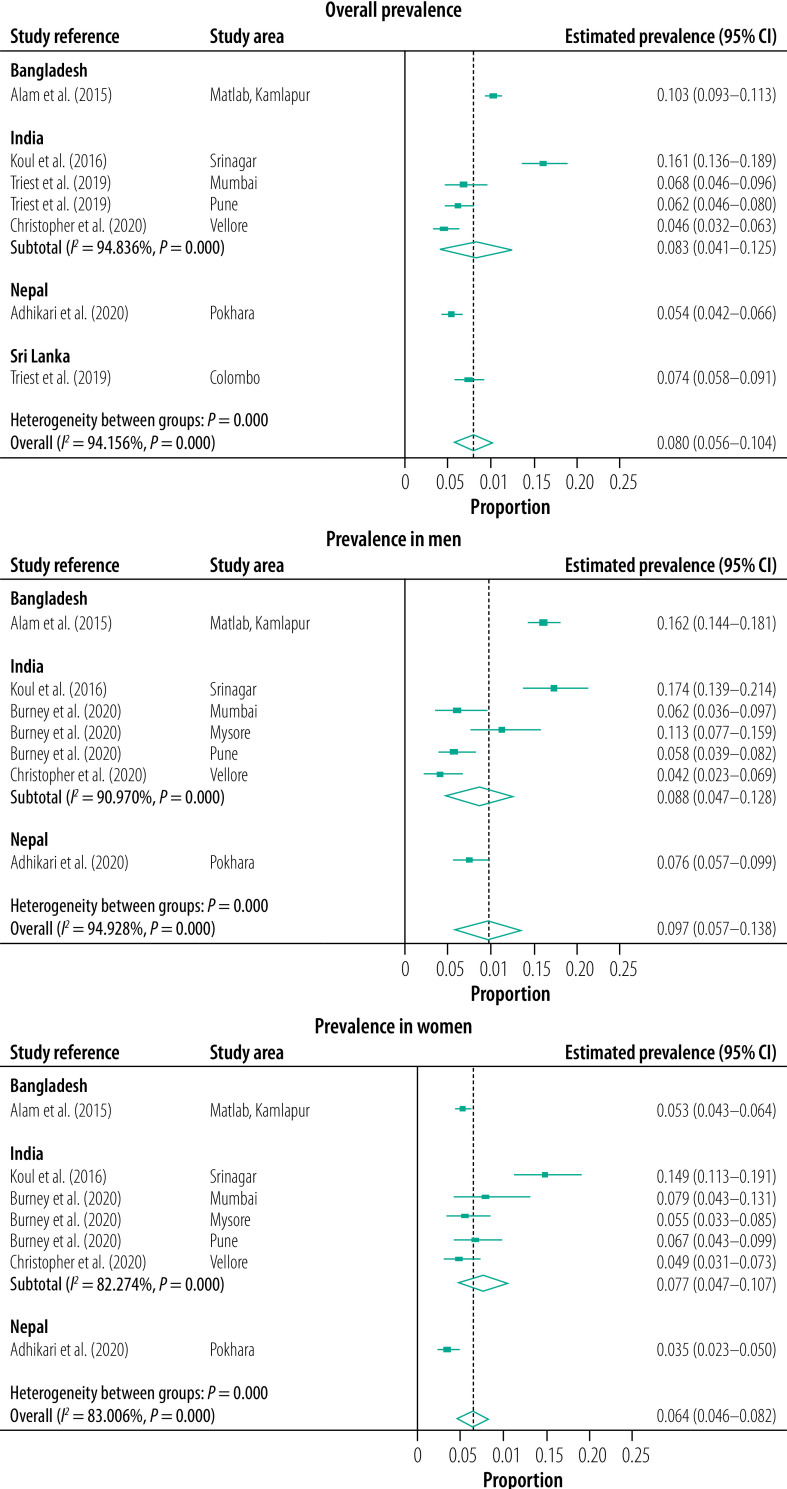
The estimated pooled prevalence of COPD in the South Asian countries included in our study was 11.1% (95% CI: 7.4–14.8%) using the fixed criteria21,25,26,28,30,38 (Fig. 2) and 8.0% (95% CI: 5.6–10.4%) using the lower limit of normal criteria (Fig. 3).21,22,25,28,30 The study outcomes had considerable and statistically significant heterogeneity across South Asia (fixed criteria I2: 96.83%, P < 0.001; lower limit of normal criteria I2: 94.16%, P < 0.01), and within India with lower limit of normal criteria I2: 94.84%, P < 0.001.
Fig. 2.
Estimated pooled prevalence of COPD overall and by sex, assessed by the GOLD fixed criteria, Bangladesh, India, Nepal, 2021
COPD: chronic obstructive pulmonary disease; GOLD: Global Initiative for Chronic Obstructive Lung Disease.
Notes: Dashed vertical line represents the pooled effect estimate. For subgroups with three or less studies, statistical analyses were impossible. GOLD fixed criteria is a post-bronchodilator ratio of forced expiratory volume in one second (FEV1) to the forced vital capacity (FVC), FEV1/FVC < 0.70.
Fig. 3.
Estimated pooled prevalence of COPD overall and by sex, assessed by the lower limit of normal criteria, Bangladesh, India, Nepal, Sri Lanka, 2021
COPD: chronic obstructive pulmonary disease.
Notes: Dashed vertical line represents the pooled effect estimate. The lower limit of normal criteria is defined as a post-bronchodilator FEV1/FVC below the lower limit of normal, i.e. the lower fifth centile of values from a reference population.
The estimated pooled prevalence of COPD was highest in Bangladesh (13.1%; 95% CI: 12.1–14.0%) followed by India (11.1%; 95% CI: 3.5–18.7) and Nepal (8.5%; 95% CI: 7.2–10.0%; Fig. 2).
In India, the prevalence of COPD varied, with rural Srinagar in northern India having the highest overall prevalence reported in adults aged 40 years or older (19.3% according to the fixed criteria; 16.1% according to the lower limit of normal criteria)21 and the lowest prevalence reported in south India (4.1% according to the fixed criteria; Table 1).28 A higher prevalence of COPD was also reported in Bangladeshi men from rural Matlab and suburban Kamlapur (22.0% based on fixed criteria; 16.2% based on lower limit of normal criteria; Table 1).25
COPD prevalence was found to be significantly higher in men than women in four studies, in Delhi38 and Srinagar21 according to the fixed criteria, and in Nepal30 and rural Matlab, Bangladesh25 according to both spirometry criteria. Only one study in Bangladesh compared rural versus non-rural samples and reported a significantly higher prevalence of COPD in rural dwellers (17.0% by fixed criteria and 12.5% by lower limit of normal criteria) than suburban dwellers (9.9% by fixed criteria and 8.0% by lower limit of normal criteria).25
Prevalence of chronic bronchitis
Thirteen studies reported the prevalence of chronic bronchitis and overall prevalence ranged from 2.9% to 9.1% (Table 2).31–35,43,47,51–56 Studies varied according to residence (three both in rural and urban areas,32,33,51 nine in rural areas only,31,34,35,43,47,52–55 and one was unspecified)56 and sex, with eight studies conducted among both men and women,32,33,47,51,52,54–56 four studies restricted to women31,34,43,53 and one study was restricted to men.35 In two multicentre studies in India, the overall prevalence of chronic bronchitis was 4.1% (5.0% in men and 3.2% in women) in 200632 and 3.5% in 2012.33 The prevalence of chronic bronchitis was higher in men32,47,51,54 and rural dwellers (Table 2).32,51
Table 2. Sample characteristics and study outcomes of research reporting the prevalence of chronic bronchitis, India, Pakistan, 2021.
| Country and study (year data collected) | Study area | Age, years | Sex | Sample size | Prevalence of chronic bronchitis, % |
||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Male | Female | Rural | Urban | |||||
| Chronic bronchitis based on standardized criteria included in qualitative summary and/or meta-analysis | |||||||||
| India | |||||||||
| Dutta et al., 2015 (2010–2012)31 | Rural Wardha (Maharashtra) | ≥ 20 | Female | 1650 | NA | NA | 2.7 | NA | NA |
| Goel et al., 2007 (2001–2002)51 | Urban and rural Shimla (Himachal Pradesh) | > 18 | Overall, male, female | 1330 | 9.1 | 11.1 | 6.1 | 13.5 | 4.7 |
| Jindal et al., 2006 (NR)32 | Urban and rural Chandigarh, Delhi, Kanpur (Uttar Pradesh), Bengaluru (Karnataka) | ≥ 35 | Overall, male, female | 35 295 | 4.1 | 5.0 | 3.2 | 4.4 | 3.7 (semi-urban: 6.5) |
| Jindal et al., 2012 (2007–2009)33 | Urban and rural: Shimla, Chandigarh, Bikaner, Ahmedabad, Nagpur, Mumbai, Mysore, Trivandrum, Chennai, Secunderabad, Behrampur, Kolkata, Guwahati | ≥ 35 | Overall | 169 575 | 3.5 | NR | NR | NR | NR |
| Mahesh et al., 2013 (2006–2009)34 | Rural Mysuru (Karnataka) | > 30 | Female | 3953 | NA | NA | 3.4 | NA | NA |
| Mahesh et al., 2014 (2006–2009)35 | Rural Mysuru | ≥ 30 | Male | 2322 | NA | General: 1.7; Smokers: 2.1; Non-smokers: 1.1 | NA | NA | NA |
| Rural Nanjangud (Karnataka) | ≥ 30 | Male | 2182 | NA | General: 21.6; Smokers: 44.8; Non-smokers: 2.0 | NA | NA | NA | |
| Panigrahi et al., 2018 (NR)43 | Rural Khorda (Odisha) | 18–49 | Femalea,b | 1120 | NA | NA | 7.3 | NA | NA |
| Sharma et al., 2016 (2012–2013)47 | Rural Jammu | > 20 | Overall, male, female | 2018 | 3.4 | 4.9 | 1.7 | NA | NA |
| Spon et al., 2014 (NR)52 | Rural Kashmir | > 18 | Overall, male, female | 912 | 5.4 | 8.0 | 3.5 | NA | NA |
| Sukhsohale et al., 2013 (NR)53 | Rural Nagpur (Maharashtra) | ≥ 15 | Femalea,b,c | 760 | NA | NA | 12.5 | NA | NA |
| Viswanathan et al., 2018 (2014–2015)54 | Rural Kollam (Kerala) | > 15 | Overall, male, female | 12 556 | 6.2 (95% CI: 5.8–6.6) | 6.7 | 5.7 | NA | NA |
| Pakistan | |||||||||
| Akhtar et al., 2007 (2003–2004)55 | Rural Peshawar | ≥ 10 | Overall | 2557 | 5.2 | NR | NR | NA | NA |
| Tageldin et al., 2012 (2010–2011)56 | Not given | ≥ 40 | Overall | 3654 | 2.9 | NR | NR | NR | NR |
| Additional studies on the prevalence of chronic bronchitis | |||||||||
| India | |||||||||
| Akhtar et al., 1999 (NR)57 | Urban Kashmir | > 30 | Overall, male, female | 1140 | 5.7 | 6.7 | 4.5 | NA | NA |
| Arora et al., 2018 (2015)40 | Urban Delhi | 18–59 | Female | 500 | NA | NA | NR | NA | NA |
| Chhabra et al., 2001 (NR)58 | Urban Delhi | > 18 | Male, femalea | 4171 | NA | 3.1d,e | 2.1d,e | NA | NA |
| NA | 0.8d,f | 0.7d,f | NA | NA | |||||
| NA | 1.8d,g | 0.3d,g | NA | NA | |||||
| NA | 3.2e,h | 5.9e,h | NA | NA | |||||
| NA | 4.6f,h | 1.1f,h | NA | NA | |||||
| NA | 0.5g,h | 1.7g,h | NA | NA | |||||
| Jindal, 1993 (NR)59 | Urban and rural Chandigarh | NR | Overall, male, female | 1475 | 2.4 | 2.1 | 1.6 | NR | NR |
| Mahesh et al., 2009 (NR)60 | Rural Mysuru (Karnataka) | > 40 | Overall, male, female | 900 | 7.1i | 11.1i | 4.5i | NA | NA |
| Pandita et al., 2017 (NR)61 | Urban Dehradun (Uttarakhand) | ≥ 60 | Male, female | 520 | 25.0j | NR | NR | NA | NA |
| Qureshi, 1994 (NR)62 | Rural Gandarbal (Kashmir) | > 15 | Male, female | 560 | 7.7 | NR | NR | NA | NA |
| Shanmugananth et al., 2019 (NR)46 | Chennai (Tamil Nadu), Surendranagar (Gujarat), Hisar (Haryana) | > 30 | Male, female | 1000 | 4.1k | NR | NR | NA | NA |
CI: confidence interval; COPD: chronic obstructive pulmonary disease; NA: not applicable; NR: not reported.
a Non-smokers.
b Involved in cooking.
c Not pregnant.
d Low pollution zone.
e Low socioeconomic status.
f Middle socioeconomic status.
g High socioeconomic status.
h High pollution zone.
i Validation study followed by pilot study.
j Conducted among elderly and chronic bronchitis/COPD definitions not mentioned.
k Purposive sampling used.
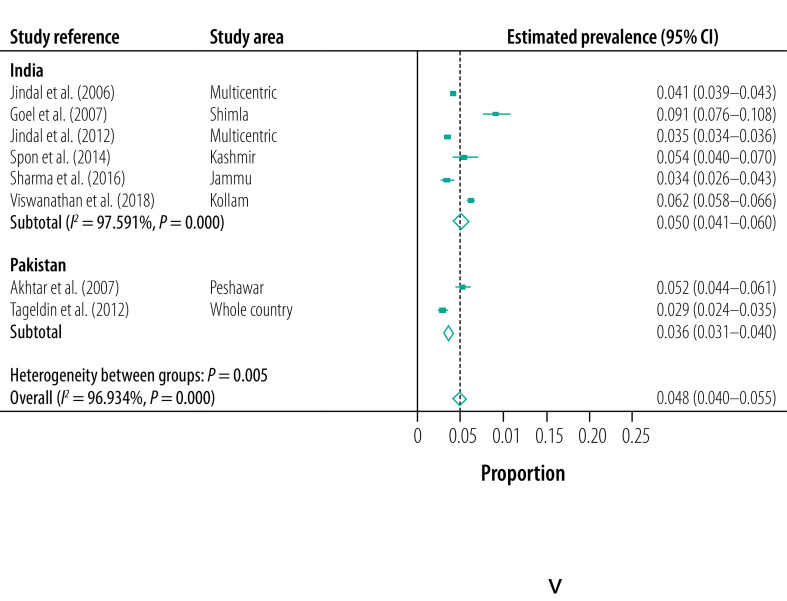
Eight studies (six from India and two from Pakistan), which diagnosed chronic bronchitis using standardized criteria and provided the overall prevalence of chronic bronchitis, were used in the meta-analysis (Table 2).32,33,47,51,52,54–56 The overall estimated pooled prevalence of chronic bronchitis was 4.8% (95% CI: 4.0–5.5%): 5.0% (95% CI: 4.1–6.0%) in India and 3.6% (95% CI: 3.1–4.0) in Pakistan (Fig. 4). Statistical heterogeneity was very high and statistically significant for all the studies included (I2: 96.93%; P < 0.001) and for studies undertaken in India (I2: 97.59%; P < 0.01). There was an insufficient number of relevant studies to calculate statistical heterogeneity in Pakistan.
Fig. 4.
Estimated pooled prevalence of chronic bronchitis, India, Pakistan, 2021
Notes: Dashed vertical line represents the pooled effect estimate. For subgroups with three or less studies, statistical analyses were impossible.
Common risk factors associated with COPD and chronic bronchitis were age, smoking, lower socioeconomic status, exposure to environmental tobacco smoke, exposure to biomass fuel, exposure to dust, history of tuberculosis and history of allergy and/or asthma.20,21,24–26,28,30,32,33,36–38,51,55,56
Discussion
Here we report on the prevalence of COPD and chronic bronchitis in South Asia. A substantial regional variation was seen in the prevalence of COPD and chronic bronchitis, with higher prevalence estimates reported by studies in north India21 and Bangladesh.25 While tobacco smoking and indoor air pollution were the most common risk factors assessed for their association with COPD, no population-based studies were found in the area that determined the association of COPD with other important risk factors such as ambient air pollution and occupational hazards.
Within-country and between-country variations in the prevalence of COPD have been reported previously due to differences in the prevalence of risk factors, especially tobacco smoking.63,64 The higher prevalence of COPD in north India (Kashmir) was mainly ascribed to tobacco smoking using traditional hookahs and higher exposure to indoor air pollution.21 The high prevalence of COPD in Bangladesh was also attributed to the high prevalence of tobacco smoking, particularly among men.25 Traditional norms of offering smoking products, low awareness of the harmful effects of smoking among low-income groups and people living in rural areas, suboptimal implementation of tobacco control measures and limited access to cessation services may account for high prevalence estimates in these areas.65 Thus, these relevant regional, sociocultural and economic factors need to be considered while planning strategies to reduce smoking, decrease the COPD burden and improve population lung health.65
Indoor air pollution is one of the main causes of COPD, especially in South Asian women.66 Although many governments have scaled up access to cleaner cooking fuels, the reach and change to cleaner fuels is suboptimal. Furthermore, the effect of this transition on the COPD burden, especially among women in South Asia, remains unclear and warrants further evaluation.67 More specific approaches are required to understand the role of various indoor air pollutants, such as particulate matter, nitrogen dioxide, carbon monoxide, sulfur oxides, polycyclic organic matter and formaldehyde which are produced by combustion of biomass fuels, in the development of COPD and chronic bronchitis.68,69
Ambient air pollution is one of the main risk factors for COPD mortality and disability-adjusted life years lost, with the highest burden reported in South Asia.8,70 In 2015, Bangladesh, India and Nepal had the highest burden of particulate matter 2.5 (PM2.5) and Bangladesh, India and Pakistan had the highest increase in the ozone levels and the highest mortality due to ambient PM2.5 observed.71 Increased air pollution aggravates COPD symptoms, known as COPD exacerbations, and increases hospitalizations and mortality.72 Although a few studies from large cities in India have shown the short-term effect of increased ambient air pollution and increased hospital visits due to respiratory problems,73,74 the long-term effects of extended exposure to ambient air pollution and its effects on lung function, morbidity and mortality need to be studied.69,75 Educating health-care providers and patients about the adverse health effects of ambient air pollution and simple measures that can be taken to reduce exposure, such as avoiding going out during periods of high pollution and wearing masks outdoors, is needed.76
Few studies have assessed the association between COPD and respiratory infections in South Asia, such as lower respiratory tract infections and tuberculosis.21,24,30 Given that many people in the area have been affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),77 the long-term effects of this virus on the burden of COPD and other chronic respiratory diseases need to be assessed. Poor lung function associated with SARS-COV-2 and respiratory infections also requires monitoring to assess their long-term effects and the attributable risk for the development of COPD.
We also found that none of the included studies had assessed the presence of chronic bronchitis with airway obstruction in COPD. Chronic bronchitis in people with airway obstruction is associated with more severe disease, poor general health status, greater limitations on physical activity and higher mortality.78 Therefore, COPD should be assessed together with the presence of chronic bronchitis in both clinical and research settings.
In our study, we tried to assess the current prevalence of COPD in South Asian countries. The strengths of our study include selection of recent studies, a comprehensive literature search using explicit definitions for COPD and chronic bronchitis according to international guidelines and a comprehensive quality assessment of studies selected for the review.
Our study has some limitations. First, because few studies in the area were available, our pooled prevalence estimates of COPD cannot be generalized to all South Asian countries. Only one study each from Bangladesh and India assessed the prevalence of COPD in urban areas and the only study from Nepal assessed the prevalence in a semi-urban area. Moreover, due to within-country differences in the burden of risk factors, especially in India,79 the generalizability of the prevalence estimates may be limited to the states or areas where the studies were conducted. There were too few studies to calculate statistical heterogeneity within Bangladesh, Nepal and Sri Lanka, and to identify predictors of heterogeneity using meta-regression. However, visual inspection of the forest plots suggests variations in the prevalence of COPD by country, geographical location and sex. Second, most studies included in the systematic review had collected data more than 5 years ago. In recent times, the burden of risk factors for COPD and chronic bronchitis has changed considerably. For example, smoking rates decreased from 14.0% to 10.7% between 2009–2010 and 2016–2017 in India,80 while the ambient air pollution has increased in South Asia.81 These limitations highlight the lack of research to determine the accurate burden of COPD in the area and the need for large population-based studies with rigorous methods to generate accurate prevalence estimates in various population subgroups as well as attributable risks of common risk factors, including outdoor air pollution, occupational exposure and infections. Such data are important to inform the development and implementation of context-relevant policies and programmes for reducing the increasing burden of COPD and its risk factors.
Estimation of the prevalence of COPD is challenging for researchers in low- and middle-income countries. As a result, there is likely a large burden of undetected COPD. COPD is a complex disease with varied presentations and new phenotypes being identified.82,83 Conducting post-bronchodilation spirometry, which is required for the confirmation of a diagnosis of COPD, is resource intensive and requires a high level of quality control.1,8 Different spirometry criteria for diagnosing COPD result in within-study differences in the prevalence estimates, which further complicates the interpretation of the disease burden, especially for policy-makers who need to allocate resources for COPD control. This problem emphasizes the need for standardized COPD diagnosing criteria that can be implemented in low- and middle-income countries.
Some studies have suggested screening strategies for early detection of COPD.84,85 However, COPD screening needs further evaluation and feasibility studies and should be supported by strengthening of public health infrastructure for the confirmation of diagnosis, early initiation of pharmacological and non-pharmacological treatment including pulmonary rehabilitation.85
In conclusion, given the paucity of studies on the current burden of COPD and its risk factors in most South Asian countries, future research in these countries should ensure that standardized diagnostic criteria are used to examine the contribution of exposure to context-relevant risk factors to inform COPD prevention and control policies.
Funding:
This study was supported by a PhD scholarship grant under the Deakin India Research Initiative to PJ. PJ was also partly supported by the Public Health Foundation of India and by a VECD Global Health Fellowship, funded by the Fogarty International Centre (FIC) of the NIH (3D43TW009337-09S3).
Competing interests:
None declared.
References
- 1.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2021 report [internet]. Global Initiative for Chronic Obstructive Lung Disease; 2021 Available from: https://goldcopd.org/ [cited 2021 Aug 8].
- 2.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. ; BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007. Sep 1;370(9589):741–50. 10.1016/S0140-6736(07)61377-4 [DOI] [PubMed] [Google Scholar]
- 3.Chronic obstructive pulmonary disease (COPD) in Vietnam [internet]. Geneva: World Health Organization; 2021. Available from: https://www.who.int/vietnam/health-topics/chronic-obstructive-pulmonary-disease-copd [cited 2021 Oct 20].
- 4.Chronic obstructive pulmonary disease (COPD). Geneva: World Health Organization; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) [cited 2021 Oct 20].
- 5.Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, et al. ; Global Health Epidemiology Reference Group (GHERG). Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015. Dec;5(2):020415. 10.7189/jogh.05.020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.South Asia [internet]. Washington, DC: World Bank Group; 2021. Available from: https://www.worldbank.org/en/region/sar [cited 2021 Oct 20].
- 7.Siegel KR, Patel SA, Ali MK. Non-communicable diseases in South Asia: contemporary perspectives. Br Med Bull. 2014. Sep;111(1):31–44. 10.1093/bmb/ldu018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soriano JB, Kendrick PJ, Paulson KR, Gupta V, Abrams EM, Adedoyin RA, et al. ; GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020. Jun;8(6):585–96. 10.1016/S2213-2600(20)30105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciapponi A, Alison L, Agustina M, Demián G, Silvana C, Edgardo S. The epidemiology and burden of COPD in Latin America and the Caribbean: systematic review and meta-analysis. COPD. 2014. Jun;11(3):339–50. 10.3109/15412555.2013.836479 [DOI] [PubMed] [Google Scholar]
- 10.Finney LJ, Feary JR, Leonardi-Bee J, Gordon SB, Mortimer K. Chronic obstructive pulmonary disease in sub-Saharan Africa: a systematic review. Int J Tuberc Lung Dis. 2013. May;17(5):583–9. 10.5588/ijtld.12.0619 [DOI] [PubMed] [Google Scholar]
- 11.McKay AJ, Mahesh PA, Fordham JZ, Majeed A. Prevalence of COPD in India: a systematic review. Prim Care Respir J. 2012. Sep;21(3):313–21. 10.4104/pcrj.2012.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006. Jan;27(1):188–207. 10.1183/09031936.06.00024505 [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009. Jul 21;339 jul21 1:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamed Hoesein FAA, Zanen P, Lammers J-WJ. Lower limit of normal or FEV1/FVC < 0.70 in diagnosing COPD: an evidence-based review. Respir Med. 2011. Jun;105(6):907–15. 10.1016/j.rmed.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 15.Definition and classification of chronic bronchitis for clinical and epidemiological purposes. A report to the Medical Research Council by their Committee on the Aetiology of Chronic Bronchitis. Lancet. 1965. Apr 10;1(7389):775–9. [PubMed] [Google Scholar]
- 16.Jarhyan P, Hutchinson A, Khaw D, Dorairaj P, Sailesh M. Supplementary material. London: figshare; 2021. 10.6084/m9.figshare.16945234.v1 10.6084/m9.figshare.16945234.v1 [DOI]
- 17.Covidence systematic review software. Melbourne: Veritas Health Innovation; 2021. Available from: www.covidence.org [cited 2021 Nov 4].
- 18.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014. Aug 13;3(3):123–8. 10.15171/ijhpm.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014. Nov 10;72(1):39. 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burney P, Jithoo A, Kato B, Janson C, Mannino D, Niżankowska-Mogilnicka E, et al. ; Burden of Obstructive Lung Disease (BOLD) Study. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty–a BOLD analysis. Thorax. 2014. May;69(5):465–73. 10.1136/thoraxjnl-2013-204460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koul PA, Hakim NA, Malik SA, Khan UH, Patel J, Gnatiuc L, et al. Prevalence of chronic airflow limitation in Kashmir, North India: results from the BOLD study. Int J Tuberc Lung Dis. 2016. Oct;20(10):1399–404. 10.5588/ijtld.15.0968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Triest FJJ, Studnicka M, Franssen FME, Vollmer WM, Lamprecht B, Wouters EFM, et al. Airflow obstruction and cardio-metabolic comorbidities. COPD. 2019. Apr;16(2):109–17. 10.1080/15412555.2019.1614550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townend J, Minelli C, Mortimer K, Obaseki DO, Al Ghobain M, Cherkaski H, et al. The association between chronic airflow obstruction and poverty in 12 sites of the multinational BOLD study. Eur Respir J. 2017. Jun 1;49(6):1601880. 10.1183/13993003.01880-2016 [DOI] [PubMed] [Google Scholar]
- 24.Burney P, Patel J, Minelli C, Gnatiuc L, Amaral AFS, Kocabaş A, et al. Prevalence and population-attributable risk for chronic airflow obstruction in a large multinational study. Am J Respir Crit Care Med. 2020. Nov 10;203(11):1353–65. 10.1164/rccm.202005-1990OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alam DS, Chowdhury MA, Siddiquee AT, Ahmed S, Clemens JD. Prevalence and determinants of chronic obstructive pulmonary disease (COPD) in Bangladesh. COPD. 2015;12(6):658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Islam MS, Hossain MM, Pasha MM, Azad AK, Murshed KM. Prevalence and risk factors of chronic obstructive pulmonary disease (COPD) in Dhaka city population. Mymensingh Med J. 2013. Jul;22(3):547–51. [PubMed] [Google Scholar]
- 27.Grigsby M, Siddharthan T, Chowdhury MA, Siddiquee A, Rubinstein A, Sobrino E, et al. Socioeconomic status and COPD among low- and middle-income countries. Int J Chron Obstruct Pulmon Dis. 2016. Oct 5;11:2497–507. 10.2147/COPD.S111145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christopher DJ, Oommen AM, George K, Shankar D, Agrawal A, Thangakunam B. Prevalence of airflow obstruction as measured by spirometry, in rural southern Indian adults. COPD. 2020. Apr;17(2):128–35. 10.1080/15412555.2020.1723074 [DOI] [PubMed] [Google Scholar]
- 29.Johnson P, Balakrishnan K, Ramaswamy P, Ghosh S, Sadhasivam M, Abirami O, et al. Prevalence of chronic obstructive pulmonary disease in rural women of Tamilnadu: implications for refining disease burden assessments attributable to household biomass combustion. Glob Health Action. 2011;4(1):7226. 10.3402/gha.v4i0.7226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adhikari TB, Acharya P, Högman M, Neupane D, Karki A, Drews A, et al. Prevalence of chronic obstructive pulmonary disease and its associated factors in Nepal: findings from a community-based household survey. Int J Chron Obstruct Pulmon Dis. 2020. Sep 29;15:2319–31. 10.2147/COPD.S268110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutta S, Deshmukh PR. Prevalence and determinants of self-reported chronic bronchitis among women in rural central India. Med J Armed Forces India. 2015. Jan;71(1):48–52. 10.1016/j.mjafi.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jindal SK, Aggarwal AN, Chaudhry K, Chhabra SK, D’Souza GA, Gupta D, et al. ; Asthma Epidemiology Study Group. A multicentric study on epidemiology of chronic obstructive pulmonary disease and its relationship with tobacco smoking and environmental tobacco smoke exposure. Indian J Chest Dis Allied Sci. 2006. Jan-Mar;48(1):23–9. [PubMed] [Google Scholar]
- 33.Jindal SK, Aggarwal AN, Gupta D, Agarwal R, Kumar R, Kaur T, et al. Indian study on epidemiology of asthma, respiratory symptoms and chronic bronchitis in adults (INSEARCH). Int J Tuberc Lung Dis. 2012. Sep;16(9):1270–7. 10.5588/ijtld.12.0005 [DOI] [PubMed] [Google Scholar]
- 34.Mahesh PA, Jayaraj BS, Prabhakar AK, Chaya SK, Vijaysimha R. Identification of a threshold for biomass exposure index for chronic bronchitis in rural women of Mysore district, Karnataka, India. Indian J Med Res. 2013. Jan;137(1):87–94. [PMC free article] [PubMed] [Google Scholar]
- 35.Mahesh PA, Jayaraj BS, Chaya SK, Lokesh KS, McKay AJ, Prabhakar AK, et al. Variation in the prevalence of chronic bronchitis among smokers: a cross-sectional study. Int J Tuberc Lung Dis. 2014. Jul;18(7):862–9. 10.5588/ijtld.13.0048 [DOI] [PubMed] [Google Scholar]
- 36.Biswas RS, Paul S, Rahaman MR, Sayeed MA, Hoque MG, Hossain MA, et al. Indoor biomass fuel smoke exposure as a risk factor for chronic obstructive pulmonary disease (COPD) for women of rural Bangladesh. Chattagram Maa-O-Shishu Hospital Med Coll J. 2016. Jul 17;15(1):8–11. 10.3329/cmoshmcj.v15i1.28753 [DOI] [Google Scholar]
- 37.Mukhmohit S, Bhardwaj A, Saini S, Mukherjee AK, Kannan R. COPD–prevalence and risk study among females of rural area, district Ambala, Haryana, India. J Evol Med Dent Sci. 2014;3(16):4183–92. [Google Scholar]
- 38.Sinha B, Vibha, Singla R, Chowdhury R. An epidemiological profile of chronic obstructive pulmonary disease: a community-based study in Delhi. J Postgrad Med. 2017. Jan–Mar;63(1):29–35. 10.4103/0022-3859.194200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahesh PA, Lokesh KS, Madhivanan P, Chaya SK, Jayaraj BS, Ganguly K, et al. The Mysuru studies of determinants of health in rural adults (MUDHRA), India. Epidemiol Health. 2018. Jun 23;40:e2018027. 10.4178/epih.e2018027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arora S, Rasania SK, Bachani D, Gandhi A, Chhabra SK. Air pollution and environmental risk factors for altered lung function among adult women of an urban slum area of Delhi: a prevalence study. Lung India. 2018. May-Jun;35(3):193–8. 10.4103/lungindia.lungindia_263_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chaturvedi R, Muzammil K, Singh N, Davey S, Singh JV. Prevalence of COPD in rural population, Muzaffarnagar. Indian J Community Health. 2015;27(4):467–71. [Google Scholar]
- 42.Mukherjee S, Roychoudhury S, Siddique S, Banerjee M, Bhattacharya P, Lahiri T, et al. Respiratory symptoms, lung function decrement and chronic obstructive pulmonary disease in pre-menopausal Indian women exposed to biomass smoke. Inhal Toxicol. 2014. Dec;26(14):866–72. 10.3109/08958378.2014.965560 [DOI] [PubMed] [Google Scholar]
- 43.Panigrahi A, Padhi BK. Chronic bronchitis and airflow obstruction is associated with household cooking fuel use among never-smoking women: a community-based cross-sectional study in Odisha, India. BMC Public Health. 2018. Jul 27;18(1):924. 10.1186/s12889-018-5846-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parasuramalu BG, Huliraj N, Prashanth Kumar SP, Gangaboraiah, Ramesh Masthi NR, Srinivasa Babu CR. Prevalence of chronic obstructive pulmonary disease and its association with tobacco smoking and environmental tobacco smoke exposure among rural population. Indian J Public Health. 2014. Jan-Mar;58(1):45–9. 10.4103/0019-557X.128166 [DOI] [PubMed] [Google Scholar]
- 45.Pathak U, Kumar R, Suri TM, Suri JC, Gupta NC, Pathak S. Impact of biomass fuel exposure from traditional stoves on lung functions in adult women of a rural Indian village. Lung India. 2019. Sep-Oct;36(5):376–83. 10.4103/lungindia.lungindia_477_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shanmugananth E, Singh S, Ahlawat R, Nambi G, Sperjan G, Abraham M, et al. Prevalence of chronic obstructive pulmonary disease (COPD) among Indian population. Res J Pharm Technol. 2019;12(11):5285–9. 10.5958/0974-360X.2019.00915.6 [DOI] [Google Scholar]
- 47.Sharma V, Gupta RK, Jamwal DS, Raina SK, Langer B, Kumari R. Prevalence of chronic respiratory disorders in a rural area of North West India: a population-based study. J Family Med Prim Care. 2016. Apr-Jun;5(2):416–9. 10.4103/2249-4863.192342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma AK, Kalra OP, Saini NK, Kelkar H. Pilot study of chronic obstructive pulmonary disease in an industrial town in India. J Health Pollut. 2019. March 7;9(21):190304. 10.5696/2156-9614-9.21.190304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dhimal M, Karki KB, Sharma SK, Aryal KK, Shrestha N, Poudyal A, et al. Prevalence of selected chronic non-communicable diseases in Nepal. J Nepal Health Res Counc. 2019. November 14;17(3):394–401. 10.33314/jnhrc.v17i3.2327 [DOI] [PubMed] [Google Scholar]
- 50.Kurmi OP, Devereux GS, Smith WC, Semple S, Steiner MF, Simkhada P, et al. Reduced lung function due to biomass smoke exposure in young adults in rural Nepal. Eur Respir J. 2013. Jan;41(1):25–30. 10.1183/09031936.00220511 [DOI] [PubMed] [Google Scholar]
- 51.Goel S, Gupta BP, Kashyap S, Bhardwaj AK. Epidemiological aspects of chronic bronchitis in Shimla hills. Indian J Chest Dis Allied Sci. 2007;49:144–7. [Google Scholar]
- 52.Spon RA, Bilques S, Hussain I, Khan SS, Ul Haq I, Dolma Y. Prevalence of chronic bronchitis in selected districts of Kashmir Valley. J Adv Res Med. 2014;1(1):27–32. [Google Scholar]
- 53.Sukhsohale ND, Narlawar UW, Phatak MS. Indoor air pollution from biomass combustion and its adverse health effects in central India: an exposure–response study. Indian J Community Med. 2013. Jul;38(3):162–7. 10.4103/0970-0218.116353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viswanathan K, Rakesh PS, Balakrishnan S, Shanavas A, Dharman V. Prevalence of chronic respiratory diseases from a rural area in Kerala, southern India. Indian J Tuberc. 2018. Jan;65(1):48–51. 10.1016/j.ijtb.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 55.Akhtar T, Ullah Z, Khan MH, Nazli R. Chronic bronchitis in women using solid biomass fuel in rural Peshawar, Pakistan. Chest. 2007. Nov;132(5):1472–5. 10.1378/chest.06-2529 [DOI] [PubMed] [Google Scholar]
- 56.Tageldin MA, Nafti S, Khan JA, Nejjari C, Beji M, Mahboub B, et al. ; BREATHE Study Group. Distribution of COPD-related symptoms in the Middle East and North Africa: results of the BREATHE study. Respir Med. 2012. Dec;106 Suppl 2:S25–32. 10.1016/S0954-6111(12)70012-4 [DOI] [PubMed] [Google Scholar]
- 57.Akhtar MA, Latif PA. Prevalence of chronic bronchitis in urban population of Kashmir. J Indian Med Assoc. 1999. Sep;97(9):365–6, 369. [PubMed] [Google Scholar]
- 58.Chhabra SK, Chhabra P, Rajpal S, Gupta RK. Ambient air pollution and chronic respiratory morbidity in Delhi. Arch Environ Health. 2001. Jan-Feb;56(1):58–64. 10.1080/00039890109604055 [DOI] [PubMed] [Google Scholar]
- 59.Jindal SK. A field study on follow up at 10 years of prevalence of chronic obstructive pulmonary disease & peak expiratory flow rate. Indian J Med Res. 1993. Feb;98:20–6. [PubMed] [Google Scholar]
- 60.Mahesh PA, Jayaraj BS, Prahlad ST, Chaya SK, Prabhakar AK, Agarwal AN, et al. Validation of a structured questionnaire for COPD and prevalence of COPD in rural area of Mysore: A pilot study. Lung India. 2009. Jul;26(3):63–9. 10.4103/0970-2113.53226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pandita AK, Roy D, Saxena V. A study on morbidity pattern among geriatric population of an urban slum, Dehradun, India. Indian J Community Health. 2017;29(4):402–9. [Google Scholar]
- 62.Qureshi KA. Domestic smoke pollution and prevalence of chronic bronchitis/asthma in a rural area of Kashmir. Indian J Chest Dis Allied Sci. 1994. Apr-Jun;36(2):61–72. [PubMed] [Google Scholar]
- 63.Jaganath D, Miranda JJ, Gilman RH, Wise RA, Diette GB, Miele CH, et al. ; CRONICAS Cohort Study Group. Prevalence of chronic obstructive pulmonary disease and variation in risk factors across four geographically diverse resource-limited settings in Peru. Respir Res. 2015. Mar 18;16(1):40. 10.1186/s12931-015-0198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leung C, Bourbeau J, Sin DD, Aaron SD, FitzGerald JM, Maltais F, et al. ; CanCOLD Collaborative Research Group. The prevalence of chronic obstructive pulmonary disease (COPD) and the heterogeneity of risk factors in the Canadian population: results from the Canadian Obstructive Lung Disease (COLD) Study. Int J Chron Obstruct Pulmon Dis. 2021. Feb 12;16:305–20. 10.2147/COPD.S285338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iqbal S, Barolia R, Ladak L, Petrucka P. Smoking cessation interventions in South Asian countries: protocol for scoping review. BMJ Open. 2021. Feb 9;11(2):e038818. 10.1136/bmjopen-2020-038818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shetty BSP, D’Souza G, Padukudru Anand M. Effect of indoor air pollution on chronic obstructive pulmonary disease (COPD) deaths in southern Asia – a systematic review and meta-analysis. Toxics. 2021. Apr 16;9(4):85. 10.3390/toxics9040085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farabi-Asl H, Taghizadeh-Hesary F, Chapman A, Bina SM, Itaoka K. Energy challenges for clean cooking in Asia, the background, and possible policy solutions. ADBI working paper 1007. Tokyo: Asian Development Bank Institute; 2019. Available from: https://www.adb.org/publications/energy-challenges-clean-cooking-asia [cited 2021 Nov 4]. [Google Scholar]
- 68.Tran VV, Park D, Lee YC. Indoor air pollution, related human diseases, and recent trends in the control and improvement of indoor air quality. Int J Environ Res Public Health. 2020. Apr 23;17(8):2927. 10.3390/ijerph17082927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. Environmental and health impacts of air pollution: a review. Front Public Health. 2020. Feb 20;8:14. 10.3389/fpubh.2020.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang X, Zhang T, Zhang Y, Chen H, Sang S. Global burden of COPD attributable to ambient PM2.5 in 204 countries and territories, 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Sci Total Environ. 2021. Nov 20;796:148819. 10.1016/j.scitotenv.2021.148819 [DOI] [PubMed] [Google Scholar]
- 71.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017. May 13;389(10082):1907–18. 10.1016/S0140-6736(17)30505-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang XQ, Mei XD, Feng D. Air pollution and chronic airway diseases: what should people know and do? J Thorac Dis. 2016. Jan;8(1):E31–40. 10.3978/j.issn.2072-1439.2015.11.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maji S, Ghosh S, Ahmed S. Association of air quality with respiratory and cardiovascular morbidity rate in Delhi, India. Int J Environ Health Res. 2018. Oct;28(5):471–90. 10.1080/09603123.2018.1487045 [DOI] [PubMed] [Google Scholar]
- 74.Chhabra SK, Chhabra P, Rajpal S, Gupta RK. Ambient air pollution and chronic respiratory morbidity in Delhi. Arch Environ Health. 2001. Jan-Feb;56(1):58–64. 10.1080/00039890109604055 [DOI] [PubMed] [Google Scholar]
- 75.Berend N. Contribution of air pollution to COPD and small airway dysfunction. Respirology. 2016. Feb;21(2):237–44. 10.1111/resp.12644 [DOI] [PubMed] [Google Scholar]
- 76.Rhee CK, Chau NQ, Yunus F, Matsunag K, Perng DW; on behalf the COPD Assembly of the APSR. Management of COPD in Asia: a position statement of the Asian Pacific Society of Respirology. Respirology. 2019. Oct;24(10):1018–25. 10.1111/resp.13633 [DOI] [PubMed] [Google Scholar]
- 77.Babu GR, Khetrapal S, John DA, Deepa R, Narayan KMV. Pandemic preparedness and response to COVID-19 in South Asian countries. Int J Infect Dis. 2021. Mar;104:169–74. 10.1016/j.ijid.2020.12.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim V, Criner GJ. The chronic bronchitis phenotype in chronic obstructive pulmonary disease: features and implications. Curr Opin Pulm Med. 2015. Mar;21(2):133–41. 10.1097/MCP.0000000000000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salvi S, Kumar GA, Dhaliwal RS, Paulson K, Agrawal A, Koul PA, et al. ; India State-Level Disease Burden Initiative CRD Collaborators. The burden of chronic respiratory diseases and their heterogeneity across the states of India: the Global Burden of Disease Study 1990–2016. Lancet Glob Health. 2018. Dec;6(12):e1363–74. 10.1016/S2214-109X(18)30409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tata Institute of Social Sciences (TISS). Global Adult Tobacco Survey GATS 2 India 2016–17. New Delhi: Ministry of Health and Family Welfare, Government of India; 2018. Available from: https://ntcp.nhp.gov.in/assets/document/surveys-reports-publications/Global-Adult-Tobacco-Survey-Second-Round-India-2016-2017.pdf [cited 2021 Nov 4].
- 81.Shaddick G, Thomas ML, Mudu P, Ruggeri G, Gumy S. Half the world’s population are exposed to increasing air pollution. NPJ Clim Atmos Sci. 2020;3(23):1–5. 10.1038/s41612-020-0124-2 [DOI] [Google Scholar]
- 82.Sun L, Chen Y, Wu R, Lu M, Yao W. Changes in definition lead to changes in the clinical characteristics across COPD categories according to GOLD 2017: a national cross-sectional survey in China. Int J Chron Obstruct Pulmon Dis. 2017. October 20;12:3095–102. 10.2147/COPD.S142801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Corlateanu A, Mendez Y, Wang Y, Garnica RJA, Botnaru V, Siafakas N. “Chronic obstructive pulmonary disease and phenotypes: a state-of-the-art.”. Pulmonology. 2020. Mar - Apr;26(2):95–100. 10.1016/j.pulmoe.2019.10.006 [DOI] [PubMed] [Google Scholar]
- 84.Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015. August 27;25(1):15056. 10.1038/npjpcrm.2015.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jarhyan P, Hutchinson A, Khatkar R, Kondal D, Botti M, Prabhakaran D, et al. Diagnostic accuracy of a two-stage sequential screening strategy implemented by community health workers (CHWs) to identify individuals with COPD in rural India. Int J Chron Obstruct Pulmon Dis. 2021. Apr 29;16:1183–92. 10.2147/COPD.S293577 [DOI] [PMC free article] [PubMed] [Google Scholar]