Abstract
Objective:
Patients with solid malignancies are more vulnerable to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection than the healthy population. The outcome of SARS-CoV-2 infection in highly immunosuppressed populations, such as in patients with hematological malignancies, is a point of interest. We aimed to analyze the symptoms, complications, intensive care unit admissions, and mortality rates of patients with hematological malignancies infected with SARS-CoV-2 in Turkey.
Materials and Methods:
In this multicenter study, we included 340 adult and pediatric patients diagnosed with SARS-CoV-2 from March to November 2020. Diagnosis and status of primary disease, treatment schedules for hematological malignancies, time from last treatment, life expectancy related to the hematological disease, and comorbidities were recorded, together with data regarding symptoms, treatment, and outcome of SARS-CoV-2 infection.
Results:
Forty four patients were asymptomatic at diagnosis of SARS-CoV- 2 infection. Among symptomatic patients, fever, cough, and dyspnea were observed in 62.6%, 48.8%, and 41.8%, respectively. Sixty-nine (20%) patients had mild SARS-CoV-2 disease, whereas moderate, severe, and critical disease was reported in 101 (29%), 71 (20%), and 55 (16%) patients, respectively. Of the entire cohort, 251 (73.8%) patients were hospitalized for SARS-CoV-2. Mortality related to SARS-CoV-2 infection was 26.5% in the entire cohort; this comprised 4.4% of those patients with mild disease, 12.4% of those with moderate disease, and 83% of those with severe or critical disease. Active hematological disease, lower life expectancy related to primary hematological disease, neutropenia at diagnosis of SARS-CoV-2, ICU admission, and first-line therapy used for coronavirus disease-2019 treatment were found to be related to higher mortality rates. Treatments with hydroxychloroquine alone or in combination with azithromycin were associated with a higher rate of mortality in comparison to favipiravir use.
Conclusion:
Patients with hematological malignancy infected with SARS-CoV-2 have an increased risk of severe disease and mortality.
Keywords: COVID-19, SARS-CoV-2 infection, Hematological malignancy
Abstract
Amaç:
Solid malignite hastalarının şiddetli akut solunum yolu enfeksiyonu-koronavirüs-2 (SARS-CoV-2) enfeksiyonuna sağlıklı bireylerden daha yatkın oldukları gösterilmiştir. Bu verilerin ardından oldukça yoğun immünosupresif olan hematolojik malignite hastalarında SARS-CoV-2 infeksiyonu sonuçları ilgi konusu olmuştur. Biz de bu makalede Türkiye’de hematolojik malignite tanısı ile takip ve tedavi edilirken SARS-CoV-2 enfeksiyonu saptanan hastaların semptom, komplikasyon, yoğun bakım ünitesine yatış ve mortalite oranlarını değerlendirmeyi amaçladık.
Gereç ve Yöntemler:
Çok merkezli çalışmamıza Mart-Kasım 2020 tarihleri arasında SARS-CoV-2 enfeksiyonu tanısı alan erişkin ve pediatrik 340 hastayı dahil ettik. Hastaların hematolojik malignite tanıları, hastalık statusları, tedavileri, son tedaviden enfeksiyona kadar geçen süre, komorbiditeleri, yaşam beklentileri değerlendirildi. Semptomları, oluşan komplikasyonlar ve sonuçlar analiz edildi.
Bulgular:
Kırk-dört hasta semptomsuz olarak enfeksiyonu geçirirken, semptomatik hastaların ateş, öksürük, ve dispne oranları sırasıyla %62,6, %48,8 ve %41,8 idi. Altmış altı hasta (%20) hastalığı hafif geçirirken, orta, ciddi ve kritik hastalık tanısı alanların oranı sırasıyla %29, %20 ve %16 idi. Tüm kohortta ölüm oranı %26,6 idi; ölüm hafif hastalığı olanlarda %4,4 , orta derece hastalık geçirenlerde %12 ve ciddi/kritik hastalık geçirenlerde ise %83 olarak saptandı. Aktif hematolojik hastalık olması, primer hematolojik hastalığa bağlı düşük hayat beklentisi, SARS-CoV-2 tanısı aldığında nötropenik olmak, yoğun bakıma alınmış olmak ve ilk sıra koronavirüs hastalığı-2019 tedavisi yüksek ölüm riski ile ilişkilendirilen faktörlerdendi. Tek başına veya azitromisin ile kombine olarak hidroksiklorokin kullanan hastaların sadece favipiravir kullananlara göre ölüm riskleri daha yüksek saptandı.
Sonuç:
SARS-CoV-2 infeksiyonu geçiren hematolojik malignite hastalarında daha yüksek oranda ciddi hastalık ve ölüm riski gözlenmektedir.
Introduction
Millions of people have been infected with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) worldwide. Comorbidities like diabetes mellitus, hypertension, and chronic renal failure as well as older age have been identified as risk factors for the ensuing severity of coronavirus disease-2019 (COVID-19) [1,2,3,4]. Cancer patients were also found to be more vulnerable to SARS-CoV-2 infection than the healthy population in studies that mostly included solid malignancies [5,6,7].
The increased risk of viral infections of the respiratory tract in patients with hematological malignancy and hematopoietic stem cell transplantation (HSCT) has been previously reported [8,9,10]. The underlying diagnosis and the treatments may both influence humoral and cellular immune functions negatively and result in poor outcome. The clinical characteristics and risk factors that may be predictive for severity or mortality of COVID-19 in cases of hematological malignancy need to be addressed.
In this registry data analysis, we aimed to evaluate the symptoms, complications, intensive care unit (ICU) admissions, and mortality rates of SARS-CoV-2 infection in patients with underlying hematological malignancies and to clarify the risk factors associated with mortality in COVID-19 in Turkey. Additionally, the influence of national treatment protocols for SARS-CoV-2 infection on outcomes was analyzed.
Materials and Methods
On behalf of the Turkish Society of Hematology’s Infectious Complications and Supportive Care Working Party, we retrospectively collected data from 25 centers in Turkey from March to November 2020. The study was approved by both the Turkish Ministry of Health and the Ethics Committee of İstanbul University-Cerrahpaşa, Cerrahpaşa Faculty of Medicine (22-Sep-2020/80350), as well as locally by the participating centers.
Patients were included in the study according to the following criteria: a) SARS-CoV-2 polymerase chain reaction (PCR) positivity via nasal swabs or b) negative PCR results but symptoms related to SARS-CoV-2 with highly suggestive thoracic computerized tomography findings. Patients who were followed as both outpatients and inpatients for COVID-19 were eligible for the study. Forms for data collection were emailed to participating centers.
Diagnosis and status of the primary disease, treatment schedules for hematological malignancies, time from last treatment, and life expectancy related to the hematological disease were recorded. Data regarding symptoms related to SARS-CoV-2 infection, hospitalization and oxygen requirements, severity, complications and organ involvement, laboratory parameters on admission, and treatments given for COVID-19 were also investigated. Comorbidities, defined as diabetes mellitus, hypertension, chronic renal failure, chronic obstructive pulmonary disease, cardiovascular disease, or preexisting solid malignancy diagnosis, were also recorded.
Patients who had undergone autologous HSCT and were in the first 100 days after transplantation were grouped as “auto-HSCT.” All patients who had undergone allogeneic HSCT were grouped as “allo-HSCT” irrespective of their primary diagnosis and the status of the disease.
The severity of SARS-CoV-2 infection was classified according to World Health Organization (WHO) definitions [11] as follows. Mild disease: Symptomatic patients without findings of pneumonia or hypoxia. Moderate disease: Patients with signs of pneumonia, like cough, fever, and dyspnea, without signs of severe pneumonia or SpO2 of >90% on room air. Severe disease: Patients with symptoms of pneumonia and respiratory rate of >30/min, severe respiratory distress, or SpO2 of <90% on room air. Critical disease: Patients with acute respiratory distress syndrome (ARDS), sepsis, and septic shock.
The COVID-19 treatments of patients were determined according to guidelines released by the Turkish Ministry of Health. Due to the antiviral potency of hydroxychloroquine (HCQ), it was introduced as the initial treatment schedule alone or in combination with azithromycin and favipiravir as salvage treatment. In subsequent months of the pandemic, favipiravir was moved to first-line treatment, consistent with the updated version of the guidelines.
The primary objective of this study was to identify the clinical outcomes and complications of COVID-19 in patients with hematological malignancies and to determine the rates of hospitalization, ICU admission, and overall 45-day mortality. The secondary objective was to identify additional risk factors for mortality specifically defined for this group of immunosuppressed patients.
Descriptive statistics were calculated as median and range for continuous and percentage for categorical variables. The Cox regression model was used for univariate analysis. Parameters achieving values of p<0.20 were added to the multivariate Cox regression model and significant factors were detected with the stepwise method. Analysis was performed with SPSS 20.0.
Results
The characteristics of 335 adult and 5 pediatric patients are summarized in Table 1. The median age was 59 years (range: 7-93). COVID-19 was more frequent in males (male-to-female ratio: 1.3). The most common underlying hematological diagnosis was multiple myeloma (MM), seen in 25% of cases, followed by acute myeloid leukemia (AML) (20%) and non-Hodgkin lymphoma (NHL) (18%). The hematological disease statuses of the patients are also shown in Table 1. Twenty-eight percent of the patients had active disease, and 28 of those patients were newly diagnosed but treatment could not be started as a consequence of SARS-CoV-2 infection. The treatment schedules for hematological malignancies are also summarized in Table 1. Treatment protocols for primary disease were changed before the diagnosis of COVID-19 for 21% of these patients.
Table 1. Patient characteristics, clinical outcomes, and treatments of hematological malignancy patients infected with SARS-CoV- 2.
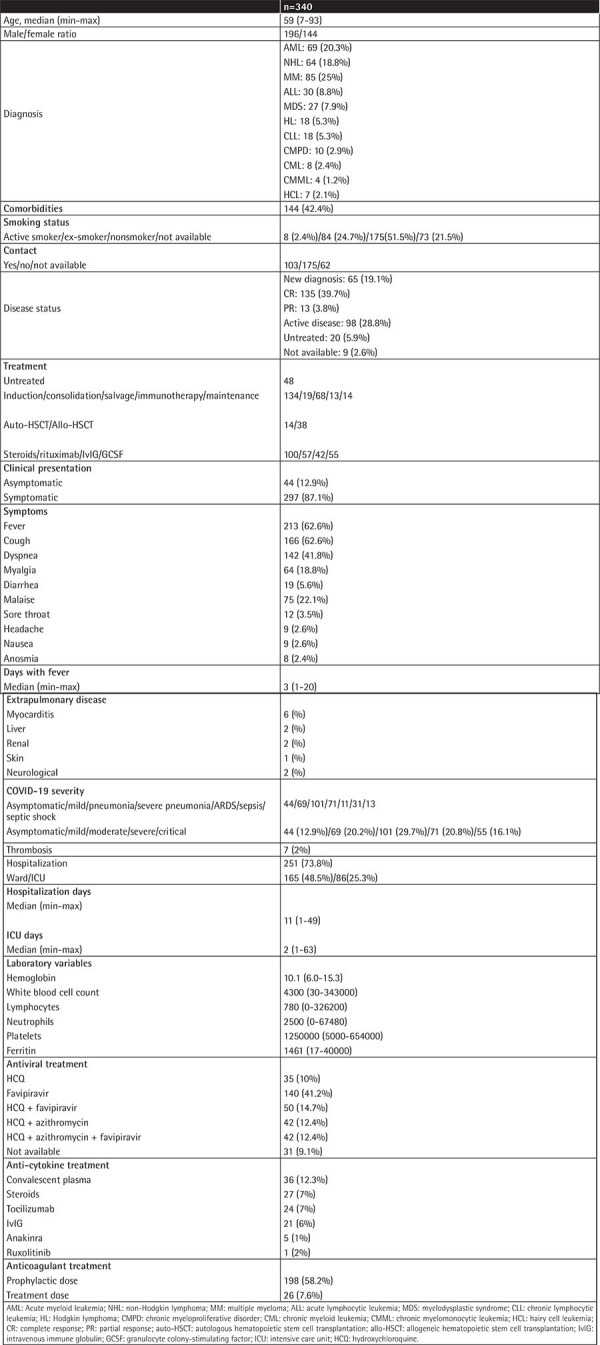
Nasopharyngeal swab PCR positivity for SARS-CoV-2 was observed in 264 of 340 (77%) patients. Forty-four (12.9%) patients were asymptomatic at diagnosis. In symptomatic patients, fever, cough, and dyspnea were observed in 62.6%, 48.8%, and 41.8%, respectively. In the allo-HSCT group, 13% of the patients were asymptomatic. Fever was present in 55%, cough in 50%, dyspnea in 28%, and myalgia and malaise in 34% and 31% of the patients, respectively. In the auto-HSCT group, 9 patients (64%) had fever, 5 (35%) patients had cough and malaise, 4 (28%) patients had dyspnea, and 1 patient (7%) was asymptomatic.
The median number of febrile days was 3 (range: 1-20). Sixty-nine (20.2%) patients had mild disease, whereas moderate, severe, and critical disease was reported in 101 (29.7%), 71 (20.8%), and 55 (16.1%) patients, respectively. ARDS was reported in 11 patients while sepsis and septic shock were observed in 31 and 13 patients, respectively. Two of 5 pediatric patients were asymptomatic; 2 had severe and 1 had critical disease. Severity of COVID-19 was not found to be related to age, comorbidities, primary disease status, malignancy treatments, HSCT, or type of COVID-19 treatment.
In the allo-HSCT group, mild, moderate, severe, and critical COVID-19 was observed in 18%, 44%, 13%, and 10% of cases, respectively. Patients with graft-versus-host disease (GVHD) had more severe and critical disease in comparison to those without GVHD (p=0.03). In patients who were diagnosed with COVID-19 in the first 30 days after auto-HSCT, mild disease was observed in 4 of 14 patients, while moderate, severe, and critical disease was observed in 3, 4, and 2 patients, respectively.
Laboratory variables of the patients are summarized in Table 1. Neutropenia and lymphopenia were observed at diagnosis in 23% and 57%, respectively.
Treatment for COVID-19 was either with HCQ or favipiravir alone or in combination with other treatments (Table 1). Favipiravir alone was given to 41.4% of the entire cohort, while it was given in combination with HCQ to 14.7% and in combination with HCQ and azithromycin to 12.4% of the patients. Ten percent of the patients received HCQ alone while 12.4% received it in combination with azithromycin.
Thirty-six patients (12.3%) received convalescent plasma and the rest of the anti-cytokine treatments are summarized in Table 1.
Of the entire cohort, 251 (73.8%) patients were hospitalized and 86 (25%) of those patients were admitted directly to the ICU. Median number of hospitalization days in the ward and ICU was 11 (range: 1-49) and 2 (range: 1-63), respectively.
Thrombotic events were observed in 7 (2%) patients. Three of them had thrombotic attacks while using prophylactic-dose low-molecular-weight heparin. One of those three patients had a history of previous pulmonary embolism.
PCR negativity could be achieved in a median of 11 days (range: 1-60). Patients who had received rituximab for the primary disease within 1 year before COVID-19 showed significantly prolonged viral shedding (median: 14 days (3-60) vs. 11 days (1-59), p=0.023).
Mortality was 26.5% in the entire cohort and 4.4% in cases of mild disease, 12.4% in cases of moderate disease, and 83% in cases of severe and critical disease. Nine of 38 (23.7%) patients who had undergone allo-HSCT and 3 (21%) of 14 patients who had undergone auto-HSCT died. No difference in mortality was observed according to the timing of HSCT or presence of GVHD.
Parameters analyzed for relationships with mortality in univariate analysis with Cox regression are shown in Table 2. Age, comorbidities, status of primary hematological disease, neutropenia and lymphopenia at diagnosis of SARS-CoV-2 infection, severity of COVID-19, hospitalization, admission to the ICU, intubation, type of COVID-19 treatment, convalescent plasma treatment, and decreased life expectancy related to primary hematological disease were all found to be associated with higher mortality rates. Patients with PCR positivity also had a higher mortality rate in comparison with patients who had only CT findings but negative PCR results (Table 2).
Table 2. Univariate analysis for mortality of the patients by Cox regression model (p<0.20 is statistically significant).

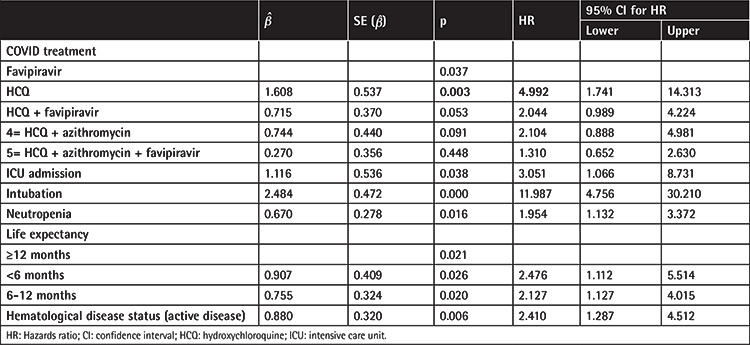
In multivariate Cox regression analysis by stepwise approach, all the significant parameters related to mortality in univariate analysis were included in multivariate analysis. Hematological disease status, decreased life expectancy related to primary hematological disease, neutropenia, ICU admission, and type of COVID-19 treatment were accordingly found to be associated with higher mortality (Table 3). Patients treated with HCQ alone had 4.9-fold higher mortality risk in comparison to patients treated with favipiravir alone, while those treated with HCQ plus favipiravir had 2.04-fold higher risk and those treated with HCQ plus azithromycin had 2.14-fold higher risk.
Table 3. Multivariate analysis for mortality of the patients by Cox regression model (p<0.20 is statistically significant).
Discussion
We have reported the outcomes of 340 hematological malignancy patients who contracted SARS-CoV-2 infection from March to November 2020 in Turkey. Patients with hematological malignancies are a high-risk population for SARS-CoV-2 infection as a result of immunosuppression arising from both the disease and its treatment. In our study, severe/critical disease as defined according to the WHO classification was observed in 36% of patients [11]. Consistent with our results, another report from Turkey that included patients from the Turkish Ministry of Health database showed more severe and critical disease among hematological malignancy patients with COVID-19 compared to patients without cancer [12]. Piñana et al. [13] included patients grouped according to different severity criteria [14] in their study and observed severe disease in 55% of non-HSCT, 36% of auto-HSCT, and 43% of allo-HSCT patients.
We could not find a relation between the severity of COVID-19 and age, comorbidities, primary disease status, malignancy treatments, HSCT, or COVID-19 treatment. In allo-HSCT patients, however, GVHD was related to severe-critical disease status. Risk factors for severe disease were reported as hypertension, baseline lymphopenia, baseline C-reactive protein of >20 mg/dL, age, and comorbidities in different series [13,15,16].
Factors related to mortality in hematological malignancy patients are still debated. The mortality rate was 26% in our study, correlated with increasing COVID-19 severity. In a meta-analysis of 34 adult and 5 pediatric studies that predominantly included hospitalized patients, the risk of death was 34% and 4%, respectively [17]. Although there were only five pediatric patients in our study, all were alive at the end of follow-up. Piñana et al. [13] showed associations between mortality and age, performance score, neutropenia, uncontrolled disease, and increased C-reactive protein. In our study, hematological disease status, life expectancy related to the primary hematological disease, neutropenia, ICU admission, and type of COVID-19 treatment were risk factors for mortality in multivariate analysis. ICU admission closely reflected the disease severity and mortality increase irrespective of age, probably related to the primary hematological disease status.
Thirteen percent of our patients were asymptomatic at diagnosis, a rate lower than that seen in the general population. The most prominent symptoms at diagnosis were fever, cough, and dyspnea. In an Italian study that included only adult patients, fever was reported in 75%, dyspnea in 51%, cough in 45%, and malaise in 39% of cases [15]. He et al. [18] observed more fever, cough, and dyspnea in hematology patients in comparison to healthcare professionals. Consistent with our findings, Piñana et al. [13] failed to show a difference in the symptoms of patients with different HSCT statuses. Hospitalization was required for 73% of our patients and 25% were admitted to the ICU, similar to the findings of other series [13,15]. In Italy, among patients with severe or critical COVID-19, those who were admitted to the ICU were younger and had a lower comorbidity index [15].
In our study, AML, NHL, and MM were the most frequent hematological malignancies, consistent with previous studies [16,17,19]. Chronic myeloproliferative neoplasms (CMPNs) were the least frequent in this patient population. In another Turkish study, in contrast to our data, besides NHL, myelodysplastic syndrome and myeloproliferative diseases were the most commonly diagnosed malignancies [12].
There are controversial results about the impact of the underlying hematological diagnosis on mortality in cases of COVID-19. We suggest that not the diagnosis but rather the disease status before COVID-19 is the significant factor. In population-based registry data analysis of 833 patients, besides age and comorbidities, the diagnosis of AML was found to be related to the highest mortality rate, whereas patients with Philadelphia-negative CMPNs had the lowest risk [16]. Passamonti et al. [15] showed worse survival in cases of uncontrolled disease and among AML, NHL, and plasma cell dyscrasia patients. In a study that included only chronic lymphocytic leukemia (CLL) patients, 79% presented with severe COVID-19 findings. No difference was observed regarding the presence of three or more comorbidities or hypogammaglobulinemia [10]. Predictors of adverse outcome in MM patients were revealed as age, high-risk MM, renal disease, and suboptimal control of the disease [20,21].
Treatment schedules for various hematological malignancies have been suggested to be modified during the pandemic in order to reduce immunosuppression and the admission of patients to the hospital [22,23,24]. In our cohort, the data revealed that hematological malignancy treatments were modified for 21% of our patients before COVID-19 diagnosis. There are controversial data about the impact of hematological malignancy treatment on COVID-19 outcomes. Vijenthira et al. [17] could not show the impact of recent hematological malignancy treatment on the risk of death irrespective of the type of therapy. In cases of CLL, the severity of COVID-19 increased among untreated patients and those who were treated within the last year, but administration of Bruton kinase inhibitors exerted a protective effect against the virus [10]. In myeloma patients, no anti-myeloma treatments including transplantation were found to be associated with outcome [25]. We could not show a significant impact of either the type or the timing of the last hematological malignancy treatment on mortality in our study.
Study Limitations
In our study, the treatment schedules designed by Turkish health authorities were followed. Favipiravir was moved to the first line of treatment as a consequence of studies that could not show benefits of HCQ [25,26,27]. Patients treated with HCQ alone had 4.9-fold increased mortality risk compared to patients treated with favipiravir alone, while those treated with HCQ plus favipiravir had 2.0-fold increased risk and those treated with HCQ plus azithromycin had 2.1-fold increased risk. The group receiving HCQ plus favipiravir mainly included patients who had received HCQ initially and favipiravir with the further progression of pneumonia. In a multicenter randomized superiority trial, conventional therapy in combination with favipiravir or arbidol was investigated and favipiravir was found to be significantly superior to arbidol in terms of 7-day clinical recovery rates [28,29]. Data on the impact of these drugs in immunosuppressive patients are limited. The lowest mortality was observed in patients who received favipiravir alone in our study, which may be a valuable finding for further studies.
Conclusion
Hematological malignancy patients infected with SARS-CoV-2 have an increased risk of mortality. Having active hematological malignancy, neutropenia, admission to the ICU, and/or lower life expectancy related to the primary disease increases the mortality rates in these patients.
Footnotes
Ethics
Ethics Committee Approval: The study was approved by both the Turkish Ministry of Health and the Ethics Committee of İstanbul University-Cerrahpaşa, Cerrahpaşa Faculty of Medicine (22-Sep-2020/80350), as well as locally by the participating centers.
Informed Consent: Retrospective study.
Authorship Contributions
Medical Practice: S.C.B., G.C.S., İ.Y.H., F.H., N.A., M.B., L.A.K., S.K.T., H.S.G., B.B.A., U.D., F.C., V.Ö., E.G., Z.T.G., Z.N.Ö., S.D., M.B., İ.İ., U.Y., H.E.K., E.A., B.Y., Ü.A., Y.G.M., V.B., F.Ö., H.Ü.T., V.G., S.Ç., R.Ç., M.Y., P.T., Ö.Ç., H.A., C.S., M.C.A., O.K.Y., S.S., C.A., A.M.D., N.G., M.K., H.T., A.D., Z.A.Y., M.K.Y., S.S., İ.Y., H.S.B., T.A., S.M., V.E., L.K., O.İ., A.Z.B., Ö.G.S., A.A., M.Ö., G.G., Ş.Ü., Y.Y., R.D.K., G.H.Ö.; Concept: S.C.B., G.H.Ö., M.C.A., M.K.Y., Ş.Ü., N.A.; Design: S.C.B., G.H.Ö., M.C.A., M.K.Y., Ş.Ü., N.A.; Data Collection: S.C.B., G.C.S.; Analysis: S.C.B., G.C.S., Y.Y.; Literature Search: S.C.B.; Writing: S.C.B., G.H.Ö., M.C.A., M.K.Y., Ş.Ü., N.A.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Bloomgarden ZT. Diabetes and COVID-19. J Diabetes. 2020;12:347–348. doi: 10.1111/1753-0407.13027. [DOI] [PubMed] [Google Scholar]
- 2.Rodgers GP, Gibbons GH. Obesity and hypertension in the time of COVID-19. JAMA. 2020;324:1163–1165. doi: 10.1001/jama.2020.16753. [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Fernández FJ. COVID-19, hypertension and angiotensin receptor-blocking drugs. J Hypertens. 2020;38:1191. doi: 10.1097/HJH.0000000000002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung JM, Niikura M, Yang CWT, Sin DD. COVID-19 and COPD. Eur Respir J. 2020;56:2002108. doi: 10.1183/13993003.02108-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, Zhang Z, You H, Wu M, Zheng Q, Xiong Y, Xiong H, Wang C, Chen C, Xiong F, Zhang Y, Peng Y, Ge S, Zhen B, Yu T, Wang L, Wang H, Liu Y, Chen Y, Mei J, Gao X, Li Z, Gan L, He C, Li Z, Shi Y, Qi Y, Yang J, Tenen DG, Chai L, Mucci LA, Santillana M, Cai H. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:e180. doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontana L, Strasfeld L. Respiratory virus infections of the stem cell transplant recipient and the hematologic malignancy patient. Infect Dis Clin North Am. 2019;33:523–544. doi: 10.1016/j.idc.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khawaja F, Chemaly RF. Respiratory syncytial virus in hematopoietic cell transplant recipients and patients with hematologic malignancies. Haematologica. 2019;104:1322–1331. doi: 10.3324/haematol.2018.215152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarfò L, Chatzikonstantinou T, Rigolin GM, Quaresmini G, Motta M, Vitale C, Garcia-Marco JA, Hernández-Rivas JÁ, Mirás F, Baile M, Marquet J, Niemann CU, Reda G, Munir T, Gimeno E, Marchetti M, Quaglia FM, Varettoni M, Delgado J, Iyengar S, Janssens A, Marasca R, Ferrari A, Cuéllar-García C, Itchaki G, Špaček M, De Paoli L, Laurenti L, Levin MD, Lista E, Mauro FR, Šimkovič M, Van Der Spek E, Vandenberghe E, Trentin L, Wasik-Szczepanek E, Ruchlemer R, Bron D, De Paolis MR, Del Poeta G, Farina L, Foglietta M, Gentile M, Herishanu Y, Herold T, Jaksic O, Kater AP, Kersting S, Malerba L, Orsucci L, Popov VM, Sportoletti P, Yassin M, Pocali B, Barna G, Chiarenza A, Dos Santos G, Nikitin E, Andres M, Dimou M, Doubek M, Enrico A, Hakobyan Y, Kalashnikova O, Ortiz Pareja M, Papaioannou M, Rossi D, Shah N, Shrestha A, Stanca O, Stavroyianni N, Strugov V, Tam C, Zdrenghea M, Coscia M, Stamatopoulos K, Rossi G, Rambaldi A, Montserrat E, Foà R, Cuneo A, Ghia P. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34:2354–2363. doi: 10.1038/s41375-020-0959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. WHO Interim Guidance. Clinical Management of COVID-19. Geneva, WHO. 2020. [Google Scholar]
- 12.Yigenoglu TN, Ata N, Altuntas F, Bascı S, Dal MS, Korkmaz S, Namdaroglu S, Basturk A, Hacıbekiroglu T, Dogu MH, Berber İ, Dal K, Erkurt MA, Turgut B, Ulgu MM, Celik O, Imrat E, Birinci S. The outcome of COVID-19 in patients with hematological malignancy. J Med Virol. 2021;93:1099–1104. doi: 10.1002/jmv.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piñana JL, Martino R, García-García I, Parody R, Morales MD, Benzo G, Gómez-Catalan I, Coll R, De La Fuente I, Luna A, Merchán B, Chinea A, de Miguel D, Serrano A, Pérez C, Diaz C, Lopez JL, Saez AJ, Bailen R, Zudaire T, Martínez D, Jurado M, Calbacho M, Vázquez L, Garcia-Cadenas I, Fox L, Pimentel AI, Bautista G, Nieto A, Fernandez P, Vallejo JC, Solano C, Valero M, Espigado I, Saldaña R, Sisinni L, Ribera JM, Jimenez MJ, Trabazo M, Gonzalez-Vicent M, Fernández N, Talarn C, Montoya MC, Cedillo A, Sureda A; Infectious Complications Subcommittee of the Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group (GETH) Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020;9:21. doi: 10.1186/s40164-020-00177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, Angelucci E, Krampera M, Cairoli R, Della Porta MG, Fracchiolla N, Ladetto M, Gambacorti Passerini C, Salvini M, Marchetti M, Lemoli R, Molteni A, Busca A, Cuneo A, Romano A, Giuliani N, Galimberti S, Corso A, Morotti A, Falini B, Billio A, Gherlinzoni F, Visani G, Tisi MC, Tafuri A, Tosi P, Lanza F, Massaia M, Turrini M, Ferrara F, Gurrieri C, Vallisa D, Martelli M, Derenzini E, Guarini A, Conconi A, Cuccaro A, Cudillo L, Russo D, Ciambelli F, Scattolin AM, Luppi M, Selleri C, Ortu La Barbera E, Ferrandina C, Di Renzo N, Olivieri A, Bocchia M, Gentile M, Marchesi F, Musto P, Federici AB, Candoni A, Venditti A, Fava C, Pinto A, Galieni P, Rigacci L, Armiento D, Pane F, Oberti M, Zappasodi P, Visco C, Franchi M, Grossi PA, Bertù L, Corrao G, Pagano L, Corradini P; ITA-HEMA-COV Investigators. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Suárez J, de la Cruz J, Cedillo Á, Llamas P, Duarte R, Jiménez-Yuste V, Hernández-Rivas JÁ, Gil-Manso R, Kwon M, Sánchez-Godoy P, Martínez-Barranco P, Colás-Lahuerta B, Herrera P, Benito-Parra L, Alegre A, Velasco A, Matilla A, Aláez-Usón MC, Martos-Martínez R, Martínez-Chamorro C, Susana-Quiroz K, Del Campo JF, de la Fuente A, Herráez R, Pascual A, Gómez E, Pérez-Oteyza J, Ruiz E, Alonso A, González-Medina J, Martín-Buitrago LN, Canales M, González-Gascón I, Vicente-Ayuso MC, Valenciano S, Roa MG, Monteliu PE, López-Jiménez J, Escobar CE, Ortiz-Martín J, Diez-Martin JL, Martinez-Lopez J; Asociación Madrileña de Hematología y Hemoterapia (AMHH) Impact of hematological malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population based registry study. J Hematol Oncol. 2020;13:133. doi: 10.1186/s13045-020-00970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, Martin Moro F, Razanamahery J, Riches JC, Zwicker JI, Patell R, Vekemans MM, Scarfo L, Chatzikonstantinou T, Yildiz H, Lattenist R, Mantzaris I, Wood WA, Hicks LK. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He W, Chen L, Chen L, Yuan G, Fang Y, Chen W, Wu D, Liang B, Lu X, Ma Y, Li L, Wang H, Chen Z, Li Q, Gale RP. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malard F, Genthon A, Brissot E, van de Wyngaert Z, Marjanovic Z, Ikhlef S, Banet A, Lapusan S, Sestilli S, Corre E, Paviglianiti A, Adaeva R, M’Hammedi- Bouzina F, Labopin M, Legrand O, Dulery R, Mohty M. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020;55:2180–2184. doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martín-Moro F, Marquet J, Piris M, Michael BM, Sáez AJ, Corona M, Jiménez C, Astibia B, García I, Rodríguez E, García-Hoz C, Fortún-Abete J, Herrera P, López-Jiménez J. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol. 2020;190:e16–e20. doi: 10.1111/bjh.16801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chari A, Samur MK, Martinez-Lopez J, Cook G, Biran N, Yong KL, Hungria VTM, Engelhardt M, Gay F, Garcia-Feria A, Oliva S, Oostvogels R, Gozzetti A, Rosenbaum CA, Kumar SK, Stadtmauer E, Einsele H, Beksac M, Weisel KC, Anderson KC, Mateos MV, Moreau P, San Miguel J, Munshi NC, Avet-Loiseau H. Clinical features associated with COVID-19 outcome in MM: first results from International Myeloma Society Dataset. Blood. 2020;36:3033–3040. doi: 10.1182/blood.2020008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terpos E, Engelhardt M, Cook G, Gay F, Mateos MV, Ntanasis-Stathopoulos I, van de Donk NWCJ, Avet-Loiseau H, Hajek R, Vangsted AJ, Ludwig H, Zweegman S, Moreau P, Einsele H, Boccadoro M, San Miguel J, Dimopoulos MA, Sonneveld P. Management of patients with multiple myeloma in the era of COVID-19 pandemic: a consensus paper from the European Myeloma Network (EMN) Leukemia. 2020;34:2000–2011. doi: 10.1038/s41375-020-0876-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrara F, Zappasodi P, Roncoroni E, Borlenghi E, Rossi G. Impact of Covid-19 on the treatment of acute myeloid leukemia. Leukemia. 2020;34:2254–2256. doi: 10.1038/s41375-020-0925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahu KK, Siddiqui AD, Cerny J. COVID-19 pandemic and impact on hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55:2193–2195. doi: 10.1038/s41409-020-0913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horby P, Mafham M, Linsell L, Bell JL, Staplin N, Emberson JR, Wiselka M, Ustianowski A, Elmahi E, Prudon B, Whitehouse T, Felton T, Williams J, Faccenda J, Underwood J, Baillie JK, Chappell LC, Faust SN, Jaki T, Jeffery K, Lim WS, Montgomery A, Rowan K, Tarning J, Watson JA, White NJ, Juszczak E, Haynes R, Landray MJ; RECOVERY Collaborative Group. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DLM, de Barros E Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O; Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, Engen NW, Cheng MP, LaBar D, Lother SA, MacKenzie LJ, Drobot G, Marten N, Zarychanski R, Kelly LE, Schwartz IS, McDonald EG, Rajasingham R, Lee TC, Hullsiek KH. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coomes EA, Haghbayan H. Favipiravir, an antiviral for COVID-19? J Antimicrob Chemother. 2020;75:2013–2014. doi: 10.1093/jac/dkaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi S, Parkar J, Ansari A, Vora A, Talwar D, Tiwaskar M, Patil S, Barkate H. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis. 2021;102:501–508. doi: 10.1016/j.ijid.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]


