Abstract
Giant cell arteritis can involve both cranial and extracranial arteries. Isolated extracranial large vessel vasculitis more often manifests with non-specific constitutional symptoms, causing a diagnostic delay. We report the case of a 57-year-old Caucasian female patient presenting with persistently elevated resting heart rate, as revealed by a smartwatch healthcare application, and non-specific constitutional symptoms. Imaging revealed inflammation of the aorta, bilateral subclavian and axillary arteries, compatible with large vessel vasculitis. Treatment with glucocorticoids and tocilizumab led to a significant improvement of her symptoms and decrease in inflammatory parameters. In sum, an unexplained elevated resting heart rate may lead to an earlier diagnosis and treatment of large vessel vasculitis, especially when other manifestations are non-specific. The use of healthcare smartwatch applications may prove useful in the future and lead to an earlier referral of patients to a physician.
Keywords: vasculitis, biological agents
Background
Giant cell arteritis (GCA) is the most common large vessel vasculitis (LVV) in patients older than 50 years and typically involves the temporal artery, the extracranial branches of the carotid artery and the thoracic aorta and its branches. The clinical presentation is less typical in cases of isolated extracranial involvement of the aorta and other large- to medium-sized arteries.1 In these cases, clinically evident vasculitis has been reported in up to 27% of the patients.2 For instance, involvement of the subclavian arteries causes arm claudication in 10%–15% of the patients.3 Much more common is the subclinical involvement of large vessels, where patients frequently present primarily with constitutional and non-specific symptoms,4 5 posing diagnostic difficulties and causing a treatment delay, potentially leading to worse prognosis and increased mortality associated with the development of aortic aneurysms.6
Nowadays, smartwatches and health applications are being increasingly used in everyday life for self-monitoring.7 They are also widely used for monitoring of diseases, which might potentially prove very useful for the monitoring of patients with chronic diseases.8
We present a case of an extracranial GCA in a female patient, presenting with non-specific constitutional symptoms and persistent elevated resting heart rate (RHR), as revealed by a smartwatch healthcare application (Fitbit Alta HR).9
Case presentation
A 57-year-old Caucasian woman presented with a persistent elevation in her RHR, compared with her baseline, and unexplained fatigue. On further questioning, she mentioned myalgias without stiffness in the neck and shoulder girdle, intermittent night sweats, weight loss of 4 kg and exertional dyspnoea of 3 months duration. She denied fever, headache, scalp tenderness, jaw pain, visual symptoms, chest pain and arm claudication. She has always been physically active and her baseline RHR prior to symptom onset was around 55 beats per minute (bpm), which is why she became increasingly worried and sought medical attention, when she noticed that this had increased to 75 bpm (figure 1).
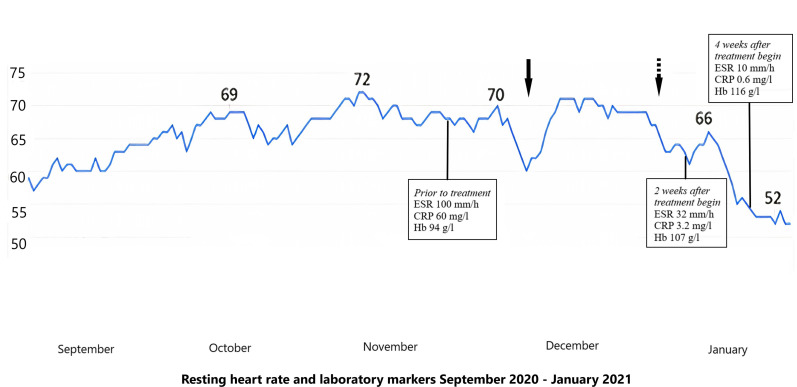
Figure 1.
Graphic representation of the resting heart rate in beats per minute since the symptom onset. The arrows show the rapid effect of treatment with glucocorticoids on the resting heart rate. CRP, C reactive protein; ESR: erythrocyte sedimentation rate; Hb haemoglobin.
Two months prior to the presentation in our department and because of the unexplained shortness of breath, elevated d-dimers and constitutional symptoms, her general practitioner ordered a CT of the chest in order to exclude pulmonary embolism and lung cancer. The examination was interpreted as normal and mild wall thickening of the aorta was missed (figure 2). The ECG, echocardiography and stress test helped exclude a cardiac aetiology of the symptoms. A colonoscopy and gynaecological examination a year ago had been normal.
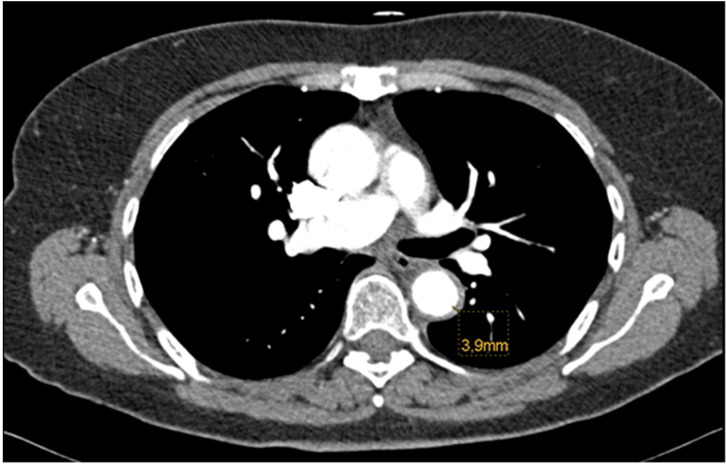
Figure 2.
CT angiogram of the thorax showing a wall thickening of the aorta (3.9 mm).
One month before the consultation in our clinic, the blood tests showed for the first time elevated erythrocyte sedimentation rate (70 mm/hour) and C-reactive protein (CRP) (51 mg/L) and the patient was empirically treated for polymyalgia rheumatica (PMR), albeit with high dose glucocorticoids (prednisone 80 mg daily) inducing prompt relief of all her symptoms and a decrease in her RHR. However, when the prednisone dose was reduced a week later to less than 20 mg per day, her symptoms returned and her heart rate increased again (figure 1, arrow). Other causes of elevated heart rate (ie, anaemia, fever, hyperthyroidism) were excluded.
Because of the resistance to glucocorticoids, the patient was then referred to our clinic with a high degree of suspicion of GCA in association with PMR. She denied any cranial symptoms or limb claudication. In the clinical examination, the temporal arteries were without abnormalities, but the auscultation of both subclavian and axillary arteries revealed loud vascular bruits. All peripheral pulses were normal and there was no difference in the blood pressure measurements in the upper extremities. There were no clinical signs of pericarditis, heart murmurs or signs of heart failure.
Investigations
The inflammatory markers where elevated (CRP=60 mg/L, erythrocyte sedimentation rate=100 mm/hour). The antinuclear antibodies and the antineutrophil cytoplasmic antibodies were negative. The serum protein electrophoresis and immunofixation were normal. The ultrasound showed no abnormalities of the temporal arteries, but demonstrated wall thickening of the subclavian and axillary arteries, which was initially interpreted by the examiner as hyperechoic and thus atypical for vasculitis and rather compatible with atherosclerosis (figure 3). Because of the ambiguous results of the ultrasound examination and the strong clinical suspicion of LLV, we performed an additional 18fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT, which demonstrated unequivocal aorta and large vessel circumferential wall thickening with increased metabolic activity compatible with LVV (figure 4). A temporal artery biopsy was not performed because the patient denied cranial symptoms, we did not find any abnormalities of the temporal arteries in the clinical examination and the ultrasound did not show any changes in the temporal arteries.
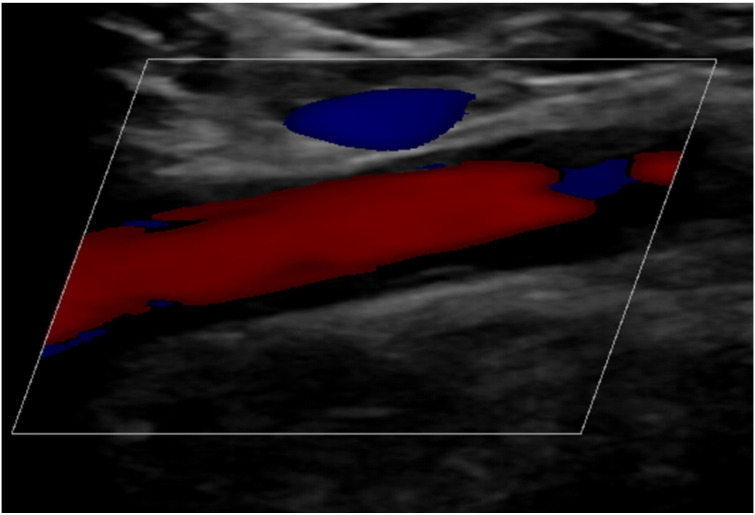
Figure 3.
Ultrasound examination of the right subclavian artery showing wall thickening of the vessel.
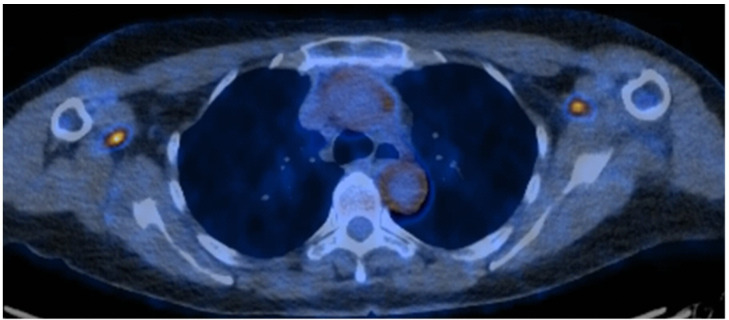
Figure 4.
18FDG-PET/CT showing aorta and large vessel (subclavian and axillary arteries) circumferential wall thickening with increased metabolic activity. 18FDG-PET/CT: 18 fluorodeoxyglucose (FDG)-positron emission tomography (PET) computed tomography (CT).
Treatment
The patient was then treated with high-dose prednisone (50 mg daily) and her symptoms subsided within a few days. Her RHR returned to normal within 10 days (figure 1, the dotted arrow corresponds to the introduction of treatment) and the inflammatory markers normalised 14 days later. We started a glucocorticoid tapering regimen according to the Giant-Cell Arteritis Actemra (GiACTA)-trial,10 and introduced a steroid-sparing treatment with intravenous tocilizumab 8 mg/kg 2 weeks later.
Outcome and follow-up
At the last follow-up, 4 weeks after treatment with glucocorticoids was introduced, she reported being well and denied any other symptoms than a weight gain of 2 kg, due to glucocorticoid-induced fluid retention. Her RHR remained within her baseline values and the lab tests were normal (figure 1).
Discussion
The diagnosis of isolated extracranial GCA can be challenging, since patients usually present with non-specific general symptoms. While clinical manifestation of large vessel involvement is less common, subclinical inflammation is present in most patients with GCA. According to a review of Koster et al histological evidence of LVV has been demonstrated for 80% in autopsy studies and up to 83% of patients with GCA in imaging studies.11 Berger et al emphasised the role of imaging in the one third of the GCA patients with no involvement of the temporal arteries.12 The subclavian arteries were involved in 42% of 187 patients with GCA in a recent prospective longitudinal study.5 The role of imaging proved to be of great importance in our patient who did not have symptoms or signs of cranial involvement. Unfortunately, the diagnosis was delayed because the ultrasound and the CT findings were initially interpreted as rather compatible with atherosclerotic disease, which would be unexpected in an athletic patient with healthy lifestyle, no history of active or passive smoking or family history of premature atherosclerosis.
The persistently elevated RHR led the patient to seek medical advice and contributed to an earlier diagnosis of a potentially severe disease. A numerically slightly elevated RHR of 20 bpm can be easily overlooked, but comparison with previous baseline RHR from her smartwatch showed that it corresponded to an increase of almost 50% compared with baseline RHR before symptom onset. Interestingly, the RHR normalised quickly during the first treatment with glucocorticoids initiated by her general practitioner, increased again when the dose was reduced to less than 20 mg daily and subsequently normalised quickly after the high-dose treatment (50 mg daily) was introduced for the second time.
This is not the first time that a smartwatch application leads to an early diagnosis of a potentially life threatening disease. Walsh et al recently described a case of a 54-year-old female patient with cardiac sarcoidosis who presented with a decreased heart rate.13 Moreover, a recent retrospective smartwatch data analysis of patients with COVID-19 showed that 63% of the patients would have been detected during the pre-symptomatic phase of the infection by means of an elevated RHR.14 However, we could not find in the literature any descriptions of similar cases of increased RHR without evident myocardial injury as one of the presenting manifestations of GCA. Lazzerini et al reported two cases of Torsades de pointes in patients with PMR and suggested a possible role of active inflammation and elevated interleukin-6 (IL-6) levels for arrhythmogenesis in PMR/GCA patients.15 We did not measure IL-6 in our patient but the other inflammatory markers were highly elevated, indicating a systemic inflammatory response. A possible pathophysiological mechanism for the increased heart rate is subclinical myocardial injury. A study by Bechman et al showed that clinically significant myocarditis in LVV is rare, especially in patients with GCA.16 Nair et al described a case of clinically evident myopericarditis in a patient with GCA.17 The ECG of our patient showed no arrhythmias, the echocardiography was unremarkable and the troponin was normal, making a myocarditis unlikely. However, we did not perform a cardiac MRI, which could have possibly showed signs of subclinical myocarditis. Future studies assessing subclinical myocardial injury in patients with GCA would be needed. Other possible mechanisms include a direct effect of the systemic inflammatory response on the myocardium and autonomic nervous system dysfunction, as described in some cases of autoimmune rheumatic diseases.18–20 Moreover, Syngle et al reported a case of improvement of autonomic dysfunction in a patient with severe rheumatoid arthritis following treatment with IL-6 blockade with tocilizumab.21 The exact mechanism remains unclear and further investigation might shed light on this topic.
Smartwatch applications may also have a potential role in monitoring of patients with an established diagnosis of LVV. The inflammatory burden associated with a relapse of a systemic inflammatory disease could be mirrored by rises in RHR, which might even occur during the preclinical stage of a relapse. However, even if this is true, this would not necessarily mean that those patients need an adjustment of their treatment. In any case, we believe that being able to early identify patients at imminent risk for clinical relapse using smartwatch applications could potentially be a cost-effective strategy in the management of LVV.
In our patient, the persistently elevated RHR, the constitutional symptoms and the high degree of suspicion led to the diagnosis of GCA. We suggest that a persistently elevated RHR compared with baseline, though non-specific, may induce systematic work-up and lead to an early diagnosis of a life-threatening, systemic inflammatory disease, such as LVV, especially when other symptoms are not typical. It remains to be seen to what extent smartwatch healthcare applications will be incorporated in the diagnostic armamentarium in the future.
Patient’s perspective.
Until I was finally diagnosed with vasculitis I was feeling constantly exhausted, I knew something was wrong and I thought that I must be ill. The application in my smartwatch had been recording a high resting heart rate for many weeks, which I found very worrying, given the fact that I have always been physically active and my normal values were around 50 beats per minute. I am happy that the cause of my symptoms was finally found and that a treatment could be started. My symptoms completely disappeared as early as 2 days after I started taking prednisolone. I finally feel healthy again after many months. I am looking forward to reducing the dose of the steroids, because I gained some weight even if I try to avoid salt and sugar as much as possible.
Learning points.
Persistent abnormalities in health monitoring applications should be further investigated, especially when associated with unexplained symptoms.
A persistently elevated resting heart rate, as revealed by smartwatch healthcare applications, may lead to an early diagnosis of a life threatening, systemic inflammatory disease, such as large vessel vasculitis.
Most patients with giant cell vasculitis have at least subclinical inflammation of the large vessels.
Imaging is of paramount importance in diagnosing large vessel vasculitis, especially when patients present with isolated involvement of the extracranial arteries and without typical cranial manifestations.
Footnotes
Contributors: NG and MW treated the patient. NG wrote the case report; MW, OD and MB supervised the writing and made some major changes in manuscript after reviewing the first versions. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.González-Gay MA, Prieto-Peña D, Martínez-Rodríguez I, et al. Early large vessel systemic vasculitis in adults. Best Pract Res Clin Rheumatol 2019;33:101424. 10.1016/j.berh.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 2.Nuenninghoff DM, Hunder GG, Christianson TJH, et al. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum 2003;48:3522–31. 10.1002/art.11353 [DOI] [PubMed] [Google Scholar]
- 3.Salvarani C, Cantini F, Boiardi L, et al. Polymyalgia rheumatica and giant-cell arteritis. N Engl J Med 2002;347:261–71. 10.1056/NEJMra011913 [DOI] [PubMed] [Google Scholar]
- 4.Muratore F, Kermani TA, Crowson CS, et al. Large-vessel giant cell arteritis: a cohort study. Rheumatology 2015;54:463–70. 10.1093/rheumatology/keu329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kermani TA, Diab S, Sreih AG, et al. Arterial lesions in giant cell arteritis: a longitudinal study. Semin Arthritis Rheum 2019;48:707–13. 10.1016/j.semarthrit.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kermani TA, Warrington KJ, Crowson CS, et al. Large-vessel involvement in giant cell arteritis: a population-based cohort study of the incidence-trends and prognosis. Ann Rheum Dis 2013;72:1989–94. 10.1136/annrheumdis-2012-202408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reeder B, David A. Health at hand: a systematic review of smart watch uses for health and wellness. J Biomed Inform 2016;63:269–76. 10.1016/j.jbi.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 8.King CE, Sarrafzadeh M. A survey of Smartwatches in remote health monitoring. J Healthc Inform Res 2018;2:1–24. 10.1007/s41666-017-0012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitbit. Available: https://www.fitbit.com
- 10.Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2016;387:1921–7. 10.1016/S0140-6736(16)00560-2 [DOI] [PubMed] [Google Scholar]
- 11.Koster MJ, Matteson EL, Warrington KJ. Large-vessel giant cell arteritis: diagnosis, monitoring and management. Rheumatology 2018;57:ii32–42. 10.1093/rheumatology/kex424 [DOI] [PubMed] [Google Scholar]
- 12.Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly 2018;148:w14661. 10.4414/smw.2018.14661 [DOI] [PubMed] [Google Scholar]
- 13.Walsh KA, Lin D. A Smartwatch heart rate monitor prompts an unusual diagnosis. JACC Case Rep 2020;2:431–3. 10.1016/j.jaccas.2019.11.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra T, Wang M, Metwally AA, et al. Pre-symptomatic detection of COVID-19 from smartwatch data. Nat Biomed Eng 2020;4:1208–20. 10.1038/s41551-020-00640-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazzerini PE, Bertolozzi I, Acampa M, et al. Torsades de pointes in patients with polymyalgia rheumatica. Curr Pharm Des 2018;24:323–40. 10.2174/1381612824666180111111124 [DOI] [PubMed] [Google Scholar]
- 16.Bechman K, Gopalan D, Nihoyannopoulos P, et al. A cohort study reveals myocarditis to be a rare and life-threatening presentation of large vessel vasculitis. Semin Arthritis Rheum 2017;47:241–6. 10.1016/j.semarthrit.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 17.Nair JR, Somauroo JD, Over KE. Myopericarditis in giant cell arteritis: case report of diagnostic dilemma and review of literature. BMJ Case Rep 2012;2012:bcr1220115469. 10.1136/bcr.12.2011.5469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syngle A, Verma I, Krishan P. Autonomic dysfunction in rheumatoid arthritis improves with disease modifying anti rheumatic drugs. Arthritis Rheum-Us 2013;65:S169-S. [Google Scholar]
- 19.Stojanovich L. Autonomic dysfunction in autoimmune rheumatic disease. Autoimmun Rev 2009;8:569–72. 10.1016/j.autrev.2009.01.018 [DOI] [PubMed] [Google Scholar]
- 20.Ingegnoli F, Buoli M, Antonucci F, et al. The link between autonomic nervous system and rheumatoid arthritis: from bench to bedside. Front Med 2020;7:589079. 10.3389/fmed.2020.589079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Syngle A, Verma I, Krishan P. Interleukin-6 blockade improves autonomic dysfunction in rheumatoid arthritis. Acta Reumatol Port 2015;40:85–8. [PubMed] [Google Scholar]