Abstract
A 21-year-old patient presented with sudden-onset headache, visual disturbance and left hand incoordination. She was diagnosed with a left vertebral artery dissection of the V3 segment resulting in multiple cerebellar and cerebral infarcts. There were no risk factors for dissection other than recent COVID-19 infection. She was treated initially with antiplatelets, followed by anticoagulation, but experienced recurrent ischaemia. Although guidance suggests endovascular repair may be beneficial for patients with cerebral artery dissection (CAD) who experience recurrent strokes on medical therapy, evidence is limited. After multidisciplinary team consideration of the individual patient anatomy and risks and benefits of different endovascular techniques, the patient was treated with endovascular coiling. At 10 months follow-up, she had no further strokes and improving neurological symptoms. The case highlighted COVID-19 as a potential trigger for CAD and the use of endovascular coiling in preventing catastrophic cerebral ischaemia in CAD refractive to medical therapy.
Keywords: neurology, neuroimaging, stroke, interventional radiology
Background
Cerebral artery dissection (CAD) accounts for up to 15% of ischaemic strokes in young patients.1 Although the most common cause is neck trauma, many other triggers have been identified, including infection.2–6 While COVID-19 has been well described as a risk factor for ischaemic stroke in young patients, only two case studies have described COVID-19 infection as a potential trigger for CAD.7–9 Our case adds to the literature regarding COVID-19 as a risk factor for CAD in young patients with no other identifiable risk factors.
Our case also describes the challenge of managing patients with CAD who experience recurrent strokes on optimum medical management. Clinical guidance recommends initial treatment with anticoagulation and/or antiplatelet therapy.10–13 The Cervical Artery Dissection in Stroke Study (CADISS) study reported that recurrent strokes only affect 2.5% of patients with CAD who receive antiplatelet or anticoagulation therapy.14 Although guidelines recommend endovascular therapy for patients with recurrent ischaemia on medical therapy,10 the evidence base is limited to case studies and case series. Further, no studies to date have compared the efficacy and safety of different endovascular techniques. Our case describes the difficulty in selecting an appropriate endovascular technique based on the limited available evidence along with consideration of individual anatomy and location of the dissection. This case also highlights the value of a multidisciplinary team approach of neurologists, neurosurgeons and interventional radiologists in the management of rare complex cases where clinical evidence is lacking.
Case presentation
A 21-year-old woman presented with a 2-week history of intermittent visual disturbances, followed by a sudden-onset severe, sharp headache and left hand incoordination. She deteriorated 2 days later with worsening headache, photophobia, neck stiffness and worsening left arm incoordination. On examination, there was reduced peripheral vision in all four quadrants bilaterally, with macular sparing. Eye movements and pupillary reflexes were normal, and ophthalmoscopy showed healthy optic discs and retinal vessels. On upper limb examination, there was dysmetria and dysdiadochokinesia while lower limb examination was unremarkable.
Investigations
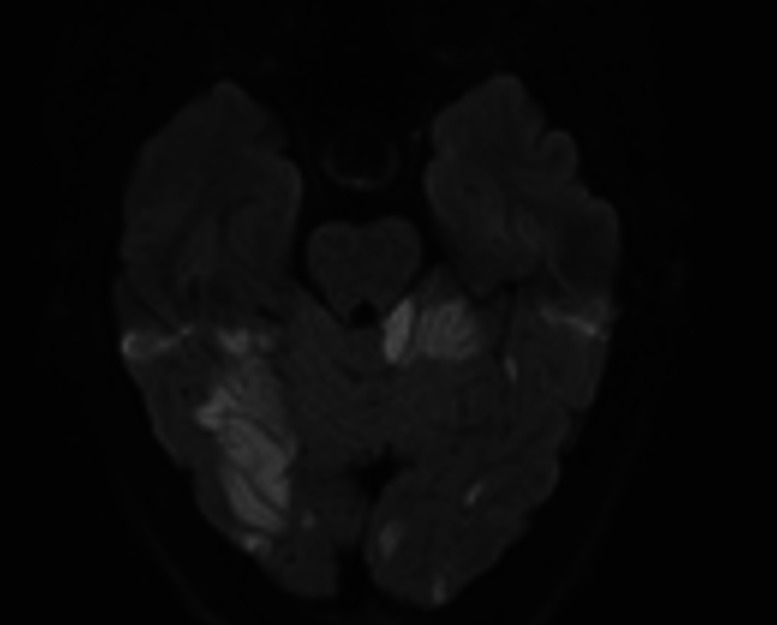
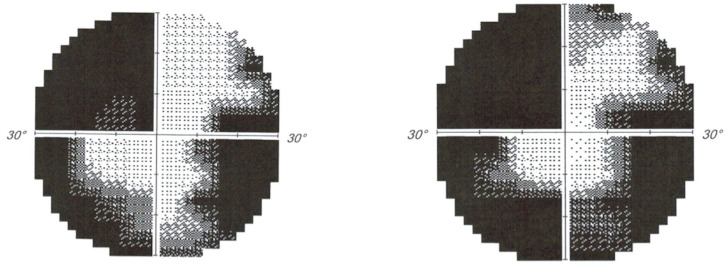
Initial CT showed low attenuation in the occipital lobes bilaterally while an MRI demonstrated acute ischaemic changes in the posterior circulation bilaterally (figure 1). Visual field testing revealed reduced peripheral vision with congruous loss of superior fields and thirty degree field loss inferiorly (figure 2). A vertebral artery dissection was suspected and this was confirmed on CT angiogram, which revealed focal narrowing of the left V3 segment, in keeping with a dissection flap or thrombus.
Figure 1.
Diffusion-weighted imaging of the brain demonstrating bilateral posterior cerebral artery territory acute restricted diffusion including cerebellar ischaemic areas.
Figure 2.
Formal visual field analysis.
The patient was started on 300 mg aspirin in line with current clinical evidence,14 however, 5 days later, she experienced further headache and worsening in her vision. Repeat MRI head confirmed evolution of the bilateral occipital lobe infarcts and new left cerebellar hemispheric infarction.
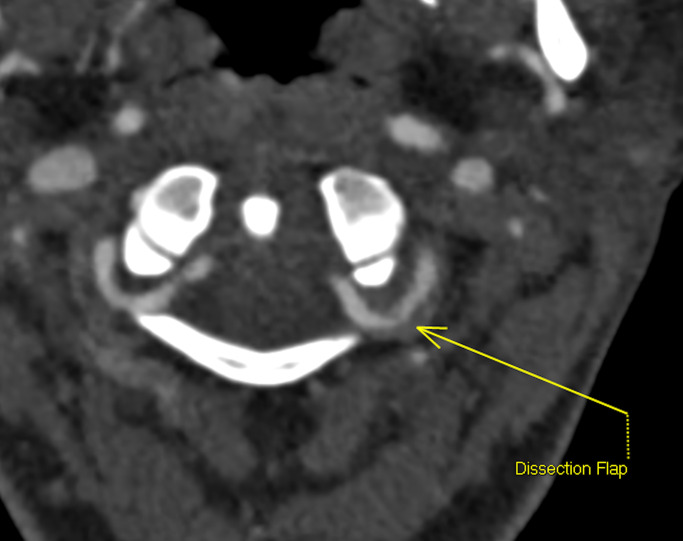
Due to recurrent infarcts while on aspirin, she was commenced on an intravenous heparin infusion. This was preferable to LMWH due to its short half-life and rapid reversibility in the event of haemorrhagic transformation. She was switched to low molecular weight heparin after 72 hours of clinical stability however experienced worsening visual fields and headache and a repeat MRI demonstrated the presence of a new left posterior thalamic stroke and microhaemorrhages in the left occipital region. She was again restarted on an intravenous heparin infusion. The activated partial thromboplastin time (APTT) ratio was maintained at a target ratio of 2–2.5. An MR angiogram 2 days later confirmed a dissection flap of the left V3 segment and identified further infarction in the right superior cerebellar artery territory (figure 3).
Figure 3.
CT angiogram demonstrating the dissection flap at the left vertebral artery distal V3 segment.
Differential diagnosis
The aetiological cause for ongoing ischaemia was felt to be embolic thrombi from an unstable vertebral artery dissection. The cause of this patient’s vertebral artery dissection was unclear in the absence of a history of trauma. Cereberospinal Fluid (CSF) studies were normal. Blood investigations showed normal clotting, autoimmune screen, thrombophilia and antiphospholipid screens. Infective and cardiological causes were also investigated with no cause found. The patient reported infection with COVID-19 5 months previously, which has been reported to increase the risk of thrombotic events.15 16
Treatment
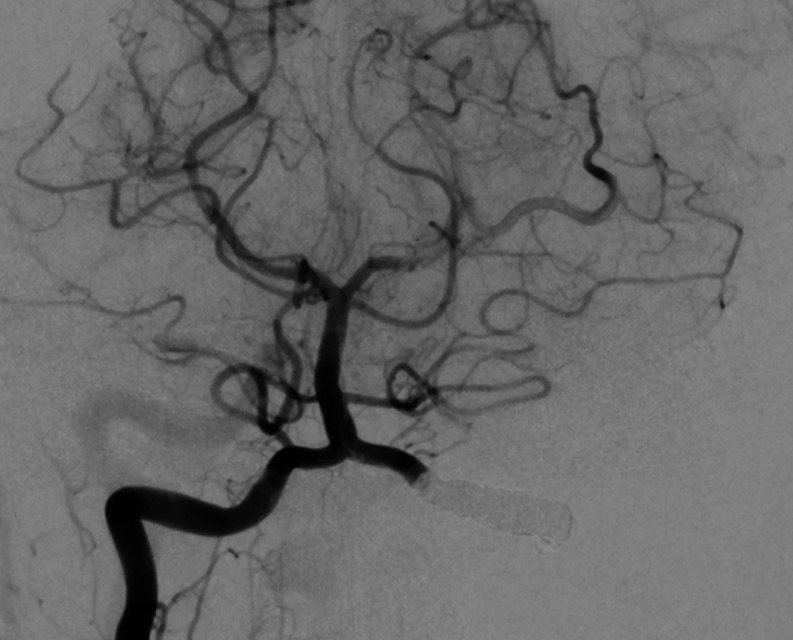
In view of ongoing ischaemic embolic strokes despite maximal anticoagulation and the risk of a potential catastrophic brainstem infarction she was consented for a left vertebral artery coiling of the V3 segment as a definitive treatment of the dissection flap. Postprocedure angiography is shown in figure 4. Postprocedure she was commenced on aspirin 75 mg to continue for a minimum of 6 months.
Figure 4.
Right vertebral artery angiogram demonstrating the left V3/4 segment occlusion with coils.
Outcome and follow-up
The patient remained well and on follow-up 10 months after the procedure she had noticed an improvement in her coordination and visual fields, with no further ischaemic cerebrovascular events. Follow-up MRI scan at 8 months confirmed multiple mature infarcts in the cerebellum and occipital lobes bilaterally with occlusion of the sacrificed left vertebral segment. No new infarctions were identified.
Discussion
CAD is a well-recognised cause of acute ischaemic stroke, particularly in younger patients where it accounts for up to 15% of cases.1 Studies have shown that up to 40% of spontaneous CADs are associated with trauma or other mechanical events.2 Other common aetiological factors include connective tissues disease3 and vascular disorders; fibromuscular disorder in particular has been shown to be accountable for up to 40% of CADs.6 Other recognised risk factors include smoking, hypertension, migraine and recent infection.4 5 Despite investigation no obvious risk factors for CAD were identified in the patient, however, she did report infection with COVID-19 5 months previously. The link is tenuous although it is important to consider. Since the start of the COVID-19 pandemic multiple reviews have highlighted a link between the infection and ischaemic stroke.17–20 Other published case reports have suggested a link between CAD and COVID-19 infection; two vertebral artery dissections7 8 and one carotid dissection.9 Similar to our patient, both patients with vertebral artery dissection (VAD) were young women with no other risk factors. In contrast to our case, however, the onset of COVID-19 infection and CAD was a matter of days to weeks for one patient rather than 5 months in our patient.7 The onset of COVID-19 infection relative to CAD was less clear for the other VAD case, as the patient was not confirmed as COVID-19 positive until several days after admission with CAD.8 Unlike our patient, both cases did not experience secondary ischaemic events and were successfully managed with medical therapy. It is increasingly recognised that COVID-19 increases the risk of thrombotic events, and various case series have demonstrated an increased risk of stroke in patients with COVID-19 infection.15 16 The suggested pathological processes include an exaggerated inflammatory response leading to vascular endothelial dysfunction and coagulopathy.14 It is unclear whether COVID-19 contributed to the development of CAD in our patient, especially given the substantial time gap of 5 months from COVID-19 infection to symptomatic dissection. More long-term studies are required to identify long term risk for cerebrovascular disease in patients who have had a history of COVID-19.
The aims of treatment for CAD are to prevent arterial rupture, further ischaemia and infarction, while preserving blood supply to the associated vascular territory.21 Current guidelines recommend initial therapy with antiplatelets and/or anticoagulants.10–13 The randomised CADISS clinical trial showed no significant difference between antiplatelet and anticoagulant treatments in rates of further ischaemic stroke, subarachnoid haemorrhage and death at 3 months.14 In the more recent TREAT-CAD trial, aspirin was not shown to be non-inferior to vitamin K agonists at preventing ischaemic events following a CAD.22
Unfortunately, despite optimal medical management our patient continued to have new infarctions, creating a clinical challenge. Recurrent ischaemia despite anticoagulation remains a rare event, with the CADISS study reporting that approximately 2.5% of patients develop recurrent ischaemia.14 The TREAT-CAD trial suggested that new ischaemic events with symptoms occur in 10% of patients treated with Aspirin and 6% treated with vitamin K antagonists following a CAD.22 Guidance recommends that endovascular therapy may be considered for patients with CAD who experience recurrent ischaemia despite antiplatelet or anticoagulation,10 but data are limited to case reports and case series. Therefore, the management of patients with CAD and recurrent ischaemia on optimal medical therapy presents a clinical management challenge, with no large randomised control trials investigating the optimal way to manage these patients.
Interventional neuroradiological techniques have been trialled and reported in various case reports and case series. In one case series of carotid artery dissection, seven patients underwent endovascular stent angioplasty and all showed clinical improvement post- procedure, except one who developed haemorrhagic transformation of a large ischaemic stroke.23 In a case series of 27 patients with VAD, those with hypoplastic or codominant vertebral arteries (such as our patient) were treated with deconstruction (coiling) and those with dominant vertebral arteries underwent reconstruction (stenting).24 Twenty-two patients had favourable outcomes with similar rates of post-treatment infarct for reconstructive and deconstructive treatment, and one fatal intracerebral haemorrhage in a patient who underwent pipeline placement.24 The American Heart Association (AHA) recommend considering management with angioplasty and stenting in patients who have not responded to antithrombic therapies following CAD.14
In our patient, a multidisciplinary team comprising neurology, neurosurgery and interventional radiology was employed to consider the cervical anatomy, location of dissection flap and available endovascular techniques to decide on the optimal management for this patient. Coiling was chosen rather than stenting as the patient’s dissection flap was located in the horizontal distal part of the vertebral artery V3 segment. Therefore, the risk of intradural extension of the dissection into the V4 and basilar artery with stenting was deemed to be high. The risk of post stroke haemorrhage was also deemed to be higher with stenting as the patient would require dual antiplatelet treatment as opposed to aspirin treatment long term. In our patient, a positive outcome was seen at 10 months follow-up with improving neurological symptoms and no further ischaemic events. Well-designed and high powered clinical trials are required to investigate optimal endovascular treatment in patients with cerebral ischaemia secondary to CAD.
Learning points.
Vertebral artery dissection with ischaemic emboli should be considered in the differential diagnosis of acute headache with focal neurological deficit.
Interventional radiological procedures, such as vertebral artery coiling, can be instrumental in avoiding potentially catastrophic embolisation in vertebral artery dissection.
COVID-19 may contribute to the aetiology of acute vertebral artery dissection in patients with no other risk factors although more research is required to investigate long-term risks.
Footnotes
NM, LMW and MB contributed equally.
Contributors: NM and LMW conceived and drafted the manuscript. These authors contributed equally to the work. MB edited the manuscript. AC provided the imaging review and description.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
References
- 1.Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol 2009;8:668–78. 10.1016/S1474-4422(09)70084-5 [DOI] [PubMed] [Google Scholar]
- 2.Engelter ST, Grond-Ginsbach C, Metso TM, et al. Cervical artery dissection: trauma and other potential mechanical trigger events. Neurology 2013;80:1950–7. 10.1212/WNL.0b013e318293e2eb [DOI] [PubMed] [Google Scholar]
- 3.Grond-Ginsbach C, Debette S. The association of connective tissue disorders with cervical artery dissections. Curr Mol Med 2009;9:210–4. 10.2174/156652409787581547 [DOI] [PubMed] [Google Scholar]
- 4.Guillon B, Berthet K, Benslamia L, et al. Infection and the risk of spontaneous cervical artery dissection: a case-control study. Stroke 2003;34:29. 10.1161/01.STR.0000078309.56307.5C [DOI] [PubMed] [Google Scholar]
- 5.Micheli S, Paciaroni M, Corea F, et al. Cervical artery dissection: emerging risk factors. Open Neurol J 2010;4:50–5. 10.2174/1874205X01004010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talarowska P, Dobrowolski P, Klisiewicz A, et al. High incidence and clinical characteristics of fibromuscular dysplasia in patients with spontaneous cervical artery dissection: the ARCADIA-POL study. Vasc Med 2019;24:112–9. 10.1177/1358863X18811596 [DOI] [PubMed] [Google Scholar]
- 7.Dakay K, Kaur G, Gulko E, et al. Reversible cerebral vasoconstriction syndrome and dissection in the setting of COVID-19 infection. J Stroke Cerebrovasc Dis 2020;29:105011. 10.1016/j.jstrokecerebrovasdis.2020.105011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel P, Khandelwal P, Gupta G, et al. "COVID-19 and cervical artery dissection- A causative association?". J Stroke Cerebrovasc Dis 2020;29:105047. 10.1016/j.jstrokecerebrovasdis.2020.105047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrasi M, Bigni B, Cobelli M. Bilateral carotid artery dissection in a SARS-CoV-2 infected patient: causality or coincidence? J Neurol 2020;12:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines, and the American stroke association, American association of neuroscience nurses, American association of neurological Surgeons, American College of radiology, American Society of Neuroradiology, Congress of neurological Surgeons, society of atherosclerosis imaging and prevention, Society for cardiovascular angiography and interventions, society of interventional radiology, society of NeuroInterventional surgery, Society for vascular medicine, and Society for vascular surgery. J Am Coll Cardiol 2011;57:e16–94. 10.1016/j.jacc.2010.11.006 [DOI] [PubMed] [Google Scholar]
- 11.NICE guideline [NG128] . Stroke and transient ischaemic attack in over 16s: diagnosis and initial management, 2019. [PubMed] [Google Scholar]
- 12.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke 2018;49:e46–110. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 13.Royal College of Physicians . National clinical guideline for stroke, 2016. Available: http://www.strokeaudit.org [Accessed 13 Feb 2021].
- 14.Markus HS, Levi C, King A. Cervical artery dissection in stroke study (CADISS) Investigators. antiplatelet therapy vs anticoagulation therapy in cervical artery dissection: the cervical artery dissection in stroke study (CADISS) randomized clinical trial final results. JAMA Neurol 2019;76:657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oxley TJ, Mocco J, Majidi S, et al. Large-Vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med 2020;382:e60. 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Li M, Wang M, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol 2020;5:279–84. 10.1136/svn-2020-000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol 2020;19:767–83. 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fifi JT, Mocco J. COVID-19 related stroke in young individuals. Lancet Neurol 2020;19:713–5. 10.1016/S1474-4422(20)30272-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogrig A, Gigli GL, Bnà C, et al. Stroke in patients with COVID-19: clinical and neuroimaging characteristics. Neurosci Lett 2021;743:135564. 10.1016/j.neulet.2020.135564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nannoni S, de Groot R, Bell S, et al. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke 2021;16:137–49. 10.1177/1747493020972922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng J, Liu Z, Luo C, et al. Treatment of cervical artery dissection: antithrombotics, thrombolysis, and endovascular therapy. Biomed Res Int 2017;2017:3072098 10.1155/2017/3072098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelter ST, Traenka C, Gensicke H, et al. Aspirin versus anticoagulation in cervical artery dissection (TREAT-CAD): an open-label, randomised, non-inferiority trial. Lancet Neurol 2021;20:341–50. 10.1016/S1474-4422(21)00044-2 [DOI] [PubMed] [Google Scholar]
- 23.Edgell RC, Abou-Chebl A, Yadav JS. Endovascular management of spontaneous carotid artery dissection. J Vasc Surg 2005;42:854–60. 10.1016/j.jvs.2005.06.029 [DOI] [PubMed] [Google Scholar]
- 24.Gupta G, Eckstein DA, Narayan V, et al. Endovascular management of intracranial vertebral artery dissection: technical nuances for the preservation of posterior inferior cerebellar artery and basilar artery. Oper Neurosurg 2020;19:241–8. 10.1093/ons/opaa174 [DOI] [PubMed] [Google Scholar]