Abstract
Methaemoglobinaemia is a potentially life-threatening condition characterised by hypoxaemia, cyanosis, pallor, fatigue, metabolic acidosis, headache and in severe cases, coma or death. Topical anaesthetics have been reported to cause methaemoglobinaemia. Topical benzocaine was specifically implicated in roughly 66% of anesthetic-induced methaemoglobinaemia cases in a large systematic review in adults. This complication has occurred often in adult patients with pre-existing comorbidities resulting in diminished use in children overall with only few paediatric cases reported worldwide. Additionally, there is growing evidence of a link between sepsis and methaemoglobinaemia due to increased circulating nitrous oxide from infectious pathogen metabolism. In this report, we discuss a case of a 16-year-old young boy, being evaluated for suspected endocarditis, presenting with acute methaemoglobinaemia after use of topical benzocaine spray for transesophageal echocardiogram. This case exemplifies the importance of blood gas with co-oximetry testing in all cases of refractory hypoxemia who have had procedures requiring topical anaesthetics.
Keywords: paediatrics (drugs and medicines), neonatal and paediatric intensive care, paediatrics, paediatric intensive care
Background
Methaemoglobinaemia is a relatively uncommon but life-threatening condition involving an abnormal accumulation of methaemoglobin in the blood. Symptoms include hypoxaemia, cyanosis, pallor, fatigue, metabolic acidosis, headache, and in severe cases, coma or death. It can manifest because of inherited enzyme mutations or deficiencies (congenital methaemoglobinaemia) or from acquired exposure to a causative agent such as certain types of anaesthetics.1 Congenital methaemoglobinaemia occurs due to a deficiency in Nicotinamide Adenine Dinucleotide Hydrogen (NADH)-cytochrome b5 reductase and is more rare compared with the acquired form.2 Acquired methaemoglobinaemia can be caused by topical anaesthetics, sulfonamides, and certain types of antibiotics.3 The true incidence of acquired methaemoglobinaemia from topical anaesthetics is difficult to ascertain but exceedingly rare.
Although any topical anaesthetic can cause methaemoglobinaemia, benzocaine had been implicated as the culprit in many cases. The implication of benzocaine in the causation of methaemoglobinaemia is to such a large extent that the US Food and Drug Administration has released several statements emphasising the risk of methaemoglobinaemia with use of benzocaine containing over the counter drugs.4 Benzocaine still is commonly used as a topical oropharyngeal anaesthetic prior to routine inpatient and outpatient endoscopic procedures such as transoesophageal echocardiography (TEE) and bronchoscopies, dental procedures in the hospital. Benzocaine-induced methaemoglobinaemia after transoesophageal echocardiogram had been described in adult literature with few case reports but extremely rare in children and adolescents.5–9
Although the incidence of methaemoglobinaemia is exceedingly rare and generally safe, if left untreated methaemoglobinaemia can lead to cardiopulmonary compromise, neurological sequelae and even death.10 In this report, we describe an adolescent male, initially admitted for complications of methicillin-sensitive Staphylococcus aureus (MSSA) infection, presenting with acute methaemoglobinaemia that occurred after topical benzocaine spray administration for transoesophageal echocardiogram. Review of literature reveals only two cases in paediatrics that were described to have developed methaemoglobinaemia from use of topical benzocaine during endoscopies.11 12 This case is being reported in view of its rarity and relative underappreciation of the true incidence in paediatric literature.
Case presentation
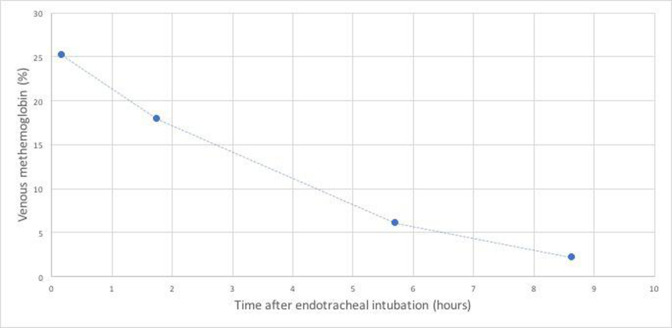
We report a case of a 16-year-old man admitted to our hospital as a transfer from an outside facility for management of left orbital cellulitis and right hip pain. Five days prior while working on demolition in an old pigeon barn, he had stepped on a nail through his sneaker. He is otherwise a healthy adolescent with an up-to-date immunisation status (including tetanus) and no history of allergies, frequent infections or immunodeficiency within the family. Peripheral blood cultures were negative for growth. He underwent orbital washout and functional endoscopic sinus surgery by otolaryngology (Ear Nose Throat doctor) and ophthalmology. These cultures revealed MSSA. Physical examination of his right hip was significant for tenderness and pain with range of motion, but negative for joint effusion subsequently confirmed by hip ultrasound, so arthritis was unlikely. Right hip MRI showed no evidence of osteomyelitis, but extensive pyomyositis with abscess (figure 1). Resultant cultures from irrigation and debridement were consistent with MSSA. Due to a potential vascular focus that could be seeding to other sites in addition to a newly auscultated heart murmur, a transthoracic echocardiography was performed to rule out endocarditis. Although the study was grossly normal, the patient’s body habitus with a body mass index of 33 kg/m2 made for difficult visualisation. As a result, a TEE was performed, which revealed no vegetations with good valve motion and normal biventricular function. Less than 2 hours after the TEE was performed, a rapid response was called for syncope after going to the bathroom, mild respiratory distress and oxygen desaturation to 75%. He was put on 100% non-rebreather which increased his oxygen saturation to low 80s. The patient was transferred to the paediatric intensive care unit (PICU) due to persistent hypoxia. It was hard to appreciate any air entry in his bilateral lower lung fields in the setting of his obesity. His chest X-ray did not show any focal opacities or pneumothorax. Due to his persistent hypoxia in low 80s and no response to high flow nasal cannula with 100% oxygen, bilevel positive airway pressure was initiated once pneumothorax or pneumomediastinum were ruled out. Despite escalating respiratory support, he remained hypoxic with some respiratory distress and so was electively intubated. Due to his persistent hypoxaemia and body habitus and relative immobility from right hip pyomyositis, CT pulmonary angiogram (figure 2) was performed to rule out pulmonary embolism which came back negative. Venous blood gas obtained after intubation resulted a methaemoglobin of 25%. He did not exhibit any cyanosis at any point. All the medications that he had received were investigated as possible causes for his acquired methaemoglobinaemia. On further investigation into his chart and conversation with the adult cardiologist who performed the TEE, we learnt that he was given several sprays of 20% benzocaine (Hurricaine spray) along with viscous lidocaine 2% prior to the procedure. Of note, we also noticed that he had a peripherally inserted central catheter (PICC) line placement that morning before the TEE, where he required eutetic mixture of local anaesthetics (EMLA) cream which is a mixture of 2.5% lidocaine and 2.5% prilocaine for local anaesthesia. Repeat blood gas within 2 hours after intubation showed his methaemoglobin trended down to 18% (figure 3), and he was extubated 4 hours after intubation without complications or further need for respiratory support.
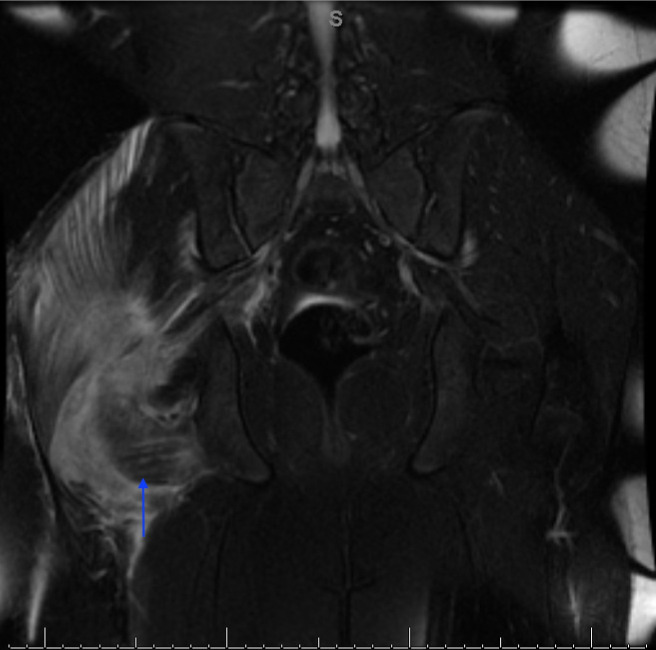
Figure 1.
MRI of the hip: coronal fat-saturated T2-weighted image at the level of the right gluteus intermedius muscle illustrating pyomyositis as hyperintense 8.2×2.8×5.3 cm intramuscular abscess (arrow).
Figure 2.
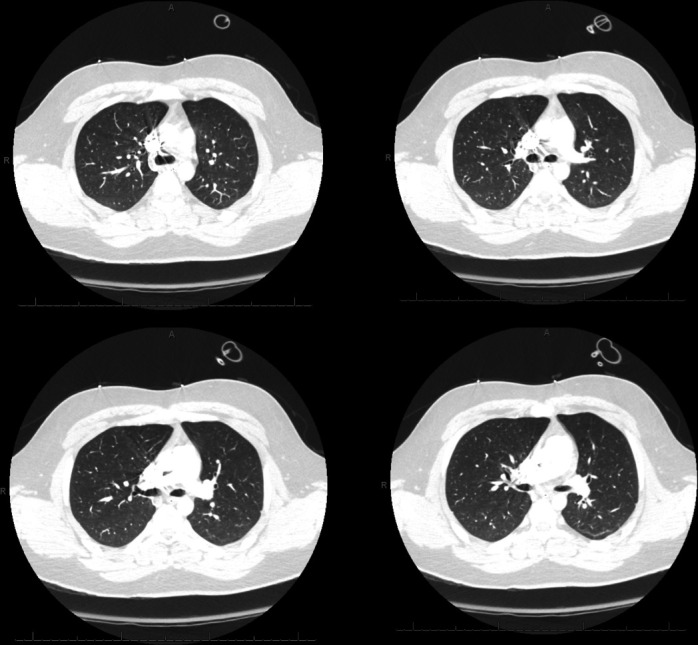
CT pulmonary angiogram of the chest showing no thrombus in the pulmonary artery.
Figure 3.
Spontaneous down-trending of our patient’s venous methaemoglobin levels.
Differential diagnosis
Methaemoglobin levels of 10%–25% are usually associated with cyanosis.13 Our patient was not cyanotic on exam but had other symptoms of dizziness, fatigue, mild dyspnoea and hypoxia. Our differential diagnoses in our patient post-TEE for hypoxaemia included hypoventilation, aspiration pneumonitis, right-to-left shunting, cardiogenic shock, pneumothorax or pneumomediastinum from oesophageal perforation, atelectasis, pulmonary oedema and pulmonary embolus. These were ruled out based on a normal chest X-ray, echocardiogram, clinical examination and haemodynamic parameters. Due to the rarity of use of benzocaine in paediatrics, methaemoglobinaemia was not included in our differential. The diagnosis of methaemoglobinaemia was not concluded until the venous co-oximetry in the blood gas revealed a methaemoglobin level of 25% and confirming with the cardiologist about the use of benzocaine for TEE. Co-oximetry testing can accurately detect the presence of abnormal and normal forms of haemoglobin by measuring the methaemoglobin, carboxyhaemoglobin, and oxyhaemoglobin and reporting them as a percentage of the total haemoglobin concentration. Our case underscores the importance of blood gas co-oximetry in cases of persistent hypoxaemia unresponsive to oxygen therapy.
Treatment
Knowing the cause for his hypoxia, he was extubated subsequently without any complications. We continued supportive treatment with oxygen until his methaemoglobin levels normalised by the next day. As per toxicology and poison control recommendations, our patient was monitored with supportive measures, with serial methaemoglobin levels while not requiring any methylene blue treatment. He was also investigated for possible glucose-6-phosphate dehydrogenase deficiency which was negative. He was observed in the PICU until his methaemoglobin levels normalised 9 hours later and was transferred back to the general paediatric hospital ward.
Outcome and follow-up
Our patient was discharged home after 8-day hospital stay on oxacillin to complete a total of 4-week course for left periorbital cellulitis/subperiosteal abscess associated with MSSA. He was followed by ENT, ophthalmology, orthopaedics and paediatric infectious disease as an outpatient. At 4-week follow-up, the patient was doing great with normal eye exam, full range of motion of his hip and gait with no pain, and normalised inflammatory markers indicating resolved infection.
Discussion
Methaemoglobinaemia is conventionally defined by a blood methaemoglobin level greater than 5%, with levels above 50% often considered fatal.14 Although relatively rare, the incidence of acquired methaemoglobinaemia is higher than congenital forms. Drugs that may induce methaemoglobinaemia are widely used in clinical settings. Acquired methaemoglobinaemia is often unrecognised and thus untreated. Topical anaesthetics, sulfonamides, nitrates and certain types of antibiotics can precipitate methaemoglobinaemia.15 Topical oropharyngeal anaesthetics are routinely used in inpatient and outpatient endoscopic procedures such as TEE and bronchoscopies, dental procedures in the hospital. Although nearly all topical anaesthetic preparations have been associated with methaemoglobinaemia, benzocaine spray is the most common culprit reported in the literature.15 One large systematic review of 242 cases of local anesthetic-related methaemoglobinaemia demonstrated that benzocaine was implicated in 65.7% of total cases. Specifically, benzocaine was used in 51.3% of children and 77% of adults presenting with local-anaesthetic induced methaemoglobinaemia.16 Previous studies abundantly demonstrate that benzocaine is one of the major causative agents of methaemoglobinaemia and should be avoided or used with abundant caution in both paediatric and adult care settings.
Benzocaine spray is available as 14% Cetacaine and, more commonly, 20% Hurricaine.5 Twenty per cent benzocaine spray is routinely used in the hospitals. The exact amount of spray delivered to each individual has not been standardised, although the recommended dose of a 1 s spray is the usual practice. Our patient had two or more Hurricaine sprays along with 2% viscous lidocaine contributing to methaemoglobinaemia. Of note, our patient also received EMLA topical anaesthetic cream for PICC line which might have added to the causation. There have been case reports of EMLA topical anaesthetic causing methaemoglobinaemia in literature.17–21
There is a growing body of evidence demonstrating a link between methaemoglobinaemia and sepsis.22 23 In an infectious process leading to sepsis, large quantities of nitric oxide are released by endothelial cells of the vasculature in response to proinflammatory cytokines due to infection, which subsequently reacts with haemoglobin to form methaemoglobin. One study over 2 years outlines how infants with sepsis, most commonly Staphylococcus, had significantly higher circulating methaemoglobin levels compared with their age and weight-matched healthy counterparts.24 The study also demonstrated that risk factors including, but not limited to, anaemia, cardiovascular anomalies, acidaemia, impaired intestinal motility and imbalance in natural gut flora can predispose infants to formation of nitric oxide and free radicals, increasing the risk of accumulating methaemoglobin.
The biochemistry of methaemoglobin is well documented. Substances that oxidise ferrous (Fe2+) into ferric (Fe3+) make methaemoglobin, which is incapable of carrying oxygen. Further methaemoglobinaemia prevents oxygen release to the tissues aggravating the tissue hypoxia. There is a spontaneous slow rate of methaemoglobin synthesis, due to inappropriate dissociation of a superoxide radical from haemoglobin. As a result, most adults normally have between 0% and 3% methaemoglobin.10 Risk factors for developing methaemoglobinaemia can vary depending on age, pre-existing medical conditions and those who are intolerant to reduction in oxygen carrying capacity.25 Cytochrome b5 reductase is the enzyme responsible for converting ferric methaemoglobin to ferrous oxyhaemoglobin. This enzyme is deficient in congenital methaemoglobinaemia. Even in healthy infants, there is half the erythrocyte cytochrome b5 reductase activity as seen in adults. As a result, infants are more at risk for developing methaemoglobinaemia.26 Conditions such as cardiovascular disease, anaemia and acidosis put individuals at increased risk of developing methaemoglobinaemia when exposed to different oxidising agents.3
From a diagnostic standpoint, co-oximetry via arterial blood gas acquisition determines the extent of patients’ hypoxia by measuring the true fractional oxygen saturation (SaO2). Arterial blood gas acquisition is invasive and inherently involves delays in laboratory quantification of values. Conversely, pulse oximetry is noninvasive and can yield point-of-care oxygen saturation values, but readings provide a less accurate depiction of a patient’s hypoxia compared with arterial blood gas samples. This is an important consideration in critically ill patients receiving supplemental oxygen such as premature neonates at risk for retinopathy of prematurity, or in patients with pulmonary hypertension receiving nitric oxide. Other limitations of pulse oximetry include the oxyhaemoglobin dissociation curve, low perfusion states, motion artefact or presence of nail polish.14 More novel multiwavelength pulse oximeters have been recently developed that can detect methaemoglobinaemia with an accuracy comparable to that achievable with laboratory co-oximeters.27 28 Two studies of healthy adult volunteers with sodium nitrite-induced mild-to-moderate methaemoglobinaemia found that a multiwavelength oximeter (Masimo Rad-57) accurately depicted methaemoglobin levels measured by standard laboratory co-oximetry, with measurement uncertainties of 0.45% and 0.83%.29 30 Another study found that the multiwavelength oximeter, Masimo Radical-7, overestimated true values of methaemoglobin by 10%–45% when SaO2 on radial arterial blood gas sampling dropped below 95%, indicating variability among use of different multiwavelength oximeters.28 Nevertheless, multiwavelength pulse oximeters have more recently able to provide variables (oxygen reserve index) to reflect oxygenation in moderate hyperoxic states.31
Treatment and management of methaemoglobinaemia includes removal of the agent implicated in precipitating methaemoglobinaemia and administration of intravenous methylene blue, which when administered, is metabolised into leukomethylene blue. This metabolite of methylene blue acts as an electron donor, reducing methaemoglobin to functional haemoglobin. Methylene blue infusion is used typically when methaemoglobin levels are 30% for asymptomatic and 20% for symptomatic patients.25 Methylene blue, although highly effective, can also precipitate refractory methaemoglobinaemia in high doses and harbours a risk of inciting serotonin syndrome in patients using serotonergic agents (due to its inhibitory effects on MAO-A). Other treatment options include the use of high-dose ascorbic acid, exchange transfusions and hyperbaric oxygen therapy.10 Our patient required only supportive treatment without any methylene blue required as the levels quickly decreased to less than 20% within the few hours of the diagnosis with continued down-trending. To our knowledge, spontaneous resolution of methaemoglobinaemia without treatment intervention has only been described in two other case reports.17 32 We believe that the rapid decline in methaemoglobin levels in our patient could be due to re-emergence from transient suppression of haemoglobin-reducing systems by the oxidants (benzocaine) back to their normal function.
In our patient, we hypothesise that methaemoglobinaemia was caused by generous use of benzocaine and viscous lidocaine for his TEE procedure preceded by the application of EMLA topical cream for PICC line placement, in combination with sepsis due to MSSA infection as a contributing factor. Blood gas co-oximetry for patients with an unclear aetiology of refractory hypoxaemia serves as an essential part of the workup, as it was crucial to effective diagnosis and management in this case.
Patient’s perspective.
When my son had eye pain and hip pain from his infections, I was first really scared about the surgeries. But when he had to go to the PICU for respiratory failure, it was so unexpected and I was worried that he was going to die. I’m glad the team of doctors could figure out the reason.
After his long antibiotic treatment, his health has been back to normal and he’s finishing high school this year.
Learning points.
Acquired methaemoglobinaemia causes significant morbidity and even mortality if untreated.
The purpose of this report is to illustrate importance of blood gas with co-oximetry testing in all cases of refractory hypoxaemia who had undergone procedures requiring any type of topical anaesthetics.
Paediatric providers performing transesophageal echocardiography, as well as those responsible for postprocedural care of these patients, should be aware of this rare but fatal complication as timely recognition and treatment will improve outcomes.
Footnotes
Contributors: AJ compiled the available literature, drafted the initial manuscript, reviewed and revised the manuscript. DCR reviewed the existing literature, drafted, reviewed and revised the manuscript. JS provided clinical care of the study patient, participated in the intellectual content and conception of this case report, critically reviewed and revised the manuscript. SA managed the clinical care of the study patient, conceptualised the idea of the case report, reviewed the existing literature, drafted the discussion section of the manuscript, reviewed and revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s)
References
- 1.Ludlow JT, Wilkerson RG, Nappe TM. Methemoglobinemia. StatPearls. Treasure Island (FL): StatPearls Publishing, 2021. https://www.ncbi.nlm.nih.gov/books/NBK537317/ [PubMed] [Google Scholar]
- 2.Viršilas E, Timukienė L, Liubšys A. Congenital methemoglobinemia: rare presentation of cyanosis in newborns. Clin Pract 2019;9:106–8. 10.4081/cp.2019.1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warren OU, Blackwood B. Acquired methemoglobinemia. N Engl J Med 2019;381:1158. 10.1056/NEJMicm1816026 [DOI] [PubMed] [Google Scholar]
- 4.Veltri KT, Rudnick E. Benzocaine-Induced methemoglobinemia: a case report. P T 2016;41:180–91. [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhary S, Bukoye B, Bhansali AM, et al. Risk of topical anesthetic-induced methemoglobinemia: a 10-year retrospective case-control study. JAMA Intern Med 2013;173:771–6. 10.1001/jamainternmed.2013.75 [DOI] [PubMed] [Google Scholar]
- 6.Bayard M, Farrow J, Tudiver F. Acute methemoglobinemia after endoscopy. J Am Board Fam Pract 2004;17:227–9. 10.3122/jabfm.17.3.227 [DOI] [PubMed] [Google Scholar]
- 7.Buckley AB, Newman A. Methemoglobinemia occurring after the use of a 20% benzocaine topical anesthetic prior to gastroscopy. Gastrointest Endosc 1987;33:466–7. 10.1016/S0016-5107(87)71702-7 [DOI] [PubMed] [Google Scholar]
- 8.Vallurupalli S, Manchanda S. Risk of acquired methemoglobinemia with different topical anesthetics during endoscopic procedures. Local Reg Anesth 2011;4:25–8. 10.2147/LRA.S22711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filipiak-Strzecka D, Kasprzak JD, Wiszniewska M, et al. The influence of lidocaine topical anesthesia during transesophageal echocardiography on blood methemoglobin level and risk of methemoglobinemia. Int J Cardiovasc Imaging 2015;31:727–31. 10.1007/s10554-015-0608-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skold A, Cosco DL, Klein R. Methemoglobinemia: pathogenesis, diagnosis, and management. South Med J 2011;104:757–61. 10.1097/SMJ.0b013e318232139f [DOI] [PubMed] [Google Scholar]
- 11.Dahshan A, Donovan GK. Severe methemoglobinemia complicating topical benzocaine use during endoscopy in a toddler: a case report and review of the literature. Pediatrics 2006;117:e806–9. 10.1542/peds.2005-1952 [DOI] [PubMed] [Google Scholar]
- 12.So T-Y, Farrington E, EJJoPHC F. Topical benzocaine-induced methemoglobinemia in the pediatric population. J Pediatr Health Care 2008;22:335–9. 10.1016/j.pedhc.2008.08.008 [DOI] [PubMed] [Google Scholar]
- 13.Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med 1999;34:646–56. 10.1016/S0196-0644(99)70167-8 [DOI] [PubMed] [Google Scholar]
- 14.Chan ED, Chan MM, Chan MM. Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respir Med 2013;107:789–99. 10.1016/j.rmed.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 15.Wills BK, Cumpston KL, Downs JW, et al. Causative agents in clinically significant methemoglobinemia: a national poison data system study. Am J Ther 2020;28:e548–51. 10.1097/MJT.0000000000001277 [DOI] [PubMed] [Google Scholar]
- 16.Guay J. Methemoglobinemia related to local anesthetics: a summary of 242 episodes. Anesth Analg 2009;108:837–45. 10.1213/ane.0b013e318187c4b1 [DOI] [PubMed] [Google Scholar]
- 17.Selder JL, Veenstra J. Methaemoglobinaemia after using EMLA cream, 2013. Nederlands Tijdschrift voor Geneeskunde. Available: https://www.ntvg.nl/artikelen/methemoglobinemie-na-gebruik-van-emla-crème [PubMed]
- 18.Lerner RP, Lee E. EMLA-induced methemoglobinemia after laser-assisted hair removal procedure. Am J Emerg Med 2019;37:2119.e1–2119.e2. 10.1016/j.ajem.2019.158415 [DOI] [PubMed] [Google Scholar]
- 19.Hahn I-H, Hoffman RS, Nelson LS. EMLA-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med 2004;26:85–8. 10.1016/j.jemermed.2003.03.003 [DOI] [PubMed] [Google Scholar]
- 20.Eisner P, Dummer R. Signs of methaemoglobinaemia after topical application of EMLA® cream in an infant with haemangioma. Dermatology 1997;195:153–4. 10.1159/000245720 [DOI] [PubMed] [Google Scholar]
- 21.Schmitt C, Matulic M, Kervégant M. Methaemoglobinaemia in a child treated with Emla cream: circumstances and consequences of overdose. Science Direct 2012;12 https://linkinghub.elsevier.com/retrieve/pii/S0151-9638 [DOI] [PubMed] [Google Scholar]
- 22.Ohashi K, Yukioka H, Hayashi M, et al. Elevated methemoglobin in patients with sepsis. Acta Anaesthesiol Scand 1998;42:713–6. 10.1111/j.1399-6576.1998.tb05306.x [DOI] [PubMed] [Google Scholar]
- 23.Schuerholz T, Irmer J, Simon TP, et al. Methemoglobin level as an indicator for disease severity in sepsis. Crit Care 2008;12:P448. 10.1186/cc6669 [DOI] [Google Scholar]
- 24.Schierz IAM, Pinello G, Piro E, et al. Methemoglobinemia associated with late-onset neonatal sepsis: a single-center experience. Am J Perinatol 2019;36:1510–3. 10.1055/s-0039-1678556 [DOI] [PubMed] [Google Scholar]
- 25.Cefalu JN, Joshi TV, Spalitta MJ, et al. Methemoglobinemia in the operating room and intensive care unit: early recognition, pathophysiology, and management. Adv Ther 2020;37:1714–23. 10.1007/s12325-020-01282-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fossen Johnson S. Methemoglobinemia: infants at risk. Curr Probl Pediatr Adolesc Health Care 2019;49:57–67. 10.1016/j.cppeds.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 27.Annabi EH, Barker SJ. Severe methemoglobinemia detected by pulse oximetry. Anesth Analg 2009;108:898–9. 10.1213/ane.0b013e318172af73 [DOI] [PubMed] [Google Scholar]
- 28.Feiner JR, Bickler PE, Mannheimer PD. Accuracy of methemoglobin detection by pulse CO-oximetry during hypoxia. Anesth Analg 2010;111:143–8. 10.1213/ANE.0b013e3181c91bb6 [DOI] [PubMed] [Google Scholar]
- 29.Barker SJ, Curry J, Redford D, et al. Measurement of carboxyhemoglobin and methemoglobin by pulse oximetry: a human volunteer study. Anesthesiology 2006;105:892–7. 10.1097/00000542-200611000-00008 [DOI] [PubMed] [Google Scholar]
- 30.Feiner JR, Bickler PE. Improved accuracy of methemoglobin detection by pulse CO-oximetry during hypoxia. Anesth Analg 2010;111:1160–7. 10.1213/ANE.0b013e3181f46da8 [DOI] [PubMed] [Google Scholar]
- 31.Scheeren TWL, Belda FJ, Perel A. The oxygen reserve index (ori): a new tool to monitor oxygen therapy. J Clin Monit Comput 2018;32:379–89. 10.1007/s10877-017-0049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mortamet G, Oualha M, Renolleau S, et al. Methemoglobinemia following Monolinuron ingestion: a case report in a child. Pediatr Emerg Care 2018;34:e55. 10.1097/PEC.0000000000000745 [DOI] [PubMed] [Google Scholar]