Abstract
Little is known about whether or how plant cells regulate the position of heavy organelles that sediment toward gravity. Dark-grown protonemata of the moss Ceratodon purpureus displays a complex plastid zonation in that only some amyloplasts sediment along the length of the tip cell. If gravity is the major force determining the position of amyloplasts that sediment, then these plastids should be randomly distributed in space. Instead, amyloplasts were clustered in the subapical region in microgravity. Cells rotated on a clinostat on earth had a roughly similar non-random plastid distribution. Subapical clusters were also found in ground controls that were inverted and kept stationary, but the distribution profile differed considerably due to amyloplast sedimentation. These findings indicate the existence of as yet unknown endogenous forces and mechanisms that influence amyloplast position and that are normally masked in stationary cells grown on earth. It is hypothesized that a microtubule-based mechanism normally compensates for g-induced drag while still allowing for regulated amyloplast sedimentation.
The regulation of organelle position is a fundamental feature of eukaryotic cells, a control that is often mediated by the cytoskeleton. In general, the influence of gravity or other mechanical forces on organelle position is poorly understood. Gravity has a negligible effect on organelles whose density (mass per unit volume) is close to that of the surrounding cytoplasm (Björkman, 1988). Denser organelles such as amyloplasts and nuclei usually do not sediment, presumably because they are mechanically associated with the cytoskeleton (Todd, 1989; Chicurel et al., 1998; Morris, 2000). Amyloplast sedimentation is only known to occur in specialized cells, cells that are thought to sense gravity using amyloplast mass (Sack, 1991, 1997).
One cell type specialized for amyloplast sedimentation is the dark-grown apical cell of protonemata of some mosses (Sack, 1997; Sack et al., 1998). This gravitropic cell grows upward by oriented tip growth. In Ceratodon purpureus, the mass of the amyloplasts that sediment probably participates in gravitropic sensing (Kuznetsov et al., 1999). However, only a subset of amyloplasts sediment and only incompletely in vertical cells (Schwuchow and Sack, 1993). In contrast, in other known cell types with sedimentation, all the amyloplasts fall completely to the bottom of the cell (Sack, 1991).
Several lines of evidence suggest that forces in addition to gravity operate on apical cell plastids. Indirect data show that microtubules restrict plastid sedimentation in these cells (Schwuchow and Sack, 1994). Moreover, the zonation in the apical cell is maintained despite the constant acropetal and basipetal movement of most plastids (Young and Sack, 1992).
A unique way to assess the significance of these mechanical forces for amyloplast positioning is to grow the cells in microgravity. If gravity is the major determinant of the position of amyloplasts that sediment at 1g, then plastids should be randomly distributed in space. To test this, the distribution of plastids was analyzed in moss cultures that were grown on an orbiting National Aeronautics and Space Administration (NASA) Space Shuttle. These distributions were compared with those in cells that were upright, inverted, or rotated on a clinostat on earth. Here, we show that microgravity and the randomization of the g-vector on the clinostat result in the clustering of amyloplasts that would otherwise sediment in a constant g-vector. This suggests that endogenous forces act on these amyloplasts in addition to gravity.
RESULTS
Plastid Zonation in Stationary Cells
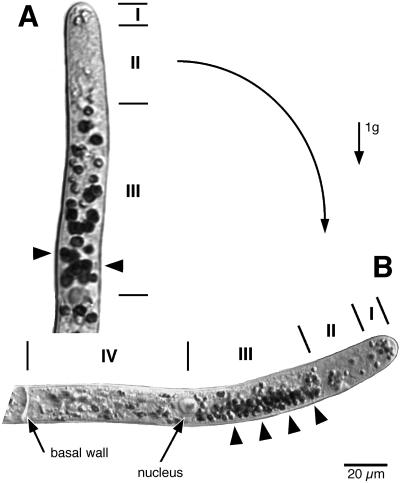
Apical cells can be divided into four zones of plastids based on their distribution and the extent of sedimentation in stationary cells at 1g (Fig. 1; Walker and Sack, 1990). Zone I, the tipmost 10 μm, contains a group of non-sedimenting plastids. Behind this is a plastid-free zone (II, 10–20 μm). The sedimentation zone (III) extends 40 μm from the plastid-free zone to the nucleus. Sedimentation is absent from zone IV, which encompasses the rest of the cell between the nucleus and the basal cell wall. The length of this zone varies from 50 to 150 μm, largely due to cell growth during the cell cycle.
Figure 1.
Plastid zones and gravitropism of the apical cell of C. purpureus protonemata. A, Upright cell. B, Cell that was horizontal for 3 h and was curving upward. Plastid starch stained with I2KI. See text for description of zones I to IV. Note sedimentation (arrowheads) toward base of zone III in A and toward side wall in B. Scale bar = 20 μm.
The pattern of amyloplast sedimentation in zone III is complex (Sack et al., 1998). In upright cells, the plastids sediment along the length of the cell toward the nucleus (arrowheads in Fig. 1A). But not all plastids sediment, and those that sediment fall to varying degrees. This is also true for inverted cells where plastids sediment toward the bottom of the sedimentation zone i.e. toward the plastid free zone (Fig. 2E). When an upright cell is turned to the horizontal, the amyloplasts sediment toward the lateral wall, especially in the apical part of zone III (Fig. 1B). Sedimentation to the lateral wall precedes and is associated with gravitropic curvature.
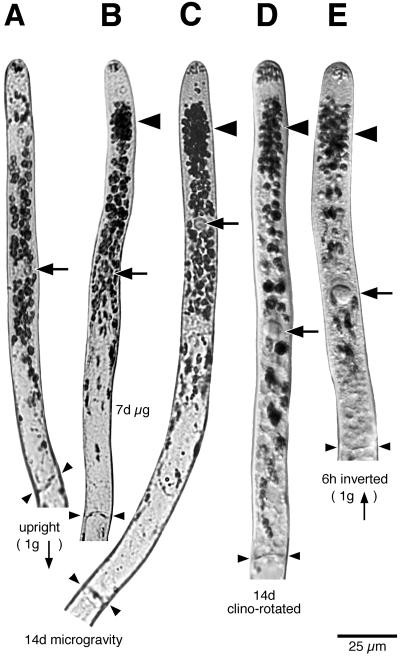
Figure 2.
Plastid distribution in cells grown under different conditions and then chemically fixed. The large arrows indicate nuclei, the small arrows (below A and E) point to the gravity vector; the large arrowheads indicate plastid clusters, and the paired small arrowheads show the location of the basal cell wall. Note the different locations of plastid sedimentation in upright (A) and inverted (E) cells. Plastid clusters were present close to the plastid-free zone in cells grown in microgravity for 7 (B) or 14 d (C), as well as in cells that were rotated on a clinostat on earth (D). Scale bar = 25 μm.
Microgravity
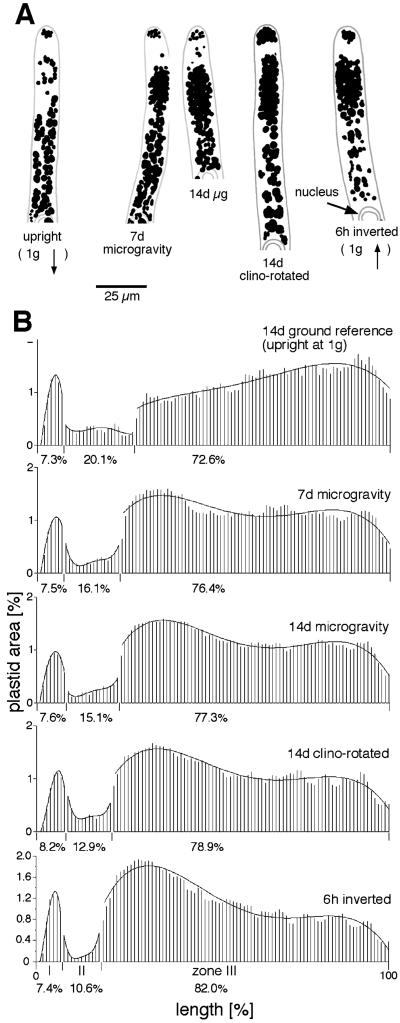
Protonemata were grown in microgravity in the dark for 7 and 14 d before fixation in situ. Plastid zonation was maintained in microgravity as evidenced by the presence of zones I, a group of plastids in the tip, and II, the plastid-free zone (Figs. 2, B and C, and 3A). Dense, oval-shaped plastid clusters were found at the apical end of zone III (Fig. 4A). Mean plastid area was determined in zones I to III from populations of cells (Fig. 3B). In upright cells grown on the ground, plastid area was highest close to the nucleus, a distribution that reflects the effect of sedimentation (Fig. 3B, top). In contrast, the region containing the highest plastid area in microgravity-grown cells was located in the part of zone III that was closest to the plastid-free zone.
Figure 4.
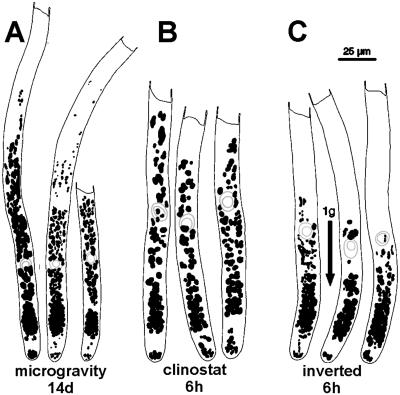
Cluster shapes and plastids in zone IV. A, Dense plastid clusters are present after 14 d in microgravity. B, Looser clusters develop after 6 h of clinostat rotation. C, Elongated clusters in cells inverted for 6 h. Note scarcity of plastids above nucleus. Scale bar = 25 μm.
Figure 3.
Distribution of plastid area in stationary, rotated, and space-grown cells. A, Tracings from micrographs in Figure 2. Scale bar = 25 μm. B, Each bar shows the percentage of total plastid area found in each sector. Values are means for 19 to 34 apical cells for each treatment. The data were normalized for the distance between the cell tip and the center of the nucleus by dividing this distance into 100 sectors. The percentages below each abscissa are the relative plastid areas in zones I to III, respectively. The curves were derived from polynomials expressed to the fourth degree. Note the greater plastid area at the apical part of zone III in space-grown and clino-rotated cells and the opposite curves of upright compared with inverted cells.
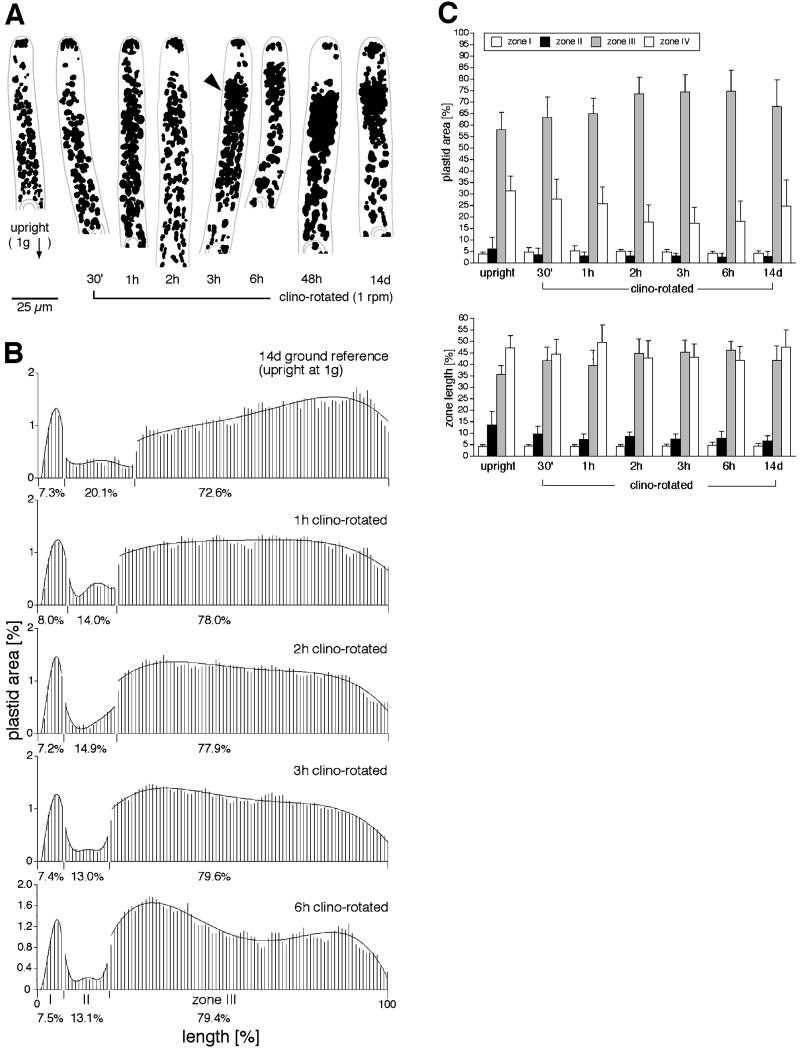
Clinostat Rotation
Cells that are rotated horizontally on a clinostat experience continuously changing orientations with respect to the gravity vector. Apical cells from cultures rotated for 7 or 14 d displayed subapical groupings of amyloplasts that mostly resembled those found in microgravity (Fig. 3A). In some cells grown on a clinostat, the clusters were less compact and oval than those formed in space (Fig. 2D). The development of clusters on a clinostat was independent of the direction of rotation or of whether the long axis of the protonemata (resulting from gravitropism in 7 d prior to placement on clinostat) was perpendicular or parallel to the axis of clinostat rotation.
To control for the effects of rotation, other cultures were rotated around a vertical axis. These cells were maintained in an upright orientation and did not experience changes in the g-vector. This treatment did not induce cluster formation (data not shown). Thus, cluster formation does not result from the mechanical or environmental effects associated with clinostat rotation itself.
To determine how short a period of rotation is needed for clusters to develop, cells were fixed at different times and mean plastid area was determined (Fig. 5). After 1 h, a fraction of the plastids located close to the nucleus had redistributed to the apical end of zone III, resulting in a more even distribution than in upright, stationary cells (Fig. 5B, top two curves). Clusters started to appear in some cells by approximately 3 h (arrowhead in Fig. 5A). By 6 h, the area profile resembled that of cells that had been rotated for 14 d (Figs. 3B and 5B) as well those rotated for periods of 0.5, 1, 2, 7, and 42 d (data not shown).
Figure 5.
Time course of the effect of clinostat rotation on the development of subapical plastid clusters. A, Tracing of the apical region of representative cells. Clusters start to appear in some cells after about 3 h of rotation (arrowhead). Scale bar = 25 μm. B, Histograms of plastid area as described in Figure 3. Plastids located near the nucleus move toward the apical end of zone III resulting in a local enrichment in plastid area after 6 h of rotation; n = 13 to 25 cells for each time point. C, Neither plastid area nor zone length changed significantly during the time course.
The increase in plastid area in the apical region of zone III is probably not due to the movement of plastids from zone IV (between the nucleus and the basal wall) into zone III. Figure 5C (top) shows that plastid area in each zone did not change through time. The length of each zone (normalized as a percentage) also did not change (Fig. 5C, bottom). This suggests that clusters form as result of the redistribution of plastids within zone III, and that the population of plastids that move acropetally probably includes those that sediment along the length of the cell at 1g.
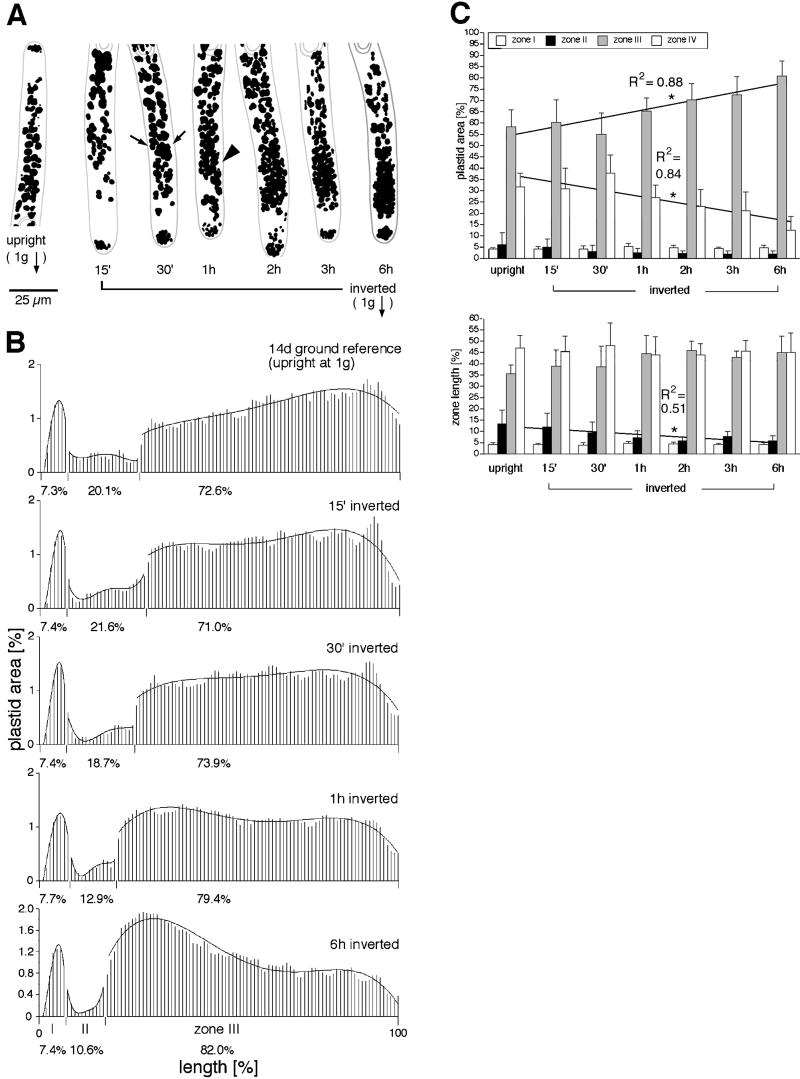
Inversion
In inverted cells, amyloplasts accumulate close to the plastid-free zone as a result of sedimentation (Fig. 2E; Schwuchow and Sack, 1993). The location of this plastid enrichment is somewhat similar to that of clusters produced in space and on a clinostat. However, plastid area in this region is greater in inverted cells. In addition, plastid area decreases more sharply toward the nucleus in inverted cells compared with those grown in microgravity or on a clinostat (Fig. 3B). Also, in inverted cells, the clusters are more elongated and less dense than the clusters in microgravity (Fig. 4). It is not possible to study plastid position in cells inverted for periods longer than 6 h because most cells start to curve upward after that time.
The enrichment in plastid area at the apical end of zone III starts after approximately 1 h of inversion and becomes pronounced after 6 h (Fig. 6, A and B). This peak is probably higher than in other treatments because during inversion plastids move from zone IV into zone III. This migration is shown by a 61% decrease in plastid area in zone IV and a 39% increase in zone III (Fig. 6C, top; n = 11–22 cells). It is likely that zone IV plastids fall around the nucleus into zone III, as some inverted cells had very few plastids distal to the nucleus (Fig. 4C). In contrast, in other treatments, plastids were constantly present in zone IV (Fig. 4, A and B).
Figure 6.
Time course of plastid sedimentation following inversion. A, Tracings of representative cells. Scale bar = 25 μm. Sedimentation can be detected after 30 min (arrows), and a higher concentration of plastids in the apical end of zone III can be seen as early as 1 h (arrowhead). B, Histograms of plastid area using methods described in the legend to Figure 3; n = 19 to 24 cells for each time point. C, Plastid area increases in zone III and decreases in zone IV (R2 values of linear regressions shown). Neither zone changes in length over time.
The four different treatments had relatively little effect on cellular parameters other than plastid distribution. The mean distance between the nucleus and the cell tip ranged from approximately 49% to 55% of the cell length for upright, inverted, space-grown, and rotated cells (Table I). Mean apical cell length was approximately 180 μm except for cells rotated on a clinostat, which were approximately 15% longer. The cultures grew robustly in space as well as in ground treatments.
Table I.
Parameters of apical cells in four different g-related treatments
| Treatment | Cell Widtha | Cell Lengtha | Distance Tip To Nucleus | Percentb |
|---|---|---|---|---|
| μm | ||||
| Upright | 12.9 ± 1.3 | 177.2 ± 37.3 | 91.6 ± 14.1 | 51.7 |
| Microgravity | 11.7 ± 1.1c | 179.6 ± 44.0 | 83.2 ± 11.3c | 46.3 |
| Clino-rotated | 16.3 ± 2.0c | 206.8 ± 49.2c | 109.7 ± 14.7c | 53.0 |
| Inverted | 15.0 ± 1.3c | 183.7 ± 32.3 | 99.5 ± 9.0c | 54.2 |
Moss cells were grown in an upright stationary position, or rotated on a clinostat, or were grown in microgravity for 14 d. Other cells were inverted for 6 h. The sample included between 34 and 145 cells in each treatment.
Mean ± sd.
Third data column divided by second column expressed as a percentage.
Different statistically from upright cells (P ≥ 0.95).
DISCUSSION
To analyze the forces that act on plastids that sediment at 1g in moss protonemata, we compared the effects of different gravity-related treatments on plastid distribution. The presence of a non-random distribution in space indicates the existence of as yet unknown endogenous forces and mechanisms that influence amyloplast position and that are normally masked in stationary cells grown on earth.
Nonrandom Plastid Distributions in All Treatments
Although plastids are present throughout much of the protonemal apical cell, sedimentation is largely confined to a subapical zone that extends to the nucleus (zone III; Walker and Sack, 1990; Schwuchow and Sack, 1993). Within this zone, only some plastids sediment along the cell axis. Because gravity regulates their position, one prediction for microgravity was that plastids would be randomly distributed, i.e. that they would occur with roughly equal frequency throughout zone III. Instead, the presence of plastid clusters at the apical end of this zone indicates that plastids are non-randomly distributed in microgravity. Rotating cells in a clinostat on earth mostly mimicked this non-random distribution.
These non-random distributions appear to result from the acropetal movement of plastids within zone III. Although it was not possible to follow a time course of cluster formation in space (due to the absence of an in-flight 1g centrifuge), this analysis was feasible with clinostat-rotated cells. Because total plastid area in each zone was constant throughout the period of rotation, there was no significant net movement of plastids between zones. Before clinostat rotation, plastid area peaked close to the nucleus in upright cells due to axial sedimentation in upright cells. This peak dropped sharply within 1 h of clinostat rotation. Together these data indicate that the clusters result mostly from the redistribution of previously sedimented plastids within zone III.
In contrast to rotation, inversion caused a significant redistribution of plastids between zones in that plastids in zone IV moved acropetally around the nucleus into zone III. This suggests that the clusters in inverted cells are produced by a different mechanism than those produced by clinostat rotation or microgravity. In addition, if the plastid redistribution in inverted cells results from sedimentation, then sedimentation in upright cells probably occurs over more of the cell length than previously thought (Schwuchow and Sack, 1993).
Endogenous Forces as Well as Gravity Influence Plastid Position
It is likely that endogenous, tip-directed forces act constitutively on plastids in apical cells. This would generate subapical clusters in microgravity and might explain why sedimentation is incomplete in upright cells on earth. As mentioned, plastid sedimentation is restricted along the cell axis because plastids do not fall all the way to the bottom of the cell.
Microtubules probably contribute to these endogenous forces. While both microtubules and microfilaments are abundant near plastids, only the depolymerization of the former enhances plastid sedimentation in vertical apical cells (Schwuchow et al., 1990; Schwuchow and Sack, 1994; Walker and Sack, 1995). Because microtubules may be load bearing for plastids, they may generate or transmit forces that cause clustering.
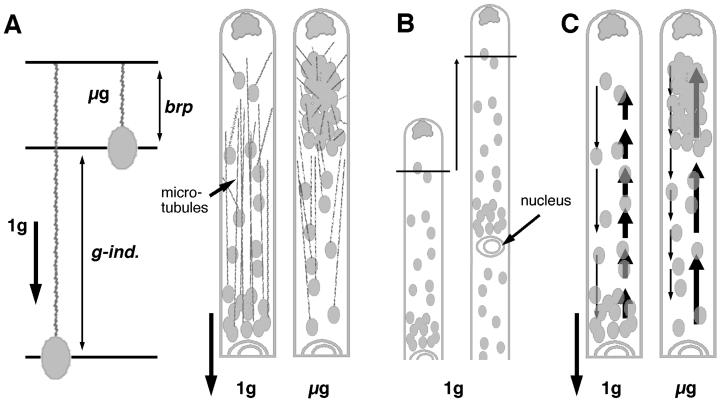
One model for how microtubules could contribute to plastid clustering is that g-induced strain affects microtubule length (Fig. 7A). Both theoretical considerations and empirical data suggest that tension might control microtubule assembly (Zheng et al., 1993). The pull of gravity on the plastid might stimulate tubulin polymerization above basal levels so that heavier plastids would be associated with longer microtubules. This would allow plastids to sediment as far as the length of the microtubule allowed. In microgravity, microtubules would be shorter, and plastids would be closer to the cell tip.
Figure 7.
Two models of how plastid clusters develop in microgravity and the relationship between growth and plastid position. A, Model 1. Microtubules polymerize at a basal rate (brp) in microgravity (left). The pull of gravity on the plastid produces a g-induced strain (g-ind.) on the microtubule that stimulates polymerization. Microtubules are thus shorter in microgravity (μg) than on earth (1g), resulting in plastid clusters (right). B, Diagram showing that apical cells grow at the same rate that plastid zones and the nucleus move toward the tip. C, Model 2. Acropetal movement (bold arrows) is stronger than basipetal plastid movement (thinner arrows). The rate of plastid movement (arrow length) is faster basipetally than acropetally in upright cells due to gravity, but the reverse takes place in microgravity producing clusters.
An additional consideration is that there are two types of dynamism seen in stationary apical cells. First, most plastids exhibit slow (approximately 1 μm min−1) acropetal and basipetal movements (Young and Sack, 1992). Second, entire plastid zones move acropetally at the same rate as tip growth so that the distance between the cell tip and each zone remains more or less constant (Fig. 7B; Young and Sack, 1992). Plastid sedimentation must be superimposed on these movements, e.g. in upright cells acropetal plastid movement occurs away from gravity.
A second model for how clusters arise suggests that acropetal plastid movement is intrinsically stronger than basipetal movement (Fig. 7C). This differential could derive from variations in the quantity or types of microtubule motors on plastids. Stronger acropetal movement would partially offset a g-induced drag in upright cells, but movement would still be slower acropetally than basipetally. Some plastids would sediment but acropetal movement would prevent complete stratification. Clusters would form because acropetal movement would be faster and basipetal movement would be slower in microgravity compared with upright cells.
Much work is needed to test these models including establishing the identity of the cytoskeletal motors on plastids, measuring the relative rates of acropetal and basipetal plastid movements, and determining whether microtubules are anchored and whether their polarity is coordinated. Moreover, the relationship between microtubules and plastids might be more complex than hypothesized above. For example, microtubules might be part of a cytoskeletal network that is anchored and prestressed (Ingber, 1999) in which case the g-induced stresses would be distributed throughout. Nevertheless, the presence of clustering in microgravity indicates that gravity normally interacts with endogenous forces that probably involve the cytoskeleton and thus both intrinsic and extrinsic forces control the position of these dense organelles.
The Position of Dense Organelles in Different Cell Types
Heavy organelles such as amyloplasts are only known to sediment in specialized types of cells, those that probably function in gravity sensing (Sack, 1991, 1997). There are several different types of these cells, and the effects of microgravity on organelle distribution have been studied in each type.
In the first type, such as central rootcap cells, all the amyloplasts fall to the bottom of the cell at 1g. In microgravity, the amyloplasts can group together toward the cell center (Smith et al., 1997; Volkmann et al., 1999). In the second type, algal rhizoids in the Characeae, all barium sulfate vesicles sediment to a subapical region of the downward-growing cell tip. These organelles disperse in a basipetal direction in microgravity (Braun et al., 1999). Dark-grown C. purpureus apical cells (this study) exemplify the third type.
The different responses to microgravity in each cell type probably reflect variations in cell organization and in interactions between the cytoskeleton and organelles (Braun et al., 1999; Volkmann et al., 1999). Despite these variations, all three cell types display non-random distributions of organelles in space. This indicates that these cells are specialized to regulate organelle position with respect to gravity in two ways. First, they allow sedimentation, and second, this sedimentation is superimposed upon counteracting forces. These forces are revealed when the g-vector is removed or randomized. Thus, these forces act constitutively at 1g to maintain the distribution of dense organelles in a dynamic equilibrium. Moreover, it is likely that these counteracting forces function directly or indirectly in gravitropic sensing.
Most other cells contain at least one heavy organelle, the nucleus, that does not sediment (the nucleolus is denser than starch; Todd, 1989). While much is known about how nuclear position is regulated, the relationship of this regulation to gravity and to the evolution of organelle positioning in eukaryotic cells is poorly understood (Ingber, 1999; Morris, 2000). Some storage cells similarly have large amyloplasts that do not sediment (Sack, 1991). Analysis of how sedimentation is regulated in cells specialized for sedimentation is relevant to understanding how sedimentation is prevented in other eukaryotic cells.
MATERIAL AND METHODS
Plant Material and Culture
All experiments used the wild-type 4 strain of Ceratodon purpureus (Hedw.) Brid. (Walker and Sack, 1990). Protonemata were cultured and vegetatively propagated as in Kern and Sack (1999).
Space Flight
Purpose-built hardware allowed for growth of moss cultures in Petri dishes in darkness and for chemical fixation in microgravity (Kern and Sack, 1999; Kern et al., 1999). The SPM-A (space moss) experiment flew on the Space Shuttle Columbia (STS-87) between November 19 and December 5, 1997. Sterile cultures were grown in a approximately 1-mm-thick layer of optically clear agarose (A-4679, Sigma, St. Louis). Prior to inoculation, the agarose was overlaid onto a sheet of canning-grade cellophane (Kern and Sack, 1999), which prevented the filaments from growing into the subjacent agar.
Cultures were launched into space approximately 30 h after inoculation to ensure that almost all cells developed in microgravity. Three Petri dishes, each containing a single C. purpureus inoculum, were incubated in darkness in space at 23°C to 26°C for 7 d. They were then fixed in position in 1% (w/v) paraformaldehyde, 2% (v/v) glutaraldehyde, 50 mm PIPES (1,4-piperazinediethanesulfonic acid), and 5 mm CaCl2, at pH 7. The cultures were maintained in fixative until landing. Another three dishes were grown for 14 d and then fixed. Approximately 2 d later the dishes were retrieved shortly after landing. The agarose was removed and rinsed in buffer. Pieces of agarose containing apical cells were examined by microscopy and photographed at Kennedy Space Center (FL) within a day of landing.
Ground Experiments
Upright cells were grown and processed as in the space-flight experiment. The hardware was identical to that used for flight. Cultures were maintained under environmental conditions (especially temperature) that matched those in flight (with a 48-h delay). These controls were housed in the Orbiter Environmental Simulator at Kennedy Space Center.
Additional experiments were performed at Ohio State University using the same culture method, but the cultures were not contained by space-flight hardware. Freshly inoculated Petri dishes were incubated in darkness for 7 d at 21°C to 24°C, after which hundreds of upward-growing protonemata were present in each dish. Some dishes were then inverted for periods ranging from 0.25 to 6 h and then fixed (Kern and Sack, 1999). Other dishes were then rotated at 1 rpm on custom-built clinostats (Kern and Sack, 1999). These dishes were rotated around a horizontal axis.
Microscopy and Data Analysis
Because essentially all apical cell plastids contain at least some starch, fixed protonemata were stained with I2KI and visualized as in Kern and Sack (1999). The distribution of plastids in chemically fixed cells has been shown to be comparable with that in living apical cells of C. purpureus (Walker and Sack, 1990; Young and Sack, 1992). Tracings were made from light micrographs to show the outlines of apical cells, nuclei, and plastids. Plastid outlines were filled with black using Photoshop (Adobe, Mountain View, CA) so that plastid position could be determined digitally. In combination with NIH Image software (developed at the United States National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image), a customized software macro (NASA Ames Research Center, Moffett Field, CA) was used to determine the number of black pixels as a percentage of all pixels for 100 sectors. These sectors were arranged in series from the tip of the cell to the center of the nucleus. Each sector was defined as 1% of the distance between the cell tip and the nucleus. The mean distance between the cell tip and the nucleus was roughly comparable between the different treatments (Table I). This method was used for Figures 3, 5, and 6.
Linear regressions were derived (95% confidence level) to analyze the time courses (Figs. 5 and 6). Regressions were derived from the means for each time point because the position of the plastid zones varied cell to cell.
ACKNOWLEDGMENTS
Thanks to Nathan White, Jaclyn O'Connor, Chanda McGlaughn, and Ben Sayers for technical assistance. Thanks also to Jeanette Nadeau for comments on the manuscript, to the many Kennedy Space Center employees that helped develop the hardware and prepare the flight experiment, and to astronauts Leonid Kadenyuk and Yaroslav Pustovyi for performing the space and ground control experiments.
Footnotes
This work was supported by the National Aeronautics and Space Administration (grant no. NAG10–0179 to F.S.).
LITERATURE CITED
- Björkman T. Perception of gravity by plants. Adv Bot Res. 1988;15:1–41. doi: 10.1016/0273-1177(92)90283-4. [DOI] [PubMed] [Google Scholar]
- Braun M, Buchen B, Sievers A. Electron microscopic analysis of gravisensing Chara rhizoids developed under microgravity conditions. FASEB J Suppl. 1999;13:S113–S120. doi: 10.1096/fasebj.13.9001.s113. [DOI] [PubMed] [Google Scholar]
- Chicurel ME, Chen CS, Ingber DE. Cellular control lies in the balance of forces. Curr Opin Cell Biol. 1998;10:232–239. doi: 10.1016/s0955-0674(98)80145-2. [DOI] [PubMed] [Google Scholar]
- Ingber DE. How cells (might) sense microgravity. FASEB J Suppl. 1999;13:S3–S15. doi: 10.1096/fasebj.13.9001.s3. [DOI] [PubMed] [Google Scholar]
- Kern VD, Sack FD. Irradiance-dependent regulation of gravitropism by red light in protonemata of the moss Ceratodon purpureus. Planta. 1999;209:299–307. doi: 10.1007/s004250050636. [DOI] [PubMed] [Google Scholar]
- Kern VD, Sack FD, White NJ, Anderson K, Wells W, Martin C. Spaceflight hardware allowing unilateral irradiation and chemical fixation in situ in petri dishes. Adv Space Res. 1999;24:775–778. doi: 10.1016/s0273-1177(99)00412-3. [DOI] [PubMed] [Google Scholar]
- Kuznetsov O, Schwuchow JM, Sack FD, Hasenstein KH. Curvature induced by amyloplast magnetophoresis in protonemata of the moss Ceratodon purpureus. Plant Physiol. 1999;119:645–650. doi: 10.1104/pp.119.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NR. Nuclear migration: from fungi to the mammalian brain. J Cell Biol. 2000;148:1097–1101. doi: 10.1083/jcb.148.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack FD. Plant gravity sensing. Intern Rev Cytol. 1991;127:193–252. doi: 10.1016/s0074-7696(08)60695-6. [DOI] [PubMed] [Google Scholar]
- Sack FD. Plastids and gravitropic sensing. Planta Suppl. 1997;203:S63–S68. doi: 10.1007/pl00008116. [DOI] [PubMed] [Google Scholar]
- Sack FD, Wagner TA, Kern VD. Gravitropism in moss protonemata. In: Bates JW, Ashton NW, Duckett JG, editors. Bryology for the Twenty-First Century. Leeds, UK: Maney Publishing and The British Bryological Society; 1998. pp. 247–260. [Google Scholar]
- Schwuchow J, Hartmann E, Sack FD. Microtubule distribution in gravitropic protonema of the moss Ceratodon. Protoplasma. 1990;159:60–69. doi: 10.1007/BF01326635. [DOI] [PubMed] [Google Scholar]
- Schwuchow J, Sack FD. Effects of inversion on plastid position and gravitropism in Ceratodon protonemata. Can J Bot. 1993;71:1243–1248. doi: 10.1139/b93-147. [DOI] [PubMed] [Google Scholar]
- Schwuchow J, Sack FD. Microtubules restrict plastid sedimentation in protonemata of the moss Ceratodon. Cell Motil Cytoskel. 1994;29:366–374. doi: 10.1002/cm.970290409. [DOI] [PubMed] [Google Scholar]
- Smith JD, Todd P, Staehelin LA. Modulation of statolith mass and grouping in white clover (Trifolium repens) grown in 1-g, microgravity and on the clinostat. Plant J. 1997;12:1361–1373. doi: 10.1046/j.1365-313x.1997.12061361.x. [DOI] [PubMed] [Google Scholar]
- Todd P. Gravity-dependent phenomena at the scale of the single cell. Am Soc Gravit Space Biol Bull. 1989;2:95–113. [PubMed] [Google Scholar]
- Volkmann D, Baluska F, Lichtschiedl I, Driss-École D, Perbal G. Statoliths motions in gravity-perceiving plant cells: does actomyosin counteract gravity? FASEB J Suppl. 1999;13:S143–S147. doi: 10.1096/fasebj.13.9001.s143. [DOI] [PubMed] [Google Scholar]
- Walker LM, Sack FD. Amyloplasts as possible statoliths in gravitropic protonemata of the moss Ceratodon purpureus. Planta. 1990;181:71–77. [PubMed] [Google Scholar]
- Walker LM, Sack FD. Microfilament distribution in protonemata of the moss Ceratodon. Protoplasma. 1995;189:229–237. doi: 10.1007/BF01280177. [DOI] [PubMed] [Google Scholar]
- Young JC, Sack FD. Time-lapse analysis of gravitropism in Ceratodon protonemata. Am J Bot. 1992;79:1348–1358. [PubMed] [Google Scholar]
- Zheng J, Buxbaum RE, Heidemann SR. Investigation of microtubule assembly and organization accompanying tension-induced neurite initiation. J Cell Sci. 1993;104:1239–1250. doi: 10.1242/jcs.104.4.1239. [DOI] [PubMed] [Google Scholar]