Abstract
Objectives
This systematic review aims to evaluate the evidence of non-pharmacological strategies to improve blood pressure (BP) control in patients with hypertension from African countries.
Design
We performed a systematic review and searched Medline, Central, CINAHL and study registers until June 2020 for randomised studies on interventions to decrease BP of patients with hypertension in African countries. We assessed the study quality using the Cochrane risk of bias tool and narratively synthesised studies on non-pharmacological hypertension interventions.
Setting
We included studies conducted in African countries.
Participants
Adult African patients with a hypertension diagnosis.
Interventions
Studies on non-pharmacological interventions aiming to improve BP control and treatment adherence.
Outcomes
Main outcomes were BP and treatment adherence.
Results
We identified 5564 references, included 23 with altogether 18 153 participants from six African countries. The studies investigated educational strategies to improve adherence (11 studies) and treatment by healthcare professionals (5 studies), individualised treatment strategies (2 studies), strategies on lifestyle including physical activity (4 studies) and modified nutrition (1 study). Nearly all studies on educational strategies stated improved adherence, but only three studies showed a clinically relevant improvement of BP control. All studies on individualised strategies and lifestyle changes resulted in clinically relevant effects on BP. Due to the type of interventions studied, risk of bias in domain blinding of staff/participants was frequent (83%). Though incomplete outcome data in 61% of the studies are critical, the general study quality was reasonable.
Conclusions
The identified studies offer diverse low-cost interventions including educative and task-shifting strategies, individualised treatment and lifestyle modifications to improve BP control. Especially trialled physical activity interventions show clinically relevant BP changes. All strategies were trialled in African countries and may be used for recommendations in evidence-based guidelines on hypertension in African settings.
PROSPERO registration number
CRD42018075062.
Keywords: internal medicine, preventive medicine, quality in health care, primary care, public health, hypertension, healthcare
Strengths and limitations of this study.
This systematic review summarises evidence on a wide range of different non-pharmacological interventions, adding a comprehensive overview to the literature that can support physicians and healthcare policymakers in the African setting.
Most of the included studies were conducted in urban areas of few Western and Southern African countries leading to a lack of generalisability to other African regions and showing a need of future research in rural areas.
A main limitation of this systematic review occurs through deviations from the protocol. Due to the amount of search results for the initially planned more general scope on cardiovascular diseases, we decided to focus on hypertension.
Nevertheless, this review was limited to studies with the highest level of evidence to investigate the benefits and harms of non-pharmacological interventions on blood pressure control in African patients with hypertension.
This review adds to the scope of a recently published systematic review on the efficacy of common pharmacological treatment for patients with hypertension in sub-Saharan Africa.
Background
Hypertension is a major public health problem and affects the lives of about 1.13 billion people.1 The highest blood pressure (BP) levels shifted from high to low-income countries in South Asia and sub-Saharan Africa (SSA)2 with a prevalence of 57% in older adults in African countries.3 4 The estimated number of adults with raised BP in SSA rose from 30 million in 1975 to over 100 million in 2016 due to population growth, ageing and westernisation of lifestyle.2 Hypertension is a leading risk factor of cardiovascular disease (CVD), chronic kidney disease and diabetes.1 Studies show that black people suffer from more severe forms of hypertension associated with more frequent treatment failure and more severe and earlier target organ damage, all resulting in higher morbidity and mortality.5 6 Hypertension is a major contributor to devastating health events like stroke or heart failure,7–9 which can be catastrophic to both individuals and healthcare systems in which resources are scarce.
Tackling and reducing the burden of premature mortality due to non-communicable diseases (NCDs) through prevention and treatment has been a designated goal within the United Nations (UN) 2030 Agenda.10 The Pan-African Society of Cardiology developed an algorithm including recommendations on screening, diagnosis and treatment to achieve 25% hypertension control in Africa by 2025 with a treatment target value of less than 140/90 mm Hg. Screening programmes are proposed to be carried out in healthcare facilities as well as public places like markets and churches. The treatment starts with lifestyle modifications, is intensified through a monotherapy and a subsequent combination of two or three medications in higher stages and resistant forms of hypertension. In some cases, the assessment of secondary causes by specialists is recommended.9
However, the awareness of hypertension remains relatively low in many parts of Africa, hindering adequate screening, treatment and control to lower the long-term risks.11–13 Extensive counselling and education of patients and healthcare providers on the importance of adherence to medications and lifestyle modifications is necessary in order to improve hypertension control.14 15 Especially patients with multiple medications benefit from the support of their healthcare providers to understand the treatment’s purpose.16
Evidence is needed detailing regional differences in hypertension incidences, risk factors, and, as subject of this review, treatment strategies in different, transitioning populations on the African continent. Seeley et al recently published a systematic review on the efficacy of common pharmacological treatment for patients with hypertension in SSA.17 These interventions do not include treatment strategies like lifestyle modifications (eg, nutritional modifications, physical activity) or educational strategies, which can be summarised as non-pharmacological interventions.18 The main aim of this systematic review is to summarise the best available evidence on the effectiveness of non-pharmacological strategies on BP control in African patients with hypertension.
Methods
A protocol of this systematic review was prospectively registered on PROSPERO (CRD42018075062) following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline19. We initially planned to include randomised controlled trials (RCTs) on all CVDs. Due to the high number and heterogeneity of eligible studies, we decided to focus this review on patients with hypertension as one of the main risk factors for other CVDs. We aim to describe all non-pharmacological hypertension interventions in detail in order to broaden the scope of the existing evidence.
Patient and public involvement
The conception of this systematic review was discussed in detail with members and students at the Addis Ababa School of Public Health in order to consider the priorities in the African context. Consensus was to gather evidence on hypertension treatment as a measure of tackling the burden of NCDs which is part of the UN 2030 Agenda.10 No patients were involved.
Inclusion and exclusion criteria
We included full-text publications on RCTs20 including cross-over RCTs and cluster RCTs on non-pharmacological interventions with adult patients with hypertension in African countries and reported results on BP. The study aims were improvement of prevention, diagnoses and treatment of hypertension in African countries. Studies on primary prevention were excluded due to the high variety of possible participants and interventions. International multicentre studies were included if more than 50% of centres were set in African countries. For detailed inclusion criteria, see table 1.
Table 1.
Inclusion and exclusion criteria
| Design | RCTs conducted in African countries, in international studies with at least 50% African countries |
| Population | African adult patients in secondary and tertiary prevention, diagnosis and treatment of hypertension Exclusion of patients with gestational diabetes, pre-eclampsia or eclampsia |
| Intervention | All non-pharmacological strategies to improve adequate diagnoses, prevention and treatment of hypertension |
| Control |
|
| Outcome | Blood pressure (SBP, DBP, MAP) and adherence to recommendations (medications and lifestyle changes) within longest follow-up |
| Publication | Full-text publications according to CONSORT in English or German |
CONSORT, Consolidated Standards of Reporting Trials; DBP, diastolic blood pressure; MAP, mean arterial pressure; RCTs, randomised controlled trials; SBP, systolic blood pressure.
Literature search and study selection
Two electronic databases (Medline Ovid, Central) and registers of ongoing and completed studies (International Clinical Trials Registry Platform) were searched to identify all relevant studies (see online supplemental file 1). We added a search in CINAHL to cover nursing interventions. The main keywords of the search strategy included hypertension, high blood pressure, blood pressure control, Africa, a list of all African countries and randomized controlled trials. The first searches in 2017 included all CVDs, while updated strategies were limited to hypertension. The last search was conducted in June 2020. All searches were done without time frame constrictions. The study selection process was described in a flow chart according to the PRISMA statement.19 We exported articles retrieved from the literature search into a reference manager software (EndNote21). Duplicate references were identified in case of congruence of authors, title, year, and journal and deleted.
bmjopen-2020-048079supp001.pdf (127.7KB, pdf)
Titles, abstracts and full texts of potentially eligible articles were independently screened by three authors (MC, ESK and SU). Disagreements were resolved through consensus.
Interventions
This systematic review compares non-pharmacological interventions to improve adequate diagnoses, prevention and treatment of patients with hypertension with standard care, no intervention or another, less intensive or frequent intervention (table 1). Non-pharmacological interventions are considered non-medication treatment strategies such as educational programmes for patients or health professionals, individualised treatment, physical activity or nutrition-modification strategies.18
Outcomes
The main goal of non-pharmacological interventions for patients with hypertension is to improve BP control through the implementation of recommended lifestyle changes, attendance to follow-up visits and interventions promoting adherence to take hypertensive medications. We therefore report results on BP and adherence (table 1).
Data extraction and management
One author (MC or SU) extracted and a second author (SU or ESK) checked all information on study design and setting, participants, interventions and main results by using an assessment form in Excel. The form was especially designed for this systematic review and piloted for the first five studies.
We extracted information on the publication (study name consisting of the name of first author and year of the first publication of final results, registration and additional publications), study characteristics (design, country and region in which the study was conducted, duration, preplanned outcomes), participants (with inclusion/exclusion criteria, randomised sample size, prevention level, grade of hypertension, mean age, baseline BP), a short description of the intervention and control groups, and the main results on BP and adherence within the longest follow-up periods. The grade of hypertension was described as mild (grade 1, 140–159/90–99 mm Hg), moderate (grade 2, 160–179/100–109 mm Hg) or severe (grade 3, ≥180/≥110 mm Hg).15 If BP was reported in standing and supine position, we extracted results for supine position.
All effect sizes were reported with their corresponding CIs. They were calculated either on the basis of mean and SD for metric outcomes or by comparing the frequencies of better adherence or BP control. Positive mean differences (MDs) describe a positive treatment effect on BP with lower mean values or higher decrease in the intervention group. Relative risks (RRs), HRs and ORs compare the frequency of good adherence or BP control. Effect measures greater than 1 describe a better adherence or BP control in the intervention group.
Quality assessment and risk of bias
Risk of bias was evaluated for all studies based on the Cochrane risk of bias tool.22 Two investigators (MC or ESK and SU) independently assessed the risk of bias in seven domains (sequence generation, allocation concealment, blinding of personal and participants, blinding of outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias). Risk of bias due to selective outcome reporting was judged as low, when the study protocol was available and results on all preplanned outcomes were reported. Incomplete outcome data were judged as high, when more than 10% of randomised participants dropped out. Other sources of bias were reported to be high in cases of missing sample size calculation, no definition of the primary endpoint or no reporting of baseline values.
Data synthesis
The main aim of this review is a narrative synthesis of studies with their participants, different types of interventions and resulting outcomes. We added a figure visualising the effect sizes on BP of different types of interventions in forest plots using RevMan.23 Due to the high clinical heterogeneity between included studies with their different settings, interventions, control groups and lengths of follow-up, we did not pool any results.
Treatment effects were described as statistically significant or clinically relevant. Statistically significant results on BP with MD over 5 mm Hg were defined as clinically relevant.
Results
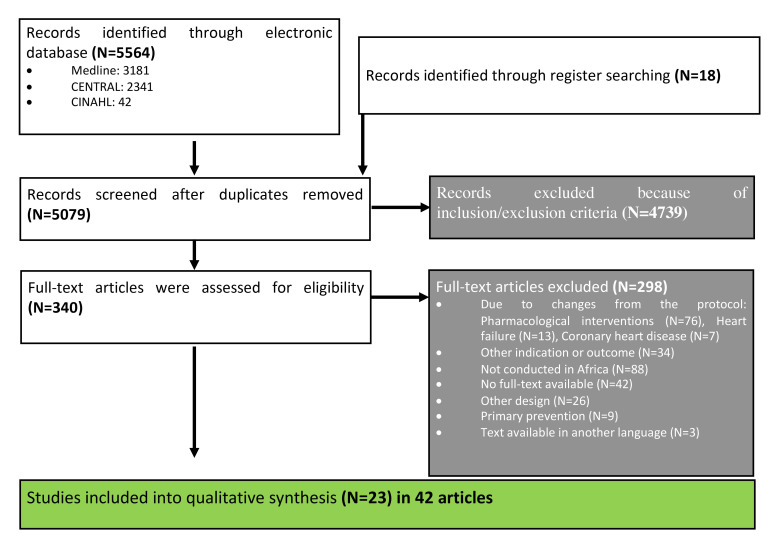
We identified a total of 5564 references from electronic databases and 18 references from the International Clinical Trials Registry Platform. Three hundred forty articles were potentially eligible and full texts were assessed for the inclusion and exclusion criteria. Of those, 298 articles were excluded including 13 articles on studies to treat heart failure, 7 articles on coronary heart diseases and 76 articles on pharmacotherapy for hypertension (see list of excluded studies in the online supplemental material 1). Twenty-three studies (reported in 42 articles)24–66 on non-pharmacological strategies to treat patients with hypertension matched the inclusion criteria and were included in this systematic review (figure 1 and list of included studies in the online supplemental material 2). The characteristics and main results of these studies were summarised in table 2.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart describing the process of study selection.
Table 2.
Study characteristics
| Participants | Intervention (IG) vs control (CG) group | Results on adherence and BP | |||||
| Name (design) | Country | n | Age (years)/females | Hypertension; SBP/DBP (mm Hg) | Description | Follow-up (months) | IG vs CG; treatment effect (95% CI) |
| Educational strategies for patients (11 RCTs) | |||||||
| Adeyemo et al24 (RCT) |
Nigeria (mixed) | 668 | 62.7±10.0/66% | Mild to moderate 167.4±19.2/91.8±12.3 |
Home visits by nurses and clinic management (community based, nurse-led treatment programme with physician backup; facilitation of clinic visits and health education; use of diuretics and a beta blocker as needed) vs clinic management | 6 |
Excellent adherence (missed ≤2 pills per month): worse in IG: 72.5% vs 79.0%; ORa 0.524 (0.30 to 0.75) BP control: no difference 65.0% vs 66.3%; RR 0.98 (0.87 to 1.11) |
| Ayodapo and Olukokun27 (RCT) |
Nigeria (mixed) | 322 | 60.9±10.0/51% | MAP: 106.4±8.3 | Counselling on lifestyle behaviours (physical activity, fruit and vegetable consumption, alcohol consumption, smoking) over 30–45 min, reminders (telephone calls/SMS) vs usual care | 3 |
Met recommendations on: physical activity: better in IG: 22.4% vs 6.2%; RR 3.60 (1.85 to 7.00) fruit and vegetable consumption: better in IG: 71.4% vs 66%; RR 1.74 (1.41 to 2.15) alcohol consumption: better in IG: 100% vs 87.6%; RR 1.14 (1.08 to 1.21) smoking: no difference: 83.9% vs 78.5%; RR 1.05 (0.95 to 1.17) BP: MAP: lower in IG: 94.6±8.1 vs 106.2±7.6 mm Hg; MD −9.8 (−11.5 to −8.1) |
| Bobrow et al28 (PACTR2014 11000724141) (RCT) |
South Africa (urban) |
1372 | 54.3±11.5/72% | Mild to moderate 135.4±17.5/83.4±12.1 |
Mobile phone text messages on behaviour change techniques (IG2: interactive with information and possibility to response vs IG1: only information on hypertension, motivation to take medications and reminders) vs usual care | 12 |
Adherence (days with medication ≥80%): higher with IG: 59.7% vs 62.8% vs 49.4%; RR 1.12 (1.01 to 1.23) IG2 vs CG: ORa 1.86 (1.39 to 2.49) IG1 vs CG: ORa 1.60 (1.20 to 2.16) BP: slightly lower with IG1 SBP: 132.7±17.5 vs 132.1±16.6 vs 134.3±17.3 mm Hg IG2 vs CG: MDa −1.6 mm Hg (−3.7 to 0.6) IG1 vs CG: MDa −2.2 mm Hg (−4.4 to −0.04) BP control: slightly better with IG: 65% vs 65% vs 58% IG1 vs CG: ORa 1.42 (1.03 to 1.95) IG2 vs CG: ORa 1.41 (1.02 to 1.95) |
| Bolarinwa et al29 (PACTR2016 06001671335) (RCT) |
Nigeria (urban) | 299 | 61.1±10.8/77% | 140.0±22.9/86.9±11.9 | Task-shifting (driven by trained and professionally competent nurses) home-based follow-up care (BP and BMI monitoring, medical advice and counselling at home) vs usual care | 12 | Medical adherence: better with IG: low: 4% vs 16.6%, medium: 17.5% vs 34.7%, high: 78.5% vs 48.7% BP control: better with IG: 85.9% vs 76.7%; RR 1.12 (1.00 to 1.25) |
| Labhardt et al36 (cluster RCT) | Cameroon (rural) | 187 | 59.9±12.5/64% | Mild to moderate 175.8/100.7 |
Reminder letters in case of missing follow-up (IG2) vs financial incentive (1 month free treatment for regular attenders) (IG1) vs usual care in nurse-led facilities | 12 |
Adherence: retention rate: 60% vs 65% vs 29%; lower risk of loss to follow-up from the programme and better adherence in IG IG2 vs CG: HRa: 0.38 (0.24 to 0.61) IG1 vs CG: HRa: 0.44 (0.27 to 0.72) Adherence (≥80%): 38% vs 35% vs 10% IG2 vs CG: MDa: 28% (14% to 42%) IG1 vs CG: MDa: 25% (13% to 37%) BP: no differences in SBP in retained patients Costs: In IG1: average monthly cost per patient for antihypertensive medication: €1.1±0.9, transport: €1.1±1.0 |
| Owolabi et al56 (NCT01900756) (RCT) |
Nigeria (mixed) |
400* | 57.2±11.7/37% | All stroke (n=400); 138.3±23.6/83.0±15.2 stroke and uncontrolled hypertension (SBP/DBP >140/90 mm Hg) (n=168) 158.7±21.7/92.5±15.6 |
Chronic care model components of delivery system redesign (increased follow-up visits, pre-appointment phone texts), self-management support (patient report card, post-clinic follow-up phone texts, waiting room educational video) and clinical information systems (patient report card as part of medical chart, hospital registry) vs standardised usual care (risk factor identification and control) and phone contact information | 12 |
BP: No difference for all patients after stroke: SBP: 136.5±22.3 vs 136.2±21.2 mm Hg Patients with uncontrolled hypertension: SBP: 145.1±22.6 vs 148.5±22.8 mm Hg |
| Sarfo et al58 (NCT02568137) (cluster RCT) |
Ghana (urban) |
60* | 55±13/35% | Stroke and uncontrolled hypertension; 143.8±26.7/90.5±15.7 |
Nurse-led, multilevel approach with m-Health technology for monitoring and reporting BP measurement and tailored motivational text messages vs usual care | 9 |
Adherence: modified MMA score: no difference: 13±1.5 vs 13±1.7 BP: BP control: no difference: 47% vs 40%; ORa: 1.24 (0.83 to 1.84) SBP <140 mm Hg: better in IG: 73% vs 43% DBP <90 mm Hg: better in CG: 47% vs 77% |
| Saunders et al60 (RCT) |
South Africa (urban) |
224 | 65% between 40 and 50/73% | Mild to moderate; n.r. 116.6 |
Reminder letters and home visits by fieldworkers and patient-retained records for self-monitoring of medication compliance and BP control vs usual care (appointment system and health education) | 6 |
Adherence (treatment received) over 6 months: higher for newly treated (135.5±48.9 vs 95.0±60.0 days) and infrequent attenders (168.4±16.4 vs 116.7±56.9 days) of 180 days >80% of treatment: better for newly treated (59% vs 29%; p<0.001) and infrequent attenders (87% vs 42%; p<0.001) BP: DBP: lower for newly treated patients (93.4 vs 100.5 mm Hg; MD: 7.1 mm Hg (0.5 to 13.7), no difference for infrequent attenders: 97.5 vs 94.7 mm Hg; MD: −2.8 mm Hg (−6.9 to 1.3)) |
| Stewart et al61 (RCT) |
South Africa (urban) | 83 | Late middle-aged/n.r. | All hypertensives; 146.4±18.5/93.5±11.1 |
Telephonic intervention (educational and home-based exercise programme+support of a healthcare practitioner and a family member) vs control group (educational and home-based exercise programme only) | 6 |
Adherence: better with IG: 62.8%±34.5% vs 39.3±42.8%; p=0.007 BP: no difference: SBP: 142±16 vs 144±20 mm Hg; MD: −2 mm Hg (−10.3 to 6.3) DBP: 92±12 vs 91±10 mm Hg, change: MD: 1 mm Hg (−4.0 to 6.0) |
| Vedanthan et al64 (NCT01844596) (cluster RCT) |
Kenya (rural) | 1460 | 54.2±16.4/58% | All hypertensives; 159.4±19.5/89.7±12 |
Tailored behavioural communication (smartphone (IG2) or paper-based (IG1)) vs usual care | 12 |
Adherence (linkage to care): best results with IG2, worse with IG1: IG2 vs CG: ORa: 1.21 (0.70 to 2.01) IG1 vs CG: ORa: 0.64 (0.43 to 0.91) IG2 vs IG1: ORa: 1.95 (1.23 to 3.01) BP: no difference SBP: 149.4±20.8 vs 150.2±21.6 vs 150.0±22.9 mm Hg, change: −13.1±20.5 vs −8.4±24.0 vs −9.7±25.1 mm Hg IG2 vs CG: MDa: −2.13 mm Hg (−4.89 to 0.42) IG1 vs CG: MDa: −0.06 mm Hg (−3.61 to 3.20) IG2 vs IG1: MDa: −2.07 mm Hg (−5.14 to 1.12) DBP: no difference: 91.3±12.7 vs 91.0±14.1 vs 90.1±13.7 mm Hg, change: 1.5±12.7 vs 0.4±15.2 vs 0.1±14.7 mm Hg BP control: no difference: IG2 vs CG: ORa: 0.95 (0.61 to 1.38) IG1 vs CG: ORa: 0.97 (0.63 to 1.42) IG2 vs IG1: ORa: 1.00 (0.69 to 1.40) |
| Wahab et al66 (RCT) |
Nigeria (urban) | 35* | 58.1±10.5/34% | All patients with stroke; 138.3±24.2/85.0±12.4 |
Feasibility of a nurse-led Intervention (education and skill building, BP monitor with review, phone calls) vs usual care | 0.5 |
Adherence: no difference, but improvement in both groups: MMA score: 7.32±0.93 vs 7.03±1.36 BP: no difference SBP: 137.5±23.0 vs 133.1±18.2 mm Hg; MD: 4.40 mm Hg (−9.4 to 18.2) DBP: 84.1±9.7 vs 84.2±13.1 mm Hg; MD −0.1 mm Hg (−7.7 to 7.5) |
| Educational strategies for healthcare professionals (5 RCTs) | |||||||
| Fairall et al32 (ISRCTN20283604) (cluster RCT) |
South Africa (rural) | 4393 | 52 (IQR 43–62)/73% | Mild to moderate 139±23.6 90±13.2 |
Education of nurses on NCD care (nurse training in educational outreach sessions with a primary care programme to expand their role in NCD care, authorisation to prescribe an expanded range of drugs on NCDs) vs usual training | 14 |
Adherence: no difference BP: BP controlled: no difference: 33% vs 32%; RR 1.01 (0.2 to 1.8) |
| Goudge et al34 (ISRCTN12128227) (cluster RCT) |
South Africa (rural) | 4722 | 56.6±19.4/56% | Hypertension: 46.6%, of them: 53.4%, on treatment and controlled: 8.6%, on treatment and uncontrolled: 9%, not on treatment: 29% | Support of nurses by health workers (eg, assistance with booking appointments, retrieve and fill patient files, health education, measurements in the vital signs queue, prepacking of medications, reminders to appointment for patients) to provide chronic disease care vs usual care | 18 | No hypertension: 50.9% vs 52.9% Adherence and BP: no difference on treatment and controlled: 11.3% vs 11.2% on treatment and uncontrolled: 13.0% vs 13.2% not on treatment: 24.9% vs 22.7% undiagnosed: 24.1% vs 22.2% taking medication: 24.3% vs 24.4% |
| Gyamfi et al35 (NCT01802372) (cluster RCT) |
Ghana (mixed) | 757 | 58.0±12.4/60% | Mild to moderate 155.9±12.1/89.6±10.8 |
Training of nurses in task-shifting for hypertension control+health insurance coverage vs health coverage | 12 | BP: improvement in both groups, but no difference between groups: SBP: 137.1±27.5 vs 138.4±27.3 mm Hg; change: −19.5±18.0 vs −16.6±17.9 mm Hg; MD: −2.9 mm Hg (−6.9 to 1.0) DBP: 79.8±22.9 vs 81.8±22.8 mm Hg; change −9,3±11.5 vs 8.7±18.7 mm Hg; MD −0.6 mm Hg (−2.9 to 1.7) BP control: 55.2% vs 49.9% (MD 5.2% (−1.8% to 12.4%)) |
| Mendis et al52 (cluster RCT) | Nigeria (mixed) | 1188 | 55±4.7/58% | Mild to moderate 153.2±12.4/94±9.7 |
Education of healthcare workers and patients with a simple cardiovascular risk management package vs usual care | 12 |
Adherence: higher with IG Attended visits: 90.1% vs 74.5% quit smoking: 100% vs 74.4% (p=0.023) Increased fruit consumption: 93.4% vs 18.8% (p<0.0001) Increased vegetable consumption: 14.2% vs 7.0% (p=0.0002) BP: higher decrease in IG SBP: −11.0±15.4 vs −6.6±20.6 mm Hg; MD −4.4 mm Hg (−6.7 to −2.1) DBP: −5.4±10.0 vs −2.0±13.2 mm Hg; MD −3.4 mm Hg (−4.9 to −1.9) |
| Steyn et al62 (PACTR2013 03000493351) (cluster RCT) |
South Africa (urban) |
920 | 60.3±11.1/79% | All hypertensives 151.2±26.7/87.1±12.4 |
Multifaced intervention to implement national guidelines (structured record of national guidelines and visits to train clinicians) vs usual care (passive dissemination) at primary care level | 12 |
BP: no difference SBP: 161±28.9 vs 158.2±29.5 mm Hg; MD 2.8 mm Hg (−1.2 to 6.8) DBP: 88.1±13 vs 87.1±12.6 mm Hg; MD 1.00 mm Hg (−0.73 to 2.73) controlled BP: 23.1% vs 26% |
| Individualised treatment (3 RCTs) | |||||||
| Akintunde et al25 (ISRCTN69440037) (RCT) {Akintunde, 2017 #4980} (ISRCTN69440037) |
Nigeria, Kenya, South Africa (urban) | 105 | 56.6±14.3/53% | Uncontrolled 170.9±19.2/85.6±21.8 |
Physiologically individualised care (guided by their physiological phenotype, based on plasma renin and aldosterone) vs usual care | 12 |
BP: lower in IG SBP: 139.4±17.4 vs 152.6±12.3 mm Hg; MD −13.2 mm Hg (−19.4 to −7.0) DBP: 84.0±11.0 vs 89.6±7.0 mm Hg; MD −5.6 mm Hg (−9.4 to −1.8) BP control: 50.0% vs 11.1% (p=0.0001) |
| Okeahialam et al55 (RCT) |
Nigeria (urban) | 181 | 49.7±14.2/61% | Mild to moderate 150.3±14.8/93.7±9.6 |
Chronotherapy: drug intake in the night (22:00) vs drug intake in the morning (10:00) | 3 |
BP: higher decrease in IG SBP: −18.1±17.9 vs −14.1±14.7 mm Hg; MD −4.0 mm Hg (−9.0 to 1.0) DBP: −15.6±12.2 vs −8.7±10.2 mm Hg; MD −6.9 mm Hg (−10.4 to −3.4) |
| Physical activity (4 RCTs) | |||||||
| Aweto et al26 (RCT) |
Nigeria (urban) |
50 | 45±12.3/58% | Mild to moderate 138.7±10.9/79.9±9.3 |
Dance movement therapy (50 min) vs educational sessions, both 2×/week over 4 weeks | 1 |
BP: lower in IG SBP: 119.9±8.3 vs 135.5±11.6 mm Hg; MD −15.6 mm Hg (−22.4 to −8.8) DBP: 70.9±7.2 vs 74.1±7.7 mm Hg; MD −3.2 mm Hg (−8.1 to 1.7) |
| Lamina37 (RCT) |
Nigeria (urban) |
485 | 58.5±6.8/0% | Mild to moderate, stable 165.4±13.2/98.1±4.6 |
Training programmes on bicycle ergometer, 3×/week, 45–60 min: interval training (IG2) vs continuous training (IG1) vs usual care over 8 weeks | 2 |
BP: lower in IG SBP: 150.4±16.7 vs 154.4±12.6 vs 163.5±14.9 mm Hg; MD −11.1 mm Hg (−14.8 to −7.4) DBP: 95±5 vs 94.4±8.8 vs 96.1±2.7 mm Hg; MD −1.4 mm Hg (−2.6 to −0.2) |
| Maruf et al51 (ISRCTN81952488) (RCT) |
Nigeria (urban) |
120 | 52.8±8.4 (range 38–65)/71% | Mild to moderate, 155.7±11.4/93±10 |
Aerobic dance training (3×/week, 45 min) vs usual care over 12 weeks | 3 |
BP: lower in IG SBP: 135.3±5.6 vs 142.4±4.7 mm Hg; MD: −7.1 mm Hg (−9.3 to −4.9) DBP: 82.2±3.4 vs 83.9±2.8 mm Hg; MD: −1.7 mm Hg (−3.0 to −0.4) |
| Khalid et al63 (RCT) |
Egypt (urban) |
30 | 52.8±2.4, 40–50/100% | Postmenopausal hypertensives 151±6.2/94.5±4.2 |
Moderate aerobic exercise training (40 min, 3×/week) by walking on a treadmill vs usual care over 8 weeks | 2 |
BP: lower in IG SBP: 124±5.6 vs 145±6.7 mm Hg; MD: −21.0 mm Hg (−25.8 to −16.2) DBP: 85±5.4 vs 95±3.7 mm Hg; MD: −10.0 mm Hg (−13.7 to −6.3) |
| Modified nutrition (1 RCT) | |||||||
| Charlton et al31 (RCT) |
South Africa (urban) | 92 | 61.1±7/84% | Mild to moderate 134.6±15.7/81.1±8.1 |
Food-based dietary strategy (modified food, salt replacement, +500 mL of maas (fermented milk) vs control (same quantities of the targeted foods of standard commercial composition, 500 mL/day artificially sweetened cool drink) | 2 |
BP: lower in IG SBP: 132.5±15.8 vs 127.5±15.8 mm Hg; MDa: −6.2 mm Hg (−11.4 to −0.9) DBP: 82.2±9.5 vs 79.2±11.4 mm Hg; MDa: −0.6 mm Hg (−3.0 to 1.8) |
*Tertiary prevention.
BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; MD, mean difference; MDa, adjusted mean difference; MMA, Morisky medication adherence; n, number of randomised participants; NCD, non-communicable disease; n.r, not reported; ORa, adjusted OR; RCT, randomised controlled trial; RR, relative risk; SBP, systolic blood pressure; SMS, short message service.
bmjopen-2020-048079supp002.pdf (226.2KB, pdf)
bmjopen-2020-048079supp003.pdf (81.7KB, pdf)
Study characteristics
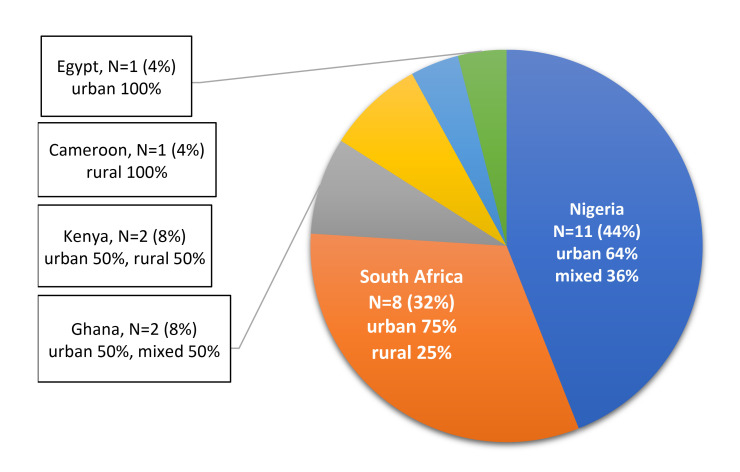
We identified 15 studies with two or more independent parallel groups and individual randomisation of patients and eight cluster RCTs with randomisation of different observation units, such as independent villages, healthcare facilities or different geographical regions (table 2). Most of the included studies were conducted in Nigeria (11 studies) and South Africa (8 studies), others in Ghana, Kenya, Cameroon and Egypt. One of the studies25 25 recruited patients in three countries (South Africa, Nigeria and Kenya). Nine studies (39%) were at least partly conducted in rurally located healthcare facilities (figure 2).24 27 32 34–36 52 56 64 The included studies were published between 1991 and 2019. Only three of the studies, all conducted in South Africa, were published before 2010.31 60 61
Figure 2.
Spatial distribution of countries in which randomised studies were conducted.
Participants
The total sample size ranged from 30 to 4722 participants with a total number of 18 153 participants (table 2). Eighteen studies (78%) randomised more than 100 participants. The mean age was between 45 and 63 years. Most studies (n=19) included more women. Two studies to enhance physical activity included women (Khalid et al)63 or men (Lamina)37 only. Mean systolic BP (SBP) at baseline was between 128 and 175 mm Hg, mean diastolic BP (DBP) between 76 and 117 mm Hg. Most studies included patients in secondary prevention with mild to moderate hypertension. Three studies56 58 66 included patients with hypertension post-stroke.
Intervention
Studies investigated educational strategies to improve adherence of patients and treatment by healthcare professionals (16 studies), to individualise treatment (2 studies), and to change lifestyle via enhanced physical activity (4 studies) or modified nutrition (1 study) (table 2).
Educational strategies to improve adherence
Sixteen studies (17 090 participants), with follow-up periods from 2 weeks in a short-term feasibility study (Wahab et al)66 up to 18 months (Goudge et al),34 were published between 1991 and 2019.
The main aim of 11 studies was the improvement of patients’ knowledge on hypertension and adherence to self-monitoring of BP, recommendations on medication, lifestyle changes and regular attendance at healthcare facilities.24 27–29 36 56 58 60 61 64 66 Five studies investigated strategies to improve adequate treatment of patients with hypertension by clinicians, nurses and healthcare workers.32 34 35 52 62
Eight studies27 28 36 56 58 60 61 64 investigated the efficacy of adherence promotion via counselling and phone or letter-based interventions. Seven studies24 29 32 34 35 52 66 investigated the efficacy of interventions on the basis of training measures with subsequent task-shifting to nurses or health workers for home visits and patient education. One study (Steyn et al)62 tested a multifaced intervention to implement national South African guidelines into primary care of patients with hypertension or diabetes. Another two studies investigated the efficacy of financial incentives as an additional health insurance coverage (Gyamfi et al)35 or free treatment (Labhardt et al),36 respectively.
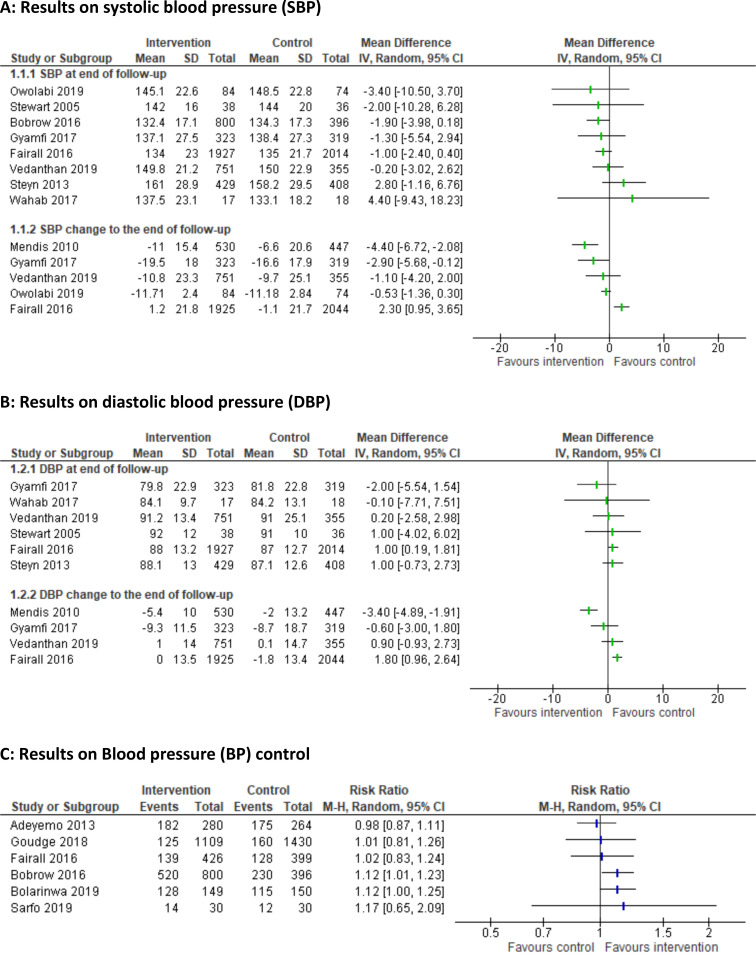
Nearly all studies stated improved medication adherence,24 28 29 36 60 61 implementation of lifestyle recommendations (Ayodapo and Olukokun, Mendis et al),27 52 linkage to care,36 52 64 or knowledge and practical skills of healthcare professionals (Fairall et al, Gyamfi et al).32 35 In only three studies,27–29 these improvements resulted in modest benefits on BP (table 2 and figure 3A–C). In the study by Ayodapo and Olukokun,27 counselling had a positive impact on lifestyle behaviour and resulted in a clinically relevant decrease of mean arterial BP (−9.8 mm Hg; 95% CI −11.5 to −8.1). Bobrow et al28 assessed the effect of automated treatment adherence support delivered via mobile phone short messages. Bolarinwa et al29 trialled home-based follow-up care with education and counselling of patients and modifications of environmental characteristics. Both studies achieved a 12% higher BP control with SBP <140 mm Hg and DBP <90 mm Hg in participants of the intervention compared with the control groups (RR: 1.12; 95% CI 1.01 to 1.23 and 1.12; 95% CI 1.00 to 1.25) (figure 3).
Figure 3.
Results of educational strategies to improve adherence (3a Results on systolic blood pressure; 3b Results on diastolic blood pressure; 3c Results on blood pressure control)
Individualised treatment strategies
Two studies (286 participants) with follow-up periods of 3 and 12 months were published in 2011 and 2017 (Akintunde et al, Okeahialam et al).25 55 Both investigated strategies on the efficacy of an individualised therapy. Therapy individualisation based on the patients’ renin/aldosterone profile (Akintunde et al)25 resulted in more appropriate prescriptions and a relevant decrease of SBP (MD: −13.2 mm Hg; 95% CI −19.4 to −7.0) and DBP (MD: −5.6; 95% CI −9.4 to −1.8) in patients with uncontrolled hypertension. The second study (Okeahialam et al)55 showed a higher reduction of DBP in patients using their anti-hypertensives at night compared with a morning intake (MD: −6.9 mm Hg; 95% CI −10.4 to −3.4) but stated no change in SBP (table 2).
Strategies with physical activity
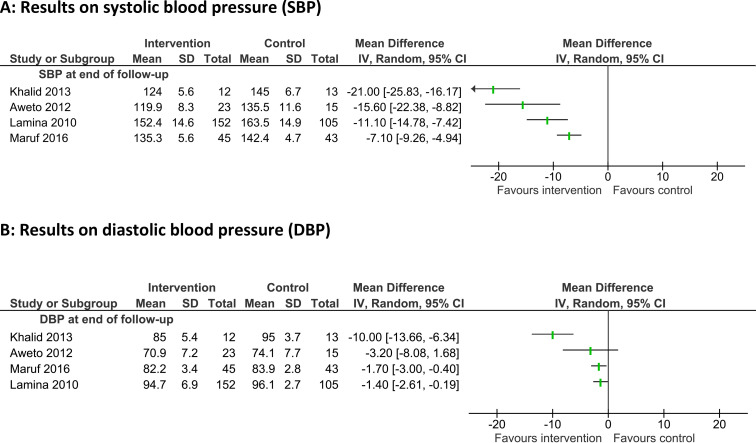
Four studies26 37 51 63 (685 participants), published between 2010 and 2016, investigated the BP-lowering effect of different aerobic training strategies over 4–12 weeks. Enhanced physical activities were performed two or three times a week and included dance training (Aweto et al, Maruf et al)26 51 and exercise training on an ergometer (Lamina)37 or treadmill (Khalid et al).63
All studies stated a clinically relevant benefit with mean reductions of SBP between 21 and 7.1 mm Hg and DBP between 10 and 1.4 mm Hg (figure 4). The highest BP decrease was achieved in a study on the effect of moderate aerobic exercise training by walking on a treadmill in postmenopausal women with hypertension (Khalid et al)63 (MD: −21 mm Hg; 95% CI −25.8 to −16.2).
Figure 4.
Results of strategies to enhance physical activity (4a Results on systolic blood pressure; 4b Results on diastolic blood pressure).
Modified nutrition strategies
Charlton et al31 tested a food-based dietary strategy (reduced salt consumption) in 92 patients with mild to moderate hypertension from a low socioeconomic background, stating a clinically relevant decrease in SBP after 2 months (MD: −6.2 mm Hg; 95% CI −11.4 to −0.9), but no effect on DBP (table 2).
Potential biases
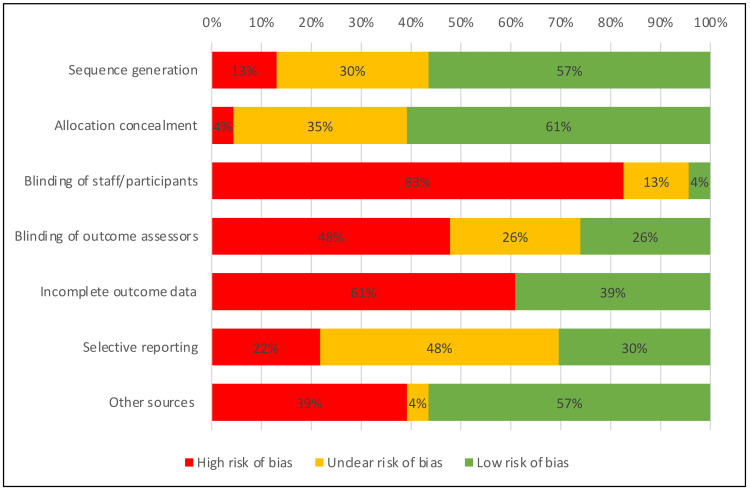
The greatest restriction of study quality was a high risk of bias in the blinding of staff and study participants in 19 studies. Especially educational strategies were not examined in double-blinded studies, however three of these studies34 56 58 reported a quality assurance against detection bias with blinded measurement of BP. Two studies on physical activity enhancement in comparison with usual care (Lamina, Maruf et al)37 51 were described as double blinded without reporting further details. Only the study on modified nutrition (Charlton et al)31 adequately reported detailed methods to ensure blinding of participants and fieldworkers. Another frequent problem was incomplete outcome data in 14 studies with loss to follow-up of over 10% of randomised participants or per-protocol analyses. Selective reporting was checked in all 13 studies with a published protocol. Of those, five studies29 35 36 51 56 did not report all preplanned outcomes. Problems concerning randomisation were identified in three studies with a non-random component in sequence generation or allocation concealment.25 37 52 Other sources of bias include missing sample size calculations, reporting of intermediate results only and relevant differences at baseline in nine studies (table 3, figure 5).24–26 29 51 55 63 64 66
Table 3.
Risk of bias assessment
| Study | Sequence generation | Allocation concealment | Blinding of | Incomplete outcome data | Selective reporting | Other sources | |
| Personnel/participants | Outcome assessors | ||||||
| Educational strategies | |||||||
| Adeyemo et al24 |

|

|

|

|

|

|

|
| Ayodapo and Olukokun27 |

|

|

|

|

|

|

|
| Bobrow et al28 |

|

|

|

|

|

|

|
| Bolarinwa et al29 |

|

|

|

|

|

|

|
| Fairall et al32 |

|

|

|

|

|

|

|
| Goudge et al34 |

|

|

|

|

|

|

|
| Gyamfi et al35 |

|

|

|

|

|

|

|
| Labhardt et al36 |

|

|

|

|

|

|

|
| Mendis et al52 |

|

|

|

|

|

|

|
| Owolabi et al56 |

|

|

|

|

|

|

|
| Sarfo et al58 |

|

|

|

|

|

|

|
| Saunders et al60 |

|

|

|

|

|

|

|
| Stewart et al61 |

|

|

|

|

|

|

|
| Steyn et al62 |

|

|

|

|

|

|

|
| Vedanthan et al64 |

|

|

|

|

|

|

|
| Wahab et al66 |

|

|

|

|

|

|

|
| Standardised treatment | |||||||
| Akintunde et al25 |

|

|

|

|

|

|

|
| Okeahialam et al55 |

|

|

|

|

|

|

|
| Physical activity | |||||||
| Aweto et al26 |

|

|

|

|

|

|

|
| Lamina37 |

|

|

|

|

|

|

|
| Maruf et al51 |

|

|

|

|

|

|

|
| Khalid et al63 |

|

|

|

|

|

|

|
| Modified nutrition | |||||||
| Charlton et al31 |

|

|

|

|

|

|

|
 : low;
: low;  : unclear;
: unclear;  : high risk of bias.
: high risk of bias.
Figure 5.
Summary of risk of bias.
Discussion
This systematic review describes interventions and treatment effects of 23 studies with a total of 18 153 participants with hypertension from six African countries. Most of the studies investigated successful low-cost concepts to improve BP control through improved adherence to medical treatment and lifestyle changes.
While lower-income and middle-income countries’ CVD mortality remained unchanged over the last decades, high-income countries have reduced the CVD mortality by more than 50% since 1990,67 largely by using country-specific guidelines, evidence-based policy interventions to reduce risk factor levels, strengthening the health system at the primary care level and improving acute care with attention to early initiation of treatment. However, policies to reduce population-wide risk factors of hypertension have not been widely adopted in low-income and middle-income countries.68
Pharmacotherapy with the well-established anti-hypertensive medications is the mainstay of hypertension management.15 69 Nevertheless, treatment recommendations on adherence to medication and changed lifestyle habits are often only incompletely applied in practice.70–72 Patients are frequently unwilling to take drugs due to possible side effects. They may benefit from adequate knowledge as well as a higher motivation to take their prescribed medications and to implement sustainable lifestyle changes.73–75 Despite the frequent lack of acute symptoms, uncontrolled BP may result in severe long-term outcome and increased mortality. The risk increases in cases of inadequate treatment and low patient adherence as well as inconsistent follow-up on BP control.7 Therefore, all strategies with the aim to increase knowledge, awareness and adherence are essential to lowering BP levels and improving the prognoses of patients.69 76 Due to the short-term follow-up, no study reported long-term outcomes on mortality, and we interpreted available results on BP changes and treatment adherence.
Several strategies to improve health-related behaviour concerning hypertension with convincing results were examined. We identified eight studies that investigated the efficacy of phone or letter-based interventions (eg, via short message service) to improve knowledge on hypertension, with adherence support or reminder letters for follow-up.27 28 36 56 58 60 61 64 All these studies showed strong effects of the intervention concerning self-reported behavioural changes, but only two of these studies showed improved BP during follow-up (Ayodapo and Olukokun, Bobrow et al).27 28 Three studies29 35 52 reported improved adherence and two of those a decreased BP level through nurse-led interventions (Bolarinwa et al, Mendis et al).29 52 These studies demonstrated the efficacy of task-shifting interventions in a low-resource setting. Furthermore, low-cost interventions suited to the environment, including financial incentives for adherent patients with minimal additional resources, can significantly improve the adherence of patients (Labhardt et al)36 and thus potentially influence BP control.
Even though cost-effective interventions are globally available, there are major gaps in their implementation, particularly in limited-resource settings.68 Two large multilevel studies that combined phone or letter-based interventions with task-shifting to nurses or health workers were not successful in achieving a relevant improvement in adherence and BP control (Fairall et al, Goudge et al).32 34 On the other hand, no harm was observed after the expansion of the nurses’ roles (Fairall et al).32 Thus, the intervention might be a practical and acceptable tool to expand the scope of non-physician clinicians into primary care of patients with common NCDs. There is a generally good access to essential medications in four countries where the included studies have been conducted (South Africa, Egypt, Kenya and Ghana). The access is not as widespread in Cameroon and Nigeria.77 Nevertheless, one study conducted in rural parts of South Africa between 2014 and 2015 (Goudge et al)34 reported insufficient or unavailable equipment and medication shortage. Moreover, increasing numbers of patients with NCD require an adequate number of nursing personnel as well as healthcare facilities. Similar factors contributed to the poor results of the implementation of national guidelines in resource-scarce primary healthcare settings in South Africa,62 which did not show improved outcomes in patients with hypertension and diabetes. In studies with follow-up-periods of less than 1 year, the time frame might have been too short to reach a clinically relevant BP control through improved knowledge and awareness, since lifestyle changes are oftentimes challenging and should be applied over a long time.24 61 66 Generally, the results of the systematic review are consistent with existing evidence on the importance of long-acting patient-centred interventions. Unfortunately, these interventions do not reach all patients and often, a full benefit of medical treatment on clinically important outcomes cannot be achieved.78
Most studies in this review included participants in secondary prevention with mild to moderate hypertension. In contrast, observational studies and conclusions from a systematic review on pharmacological treatment generally concerned participants with higher grades of hypertension.5 7 79 Interventions for patients with severe or uncontrolled hypertension and potentially target-organ damage are under-represented. Interventions for high-risk patients are especially necessary due to the high frequency of late first diagnosis7 and high prevalence of severe forms of hypertension at an early age in African patients.6 A multicentre study on patients with uncontrolled hypertension in clinics in Nigeria, Kenya and South Africa stated the efficacy of an individualised therapy based on phenotyping with plasma renin and aldosterone to improve BP control (Akintunde et al).25 The researchers suggest testing this approach in African Americans and patients of any race with therapy-resistant hypertension. Three studies56 58 66 investigated the implementation of multilevel approaches including educational, telephone-based, nurse-led, self-management supporting interventions, as well as BP monitoring for stroke survivors. These studies were not successful in sufficiently improving BP control, possibly due to short follow-up periods.
Regarding the different grades of hypertension, low-risk patients with grade 1 hypertension benefit from lifestyle modifications including regular physical activity, sodium restriction, weight reduction, smoking cessation, moderation of alcohol consumption and other dietary changes. These are recommended as initial strategies to reduce BP levels in order to prevent or delay the use of pharmacotherapy.14 15 Nevertheless, even for patients with higher grades of hypertension, lifestyle modifications remain important in addition to pharmacotherapy.14 15 69 80 The clinically accepted relevant BP-lowering effect of medium-intensity to high-intensity physical activity as a single or additive treatment for hypertension81 was demonstrated in four of the included studies.26 37 51 63 Only one study from South Africa investigated the effect of a modified nutrition strategy (reduction of salt intake) and stated a clinically relevant effect on SBP (Charlton et al).31 To the authors’ knowledge, no randomised study investigated the efficacy of other recommended lifestyle interventions, like smoking cessation or weight reduction, in patients with hypertension in an African country.
Strengths and limitations of this review
We were able to generate evidence on a wide range of different non-pharmacological interventions, adding a comprehensive overview to the literature that can support physicians and healthcare policymakers in the African setting.
A main limitation occurs through deviations from the protocol. We planned a comprehensive summary of all RCTs to prevent, diagnose and treat patients with CVDs in African countries. Due to a high number of eligible studies in the first systematic search, we decided to focus on published studies on hypertension. We therefore had to change the preplanned outcomes and instead focus on BP and additionally describe results on medication adherence and lifestyle changes. The preplanned outcomes mortality, New York Heart Association (NYHA) classification and hospital admission were dropped. Due to the recently published systematic review by Seeley et al,17 this publication describes non-pharmacological strategies. The complete results, including pharmacological interventions, were summarised in a doctoral thesis paper.82
Nevertheless, this review was limited to studies with the highest level of evidence to investigate the benefits and harms of non-pharmacological interventions for hypertension. The randomised allocation ensures the comparability of participants across intervention groups. However, the unfeasibility of double blinding might restrict the internal validity of results.
The external validity might be limited by our restriction to studies published in the English language and the disproportionally high number of studies conducted in urban areas in some Western and Southern African countries. According to the UN, there are currently 54 African countries. RCTs have been conducted in only six of those countries. Inhabitants of these countries (approximately 480 million) represent only a fraction of the African population of about 1.34 billion.83 Especially Central and Northern Africa were under-represented. There are high levels of diversity within and between African populations. Subpopulations with genetic variants are living in geographically distant areas with specific local lifestyle or environmental conditions, which may be associated with a susceptibility to specific NCDs.84 Therefore, it is uncertain whether our results can be extrapolated to patients living in other areas than those studied. A significant amount of the African population lives in rural areas while the majority of studies were conducted in urban settings. However, it is crucial to make health service available as close as possible to the population in order to achieve the most comprehensive care. Thus, research on non-pharmacological interventions such as educational strategies to improve adherence and lifestyle modification should be expanded across all parts of Africa. Research must be conducted especially in rural areas to ensure a higher generalisability, quality of services and resulting improvement of the African people’s health.
Conclusion
This systematic review shows that even though hypertension is a critical health problem, there are still few randomised studies on non-pharmacological treatment of hypertension conducted on the African continent. Available studies do not represent all Africans since they were conducted in only six countries, many in urban settings only. It is advisable to plan and implement studies on patients with hypertension and healthcare professionals in rural areas as well as Northern and Central African countries.
An improvement in the prognosis of patients with high BP in Africa requires the implementation of practical and effective solutions to diagnose, treat and control hypertension in specific settings.9 The identified studies describe diverse approaches tested in African countries that may be used to generate local African evidence-based guidelines on hypertension treatment. Especially trialled physical activity interventions and individualised treatment strategies show clinically relevant BP changes. Educational strategies for patients and medical personnel show mixed results and offer a comprehensive insight into trialled approaches as well as a basis for future research opportunities. This review summarises miscellaneous low-cost interventions including task-shifting, education individualised treatment and lifestyle modifications to improve BP control.
Supplementary Material
Footnotes
Contributors: MC was involved in all steps to plan this systematic review including the protocol; wrote the draft of this manuscript; screened titles, abstracts, and full texts; and did data extraction and quality assessment. ESK wrote the draft of this manuscript; screened titles, abstracts and full texts; did data extraction and quality assessment of the last update; and submitted the manuscript. TD was involved in all steps to plan this systematic review including the protocol; provided expertise and discussed the results in the African context; and commented on the manuscript. TF provided expertise on primary care aspects of hypertension treatment, discussed the results and commented on the manuscript. SG provided expertise on CVD epidemiology and public health, discussed the results in the African context and commented on the manuscript. EJK was involved in all steps to plan this systematic review including the protocol; provided expertise on the needs of evidence in the African context; and commented on the manuscript. EN provided expertise on public health and African guideline work, discussed the results in the African context, commented on the manuscript, provided expertise and discussed the reviewer comments and supported the draft of the revised manuscript. SU is responsible for the overall content as the guarantor and was involved in all steps to plan this systematic review including the protocol; wrote the draft of this manuscript; made systematic search; screened titles, abstracts and full texts; did data extraction and quality assessment; and submitted the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplemental information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
No ethics approval and consent to participate was necessary.
References
- 1.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration . Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol 2014;2:634–47. 10.1016/S2213-8587(14)70102-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017;389:37–55. 10.1016/S0140-6736(16)31919-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaze AD, Schutte AE, Erqou S, et al. Prevalence of hypertension in older people in Africa: a systematic review and meta-analysis. J Hypertens 2017;35:1345–52. 10.1097/HJH.0000000000001345 [DOI] [PubMed] [Google Scholar]
- 4.Bosu WK, Reilly ST, Aheto JMK, et al. Hypertension in older adults in Africa: a systematic review and meta-analysis. PLoS One 2019;14:e0214934. 10.1371/journal.pone.0214934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewster LM, Seedat YK. Why do hypertensive patients of African ancestry respond better to calcium blockers and diuretics than to ACE inhibitors and β-adrenergic blockers? A systematic review. BMC Med 2013;11:141. 10.1186/1741-7015-11-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brewster LM, van Montfrans GA, Oehlers GP, et al. Systematic review: antihypertensive drug therapy in patients of African and South Asian ethnicity. Intern Emerg Med 2016;11:355–74. 10.1007/s11739-016-1422-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oladapo OO, Salako L, Sadiq L, et al. Target-organ damage and cardiovascular complications in hypertensive Nigerian Yoruba adults: a cross-sectional study. Cardiovasc J Afr 2012;23:379–84. 10.5830/CVJA-2012-021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelissen HE, Hendriks ME, Wit FWNM, et al. Target organ damage among hypertensive adults in rural Nigeria: a cross-sectional study. J Hypertens 2014;32:487–94. 10.1097/HJH.0000000000000056 [DOI] [PubMed] [Google Scholar]
- 9.Dzudie A, Rayner B, Ojji D, et al. Roadmap to achieve 25% hypertension control in Africa by 2025. Cardiovasc J Afr 2017;28:262–72. 10.5830/CVJA-2017-040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.United Nations . Sustainable development goals. Goal 3: ensure healthy living and promote well-being for all at all ages, 2019. Available: https://www.un.org/sustainabledevelopment/health/
- 11.Gómez-Olivé FX, Ali SA, Made F, et al. Regional and sex differences in the prevalence and awareness of hypertension: an H3Africa AWI-Gen study across 6 sites in sub-Saharan Africa. Glob Heart 2017;12:81–90. 10.1016/j.gheart.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lloyd-Sherlock P, Beard J, Minicuci N, et al. Hypertension among older adults in low- and middle-income countries: prevalence, awareness and control. Int J Epidemiol 2014;43:116–28. 10.1093/ije/dyt215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guwatudde D, Nankya-Mutyoba J, Kalyesubula R, et al. The burden of hypertension in sub-Saharan Africa: a four-country cross sectional study. BMC Public Health 2015;15:1211. 10.1186/s12889-015-2546-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive summary: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Hypertension 2018;71:1269–324. 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 15.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–104. 10.1093/eurheartj/ehy339 [DOI] [PubMed] [Google Scholar]
- 16.Fredericksen RJ, Gibbons L, Brown S, et al. Medication understanding among patients living with multiple chronic conditions: implications for patient-reported measures of adherence. Res Social Adm Pharm 2018;14:540–4. 10.1016/j.sapharm.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeley A, Prynn J, Perera R, et al. Pharmacotherapy for hypertension in sub-Saharan Africa: a systematic review and network meta-analysis. BMC Med 2020;18:1–11. 10.1186/s12916-020-01530-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmood S, Shah KU, Khan TM, et al. Non-pharmacological management of hypertension: in the light of current research. Ir J Med Sci 2019;188:437–52. 10.1007/s11845-018-1889-8 [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutron I, Altman DG, Moher D, et al. Consort statement for randomized trials of nonpharmacologic treatments: a 2017 update and a consort extension for nonpharmacologic trial Abstracts. Ann Intern Med 2017;167:40–7. 10.7326/M17-0046 [DOI] [PubMed] [Google Scholar]
- 21.Team TE . EndNote. EndNote X9 ed. Philadelphia, PA: Clarivate, 2013. [Google Scholar]
- 22.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collaboration Copenhagen TC . Review Manager (RevMan) [Computer program]. Version 5.3, 2014. [Google Scholar]
- 24.Adeyemo A, Tayo BO, Luke A, et al. The Nigerian antihypertensive adherence trial: a community-based randomized trial. J Hypertens 2013;31:201–7. 10.1097/HJH.0b013e32835b0842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akintunde A, Nondi J, Gogo K, et al. Physiological phenotyping for personalized therapy of uncontrolled hypertension in Africa. Am J Hypertens 2017;30:923–30. 10.1093/ajh/hpx066 [DOI] [PubMed] [Google Scholar]
- 26.Aweto HA, Owoeye OBA, Akinbo SRA, et al. Effects of dance movement therapy on selected cardiovascular parameters and estimated maximum oxygen consumption in hypertensive patients. Nig Q J Hosp Med 2012;22:125–9. [PubMed] [Google Scholar]
- 27.Ayodapo AO, Olukokun TAV. Lifestyle counselling and behavioural change: role among adult hypertensives in a rural tertiary institution. South African Family Practice 2019;61:91–6. 10.1080/20786190.2019.1569453 [DOI] [Google Scholar]
- 28.Bobrow K, Farmer AJ, Springer D, et al. Mobile Phone Text Messages to Support Treatment Adherence in Adults With High Blood Pressure (SMS-Text Adherence Support [StAR]): A Single-Blind, Randomized Trial. Circulation 2016;133:592–600. 10.1161/CIRCULATIONAHA.115.017530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolarinwa OA, Juni MH, Nor Afiah MZ, et al. Mid-term impact of home-based follow-up care on health-related quality of life of hypertensive patients at a teaching hospital in Ilorin, Nigeria. Niger J Clin Pract 2019;22:69–78. 10.4103/njcp.njcp_246_17 [DOI] [PubMed] [Google Scholar]
- 30.Cappuccio FP, Kerry SM, Micah FB, et al. A community programme to reduce salt intake and blood pressure in Ghana [ISRCTN88789643]. BMC Public Health 2006;6:13. 10.1186/1471-2458-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charlton KE, Steyn K, Levitt NS, et al. A food-based dietary strategy lowers blood pressure in a low socio-economic setting: a randomised study in South Africa. Public Health Nutr 2008;11:1397–406. [Erratum appears in Public Health Nutr 2009;12:284]. 10.1017/S136898000800342X [DOI] [PubMed] [Google Scholar]
- 32.Fairall LR, Folb N, Timmerman V, et al. Educational outreach with an integrated clinical tool for nurse-led non-communicable chronic disease management in primary care in South Africa: a pragmatic cluster randomised controlled trial. PLoS Med 2016;13:e1002178. 10.1371/journal.pmed.1002178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folb N, Timmerman V, Levitt NS, et al. Multimorbidity, control and treatment of noncommunicable diseases among primary healthcare attenders in the Western Cape, South Africa. S Afr Med J 2015;105:642–7. 10.7196/samjnew.8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goudge J, Chirwa T, Eldridge S, et al. Can lay health workers support the management of hypertension? Findings of a cluster randomised trial in South Africa. BMJ Glob Health 2018;3:e000577. 10.1136/bmjgh-2017-000577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gyamfi J, Plange-Rhule J, Iwelunmor J, et al. Training nurses in task-shifting strategies for the management and control of hypertension in Ghana: a mixed-methods study. BMC Health Serv Res 2017;17:104. 10.1186/s12913-017-2026-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labhardt ND, Balo J-R, Ndam M, et al. Improved retention rates with low-cost interventions in hypertension and diabetes management in a rural African environment of nurse-led care: a cluster-randomised trial. Trop Med Int Health 2011;16:1276–84. 10.1111/j.1365-3156.2011.02827.x [DOI] [PubMed] [Google Scholar]
- 37.Lamina S. Effects of continuous and interval training programs in the management of hypertension: a randomized controlled trial. J Clin Hypertens 2010;12:841–9. 10.1111/j.1751-7176.2010.00315.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamina S. Comparative effect of interval and continuous training programs on serum uric acid in management of hypertension: a randomized controlled trial. J Strength Cond Res 2011;25:719–26. 10.1519/JSC.0b013e3181d09edf [DOI] [PubMed] [Google Scholar]
- 39.Lamina S, Okoye CG. Effect of low intensity continuous training programme on serum uric acid in the non pharmacological management of hypertension: a randomized controlled trial. Niger J Med 2010;19:77–86. 10.4314/njm.v19i1.52485 [DOI] [PubMed] [Google Scholar]
- 40.Lamina S, Okoye CG. Uricaemia as a cardiovascular events risk factor in hypertension: the role of interval training programme in its downregulation. J Assoc Physicians India 2011;59:23–8. [PubMed] [Google Scholar]
- 41.Lamina S, Okoye CG. Effect of interval training program on white blood cell count in the management of hypertension: a randomized controlled study. Niger Med J 2011;52:271–7. 10.4103/0300-1652.93803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamina S, Okoye CG, Dagogo TT. Managing erectile dysfunction in hypertension: the effects of a continuous training programme on biomarker of inflammation. BJU Int 2009;103:1218-–21. 10.1111/j.1464-410X.2008.08254.x [DOI] [PubMed] [Google Scholar]
- 43.Lamina S, Okoye CG, Dagogo TT. Therapeutic effect of an interval exercise training program in the management of erectile dysfunction in hypertensive patients. J Clin Hypertens 2009;11:125–9. 10.1111/j.1751-7176.2009.00086.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamina S, Okoye CG, Hanif SM. Randomised controlled trial: effects of aerobic exercise training programme on indices of adiposity and metabolic markers in hypertension. J Pak Med Assoc 2013;63:680–7. [PubMed] [Google Scholar]
- 45.Lamina S, Okoye CG, Hanif SM. Effects of interval exercise training programme on the indices of adiposity and biomarker of inflammation in hypertension: a randomised controlled trial. Niger Postgrad Med J 2014;21:136–43. [PubMed] [Google Scholar]
- 46.Lamina S, Okoye G. Effects of aerobic exercise training on psychosocial status and serum uric acid in men with essential hypertension: a randomized controlled trial. Ann Med Health Sci Res 2012;2:161–8. 10.4103/2141-9248.105665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamina S, Okoye GC. Therapeutic effect of a moderate intensity interval training program on the lipid profile in men with hypertension: a randomized controlled trial. Niger J Clin Pract 2012;15:42–7. 10.4103/1119-3077.94096 [DOI] [PubMed] [Google Scholar]
- 48.Lamina S, Okoye GC. Effect of interval exercise training programme on C-reactive protein in the non-pharmacological management of hypertension: a randomized controlled trial. Afr J Med Med Sci 2012;41:379–86. [PubMed] [Google Scholar]
- 49.Leon N, Surender R, Bobrow K, et al. Improving treatment adherence for blood pressure lowering via mobile phone SMS-messages in South Africa: a qualitative evaluation of the SMS-text adherence support (StAR) trial. BMC Fam Pract 2015;16:80. 10.1186/s12875-015-0289-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maruf FA, Akinpelu AO, Salako BL. Self-reported quality of life before and after aerobic exercise training in individuals with hypertension: a randomised-controlled trial. Appl Psychol Health Well Being 2013;5:209–24. 10.1111/aphw.12005 [DOI] [PubMed] [Google Scholar]
- 51.Maruf FA, Akinpelu AO, Salako BL, et al. Effects of aerobic dance training on blood pressure in individuals with uncontrolled hypertension on two antihypertensive drugs: a randomized clinical trial. J Am Soc Hypertens 2016;10:336–45. 10.1016/j.jash.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 52.Mendis S, Johnston SC, Fan W, et al. Cardiovascular risk management and its impact on hypertension control in primary care in low-resource settings: a cluster-randomized trial. Bull World Health Organ 2010;88:412–9. 10.2471/BLT.08.062364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ogedegbe G, Plange-Rhule J, Gyamfi J, et al. Health insurance coverage with or without a nurse-led task shifting strategy for hypertension control: a pragmatic cluster randomized trial in Ghana. PLoS Med 2018;15:e1002561. 10.1371/journal.pmed.1002561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogedegbe G, Plange-Rhule J, Gyamfi J, et al. A cluster-randomized trial of task shifting and blood pressure control in Ghana: study protocol. Implement Sci 2014;9:73. 10.1186/1748-5908-9-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okeahialam B, Ohihoin E, Ajuluchukwu J. Chronotherapy in Nigerian hypertensives. Ther Adv Cardiovasc Dis 2011;5:113–8. 10.1177/1753944711402119 [DOI] [PubMed] [Google Scholar]
- 56.Owolabi MO, Gebregziabher M, Akinyemi RO, et al. Randomized trial of an intervention to improve blood pressure control in stroke survivors. Circ Cardiovasc Qual Outcomes 2019;12:e005904. 10.1161/CIRCOUTCOMES.119.005904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarfo FS, Ovbiagele B. Stroke minimization through additive anti-atherosclerotic agents in routine treatment (SMAART): a pilot trial concept for improving stroke outcomes in sub-Saharan Africa. J Neurol Sci 2017;377:167–73. 10.1016/j.jns.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 58.Sarfo FS, Treiber F, Gebregziabher M, et al. Phone-based intervention for blood pressure control among Ghanaian stroke survivors: a pilot randomized controlled trial. Int J Stroke 2019;14:630–8. 10.1177/1747493018816423 [DOI] [PubMed] [Google Scholar]
- 59.Sarfo FS, Treiber F, Jenkins C, et al. Phone-based intervention under nurse guidance after stroke (PINGS): study protocol for a randomized controlled trial. Trials 2016;17:436. 10.1186/s13063-016-1557-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saunders LD, Irwig LM, Gear JS, et al. A randomized controlled trial of compliance improving strategies in Soweto hypertensives. Med Care 1991;29:669–78. 10.1097/00005650-199107000-00007 [DOI] [PubMed] [Google Scholar]
- 61.Stewart A, Noakes T, Eales C, et al. Adherence to cardiovascular risk factor modification in patients with hypertension. Cardiovasc J S Afr 2005;16:102–7. [PubMed] [Google Scholar]
- 62.Steyn K, Lombard C, Gwebushe N, et al. Implementation of national guidelines, incorporated within structured diabetes and hypertension records at primary level care in Cape Town, South Africa: a randomised controlled trial. Glob Health Action 2013;6:20796. 10.3402/gha.v6i0.20796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khalid T, Nesreen E, Ramadhan O. Effects of exercise training on postmenopausal hypertension: implications on nitric oxide levels. Med J Malaysia 2013;68:459–64. [PubMed] [Google Scholar]
- 64.Vedanthan R, Kamano JH, DeLong AK, et al. Community Health Workers Improve Linkage to Hypertension Care in Western Kenya. J Am Coll Cardiol 2019;74:1897–906. 10.1016/j.jacc.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vedanthan R, Kamano JH, Naanyu V, et al. Optimizing linkage and retention to hypertension care in rural Kenya (LARK hypertension study): study protocol for a randomized controlled trial. Trials 2014;15:143. 10.1186/1745-6215-15-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wahab KW, Owolabi M, Akinyemi R, et al. Short-term pilot feasibility study of a nurse-led intervention to improve blood pressure control after stroke in Nigeria. J Neurol Sci 2017;377:116–20. 10.1016/j.jns.2017.04.005 [DOI] [PubMed] [Google Scholar]
- 67.Jagannathan R, Patel SA, Ali MK, et al. Global updates on cardiovascular disease mortality trends and attribution of traditional risk factors. Curr Diab Rep 2019;19:44. 10.1007/s11892-019-1161-2 [DOI] [PubMed] [Google Scholar]
- 68.Jeemon P, Gupta R, Onen C. In: Prabhakaran D, Anand S, Gaziano TA, eds. Management of hypertension and dyslipidemia for primary prevention of cardiovascular diseases. World Bank Publications, 2017: 444. [PubMed] [Google Scholar]
- 69.Noone C, Leahy J, Morrissey EC, et al. Comparative efficacy of exercise and anti-hypertensive pharmacological interventions in reducing blood pressure in people with hypertension: a network meta-analysis. Eur J Prev Cardiol 2020;27:247–55. 10.1177/2047487319879786 [DOI] [PubMed] [Google Scholar]
- 70.Burnier M. Drug adherence in hypertension. Pharmacol Res 2017;125:142–9. 10.1016/j.phrs.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 71.GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nielsen J Ø, Shrestha AD, Neupane D, et al. Non-adherence to anti-hypertensive medication in low- and middle-income countries: a systematic review and meta-analysis of 92443 subjects. J Hum Hypertens 2017;31:14–21. 10.1038/jhh.2016.31 [DOI] [PubMed] [Google Scholar]
- 73.Ajiboye RO, Okafor NA, Abiodun IO. Knowledge and practice of lifestyle modification among hypertensive patients in a general Hospital Lagos. Indian J Commun Health 2015;27:143–9. [Google Scholar]
- 74.Tedla YG, Bautista LE. Drug side effect symptoms and adherence to antihypertensive medication. Am J Hypertens 2016;29:772–9. 10.1093/ajh/hpv185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goel MK. Non-adherence to anti-hypertensive treatment. Indian J Commun Health 2020;32:126–9. [Google Scholar]
- 76.Xu T, Yu X, Ou S, et al. Adherence to antihypertensive medications and stroke risk: a dose-response meta-analysis. J Am Heart Assoc 2017;6. 10.1161/JAHA.117.006371. [Epub ahead of print: 25 Jul 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.WHO . Noncommunicable diseases country profiles. Geneva: World Health Organization, 2018. [Google Scholar]
- 78.Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014;11:CD000011. 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ayodele OE, Alebiosu CO, Akinwusi PO, et al. Target organ damage and associated clinical conditions in newly diagnosed hypertensives attending a tertiary health facility. Niger J Clin Pract 2007;10:319–25. [PubMed] [Google Scholar]
- 80.Hypertension guideline working group, Seedat YK, Rayner BL, et al. South African hypertension practice guideline 2014. Cardiovasc J Afr 2014;25:288–94. 10.5830/CVJA-2014-062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Börjesson M, Onerup A, Lundqvist S, et al. Physical activity and exercise lower blood pressure in individuals with hypertension: narrative review of 27 RCTs. Br J Sports Med 2016;50:356–61. 10.1136/bjsports-2015-095786 [DOI] [PubMed] [Google Scholar]
- 82.Cernota MD. Randomized controlled trials on prevention, diagnosis and treatment of hypertension in Africa, a systematic review 2020.
- 83.United Nations . World population prospects, 2019. Department of economic and social Affairs, population division. Available: https://population.un.org/wpp/Download/Standard/Population/ [Accessed 21 Sep 2020].
- 84.Gomez F, Hirbo J, Tishkoff SA. Genetic variation and adaptation in Africa: implications for human evolution and disease. Cold Spring Harb Perspect Biol 2014;6:a008524. 10.1101/cshperspect.a008524 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-048079supp001.pdf (127.7KB, pdf)
bmjopen-2020-048079supp002.pdf (226.2KB, pdf)
bmjopen-2020-048079supp003.pdf (81.7KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplemental information.