Abstract
Introduction
One anastomosis gastric bypass (OAGB) leads to improvement in glucose homeostasis; however, the mechanism of this beneficial effect is not fully understood. Increased serum free fatty acid (FFA) concentrations in obese subjects contribute to the development of insulin resistance and type 2 diabetes.
Aim
The authors hypothesized that improvement in glucose homeostasis after OAGB may be associated with a decrease in FFA concentration.
Material and methods
Serum FFA levels were measured by gas chromatography-mass spectrometry before and 3 months after OAGB and, for comparison, in patients who underwent laparoscopic sleeve gastrectomy (LSG). Serum insulin was assayed by immunoenzymatic method, and other parameters by standard laboratory methods.
Results
OAGB resulted in a large decrease in FFA levels and great improvement in insulin sensitivity. These effects in patients after LSG were less prominent.
Conclusions
Results suggest that decreased serum FFA levels after OAGB contribute to resolution of insulin sensitivity after this type of bariatric surgery.
Keywords: glucose homeostasis, free fatty acids, one-anastomosis gastric bypass
Introduction
The levels of serum free fatty acids (FFAs), also known as non-esterified fatty acids, are chronically elevated in obese individuals as a result of dysfunctional lipid metabolism and increased lipolysis in adipose tissue [1]. The storage of fatty acids in the form of triglycerides in adipocytes protect other tissues from their toxic effects. Increased FFA levels in the blood lead to an inflammatory state, lipotoxicity, and hypertriglyceridaemia and increases insulin resistance, mainly in the liver and muscles [2]. Currently, surgical intervention(s) can achieve sustained weight loss, and a reduction in mortality and obesity-related comorbidities. One-anastomosis gastric bypass (OAGB) is an increasingly popular bariatric technique, whereas laparoscopic sleeve gastrectomy (LSG) is one of the most commonly performed bariatric operations worldwide [3, 4]. OAGB, a simplified malabsorption and restrictive technique, is a safe and effective procedure. Its advantage is that it is technically simple, and has lower morbidity and mortality rates compared with vertical sleeve gastrectomy and Roux-en-Y gastric bypass (RYGB) [4]. LSG is a restrictive bariatric procedure, which reduces the concentration of ghrelin by removing the gastric fundus, which is a major source of ghrelin that regulates appetite and food-intake [5]. LSG and OAGB have been proposed as alternatives to RYGB, with comparable short-term weight loss and metabolic effects. All these procedures lead to weight loss, resolution of hyperglycaemia, insulin resistance, and type 2 diabetes mellitus; however, the mechanisms of their beneficial effects are not fully understood. Currently available data suggest that various bariatric procedures reduce plasma FFA levels. It is known that in patients who undergo LSG, fatty acid levels decrease after 3 months, but return to preoperative levels approximately 1 year after surgery [6]. Some authors presented improved lipidogram after OAGB [7], but data on serum FFA concentrations after OAGB are not available.
Aim
The aim of this study was to evaluate to the effect of OAGB on serum FFA levels. We also compared it with the effect of LSG. Moreover, we studied how these bariatric procedures influenced glucose metabolism.
Material and methods
Thirty patients with obesity underwent bariatric surgery: OAGB, n = 15; LSG, n = 15. The patients were qualified for surgery according to the IFSO guidelines (International Federation for the Surgery of Obesity and Metabolic Disorders) [8] and Polish standards of care in bariatric and metabolic surgery [9]. Briefly, the indication for the surgical treatment of morbid obesity are the patient’s highest documented body mass index (BMI) values: a) BMI ≥ 40 kg/m2, b) BMI in the range 35–40 kg/m2 in patients in whom surgically induced weight reduction can bring potential improvement in diseases caused by obesity alone, and c) BMI > 30 kg/m2 in patients with diabetes type 2 who do not respond to drug treatment.
The patients were qualified for various types of surgery by a team of experienced bariatric surgeons. Patients who were mainly expected to have a bariatric effect were considered eligible for LSG. Patients who were mainly expected to have a metabolic effect and did not have the contraindications listed below were considered eligible for OAGB. Contraindications for OAGB were iron and protein deficiencies, advanced fatty liver, gastric polyps, family history of gastric cancer, gallstones, and lack of full cooperation.
In the study groups, the treatments were performed briefly as follows:
Laparoscopic sleeve gastrectomy (LSG)
Small incisions were made in the abdominal wall for the insertion of small trocars (15 mm trocar, 2 × 5 mm, and 12 mm). The stomach was inspected. The blood vessels to the lateral side of stomach were divided using a cutting device like a harmonic scalpel or LigaSure. A 34F Bougie tube was inserted into the stomach, which served as a sizer for the size of the new stomach. Starting from 4–6 cm from the pylorus a stapler was used to divide the stomach into 2 parts. Continuous firing of the stapler was used to divide the stomach. After the first fire, the stapler was very close to the Bougie tube and made a small “banana shaped” new stomach. The stomach was completely divided into 2 parts. The removed larger part comprised about 75–80% of the original stomach volume. Once this part of stomach was decompressed, it was removed through an optical trocar incision.
One-anastomosis gastric bypass (OAGB)
The upper part of the stomach was divided into a tube, similar to the top three-quarters of a sleeve (based on a 36F calibration tube), and then joined to a loop of intestine. This new stomach was then joined to the middle part of the small intestine (the jejunum), skipping the first 150–180 cm of the intestine. The unused part of the stomach and intestine remained in the body but were no longer involved in food digestion. It is an operation that combines food intake restriction with some calorie malabsorption, resulting in good weight loss with great quality of life. To count the length of the loop, we used Storz surgical tools with a 10 cm line marked on the shaft – so we could use the tool as a ruler and measure the desired length.
Serum concentrations of FFA were measured using Wako NEFA-HR(2) reagent (FUJIFILM Wako Chemicals Europe GmbH, Neuss, Germany). Briefly, fatty acid oxidation leads to the formation of hydrogen peroxide, which, by quantitative oxidative condensation with 3-methyl-N-ethyl-N-(β-hydroxyethyl)-aniline and 4-aminoantipyrine, forms a blue-purple pigment. The FFA concentration is determined by measuring the absorbance of this pigment at 550 nm. Serum glucose, triglyceride, high-density lipoprotein (HDL), low-density lipoprotein, total cholesterol, high-sensitivity C-reactive protein, albumin, and total protein levels were estimated using a laboratory analyser (Erba Diagnostics Mannheim Gmbh, XL-100, Mannheim, Germany). Insulin was assessed using the Human Insulin ELISA Kit (Abcam, Fluorescent ab229416, Cambridge, United Kingdom). The Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) ratio was calculated using the equation: HOMA-IR = [fasting insulin (μU/ml) × fasting glucose (mg/dl)]/405.
Ethics
The study was performed in agreement with the principles of the Declaration of Helsinki of the World Medical Association. The study protocol was approved by the Local Bioethics Committee (decision no. NKBBN/493/2016), and written informed consent was obtained from all participants.
Statistical analysis
Statistical analysis of pre- and postoperative changes was performed using Statistica version 13.3 (Tibco Software Inc., Palo Alto, CA, USA) and the Wilcoxon matched pair test or paired t-test. To examine differences between the 2 bariatric procedures, the Mann-Whitney or 2-tailed t-test was used. Data are expressed as mean ± SD, and differences with p < 0.05 were considered to be statistically significant.
Results
Bariatric surgery (both OAGB and LSG) resulted in a significant decrease in body mass index and insulin concentration, but the decrease of serum insulin was more pronounced in patients after OAGB (Table I). Serum glucose concentrations decreased slightly only after OAGB. After LSG, triglyceride levels decreased, HDL cholesterol increased; total cholesterol concentrations were slightly increased 3 months after surgery (Table I). No significant changes in lipidogram were found after OAGB.
Table I.
Selected biochemical and anthropometric characteristics of the study subjects before and 3 months after bariatric surgery
| Parameter | Pre-OAGB | 3 m Post-OAGB | Pre-LSG | 3 m Post-LSG | P (pre- vs. post-OAGB) | P (pre- vs. post-LSG) | P (pre- OAGB vs. pre-LSG) | P (post- OAGB vs. post-LSG) |
|---|---|---|---|---|---|---|---|---|
| N | 15 | 15 | 15 | 15 | – | – | – | – |
| Sex (F/M) | 13/2 | – | 12/3 | – | – | – | – | – |
| Age [years] | 43.4 ±7.54 | – | 37.3 ±9.96 | – | – | – | – | – |
| BMI [kg/m2] | 36.8 ±2.42 | 29.6 ±2.84 | 41.6 ±8.73 | 35.1 ±8.10 | 0.001 | 0.001 | 0.331 | 0.070 |
| Triglycerides [mg/dl] | 124 ±47.2 | 126 ±61.8 | 157 ±67.5 | 121 ±45.8 | 0.534 | 0.036 | 0.132 | 0.959 |
| HDL [mg/dl] | 37.3 ±6.64 | 40.1 ±7.05 | 42.5 ±5.92 | 55.1 ±13.8 | 0.534 | 0.001 | 0.077 | 0.002 |
| LDL [mg/dl] | 116 ±24.3 | 123 ±52.0 | 124 ±32.1 | 134 ±32.6 | 0.152 | 0.173 | 0.485 | 0.177 |
| Total cholesterol [mg/dl] | 167 ±32.1 | 194 ±60.3 | 186 ±38.8 | 210 ±44.2 | 0.196 | 0.027 | 0.102 | 0.214 |
| Hs-CRP [mg/l] | 2.77 ±1.56 | 2.20 ±0.69 | 4.36 ±4.50 | 2.08 ±0.93 | 0.445 | 0.004 | 0.371 | 0.637 |
| Albumin [g/l] | 35.2 ±3.31 | 39.3 ±3.54 | 40.7 ±2.73 | 41.7 ±4.83 | 0.010 | 0.496 | < 0.001 | 0.070 |
| Total protein [g/dl] | 6.71 ±0.78 | 7.42 ±0.86 | 7.65 ±0.55 | 7.88 ±1.18 | 0.114 | 0.427 | 0.002 | 0.233 |
| Glucose [mg/dl] | 110 ±14.7 | 99.7 ±16.6 | 100 ±24.0 | 104 ±20.5 | 0.041 | 0.955 | 0.062 | 0.371 |
| Insulin [μU/ml] | 14.0 ±3.08 | 9.74 ±2.84 | 17.4 ±5.76 | 14.0 ±5.18 | 0.010 | 0.031 | 0.061 | 0.003 |
Data are presented as mean ± standard deviation. BMI – body mass index, HOMA-IR – homeostasis model assessment for insulin resistance, LDL – low-density lipoprotein, HDL – high-density lipoprotein cholesterol, hs-CRP – highly sensitive C-reactive protein.
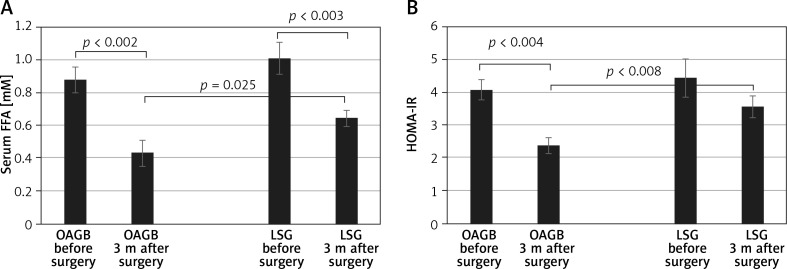
We have compared the baseline FFA concentrations and HOMA between OAGB and LSG groups and they were not statistically different. Three months after the bariatric procedures, a significant reduction (p < 0.01) in FFA levels was found in both groups (i.e. OAGB and LSG) compared with pre-surgery levels (Figure 1 A). However, a larger decrease was observed in those who underwent OAGB (51%) compared with LSG (36%). In addition, there was a significant difference (p = 0.025) in FFA levels between patients in the LSG and OAGB groups 3 months after the procedure (Figure 1 A). HOMA-IR also decreased 3 months after both interventions; however, a statistically significant decrease was observed only in the group that underwent OAGB. The decrease in HOMA-IR after OAGB was also larger than after LSG (41% vs. 20%, respectively) (Figure 1 B). In addition, as in the case of FFA, there was a significant difference (p = 0.008) in HOMA-IR between patients in the LSG and OAGB groups after the procedure (Figure 1 B).
Figure 1.
Changes in plasma free fatty acid (FFA) levels (A) and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) (B) 3 months after bariatric surgery
Discussion
For both interventions, the reduction in food intake after surgery seems to be one of the reasons for the decrease in serum FFA levels. A greater decrease in FFA concentration after OAGB may be associated with the reduction in the distance of food passage in the intestine and, accordingly, to a decrease in fat absorption [2, 3, 7]. The differences between the effect of OAGB and LSG on serum FFA levels may also be due to the length of the excluded part of the digestive tract, as well as the exclusion of the duodenum and part of the jejunum in OAGB, which plays a greater role in fatty acid absorption than the excised part of the stomach in the case of LSG [10]. Both bariatric procedures are known to have a positive effect on insulin resistance [3, 4, 11]. Considering the well-established association between increased serum FFA levels and the development of insulin resistance [2], we postulate that decreased levels of FFA after OAGB and LSG contribute to the resolution of insulin resistance. Moreover, the effect of OAGB on serum FFA levels and HOMA-IR was significantly stronger than from LSG (Figure 1). Also, it should be noted that a slight but significant decrease in glucose levels (p < 0.041) was observed only in patients referred for OAGB, and that the decrease in insulin concentration after OAGB was larger than after LSG (31% vs. 20%, respectively). To our knowledge, the present study is the first to demonstrate the considerable effect of OAGB on serum FFA levels, and to suggest that it may significantly contribute to improvement in glucose homeostasis after this type of bariatric surgery.
Another possible mechanism explaining the favourable metabolic effect observed in patients after OAGB may be associated with a significant increase in the undiluted concentration of bile in the intestine and a longer bile loop, which leads to more efficient absorption of bile acids and an increase in their blood levels, as well as the direct effect of bile acids on receptors in the intestinal tract [3, 12]. Possible mechanisms that may explain the differences in the results of the different bariatric procedures include the following: microbial alterations in the gut [13]; changes in intestinal metabolism itself [14]; more significant changes in ghrelin, obestatin, and glucagon-like peptide 1 concentrations (hindgut hypothesis); decreased hydrochloride production after LSG; and different postprandial nutrient delivery time, significantly affecting the speed of production and the intensity of action of gastric, intestinal, and adipose tissue hormones at the cellular or tissue levels [1, 10, 12].
A limitation of our study was the small study cohort. However, even with this sample size, the reported results are statistically significant. Because both groups were not different in FFA concentrations and HOMA index at baseline, we believe that these results are convincing.
Conclusions
Our study demonstrated, for the first time, that OAGB results in a considerable decrease in serum FFA levels 3 months after surgery, and this effect was more prominent than in patients after LSG. This effect may be added to other metabolic improvements that lead to resolution of insulin resistance after this type of bariatric surgery.
Acknowledgments
This study was supported by the National Science Centre of Poland [grant number NCN 2016/21/D/NZ5/00219].
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Carswell KA, Belgaumkar AP, Amiel SA, Patel AG. A systematic review and meta-analysis of the effect of gastric bypass surgery on plasma lipid levels. Obes Surg. 2016;26:843–55. doi: 10.1007/s11695-015-1829-x. [DOI] [PubMed] [Google Scholar]
- 2.Mika A, Sledzinski T. Alterations of specific lipid groups in serum of obese humans: a review. Obes Rev. 2017;18:247–72. doi: 10.1111/obr.12475. [DOI] [PubMed] [Google Scholar]
- 3.Mika A, Kaska L, Proczko-Stepaniak M, et al. Evidence that the length of bile loop determines serum bile acid concentration and glycemic control after bariatric surgery. Obes Surg. 2018;28:3405–14. doi: 10.1007/s11695-018-3314-9. [DOI] [PubMed] [Google Scholar]
- 4.Taha O, Abdelaal M, Abozeid M, et al. Outcomes of omega loop gastric bypass, 6-years experience of 1520 cases. Obes Surg. 2017;27:1952–60. doi: 10.1007/s11695-017-2623-8. [DOI] [PubMed] [Google Scholar]
- 5.Haluzíková D, Lacinová Z, Kaválková P, et al. Laparoscopic sleeve gastrectomy differentially affects serum concentrations of FGF-19 and FGF-21 in morbidly obese subjects. Obesity. 2013;21:1335–42. doi: 10.1002/oby.20208. [DOI] [PubMed] [Google Scholar]
- 6.Farey JE, Preda TC, Fisher OM, et al. Effect of laparoscopic sleeve gastrectomy on fasting gastrointestinal, pancreatic, and adipose-derived hormones and on non-esterified fatty acids. Obes Surg. 2017;27:399–407. doi: 10.1007/s11695-016-2302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbajo MA, Fong-Hirales A, Luque-de-León E, et al. Weight loss and improvement of lipid profiles in morbidly obese patients after laparoscopic one-anastomosis gastric bypass: 2-year follow-up. Surg Endosc. 2017;31:416–21. doi: 10.1007/s00464-016-4990-y. [DOI] [PubMed] [Google Scholar]
- 8.De Luca M, Angrisani L, Himpens J, et al. Indications for surgery for obesity and weight-related diseases: position statements from the international federation for the surgery of obesity and metabolic disorders (IFSO) Obes Surg. 2016;26:1659–96. doi: 10.1007/s11695-016-2271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szeliga J, Wyleżoł M, Major P, et al. Metabolic and bariatric surgery chapter of the Association of Polish Surgeons. Bariatric and metabolic surgery care standards. Videosurgery Miniinv. 2020;15:391–4. doi: 10.5114/wiitm.2020.97935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaska Ł, Proczko M, Wiśniewski P, et al. A prospective evaluation of the influence of three bariatric procedures on insulin resistance improvement. Should the extent of undiluted bile transit be considered a key postoperative factor altering glucose metabolism? Videosurgery Miniinv. 2015;10:213–28. doi: 10.5114/wiitm.2015.52062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Zhu C, Wen X, et al. Laparoscopic sleeve gastrectomy improves body composition and alleviates insulin resistance in obesity related acanthosis nigricans. Lipids Health Dis. 2017;16:209. doi: 10.1186/s12944-017-0598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faraj M, Havel PJ, Phélis S, et al. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 13.Damms-Machado A, Mitra S, Schollenberger AE, et al. Effects of surgical and dietary weight loss therapy for obesity on gut microbiota composition and nutrient absorption. Biomed Res Int. 2015;2015:806248. doi: 10.1155/2015/806248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavin JB, Voitellier E, Cluzeaud F, et al. Malabsorption and intestinal adaptation after one anastomosis gastric bypass compared with Roux-en-Y gastric bypass in rats. Am J Physiol Gastrointest Liver Physiol. 2016;311:G492–500. doi: 10.1152/ajpgi.00197.2016. [DOI] [PubMed] [Google Scholar]