Abstract
Based on findings from cognitive science, it has been theorized that the reductions in motivation and goal-directed behavior in people with psychosis could stem from impaired episodic memory. In the current meta-analysis, we investigated this putative functional link between episodic memory deficits and negative symptoms. We hypothesized that episodic memory deficits in psychosis would be related to negative symptoms in general but would be more strongly related to amotivation than to reduced expressivity. We included 103 eligible studies (13,622 participants) in the analyses. Results revealed significant, moderate negative associations of episodic memory with negative symptoms in general (k = 103; r = −.23; z = −13.40; P ≤ .001; 95% CI [−.26; −.20]), with amotivation (k = 16; r = −.18; z = −6.6; P ≤ .001; 95% CI [−.23; −.13]) and with reduced expressivity (k = 15; r = −.18; z = −3.30; P ≤.001; 95% CI[−.29; −.07]). These associations were not moderated by sociodemographic characteristics, positive symptoms, depression, antipsychotic medication or type of negative symptom scale. Although these findings provide sound evidence for the association between episodic memory deficits and amotivation, the rather small magnitude and the unspecific pattern of this relationship also indicate that episodic memory deficits are unlikely to be the only factor relevant to amotivation. This implicates that future research should investigate episodic memory in conjunction with other factors that could account for the association of episodic memory deficits and amotivation in psychosis.
Keywords: schizophrenia, avolition, apathy, experimental negative symptoms, anhedonia, deficit syndrome, prospection
Introduction
Anhedonia, apathy, social withdrawal, blunted affect and alogia are subsumed under the umbrella of negative symptoms1 and are evident in approximately 60% of people with psychosis.2 These can be referred to as either five distinct symptom domains,3 or can be summarized to amotivation (i.e., anhedonia, apathy and social withdrawal) and reduced expressivity (i.e., blunted affect and alogia).4 Given that particularly amotivation has been found to predict low subjective quality of life5 and reduced psychosocial functioning,6 both practitioners7 and patients8 prioritize the reduction of amotivation as a treatment goal for recovery. However, antipsychotic medication9–11 and available psychological interventions12 have been found to show rather small effects on amotivation. This lack of efficacy is most likely due to our limited understanding of the factors underlying amotivation.
One factor that is likely to drive amotivation is reduced anticipatory pleasure as it has been found to predict the reductions in goal-directed activities in daily-life13 and to be associated with behavioral avoidance.14 Anticipatory pleasure has been proposed to include four interrelated processes: (1) reward prediction (i.e., ability to form associations between cues predicting potential rewards and outcomes themselves), (2) prospection (i.e., mental simulation of future events by drawing upon memories), (3) anticipatory affect (i.e., momentary hedonic affective experience in anticipation of future events) and (4) affective forecasting (i.e., expectation of how a specific future event will feel).15 Findings from previous research on amotivation revealed that people with psychosis differed from healthy controls in each of these processes. For instance, they revealed deficient prediction16 and value representations of positive reinforcement17 despite intact hedonic reactions to reward, less vivid prospections,18 reduced anticipatory pleasure19 and negative expectations regarding the pleasurableness of future events.20 Given that the association between stimuli and rewarding experiences needs to be encoded, retained and flexibly reconstructed to build mental representations of the future, a common denominator of these processes could be memory. This assumption converges with findings from basic cognitive psychology, which indicate that people consciously and unconsciously draw on information about their past experiences to stimulate motivational processes by imagining future events or activities.21–23 This information is stored in episodic memory and comprises context-based knowledge of temporally dated and spatially located events of ones’ past experiences.24 Recalling information about positive experiences has been found to induce both current positive affect25 and the expectation of future positive affect,26 which both motivate behavior.27,28 Based on this evidence, it has been theorized that the reductions in motivation and goal-directed behavior in psychosis could stem from impaired episodic memory.29,30
Indeed, several meta-analyses point to episodic memory deficits in people with psychosis.31–38 These meta-analyses—mostly with a broad focus on impaired neurocognition—found that compared to healthy controls, those diagnosed with psychosis showed moderately to strongly reduced performance in tests of verbal and visual memory as well as logical and visuo-spatial or autobiographical memory. The performance in these tests taps to a varying extent into features of episodic memory, namely encoding and retrieval of context information, coding of spatiotemporal relations and free recall of past experiences.39 Most commonly, episodic memory is operationalized by the number of correctly recalled items of a set of neutral stimuli (e.g., word lists, geometrical figures, etc.) presented prior to a standardized delay interval. In autobiographical memory tests, memory performance is operationalized by the amount and detail of spontaneously recalled experiences in relation to a list of cues (e.g., list of emotional words or pictures). Notably, within the range of neurocognitive impairments found in psychosis, episodic memory deficits fall amongst the most severely impaired neurocognitive functions and are more pronounced than deficits in other memory domains (e.g., working memory).31 Moreover, Bora et al38 found that individuals who met the criteria of the so called deficit syndrome (i.e., a psychosis syndrome characterized by primary and enduring negative symptoms40) showed more severe deficits in verbal (k = 12; d = 0.34) and in visual memory (k = 10; d = 0.27) than those without deficit syndrome. Similarly, a correlational meta-analysis found that negative symptoms, but not positive symptoms were related to deficits in verbal (k = 23; r = −.21) and in visual memory (k = 8; r = −.16).41 Taken together, although the effect sizes were rather small, the meta-analytic evidence indicates specific associations of episodic memory deficits and negative symptoms. However, despite its recency and sound rationale, the meta-analysis by Bora et al38 only included studies referring to the deficit syndrome. Also, there has been a considerable increase of publications since Ventura et al41 completed their literature search in 2006. Accordingly, previous meta-analyses have only covered a part of the relevant studies available today and do not reflect the recent advances in negative symptom research. This includes, for instance, the so-called “second-generation negative symptom scales,” which have been available since 201142 and were developed to improve the assessment of amotivation.43 In addition, it is important to note that most of the previous meta-analyses were more broadly focused on investigating neurocognitive deficits in general. Consequently, the reported associations between episodic memory deficits and negative symptoms result from a series of sub-analyses and multiple significance testing, which could have been biased by an alpha error inflation. Therefore, an updated meta-analysis that focuses on episodic memory specifically is necessary to gain a more reliable picture of the relationship between episodic memory and negative symptoms.
A further question that has not been addressed by previous meta-analyses is whether episodic memory deficits are specifically related to amotivation or to reduced expressivity. Given the findings from functional neuroimaging studies, suggesting that amotivation and reduced expressivity relate to distinct neural networks,44 there is reason to expect differential associations of amotivation and reduced expressivity with certain neurocognitive functions. Also, theoretical accounts have emphasized different putative neuropsychological underpinnings to explain reductions in expressivity versus amotivation. While reduced expressivity has been theorized to be a consequence of impairments in attention and working memory,45,46 amotivation has been traced to deficits in episodic memory.30,47 Therefore, one would expect deficits in episodic memory to show a stronger association to amotivation than to reduced expressivity. However, research findings have been inconsistent in this regard with some studies reporting either specific associations of episodic memory deficits with amotivation,48 or with reduced expressivity49 and others reporting unspecific associations with both.50 A meta-analysis examining the specific association between episodic memory deficits and amotivation versus reduced expressivity would be helpful to gain a clearer picture of whether existing research supports the notion of a functional link between episodic memory deficits and impaired motivational processes.
We therefore provide an updated examination of the association between episodic memory and negative symptoms in psychosis. We hypothesized that (1) episodic memory deficits would be significantly related to the severity of negative symptoms in people with psychosis, and (2) that the association between episodic memory deficits and amotivation would be stronger in magnitude than the association between episodic memory deficits and reduced expressivity.
Method
Reporting Guidelines and Registry
The meta-analysis was conducted in accord with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).51 The protocol was preregistered with PROSPERO (Record ID: CRD42020214555, accessible at https://www.crd.york.ac.uk/PROSPERO/).
Literature Search
The systematic literature search was conducted in December 2020. It included the databases PubMed, PychInfo and Web of Science and the following key words: schizophreni*, psychotic disorders, schizoaffective, negative symptoms, anhedoni*, avolition, amotivation, apath*, anticipatory pleasure, asociality, negative schizophrenia, deficit syndrome, deficit schizophrenia, blunted affect, alogia, neurocogn*, cognitive impairment, memor*. Searches were restricted to articles published in either English or German between 1989 and 2021 and included peer-reviewed articles and published dissertations. Google scholar and reference lists of previous meta-analyses on neurocognition in schizophrenia31–38,41 were used for additional manual searches.
Study Selection and Eligibility Criteria
M.P. and L.B. independently conducted the title and abstract screening. M.P. and K.K. applied the eligibility criteria after full-text screening. Disagreements were resolved by discussion. If required, further information was requested from study authors.
Studies were eligible if they (1) assessed episodic memory (e.g., immediate and delayed recall in either verbal or visual learning or visuo-spatio-temporal or logical memory) with a validated and standardized test, (2) assessed negative symptoms with established scales, and (3) reported a correlation coefficient or any other effect size measure (e.g., Cohen’s d) on the relationship between episodic memory and negative symptoms. Intervention studies were only eligible if these reported baseline correlations. M.P., L.B., and K.K. independently checked the eligible studies for overlapping samples and excluded any duplicates.
Quality Assessment
Study quality was evaluated with a version of the Quality Assessment Tool for Quantitative Studies (QATQ)52 that had been adjusted for correlational studies.53 This version of the QATQ consists of five subscales to assess the quality of the sample selection (e.g., participants referred from multiple settings), the study design (e.g., a priori hypothesis stated), data collection methods (e.g., reliable instruments are used), missing data reporting (e.g., numbers and reasons for missing data are reported) and quality of analyses (e.g., significance level is adjusted for multiple testing). We extended this adjusted version of the QATQ by a further item on reporting bias. On this item, a study was rated as of “high quality” if the authors had reported all correlation coefficients of the associations that were examined in the respective study and of “low quality” if only a subset (e.g., only statistically significant coefficients) had been reported (see supplement S1 for full rating criteria). Each subscale of the QATQ was rated on a three-point scale ranging from 1 (“low quality/high risk of bias”) to 3 (“high quality/low risk of bias”). The sum scores of the QATQ ratings had a possible range from 5 (“poor quality”) to 16 (“high quality”). MP rated the quality of all studies and KK rated a random sample of 20% (k=21) for independent ratings. Rating discrepancies (≥3 on the total QATQ; n = 3) were discussed until consensus was reached. The ratings showed a good reliability with ICC = 0.79 (95% CI [.49;.91]).
Data Extraction and Effect Size Calculation
M.P. and L.B. independently extracted data and double-checked the datasets for inconsistencies. In case of missing data, study authors were contacted to obtain missing data. K.K. checked the final dataset and the calculated effect sizes. Inconsistencies were resolved by discussion with M.P.
Effect sizes were calculated based on Fisher’s z-transformed Pearson’s correlation coefficients. If a study reported another type of correlation coefficient (e.g., Spearman’s p) or effect size (e.g., Cohen’s d), these were transformed into Pearson’s r prior to Fisher’s z-transformation. If a study reported more than one relevant effect size (e.g., multiple indices of a memory test, multiple subsamples or longitudinal data), these were summarized at study level by calculating weighted average scores across subtests, subsamples, or measurements, respectively. The variance at the study level outcomes was calculated as 1⁄(n − 3) as described in Borenstein et al.54
Coding of Sample Characteristics and Covariates
Sample characteristics included age, gender, years of education, diagnoses, chlorpromazine equivalent doses, severity of positive symptoms, and depressive symptoms. We further coded number of data points that were synthesized in the effect size at study-level, name, and indices used to measure episodic memory, name of the scales used to assess negative symptoms, positive and depressive symptoms as well as type of effect size measure and significance level.
Statistical Analysis
The metafor package55 implemented in RStudio was used to conduct the analyses. We used random-effect models to calculate the summary effect sizes based on 95% confidence intervals. To test for specificity of the association between episodic memory and amotivation, we calculated pairwise differences between the effect sizes in those studies that reported associations of episodic memory with both amotivation and reduced expressivity. Negative values of these effect sizes indicate a stronger association of episodic memory with reduced expressivity than with amotivation. Effect sizes of r = ±.10; ±.20, and ±.30 were considered small, moderate and large, respectively.56
Heterogeneity of effect sizes was evaluated using the Q statistic and the I²-Index.57,58 Where significant between-study variability was indicated (i.e., significant Q-statistic and a I2 index value ≥ 25%),59 we conducted moderator analyses by calculating meta-regression random-effect models with restricted maximum likelihood estimation. We predefined the following covariates for the test of moderation effects: Gender, age, years of education, chlorpromazine equivalent doses, positive symptoms, depressive symptoms, number of synthesized data points per study, study quality ratings, and type of negative symptom scale (first vs second generation).
Publication bias was assessed by visual inspection of funnel plots and by using the rank correlation test60 and Egger’s regression test.61
Results
Study Characteristics and Participants
A total of 101 studies met our inclusion criteria, of which 64 were not included in the meta-analysis by Ventura et al41 and 87 were not included in the meta-analysis by Bora et al38 (see figure 1 for study retrieval flow diagram). Within the 101 studies, we identified 103 independent samples that were included in the analyses. Across all studies, a total of 13 622 participants (31% female) diagnosed with a psychotic disorder were included (89% schizophrenia). Participants had a mean age of 36.29 (SD = 9.76) years and reported a mean of 11.71 (SD = 1.46) years of education. Sixteen studies (15%) reported effect sizes of the relationship between episodic memory and amotivation and 15 studies (14%) reported effect sizes of the relationship between episodic memory and reduced expressivity. Ten studies (10%) reported both the association of episodic memory with amotivation and with reduced expressivity. The most frequently used scales to assess negative symptoms were the Positive and Negative Syndrome Scale62 (PANSS; 44%), the Scale for the Assessment of Negative Symptoms63 (SANS; 30%), and the Schedule for the Deficit Syndrome64 (SDS; 12%). Amotivation and reduced expressivity were predominantly assessed with the SANS (63% and 67%, respectively). Only one study reporting on the association between episodic memory and reduced expressivity used PANSS item “blunted affect.” Across all studies, 96 (92%) used scales of the first generation and eight (8%) used scales of the second generation to assess negative symptoms (see supplement S2 for detailed study characteristics).
Fig. 1.
PRISMA flow chart of study selection.
Measurement of Episodic Memory
Table 1 depicts the tests and subtests used to measure episodic memory performance in the included studies. Across studies, 12 tests of verbal memory, 12 tests of visual or visual-spatial memory, and one test of autobiographical memory were used. Sixty-four percent of the studies reported an effect size of the association between verbal memory and negative symptoms. Eight percent reported an effect size of the association between visual memory and negative symptoms, 27% reported effect sizes of the association of both verbal and visual memory with negative symptoms and 1% of the association between autobiographical memory and negative symptoms.
Table 1.
Episodic memory tests included in meta-analyses
| Cognitive domain | Neurocognitive test | Subtest | Brief description |
|---|---|---|---|
| Verbal learning and memory | Wechsler Memory Scale (WMS, WMS-R, WMS-III) | Logical Memory | Subjects are asked to recall the contents of two short stories immediately after presentation and again after a 30-minute delay. |
| Verbal Paired Associates | Subjects are presented with 5 trials of paired word presentations and are asked to recall the list immediately after presentation and again after a 30-minute delay. | ||
| Hopkins Verbal Learning Test (HVLT) | A list of words belonging to different semantic categories is presented verbally for three trials. Subjects are asked to recall as many words as possible immediately and again after a delay. | ||
| California Verbal Learning Test (CVLT) | Subjects are verbally presented with a 16-word list for five immediate recall trials, followed by a single presentation and recall of a second 16-word ‘interference’ list. Subjects give free- and category-cued recall immediately after presentation and after a 20-minute delay interval. | ||
| Brief Assessment of Cognition in Schizophrenia (BACS) | Verbal Memory Subtest | Subjects are presented with a list of 15 words and then asked to recall as many as possible in five consecutive trials. | |
| Auditory Verbal Learning Test (AVLT) | Subjects are given five presentations of a 15-word list (list A), each followed by an immediate recall. This is followed by a 15-word interference list (list B), followed by an immediate and delayed recall of list A. | ||
| Penn Word Memory Test (PWMT) | Subjects are presented with 20 target words that are then mixed with 20 distractors. The subjects' score reflects the number of correctly recognized targets and correctly rejected foils. A 20-minute delayed recall procedure is administered to measure episodic memory. | ||
| Consortium to Establish a Registry for Alzheimer's Disease (CERAD) |
Word List Memory Subtest | A 10-item word list is presented over three trials in altering order. The subject is asked to recall as many words as possible. After a short delay of five minutes the subject is again asked to recall as many words as possible. | |
| Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) | Delayed Recall-List (Recall) | Subjects are asked to learn and to recall a 10-item list of semantically unrelated words over four trials. | |
| Hong Kong List Learning Test (HKLLT) | Subjects are asked to recall as many words as possible from a 16-item Chinese word list immediately after three learning trials, after 10-minute and 30-minute delay intervals. | ||
| Groningen Word Learning Task (WLT) |
Subjects are presented with a 15-item word list and are subsequently asked to recall as many of the previously learned words out of an extended list containing additional and similar words as distractors. | ||
| International Shopping List Task (ISLT) | Subjects are presented with shopping list items in three trials and are asked to remember each item. Subjects are asked to recall as many words as possible immediately after acquisition and after a 15-minute delay interval. | ||
| Visual learning and memory |
Post Graduate Institute Battery of Brain Dysfunction (PGI-MS) | Memory Scale | Includes a number of subtests such as delayed recall of a word list, immediate recall of sentences, retention of similar word pairs, retention of dissimilar pairs, and visual retention. |
| Rey–Osterreith Complex Figures Test (ROCFT) |
Subjects are asked to copy a stimulus figure and are asked to draw the figure from memory after a 3-minute and a 30-minute delay interval. | ||
| Wechsler Memory Scale (WMS, WMS-R, WMS-III) | Visual Reproduction | Subjects are asked to look at five figures for 10 seconds each and to “draw the design” from memory immediately and after a 25-minute delay interval. | |
| Visual Paired Associates Subtest | Subjects are asked to learn the color associated with each of six abstract line drawings within up to six learning trials. Subjects are asked to recall the associates immediately after each trial and after a 30-minute delay interval. | ||
| Figural Memory Subtest | Subjects are presented with a set of abstract designs, and are subsequently asked to identify the target designs within a large group of designs. | ||
| Brief Visuospatial Memory Test (BVMT) | Subjects are asked to learn six geometric figures within three learning trials and to reproduce the drawings immediately after each trial and after a 30-minute delay interval. All reproductions are scored according to standardized criteria. | ||
| Cambridge Neuropsychological Test Automated Battery (CANTAB) | Paired Associates Learning Task (PAL) |
Subjects are presented with a number of boxes that are simultaneously displayed on a screen and are “opened” in a randomized order with one of them containing a target pattern. The subject is then asked to select the box in which the target pattern was originally located. The number of correct identifications is analyzed. | |
| Face Recognition Task (FRT) | Subjects are presented with a series of 10 faces. After the learning trial, subjects are asked to identify these 10 original faces out of pairwise presented faces of which one is a distractor stimulus. | ||
| Picture Memory Interference Test (PMIT) | Subjects are asked to select between two previously visually presented pictures. Recognition memory tasks are the presentation of previously presented pictures with distractor items not initially presented. The number of correctly identified target items is analyzed as index of recognition performance. | ||
| Penn Face Memory Test (PFMT) | Subjects are presented with 20 faces that are then mixed with 20 distractors matched for age, gender and ethnicity. Subjects are asked to identify the target faces immediately after the learning trial and after a 20-minute delay. The subjects' score reflects the number of correctly recognized targets and correctly rejected foils. | ||
| Visual Object Learning Test (VOLT) | Subjects are presented with 20 Euclidean shapes as learning stimuli over four learning trials, followed by short and long delay recall test. | ||
| Benton Visual Retention Test (BVRT) | Subjects are presented with 10 designs, one at a time, and asked to reproduce each one as precisely as possible from memory. | ||
| Serial Digit Learning Test (SDLT) | Subjects are presented with a mixed series of numbers ranging from 1 to 9. Subjects are asked to remember and to recall the number set verbally in the correct order. The number of recall trials needed until correct recall is analyzed as an index of memory performance. | ||
| Autobiographical memory | Autobiographical Memory Test (AMT) | Subjects are presented with 10 cue words printed on cards (5 × positive; 5 × negative) and are asked to recall a specific autobiographical memory related to each cue word within 60 seconds. |
Quality Ratings
The mean quality of the studies was “moderate” (M = 11.08, median = 11.5, SD = 1.72, range 7–16). Studies scored “moderate” for sample selection with 38% of studies recruiting from at least two settings. Fifty-seven percent of the studies did not specify a priori hypothesis, which reduced the quality of the respective studies to “low.” Due to our inclusion criteria (eligible studies needed to employ a validated measure of episodic memory and of negative symptoms), the quality of the data collection methods was generally rated as “high.” Study quality in terms of missing data reporting was rated as “low” as only 5% of the studies reported on missing data. Statistical analyses of the studies were rated as “moderate” since 74% of the studies did not test preconditions for the respective statistical test and 63% did not adjust the significance level for multiple testing. Finally, reporting of results was rated as “good,” with only 11% of the studies not reporting the non-significant coefficients.
Main Analyses
Episodic memory and negative symptoms in general showed a significant negative association with a moderate effect size (k = 103; r = −.23; z = −13.31; P ≤ .001; 95% CI [−.26; −.20]). The heterogeneity analysis revealed a significant Q-statistic (Q = 284; P ≤ .001) and an I2 index of 64% (see table 2 for individual study-level effect sizes).
Table 2.
Study characteristics and measurement information
| Study | Study ES (VZ) | N | Data points | Negative symptom scale | Memory test | Study quality |
|---|---|---|---|---|---|---|
| Addington & Addington65 | −0.26 (0.01) | 80 | 2 | PANSS | WMS-R, ROCFT | 14 |
| Addington et al66 | −0.13 (0.03) | 38 | 5 | SANS | WMS, ROCFT | 10 |
| Bagney et al67 | −0.16 (0.01) | 80 | 2 | PANSS | HVLT-R, BVMT-R | 12 |
| Balogh et al68 | −0.61 (0.03) | 42 | 2 | PANSS | CANTAB | 10 |
| Basso et al69 | −0.42 (0.02) | 62 | 2 | SANS | WMS-R | 15 |
| Bell & Mishara70 | −0.08 (0.01) | 151 | 7 | SANS | HVLT, WMS-R | 14 |
| Berenbaum et al71 | −0.10 (0.02) | 47 | 2 | SANS, UPS | WMS-R, FRT | 11 |
| Berman et al72 | 0.17 (0.04) | 30 | 2 | PANSS | WMS-R | 13 |
| Bilder et al49 | −0.23 (0.01) | 94 | 4 | SANS | WMS-R, CVLT, ROCFT | 12 |
| Bismarck et al73 | −0.21 (0.03) | 36 | 2 | SANS | HVLT-R, BVMT-R | 11 |
| Bodapati et al74 | −0.26 (0.03) | 38 | 4 | CAS | HVLT-R, BVMT-R | 11 |
| Boeker et al75 | −0.43 (0.05) | 22 | 1 | SANS | WMS-R | 10 |
| Bozikas et al76 | −0.30 (0.02) | 53 | 7 | PANSS | CVLT, ROCFT | 11 |
| Brazo et al77 | −0.51 (0.04) | 26 | 3 | SDS | CVLT | 10 |
| Bryson et al 78 | −0.12 (0.01) | 90 | 5 | SDS | HVLT, WMS-R | 11 |
| Buchanan et al79 | −0.20 (0.03) | 39 | 3 | SDS | WMS-R | 10 |
| Buchanan et al80 | −0.46 (0.03) | 33 | 1 | SANS | WMS-R | 9 |
| Cammisuli et al81 | 0.65 (0.04) | 30 | 2 | PANSS | WMS-IV | 8 |
| Cascella et al82 | −0.12 (0.01) | 105 | 4 | SDS | HVLT-R, BVMT-R | 10 |
| Chan et al83 | −0.15 (0.01) | 145 | 4 | SDS | WMS-R | 8 |
| Chang et al84 | −0.37 (0.01) | 84 | 2 | PANSS | WMS-R | 10 |
| Chang et al85 | −0.24 (0.01) | 93 | 8 | HEN | WMS-R | 14 |
| Chang et al48 | −0.10 (0.003) | 321 | 2 | SANS | WMS-R | 13 |
| Chen et al86 | −0.14 (0.01) | 157 | 3 | HEN | WMS-R | 10 |
| Chen et al87 | −0.14 (0.04) | 175 | 8 | PANSS | ISLT, CPAL | 10 |
| Chkonia & Tsverava88 | −0.45 (0.06) | 20 | 6 | SANS | CVLT | 10 |
| Cohen et al89 | −0.19 (0.02) | 45 | 5 | SDS | WMS-R | 12 |
| Dorofeikova et al90 | −0.31 (0.01) | 125 | 1 | PANSS | ROCFT | 7 |
| Eckman et al91 | −0.07 (0.02) | 51 | 1 | SANS | WMS-R | 11 |
| Ehmann et al92 | −0.02 (0.05) | 37 | 4 | PANSS | WMS-R | 10 |
| Faerden et al93 | −0.16 (0.02) | 71 | 2 | AES-C | CVLT-II, ROCFT | 12 |
| Fonseca et al94 | 0.02 (0.01) | 99 | 2 | PANSS | HVLT-R, BVMT-R | 14 |
| Foussias et al95 | −0.31 (0.02) | 69 | 1 | SANS, AES-C | BACS | 12 |
| Frydecka et al96 | −0.35 (0.01) | 85 | 3 | PANSS | AVLT | 12 |
| Galderisi et al97 | −0.06 (0.01) | 112 | 2 | SDS | AVLT, PMIT | 12 |
| Galderisi et al98 | −0.05 (0.01) | 160 | 2 | PANSS | AVLT | 8 |
| González-Blanch et al99 | −0.02 (0.01) | 131 | 2 | SANS | AVLT, ROCFT | 12 |
| Good et al100 | −0.20 (0.01) | 153 | 4 | PANSS | AVLT, WMS-R | 12 |
| Guillem et al101 | 0.02 (0.04) | 27 | 4 | SANS | WMS-R | 11 |
| Gur et al102 (a) | −0.38 (0.004) | 328 | 3 | SANS | PWMT, PFMT, VOLT | 12 |
| Gur et al102 (b) | −0.10 (0.001) | 1195 | 3 | SANS | PWMT, PFMT, VOLT | 12 |
| Hammer et al103 | −0.22 (0.02) | 65 | 2 | SANS | AVLT, BVRT | 9 |
| Harrison & Fowler104 | −0.45 (0.03) | 36 | 1 | PANSS | AMT | 13 |
| Hartmann-Riemer et al50 | −0.39 (0.02) | 47 | 2 | BNSS | AVLT | 13 |
| Harvey et al105 | −0.45 (0.01) | 174 | 12 | PANSS | CERAD | 11 |
| Hedge et al106 | −0.35 (0.02) | 49 | 3 | PANSS | AVLT, ROCFT | 11 |
| Heydebrand et al107 | −0.30 (0.004) | 254 | 1 | PANSS | AVLT, WMS-R | 14 |
| Hintze & Borkowska108 | −0.16 (0.03) | 33 | 2 | PANSS | AVLT | 11 |
| Horan & Blanchard109 | −0.15 (0.02) | 45 | 4 | SDS | WMS-R | 11 |
| Hornig et al110 | −0.42 (0.06) | 20 | 4 | SANS | WMS-R | 8 |
| Hovington et al111 | −0.34 (0.01) | 136 | 4 | SANS | WMS-R | 11 |
| Jhung et al112 | −0.22 (0.05) | 23 | 6 | CAS-R | CVLT | 12 |
| Kanchanatawan et al113 | −0.48 (0.01) | 80 | 6 | SDS | CERAD | 11 |
| Keefe et al114 | −0.24 (0.01) | 1332 | 1 | PANSS | HVLT-R | 13 |
| Khalil et al115 | −0.21 (0.01) | 109 | 2 | PANSS | WMS-R | 11 |
| Klingberg et al116 | −0.17 (0.01) | 151 | 1 | PANSS | AVLT, ROCFT | 12 |
| Konstantakopoulos et al117 | −0.07 (0.03) | 36 | 2 | AES-C | AVLT, ROCFT | 11 |
| Krishnadas et al118 | 0.08 (0.05) | 25 | 8 | SANS | PGIMS, ROCFT | 10 |
| Lee et al119 | −0.31 (0.01) | 160 | 1 | PANSS | WMS-R | 10 |
| Li et al120 | −0.20 (0.01) | 360 | 2 | SANS | WMS-R | 10 |
| Lin et al121 | −0.33 (0.003) | 302 | 2 | SANS | WMS-III | 13 |
| Lindsberg et al122 | −0.22 (0.01) | 92 | 6 | PANSS | WMS-R | 9 |
| Lipkovich et al123 | −0.07 (0.003) | 395 | 1 | PANSS | AVLT | 9 |
| Liu et al124 | −0.28 (0.01) | 78 | 2 | PANSS | HVLT-R, BVMT-R | 12 |
| Manglam & Das125 | −0.12 (0.01) | 78 | 3 | SANS | AVLT | 12 |
| McCraedie et al126 (a) | −0.41 (0.06) | 19 | 3 | PANSS | WMS | 12 |
| McCraedie et al126 (b) | −0.24 (0.05) | 25 | 3 | PANSS | WMS | 12 |
| McDaniel et al127 | −0.19 (0.03) | 35 | 2 | SANS | WMS-R | 11 |
| Mingrone et al128 | −0.32 (0.004) | 276 | 1 | PANSS | CVLT | 11 |
| Minzenberg et al129 | −0.05 (0.02) | 57 | 1 | PANSS | CVLT | 14 |
| Moritz et al130 | −0.45 (0.05) | 25 | 6 | PANADSS | AVLT | 12 |
| Morrison-Stewart et al131 | −0.44 (0.04) | 30 | 1 | SANS | WMS | 10 |
| Mu et al132 | −0.47 (0.004) | 251 | 2 | PANSS | HVLT-R, BVMT-R | 11 |
| Newcomer et al133 | −0.37 (0.07) | 17 | 2 | BPRS | AVLT, BVRT | 10 |
| Norman et al134 | −0.13 (0.01) | 87 | 4 | SANS | AVLT, BVRT, ROCFT, WMS-R | 15 |
| O’Leary et al135 | −0.22 (0.01) | 110 | 8 | SANS | AVLT, BVRT, ROCFT, WMS-R | 13 |
| Pandina et al136 | −0.06 (0.003) | 300 | 1 | PANSS | ROCFT | 10 |
| Pegoraro et al137 | −0.21 (0.01) | 73 | 1 | SDS | ROCFT | 11 |
| Perlick et al138 | −0.19 (0.003) | 309 | 1 | PANSS | RBANS | 10 |
| Puig et al139 | −0.38 (0.04) | 29 | 1 | PANSS | WMS-III | 11 |
| Putman & Harvey140 (a) | −0.43 (0.02) | 59 | 3 | SDS | CERAD | 10 |
| Putman & Harvey140 (b) | −0.42 (0.01) | 174 | 3 | SDS | CERAD | 10 |
| Quinlan et al141 | −0.15 (0.01) | 179 | 1 | SANS | HVLT | 12 |
| Raffard et al142 | −0.26 (0.01) | 137 | 1 | LARS | CVLT | 10 |
| Rémillard et al143 | −0.33 (0.04) | 28 | 3 | PANSS | CVLT | 10 |
| Réthelyi et al144 | −0.33 (0.004) | 266 | 1 | SDS | AVLT | 9 |
| Rhinewine et al145 | −0.16 (0.02) | 54 | 1 | SANS | CVLT | 12 |
| Rocca et al146 | −1.01 (0.01) | 78 | 1 | PANSS | WMS-III | 9 |
| Rund et al147 | −0.09 (0.01) | 207 | 2 | PANSS | CVLT | 13 |
| Sergi et al148 | −0.16 (0.01) | 100 | 4 | SANS | CVLT | 10 |
| Smith et al149 | −0.28 (0.01) | 72 | 1 | SANS | WMS-III | 11 |
| Srinivasan et al150 | −0.27 (0.01) | 100 | 3 | PANSS | WMS-R | 8 |
| Strauss et al151 | −0.15 (0.01) | 100 | 2 | BNSS | HVLT-R, BVMT-R | 13 |
| Tanaka et al152 | −0.42 (0.02) | 61 | 1 | PANSS | BACS | 12 |
| Tong et al153 | −0.32 (0.02) | 60 | 2 | PANSS | HKLLT | 10 |
| Tregellas et al154 | −0.49 (0.04) | 28 | 1 | SANS | HVLT-R | 9 |
| van der Werf et al155 | −0.06 (0.001) | 1053 | 4 | PANSS | WLT | 12 |
| Villalta-Gil et al156 | −0.32 (0.01) | 94 | 1 | PANSS | CVLT | 17 |
| Wang et al157 | −0.06 (0.01) | 123 | 2 | SDS | WMS-R | 11 |
| Wittorf et al158 | −0.71 (0.08) | 15 | 1 | PANSS | AVLT, ROCFT | 8 |
| Woodward et al159 | −0.24 (0.02) | 68 | 10 | SSPI | AVLT, BVMT-R | 9 |
| Yazihan & Yetkin160 | −0.61 (0.08) | 15 | 2 | PANSS | AVLT, SDLT | 11 |
| Zakzanis161 | 0.36 (0.04) | 27 | 1 | BPRS | CVLT | 10 |
Note: Study ES (Vz), Fischer’s Z transformed study effect size (study-level variance); BPRS, Brief Psychiatric Rating Scale; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms; CAS, Chapman Anhedonia scale, CAS-R, Chapman Anhedonia Scale – Revised; SPPI, Standardized Polyvalent Psychiatric Interview; SDS, Schedule for the Deficit Syndrome; BNSS, Brief Negative Symptom Scale; LARS, Lille Apathy Rating Scale; PANADSS, Positive and Negative and Disorganized Symptoms Scale; VFE, Verbal Fluency Examinations; AES-C, Apathy Evaluation Scale - Clinician version; HEN, High Royds Evaluation of Negativity Scale; SSPI, Signs and Symptoms of Psychotic Illness; UPS, Urbana Pleasure Scale; WMS-R, Wechsler Memory Scale - Revised; WMS, Wechsler Memory Scale; ROCFT, Rey–Osterrieth Complex Figure Test; HVLT, Hopkins Verbal Learning Test; HVLT-R, Hopkins Verbal Learning Test – Revised; CVLT, California Verbal Learning Test; BVMT-R, Brief Visuospatial Memory Test – Revised; BACS, Brief Assessment of Cognition in Schizophrenia; CANTAB, Cambridge Neuropsychological Test Automated Battery; AVLT, Auditory Verbal Learning Test; PMIT, Picture Memory and Interference Test; PWMT, Penn Word Memory Test; PFMT, Penn Face Memory Test; VOLT, Visual Object Learning Test; AMT, Autobiographical Memory Test; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; PGIMS, Post Graduate Institute Memory Scale; BVRT, Benton Visual Retention Test; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; HKLLT, Hong Kong List Learning Test; WLT, Groningen Word Learning Task; SDLT, Serial Digit Learning Test; ISLT, International Shopping List Task; CPAL, Continuous Paired Association Learning Task.
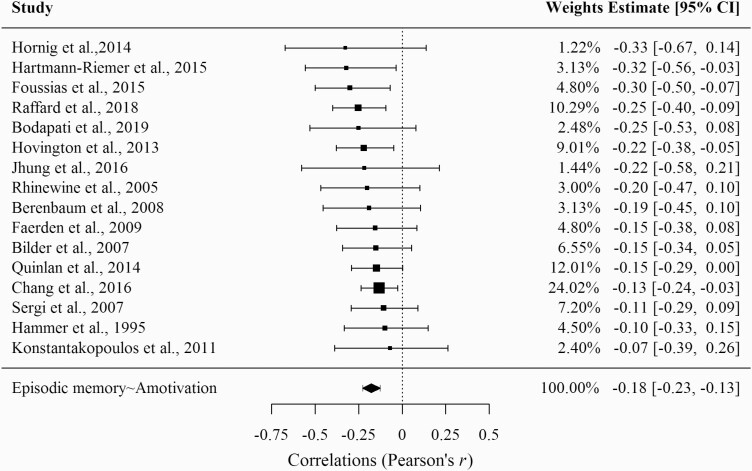
As can be seen in figure 2, amotivation and episodic memory showed a significant negative association with a small effect size (k = 16; r = −.18; z = −6.6; P ≤ .001; 95% CI [−.23; −.13]). The heterogeneity analysis revealed a non-significant Q-statistic (Q = 6.28; P = .98) and an I2 index of 0%.
Fig. 2.
Forest plot of the correlation between episodic memory deficits and amotivation.
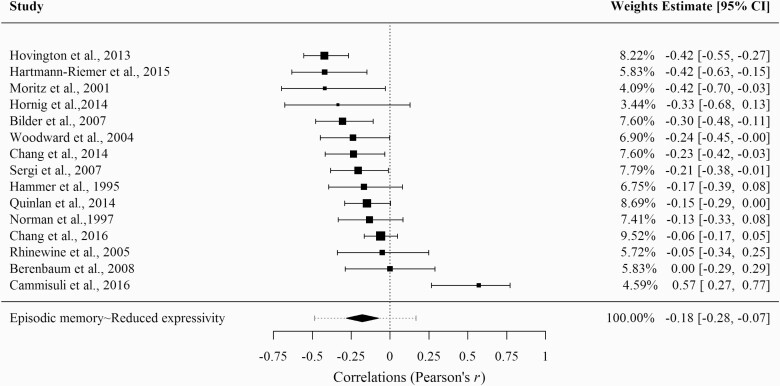
As can be seen in figure 3, reduced expressivity showed a significant negative association with a small effect size (k = 15; r = −.18; z = −3.30; P ≤ .001; 95% CI [−.29; −.07]). The heterogeneity analysis revealed a significant Q-statistic (Q = 42.43; P ≤ .001) and an I2 index of 70%.
Fig. 3.
Forest plot of the correlation between episodic memory deficits and reduced expressivity.
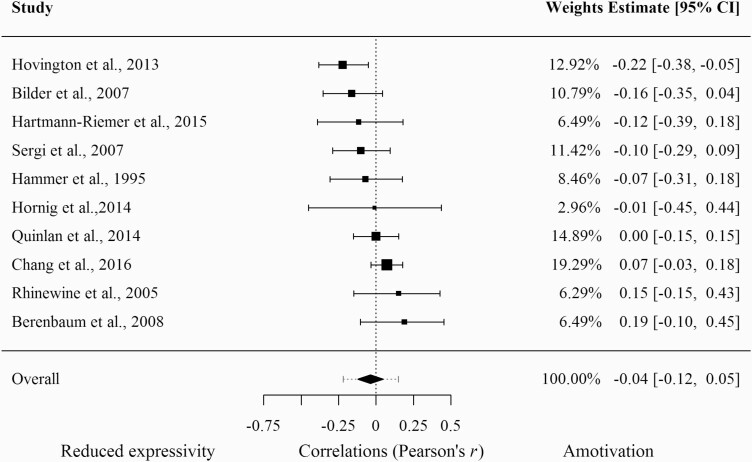
As can be seen in figure 4, the model testing whether episodic memory is more strongly related to amotivation than to reduced expressivity was not significant (k = 10; r = − .04; z = −.82; P =.41; 95% CI [−.12;.05]). The heterogeneity analysis revealed a non-significant Q-statistic (Q = 14.59; P =.10) and an I2 index of 40%.
Fig. 4.
Forest plot of the difference in magnitude of the association between episodic memory deficits with amotivation compared to reduced expressivity.
Moderation Analyses
Results of the moderation analyses are depicted in table 3. As we found significant between study variability in the model on negative symptoms in general and in the model on reduced expressivity, we calculated meta-regressions on these two models. The results revealed that none of the predefined covariates moderated the association between episodic memory and negative symptoms or reduced expressivity.
Table 3.
Moderator analyses
| Association | Moderator | k | β | 95% CI | z | I 2 |
|---|---|---|---|---|---|---|
| Episodic memory ~ Negative symptoms in general | Study quality | 104 | .02 | [−.02;.05] | 1.11 | 63.3 |
| Data points | 104 | .01 | [−.03;.04] | −.01 | 63.74 | |
| Age | 104 | −.02 | [−.05;.01] | −1.23 | 62.00 | |
| Gender (n male) | 103 | .02 | [−.01;.05] | 1.62 | 60.19 | |
| Education (years) | 69 | −.01 | [−.04;.03] | −.07 | 51.46 | |
| Diagnosis | 97 | .02 | [−.01;.05] | 1.19 | 62.8 | |
| CPZ equivalent doses | 42 | .01 | [−.04;.05] | −.34 | 41.03 | |
| Positive symptoms | 72 | −.002 | [−.05;.04] | −.10 | 70.32 | |
| Depressive symptoms | 25 | .02 | [−.06;.10] | .52 | 30.67 | |
| Negative symptom scale | 104 | .01 | [−.02;.05] | .67 | 63.15 | |
| Episodic memory ~ Reduced expressivity | Study quality | 15 | −.06 | [−.18;.06] | −.97 | 72.77 |
| Data points | 15 | −.07 | [−.18;.04] | −1.19 | 71.72 | |
| Age | 15 | .08 | [−.02;.19] | 1.66 | 65.24 | |
| Gender (n male) | 15 | −.01 | [−.13;.10] | −.22 | 72.30 | |
| Education (years) | 11 | −.09 | [−.24;.06] | −1.18 | 75.59 | |
| Diagnosis | 15 | −.01 | [−.12;.11] | −.08 | 72.4 | |
| CPZ equivalent doses | 8 | .02 | [−.12;.17] | .32 | 49.84 | |
| Positive symptoms | 7 | −.08 | [−.19;.04] | −1.27 | 57.36 | |
| Depressive symptoms | 2 | NA | NA | NA | NA | |
| Negative symptom scale | 15 | −.11 | [−.36;.15] | −.08 | 53.65 |
Note: Diagnosis = percentage of participants diagnosed with schizophrenia in relation to schizoaffective and other psychotic disorders. NA = as only two studies of those reporting on the association between episodic memory deficits and reduced expressivity reported data on depressive symptoms, the meta-regression including depressive symptoms could not be calculated.
Publication Bias
Visual inspection of the funnel plots (see supplement S3–S5) indicated a potential publication bias in the analysis of the relationship between episodic memory and negative symptoms in general as well as in the analysis of reduced expressivity, but not in the analyses of the relationship of episodic memory with amotivation. For the model testing the association between episodic memory and negative symptoms in general, both the rank correlation test (P =.16) and Egger’s regression test (P =.18) were not significant. For the model on reduced expressivity, neither the rank correlation test (P =.88) nor Egger’s regression test (P =.64) were significant. We therefore did not apply any correction for publication bias.
Additional Analyses
To gain a more detailed picture of the association between episodic memory and negative symptoms, we searched the included publications for studies reporting associations of episodic memory with the domains of the five-factor model of negative symptoms3 separately. Our literature search did not reveal a single study referring to the five-factor model, but six studies reporting separate correlations of episodic memory with the SANS scales “Affective flattening,” “Alogia,” “Avolition-Apathy,” and “Anhedonia-Asociality.” Episodic memory showed significant negative associations with “Affective flattening” (k = 6; r = −.23; z = −3.73; P ≤ .001; 95% CI [−.36; −.11]; I2 = 38%), “Alogia” (k = 6; r = −.32; z = −5.35; P ≤ .001; 95% CI [−.44; −.21]; I2 = 34%), “Avolition-Apathy” (k = 6; r = −.17; z = −3.19; P ≤ .01; 95% CI [−.28; −.07]; I2 = 21%), and “Anhedonia-Asociality” (k = 6; r = −.15; z = −3.27; P ≤ .01; 95% CI [−.25; −.06]; I2 = 0%).
We also reviewed the studies included in the subgroups for differences in sample, setting or design characteristics that could account for the slightly reduced effect size in the subgroup analyses. Compared to the total sample in which 22% were first-episode patients, the number of first-episode patients was slightly higher in the amotivation (38%) and in the reduced expressivity (27%) subgroup. However, the association between episodic memory and negative symptoms was not moderated by first-episode status (k = 103; r =.03; z =.68; P = .50; 95% CI [−.05;.11]).
Discussion
This meta-analysis confirmed the expected significant associations, both of episodic memory deficits with negative symptoms in general and of episodic memory deficits with amotivation and reduced expressivity. Our findings did not confirm the expectation that episodic memory deficits would show stronger associations with amotivation than with reduced expressivity.
The significant moderate association between performance in episodic memory tests and negative symptoms was robust across the 103 included studies as indicated by the small confidence interval of the overall effect, substantiating the findings of earlier meta-analyses.34,38,41 Moreover, the absence of a significant moderation effect indicates that the association between episodic memory and negative symptoms is neither driven by sociodemographic variables, such as gender, age or education, nor by positive symptoms, depressive symptoms or antipsychotic medication.
By contrast, the small effect size of the association between episodic memory deficits and amotivation and the absence of its specificity do not provide strong support the theoretical models positing that motivational deficits in psychosis mainly result from deficits in episodic memory.29,30 However, this finding should be interpreted with the caveat that the latent structure of negative symptoms has recently been shown to be best described in relation to the five symptom domains rather than by the two-factor model of amotivation and reduced expressivity.3 The nature of reporting in the existing literature precluded us from analyzing associations between episodic memory and the domains of the five-factor model of negative symptoms. In light of previous findings showing that recalling positive events elicits current positive affect and anticipatory pleasure,26,162 one would expect particularly anhedonia to show a relationship with episodic memory deficits. However, the available evidence for this expectation has been rated as inconclusive due to heterogeneous findings and methodological limitations.163 In contrast, our explorative analyses suggest that episodic memory deficits and anhedonia are related in people with psychosis. Nevertheless, one has to be aware that these analyses were based on a small number of studies (k = 6) and that the aggregation of ‘anhedonia’ and ‘asociality’ in the SANS does not converge with the proposed five-factor model of negative symptoms, in which anhedonia and asociality constitute two distinct factors.3 Further, it also needs to be taken into account that particularly anticipatory anhedonia might not be well captured by the scales that were employed in the included studies, of which only few used scales that have been designed to assess anticipatory anhedonia (e.g., The Brief Negative Symptom Scale151). Therefore, the specific relationship between episodic memory and anticipatory pleasure remains subject to future research.
Although autobiographical memory can be referred to as a specific taxonomic facet of episodic memory,164 one might question whether the conscious retrieval of emotionally neutral stimuli in tests of verbal and visual memory can be generalized to the unconscious retrieval of personally meaningful experiences in autobiographical memory tests. More specifically, it is conceivable that recalling emotional and personally meaningful experiences may have a stronger impact on motivation than recalling neutral stimuli. However, previous research has found significant correlations between the performance in verbal and autobiographical memory tests.165 Also, both retrieval in verbal memory tests166 and from autobiographical memory167 are associated with neural activation in the medial temporal lobes and the hippocampus. This could indicate that the performance in verbal, visual and autobiographical memory tests relies on the same neurocognitive capabilities, namely retaining and recalling context-based knowledge of temporally dated and spatially located events. Compared to autobiographical memory tests, verbal and visual memory tests however, hold the advantage that they control for the valence of the encoded stimulus material and for the duration of the delay interval between encoding and retrieval.
The significant association between episodic memory deficits and reduced expressivity could also be explained by the notion that reduced expressivity reflects a behavioral expression of the internal experience of amotivation as proposed by White et al.168 This matches the accounts of people with lived experience of negative symptoms. For instance, some report that they attribute their apparent emotional withdrawal and reduced expressivity to the avoidance of feared rejection by others and thus to motivational processes.169,170 The significant amount of shared variance between the amotivation and reduced expressivity factor provides further empirical support for this notion.171,172
In sum, our findings nevertheless clearly support the assumption that episodic memory deficits are related to amotivation. However, the rather small effect size questions the theoretical assumption that episodic memory deficits are the sole driver of reduced motivation in psychosis. Rather, episodic memory deficits could exert their influence on amotivation in conjunction with the processes involved in anticipatory pleasure. First, deficits in episodic memory may impede the ability to anticipate rewards because previously learned associations between reward predicting cues and the outcomes themselves cannot be readily retrieved. This might attenuate the capability to generate positive and vivid mental simulations of one’s personal future (i.e., prospections) and thereby blunt anticipatory pleasure. Because prospection has been found to induce a current experience of pleasure while anticipating future events (i.e., anticipatory affect)173 it is seen as a core component of motivation.174 In people with psychosis, prospections have been found to be less specific and less vivid than in healthy controls, which was associated with apathy.175 Also, they were found to be less likely to explicitly reference the past in their prospections and subsequently to anticipate less pleasure than healthy controls.176 This suggests that amotivation in psychosis could be driven by poor transition of episodic memories into prospections and anticipatory pleasure. Thus, solely recalling positive experiences does not seem to stimulate motivation. Rather, these memories have to be translated into positive prospections, which in turn motivate behavior by eliciting anticipatory pleasure. If these memories are not readily retrieved or cannot be translated into positive prospections, this may be compensated for with information from semantic memory, namely beliefs30,177, which have been found to be demotivating in nature in people with negative symptoms20,178 and to impede the translation of personal goals into goal-directed behavior by reducing anticipatory pleasure.13
Future research should therefore examine the nature of prospections in psychosis and how these relate to memory, motivational processes and goal-directed behavior. For instance, investigating whether people with psychosis differ from healthy controls in patterns of neural activation as well as sensory and emotional experience while simulating positive events could inform our understanding of the nature of prospection difficulties in psychosis. This knowledge could then be used to investigate whether these difficulties are linked to anticipatory affect, reward anticipation and goal-directed behavior. This might also help to extend our understanding of the reward processing deficits that have been observed in psychosis and in relation to negative symptoms. Specifically, deficits in episodic memory and prospections could explain the blunted responses to reward predictive cues179 and the deficits in generating and maintaining representations of the expected value of pleasurable outcomes.17 With regard to therapeutic implications, future research should also investigate whether specific episodic memory trainings can enhance episodic memory performance and thereby improve positive prospections, anticipatory anhedonia and motivation in people with negative symptoms. The Memory Specificity Training (MeST)180 constitutes a promising example for such interventions. Based on the idea that people with motivational deficits recall positive experiences in an overgeneralized and less detailed manner, participants in this structured training program are guided to recall positive experiences with increased detail and specificity. Recent meta-analytic evidence has confirmed that MeST was effective in increasing memory specificity for the recall of positive events.181 Most interestingly, it was also found that the guided recall of positive events improved the prospections of future events and that both recall and prospection increased anticipatory pleasure as well as intentions to engage in the imagined future events in a small community sample.182 If these effects can be confirmed for people with negative symptoms, interventions targeting deficits in memory recall and prospections could be a promising new direction for the treatment of negative symptoms.
The current meta-analysis has some limitations that need to be considered. First, we were unable to account for the between-study heterogeneity, which limits the confidence of interpretation of our findings. Future studies on the association between episodic memory and negative symptoms should therefore take further potential moderators of this relationship into account. Second, the vast majority of included studies used negative symptom scales of the first generation (i.e., PANSS, SANS and SDS), which have been criticized for containing outdated item content (e.g., ‘stereotyped thinking’), imprecise assessment of anhedonia (i.e., lack of differentiation between asociality, consummatory and anticipatory pleasure) and for relying on largely behavioral referents to assess internal experiences (e.g., lack of pleasure/motivation).42 Therefore, the available data does not allow to draw final conclusions regarding the impact of the type of negative symptom scale on the association between episodic memory and negative symptoms. Third, our findings are based on correlational data and the conscious retrieval in episodic memory tests can also be seen as effortful behavior. Therefore, we cannot draw any conclusions about causation and cannot exclude the possibility that the low motivation to perform well in neurocognitive tests that has been found in people with psychosis,183 could have confounded our results by impeding performance in episodic memory tests. One may also question whether the conscious recall in memory tests can be generalized to the unconscious retrieval from episodic memory that occurs in daily life and is hypothesized to drive motivation.
Conclusion
In sum, our findings indicate that episodic memory deficits are related to motivational impairments in psychosis. However, episodic memory deficits were also significantly associated with reduced expressivity. Thus, for now, one can conclude that difficulties in retrieving information from episodic memories alone do not sufficiently explain the motivational problems of people with negative symptoms. Rather, they are more likely to exert their influence in conjunction with other factors.
Conflict of Interest Disclosure
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Supplementary Material
References
- 1. Strauss GP, Cohen AS. A transdiagnostic review of negative symptom phenomenology and etiology. Schizophr Bull. 2017;43(4):712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bobes J, Arango C, Garcia-Garcia M, Rejas J; CLAMORS Study Collaborative Group . Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS study. J Clin Psychiatry. 2010;71(3):280–286. [DOI] [PubMed] [Google Scholar]
- 3. Strauss GP, Nuñez A, Ahmed AO, et al. The latent structure of negative symptoms in schizophrenia. JAMA Psychiatry. 2018;75(12):1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaiser S, Lyne J, Agartz I, Clarke M, Mørch-Johnsen L, Faerden A. Individual negative symptoms and domains – relevance for assessment, pathomechanisms and treatment. Schizophr Res. 2017;186:39–45. [DOI] [PubMed] [Google Scholar]
- 5. Savill M, Orfanos S, Reininghaus U, Wykes T, Bentall R, Priebe S. The relationship between experiential deficits of negative symptoms and subjective quality of life in schizophrenia. Schizophr Res. 2016;176(2–3):387–391. [DOI] [PubMed] [Google Scholar]
- 6. Fervaha G, Foussias G, Agid O, Remington G. Motivational deficits in early schizophrenia: prevalent, persistent, and key determinants of functional outcome. Schizophr Res. 2015;166(1–3):9–16. [DOI] [PubMed] [Google Scholar]
- 7. Kirkpatrick B, Mucci A, Galderisi S. Primary, enduring negative symptoms: an update on research. Schizophr Bull. 2017;43(4):730–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sterk B, Winter van Rossum I, Muis M, de Haan L. Priorities, satisfaction and treatment goals in psychosis patients: an online consumer’s survey. Pharmacopsychiatry. 2013;46(3):88–93. [DOI] [PubMed] [Google Scholar]
- 9. Artaloytia JF, Arango C, Lahti A, et al. Negative signs and symptoms secondary to antipsychotics: a double-blind, randomized trial of a single dose of placebo, haloperidol, and risperidone in healthy volunteers. Am J Psychiatry. 2006;163(3):488–493. [DOI] [PubMed] [Google Scholar]
- 10. Aleman A, Lincoln TM, Bruggeman R, et al. Treatment of negative symptoms: where do we stand, and where do we go? Schizophr Res. 2017;186:55–62. [DOI] [PubMed] [Google Scholar]
- 11. Zhou Y, Li G, Li D, Cui H, Ning Y. Dose reduction of risperidone and olanzapine can improve cognitive function and negative symptoms in stable schizophrenic patients: a single-blinded, 52-week, randomized controlled study. J Psychopharmacol. 2018;32(5):524–532. [DOI] [PubMed] [Google Scholar]
- 12. Riehle M, Böhl MC, Pillny M, Lincoln TM. Efficacy of psychological treatments for patients with schizophrenia and relevant negative symptoms: a meta-analysis. Clin Psychol Eur. 2020;2(3):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pillny M, Schlier B, Lincoln TM. “I just don’t look forward to anything”. How anticipatory pleasure and negative beliefs contribute to goal-directed activity in patients with negative symptoms of psychosis. Schizophr Res. 2020;222:429–436. [DOI] [PubMed] [Google Scholar]
- 14. Engel M, Fritzsche A, Lincoln TM. Anticipatory pleasure and approach motivation in schizophrenia-like negative symptoms. Psychiatry Res. 2013;210(2):422–426. [DOI] [PubMed] [Google Scholar]
- 15. Frost KH, Strauss GP. A review of anticipatory pleasure in schizophrenia. Curr Behav Neurosci Rep. 2016;3(3):232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dowd EC, Barch DM. Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PLoS One. 2012;7(5):e35622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gold JM, Waltz JA, Matveeva TM, et al. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch Gen Psychiatry. 2012;69(2):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hallford DJ, Austin DW, Takano K, Raes F. Psychopathology and episodic future thinking: a systematic review and meta-analysis of specificity and episodic detail. Behav Res Ther. 2018;102:42–51. [DOI] [PubMed] [Google Scholar]
- 19. Visser KF, Chapman HC, Ruiz I, Raugh IM, Strauss GP. A meta-analysis of self-reported anticipatory and consummatory pleasure in the schizophrenia-spectrum. J Psychiatr Res. 2020;121:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pillny M, Krkovic K, Lincoln TM. Development of the Demotivating Beliefs Inventory and test of the cognitive triad of amotivation. Cognit Ther Res. 2018;42(6):867–877. [Google Scholar]
- 21. Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8(9):657–661. [DOI] [PubMed] [Google Scholar]
- 22. Biderman N, Bakkour A, Shohamy D. What are memories for? The hippocampus bridges past experience with future decisions. Trends Cogn Sci. 2020;24(7):542–556. [DOI] [PubMed] [Google Scholar]
- 23. Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayes AR, Roberts N. Theories of episodic memory. Philos Trans R Soc Lond B Biol Sci. 2001;356(1413):1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gillihan SJ, Kessler J, Farah MJ. Memories affect mood: evidence from covert experimental assignment to positive, neutral, and negative memory recall. Acta Psychol. 2007;125(2):144–154. [DOI] [PubMed] [Google Scholar]
- 26. Morewedge CK, Gilbert DT, Wilson TD. The least likely of times: how remembering the past biases forecasts of the future. Psychol Sci. 2005;16(8):626–630. [DOI] [PubMed] [Google Scholar]
- 27. Lang PJ, Bradley MM. Emotion and the motivational brain. Biol Psychol. 2010;84(3):437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Izard CE. The many meanings/aspects of emotion: definitions, functions, activation, and regulation. Emot Rev. 2010;2(4):363–370. [Google Scholar]
- 29. Kring AM, Caponigro JM. Emotion in schizophrenia: where feeling meets thinking. Curr Dir Psychol Sci. 2010;19(4):255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169(4):364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. [DOI] [PubMed] [Google Scholar]
- 33. Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychol Bull. 2007;133(5):833–858. [DOI] [PubMed] [Google Scholar]
- 34. Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156(9):1358–1366. [DOI] [PubMed] [Google Scholar]
- 35. Berna F, Potheegadoo J, Aouadi I, et al. A meta-analysis of autobiographical memory studies in schizophrenia spectrum disorder. Schizophr Bull. 2016;42(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. [DOI] [PubMed] [Google Scholar]
- 37. Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev. 2005;15(2):73–95. [DOI] [PubMed] [Google Scholar]
- 38. Bora E, Binnur Akdede B, Alptekin K. Neurocognitive impairment in deficit and non-deficit schizophrenia: a meta-analysis. Psychol Med. 2017;47(14):2401–2413. [DOI] [PubMed] [Google Scholar]
- 39. Cheke LG, Clayton NS. Do different tests of episodic memory produce consistent results in human adults? Learn Mem. 2013;20(9):491–498. [DOI] [PubMed] [Google Scholar]
- 40. Carpenter WT Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145(5):578–583. [DOI] [PubMed] [Google Scholar]
- 41. Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr Res. 2009;113(2-3):189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blanchard JJ, Kring AM, Horan WP, Gur R. Toward the next generation of negative symptom assessments: the collaboration to advance negative symptom assessment in schizophrenia. Schizophr Bull. 2011;37(2):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lincoln TM, Dollfus S, Lyne J. Current developments and challenges in the assessment of negative symptoms. Schizophr Res. 2017;186:8–18. [DOI] [PubMed] [Google Scholar]
- 44. Bègue I, Kaiser S, Kirschner M. Pathophysiology of negative symptom dimensions of schizophrenia – current developments and implications for treatment. Neurosci Biobehav Rev. 2020. doi: 10.1016/j.neubiorev.2020.06.004 [DOI] [PubMed] [Google Scholar]
- 45. Cohen AS, McGovern JE, Dinzeo TJ, Covington MA. Speech deficits in serious mental illness: a cognitive resource issue? Schizophr Res. 2014;160(1-3):173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. García-Mieres H, Lundin NB, Minor KS, et al. A cognitive model of diminished expression in schizophrenia: the interface of metacognition, cognitive symptoms and language disturbances. J Psychiatr Res. 2020;131:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kring AM, Elis O. Emotion deficits in people with schizophrenia. Annu Rev Clin Psychol. 2013;9:409–433. [DOI] [PubMed] [Google Scholar]
- 48. Chang WC, Kwong VWY, Hui CLM, Chan SKW, Lee EHM, Chen EYH. Relationship of amotivation to neurocognition, self-efficacy and functioning in first-episode psychosis: a structural equation modeling approach. Psychol Med. 2017;47(4):755–765. [DOI] [PubMed] [Google Scholar]
- 49. Bilder R, Goldman R, Robinson D, et al. First-episode schizophrenia: characterization and clinical correlates. Neuropsychol Trends. 2007;2:7–30. [Google Scholar]
- 50. Hartmann-Riemer MN, Hager OM, Kirschner M, et al. The association of neurocognitive impairment with diminished expression and apathy in schizophrenia. Schizophr Res. 2015;169(1–3):427–432. [DOI] [PubMed] [Google Scholar]
- 51. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs. 2004;1(3):176–184. [DOI] [PubMed] [Google Scholar]
- 53. Edwards CJ, Garety P, Hardy A. The relationship between depressive symptoms and negative symptoms in people with non-affective psychosis: a meta-analysis. Psychol Med. 2019:1–13. doi: 10.1017/S0033291719002381 [DOI] [PubMed] [Google Scholar]
- 54. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR.. Introduction to Meta-Analysis. West Sussex: Wiley; 2009. [Google Scholar]
- 55. Viechtbauer W. Conducting meta-analyses in R with the metafor. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 56. Gignac GE, Szodorai ET. Effect size guidelines for individual differences researchers. Pers Individ Dif. 2016;102:74–78. [Google Scholar]
- 57. Hedges LV, Olkin I.. Statistical Methods for Meta-Analysis. New York: Academic Press; 1985. [Google Scholar]
- 58. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11(2):193–206. [DOI] [PubMed] [Google Scholar]
- 60. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 61. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 63. Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry. 1989;155(7):49–52. [PubMed] [Google Scholar]
- 64. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT Jr. The schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30(2):119–123. [DOI] [PubMed] [Google Scholar]
- 65. Addington J, Addington D. Neurocognitive and social functioning in schizophrenia. Schizophr Bull. 1999;25(1):173–182. [DOI] [PubMed] [Google Scholar]
- 66. Addington J, Addington D, Maticka-Tyndale E. Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophr Res. 1991;5(2):123–134. [DOI] [PubMed] [Google Scholar]
- 67. Bagney A, Dompablo M, Santabárbara J, et al. Are negative symptoms really related to cognition in schizophrenia? Psychiatry Res. 2015;230(2):377–382. [DOI] [PubMed] [Google Scholar]
- 68. Balogh N, Égerházi A, Berecz R. Neurocognitive changes in patients with schizophrenia during relapse and early remission. Eur J Psychiatry. 2015;29:199–209. [Google Scholar]
- 69. Basso MR, Nasrallah HA, Olson SC, Bornstein RA. Neuropsychological correlates of negative, disorganized and psychotic symptoms in schizophrenia. Schizophr Res. 1998;31(2-3):99–111. [DOI] [PubMed] [Google Scholar]
- 70. Bell MD, Mishara AL. Does negative symptom change relate to neurocognitive change in schizophrenia? Implications for targeted treatments. Schizophr Res. 2006;81(1):17–27. [DOI] [PubMed] [Google Scholar]
- 71. Berenbaum H, Kerns JG, Vernon LL, Gomez JJ. Cognitive correlates of schizophrenia signs and symptoms. II. Emotional disturbances. Psychiatry Res. 2008;159(1–2):157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Berman I, Viegner B, Merson A, Allan E, Pappas D, Green AI. Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophr Res. 1997;25(1):1–10. [DOI] [PubMed] [Google Scholar]
- 73. Bismark AW, Thomas ML, Tarasenko M, et al. Relationship between effortful motivation and neurocognition in schizophrenia. Schizophr Res. 2018;193:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bodapati AS, Jenkins LM, Sharma RP, Rosen C. Visual memory uniquely predicts anhedonia in schizophrenia but not bipolar disorder. J Neuropsychol. 2019;13(1):136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Boeker H, Kleiser M, Lehman D, Jaenke L, Bogerts B, Northoff G. Executive dysfunction, self, and ego pathology in schizophrenia: an exploratory study of neuropsychology and personality. Compr Psychiatry. 2006;47(1):7–19. [DOI] [PubMed] [Google Scholar]
- 76. Bozikas VP, Kosmidis MH, Kioperlidou K, Karavatos A. Relationship between psychopathology and cognitive functioning in schizophrenia. Compr Psychiatry. 2004;45(5):392–400. [DOI] [PubMed] [Google Scholar]
- 77. Brazo P, Marié RM, Halbecq I, et al. Cognitive patterns in subtypes of schizophrenia. Eur Psychiatry. 2002;17(3):155–162. [DOI] [PubMed] [Google Scholar]
- 78. Bryson G, Whelahan HA, Bell M. Memory and executive function impairments in deficit syndrome schizophrenia. Psychiatry Res. 2001;102(1):29–37. [DOI] [PubMed] [Google Scholar]
- 79. Buchanan RW, Strauss ME, Kirkpatrick B, Holstein C, Breier A, Carpenter WT Jr. Neuropsychological impairments in deficit vs nondeficit forms of schizophrenia. Arch Gen Psychiatry. 1994;51(10):804–811. [DOI] [PubMed] [Google Scholar]
- 80. Buchanan RW, Holstein C, Breier A. The comparative efficacy and long-term effect of clozapine treatment on neuropsychological test performance. Biol Psychiatry. 1994;36(11):717–725. [DOI] [PubMed] [Google Scholar]
- 81. Cammisuli DM, Sportiello MT. Cognitive psychopathology in Schizophrenia: comparing memory performances with obsessive-compulsive disorder patients and normal subjects on the Wechsler Memory Scale-IV. Psychiatr Danub. 2016;28(2):118–126. [PubMed] [Google Scholar]
- 82. Cascella NG, Testa SM, Meyer SM, et al. Neuropsychological impairment in deficit vs. non-deficit schizophrenia. J Psychiatr Res. 2008;42(11):930–937. [DOI] [PubMed] [Google Scholar]
- 83. Chan RCK, Geng FL, Lui SSY, et al. Course of neurological soft signs in first-episode schizophrenia: relationship with negative symptoms and cognitive performances. Sci Rep. 2015;5:11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chang WC, Hui CL, Tang JY, et al. Impacts of duration of untreated psychosis on cognition and negative symptoms in first-episode schizophrenia: a 3-year prospective follow-up study. Psychol Med. 2013;43(9):1883–1893. [DOI] [PubMed] [Google Scholar]
- 85. Chang WC, Hui CL, Chan SK, Lee EH, Wong GH, Chen EY. Relationship between diminished expression and cognitive impairment in first-episode schizophrenia: a prospective three-year follow-up study. Schizophr Res. 2014;152(1):146–151. [DOI] [PubMed] [Google Scholar]
- 86. Chen EY, Lam LC, Chen RY, Nguyen DG. Negative symptoms, neurological signs and neuropsychological impairments in 204 Hong Kong Chinese patients with schizophrenia. Br J Psychiatry. 1996;168(2):227–233. [DOI] [PubMed] [Google Scholar]
- 87. Chen C, Jiang W, Zhong N, et al. Impaired processing speed and attention in first-episode drug naive schizophrenia with deficit syndrome. Schizophr Res. 2014;159(2–3):478–484. [DOI] [PubMed] [Google Scholar]
- 88. Chkonia E, Tsverava L. Investigation of explicit verbal memory in patient with paranoid schizophrenia and their first degree relatives. Georgian Med News. 2007;( 150):14–17. [PubMed] [Google Scholar]
- 89. Cohen AS, Saperstein AM, Gold JM, Kirkpatrick B, Carpenter WT Jr, Buchanan RW. Neuropsychology of the deficit syndrome: new data and meta-analysis of findings to date. Schizophr Bull. 2007;33(5):1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dorofeikova M, Neznanov N, Petrova N. Cognitive deficit in patients with paranoid schizophrenia: its clinical and laboratory correlates. Psychiatry Res. 2018;262:542–548. [DOI] [PubMed] [Google Scholar]
- 91. Eckman PS, Shean GD. Impairment in test performance and symptom dimensions of schizophrenia. J Psychiatr Res. 2000;34(2):147–153. [DOI] [PubMed] [Google Scholar]
- 92. Ehmann TS, Khanbhai I, Macewan GW, et al. Neuropsychological correlates of the PANSS cognitive factor. Psychopathology. 2004;37(5):253–258. [DOI] [PubMed] [Google Scholar]
- 93. Faerden A, Vaskinn A, Finset A, et al. Apathy is associated with executive functioning in first episode psychosis. BMC Psychiatry. 2009;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fonseca AO, Berberian AA, de Meneses-Gaya C, et al. The Brazilian standardization of the MATRICS consensus cognitive battery (MCCB): psychometric study. Schizophr Res. 2017;185:148–153. [DOI] [PubMed] [Google Scholar]
- 95. Foussias G, Siddiqui I, Fervaha G, et al. Motivated to do well: an examination of the relationships between motivation, effort, and cognitive performance in schizophrenia. Schizophr Res. 2015;166(1–3):276–282. [DOI] [PubMed] [Google Scholar]
- 96. Frydecka D, Beszłej JA, Gościmski P, Kiejna A, Misiak B. Profiling cognitive impairment in treatment-resistant schizophrenia patients. Psychiatry Res. 2016;235:133–138. [DOI] [PubMed] [Google Scholar]
- 97. Galderisi S, Maj M, Mucci A, et al. Historical, psychopathological, neurological, and neuropsychological aspects of deficit schizophrenia: a multicenter study. Am J Psychiatry. 2002;159(6):983–990. [DOI] [PubMed] [Google Scholar]
- 98. Galderisi S, Mucci A, Bitter I, et al. ; Eufest Study Group . Persistent negative symptoms in first episode patients with schizophrenia: results from the European First Episode Schizophrenia Trial. Eur Neuropsychopharmacol. 2013;23(3):196–204. [DOI] [PubMed] [Google Scholar]
- 99. González-Blanch C, Crespo-Facorro B, Alvarez-Jiménez M, et al. Pretreatment predictors of cognitive deficits in early psychosis. Psychol Med. 2008;38(5):737–746. [DOI] [PubMed] [Google Scholar]
- 100. Good KP, Rabinowitz J, Whitehorn D, Harvey PD, DeSmedt G, Kopala LC. The relationship of neuropsychological test performance with the PANSS in antipsychotic naïve, first-episode psychosis patients. Schizophr Res. 2004;68(1):11–19. [DOI] [PubMed] [Google Scholar]
- 101. Guillem F, Bicu M, Bloom D, et al. Neuropsychological impairments in the syndromes of schizophrenia: a comparison between different dimensional models. Brain Cogn. 2001;46(1–2):153–159. [DOI] [PubMed] [Google Scholar]
- 102. Gur RC, Braff DL, Calkins ME, et al. Neurocognitive performance in family-based and case-control studies of schizophrenia. Schizophr Res. 2015;163(1–3):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hammer MA, Katsanis J, Iacono WG. The relationship between negative symptoms and neuropsychological performance. Biol Psychiatry. 1995;37(11):828–830. [DOI] [PubMed] [Google Scholar]
- 104. Harrison CL, Fowler D. Negative symptoms, trauma, and autobiographical memory: an investigation of individuals recovering from psychosis. J Nerv Ment Dis. 2004;192(11):745–753. [DOI] [PubMed] [Google Scholar]
- 105. Harvey PD, Lombardi J, Leibman M, et al. Cognitive impairment and negative symptoms in geriatric chronic schizophrenic patients: a follow-up study. Schizophr Res. 1996;22(3):223–231. [DOI] [PubMed] [Google Scholar]
- 106. Hegde S, Thirthalli J, Rao SL, Raguram A, Philip M, Gangadhar BN. Cognitive deficits and its relation with psychopathology and global functioning in first episode schizophrenia. Asian J Psychiatr. 2013;6(6):537–543. [DOI] [PubMed] [Google Scholar]
- 107. Heydebrand G, Weiser M, Rabinowitz J, Hoff AL, DeLisi LE, Csernansky JG. Correlates of cognitive deficits in first episode schizophrenia. Schizophr Res. 2004;68(1):1–9. [DOI] [PubMed] [Google Scholar]
- 108. Hintze B, Borkowska A. Associations between cognitive function, schizophrenic symptoms, and functional outcome in early-onset schizophrenia with and without a familial burden of psychosis. Isr J Psychiatry Relat Sci. 2015;52(3):6–12. [PubMed] [Google Scholar]
- 109. Horan WP, Blanchard JJ. Neurocognitive, social, and emotional dysfunction in deficit syndrome schizophrenia. Schizophr Res. 2003;65(2-3):125–137. [DOI] [PubMed] [Google Scholar]
- 110. Hornig T, Valerius G, Feige B, Bubl E, Olbrich HM, van Elst LT. Neuropsychological and cerebral morphometric aspects of negative symptoms in schizophrenia: negative symptomatology is associated with specific mnestic deficits in schizophrenic patients. BMC Psychiatry. 2014;14:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hovington CL, Bodnar M, Joober R, Malla AK, Lepage M. Impairment in verbal memory observed in first episode psychosis patients with persistent negative symptoms. Schizophr Res. 2013;147(2–3):223–229. [DOI] [PubMed] [Google Scholar]
- 112. Jhung K, Park JY, Song YY, Kang JI, Lee E, An SK. Experiential pleasure deficits in the prodrome: a study of emotional experiences in individuals at ultra-high risk for psychosis and recent-onset schizophrenia. Compr Psychiatry. 2016;68:209–216. [DOI] [PubMed] [Google Scholar]
- 113. Kanchanatawan B, Tangwongchai S, Supasitthumrong T, Sriswasdi S, Maes M. Episodic memory and delayed recall are significantly more impaired in younger patients with deficit schizophrenia than in elderly patients with amnestic mild cognitive impairment. PLoS One. 2018;13(5):e0197004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31(9):2033–2046. [DOI] [PubMed] [Google Scholar]
- 115. Khalil AH, El-Meguid MA, Bastawy M, Rabei S, Ali R, Abd Elmoneam MHE. Correlating cognitive functions to symptom domains and insight in Egyptian patients with schizophrenia. Int J Soc Psychiatry. 2020;66(3):240–248. [DOI] [PubMed] [Google Scholar]
- 116. Klingberg S, Wittorf A, Wiedemann G. Disorganization and cognitive impairment in schizophrenia: independent symptom dimensions? Eur Arch Psychiatry Clin Neurosci. 2006;256(8):532–540. [DOI] [PubMed] [Google Scholar]
- 117. Konstantakopoulos G, Ploumpidis D, Oulis P, et al. Apathy, cognitive deficits and functional impairment in schizophrenia. Schizophr Res. 2011;133(1–3):193–198. [DOI] [PubMed] [Google Scholar]
- 118. Krishnadas R, Moore BP, Nayak A, Patel RR. Relationship of cognitive function in patients with schizophrenia in remission to disability: a cross-sectional study in an Indian sample. Ann Gen Psychiatry. 2007;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lee EHM, Hui CLM, Chan KPK, et al. The role of symptoms and insight in mediating cognition and functioning in first episode psychosis. Schizophr Res. 2019;206:251–256. [DOI] [PubMed] [Google Scholar]
- 120. Li AWY, Hui CLM, Lee EHM, Chang WC, Chan SKW, Chen EYH. Gender differences in correlates of cognition in first-episode psychosis. Psychiatry Res. 2019;271:412–420. [DOI] [PubMed] [Google Scholar]
- 121. Lin CH, Huang CL, Chang YC, et al. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophr Res. 2013;146(1-3):231–237. [DOI] [PubMed] [Google Scholar]
- 122. Lindsberg J, Poutiainen E, Kalska H. Clarifying the diversity of first-episode psychosis: neuropsychological correlates of clinical symptoms. Nord J Psychiatry. 2009;63(6):493–500. [DOI] [PubMed] [Google Scholar]
- 123. Lipkovich IA, Deberdt W, Csernansky JG, Sabbe B, Keefe RS, Kollack-Walker S. Relationships among neurocognition, symptoms and functioning in patients with schizophrenia: a path-analytic approach for associations at baseline and following 24 weeks of antipsychotic drug therapy. BMC Psychiatry. 2009;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Liu Y, Wang G, Jin H, et al. Cognitive deficits in subjects at risk for psychosis, first-episode and chronic schizophrenia patients. Psychiatry Res. 2019;274:235–242. [DOI] [PubMed] [Google Scholar]
- 125. Manglam MK, Das A. Verbal learning and memory and psychopathology in schizophrenia. Asian J Psychiatr. 2013;6(5):417–420. [DOI] [PubMed] [Google Scholar]
- 126. McCreadie RG, Latha S, Thara R, Padmavathi R, Ayankaran JR. Poor memory, negative symptoms and abnormal movements in never-treated Indian patients with schizophrenia. Br J Psychiatry. 1997;171:360–363. [DOI] [PubMed] [Google Scholar]
- 127. McDaniel WF, Heindel CS, Harris DW. Verbal memory and negative symptoms of schizophrenia revisited. Schizophr Res. 2000;41(3):473–475. [DOI] [PubMed] [Google Scholar]
- 128. Mingrone C, Rocca P, Castagna F, et al. Insight in stable schizophrenia: relations with psychopathology and cognition. Compr Psychiatry. 2013;54(5):484–492. [DOI] [PubMed] [Google Scholar]
- 129. Minzenberg MJ, Poole JH, Vinogradov S, Shenaut GK, Ober BA. Slowed lexical access is uniquely associated with positive and disorganised symptoms in schizophrenia. Cogn Neuropsychiatry. 2003;8(2):107–127. [DOI] [PubMed] [Google Scholar]
- 130. Moritz S, Heeren D, Andresen B, Krausz M. An analysis of the specificity and the syndromal correlates of verbal memory impairments in schizophrenia. Psychiatry Res. 2001;101(1):23–31. [DOI] [PubMed] [Google Scholar]
- 131. Morrison-Stewart SL, Williamson PC, Corning WC, Kutcher SP, Snow WG, Merskey H. Frontal and non-frontal lobe neuropsychological test performance and clinical symptomatology in schizophrenia. Psychol Med. 1992;22(2):353–359. [DOI] [PubMed] [Google Scholar]
- 132. Mu L, Liang J, Wang H, Chen D, Xiu M, Zhang XY. Sex differences in association between clinical correlates and cognitive impairment in patients with chronic schizophrenia. J Psychiatr Res. 2020;131:194–202. [DOI] [PubMed] [Google Scholar]
- 133. Newcomer JW, Faustman WO, Whiteford HA, Moses JA Jr, Csernansky JG. Symptomatology and cognitive impairment associate independently with post-dexamethasone cortisol concentrations in unmedicated schizophrenic patients. Biol Psychiatry. 1991;29(9):855–864. [DOI] [PubMed] [Google Scholar]
- 134. Norman RM, Malla AK, Morrison-Stewart SL, et al. Neuropsychological correlates of syndromes in schizophrenia. Br J Psychiatry. 1997;170:134–139. [DOI] [PubMed] [Google Scholar]
- 135. O’Leary DS, Flaum M, Kesler ML, Flashman LA, Arndt S, Andreasen NC. Cognitive correlates of the negative, disorganized, and psychotic symptom dimensions of schizophrenia. J Neuropsychiatry Clin Neurosci. 2000;12(1):4–15. [DOI] [PubMed] [Google Scholar]
- 136. Pandina G, Nuamah I, Petersen T, Singh J, Savitz A, Hough D. Cognitive functioning in adolescents with schizophrenia treated with paliperidone extended-release: 6-Month exploratory analysis from an open-label, single-arm safety study. Schizophr Res Cogn. 2020;20:100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Pegoraro LFL, Dantas CR, Banzato CEM, Fuentes D. Correlation between insight dimensions and cognitive functions in patients with deficit and nondeficit schizophrenia. Schizophr Res. 2013;147(1):91–94. [DOI] [PubMed] [Google Scholar]
- 138. Perlick DA, Rosenheck RA, Kaczynski R, Bingham S, Collins J. Association of symptomatology and cognitive deficits to functional capacity in schizophrenia. Schizophr Res. 2008;99(1–3):192–199. [DOI] [PubMed] [Google Scholar]
- 139. Puig O, Penadés R, Gastó C, Catalán R, Torres A, Salamero M. Verbal memory, negative symptomatology and prediction of psychosocial functioning in schizophrenia. Psychiatry Res. 2008;158(1):11–17. [DOI] [PubMed] [Google Scholar]
- 140. Putnam KM, Harvey PD. Cognitive impairment and enduring negative symptoms: a comparative study of geriatric and nongeriatric schizophrenia patients. Schizophr Bull. 2000;26(4):867–878. [DOI] [PubMed] [Google Scholar]
- 141. Quinlan T, Roesch S, Granholm E. The role of dysfunctional attitudes in models of negative symptoms and functioning in schizophrenia. Schizophr Res. 2014;157(1–3):182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Raffard S, Bortolon C, Yazbek H, et al. The cognitive, affective motivational and clinical longitudinal determinants of apathy in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2019;269(8):911–920. [DOI] [PubMed] [Google Scholar]
- 143. Rémillard S, Pourcher E, Cohen H. Long-term effects of risperidone versus haloperidol on verbal memory, attention, and symptomatology in schizophrenia. J Int Neuropsychol Soc. 2008;14(1):110–118. [DOI] [PubMed] [Google Scholar]
- 144. Réthelyi JM, Czobor P, Polgár P, et al. General and domain-specific neurocognitive impairments in deficit and non-deficit schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2012;262(2):107–115. [DOI] [PubMed] [Google Scholar]
- 145. Rhinewine JP, Lencz T, Thaden EP, et al. Neurocognitive profile in adolescents with early-onset schizophrenia: clinical correlates. Biol Psychiatry. 2005;58(9):705–712. [DOI] [PubMed] [Google Scholar]
- 146. Rocca P, Bellino S, Calvarese P, et al. Depressive and negative symptoms in schizophrenia: different effects on clinical features. Compr Psychiatry. 2005;46(4):304–310. [DOI] [PubMed] [Google Scholar]
- 147. Rund BR, Melle I, Friis S, et al. Neurocognitive dysfunction in first-episode psychosis: correlates with symptoms, premorbid adjustment, and duration of untreated psychosis. Am J Psychiatry. 2004;161(3):466–472. [DOI] [PubMed] [Google Scholar]
- 148. Sergi MJ, Rassovsky Y, Widmark C, et al. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms. Schizophr Res. 2007;90(1–3):316–324. [DOI] [PubMed] [Google Scholar]
- 149. Smith MJ, Barch DM, Csernansky JG. Bridging the gap between schizophrenia and psychotic mood disorders: relating neurocognitive deficits to psychopathology. Schizophr Res. 2009;107(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Srinivasan L, Thara R, Tirupati SN. Cognitive dysfunction and associated factors in patients with chronic schizophrenia. Indian J Psychiatry. 2005;47(3):139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Strauss GP, Keller WR, Buchanan RW, et al. Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophr Res. 2012;142(1–3):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Tanaka T, Tomotake M, Ueoka Y, et al. Cognitive dysfunction in schizophrenia. Psychiatry Clin Neurosci. 2012;66(6):491–498. [DOI] [PubMed] [Google Scholar]
- 153. Tong ACY, Chang WC, Chan ANY, Lin JJ. Objective and subjective cognitive functioning in relation to psychopathology among women with early psychosis. Early Interv Psychiatry. 2019;13(5):1227–1235. [DOI] [PubMed] [Google Scholar]
- 154. Tregellas JR, Smucny J, Harris JG, et al. Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am J Psychiatry. 2014;171(5):549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. van der Werf M, Köhler S, Verkaaik M, Verhey F, van Os J; GROUP Investigators . Cognitive functioning and age at onset in non-affective psychotic disorder. Acta Psychiatr Scand. 2012;126(4):274–281. [DOI] [PubMed] [Google Scholar]
- 156. Villalta-Gil V, Vilaplana M, Ochoa S, et al. ; NEDENA Group . Neurocognitive performance and negative symptoms: are they equal in explaining disability in schizophrenia outpatients? Schizophr Res. 2006;87(1–3):246–253. [DOI] [PubMed] [Google Scholar]
- 157. Wang X, Yao S, Kirkpatrick B, Shi C, Yi J. Psychopathology and neuropsychological impairments in deficit and nondeficit schizophrenia of Chinese origin. Psychiatry Res. 2008;158(2):195–205. [DOI] [PubMed] [Google Scholar]
- 158. Wittorf A, Klingberg S, Wiedemann G. Secondary verbal memory: a potential endophenotype of schizophrenia. J Psychiatr Res. 2004;38(6):601–612. [DOI] [PubMed] [Google Scholar]
- 159. Woodward TS, Thornton AE, Ruff CC, Moritz S, Liddle PF. Material-specific episodic memory associates of the psychomotor poverty syndrome in schizophrenia. Cogn Neuropsychiatry. 2004;9(3):213–227. [DOI] [PubMed] [Google Scholar]
- 160. Yazıhan NT, Yetkin S. Sleep, sleep spindles, and cognitive functions in drug-naive patients with first-episode psychosis. J Clin Sleep Med. 2020;16(12):2079–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Zakzanis KK. Neuropsychological correlates of positive vs. negative schizophrenic symptomatology. Schizophr Res. 1998;29(3):227–233. [DOI] [PubMed] [Google Scholar]
- 162. Holmes EA, Mathews A. Mental imagery in emotion and emotional disorders. Clin Psychol Rev. 2010;30(3):349–362. [DOI] [PubMed] [Google Scholar]
- 163. Edwards CJ, Cella M, Tarrier N, Wykes T. Investigating the empirical support for therapeutic targets proposed by the temporal experience of pleasure model in schizophrenia: a systematic review. Schizophr Res. 2015;168(1–2):120–144. [DOI] [PubMed] [Google Scholar]
- 164. Squire LR, Knowlton B, Musen G. The structure and organization of memory. Annu Rev Psychol. 1993;44:453–495. [DOI] [PubMed] [Google Scholar]
- 165. Janssen SM, Kristo G, Rouw R, Murre JM. The relation between verbal and visuospatial memory and autobiographical memory. Conscious Cogn. 2015;31:12–23. [DOI] [PubMed] [Google Scholar]
- 166. Duan X, He C, Ou J, et al. Reduced hippocampal volume and its relationship with verbal memory and negative symptoms in treatment-naive first-episode adolescent-onset schizophrenia. Schizophr Bull. 2021;47(1):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11(5):219–227. [DOI] [PubMed] [Google Scholar]
- 168. White RG, Laithwaite H, Gilbert P. Negative symptoms in schizophrenia. The role of social defeat. In: Gumley A, Gillham A, Taylor K, Schwannauer M, eds. Psychosis and Emotion. The Role of Emotions in Understanding Psychosis, Therapy and Recovery. East Sussex: Routledge; 2013:177–190. [Google Scholar]
- 169. Gee B, Hodgekins J, Lavis A, et al. Lived experiences of negative symptoms in first-episode psychosis: a qualitative secondary analysis. Early Interv Psychiatry. 2019;13(4):773–779. [DOI] [PubMed] [Google Scholar]
- 170. Lievre L, Schweitzer R, Barnard A. The subjective experience of negative symptoms: characteristics of emotional withdrawal. In: Geekie J, Randal P, Lampshire D, Read J, eds. Experiencing Psychosis: Personal and Professional Perspectives. London: Routledge; 2011:187–195. [Google Scholar]
- 171. Strauss GP, Hong LE, Gold JM, et al. Factor structure of the Brief Negative Symptom Scale. Schizophr Res. 2012;142(1–3):96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Richter J, Hesse K, Schreiber L, et al. Evidence for two distinct domains of negative symptoms: confirming the factorial structure of the CAINS. Psychiatry Res. 2019;271:693–701. [DOI] [PubMed] [Google Scholar]
- 173. Gilbert DT, Wilson TD. Prospection: experiencing the future. Science. 2007;317(5843):1351–1354. [DOI] [PubMed] [Google Scholar]
- 174. Renner F, Werthmann J, Paetsch A, Bär HE, Heise M, Bruijniks SJ. Prospective mental imagery in depression: impact on reward processing and reward-motivated behaviour. Clin Psychol Eur. 2021. doi: 10.23668/psycharchives.4769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Raffard S, Esposito F, Boulenger JP, der Linden M. Impaired ability to imagine future pleasant events is associated with apathy in schizophrenia. Psychiatry Res. 2013;209(3):393–400. [DOI] [PubMed] [Google Scholar]
- 176. Painter JM, Kring AM. Toward an understanding of anticipatory pleasure deficits in schizophrenia: memory, prospection, and emotion experience. J Abnorm Psychol. 2016;125(3):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Robinson MD, Clore GL. Belief and feeling: evidence for an accessibility model of emotional self-report. Psychol Bull. 2002;128(6):934–960. [DOI] [PubMed] [Google Scholar]
- 178. Beck AT, Himelstein R, Grant PM. In and out of schizophrenia: activation and deactivation of the negative and positive schemas. Schizophr Res. 2017;203:55–61. [DOI] [PubMed] [Google Scholar]
- 179. Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psychiatry. 2011;69(5):424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Raes F, Williams JMG, Hermans D. Reducing cognitive vulnerability to depression: a preliminary investigation of MEmory Specificity Training (MEST) in inpatients with depressive symptomatology. J Behav Ther Exp Psychiatry. 2009;40(1):24–38. [DOI] [PubMed] [Google Scholar]
- 181. Barry TJ, Sze WY, Raes F. A meta-analysis and systematic review of Memory Specificity Training (MeST) in the treatment of emotional disorders. Behav Res Ther. 2019;116(November 2018): 36–51. [DOI] [PubMed] [Google Scholar]
- 182. Hallford DJ, Farrell H, Lynch E. Increasing anticipated and anticipatory pleasure through episodic thinking. Emotion. 2020. doi: 10.1037/emo0000765 [DOI] [PubMed] [Google Scholar]
- 183. Moritz S, Klein JP, Desler T, Lill H, Gallinat J, Schneider BC. Neurocognitive deficits in schizophrenia. Are we making mountains out of molehills? Psychol Med. 2017;47:2602–2612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.