Abstract
22q11.2 deletion syndrome (22q11.2DS) is a genetic neurodevelopmental disorder that represents one of the greatest known risk factors for psychosis. Previous studies in psychotic subjects without the deletion have identified a dopaminergic dysfunction in striatal regions, and dysconnectivity of striatocortical systems, as an important mechanism in the emergence of psychosis. Here, we used resting-state functional MRI to examine striatocortical functional connectivity in 22q11.2DS patients. We used a 2 × 2 factorial design including 125 subjects (55 healthy controls, 28 22q11.2DS patients without a history of psychosis, 10 22q11.2DS patients with a history of psychosis, and 32 subjects with a history of psychosis without the deletion), allowing us to identify network effects related to the deletion and to the presence of psychosis. In line with previous results from psychotic patients without 22q11.2DS, we found that there was a dorsal to ventral gradient of hypo- to hyperstriatocortical connectivity related to psychosis across both patient groups. The 22q11.2DS was additionally associated with abnormal functional connectivity in ventral striatocortical networks, with no significant differences identified in the dorsal system. Abnormalities in the ventral striatocortical system observed in these individuals with high genetic risk to psychosis may thus reflect a marker of illness risk.
Keywords: schizophrenia, functional connectivity, 22q11DS, dopaminergic systems, striatal connectivity, genetic risk to psychosis
Introduction
Chromosome 22q11.2 deletion syndrome (22q11.2DS) or velocardiofacial syndrome is a neurodevelopmental disorder caused by a microdeletion at the q11.2 locus of chromosome 22,1 which occurs in approximately 1 out of 4000 live births.2 This syndrome may present with a diverse phenotype that includes somatic, cognitive, and psychiatric features.3 Importantly, it represents one of the greatest known genetic risk factors for psychosis, with 30%–40% of patients developing schizophrenia.4,5 Moreover, duplications of the same chromosomal segment have been found to be a protective factor for schizophrenia.6 Understanding the biological underpinning of the 22q11.2DS may provide valuable insight into the mechanisms underlying schizophrenia and its vulnerability.
Dopamine dysfunction in striatal regions has been proposed as a central mechanism underlying the emergence of psychotic symptoms.7 The genetic mechanism underlying the vulnerability to psychosis in the 22q11.2DS are likely to be complex, however one should also acknowledge that the deletion includes important genes in dopamine pathways such as COMT.8,9 Growing evidence from positron emission tomography (PET) studies also suggests that patients with 22q11.2DS have a dysfunctional striatum, showing changes in dopamine transporter concentration,10 and increased presynaptic dopaminergic activity.11 In schizophrenia, these molecular abnormalities are thought to relate to dysfunction of extended cortico-striato-thalamic networks,12–14 although the function of such networks has not been extensively investigated in 22q11.2DS patients.
Two striatocortical circuits have been proposed to be relevant for psychosis: the ventral and dorsal systems.13,15 These circuits topographically connect cortical regions with the striatum, with feedback loops passing through the pallidum and thalamus.13,16 The ventral system connects the nucleus accumbens and ventral striatum with limbic regions, and its dysfunction has long been associated with psychosis.17,18 Patients with a first episode of psychosis present increased coupling in the ventral striatofrontal circuit,12 although the replication of these results remains equivocal.14 Alterations in the ventral system have been correlated with the severity of psychotic symptoms in patients (particularly connectivity between ventral caudate and left dorsolateral prefrontal cortex),12 but not with the severity of psychotic-like experiences in healthy subjects.19
The dorsal system links regions of dorsolateral prefrontal cortex and dorsal caudate/putamen, and it is involved in cognitive and associative functions.13,16 Consistent with recent evidence for a primary role of the dorsal striatum in the pathogenesis of psychosis,20 which is particularly supported by PET studies,21 reduced functional coupling of the dorsal striatocortical system has proven to track psychotic symptom expressions across a broad spectrum of severity.12,19,22,23 Accordingly, dysconnectivity of both dorsal and ventral striatocortical circuits have been proposed to represent a candidate risk phenotype. Particularly, functional decoupling of dorsal frontostriatal systems has been associated with genetic risk for psychosis, as it has been described in first-degree relatives of psychotic patients,12 and in ultra-high risk for psychosis subjects.24 On the other hand, alterations in the ventral system have been described for first-degree relatives12 but not for ultra-high risk subjects.24
We here explored striatocortical connectivity in patients with 22q11.2DS as a way to shed further light on the neural mechanisms underlying the vulnerability to psychosis. We included a group of 125 subjects comprised by healthy controls, 22q11.2DS without a history of psychosis, 22q11.2DS with a history of psychosis, and subjects with a history of psychosis without the deletion. Thus, we were able to perform a 2 × 2 factorial design, examining the effect of the 22q11.2 deletion (as a genetic vulnerability) and of psychosis.
We hypothesized that carriers of the 22q11.2 deletion would present abnormal connectivity in a dorsal striatocortical network, related to a genetic vulnerability to psychosis. We also expected to find changes in ventral circuitry related to psychosis, particularly in the connectivity between the ventral caudate and dorsolateral prefrontal cortex.
Methods
Participants
We recruited patients with 22q11.2DS who had been part of previous studies of our group25 or were in contact with support groups such as “Fundación Chilena del Niño con Síndrome Velocardiofacial.” Subjects with and without history of psychosis with the deletion were included, assessed using the Mini International Neuropsychiatric Interview (MINI).26,27 For subjects with a history of psychosis, current psychotic symptoms were measured using the Positive and Negative Syndrome Scale (PANSS).28 For those subjects with the 22q11.2DS who did not have a history of psychosis, we assessed the presence of subthreshold symptoms using the Scale of Prodromal Symptoms.29 We also recruited a group of subjects with early psychosis (within the first 2 years of the onset of psychosis, confirmed with the MINI) from the Psychiatric Institute “Dr José Horwitz Barak,” matching this group to the 22q11.2DS group with psychosis according to their symptoms (PANSS total score). Healthy control subjects without any current psychiatric disorder or lifetime history of psychotic disorder, according to the MINI, or prodromal symptoms according to the SOPS, were also included.
In all participants, comorbid affective symptoms were measured using the Hamilton Depression Rating Scale (HDRS)30 and the Young Mania Rating Scale (YMRS).31 Intelligence quotient (IQ) was assessed using the WAIS-IV test. Also, all participants underwent MLPA (multiplex ligation-dependent probe amplification) to either confirm or discard 22q11.2DS. All participants gave written consent for this study, which was approved by the Ethics committee of the Pontificia Universidad Católica de Chile.
A total of 10 subjects across all groups (1 from 22q11.2DS, 5 from early psychosis and 4 healthy controls) were excluded due to scan artifacts or failures in the preprocessing pipeline. The final sample with complete cognitive and neuroimaging data consisted in 125 subjects (38 with 22q11.2DS: 10 of which had a history of psychosis; and 87 subjects without 22q11.2DS: 55 healthy controls and 32 patients with early psychosis).
Image Acquisition and Preprocessing
Images were acquired with a Philips Ingenia 3T MRI scanner with a 16-channel brain coil. Resting-state functional MRI were acquired with the following scanning parameters: total scan time 8.33 min, single shot echo-planar imaging (EPI), repetition time (TR) 2.5 s, time to echo (TE)35 ms, flip angle of 82°, field-of-view (FOV) of 220 × 220 × 110 mm, and an isotropic spatial resolution of 2.75 mm. Subjects were asked to remain still and with their eyes opened. A structural T1-weighted image was also acquired with a voxel size of 1.0 mm3 isotropic, a inversion time delay of 965.2 ms, TE 3.6 ms, TR 7.7 ms, and flip angle of 8°.
Preprocessing of the functional images followed previously published pipelines,32 which included the following steps: (1) removal of the first 4 volumes of each acquisition; (2) slice-time correction using SPM1233; (3) 2-pass realignment of all volumes to the first volume (first pass) and then to the mean volume (second pass) using SPM12; (4) coregistration of EPI data to the structural image using ANTs34,35; (5) application of the nonlinear transform derived from the T1-weighted image processing pipeline to the coregistered EPI data using ANTs; (6) linear detrending of the spatially normalized BOLD time series; and (7) intensity normalization of the EPI data to mode 1000 units. The images were then spatially smoothed with a 6 mm FWHM Gaussian kernel, and bandpass-filtered between 0.008 and 0.08 Hz using the fast Fourier transform. Management of residual movement was performed based on an automated-ICA method: ICA-AROMA.36 Following results of Parkes et al32 for a dataset of patients with schizophrenia, regression of mean white matter and cerebrospinal fluid signals was also included as a denoising procedure.
Definition of Seeds, Regions of Interest
Following previous studies,12,37 6 bilateral striatal regions of interest (ROIs) based on 3.5-mm radius spheres were used. Coordinates for each seed are defined in the Montreal Imaging Institute (MNI) stereotaxic space.
For the caudate, 3 ROIs were seeded:
-
-
DC: dorsal caudate (x = ±13, y = 15, z = 9),
-
-
sVC: superior ventral caudate (x = ±10, y = 15, z = 0),
-
-
iVC: inferior ventral caudate/nucleus accumbens (x = ±9, y = 9, z = 28).
As for the putamen, the 3 ROIs were:
-
-
DCP: dorsocaudal putamen (x = ±28, y = 1, z = 3),
-
-
DRP: dorsorostral putamen (x = ±25, y = 8, z = 6),
-
-
VRP: ventrorostral putamen (x = ±20, y = 12, z = 23).
The dorsal striatocortical system is comprised by DC, DRP, and the DCP, whereas seeds in the ventral system are iVC (nucleus accumbens), sVC, and VRP.
Statistical Analysis
The statistical analysis was implemented as in previous studies.12,19 Mean time series of each seed (TSseed i) were used for seed-related functional connectivity mapping between such seeds and each voxel within a gray matter mask, which included all cortical regions and the thalamus.
In a first-level analysis, for each participant, a general linear model containing time series for each of the 6 seeds as covariates was used to model blood oxygen level-dependent signal fluctuations in each voxel of the gray matter mask (TSvoxel i):
| (1) |
Gray matter t-maps estimated in the first-level analysis were then passed to a second-level general linear model to generate groupwise functional connectivity maps for each seed. Group effects were estimated using a 2 × 2 factorial ANOVA model with categorical variables psychosis and 22q11.2DS (deletion), as well as their interaction. Separate models were used for each seed, and nuisance covariates were included: age, age squared, sex, IQ, and mean framewise displacement (fd) as a measure of in-scanner motion (as in Sabaroedin et al19).
| (2) |
To identify relevant changes in striatal functional connectivity, we used a clusterwise-corrected threshold of P < .01 determined using the AlphaSim permutation procedure implemented in the REST toolbox.38
Results
One hundred and twenty-five subjects were included in this study. Thirty-eight subjects were carriers of the 22q11.2 deletion, 10 of which had a history of psychosis. Eighty-seven subjects without the deletion were also included: 55 corresponded to healthy controls and 32 were patients with a history of psychosis (early psychosis) who were symptom matched to the 22q11.2DS patients with psychosis, according to their PANSS total scores (P = .5121). Demographic and clinical information of each group can be found in table 1. Groups differed in age, with analyses showing that 22q11.2DS patients were significantly older than the early psychotic group (P = .0275). There was also a significantly larger number of women in the group with the deletion compared to the early psychosis group (P = .0104). From the 28 subjects with the deletion that had no history of psychosis, 3 fulfilled criteria for attenuated psychotic syndrome. All groups displayed low levels of affective symptoms (manic and depression symptoms); however this was slightly higher in patients than healthy controls (YMRS post hoc t test for early psychosis vs healthy controls: P = .009). As expected, patients with the deletion also had a lower IQ than subjects from the early psychosis group and healthy controls (P = 4.71e−15 and P = 1.10e−23, respectively). Regarding in-scan movement, we compared mean FD across groups and found a significant difference between 22q11.2DS and healthy controls groups (P = 5.70e−5). Other clinical characteristics of the 22q11.2DS sample are reported in supplementary table 1. All subsequent reported analyses include age (as well as age squared), sex, IQ, and mean FD as covariates of no interest.
Table 1.
Demographic and Clinical Information
| Early Psychosis | 22q11.2DS (Psychosis/No Psychosis) |
Healthy Controls | F/t/χ 2 | P | ||
|---|---|---|---|---|---|---|
| N (125) | 32 | 38 (10/28) |
55 | — | — | |
| Age | 20.03 ± 3.03 | 23.66 ± 8.66 (23.4 ± 9.43/23.75 ± 8.55) |
22.91 ± 3.59 | 2.74 | .046 | |
| Sex (% female) | 25% | 55% (50%/57%) |
36% | 7.17 | .067 | |
| PANSS Total score |
43.16 ± 13.66 | 46.50 ± 14.92 | — | — | −0.66 | .512 |
| PANSS Positive symptoms |
8.03 ± 1.26 | 9.70 ± 3.56 | — | — | −2.28 | .028 |
| Fulfills attenuated-positive symptom syndrome according to SOPS (Number of subjects) |
— | — | 3 | 0 | (Fisher’s exact test) | .036 |
| HAM-D | 2.54 ± 3.56 | 2.70 ± 3.94 | 1.25 ± 2.29 | 2.816 | .06 | |
| YMRS | 1.16 ± 2.07 | 0.62 ± 1.62 | 0.13 ± 0.48 | 5.28 | .006 | |
| IQ | 94.19 ± 7.04 | 65.50 ± 14.03 | 69.75 ± 12.50 | 107.25 ± 13.82 | 76.46 | .000 |
| Mean FD | 0.43 ± 0.32 | 0.54 ± 0.28 (0.52 ± 0.28/0.55 ± 0.29) |
0.35 ± 0.16 | 6.99 | .001 | |
| Antipsychotic medication (CPZ equivalent) | 448.12 ± 263.50 | 56.91 ± 138.424 (208.75 ± 208.33/a) |
0 | 2.62 (t-stat early psychosis vs 22q11.2DS psychosis) |
.012 |
Note: CPZ, chlorpromazine; FD, framewise displacement; HAM-D, Hamilton Depression Rating Scale; PANSS, Positive and Negative Syndrome Scale; SOPS, Scale of Prodromal Symptoms; YMRS, Young Mania Rating Scale.
aOne subject in the 22q11DS group was prescribed antipsychotic medication (75 mg CPZ equivalent) for other reasons (management of impulsivity), not because of past or current psychosis.
Functional Connectivity Analyses
Analyses of striatocortical connectivity for each seed across groups replicated similar patterns as seen in previous studies (supplementary figure S1).37
22q11.2DS-Related Changes in Striatocortical Functional Connectivity
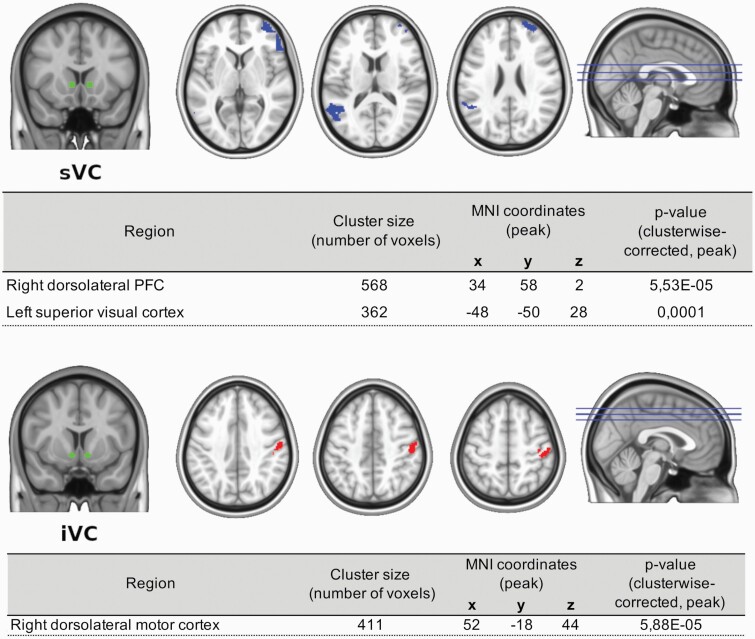
22q11.2DS was associated with changes in the ventral striatocortical system (figure 1). Carriers of the deletion showed a decreased functional connectivity between sVC and 2 voxel clusters. The first one was located at the right dorsolateral prefrontal cortex, also extending into the right ventrolateral prefrontal cortex and right ventromedial orbitofrontral cortex. The second cluster included the left superior visual cortex and temporoparietal junction.
Fig. 1.
22q11.2DS-related changes in striatocortical functional connectivity. Note: iVC, inferior ventral caudate; sVC, superior ventral caudate. Cortical regions highlighted in the upper panel represent decreased functional coupling with the sVC. For the lower panel, highlighted regions represent increased functional connectivity with iVC. All depicted regions are clusterwise corrected at a threshold of P < .01.
For the iVC, 22q11.2DS patients showed increased functional connectivity with right dorsolateral motor cortex and right dorsolateral somatosensory cortex.
Psychosis-Related Changes in Striatocortical Functional Connectivity
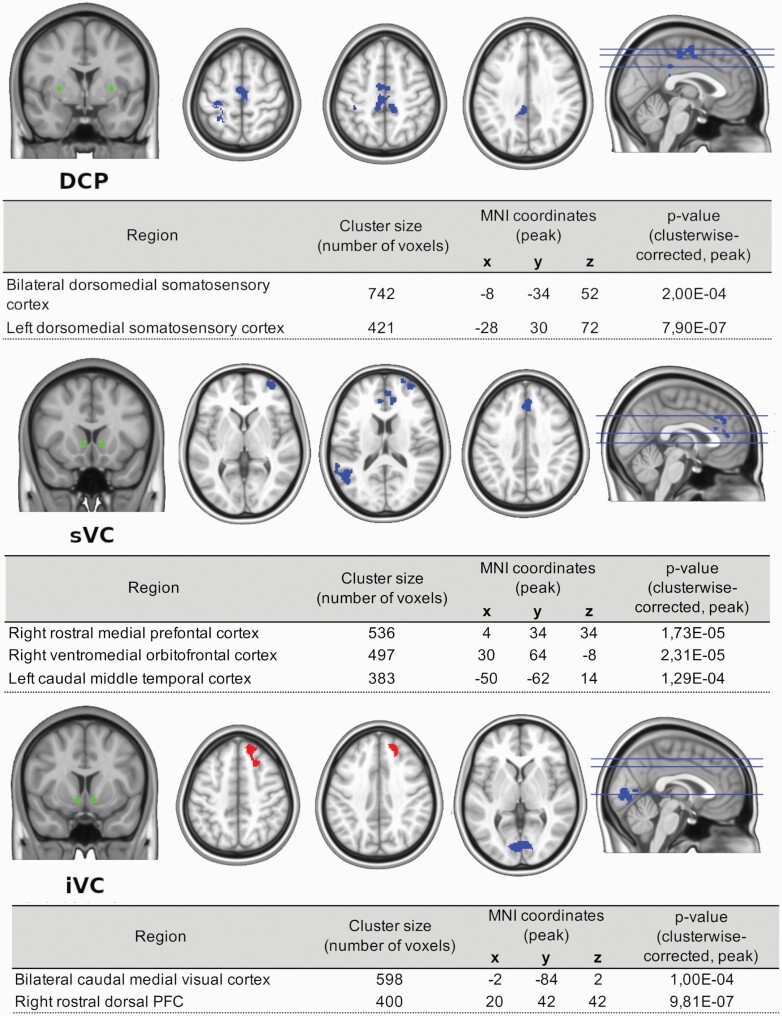
Psychosis was associated with alterations both in the dorsal and the ventral circuits (see figure 2). Reduced functional connectivity from the DCP seed was found for 2 different clusters. The first one was identified at the bilateral somatosensory and motor cortex, expanding into the bilateral cingulate cortex. The second cluster that showed hypoconnectivity with DCP in psychosis was the left somatosensory and motor cortex, with the cluster extending into the left superior parietal cortex.
Fig. 2.
Psychosis-related changes in striatocortical functional connectivity. Note: DCP, dorsocaudal putamen; iVC, inferior ventral caudate; sVC, superior ventral caudate. Cortical regions highlighted in upper and middle panels correspond to decreased functional coupling with DCP and sVC respectively. Bottom panel shows decreased cortical functional connectivity in the occipital cortex with iVC, and an increased coupling in frontal regions. All depicted regions are clusterwise corrected at a threshold of P < .01.
For the sVC seed, hypoconnectivity was found with 3 clusters. The first included the right medial, ventromedial, and dorsal prefrontal cortex, extending into the bilateral anterior cingulate cortex. The second cluster included right ventromedial orbitofrontal cortex and right dorsolateral prefrontal cortex. The third cluster encompassed left temporal and temporoparietal cortex.
For iVC seed, altered functional connectivity was found in 2 regions: bilateral medial visual cortex and cuneus, which showed hypoconnectivity; and right dorsal and dorsomedial prefrontal cortex, which showed hyperconnectivity.
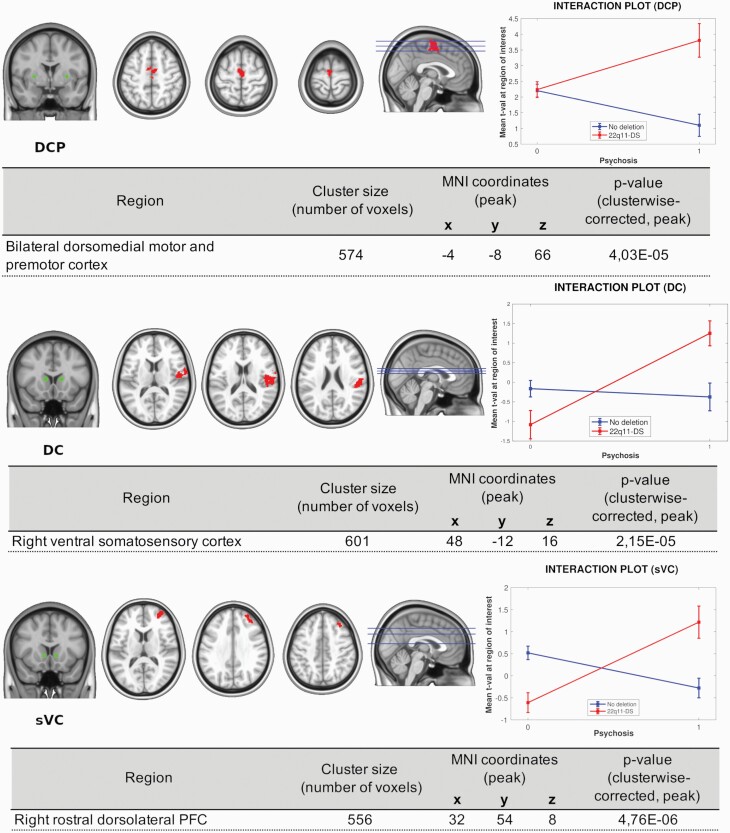
Psychosis and 22q11.2DS Interaction
We also performed an interaction analysis between psychosis and 22q11.2DS for striatocortical connectivity. Significant interactions were found for 3 seeds: DCP, DC, and sVC (figure 3). As shown in the interaction plots (figure 3, right), psychosis in the presence of the 22q11.2DS was associated with increased functional connectivity between DCP, DC, and sVC and several cortical regions. For the DCP, hyperconnectivity to bilateral dorsomedial motor and premotor cortex was found; for the DC, increased connectivity was seen with right ventral somatosensory cortex; and for the sVC seed, we found hyperconnectivity to the right dorsolateral prefrontal cortex. Increased connectivity between these regions was not seen in subjects with psychosis who were not carriers of the deletion.
Fig. 3.
Psychosis and 22q11.2DS interaction. Striatocortical functional connectivity. Cortical regions where a significant interaction was found are highlighted. The right column shows visual representations of the interactions between the effects of psychosis and 22q11.2DS at each region of interest. Note: DC, dorsal caudate; DCP, dorsocaudal putamen; sVC, superior ventral caudate. All regions showed are clusterwise corrected at a threshold of P < .01.
Effect of Antipsychotics
We repeated all the analyses above covarying for antipsychotic use. As shown in supplementary figures S2–S4, the results were very similar for the main effect of the deletion, or the interaction. The psychosis-related changes in the dorsal circuit (from DCP seeds) were no longer significant, nor were the hyperconnectivity from iVC to right dorsal pre-frontal cortex .
Discussion
We here examined striatocortical connectivity associated with a genetic change related to a very high risk to develop psychosis, namely the 22q11.2 deletion. Our main finding was a dysfunction of the ventral striatocortical system related to the deletion, which might explain the higher risk of psychosis seen in this population. Contrary to our hypothesis, we did not find significant differences in dorsal striatocortical systems in patients with 22q11.2DS. Dorsal changes were only present in subjects with a known history of psychosis, which accompanied ventral system dysconnectivity in this group. Our results point toward a dysfunction of ventral striatocortical networks in people at high genetic risk for psychosis, along with a more global dysfunction in striatocortical systems in people experiencing psychosis.
22q11.2DS was associated with decreased connectivity between the sVC seed and an extensive frontal region comprising parts of the dorsolateral prefrontal cortex, as well as ventromedial and ventrolateral prefrontal cortex. Decreases in functional connectivity were also seen between this seed and the temporoparietal junction. Previous studies have suggested an increase in ventral striatum connectivity to frontal regions and a decreased connectivity to temporoparietal regions in relatives of psychotic patients,12 although this pattern did not appear to be present in subjects with ultra-high risk to psychosis.24 It is interesting to note that these cortical regions showing abnormal connectivity with the ventral striatum have been involved in social cognition,39 and are known to be abnormal in schizophrenia.40 Their connectivity with midbrain dopaminergic regions has also been related to social amotivation in patients.41 Moreover, activation of the temporoparietal junction has been shown to be abnormal in response to a social task eliciting trust in subjects at high clinical risk of psychosis.42 Abnormalities in this system could therefore be the mechanism providing a higher risk to psychosis in patients with 22q11.2DS.
Patients with 22q11.2DS also presented increased connectivity between the iVC and sensorimotor regions. In line with this finding, subjects at ultra-high risk for psychosis also show hyperconnectivity of the ventral striatal system (ventral putamen specifically) and the postcentral gyrus.24 The association observed with motor areas resonates with findings of abnormal motor development in subjects who later develop schizophrenia,43 supporting its role in the genetic risk to the disorder. However, it might also reflect vulnerabilities to develop other disorders in the 22q11DS, like Parkinson’s disease among others.4,5,44–47
Our findings in psychosis partially support the proposed dorsal to ventral gradient of hypoconnectivity to hyperconnectivity.12 Thus, we observed decreased connectivity in the dorsal putamen with the midcingulum and nearby sensorimotor areas (although such differences were no longer significant when covarying for antipsychotic medication, as shown in supplementary figure S3). A similar pattern of reduced functional connectivity between DCP and motor regions has been described in healthy subjects with psychotic-like experiences and it was associated with the severity of such symptoms.19 In our study, psychosis was also associated with an increased connectivity between the iVC/ventral tegmental area (VTA) and the prefrontal cortex. These findings echo the functional connectivity abnormalities elicited by ketamine infusion, which have been shown to correlate with psychotic symptoms.22 However, striatal dysconnectivity in psychosis seems to be more complex than the gradient would suggest. We also found reduced connectivity between the sVC (ventral system) and the anterior cingulate cortex. Both anatomic and functional abnormalities of the anterior cingulate have been associated to psychotic disorders,48–50 and they have been implicated in the impaired integration of cognition, emotion, and motivational drive of schizophrenic patients. In the same manner, psychosis was also associated to hypoconnectivity between the iVC/VTA and occipital visual areas, alterations which are similar to those found in another study in first-episode psychotic patients.12
Several studies have shown that 22q11.2DS is associated with brain changes that are not frequently seen in patients with schizophrenia who are not carriers of the deletion, such as localized increased cortical thickness or increased fractional anisotropy.51,52 By including patients with psychosis with or without the deletion, we were also able to explore potential differences in striatal connectivity, albeit with a relatively low power. Our findings associated with psychosis across deletion and nondeletion groups, that were discussed above, show that there are many similarities. However, as our interaction analyses show, there are differences as well. Overall our results point toward a lower presence of hypoconnectivity in the dorsal striatum in psychosis in the 22q11.2DS, and an increased hyperconnectivity in the ventral striatum.
Our study is limited by its relatively small sample size. This is not unusual for a relatively rare disorder such as the 22q11.2DS. Nonetheless, our 2 × 2 factorial analysis allowed us to boost its power by looking orthogonally at the effect of psychosis and the deletion, increasing its sample size with healthy controls and patients with early psychosis. Power analysis could help us understand to what extent our study was under-powered. However, power analyses for imaging experiments require many assumptions that are not necessarily easy to make, and post hoc power analyses could be considered uninformative.53 One can still get a sense by observing that a total sample of 125 in a 2-way balanced ANOVA would have the power to detect medium size differences (Cohen’s f around 0.25). Considering the multiple correction approach used, based on the spatial clustering of significant voxels (using AlphaSim38), this would be a medium size effect that affects a volume of at least 348 voxels. The unbalanced nature of our design makes this an overestimation. It is also likely that the power for the interaction effects is particularly smaller, enough to identify large to medium differences that are clustered together. And that power might only be for disordinal or reversal interactions (as found in our study), being even smaller for ordinal or attenuation effects.54
As another limitation, the 22q11.2DS also confers a higher risk to other neuropsychiatric disorders, as well as a cognitive delay, and therefore not all brain changes might be associated with a higher vulnerability to psychosis. In order to account for some of this heterogenic presentation, we covaried for IQ as a way to control for cognitive impairment.
In summary, we here show that 22q11.2DS is related to abnormal connectivity in the ventral striatocortical network, highlighting the importance of this network in relation to the risk to psychosis.
Supplementary Material
Acknowledgments
Dr. Crossley has received personal fees from Janssen, outside the submitted work. None of the other authors has potential conflicts of interest to be disclosed.
Funding
This work was supported by the Agencia Nacional de Investigación y Desarrollo from Chile (ANID), through its grants ANILLO PIA ACT192064 and ACT1414, FONDECYT regular 1200601 and 1191710, and Millennium Nucleus for Cardiovascular Disease NCN17_129.
References
- 1. Carlson C, Sirotkin H, Pandita R, et al. Molecular definition of 22q11 deletions in 151 velo-cardio-facial syndrome patients. Am J Hum Genet. 1997;61(3):620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson DI, Cross IE, Wren C, Scambler PJ, Burn J, Goodship J. Minimum prevalence of chromosome 22q11 deletions. Am J Hum Genet. 1994;55(A169). [Google Scholar]
- 3. Cohen E, Chow EW, Weksberg R, Bassett AS. Phenotype of adults with the 22q11 deletion syndrome: a review. Am J Med Genet. 1999;86(4):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56(10):940–945. [DOI] [PubMed] [Google Scholar]
- 5. Bassett AS, Chow EW. Schizophrenia and 22q11.2 deletion syndrome. Curr Psychiatry Rep. 2008;10(2):148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rees E, Kirov G, Sanders A, et al. ; Wellcome Trust Case Control Consortium . Evidence that duplications of 22q11.2 protect against schizophrenia. Mol Psychiatry. 2014;19(1):37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35(3):549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Motahari Z, Moody SA, Maynard TM, LaMantia AS. In the line-up: deleted genes associated with DiGeorge/22q11.2 deletion syndrome: are they all suspects? J Neurodev Disord. 2019;11(1):1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gothelf D, Eliez S, Thompson T, et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8(11):1500–1502. [DOI] [PubMed] [Google Scholar]
- 10. Butcher NJ, Marras C, Pondal M, et al. Neuroimaging and clinical features in adults with a 22q11.2 deletion at risk of Parkinson’s disease. Brain. 2017;140(5):1371–1383. [DOI] [PubMed] [Google Scholar]
- 11. Rogdaki M, Devroye C, Ciampoli M, et al. Striatal dopaminergic alterations in individuals with copy number variants at the 22q11.2 genetic locus and their implications for psychosis risk: a [18F]-DOPA PET study. Mol Psychiatry. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fornito A, Harrison BJ, Goodby E, et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70(11):1143–1151. [DOI] [PubMed] [Google Scholar]
- 13. Dandash O, Pantelis C, Fornito A. Dopamine, fronto-striato-thalamic circuits and risk for psychosis. Schizophr Res. 2017;180:48–57. [DOI] [PubMed] [Google Scholar]
- 14. White TP, Wigton R, Joyce DW, Collier T, Fornito A, Shergill SS. Dysfunctional striatal systems in treatment-resistant schizophrenia. Neuropsychopharmacology. 2016;41(5):1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17(8):524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18(1):7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr Bull. 1976;2(1):19–76. [DOI] [PubMed] [Google Scholar]
- 18. Csernansky JG, Bardgett ME. Limbic-cortical neuronal damage and the pathophysiology of schizophrenia. Schizophr Bull. 1998;24(2):231–248. [DOI] [PubMed] [Google Scholar]
- 19. Sabaroedin K, Tiego J, Parkes L, et al. Functional connectivity of corticostriatal circuitry and psychosis-like experiences in the general community. Biol Psychiatry. 2019;86(1):16–24. [DOI] [PubMed] [Google Scholar]
- 20. McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci. 2019;42(3):205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Howes OD, Bose SK, Turkheimer F, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry. 2011;168(12):1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dandash O, Harrison BJ, Adapa R, et al. Selective augmentation of striatal functional connectivity following NMDA receptor antagonism: implications for psychosis. Neuropsychopharmacology. 2015;40(3):622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pani SM, Sabaroedin K, Tiego J, Bellgrove MA, Fornito A. A multivariate analysis of the association between corticostriatal functional connectivity and psychosis-like experiences in the general community. Psychiatry Res Neuroimaging. 2021;307:111202. [DOI] [PubMed] [Google Scholar]
- 24. Dandash O, Fornito A, Lee J, et al. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr Bull. 2014;40(4):904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. León LE, Benavides F, Espinoza K, et al. Partial microduplication in the histone acetyltransferase complex member KANSL1 is associated with congenital heart defects in 22q11.2 microdeletion syndrome patients. Sci Rep. 2017;7(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lecrubier Y, Sheehan DV, Weiller E, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry. 1997;12(5):224–231. [Google Scholar]
- 27. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):20–33. [PubMed] [Google Scholar]
- 28. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 29. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. [DOI] [PubMed] [Google Scholar]
- 30. Hamilton M. The Hamilton rating scale for depression. In: Sartorius N, Ban TA, eds. Assessment of Depression. Berlin, Heidelberg: Springer; 1986:143–152. [Google Scholar]
- 31. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 32. Parkes L, Fulcher B, Yücel M, Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. Neuroimage. 2018;171:415–436. [DOI] [PubMed] [Google Scholar]
- 33. Penny W, Friston K, Ashburner J, Kiebel S, Nichols T.. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London: Elsevier Ltd; 2007. [Google Scholar]
- 34. Avants BB, Tustison N, Johnson H. Advanced Normalization Tools (ANTS) Release 2.X. 2014. https://brianavants.wordpress.com/2012/04/13/updated-ants-compile-instructions-april-12-2012/. Accessed November, 2021.
- 35. Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. [DOI] [PubMed] [Google Scholar]
- 37. Di Martino A, Scheres A, Margulies DS, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18(12):2735–2747. [DOI] [PubMed] [Google Scholar]
- 38. Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9):e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tso IF, Rutherford S, Fang Y, Angstadt M, Taylor SF. The “social brain” is highly sensitive to the mere presence of social information: an automated meta-analysis and an independent study. PLoS One. 2018;13(5):e0196503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kronbichler L, Tschernegg M, Martin AI, Schurz M, Kronbichler M. Abnormal brain activation during theory of mind tasks in schizophrenia: a meta-analysis. Schizophr Bull. 2017;43(6):1240–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xu P, Klaasen NG, Opmeer EM, et al. Intrinsic mesocorticolimbic connectivity is negatively associated with social amotivation in people with schizophrenia. Schizophr Res. 2019;208:353–359. [DOI] [PubMed] [Google Scholar]
- 42. Lemmers-Jansen ILJ, Fett AJ, Hanssen E, Veltman DJ, Krabbendam L. Learning to trust: social feedback normalizes trust behavior in first-episode psychosis and clinical high risk. Psychol Med. 2019;49(5):780–790. [DOI] [PubMed] [Google Scholar]
- 43. Burton BK, Hjorthøj C, Jepsen JR, Thorup A, Nordentoft M, Plessen KJ. Research Review: Do motor deficits during development represent an endophenotype for schizophrenia? A meta-analysis. J Child Psychol Psychiatry. 2016;57(4):446–456. [DOI] [PubMed] [Google Scholar]
- 44. Butcher NJ, Kiehl TR, Hazrati LN, et al. Association between early-onset Parkinson disease and 22q11.2 deletion syndrome: identification of a novel genetic form of Parkinson disease and its clinical implications. JAMA Neurol. 2013;70(11):1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boot E, Bassett AS, Marras C. 22q11.2 deletion syndrome-associated Parkinson’s disease. Mov Disord Clin Pract. 2019;6(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lin WC, Chen HL, Hsu TW, et al. Correlation between dopamine transporter degradation and striatocortical network alteration in Parkinson’s disease. Front Neurol. 2017;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boot E, Butcher NJ, Udow S, et al. ; International Research Group on 22q11.2DS-associated Parkinson’s Disease . Typical features of Parkinson disease and diagnostic challenges with microdeletion 22q11.2. Neurology. 2018;90(23):e2059–e2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reid MA, Stoeckel LE, White DM, et al. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2010;68(7):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fornito A, Yücel M, Wood SJ, et al. Surface-based morphometry of the anterior cingulate cortex in first episode schizophrenia. Hum Brain Mapp. 2008;29(4):478–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fornito A, Yung AR, Wood SJ, et al. Anatomic abnormalities of the anterior cingulate cortext before psychosis onset: an MRI study of ultra-high-risk individuals. Biol Psychiatry. 2008;64(9):758–765. [DOI] [PubMed] [Google Scholar]
- 51. Bakker G, Caan MW, Vingerhoets WA, et al. Cortical morphology differences in subjects at increased vulnerability for developing a psychotic disorder: a comparison between subjects with ultra-high risk and 22q11.2 deletion syndrome. PLoS One. 2016;11(11):e0159928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ching CRK, Gutman BA, Sun D, et al. Mapping subcortical brain alterations in 22q11.2 deletion syndrome: effects of deletion size and convergence with idiopathic neuropsychiatric illness. Am J Psychiatry. 2020;177(7):589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mumford JA. A power calculation guide for fMRI studies. Soc Cogn Affect Neurosci. 2012;7(6):738–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lakens D, Caldwell AR. Simulation-based power-analysis for factorial ANOVA designs. PsyArxiv. 2019. 10.31234/osf.io/baxsf [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.