Abstract
Allosteric modulation represents an important approach in drug discovery because of its advantages in safety and selectivity. SOMCL-668 is the first selective and potent sigma-1 receptor allosteric modulator, discovered in our laboratory. The present work investigates the potential therapeutic effects of SOMCL-668 on phencyclidine (PCP)-induced schizophrenia-related behavior in mice and further elucidates underlying mechanisms for its antipsychotic-like effects. SOMCL-668 not only attenuated acute PCP-induced hyperactivity and PPI disruption, but also ameliorated social deficits and cognitive impairment induced by chronic PCP treatment. Pretreatment with the selective sigma-1 receptor antagonist BD1047 blocked the effects of SOMCL-668, indicating sigma-1 receptor-mediated responses. This was confirmed using sigma-1 receptor knockout mice, in which SOMCL-668 failed to ameliorate PPI disruption and hyperactivity induced by acute PCP and social deficits and cognitive impairment induced by chronic PCP treatment. Additionally, in vitro SOMCL-668 exerted positive modulation of sigma-1 receptor agonist-induced intrinsic plasticity in brain slices recorded by patch-clamp. Furthermore, in vivo lower dose of SOMCL-668 exerted positive modulation of improvement in social deficits and cognitive impairment induced by the selective sigma-1 agonist PRE084. Also, SOMCL-668 reversed chronic PCP-induced down-regulation in expression of frontal cortical p-AKT/AKT, p-CREB/CREB and BDNF in wide-type but not sigma-1 knockout mice. Moreover, administration of the PI3K/AKT inhibitor LY294002 abolished amelioration by SOMCL-668 of chronic PCP-induced schizophrenia-related behaviors by inhibition of BDNF expression. The present data provide initial, proof-of-concept evidence that allosteric modulation of the sigma-1 receptor may be a novel approach for the treatment of psychotic illness.
Keywords: SOMCL-668, chaperone protein, allosteric modulator, schizophrenia, AKT–CREB–BDNF pathway
Introduction
Although current drugs for treating psychotic illness such as schizophrenia have efficacy in relieving positive symptoms, they show poor efficacy in treating negative symptoms and cognitive impairment.1,2 This still unmet need requires novel therapeutic approaches. The sigma-1 receptor is a Ca2+-sensitive molecular chaperone residing on the mitochondrion-associated endoplasmic reticulum membrane (MAM), where these receptors bind with immunoglobulin protein (BIP) and are inactivated.3 Upon endoplasmic reticulum stress or ligand stimulation, the sigma-1 receptor dissociates from BIP and activated sigma-1 receptors then translocate to either the plasma membrane or other subcellular counterparts. There, they interact with membrane receptors such as G-protein coupled receptors (GPCRs), ion channels and other molecules to regulate their activity.4,5 Important functional roles for sigma-1 receptors in brain have been well documented and alterations in sigma-1 receptor function have been associated with various neurological and psychiatric disorders.6–8
Clinical trials using non-selective sigma-1 receptor agonists such as fluvoxamine have indicated lack of efficacy in treating positive symptoms in schizophrenia but suggested some effect on negative symptoms; 9–11 for more extended discussion see Supplementary material. Subsequently, it was reported that sigma-1 receptors in postmortem brain from schizophrenia patients were reduced12,13 and some, but not all, genetic studies have indicated the sigma-1 receptor gene to be associated with schizophrenia.14 Moreover, accumulating preclinical evidence indicates involvement of sigma-1 receptors in schizophrenia-related processes. Animal studies indicate non-selective and selective sigma-1 receptor agonists such as donepezil, fluvoxamine, pridopidine and SA4503 ameliorate phencyclidine (PCP)-induced cognitive impairment in mice.15–17 Mice with sigma-1 receptor knockout show alterations in expression of the dopamine transporter (DAT) and in phosphorylation of the glutamate NMDA NR2B receptor.18 Also, sigma-1 receptors regulate dopaminergic neurotransmission by binding to DAT and to D1 and D2 dopamine receptors.19–21 Moreover, sigma-1 receptor activation in hippocampal neurons not only promotes expression of NR2A, NR2B and PSD95, but also mediates transport of NMDAR to the cell surface, suggesting that sigma-1 receptors may play an important role in NMDAR-mediated learning and memory.22 As abnormal dopamine and glutamate transmission play key roles in the pathobiology of schizophrenia,23 targeting sigma-1 receptors may be a promising therapeutic approach.
Compared with orthodox agonists, allosteric modulators exhibit advantages in term of safety, selectivity and controllability in pharmacological action.24 Allosteric sites are more variable than orthodox binding sites and can exert more precise regulation of the target protein, hence rational design of small molecule modulator drugs can provide more options for disease treatment.25,26 Phenytoin allosterically modulates the sigma-1 receptor by transforming it from a low-affinity to a high-affinity state,27,28 though with poor selectivity and potency.29 We recently reported that SKF83959, an atypical D1R agonist, is a potent sigma-1 receptor allosteric modulator,30 and another allosteric modulator E1R was subsequently reported.31 Based on the structure of SKF83959, we then developed the potent, selective sigma-1 receptor allosteric modulator SOMCL-668.32 This shows no affinity for D1R and other neurotransmitter receptors33 but exhibits selective allosteric regulation of sigma-1 receptor activity with 100-fold greater potency than phenytoin.32 Here, we investigate the effects of SOMCL-668 in models of antipsychotic-like activity and associated cellular mechanisms.
Materials and Methods
Animals
All animal protocols were approved by the Animal Care and Use Committee of Soochow University and were in compliance with the Guidelines for the Care and Use of Laboratory Animals (Chinese National Research Council, 2006) and ARRIVE guidelines 2.034 (Supplementary material contains animal feeding conditions and access).
Drugs
SOMCL-668 was synthesized in the Laboratory of Medicinal Chemistry (Dr N. Ye) at the College of Pharmaceutical Sciences, Soochow University, to a purity >98%, as described previously.31,32 PRE084, BD1047 and LY294002 were purchased from MedChemExpress (New Jersey, USA). PCP was synthesized by the Shanghai Institute of Materia Medica, Chinese Academy of Sciences (Supplementary material contains details on drug treatment, doses and numbers of animals per group).
Behavioral Studies
Prepulse inhibition (PPI),35 locomotor activity,35,36 social interaction (SI)35,37 and novel object recognition (NOR)38 were conducted as previously described. (See Supplementary material for details).
Electrophysiological Studies
Acute slices were prepared in choline-based dissection buffer as previously described.39 Briefly, mice (2–3 weeks) were deeply anesthetized with 0.7% sodium pentobarbital (0.14 g/kg body weight). Brains were quickly removed into ice-cold (0–4°C) choline-based cutting solution. Whole-cell recordings of cortical L2/3 pyramidal neurons were made with a HEKA EPC-10 patch clamp amplifier (HEKA Instruments Inc., Lambrecht/Pfalz, Germany) as previously described.40 The drugs PRE084 and SOMCL-668, alone and in combination, were perfused at 2.5μM and 5μM, respectively, based on previous reports41,42 (see Supplementary material for further details).
Western Blots
Tissues were homogenized and lysed with NP40 lysis buffer (Beyotime Biotechnology, Shanghai, China) supplemented with 1 mM PMSF (Beyotime Biotechnology). An aliquot of 40–80 μg protein from each sample was separated with SDS-PAGE and transferred to a nitrocellulose membrane, which was then incubated with 5% non-fat milk for 1 h at room temperature. Expression of the sigma-1 receptor (1:500, Proteintech, 15168-1-AP), p-AKT (1:500, Cell Signaling Technology, CST4060), AKT (1:1000, Cell Signaling Technology, CST9272), p-CREB (1:500, Cell Signaling Technology, CST9198), CREB (1:1000, Cell Signaling Technology, CST9197), BDNF (1:200, Santa Cruz, SC-546,) and α-tubulin (1:10 000, Sigma-Aldrich, T6074) was measured using western blotting. Membranes were incubated with the respective secondary antibody (1:10 000, Sigma-Aldrich, Goat Anti Rabbit IgG A0545, Goat Anti Mouse IgG A3682) and visualized using a ChemiScope Mini system (Clinx Science, Shanghai, China). Blots were analyzed quantitatively using ImageJ software.
Statistical Analysis
GraphPad Prism 5 software was used for statistical analysis. Data are expressed as mean ± SEM. Differences between two groups were determined using Student’s t-test, with one-way or two-way ANOVA used to analyze more than two groups. On finding significant effects on ANOVA, the Newman–Keuls test or Bonferroni test was used for post-hoc analyses, which were performed when there were no significant differences in variance. In all analyses P < .05 was considered statistically significant.
Results
SOMCL-668 Attenuates PPI Deficits and Hyperlocomotion in Acute PCP-treated Mice
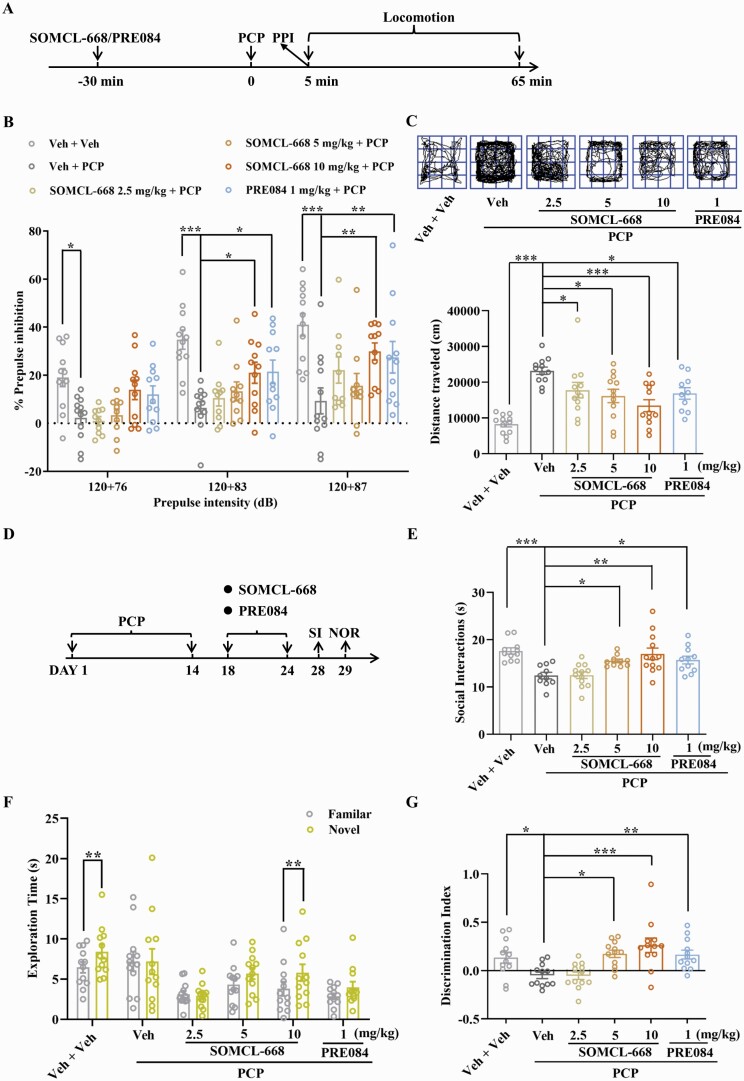
Pretreatment with SOMCL-668 ameliorated PCP-induced disruption of PPI at 83 and 87dB in a dose-dependent manner (figure 1A, B) and attenuated PCP-induced hyperlocomotion (figure 1C). Similarly, administration of the selective sigma-1 receptor agonist PRE084 (1 mg/kg i.p.) also ameliorated PCP-induced disruption of PPI (figure 1B) and attenuated PCP-induced hyperlocomotion (figure 1C). Neither SOMCL-668 nor PRE084 given alone influenced baseline locomotor activity (Supplementary figure S1A).
Fig. 1.
Effects of SOMCL-668 on acute and chronic phencyclidine-induced schizophrenia-related behaviors. (A) Experimental outline of acute studies. (B) PPI (%) was assessed using a startle stimulus intensity of 120 dB and prepulse intensities of 76, 83 and 87 dB. (C) Representative specimen traces of locomotor activity and summary bar graph of distance traveled. (D) Experimental outline of chronic studies. Social interaction (E) is presented as interaction time (s) and novel object recognition is presented as exploration time (s) for the familiar and novel objects (F) and as discrimination index (G). Data are shown as mean ± SEM; n = 10–12 mice per group. Statistical analysis was by one-way ANOVA (C, E and G) and two-way ANOVA (B and F); * P < .05, ** P < .01, *** P < .001.
SOMCL-668 Improves Social Interaction Deficits and Cognitive Impairment in Chronic PCP-treated Mice
We next tested if SOMCL-668 treatment influenced PCP-induced impairments in SI and NOR. As shown in figure 1D and E, while total SI time in PCP-treated mice was lower than in control mice, SOMCL-668 or PRE084 treatment for 1 week each dose-dependently increased SI time in PCP-treated mice (figure 1E) but not in control mice (Supplementary figure S1B). Treatment with SOMCL-668 or PRE084 for 1 week also ameliorated PCP-induced impairment in NOR in terms of both exploration time (figure 1F) and discrimination index (figure 1G); neither SOMCL-668 nor PRE084 had any effect on NOR in control mice (Supplementary figure S1C and D). These results indicated that SOMCL-668 significantly ameliorates impairment in both cognition and SI induced by chronic PCP in mice.
Sigma-1 Receptor Is Involved in Amelioration by SOMCL-668 of PCP-induced Schizophrenia-related Behaviors
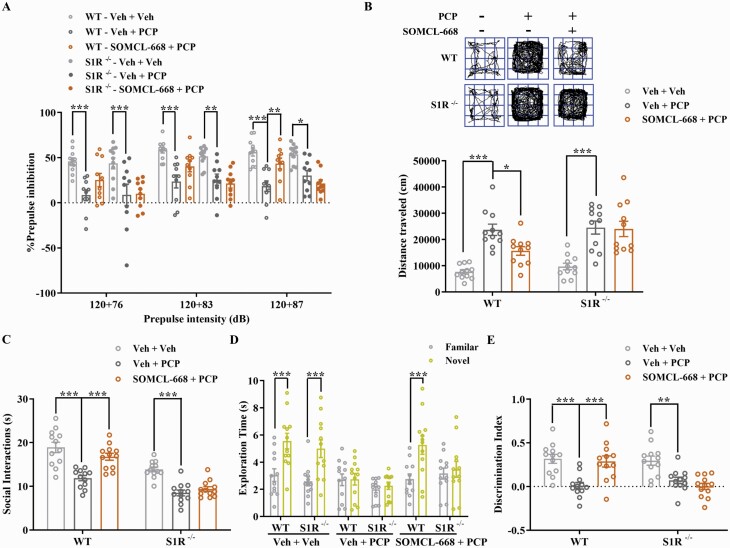
The selective sigma-1 antagonist BD1047 was used to clarify the role of the sigma-1 receptor in mediating the effect of SOMCL-668 (Supplementary figure S2A). As shown in figure S2B, %PPI induced by each of the three prepulse intensities in the PCP-treated group was smaller than those in the vehicle group and SOMCL-668 treatment significantly ameliorated this disruption. Pretreatment with BD1047 attenuated the action of SOMCL-668 to ameliorate both disruption of PPI and induction of hyperactivity by acute PCP (Supplementary figure S2B and C), indicating that these effects of SOMCL-668 were dependent on the sigma-1 receptor. In support, we found that these ameliorative effects of SOMCL-668 on disruption of PPI and induction of hyperactivity by acute PCP were absent in sigma-1 knockout mice (figure 2A and B).
Fig. 2.
SOMCL-668 fails to improve phencyclidine-induced schizophrenia-related behaviors in sigma-1 receptor knockout mice. (A) PPI (%) was assessed using a startle stimulus intensity of 120 dB and prepulse intensities of 76, 83 and 87 dB. (B) Representative specimen traces of locomotor activity and summary bar graph of distance traveled. Social interaction (C) is presented as interaction time (s) and novel object recognition is presented as exploration time (s) for the familiar and novel objects (D) and as discrimination index (E). Data are shown as mean ± SEM; n = 10–12 mice per group. Statistical analysis was by two-way ANOVA; * P < .05, ** P < .01, *** P < .001.
In preliminary experiments, chronic administration of 5 mg/kg BD1047 induced diarrhea and thus led to weight loss, whereas 2 mg/kg BD1047 treatment was well tolerated.43 At this lower dose, chronic BD1047 administered with SOMCL-668 attenuated the action of SOMCL-668 to ameliorate chronic PCP-induced impairment in both SI and the discrimination index in NOR, but was without effect when given alone (Supplementary figure S2D–G). This indicated that these effects of SOMCL-668 were dependent on the sigma-1 receptor.
On investigating these effects in sigma-1 receptor knockout mice, knockouts spent less time in basal SI than wild types, while basal NOR measures were unaltered (figure 2D). Nevertheless, we found that the ameliorative effects of SOMCL-668 on impairment in SI and NOR induced by chronic PCP were absent in sigma-1 knockout mice (figure 2C–E). This further confirmed that these ameliorative effects of SOMCL-668 were dependent on the sigma-1 receptor.
Positive Modulation of the Sigma-1 Receptor by SOMCL-668 in Brain Slices
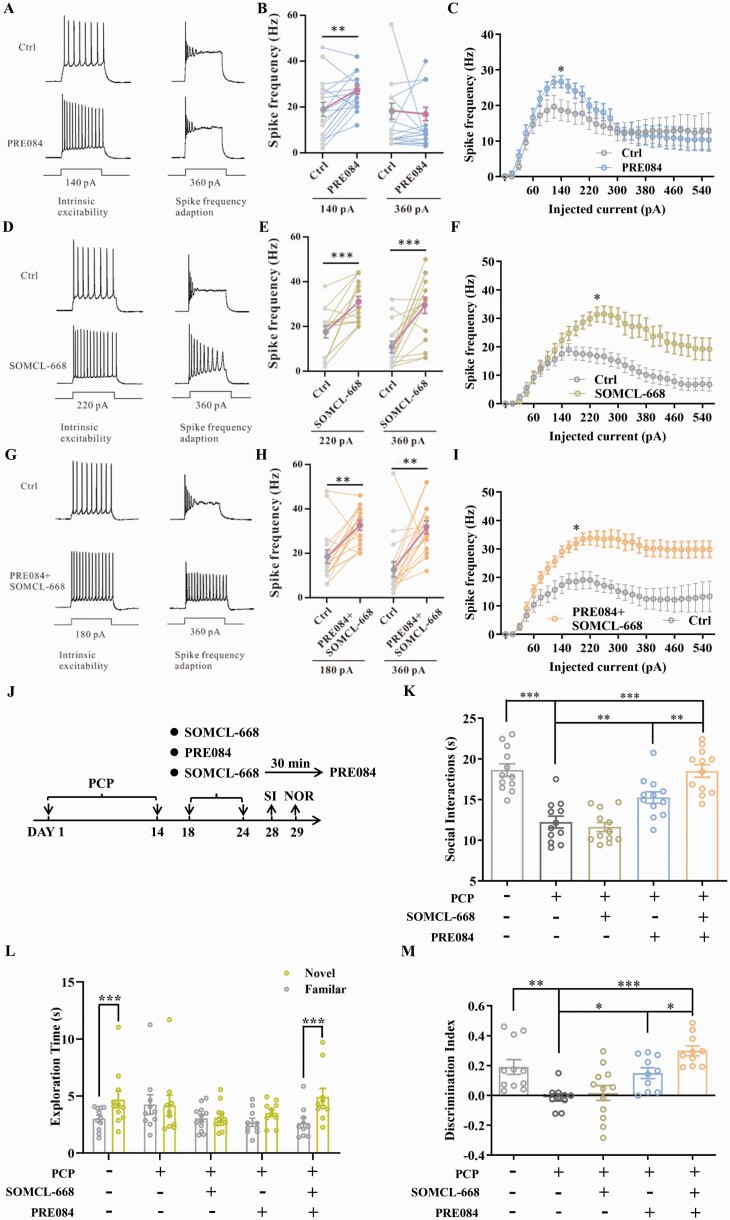
Our previous studies have established that SOMCL-668 is a novel allosteric modulator of the sigma-1 receptor.31,43 To elucidate the role of allosteric modulation of the sigma-1 receptor in SOMCL-668-induced effects, we first employed cortical brain slices. To determine if SOMCL-668 or PRE084 regulates the excitability of cortical pyramidal neurons, we first measured the rheobase, i.e. the current necessary to evoke action potentials (APs). For these experiments, pyramidal neurons were held to a standardized membrane potential of 60 mV with stepwise current injection. Under these conditions, spontaneous APs were not observed. To measure rheobase, positive current steps of 0.5 s duration and amplitudes that varied in steps of 20 pA were applied until the pyramidal neurons fired at least one AP (Supplementary figure S3A). Using this protocol, we observed no significant effects of either SOMCL-668 or PRE084 when given alone. Since allosteric modulation requires the presence of a sigma-1 receptor agonist, brain slice preparations, unlike the intact animal, may not have adequate endogenous sigma-1 receptor stimulation to reveal an allosteric effect. Therefore, we additionally perfused SOMCL-668 in the presence of the selective agonist PRE084 in cortical slices and again did not detect a significant change in the rheobase current of pyramidal neurons (Supplementary figure S3A, B).
We next explored if the sigma-1 receptor is involved in modulation of intrinsic plasticity. We found that the number of evoked APs was increased by PRE084 treatment at an injected current of 140 pA, without significant change in spike frequency adaption (figure 3A–C). Notably, SOMCL-668 increased spike frequency adaption at an injected current of 360 pA (figure 3D–F). Moreover, co-application of SOMCL-668 with PRE084 led to a leftward shift of the current–spike curve relative to PRE084 alone (figure 3G–I).
Fig. 3.
SOMCL-668 positively modulates the effect of PRE084 both in vitro and in vivo. (A) Representative traces of intrinsic excitability and spike frequency adaption. (B) Spike frequencies elicited at 140 pA and 360 pA, with or without bath application of PRE084. (C) Complete plot of spike frequency against step current (pA) with or without bath application of PRE084. (D) Representative traces of intrinsic excitability and spike frequency adaption for action potentials. (E) Spike frequencies elicited at 220 pA and 360 pA, with or without bath application of SOMCL-668. (F) Complete plot of spike frequency against step current (pA) with or without bath application of SOMCL-668. (G) Representative traces of intrinsic excitability and spike frequency adaption for action potentials. (H) Spike frequencies elicited at 180 pA and 360 pA, with or without bath application of PRE084 + SOMCL-668. (I) Complete plot of spike frequency against step current (pA) with or without bath application of PRE084 + SOMCL-668. (J) Experimental outline of behavioral study. Social interaction (K) is presented as interaction time (s) and novel object recognition is presented as exploration time (s) for the familiar and novel objects (L) and as discrimination index (M). Data are shown as mean ± SEM; n = 16–18 cells from 3–4 mice in electrophysiological tests and n = 10–12 mice in behavioral tests. Statistical analysis was by two-way ANOVA (C, F, I and L), one-way ANOVA (K and M) and Student’s paired t-test (B, E and H); * P < .05, ** P < .01, *** P < .001.
We next asked whether this was also the case in sigma-1 receptor knockout mice. The PRE084-induced increase in AP spike observed in wild-type mice was abolished in sigma-1 receptor knockout mice (Supplementary figure S3C). In addition, sigma-1 receptor knockout mice fired an average of 9 APs compared to 12 APs from neurons in wild types (Supplementary figure S3D). Taken together, it appears that the sigma-1 receptor is involved in modulation of neuronal firing and that SOMCL-668 exerts positive allosteric modulation on intrinsic plasticity induced by a sigma-1 receptor agonist.
Positive Modulation of the Sigma-1 Receptor by SOMCL-668 in Ameliorating PCP-induced Behavioral Abnormalities
We further investigated allosteric modulation by SOMCL-668 in terms of its interaction with a selective sigma-1 receptor agonist in vivo, using a lower dose of SOMCL-668 (2.5 mg/kg) that alone did not affect impairment in SI or NOR induced by chronic PCP administration (figure 3J–M). 2.5 mg/kg SOMCL-668 + 1 mg/kg PRE084 induced greater improvement in SI and NOR as compared to PRE084 alone (figure 3K–M), implicating positive modulation of the sigma-1 receptor by SOMCL-668 in vivo.
SOMCL-668 regulation of the AKT–CREB–BDNF pathway in relation to amelioration of PCP-induced impairment in social behavior and cognition
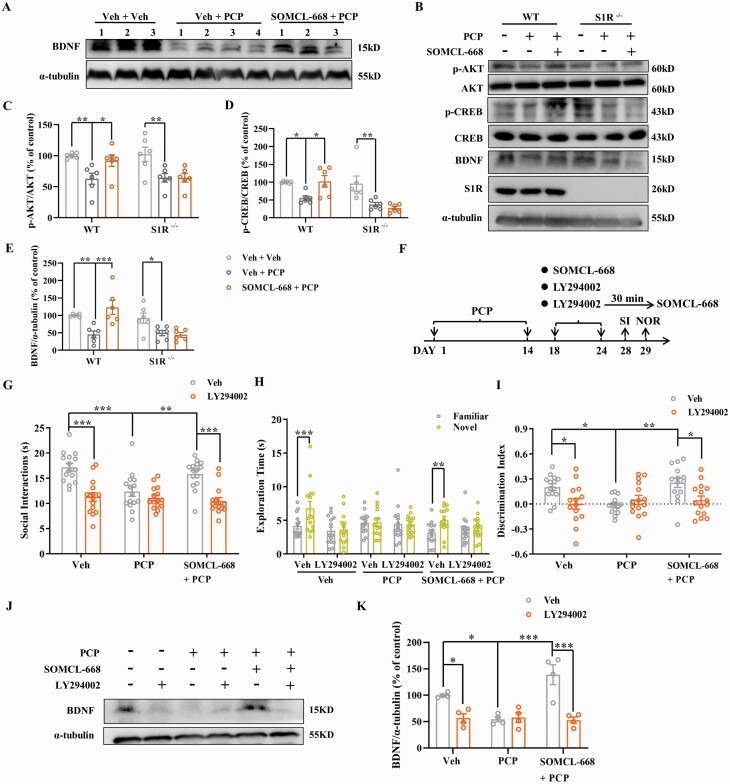
Down-regulation of brain-derived neurotrophic factor (BDNF) is known to be involved in the pathophysiology of schizophrenia and may be a potential biomarker.44–46 We and others have reported that activation of the sigma-1 receptor promotes production of BDNF.47–49 Thus, we speculated that the antipsychotic-like effects of SOMCL-668 may be attributed to its regulation of BDNF. Chronic PCP treatment indeed decreased frontal cortical expression of BDNF and this decrease was ameliorated by SOMCL-668 (figure 4A), indicating that SOMCL-668 regulates BDNF expression in vivo.
Fig. 4.
Effects of SOMCL-668 on AKT–CREB–BDNF pathway in chronic PCP-treated mice. (A) Expression of BDNF in WT treated with Veh, PCP and SOMCL-668 + PCP. (B–E) Representative immunoblots (B) and ratios of p-AKT/AKT (C), p-CREB/CREB (D) and BDNF (E) in WT and S1R-/- mice treated with PCP, SOMCL-668 and SOMCL-668 + PCP. (F) Experimental outline of chronic studies. Social interaction (G) is presented as interaction time (s) and novel object recognition is presented as exploration time (s) for the familiar and novel objects (H) and as discrimination index (I). (J, K) Representative immunoblots (J) and quantitation (K) of BDNF levels from prefrontal cortex after the behavioral studies in (I). Data are shown as mean ± SEM; n = 6 mice per group (A–E), n = 14–16 mice per group (G–I) and n = 4 mice in each group (J, K). Statistical analysis was by two-way ANOVA; * P < .05, ** P < .01, *** P < .001.
We next examined AKT/CREB phosphorylation, upstream signaling molecules in BDNF production, and found that SOMCL-668 treatment also ameliorated chronic PCP-induced decreases in expression of p-AKT and p-CREB. However, SOMCL-668 failed to alter p-AKT, p-CREB and BDNF expression in sigma-1 receptor knockout mice (figure 4B). These findings are consistent with the comparable behavioral effects (figure 2C–E) and indicate a sigma-1 receptor-dependent effect.
To further elucidate the role AKT/CREB/BDNF pathway, we employed LY294002 to inhibit PI3K/AKT-mediated expression of BDNF (figure 4J, K). Amelioration by SOMCL-668 of chronic PCP-induced social and cognitive deficits was absent when LY294002 was administered (figure 4G–I). Notably, LY294002 given alone also induced both social and cognitive deficits similar to those induced by chronic PCP administration, further supporting a role for the AKT–CREB–BDNF pathway in PCP-induced schizophrenia-related behaviors in mice.
Discussion
The sigma-1 receptor located in MAM is a Ca2+-sensitive molecular chaperone that can regulate a variety of neurotransmitter systems. There is increasing evidence that the sigma-1 receptor is involved in the pathobiology neuropsychiatric diseases such as schizophrenia and depression.6 Moreover, modulation of the sigma-1 receptor appears to contribute to the therapeutic actions of various drugs such as selective serotonin reuptake inhibitors (SSRIs), donepezil and neurosteroids.43 Alteration in brain sigma-1 receptor expression in schizophrenia patients has been reported.12,13 Early studies showed that non-selective sigma-1 receptor agonists such as fluoxetine ameliorated PCP-induced cognitive impairment.7 In the present study, we took advantage of the newly developed, selective sigma-1 receptor allosteric modulator SOMCL-66832 to investigate a potential antipsychotic-like effect.
We found that SOMCL-668 ameliorated both acute PCP-induced PPI deficiency and hyperlocomotor activity and chronic PCP-induced social deficits and cognitive impairment (figure 1). Furthermore, these effects of SOMCL-668 were confirmed to be mediated via the sigma-1 receptor, since they were reversed by pretreatment with BD1047, a selective sigma-1 receptor antagonist (figure S2), and were absent in sigma-1 receptor knockout mice (figure 2). Additionally, SOMCL-668 positively modulated both intrinsic neuronal plasticity in brain slices (figure 4A–I) and sigma-1 agonist-facilitated amelioration of social and cognitive deficits following chronic PCP treatment (figure 4J–M), indicating SOMCL-668 indeed acts functionally as an allosteric modulator. Taken together, the present data reveal, for the first time, that allosteric modulation of the sigma-1 receptor elicits antipsychotic-like effects on schizophrenia-related behaviors.
Allosteric modulation, a focus point in contemporary drug discovery, is a process by which a molecule induces a conformational or dynamic change in a targeted protein by binding to a site other than the orthosteric pocket and thereby regulates the function of that protein; as allosteric modulators do not compete with endogenous/exogenous ligands at the orthosteric site, they thus provide an alternative approach for functional modulation of receptors/enzymes.44,45 They have the potential to achieve the same efficacy as, and to reduce the adverse effects of, orthosteric ligand,46 and to delay the development of drug resistance.47,48 In addition, compared to the highly conserved orthosteric pocket, the diverse structure of allosteric sites broadens approaches for designing different small molecules for achieving precise functional regulation of a targeted protein, thus providing an improved strategy for drug discovery.25,26
We have reported the first potent sigma-1 receptor allosteric modulator SKF83959,30 a long-recognized dopamine D1 receptor agonist,49 and have subsequently developed a selective sigma-1 receptor allosteric modulator SOMCL-668 without binding affinity to D1 and other neurotransmitter receptors.32 The present data reveal that allosteric modulation of the sigma-1 receptor produced a potent antipsychotic-like effect in acute and chronic PCP-treated animals. Given the advantages of allosteric modulation, these findings may illuminate a promising novel approach to drug discovery and development for psychotic disorders such as schizophrenia.
Brain-derived neurotrophic factor (BDNF) functions via receptors enriched in the nervous system.50,51 BDNF regulates neuronal survival, growth, differentiation and neuronal plasticity. Alterations in BDNF function have been closely associated with neuropsychiatric disorders, including schizophrenia,52,53 and decreased BDNF in the brain of schizophrenia patients may serve as a potential biomarker.53–55 Activation of the sigma-1 receptor can promote BDNF expression,56–58 and we have previously reported that SOMCL-668 induces BDNF expression in experimental animals.42 The present data demonstrate that SOMCL-668 treatment reverses decreased prefrontal BDNF expression induced by chronic PCP (figure 4A–E), which parallels improved social interaction and cognitive function (figure 1).
The AKT/CREB pathway is known to be a critical modulator of BNDF and alterations in AKT/CREB activation have been implicated in the pathobiology of schizophrenia.59,60 For example, some risk genes for schizophrenia have been shown to regulate the AKT signaling pathway.60 The D2R, a key therapeutic target of currently available antipsychotic drugs, also regulates the AKT signaling pathway.61 CREB is known to be one of the downstream effectors of AKT and intracerebral injection of AAV9/CREB-S133D (phosphorylation mutation), to decrease activation of CREB, also attenuated MK801-induced schizophrenia-related behavior and upregulated brain BDNF expression in mice.62 It may not be surprising that BDNF genes are targets of the CREB family, as phosphorylated CREB binds to specific sequences in the BDNF promoter and regulates its transcription.63
The present data reveal that activation of the sigma-1 receptor by the allosteric modulator SOMCL-668 in vivo stimulated the prefrontal AKT–CREB–BDNF signaling pathway, while this effect of SOMCL-668 was absent in sigma-1 receptor knockout mice (figure 4A–E). This indicates that sigma-1 receptors mediate SOMCL-668 stimulation of AKT–CREB–BDNF signaling cascades, in parallel with improving PCP-induced disruption of social and cognitive function (figure 2). Application of the selective PI3K/AKT inhibitor LY294002, to block production of BDNF, attenuated the ameliorative effects of SOMCL-668 on chronic PCP-induced schizophrenia-related behaviors (figure 4F–K), further indicating that the sigma-1-mediated AKT–CREB–BDNF pathway may contribute to the antipsychotic-like effects of SOMCL-668.
In summary, in this study we provide the first experimental data that the sigma-1 receptor allosteric modulator SOMCL-668 ameliorates not only acute PCP-induced hyperactivity and disruption of PPI but also chronic PCP-induced social and cognitive impairment. Moreover, we further reveal that the signaling mechanism for these SOMCL-668-mediated effects may be attributed to modulation of the AKT–CREB–BDNF pathway. The present data may indicate a novel approach for drug discovery in relation to psychotic disorders such as schizophrenia. Nevertheless, though the present data involved both acute and chronic PCP, the most widely applied animal models of psychotic illness and antipsychotic-like activity, studies with additional models may further inform on this putative therapeutic role and possible adverse effects of sigma-1 allosteric modulators.
Supplementary Material
Acknowledgments
The authors declare no conflict of interest.
Funding
This work was supported by the National Science Foundation of China (No. 81373382, 81973334, 31970909, 81773702), The China Postdoctoral Science Foundation (No. 7113288521 to G.L.), the Priority Academic Program Development of the Jiangsu Higher Education Institutes (PAPD) and the Suzhou International Academician Workstation Program.
References
- 1. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCutcheon RA, Reis Marques T, Howes OD. Schizophrenia-an overview. JAMA Psychiatry. 2020;77(2):201–210. [DOI] [PubMed] [Google Scholar]
- 3. Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19(2):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kourrich S, Su TP, Fujimoto M, Bonci A. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci. 2012;35(12):762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sánchez-Fernández C, Entrena JM, Baeyens JM, Cobos EJ. Sigma-1 receptor antagonists: a new class of neuromodulatory analgesics. Adv Exp Med Biol. 2017;964:109–132. [DOI] [PubMed] [Google Scholar]
- 6. Hayashi T, Tsai SY, Mori T, Fujimoto M, Su TP. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin Ther Targets. 2011;15(5):557–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hashimoto K. Activation of sigma-1 receptor chaperone in the treatment of neuropsychiatric diseases and its clinical implication. J Pharmacol Sci. 2015;127(1):6–9. [DOI] [PubMed] [Google Scholar]
- 8. Pabba M, Sibille E. Sigma-1 and N-methyl-d-aspartate receptors: a partnership with beneficial outcomes. Mol Neuropsychiatry. 2015;1(1):47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gewirtz GR, Gorman JM, Volavka J, et al. BMY 14802, a sigma receptor ligand for the treatment of schizophrenia. Neuropsychopharmacology. 1994;10(1):37–40. [DOI] [PubMed] [Google Scholar]
- 10. Huber MT, Gotthardt U, Schreiber W, Krieg JC. Efficacy and safety of the sigma receptor ligand EMD 57445 (panamesine) in patients with schizophrenia: an open clinical trial. Pharmacopsychiatry. 1999;32(2):68–72. [DOI] [PubMed] [Google Scholar]
- 11. Niitsu T, Fujisaki M, Shiina A, et al. A randomized, double-blind, placebo-controlled trial of fluvoxamine in patients with schizophrenia: a preliminary study. J Clin Psychopharmacol. 2012;32(5):593–601. [DOI] [PubMed] [Google Scholar]
- 12. Helmeste DM, Tang SW, Bunney WE Jr, Potkin SG, Jones EG. Decrease in sigma but no increase in striatal dopamine D4 sites in schizophrenic brains. Eur J Pharmacol. 1996;314(3):R3–R5. [DOI] [PubMed] [Google Scholar]
- 13. Weissman AD, Casanova MF, Kleinman JE, London ED, De Souza EB. Selective loss of cerebral cortical sigma, but not PCP binding sites in schizophrenia. Biol Psychiatry. 1991;29(1):41–54. [DOI] [PubMed] [Google Scholar]
- 14. Watanabe Y, Nunokawa A, Kaneko N, Shibuya M, Egawa J, Someya T. Supportive evidence for the association between the Gln2Pro polymorphism in the SIGMAR1 gene and schizophrenia in the Japanese population: a case-control study and an updated meta-analysis. Schizophr Res. 2012;141(2–3):279–280. [DOI] [PubMed] [Google Scholar]
- 15. Kunitachi S, Fujita Y, Ishima T, et al. Phencyclidine-induced cognitive deficits in mice are ameliorated by subsequent subchronic administration of donepezil: role of sigma-1 receptors. Brain Res. 2009;1279:189–196. [DOI] [PubMed] [Google Scholar]
- 16. Sahlholm K, Valle-León M, Fernández-Dueñas V, Ciruela F. Pridopidine reverses phencyclidine-induced memory impairment. Front Pharmacol. 2018;9:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hashimoto K, Fujita Y, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacology. 2007;32(3):514–521. [DOI] [PubMed] [Google Scholar]
- 18. Hong J, Sha S, Zhou L, Wang C, Yin J, Chen L. Sigma-1 receptor deficiency reduces MPTP-induced parkinsonism and death of dopaminergic neurons. Cell Death Dis. 2015;6:e1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sambo DO, Lin M, Owens A, et al. The sigma-1 receptor modulates methamphetamine dysregulation of dopamine neurotransmission. Nat Commun. 2017;8(1):2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Navarro G, Moreno E, Aymerich M, et al. Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc Natl Acad Sci U S A. 2010;107(43):18676–18681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Borroto-Escuela DO, Narváez M, Romero-Fernández W, et al. Acute cocaine enhances dopamine D2R recognition and signaling and counteracts D2R internalization in Sigma1R-D2R heteroreceptor complexes. Mol Neurobiol. 2019;56(10):7045–7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pabba M, Wong AY, Ahlskog N, et al. NMDA receptors are upregulated and trafficked to the plasma membrane after sigma-1 receptor activation in the rat hippocampus. J Neurosci. 2014;34(34):11325–11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCutcheon RA, Krystal JH, Howes OD. Dopamine and glutamate in schizophrenia: biology, symptoms and treatment. World Psychiatry. 2020;19(1):15–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niello M, Gradisch R, Loland CJ, Stockner T, Sitte HH. Allosteric modulation of neurotransmitter transporters as a therapeutic strategy. Trends Pharmacol Sci. 2020;41(7):446–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grimsby J, Sarabu R, Corbett WL, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science. 2003;301(5631):370–373. [DOI] [PubMed] [Google Scholar]
- 26. Iverson C, Larson G, Lai C, et al. RDEA119/BAY 869766: a potent, selective, allosteric inhibitor of MEK1/2 for the treatment of cancer. Cancer Res. 2009;69(17):6839–6847. [DOI] [PubMed] [Google Scholar]
- 27. Cobos EJ, Baeyens JM, Del Pozo E. Phenytoin differentially modulates the affinity of agonist and antagonist ligands for sigma 1 receptors of guinea pig brain. Synapse. 2005;55(3):192–195. [DOI] [PubMed] [Google Scholar]
- 28. DeHaven-Hudkins DL, Ford-Rice FY, Allen JT, Hudkins RL. Allosteric modulation of ligand binding to [3H](+)pentazocine-defined sigma recognition sites by phenytoin. Life Sci. 1993;53(1):41–48. [DOI] [PubMed] [Google Scholar]
- 29. Vavers E, Zvejniece L, Maurice T, Dambrova M. Allosteric modulators of sigma-1 receptor: a review. Front Pharmacol. 2019;10:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guo L, Zhao J, Jin G, et al. SKF83959 is a potent allosteric modulator of sigma-1 receptor. Mol Pharmacol. 2013;83(3):577–586. [DOI] [PubMed] [Google Scholar]
- 31. Zvejniece L, Vavers E, Svalbe B, et al. The cognition-enhancing activity of E1R, a novel positive allosteric modulator of sigma-1 receptors. Br J Pharmacol. 2014;171(3):761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo L, Chen Y, Zhao R, et al. Allosteric modulation of sigma-1 receptors elicits anti-seizure activities. Br J Pharmacol. 2015;172(16):4052–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang J, Huang J, Song Z, et al. Structural manipulation on the catecholic fragment of dopamine D(1) receptor agonist 1-phenyl-N-methyl-benzazepines. Eur J Med Chem. 2014;85:16–26. [DOI] [PubMed] [Google Scholar]
- 34. Percie du Sert N, Hurst V, Ahluwalia A, et al. The arrive guidelines 2.0: updated guidelines for reporting animal research. Br J Pharmacol. 2020;177:3617–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guo Y, Zhang H, Chen X, et al. Evaluation of the antipsychotic effect of bi-acetylated l-stepholidine (l-SPD-A), a novel dopamine and serotonin receptor dual ligand. Schizophr Res. 2009;115(1):41–49. [DOI] [PubMed] [Google Scholar]
- 36. Wang P, Cao T, Chen J, et al. D2 receptor-mediated miRNA-143 expression is associated with the effects of antipsychotic drugs on phencyclidine-induced schizophrenia-related locomotor hyperactivity and with Neuregulin-1 expression in mice. Neuropharmacology. 2019;157:107675. [DOI] [PubMed] [Google Scholar]
- 37. Mouri A, Lee HJ, Mamiya T, et al. Hispidulin attenuates the social withdrawal in isolated disrupted-in-schizophrenia-1 mutant and chronic phencyclidine-treated mice. Br J Pharmacol. 2020;177(14):3210–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Loiodice S, Wing Young H, Rion B, et al. Implication of nigral dopaminergic lesion and repeated L-dopa exposure in neuropsychiatric symptoms of Parkinson’s disease. Behav Brain Res. 2019;360:120–127. [DOI] [PubMed] [Google Scholar]
- 39. Duan L, Zhang XD, Miao WY, et al. PDGFRβ cells rapidly relay inflammatory signal from the circulatory system to neurons via chemokine CCL2. Neuron. 2018;100(1):183–200.e8. [DOI] [PubMed] [Google Scholar]
- 40. Zhao R, Chen J, Ren Z, Shen H, Zhen X. GSK-3β inhibitors reverse cocaine-induced synaptic transmission dysfunction in the nucleus accumbens. Synapse. 2016;70(11):461–470. [DOI] [PubMed] [Google Scholar]
- 41. Wang M, Wan C, He T, et al. Sigma-1 receptor regulates mitophagy in dopaminergic neurons and contributes to dopaminergic protection. Neuropharmacology. 2021;196:108360. [DOI] [PubMed] [Google Scholar]
- 42. Wang Y, Guo L, Jiang HF, Zheng LT, Zhang A, Zhen XC. Allosteric modulation of sigma-1 receptors elicits rapid antidepressant activity. CNS Neurosci Ther. 2016;22(5):368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Niitsu T, Iyo M, Hashimoto K. Sigma-1 receptor agonists as therapeutic drugs for cognitive impairment in neuropsychiatric diseases. Curr Pharm Des. 2012;18(7):875–883. [DOI] [PubMed] [Google Scholar]
- 44. Schwartz TW, Holst B. Allosteric enhancers, allosteric agonists and ago-allosteric modulators: where do they bind and how do they act? Trends Pharmacol Sci. 2007;28(8):366–373. [DOI] [PubMed] [Google Scholar]
- 45. Liu J, Nussinov R. Allostery: an overview of its history, concepts, methods, and applications. PLoS Comput Biol. 2016;12(6):e1004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wootten D, Christopoulos A, Sexton PM. Emerging paradigms in GPCR allostery: implications for drug discovery. Nat Rev Drug Discov. 2013;12(8):630–644. [DOI] [PubMed] [Google Scholar]
- 47. Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468(7326):968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Corcoran RB, Dias-Santagata D, Bergethon K, Iafrate AJ, Settleman J, Engelman JA. BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal. 2010;3(149):ra84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Deveney AM, Waddington JL. Pharmacological characterization of behavioural responses to SK&F 83959 in relation to ‘D1-like’ dopamine receptors not linked to adenylyl cyclase. Br J Pharmacol. 1995;116(3):2120–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Denghien I, Joober R, Rouleau GA, Néri C. Polyglutamine tracts in schizophrenia: gaining new insights. Mol Psychiatry. 2000;5(3):236–237. [DOI] [PubMed] [Google Scholar]
- 51. Rizos EN, Papathanasiou M, Michalopoulou PG, et al. Association of serum BDNF levels with hippocampal volumes in first psychotic episode drug-naive schizophrenic patients. Schizophr Res. 2011;129(2–3):201–204. [DOI] [PubMed] [Google Scholar]
- 52. Angelucci F, Brenè S, Mathé AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10(4):345–352. [DOI] [PubMed] [Google Scholar]
- 53. Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16(9):960–972. [DOI] [PubMed] [Google Scholar]
- 54. Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8(6):592–610. [DOI] [PubMed] [Google Scholar]
- 55. Ray MT, Shannon Weickert C, Webster MJ. Decreased BDNF and TrkB mRNA expression in multiple cortical areas of patients with schizophrenia and mood disorders. Transl Psychiatry. 2014;4:e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu Q, Ji XF, Chi TY, et al. Sigma-1 receptor in brain ischemia/reperfusion: possible role in the NR2A-induced pathway to regulate brain-derived neurotrophic factor. J Neurol Sci. 2017;376:166–175. [DOI] [PubMed] [Google Scholar]
- 57. Yamaguchi K, Shioda N, Yabuki Y, Zhang C, Han F, Fukunaga K. Sa4503, a potent sigma-1 receptor ligand, ameliorates synaptic abnormalities and cognitive dysfunction in a mouse model of atr-x syndrome. Int J Mol Sci. 2018;19(9):2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yagasaki Y, Numakawa T, Kumamaru E, Hayashi T, Su TP, Kunugi H. Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J Biol Chem. 2006;281(18):12941–12949. [DOI] [PubMed] [Google Scholar]
- 59. Wang H, Xu J, Lazarovici P, Quirion R, Zheng W. cAMP Response Element-Binding protein (CREB): a possible signaling molecule link in the pathophysiology of schizophrenia. Front Mol Neurosci. 2018;11:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zheng W, Wang H, Zeng Z, et al. The possible role of the Akt signaling pathway in schizophrenia. Brain Res. 2012;1470:145–158. [DOI] [PubMed] [Google Scholar]
- 61. Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. [DOI] [PubMed] [Google Scholar]
- 62. Guo C, Liu Y, Fang MS, et al. ω-3PUFAs improve cognitive impairments through Ser133 phosphorylation of CREB upregulating BDNF/TrkB signal in schizophrenia. Neurotherapeutics. 2020;17(3):1271–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20(4):709–726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.