Abstract
Psychotic major depression (PMD) is hypothesized to be a distinct clinical entity from nonpsychotic major depression (NPMD). However, neurobiological evidence supporting this notion is scarce. The aim of this study is to identify gray matter volume (GMV) differences between PMD and NPMD and their longitudinal change following electroconvulsive therapy (ECT). Structural magnetic resonance imaging (MRI) data from 8 independent sites in the Global ECT-MRI Research Collaboration (GEMRIC) database (n = 108; 56 PMD and 52 NPMD; mean age 71.7 in PMD and 70.2 in NPMD) were analyzed. All participants underwent MRI before and after ECT. First, cross-sectional whole-brain voxel-wise GMV comparisons between PMD and NPMD were conducted at both time points. Second, in a flexible factorial model, a main effect of time and a group-by-time interaction were examined to identify longitudinal effects of ECT on GMV and longitudinal differential effects of ECT between PMD and NPMD, respectively. Compared with NPMD, PMD showed lower GMV in the prefrontal, temporal and parietal cortex before ECT; PMD showed lower GMV in the medial prefrontal cortex (MPFC) after ECT. Although there was a significant main effect of time on GMV in several brain regions in both PMD and NPMD, there was no significant group-by-time interaction. Lower GMV in the MPFC was consistently identified in PMD, suggesting this may be a trait-like neural substrate of PMD. Longitudinal effect of ECT on GMV may not explain superior ECT response in PMD, and further investigation is needed.
Keywords: psychosis, depression, magnetic resonance imaging, gray matter volume, medial prefrontal cortex
Introduction
The current diagnostic category of major depressive disorder (MDD) has been extensively criticized for the clinical and biological heterogeneity within the same diagnosis.1,2 Many efforts have been made to develop clinically and biologically plausible subgroups within the broad concept of MDD. Historically, clinicians and researchers have proposed that MDD with psychotic features (psychotic major depression [PMD]) is a distinct clinical entity from nonpsychotic major depression (NPMD).3–5 PMD is not a rare condition,6,7 and its prevalence increases across the lifespan with higher rates in late-life depression (eg, up to 45% in older hospitalized patients).6,8 Compared with NPMD, PMD has been associated with more severe cognitive impairment,9 higher recurrence rate,10 higher suicide risk,7,11 more psychosocial impairment,12 and lower quality of life.13 Moreover, PMD has shown a diagnostic stability over the course of episodes compared to other suggested subtypes (eg, agitated/retarded depression),14 and a stability of the clinical features of delusions and hallucinations over time.5 From a therapeutic point of view, PMD has shown poor response to placebo,15 and antidepressant/antipsychotic monotherapy,16 but favorable response to the combination of antipsychotics and antidepressants,16 and to electroconvulsive therapy (ECT).17–19 Notably, ECT is particularly effective in late-life PMD,20 and ECT is considered a first-line treatment for PMD in several clinical guidelines.21 Compared to this clinical and therapeutic evidence that supports the notion of PMD as a distinct clinical entity, little is known about its underlying neurobiology.
Previous structural MRI (magnetic resonance imaging) studies that conducted a direct comparison between PMD and NPMD reported inconsistent results: smaller prefrontal cortex,22–24 smaller brain stem,23 larger lateral and third ventricle,23 larger posterior sulcus,25 and thinner cortex in multiple structural networks26 in PMD compared with NPMD, whereas others did not find any differences.27–29 These inconsistencies may be due to the methodological differences, including a variety of magnetic field strengths (eg, from 0.5T to 3T), limited sample size (eg, the average number of PMD patients in previous studies was 19.8), limited brain regions investigated using regions of interests (ROIs), or age difference across studies (supplementary table 1; supplementary material).
Gray matter volume (GMV) increase, especially in the hippocampus and amygdala, has been consistently reported following ECT.30,31 Recent evidence has revealed the effect of ECT was prominent in one of the most neuroplastic brain regions (ie, dentate gyrus in the hippocampus),32,33 which is in line with neuroplastic hypothesis of ECT action.34 Several factors, including age,30 the number of ECT,31 induced electric field,35 or induced seizure duration,36 have been associated with this GMV increase following ECT. However, an effect of clinical heterogeneity (eg, PMD or NPMD) on the GMV increase following ECT has not been investigated. In addition, previous studies have revealed larger GMV increase in patients who remitted after ECT compared to those who did not remit,32,37 although the relationship between GMV increase following ECT and clinical outcome is still on debate.30,31 Since PMD is known to show superior ECT response,20 we hypothesized that PMD may show larger neuroplastic changes following ECT, and that this could be related to superior ECT response compared to NPMD.
The Global ECT-MRI Research Collaboration (GEMRIC) is a multisite consortium collecting clinical and neuroimaging data from patients who received ECT.31 Neuroimaging data are acquired longitudinally (eg, before and after ECT), which provides a unique opportunity to make assumptions about the underlying neurobiology of PMD and its stability during the treatment course. Furthermore, we have an opportunity to examine neurobiological characteristics that could explain the differences in the clinical outcome of ECT between PMD and NPMD.17–20 In addition, studying the extreme clinical subset of depressed patients (eg, those who needed ECT) may reduce etiological heterogeneity and increase statistical power.38,39
The primary aim of the current study was to identify regional GMV differences between PMD and NPMD in cross-sectional whole-brain voxel-wise comparisons at two time points (ie, before and after ECT). The secondary aim was to identify longitudinal differential effects of ECT on GMV between PMD and NPMD, which could explain the superior ECT response in PMD reported in the literature.
Methods
Participants
All GEMRIC sites contributing data received approval from their local ethics committees or institutional review board, and the centralized mega-analysis was approved by the Regional Ethics Committee South-East in Norway (2018/769). Written informed consent was obtained from all participants.
In the current study, we first selected 8 GEMRIC sites collecting 3T MRI data from both PMD and NPMD to reduce between-group scanner variability. The first author (A.T.) investigated the inclusion criteria in each site from already published papers19,32,40–45 and contacted each site’s principal investigator or GEMRIC members to confirm the inclusion of both psychotic and nonpsychotic depressed patients in each site. We included subjects aged 60 years or older (ie, late-life depression) with a diagnosis of MDD with/without psychotic features. Individuals with another major psychiatric disorder (eg, bipolar disorder, schizoaffective disorder, or schizophrenia), those with neurological disorders (eg, Parkinson’s disease, dementia), those with active physical illness, those who had contraindications to MRI, or those who received ECT at least within the past 3 months were excluded. In all but 2 sites, concurrent psychotropic medications were kept during a course of ECT.
Clinical characteristics (eg, age, sex, depression severity, and concurrent psychotropic medications) and high-resolution T1-weighted images were used for the current analyses. Depression severity was assessed by Montgomery-Asberg Depression Rating Scale (MADRS) and/or 17-item Hamilton Depression Rating Scale (HAM-D). MADRS total scores were converted to HAM-D by applying a validated equation.46 Response was defined as a decrease of 50% or more, and remission was defined as HAM-D total scores ≤7.
MRI Acquisition and Image Processing
Structural MRI data from 8 independent sites in the GEMRIC database were analyzed. High-resolution T1-weighted images were acquired before (TP1) and after (TP2) ECT in all sites. Imaging parameters at each site were provided in supplementary table 2. All images were processed using the default pipeline of the Computational Anatomy Toolbox (CAT12, http://dbm.neuro.uni-jena.de/cat/), a toolbox for Statistical Parametric Mapping software (SPM12, http://www.fil.ion.ucl.ac.uk/spm). Preprocessing included bias-correction, segmentation into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF), spatial normalization into MNI space using the DARTEL algorithm, and modulation. The isotropic voxel size of the resultant images was 1.5 mm. The CAT12 longitudinal preprocessing pipeline including additional within-subject registration and bias-correction before segmentation was used when investigating the longitudinal effect of ECT on GMV. After preprocessing, a quality check was conducted by using the “Check sample homogeneity” module implemented in CAT12, which offers a straightforward visualization of the correlation and the Mahalanobis distance between the volumes using a boxplot and correlation matrix, to identify outliers. All outliers were manually checked by 4 authors (A.T., W.B., M.C., and G.W.) and low-quality images from 3 participants were excluded in this study. Then images were smoothed with 10-mm full-width at half maximum (FWHM) Gaussian kernel. Total intracranial volume (TIV) was calculated using CAT12.
Statistical Analysis
Descriptive statistics were used to characterize the study participants. Distributions of all variables were inspected using histograms and Shapiro-Wilk tests. A whole-brain GMV analysis was conducted using SPM12. Only voxel values greater than 0.2 were analyzed using absolute threshold masking option in SPM.
First, a cross-sectional whole-brain voxel-wise GMV comparison between PMD and NPMD at TP1 was conducted. Age, sex, TIV, site, and HAM-D total scores were included as covariates. HAM-D total scores were included in the statistical model to identify regional GMV differences which relate to PMD independent of the severity of depression. Threshold-free cluster enhancement (TFCE) was used for multiple comparison corrections,47 which was demonstrated as a sensitive and stable method for a group-level analysis of voxel-based morphometry data.48 The number of permutations was set to 20 000. The statistical significance threshold was set at family-wise error (FWE)-corrected P < .05. The voxel-wise group comparisons were conducted including antidepressants, antipsychotics, and lithium use as additional covariates (categorical variable: yes = 1; no = 0) to account for the potential effects of medications. The same voxel-wise GMV comparison between PMD and NPMD was conducted using data at TP2 to investigate whether the regional GMV differences at TP1 would be also observed at TP2.
Second, we created a flexible factorial design that included a between-subject factor group (PMD vs NPMD) and a within-subject factor time (TP1 and TP2) to investigate whether the longitudinal effect of ECT on GMV would differ between PMD and NPMD. A group-by-time interaction was examined to identify the longitudinal differential effects of ECT on GMV between groups. A main effect of time was also conducted to replicate and confirm the already reported ECT-related GMV increase in widely distributed brain regions regardless of the presence of psychosis.31 In addition, paired t tests were also conducted to investigate the longitudinal effects of ECT on GMV in PMD and NPMD separately. The statistical significance threshold was set at voxel-level FWE-corrected P < .05.
Results
Clinical demographics of the participants are presented in table 1. Of the 108 patients with MDD, 56 patients were PMD, and 52 patients were NPMD. There were no significant differences in age (P = .42) nor gender distribution (P = .76) between the 2 groups. PMD showed higher HAM-D total scores than NPMD before ECT (PMD: 30.5 ± 8.3; NPMD: 24.6 ± 5.2; P = .001). PMD showed higher response rate than NPMD (92.8% vs 80.8%), although this difference did not reach statistical significance, and showed similar remission rate as NPMD (73.2% vs 71.2%).
Table 1.
Clinical Characteristics of Participants
| PMD | NPMD | P value* | |
|---|---|---|---|
| Number | 56 | 52 | |
| Age, years | .42 | ||
| Mean (SD) | 71.7 (7.7) | 70.2 (6.7) | |
| Median (IQR) | 70.0 (65.8–77.0) | 69.0 (65.0–75.3) | |
| Female | 35 | 32 | .76 |
| (62.5%) | (65.4%) | ||
| HAM-D before ECT | .001 | ||
| Mean (SD) | 30.5 (8.3) | 24.6 (5.2) | |
| Median (IQR) | 31.1 (24.2–37.6) | 24.8 (20.6–27.7) | |
| HAM-D after ECT | .38 | ||
| Mean (SD) | 5.1 (6.5) | 6.1 (7.3) | |
| Median (IQR) | 3.0 (1.0–7.9) | 4.0 (0.8–9.0) | |
| %change of HAM-D scores | .17 | ||
| Mean (SD) | 82.4 (22.8) | 74.3 (31.7) | |
| Median (IQR) | 89.3 (75.4–97.5) | 82.7 (67.1–96.8) | |
| Response rate | 51/55 | 42/52 | .10 |
| (92.8%) | (80.8%) | ||
| Remission rate | 41/56 | 37/52 | .81 |
| (73.2%) | (71.2%) | ||
| Number of ECT session | .10 | ||
| Mean (SD) | 12.2 (4.5) | 11.2 (5.0) | |
| Median (IQR) | 12.0 (9.0–14.3) | 10.5 (8.8–13.0) | |
| Electrode placement | .93 | ||
| RUL | 31 (55.4%) | 30 (57.7%) | |
| BT | 15 (26.8%) | 12 (23.1%) | |
| RUL to BT | 10 (17.9%) | 10 (19.2%) | |
| Medications | |||
| Antidepressants | 30 (53.6%) | 33 (63.5%) | .30 |
| Antipsychotics | 22 (39.3%) | 20 (38.5%) | .93 |
| Lithium | 1 (1.8%) | 3 (5.8%) | .30 |
| Benzodiazepine | 10 (17.9%) | 10 (19.2%) | 1.0 |
Abbreviations: BT, bitemporal; HAM-D, Hamilton Depression Rating Scale; IQR, interquartile range; NPMD, nonpsychotic major depression; PMD, psychotic major depression; RUL, right unilateral.
*Differences in participants’ characteristics between groups were examined using independent t test (HAM-D before ECT) or Mann-Whitney U test (age, HAM-D after ECT, %change of HAM-D scores, Number of ECT sessions) for continuous variables, and χ 2 analysis (Response rate, Remission rate, Antidepressants, Antipsychotics) or Fisher exact test (Electrode placement, Lithium) for categorical variables. Each test was selected in accordance with the distribution of each variable.
Cross-Sectional Regional GMV Differences Between PMD and NPMD at Two Time Points
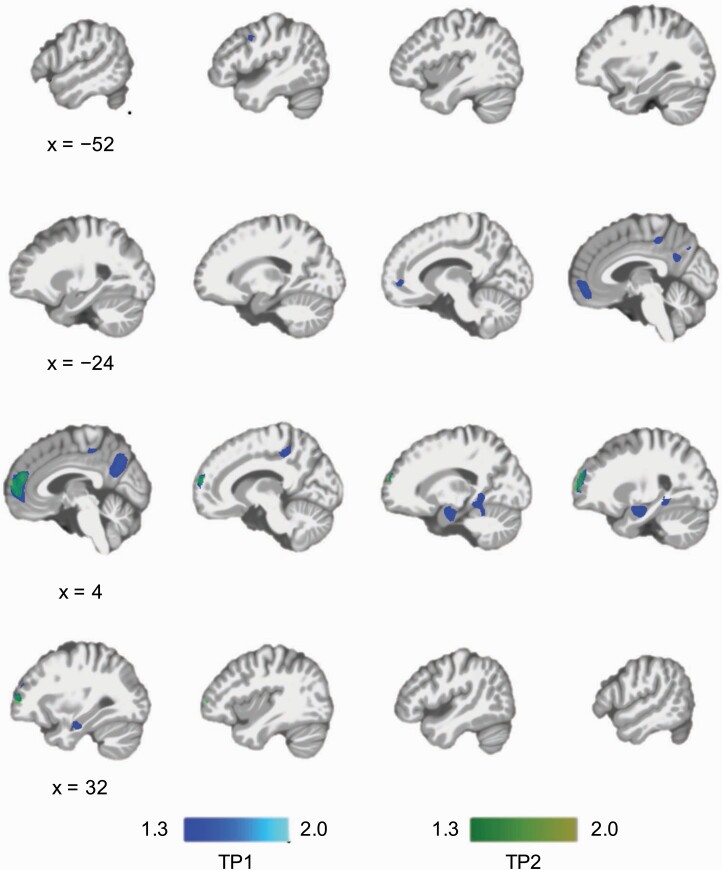
In the cross-sectional whole-brain voxel-wise analysis, PMD showed lower GMV compared with NPMD at TP1 in the medial segment of the superior frontal gyrus (SFG), left middle frontal gyrus (MFG), right amygdala/hippocampus, right lingual gyrus, medial segment of the posterior cingulate gyrus, and praecuneus (figure 1; supplementary table 3). We did not identify brain regions with greater volume in PMD than NPMD. We conducted an additional analysis excluding each site which provided less than 10 subjects to reduce potential site/scanner effects, and found similar results, showing lower GMV in PMD compared to NPMD in the fronto-temporo-parietal brain regions at TP1 (data not shown). PMD showed lower GMV in the right medial prefrontal cortex (MPFC) at TP2 (figure 1; supplementary table 4).
Fig. 1.
Cross-sectional whole-brain voxel-wise GMV comparisons between PMD and NPMD at two time points. PMD showed lower GMV in the left middle frontal gyrus, medial prefrontal cortex (MPFC), praecuneus, right amygdala/hippocampus, and right lingual gyrus at TP1 (before ECT). PMD showed lower GMV in the MPFC at TP2 (after ECT). There were no brain regions that were larger in PMD than NPMD. Significance threshold was set at family-wise error-corrected P < .05 determined by threshold-free cluster enhancement. The color bars represent –log(P) (ie, 1.3 is equivalent to “P = .05”). Abbreviations: ECT, electroconvulsive therapy; GMV, gray matter volume; NPMD, nonpsychotic major depression; PMD, psychotic major depression.
After including medication use as additional covariates, a new region (eg, left SFG medial segment) was identified as a significant larger brain region in PMD compared to NPMD, whereas some brain regions in the left hemisphere (eg, left precentral gyrus, left posterior cingulate gyrus, and left MFG) were no longer significant (supplementary figure 1; supplementary table 5). At TP2, PMD showed lower GMV in the right MPFC (supplementary table 6).
Longitudinal Effect of ECT on GMV Between Groups
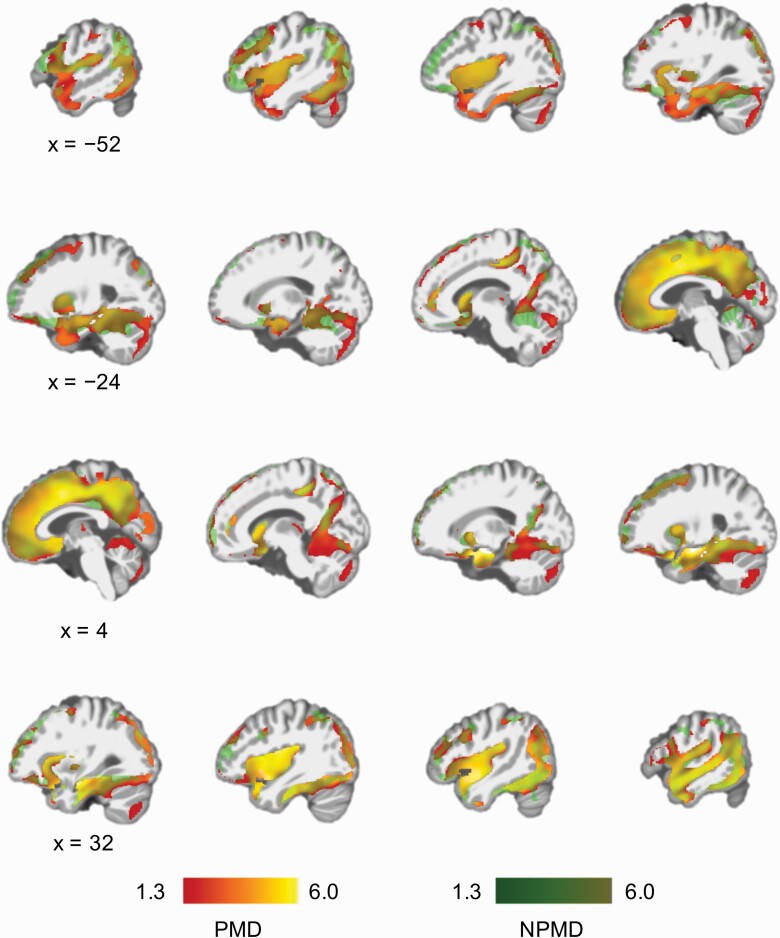
There was no significant group (PMD vs NPMD) by time (TP1 vs TP2) interaction, whereas there was a significant effect of time on GMV in widely distributed brain regions with the largest effect on the right medial temporal lobe (MTL) (supplementary figure 2). The results of the separate paired t tests in PMD and NPMD are presented in figure 2 and show that the main effect of time is present in both groups (supplementary tables 7 and 8). All identified regions showed GMV increase following ECT.
Fig. 2.
Longitudinal effects of ECT on GMV. Results of paired t tests for data at two time points (before and after ECT) in PMD and NPMD. GMV increased in widely distributed brain regions following ECT in both PMD and NPMD. Significance threshold was set at family-wise error-corrected P < .05 determined by threshold-free cluster enhancement. The color bars represent –log(P) (ie, 1.3 is equivalent to “P = .05”). Red represents regional GMV increase following ECT in PMD, and green represents regional GMV increase following ECT in NPMD. Yellow represents overlapped brain regions. Although there seems to be regional GMV increase specific to PMD or NPMD in the figure, there was no significant group-by-time interaction in a flexible factorial model. Abbreviations: ECT, electroconvulsive therapy; GMV, gray matter volume; NPMD, nonpsychotic major depression; PMD, psychotic major depression.
Discussion
To the best of our knowledge, this is the first study to investigate GMV differences between PMD and NPMD at two time points. We found that PMD showed lower GMV in the MPFC compared to NPMD both before and after ECT in this largest cohort ever, suggesting that lower GMV in the MPFC is one of the neural substrates of PMD. We also found that ECT increased GMV in widely distributed brain regions in both PMD and NPMD, but the longitudinal effect of ECT on GMV did not differ between PMD and NPMD.
Lower GMV in the MPFC in PMD
Previous studies suggest cortisol dysfunction in PMD. For example, there are evidence of excessive activity of the hypothalamic-pituitary-adrenal (HPA) axis,49 increased urinary cortisol levels,50 elevated serum adrenocorticotropic hormone,51 increased evening cortisol levels, and high rates of non-suppression of cortisol on the dexamethasone suppression test52 in patients with PMD. Genetic variation of the glucocorticoid receptor and corticotropin-releasing hormone receptor 1 has also been associated with psychotic depression.49 Preclinical studies have reported corticosterone-induced dendritic atrophy in the hippocampus53 and MPFC.54 The relationship between elevated cortisol levels and prefrontal,55 temporal and parietal atrophy56 have been reported in healthy individuals. A meta-analysis reported the relationship between increased cortisol levels and hippocampal atrophy in patients with late-life depression.57 This cortisol-GMV relationship was also reported in subjects at high risk of psychosis,58 and this interaction between cortisol and GMV reduction may be associated with vulnerability to emerging psychosis. Although speculative, the underlying mechanisms of lower prefrontal and hippocampal GMV in PMD might be related to the dysregulated cortisol system. Moreover, stress and/or glucocorticoids increase dopamine activity in the MPFC,59 which may be associated with psychotic symptoms. Future studies should focus on the complex relationships among cortisol levels, dopamine, GMV reduction, and psychotic symptoms in PMD to elucidate the underlying biology of affective psychosis.
Prefrontal Cortex and Psychosis
The development of delusion is hypothesized to be related to the content of delusional belief and the failure to reject the delusional belief despite all the evidence against it.60 The former can be different across individual patients or across psychotic disorders. For instance, delusion of guilt was more frequent in PMD compared to schizophrenia or other psychotic disorders.61 The latter factor, in other words, the dysfunction of the “belief evaluation system” may be common to the development of delusional thinking. In general, the prefrontal cortex is related to the integration of sensory inputs, spatial organization, and coordination of sensory and emotional efference.62 The lateral PFC is considered to have a role in evaluating information from other cortical areas, and rejecting those interpretations that are unreal.61 The medial PFC is associated with self-reflection, past or future events, meta-cognition, and hypothetical scenarios.63,64 We found lower GMV in the medial and lateral PFC in PMD, suggesting that these prefrontal GMV reductions may be associated with dysfunction of the belief evaluation system and with the onset of affective psychosis. Prefrontal GMV reduction has been reported in patients with schizophrenia,65 patients with bipolar disorder with delusion,66 and patients with bipolar disorder with a history of psychosis.67 Our finding of the prefrontal GMV reduction in PMD compared with NPMD may be a common neural substrate of psychosis across different disorders. Considering the differences in clinical course5,10,12–14 and the treatment strategy between PMD and NPMD21 as well as our results showing different neurobiology between PMD and NPMD, PMD could be considered as a different clinical entity from NPMD.
Default Mode Network and PMD
In addition to the MPFC, PMD showed lower GMV in the praecuneus; our results could be interpreted as lower GMV in the critical hubs of the default mode network (DMN) in PMD. The DMN is associated with self-referential thinking,68 autobiographical memory retrieval,69,70 and depressive rumination.71 Notably, the frequency of ruminative thinking was higher in PMD than NPMD.3 An abnormal DMN-related functional connectivity pattern has been reported in PMD during a remitted state compared to healthy subjects.72 Our results provide evidence that structural abnormality of the DMN regions may be related to PMD in addition to functional abnormality reported so far. However, functional abnormality of the DMN has also been reported in depression regardless of the presence of psychosis.71 A previous fMRI study did not find DMN abnormalities but found abnormal functional connectivity of the frontoparietal network compared to NPMD, although this was not replicated in another cohort.73 Further study is needed to investigate how structural abnormality of the DMN would correlate with functional abnormality and its specificity to PMD.
ECT for PMD
ECT is a recommended treatment for PMD,21 and superior ECT response in PMD compared with NPMD has been consistently reported.17–20 In this study, there was no significant group-by-time interaction in the longitudinal GMV analysis; the longitudinal effect of ECT on GMV did not differ between PMD and NPMD. Our result suggests that GMV change with ECT may not explain PMD’s superior ECT response reported in the literature. One interpretation is that ECT-related GMV change may not be related to clinical improvement as previously shown in the meta-30 and mega-analysis.31 Future investigations would benefit from multi-modal data analysis to elucidate the underlying neural mechanisms relating to superior response to ECT in PMD. Another explanation for our result might be related to older participants in this study. Older age is associated with superior ECT response,20 and indeed, 80.8% of NPMD in this study met the response criteria. There was no statistically significant difference in response nor remission rate between PMD and NPMD in our cohort, although PMD showed numerically higher response rate (eg, 92.8% vs 80.8%). Since HAM-D does not account for detailed symptom domains in PMD, the more suitable rating scale, such as the Psychotic Depression Assessment Scale,74 should be used in future studies.
Strength and Limitations
First, our study design and participants should be emphasized. Because the GEMRIC has collected clinical and MRI data from depressed patients who received ECT, we had this unique opportunity to investigate the GMV differences between PMD and NPMD at two time points and to investigate the longitudinal effect of ECT on GMV in this largest cohort ever. By this design, we provide evidence of a GMV difference in the MPFC between PMD and NPMD which was stable during a course of ECT, although ECT has a widespread effect on the GMV. Moreover, this is the largest multisite study to conduct a direct comparison between PMD and NPMD (supplementary table 1). Because of the multisite nature, there might be a scanner effect and/or unpredictable confounders between sites. However, we selected the sites which included both PMD and NPMD from the GEMRIC database to reduce the effect of scanner differences when comparing 2 groups, and we included site information as a covariate. Second, because all patients in our cohort were referred to ECT, the participants in this study may be more homogeneous than other studies regarding the clinical characteristics (eg, severity and treatment resistance) and regarding the etiology of depression.39 In addition, we focused on late-life depression to reduce age-related clinical and biological variability, which could lead to a more homogeneous cohort. In contrast, this limited inclusion could limit the generalizability of our results. However, our cohort is not uncommon because PMD is prevalent in hospitalized patients with late-life depression,8 and ECT is a recommended treatment for PMD in several guidelines.21 Moreover, focusing on extreme subset of the clinical spectrum is one reasonable approach to uncover the underlying neurobiology of depression.39 Third, PMD showed higher symptom severity than NPMD at TP1, which might affect GMV at baseline. However, we included HAM-D total scores as a covariate in the statistical model, and GMV reduction in the MPFC was also identified at TP2, when the differences in symptom severity disappeared. Fourth, although we had not collected uniform longitudinal cognitive assessments, there is a possibility that our results of the lower GMV in PMD might be associated with cognitive dysfunction. We now collect prospectively uniform cognitive assessments in the GEMRIC, and we can investigate differences in cognitive function between PMD and NPMD, and their association with GMV and/or longitudinal changes during ECT course in future study. Fifth, although we accounted for the effect of medication on brain volume, we only included it as a binary variable in our statistical models. The cumulative effect of antipsychotic medication75 might affect brain volumes and it should be investigated in future studies.
In conclusion, lower GMV in the MPFC was consistently identified in PMD both before and after ECT, suggesting this may be one of the trait-like neural substrates of PMD. The longitudinal effect of ECT on brain structure did not differ between PMD and NPMD, suggesting GMV changes following ECT may not explain superior ECT response in PMD. Future research should focus on brain functional changes to elucidate the underlying neural mechanisms of ECT action.
Supplementary Material
Conflict of Interest
The authors report no conflict of interest.
Funding
This work was supported by Keio University Medical Science Fund (A.T., grant number 99-095-0007), AMED (A.T., grant number JP20dm0307102h0003), Research Foundation Flanders (FWO) grant (L.E., F.B., M.V., grant number G0C0319N), KU Leuven Fund (L.M., F.B., M.V., grant number C24/18/095), the Sequoia Fund for Research on Ageing and Mental Health (M.V.), National Institute of Health (C.A., grant number MH125126, MH111826), Sara Borrell postdoctoral fellowships from the Carlos III Health Institute (M.C., grant number CD20/00189), Lundbeck Foundation (M.B.J., A.J., O.B.P.), and Western Norway Regional Health Authority (L.O., grant number 911986 and 912238).
Author Contribution
L.O. and H.B. conducted consortium coordination. A.T. wrote the first draft and coordinated the work. A.T., M.C., W.B., and G.V.W. analyzed data. A.T. and T.K. interpreted the data. A.T. wrote the final manuscript draft. All authors contributed data, as well as critical revision of the manuscript. All authors approved the final manuscript.
References
- 1. Ostergaard SD, Jensen SO, Bech P. The heterogeneity of the depressive syndrome: when numbers get serious. Acta Psychiatr Scand. 2011;124(6):495–496. [DOI] [PubMed] [Google Scholar]
- 2. Lynch CJ, Gunning FM, Liston C. Causes and consequences of diagnostic heterogeneity in depression: paths to discovering novel biological depression subtypes. Biol Psychiatry. 2020;88(1):83–94. [DOI] [PubMed] [Google Scholar]
- 3. Charney DS, Nelson JC. Delusional and nondelusional unipolar depression: further evidence for distinct subtypes. Am J Psychiatry. 1981;138(3):328–333. [DOI] [PubMed] [Google Scholar]
- 4. Schatzberg AF, Rothschild AJ. Psychotic (delusional) major depression: should it be included as a distinct syndrome in DSM-IV? Am J Psychiatry. 1992;149(6):733–745. [DOI] [PubMed] [Google Scholar]
- 5. Nelson JC, Bickford D, Delucchi K, Fiedorowicz JG, Coryell WH. Risk of psychosis in recurrent episodes of psychotic and nonpsychotic major depressive disorder: a systematic review and meta-analysis. Am J Psychiatry. 2018;175(9):897–904. [DOI] [PubMed] [Google Scholar]
- 6. Ohayon MM, Schatzberg AF. Prevalence of depressive episodes with psychotic features in the general population. Am J Psychiatry. 2002;159(11):1855–1861. [DOI] [PubMed] [Google Scholar]
- 7. Dold M, Bartova L, Kautzky A, et al. . Psychotic features in patients with major depressive disorder: a report from the European Group for the Study of Resistant Depression. J Clin Psychiatry. 2019;80:17m12090. [DOI] [PubMed] [Google Scholar]
- 8. Meyers BS, Greenberg R. Late-life delusional depression. J Affect Disord. 1986;11(2):133–137. [DOI] [PubMed] [Google Scholar]
- 9. Gomez RG, Fleming SH, Keller J, et al. . The neuropsychological profile of psychotic major depression and its relation to cortisol. Biol Psychiatry. 2006;60(5):472–478. [DOI] [PubMed] [Google Scholar]
- 10. Goldberg JF, Harrow M. Consistency of remission and outcome in bipolar and unipolar mood disorders: a 10-year prospective follow-up. J Affect Disord. 2004;81(2):123–131. [DOI] [PubMed] [Google Scholar]
- 11. Gournellis R, Tournikioti K, Touloumi G, et al. . Psychotic (delusional) depression and suicidal attempts: a systematic review and meta-analysis. Acta Psychiatr Scand. 2018;137(1):18–29. [DOI] [PubMed] [Google Scholar]
- 12. Coryell W, Leon A, Winokur G, et al. . Importance of psychotic features to long-term course in major depressive disorder. Am J Psychiatry. 1996;153(4):483–489. [DOI] [PubMed] [Google Scholar]
- 13. Cramer V, Torgersen S, Kringlen E. Mood disorders and quality of life. A community study. Nord J Psychiatry. 2010;64(1):58–62. [DOI] [PubMed] [Google Scholar]
- 14. Coryell W, Winokur G, Shea T, Maser JD, Endicott J, Akiskal HS. The long-term stability of depressive subtypes. Am J Psychiatry. 1994;151(2):199–204. [DOI] [PubMed] [Google Scholar]
- 15. Spiker DG, Kupfer DJ. Placebo response rates in psychotic and nonpsychotic depression. J Affect Disord. 1988;14(1):21–23. [DOI] [PubMed] [Google Scholar]
- 16. Wijkstra J, Lijmer J, Burger H, Cipiriani A, Geddes J, Nolen WA. Pharmacological treatment for psychotic depression. Cochrane Database Syst Rev. 2015;7:CD004044. [DOI] [PubMed] [Google Scholar]
- 17. Petrides G, Fink M, Husain MM, et al. . ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17(4):244–253. [DOI] [PubMed] [Google Scholar]
- 18. Birkenhäger TK, Pluijms EM, Lucius SA. ECT response in delusional versus non-delusional depressed inpatients. J Affect Disord. 2003;74(2):191–195. [DOI] [PubMed] [Google Scholar]
- 19. Dols A, Bouckaert F, Sienaert P, et al. . Early- and late-onset depression in late life: a prospective study on clinical and structural brain characteristics and response to electroconvulsive therapy. Am J Geriatr Psychiatry. 2017;25(2):178–189. [DOI] [PubMed] [Google Scholar]
- 20. van Diermen L, van den Ameele S, Kamperman AM, et al. . Prediction of electroconvulsive therapy response and remission in major depression: meta-analysis. Br J Psychiatry. 2018;212(2):71–80. [DOI] [PubMed] [Google Scholar]
- 21. Leadholm AK, Rothschild AJ, Nolen WA, Bech P, Munk-Jørgensen P, Ostergaard SD. The treatment of psychotic depression: is there consensus among guidelines and psychiatrists? J Affect Disord. 2013;145(2):214–220. [DOI] [PubMed] [Google Scholar]
- 22. Kim DK, Kim BL, Sohn SE, et al. . Candidate neuroanatomic substrates of psychosis in old-aged depression. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(5):793–807. [DOI] [PubMed] [Google Scholar]
- 23. Simpson S, Baldwin RC, Jackson A, Burns A. The differentiation of DSM-III-R psychotic depression in later life from nonpsychotic depression: comparisons of brain changes measured by multispectral analysis of magnetic resonance brain images, neuropsychological findings, and clinical features. Biol Psychiatry. 1999;45(2):193–204. [DOI] [PubMed] [Google Scholar]
- 24. Oudega ML, van Exel E, Stek ML, et al. . The structure of the geriatric depressed brain and response to electroconvulsive therapy. Psychiatry Res. 2014;222(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 25. Salokangas RKR, Cannon T, Van Erp T, et al. . Structural magnetic resonance imaging in patients with first-episode schizophrenia, psychotic and severe non-psychotic depression and healthy control: results of the Schizophrenia and Affective Psychoses (SAP) projects. Br J Psychiatry. 2002;181:s58–s65. [DOI] [PubMed] [Google Scholar]
- 26. Neufeld NH, Kaczkurkin AN, Sotiras A, et al. . Structural brain networks in remitted psychotic depression. Neuropsychopharmacology. 2020;45(7):1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keller J, Shen L, Gomez RG, et al. . Hippocampal and amygdalar volumes in psychotic and nonpsychotic unipolar depression. Am J Psychiatry. 2008;165(7):872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vassilopoulou K, Papathanasiou M, Michopoulos I, et al. . A magnetic resonance imaging study of hippocampal, amygdala and subgenual prefrontal cortex volumes in major depression subtypes: melancholic versus psychotic depression. J Affect Disord. 2013;146(2):197–204. [DOI] [PubMed] [Google Scholar]
- 29. Cohen JD, Nichols T, Keller J, Gomez RG, Schatzberg AF, Reiss AL. Insular cortex abnormalities in psychotic major depression: relationship to gender and psychotic symptoms. Neurosci Res. 2013;75(4):331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takamiya A, Chung JK, Liang KC, Graff-Guerrero A, Mimura M, Kishimoto T. Effect of electroconvulsive therapy on hippocampal and amygdala volumes: systematic review and meta-analysis. Br J Psychiatry. 2018;212(1):19–26. [DOI] [PubMed] [Google Scholar]
- 31. Ousdal OT, Argyelan M, Narr KL, et al. ; GEMRIC . Brain changes induced by electroconvulsive therapy are broadly distributed. Biol Psychiatry. 2020;87(5):451–461. [DOI] [PubMed] [Google Scholar]
- 32. Takamiya A, Plitman E, Chung JK, et al. . Acute and long-term effects of electroconvulsive therapy on human dentate gyrus. Neuropsychopharmacology. 2019;44(10):1805–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gryglewski G, Lanzenberger R, Silberbauer LR, et al. . Meta-analysis of brain structural changes after electroconvulsive therapy in depression. Brain Stimul. 2021;14(4):927–937. [DOI] [PubMed] [Google Scholar]
- 34. Bouckaert F, Sienaert P, Obbels J, et al. . ECT: its brain enabling effects: a review of electroconvulsive therapy-induced structural brain plasticity. J ECT. 2014;30(2):143–151. [DOI] [PubMed] [Google Scholar]
- 35. Argyelan M, Oltedal L, Deng ZD, et al. . Electric field causes volumetric changes in the human brain. eLife. 2019;8:e49115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takamiya A, Bouckaert F, Laroy M, et al. . Biophysical mechanisms of electroconvulsive therapy-induced volume expansion in the medial temporal lobe: a longitudinal in vivo human imaging study. Brain Stimul. 2021;14:1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takamiya A, Kishimoto T, Hirano J, Kikuchi T, Yamagata B, Mimura M. Association of electroconvulsive therapy-induced structural plasticity with clinical remission. Prog Neuropsychopharmacol Biol Psychiatry. 2021;110:110286. [DOI] [PubMed] [Google Scholar]
- 38. Soda T, McLoughlin DM, Clark SR, et al. . International Consortium on the Genetics of Electroconvulsive Therapy and Severe Depressive Disorders (Gen-ECT-ic). Eur Arch Psychiatry Clin Neurosci. 2020;270(7):921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clements CC, Karlsson R, Lu Y, et al. . Genome-wide association study of patients with a severe major depressive episode treated with electroconvulsive therapy. Mol Psychiatry. 2021;26(6):2429–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nordanskog P, Dahlstrand U, Larsson MR, Larsson EM, Knutsson L, Johanson A. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J ECT. 2010;26(1):62–67. [DOI] [PubMed] [Google Scholar]
- 41. Abbott CC, Jones T, Lemke NT, et al. . Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl Psychiatry. 2014;4:e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jorgensen A, Magnusson P, Hanson LG, et al. . Regional brain volumes, diffusivity, and metabolite changes after electroconvulsive therapy for severe depression. Acta Psychiatr Scand. 2016;133(2):154–164. [DOI] [PubMed] [Google Scholar]
- 43. Bouckaert F, Dols A, Emsell L, et al. . Relationship between hippocampal volume, serum BDNF, and depression severity following electroconvulsive therapy in late-life depression. Neuropsychopharmacology. 2016;41(11):2741–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cano M, Martínez-Zalacaín I, Bernabéu-Sanz Á, et al. . Brain volumetric and metabolic correlates of electroconvulsive therapy for treatment-resistant depression: a longitudinal neuroimaging study. Transl Psychiatry. 2017;7(2):e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yrondi A, Nemmi F, Billoux S, et al. . Grey matter changes in treatment-resistant depression during electroconvulsive therapy. J Affect Disord. 2019;258:42–49. [DOI] [PubMed] [Google Scholar]
- 46. Heo M, Murphy CF, Meyers BS. Relationship between the Hamilton Depression Rating Scale and the Montgomery-Asberg Depression Rating Scale in depressed elderly: a meta-analysis. Am J Geriatr Psychiatry. 2007;15(10):899–905. [DOI] [PubMed] [Google Scholar]
- 47. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. [DOI] [PubMed] [Google Scholar]
- 48. Li H, Nickerson LD, Nichols TE, Gao JH. Comparison of a non-stationary voxelation-corrected cluster-size test with TFCE for group-level MRI inference. Hum Brain Mapp. 2017;38(3):1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schatzberg AF, Keller J, Tennakoon L, et al. . HPA axis genetic variation, cortisol and psychosis in major depression. Mol Psychiatry. 2014;19(2):220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anton RF. Urinary free cortisol in psychotic depression. Biol Psychiatry. 1987;22(1):24–34. [DOI] [PubMed] [Google Scholar]
- 51. Posener JA, DeBattista C, Williams GH, Chmura Kraemer H, Kalehzan BM, Schatzberg AF. 24-Hour monitoring of cortisol and corticotropin secretion in psychotic and nonpsychotic major depression. Arch Gen Psychiatry. 2000;57(8):755–760. [DOI] [PubMed] [Google Scholar]
- 52. Nelson JC, Davis JM. DST studies in psychotic depression: a meta-analysis. Am J Psychiatry. 1997;154(11):1497–1503. [DOI] [PubMed] [Google Scholar]
- 53. Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531(1–2):225–231. [DOI] [PubMed] [Google Scholar]
- 54. Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49(3):245–253. [DOI] [PubMed] [Google Scholar]
- 55. Stomby A, Boraxbekk CJ, Lundquist A, et al. . Higher diurnal salivary cortisol levels are related to smaller prefrontal cortex surface area in elderly men and women. Eur J Endocrinol. 2016;175(2):117–126. [DOI] [PubMed] [Google Scholar]
- 56. Lebedeva A, Sundström A, Lindgren L, et al. . Longitudinal relationships among depressive symptoms, cortisol, and brain atrophy in the neocortex and the hippocampus. Acta Psychiatr Scand. 2018;137(6):491–502. [DOI] [PubMed] [Google Scholar]
- 57. Geerlings MI, Gerritsen L. Late-life depression, hippocampal volumes, and hypothalamic-pituitary-adrenal axis regulation: a systematic review and meta-analysis. Biol Psychiatry. 2017;82(5):339–350. [DOI] [PubMed] [Google Scholar]
- 58. Valli I, Crossley NA, Day F, et al. . HPA-axis function and grey matter volume reductions: imaging the diathesis-stress model in individuals at ultra-high risk of psychosis. Transl Psychiatry. 2016;6:e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vaessen T, Hernaus D, Myin-Germeys I, van Amelsvoort T. The dopaminergic response to acute stress in health and psychopathology: a systematic review. Neurosci Biobehav Rev. 2015;56:241–251. [DOI] [PubMed] [Google Scholar]
- 60. Coltheart M. The neuropsychology of delusions. Ann N Y Acad Sci. 2010;1191:16–26. [DOI] [PubMed] [Google Scholar]
- 61. Picardi A, Fonzi L, Pallagrosi M, Gigantesco A, Biondi M. Delusional themes across affective and non-affective psychoses. Front Psychiatry. 2018;9:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- 63. Nejad AB, Fossati P, Lemogne C. Self-referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci. 2013;7:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fleming SM. Relating introspective accuracy to individual differences in brain structure. Science. 2012;336:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fornito A, Yücel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108(1–3):104–113. [DOI] [PubMed] [Google Scholar]
- 66. Radaelli D, Poletti S, Gorni I, et al. . Neural correlates of delusion in bipolar depression. Psychiatry Res. 2014;221(1):1–5. [DOI] [PubMed] [Google Scholar]
- 67. Ekman CJ, Petrovic P, Johansson AG, Sellgren C, Ingvar M, Landén M. A history of psychosis in bipolar disorder is associated with gray matter volume reduction. Schizophr Bull. 2017;43(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim H. A dual-subsystem model of the brain’s default network: self-referential processing, memory retrieval processes, and autobiographical memory retrieval. Neuroimage. 2012;61(4):966–977. [DOI] [PubMed] [Google Scholar]
- 70. Zhu X, Wang X, Xiao J, et al. . Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biol Psychiatry. 2012;71(7):611–617. [DOI] [PubMed] [Google Scholar]
- 71. Hamilton JP, Farmer M, Fogelman P, Gotlib IH. Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol Psychiatry. 2015;78(4):224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Neufeld NH, Mulsant BH, Dickie EW, et al. . Resting state functional connectivity in patients with remitted psychotic depression: a multi-centre STOP-PD study. EBioMedicine. 2018;36:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Oudega ML, van der Werf YD, Dols A, et al. . Exploring resting state connectivity in patients with psychotic depression. PLoS One. 2019;14(1):e0209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Østergaard SD, Rothschild AJ, Flint AJ, et al. . Rating scales measuring the severity of psychotic depression. Acta Psychiatr Scand. 2015;132(5):335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chopra S, Fornito A, Francey SM, et al. . Differentiating the effect of antipsychotic medication and illness on brain volume reductions in first-episode psychosis: a longitudinal, randomised, triple-blind, placebo-controlled MRI study. Neuropsychopharmacology. 2021;46(8):1494–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.