Abstract
Objective
To quantify the risk and predictors of relapse among individuals with schizophrenia randomly withdrawn from antipsychotic maintenance treatment.
Methods
We re-analyzed time-to-event and baseline predictors from placebo arms in five placebo-controlled randomized trials of antipsychotics (n = 688 individuals; 173 stabilized on oral antipsychotic [OAP] and 515 on long-acting injectables [LAI]) for relapse-prevention available in the Yale Open Data Access repository. Using a survival and Cox-proportional hazards regression analyses, we estimated survival rates of “relapse-free” individuals by the end of follow-up (median = 118 days, IQR = 52.0–208.0), the rate of study-confirmed relapse, and adjusted hazard ratios (aHR, 95% confidence intervals [CI]) associated with baseline predictors. We also estimated these parameters for individuals followed for >5 half-lives of the stabilizing antipsychotic, and studied predictors of “rebound psychosis” in OAP-stabilized participants, defined as occurring within 30 days of antipsychotic withdrawal.
Results
29.9% (95%CI = 23.2–38.5) remained relapse-free by the end of follow-up, 11.1% (95%CI = 5.65–21.9) among those OAP-stabilized, 36.4% (95%CI = 28.4–46.7) among those LAI-stabilized. The study-confirmed relapse rate was 45.2%, 62.4% among those OAP-stabilized and 39.4% among those LAI-stabilized. Predictors of relapse included smoking (aHR = 1.54, 95%CI = 1.19–2.00), female sex (aHR = 1.37, 95%CI = 1.08–1.79), and having been stabilized on OAPs vs LAIs (aHR = 3.56, 95%CI = 2.68–4.72). Greater risk of relapse on OAP persisted even after sufficient time had elapsed to clear antipsychotic plasma level among LAI-stabilized (aHR = 5.0, 95%CI = 3.5–7.1). “Rebound psychosis” did not show predictors.
Conclusions and relevance
Our results corroborate the high relapse risk following antipsychotic withdrawal after symptom stabilization with limited patient-related predictors of safe treatment discontinuation. Stabilization with LAIs reduces the short-/medium-term relapse risk.
Keywords: schizophrenia, relapse, antipsychotics, individual participant data, long-acting injectables, withdrawal
Introduction
Schizophrenia is often characterized by multiple relapses.1 Each relapse interferes with recovery and is associated with personal and societal costs, and bears potential danger to self or others.2–5 Maintenance antipsychotic treatment has demonstrated efficacy in preventing relapse,6,7 decreasing morbidity and potentially mortality,8 but it is most often discontinued over time.9,10 Descriptive studies have shown that a minority of individuals may safely discontinue long-term antipsychotic treatment,11–13 however to date it is not well understood whether there are predictors to identify patients with low relapse risk following antipsychotic discontinuation.12,13
Predictors of safe antipsychotic discontinuation have been derived mainly from naturalistic cohorts, which are exposed to selection bias, confounding by indication, and greater attrition of individuals with worse prognosis. For instance, in the OPUS cohort (n = 496), 30% of participants had long-term remission despite antipsychotic discontinuation during 10-year follow-up, with female sex and absent substance abuse predicting remission off of antipsychotics.14 However, the lack of randomization to antipsychotic withdrawal render the results inconclusive. These limitations apply to similar study designs,15,16 resulting in various, mostly non-replicated predictors.17 For example, in 141 symptomatically remitted individuals with first-episode schizophrenia-spectrum disorders, randomized to 18-month open-label dose reduction/attempted antipsychotic discontinuation vs antipsychotic maintenance, 43% relapsed in the dose reduction/attempted antipsychotic discontinuation group vs 21% in the antipsychotic maintenance group (P = 0.011).18 Importantly, only 20% successfully discontinued antipsychotics, and recurrent symptoms caused another 30% to restart antipsychotics, while in the remaining 50% discontinuation was not feasible at all. During uncontrolled follow-up >18 months, relapse rate did not differ anymore at 7 years post randomization,11 but longer duration of untreated psychosis (DUP) predicted relapse in the dose reduction/attempted discontinuation group.19 In another, similarly designed study, only 23 patients (16.2%) successfully discontinued antipsychotics in the final 2 years of the 10-year study. Predictors of successful antipsychotic discontinuation included male sex, fewer schizophrenia-spectrum disorders, DUP <30 days, better baseline cognition, and better social functioning during the first 2 years, but lack of relapse post-baseline was the only significant predictor on a 10-year follow up in multivariable analyses.20 Moreover, beyond one year, individuals could move between the continuation and dose reduction/discontinuation condition depending on treatment response and patient preference, complicating interpretation of the results.
Double-blind, placebo-controlled relapse prevention randomized clinical trials (RCTs) remain the gold standard to minimize selection bias and confounding by indication when studying predictors of relapse after antipsychotic discontinuation, as allocation is randomized and concealed, and dose reduction or discontinuation is not influenced by clinical response or patient preference. In a study randomizing individuals to fluphenazine vs placebo (n = 17), poorer premorbid adjustment predicted relapse on placebo maintenance.21 In another cohort with 10-year outcomes,20 predictors of successful antipsychotic discontinuation during first year of randomized treatment (relapse-free: placebo = 21%, antipsychotic continuation = 59%) included in the placebo group (n = 171) lower blink rate and better cognition.22 A re-analysis of a double-blind, relapse-prevention RCT found that of individuals randomized to placebo (n = 204) 43.5% relapsed (median time-to-relapse = 163 days), predicted by older age and male sex.23 While these studies were less exposed to bias, heterogeneous populations and designs, and small samples prevent drawing definitive conclusions.
Furthermore, questions about the role of treatment-related factors for relapse risk upon antipsychotic discontinuation remain unanswered. For instance, data suggest that longer half-lives of antipsychotics or their formulation delay relapse risk,24,25 either due to longer antipsychotic medication exposure following withdrawal, or due to protective effects lasting beyond antipsychotic plasma clearance. Moreover, the possibility of “rebound psychosis,” ie, relapse briefly following abrupt antipsychotic discontinuation, has remained controversial.
Based on the above, we aimed to generate estimates of patient- and treatment-level relapse predictors after randomized, double-blind antipsychotic discontinuation, in order to overcome selection bias and confounding by indication.
Methods
Data Search and Access
We conducted a search in the Yale Open Data Access (“YODA”) clinical trial data repository by trial drug, first filtering for all trials conducted with antipsychotic medication. After identifying trials of antipsychotic medication, we read the documentation to identify datasets meeting eligibility criteria: (1) Adult participants diagnosed with schizophrenia or schizoaffective disorder, (2) Individuals participated in a double-blind placebo-controlled relapse prevention RCT, were treated with antipsychotics for >3 months, were clinically stabilized and then randomized to placebo; in these studies antipsychotics are discontinued immediately at randomization to placebo, (3) Period of observation >6 months after antipsychotic withdrawal, (4) Patient-level data were available on the primary outcome, psychosis relapse, and on patient and/or treatment level covariates. Identification of eligible datasets was conducted independently by two researchers (GS and JR), disagreements were resolved by consensus and the search was finalized by 11/13/2020. Once potentially eligible studies were identified, access to participant level datasets was requested following the standard procedure described in https://yoda.yale.edu.
Statistical Analyses
We first identified the cohort of interest by selecting individuals allocated to placebo in each one of the eligible relapse-prevention RCTs. Individuals from separate datasets were selected and merged into a single analytic cohort. Individuals off of antipsychotic followed during an open label extension (OLE) phase in any individual trial were included and censored at the end of the double-blind phase of the longest trial (ie, day 480). The rationale for this approach is that given the low rate of participation of individuals off of antipsychotic in any OLE phase, once the double-blind phase with the longest duration ended on day 480, there remained only 4 participants in the cohort past that time, which increased the uncertainty of estimates after that timepoint. Analyses including all observed data (ie, no censoring after day 480) were also provided in the Supplementary material (Supplementary tables 3–4).
Next, we proceeded to identify for each individual in that cohort whether they met study criteria for psychosis relapse, the time to psychosis relapse (or censoring), and the value for clinical covariates measured in each dataset at the time of antipsychotic withdrawal that would later be used as predictors. Covariates were included in our model (Supplementary table 1), as long as they were available in all eligible clinical trials to minimize missing data. We used the respective study definition of psychosis relapse. Using this dataset for the main analyses, we conducted a survival analysis and multivariable Cox regression analysis to generate survival (ie, time to relapse) estimates and adjusted hazard ratios (aHRs) with 95% confidence intervals (95%CIs).
Next, we conducted a secondary analysis, in which we compared the risk of relapse between individuals who were stabilized on oral antipsychotics (OAPs) prior to antipsychotic discontinuation vs those stabilized on long-acting injectable antipsychotics (LAIs). We excluded from the analyses individuals with time to event (ie, censoring or relapse) shorter than 5 half-lives after discontinuation of the antipsychotic that they were stabilized on, to remove the confounder of differences in the duration of residual antipsychotic action following discontinuation, and compared the risk of relapse between those stabilized on OAPs and on LAIs once sufficient time to assure antipsychotic clearance from plasma was confirmed. For oral paliperidone, once monthly and three-month paliperidone palmitate, we used 5, 176, and 365 days, respectively.26 Within this time frame almost 97% of the medication has been eliminated and no residual antipsychotic action is expected. For this model, time to event started counting after the lag allowed to clear the antipsychotic plasma level.
In another secondary analysis, we sought to address whether there is a differential role of clinical risk factors on the potential for “rebound psychosis,” which if identified would suggest rebound psychosis as a phenomenon that is distinct from relapse after longer periods following antipsychotic discontinuation, particularly for oral formulations. To do so, we measured the interaction terms of the covariates by relapse during the “rebound psychosis” period (arbitrarily defined as relapse within 30 days of antipsychotic withdrawal) vs during the time >30 days among those randomized to placebo who has been on an OAP at baseline.
Finally, two authors (JR and GS) conducted a risk of bias assessment for each individual cohort using the “Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies” 27; the choice of NOS was based on treating the included studies as cohort studies given that only the placebo arms were included. The analyses were conducted in R Studio v 1.2.5019. The code was written and double-checked independently by two researchers (JR and GS), and is publicly available in https://github.com/lorente01.
Results
Characteristics of the Study Cohort
We identified data from five placebo arms of relapse-prevention trials in schizophrenia (n = 688), conducted in 18 countries from 03/2004 to 04/2014.28–32 Participating individuals were followed for up to 480 days (median follow up = 118 days (IQR = 52.0–208.0) after antipsychotic withdrawal. The clinical and sociodemographic characteristics of the total cohort are summarized in table 1. Altogether, 515 patients (74.9%) were stabilized on LAIs and 173 (25.1%) were stabilized on OAPs. Patients received paliperidone palmitate and oral paliperidone. The median duration of prospective antipsychotic stabilization prior to discontinuation was 198.0 days (IQR = 121.3–239.0, range = 100–300 days). Risk of bias was deemed low for all except one cohort, with a mean Newcastle-Ottawa Scale score of 5.2 (maximum = 6.0) (Supplementary table 2).
Table 1.
Pooled baseline characteristics in the total study cohort (k = 5)
| Baseline Characteristics | Total cohort (n = 688) | Cohort stabilized on OAP (n = 173) | Cohort stabilized on LAI (n = 515) | p-value* | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Male | 398 | 57.8 | 94 | 54.33 | 304 | 59.0 | 0.29 |
| Region | |||||||
| Europe | 303 | 44.0 | 43 | 24.9 | 260 | 50.5 | <0.001 |
| Asia/Pacific | 193 | 28.0 | 107 | 61.8 | 86 | 16.7 | |
| North-America | 175 | 25.4 | 23 | 13.3 | 152 | 29.5 | |
| Central & South-America | 12 | 1.7 | 0 | 0.0 | 12 | 2.3 | |
| Africa | 5 | 0.7 | 0 | 0.0 | 5 | 1.0 | |
| Race | |||||||
| White | 373 | 54.2 | 61 | 35.3 | 312 | 60.6 | <0.001 |
| Asian | 153 | 22.2 | 71 | 41.0 | 82 | 15.9 | |
| African-American | 106 | 15.4 | 9 | 5.2 | 97 | 18.8 | |
| Other | 56 | 8.1 | 32 | 18.5 | 24 | 4.7 | |
| Smoking | 314 | 45.6 | 54 | 31.2 | 260 | 50.5 | <0.001 |
| At least moderate TD | 2 | 0.3 | 0 | 0.0 | 2 | 0.4 | 1.00 |
| At least moderate akathisia | 14 | 2.0 | 1 | 0.6 | 13 | 2.5 | 0.21 |
| At least moderate EPS | 77 | 11.2 | 23 | 13.3 | 54 | 10.5 | 0.33 |
| Mean | SD | Mean | SD | Mean | SD | ||
| Age | 37.8 | 11.2 | 35.2 | 11.4 | 38.64 | 10.98 | <0.001 |
| CGI-S | 2.7 | 0.7 | 2.7 | 0.71 | 2.70 | 0.72 | 0.67 |
| PANSS Total | 55.6 | 10.9 | 52.5 | 10.3 | 56.67 | 10.93 | <0.001 |
| PANSS General | 28.1 | 5.9 | 26.6 | 5.6 | 28.58 | 5.92 | <0.001 |
| PANSS Positive | 12.0 | 3.5 | 11.0 | 3.2 | 12.29 | 3.54 | <0.001 |
| PANSS Negative | 15.6 | 4.1 | 14.9 | 3.8 | 15.79 | 4.22 | 0.03 |
| PSP | 70.0 | 10.3 | 71.5 | 10.1 | 69.50 | 10.35 | 0.008 |
CGI-S: Clinical Global Impressions Severity score; EPS: Extrapyramidal symptoms; k: number of studies; n: number of individual participants; PANSS: Positive And Negative Syndrome Scale; PSP: Personal and social performance scale; SD: standard deviation; TD: Tardive dyskinesia.
*Fisher’s and Wilcoxon test for categorical and continuous variables respectively.
Risk of Relapse and Predictors
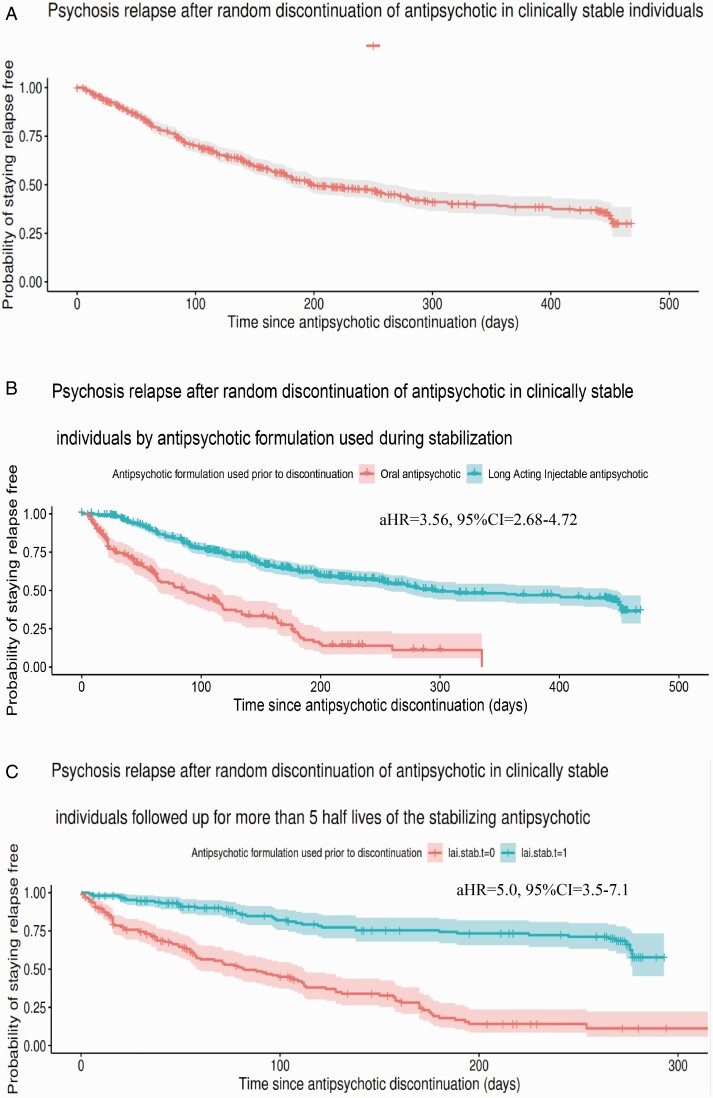
The proportion of individuals who remained relapse free by the end of the 480-day follow-up period was 29.9% (95%CI 23.2–38.5) with a median time to relapse of 199 days (IQR = 180–260) (figure 1a); of individuals stabilized on OAP, 11.1% (95%CI = 5.7–21.9) remained relapse free with a median time to relapse of 87 days (IQR = 64–117), whereas of individuals stabilized on LAI 36.4% (95%CI = 28.4–46.7) remained relapse free with a median time to relapse of 294 days (IQR = 256–444) (figure 1b). Individuals who discontinued OAPs were over three times more likely to relapse during follow-up compared to those who had discontinued an LAI (HR = 3.56, 95%CI = 2.68–4.72). The rate of study-confirmed relapse in the main cohort of 688 individuals was 45.2% (n = 311), 62.4% (n = 108) among the 173 individuals stabilized on OAPs, and 39.4% (n = 203) among the 515 individuals stabilized on LAIs (table 2).
Fig. 1.
Survival curve on time to relapse after antipsychotic withdrawal (a) in the total sample, (b) in antipsychotic formulation subgroups [oral vs long-acting injectable (LAI) antipsychotics], and (c) in a cohort of individuals with time to event (ie, censoring or relapse) longer than 5 half-lives after discontinuation of the antipsychotic. Median time to relapse: 199 days (95%CI = 180–260); relapse free at 488-day (16-month) follow-up: 29.9% (95%CI = 23.2–38.5). Median time to relapse in patients stabilized on oral antipsychotic: 49 days (IQR = 15–107.5 days); stabilized on long-acting antipsychotic: 146 days (IQR = 54–272 days); relapse-free: oral antipsychotic = 11.3% (95%CI = 5.8–22.2); long-acting injectable antipsychotic = 57.7% (95%CI = 45.4%-73.4%). aHR = adjusted hazard ratio, CI: confidence interval, IQR: interquantile range.
Table 2.
Relapse and Median Time to Relapse After Placebo-controlled Antipsychotic Withdrawal
| Total cohort n = 688 |
Stabilized on OAP n = 173 |
Stabilized on LAI n = 515 |
|
|---|---|---|---|
| Proportion of individuals relapse free by the end of follow-up (95%CI) | 29.9% (23.2%-38.5%) | 11.1% (5.6%-21.9%) | 36.4% (28.4%-46.7%) |
| Study confirmed relapse (n) | 311 | 108 | 203 |
| Study confirmed relapse (%) | 45.2 | 62.4 | 39.4 |
| Median time to study confirmed relapse in days (95%CI) | 199 (180–260) |
87 (64–117) |
294 (256–444) |
CI: confidence interval; LAI: long-acting injectable antipsychotic; OAP: oral antipsychotic.
Among the examined covariates, smoking (aHR = 1.54, 95%CI = 1.19–2.00) and female sex (aHR = 1.37, 95%CI = 1.08–1.79) were significantly associated with risk of relapse in the total cohort. In subgroup analyses by antipsychotic formulation used during the pre-discontinuation stabilization period, only nicotine smoking at the time of antipsychotic discontinuation (aHR = 1.88, 95%CI = 1.37–2.59) was associated with greater relapse risk (table 3).
Table 3.
Multivariable Cox Proportional Hazards Regression Analyses on Time to Relapse
| Total cohort (n = 688) | Cohort stabilized on OAP (n = 173) | Cohort stabilized on LAI (n = 519) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moderator Variables | aHR | Lower limit 95% CI | Upper limit 95% CI | aHR | Lower limit 95% CI | Upper limit 95% CI | aHR | Lower limit 95% CI | Upper limit 95% CI | |||
| Female sex | 1.37 | 1.08 | 1.79 | 0.82 | 0.51 | 1.30 | 1.35 | 0.99 | 1.85 | |||
| Age | 0.99 | 0.98 | 1.00 | 0.98 | 0.97 | 1.00 | 0.99 | 0.98 | 1.01 | |||
| Region North America | 0.62 | 0.15 | 2.59 | 1.09 | 0.44 | 2.69 | 0.59 | 0.14 | 2.52 | |||
| Region Asia Pacific | 2.25 | 0.47 | 10.67 | 0.68 | 0.20 | 2.35 | 3.90 | 0.39 | 39.08 | |||
| Region Central and South America | 0.30 | 0.04 | 2.25 | NA | NA | NA | 0.41 | 0.05 | 3.09 | |||
| Region Europe | 0.48 | 0.11 | 2.06 | NA | NA | NA | 0.48 | 0.11 | 2.14 | |||
| Region Africa* | NA | NA | NA | NA | NA | NA | NA | NA | NA | |||
| Race Asian | 0.48 | 0.22 | 1.04 | 0.95 | 0.37 | 2.41 | 0.21 | 0.03 | 1.65 | |||
| Race African | 1.06 | 0.51 | 2.22 | 1.15 | 0.20 | 6.54 | 1.09 | 0.43 | 2.78 | |||
| Race Caucasian | 1.28 | 0.62 | 2.63 | 1.20 | 0.30 | 4.72 | 1.09 | 0.41 | 2.92 | |||
| Race other* | NA | NA | NA | NA | NA | NA | NA | NA | NA | |||
| At least moderate tardive dyskinesia | 0.44 | 0.05 | 3.71 | NA | NA | NA | 0.38 | 0.04 | 3.29 | |||
| At least moderate akathisia | 1.03 | 0.48 | 2.19 | 0.28 | 0.03 | 2.36 | 1.17 | 0.51 | 2.67 | |||
| At least moderate extrapyramidal symptoms | 1.34 | 0.94 | 1.90 | 1.62 | 0.87 | 3.03 | 1.28 | 0.80 | 2.05 | |||
| Clinical Global Impression score | 1.08 | 0.89 | 1.32 | 1.27 | 0.85 | 1.88 | 0.97 | 0.77 | 1.23 | |||
| PANSS General score | 1.01 | 0.99 | 1.04 | 0.96 | 0.91 | 1.01 | 1.04 | 1.00 | 1.08 | |||
| PANSS Negative score | 1.01 | 0.97 | 1.04 | 0.98 | 0.91 | 1.05 | 1.01 | 0.97 | 1.06 | |||
| PANSS Positive score | 0.98 | 0.94 | 1.03 | 1.00 | 0.92 | 1.08 | 1.00 | 0.94 | 1.05 | |||
| PANSS Total score* | NA | NA | NA | NA | NA | NA | NA | NA | NA | |||
| Personal Social Performance scale score | 1.00 | 0.98 | 1.01 | 0.97 | 0.95 | 1.00 | 1.00 | 0.99 | 1.02 | |||
| Nicotine smoking | 1.53 | 1.19 | 1.99 | 1.54 | 1.19 | 2.00 | 0.79 | 0.48 | 1.31 | 1.88 | 1.37 | 2.59 |
aHR: adjusted hazard ratio; CGI-S: clinical global impression-severity; CI: confidence interval; EPS: extrapyramidal symptoms; *Results do not converge in multivariable model.
LAI: long-acting injectable antipsychotic; OAP: oral antipsychotic; PANSS: Positive and Negative Syndrome Scale.
Significant results at P < 0.05 are bolded. “NA” represents unavailable results due to insufficient events for such category.
Risk of Relapse After Sufficient Time to Expect Clearance of Antipsychotics From Plasma
Assessing only individuals who were followed up for sufficient time to expect that the antipsychotic would have been cleared from plasma (ie, >5 half-lives of the respective antipsychotic/formulation) (n = 328), among those who had been on treatment with OAPs only 11.3% (95%CI = 5.8–22.2) were relapse-free by the end of follow-up, with a median time to relapse of 49 days (IQR = 15–107), whereas for those who had been on treatment with LAIs 57.7% (95%CI = 45.4%-73.4%) remained relapse-free by the end of follow-up, with a median time to relapse of 146 days (IQR = 54–272 days) (figure 1c). Comparing time to event between the two groups, the difference was significant (HR = 5.0, 95%CI = 3.45–7.14).
Sensitivity Analysis of “Rebound” Relapse vs Relapse Later During Follow-up
For patients stabilized on OAP, there were no statistically significant interactions between clinical covariates and risk of relapse by the timing of the relapse (ie, before vs after 30 days after antipsychotic discontinuation), other than being older being related to higher relapse risk before 30 days (aHR = 1.07, 95%CI = 1.02–1.13) (table 4).
Table 4.
Interaction of Covariates with Relapse Before vs After day 30 in OAP Stabilized Sample
| Moderator Variables | aHR | Lower limit 95% CI | Upper limit 95% CI |
|---|---|---|---|
| Female sex | 1.47 | 0.56 | 3.89 |
| Age | 1.07 | 1.02 | 1.13 |
| Region North America* | NA | NA | NA |
| Region Asia Pacific* | NA | NA | NA |
| Region Central and South America* | NA | NA | NA |
| Region Europe* | NA | NA | NA |
| Region Africa* | NA | NA | NA |
| Race Asian* | NA | NA | NA |
| Race African* | NA | NA | NA |
| Race Caucasian* | NA | NA | NA |
| Race other* | NA | NA | NA |
| At least moderate tardive dyskinesia* | NA | NA | NA |
| At least moderate akathisia* | NA | NA | NA |
| At least moderate extrapyramidal symptoms | 1.15 | 0.33 | 3.95 |
| Clinical Global Impression score | 2.08 | 0.84 | 5.13 |
| PANSS General score | 0.98 | 0.87 | 1.10 |
| PANSS Negative score | 1.03 | 0.88 | 1.21 |
| PANSS Positive score | 0.87 | 0.71 | 1.06 |
| PANSS Total score* | NA | NA | NA |
| Personal Social Performance scale score | 1.03 | 0.98 | 1.09 |
| Nicotine smoking | 0.54 | 0.19 | 1.57 |
aHR: adjusted hazard ratio; CGI-S: clinical global impression-severity; CI: confidence interval; EPS: extrapyramidal symptoms;
“NA” represents unavailable results due to insufficient events for such category; OAP: oral antipsychotic; PANSS: Positive and Negative Syndrome Scale;
Significant results at P < 0.05 are bolded.
*Results do not converge in multivariable model.
Discussion
We investigated the risk of psychosis relapse among individuals previously stabilized on an antipsychotic for whom the medication had been randomly withdrawn in a double-blind fashion. Using a set of placebo-controlled randomized clinical trials, we maximize sample size while minimizing selection bias, expectation bias and confounding by indication. We estimated that less than one in three remained relapse-free by the end of follow-up over a year later. These findings highlight the high risk of relapse following antipsychotic discontinuation, in our case oral paliperidone and paliperidone palmitate, even in individuals who were symptomatically stable.
The absence of relevant predictors of relapse after antipsychotic discontinuation is consistent with the previous finding of non-replicable risk factors.17 We observed that women were somewhat at greater risk of relapse than men. The role of sex on relapse risk has been unclear, with conflicting results across studies.17 It is possible that the sources of those discrepancies result from not only selection and attrition bias, and confounding by indication, but also from unmeasured covariates not adjusted for in multivariable analyses. Given the important role of sex differences to understand not only the pathophysiological mechanisms, but also to plan interventions, whether sex as a biological variable contributes to the risk of relapse should be studied further. The other covariate that predicted relapse was nicotine smoking, which has been previously associated with risk of relapse after antipsychotic withdrawal33 and even during antipsychotic maintenance treatment.34 Given that use of other drugs of abuse was not registered in the included clinical trials, it is unclear whether the risk associated with nicotine smoking goes above and beyond comorbid substance use, which has been much better characterized as a risk factor of relapse in schizophrenia.35,36
Our results underscore the challenges in identifying for which patients with schizophrenia it is safe to interrupt maintenance treatment with information available in routine clinical practice. In order to be able to identify individuals at low risk of relapse in clinical practice, whatever characteristics are used to make that determination should have substantial sensitivity and specificity in predicting the absence of psychosis relapse. In our study we did not detect significant relapse predictors, highlighting how far we are from being able to predict reliably for whom antipsychotic drugs can be safely withdrawn with clinical information alone. Neurobiological research has provided promising initial findings in the search for prognostic biomarkers for relapse in schizophrenia. In a small sample of individuals with early phase schizophrenia treated with antipsychotics for a year and subsequently withdrawn from treatment, Kim et al. found that striatal dopamine activity differed significantly upon treatment discontinuation between those who would relapse within 12 weeks and those who would not.37 This line of work in biomarker development to adjust maintenance treatment to the patient’s needs is critical to minimize the burden of antipsychotic treatment among those who can safely be discontinued from it. In current clinical practice however, these results call for caution when discussing this strategy with patients. Prescribers should be clear about the inability to specify the prognosis once the antipsychotic is withdrawn, knowing that in the vast proportion of cases relapse will take place over time. In case of a decision to withdraw antipsychotic medication, it should be conducted very slowly, especially for individuals stabilized on OAPs, to minimize large relative decrements in plasma levels as the dose decreases.38 This recommendation to decrease antipsychotic treatment very slowly if this strategy is being pursued is supported by the results of this study, as well as by other research,24 as discontinuation of LAIs can be seen as a model of continual small decrements in antipsychotic levels, both on plasma and, presumably, in brain. These results may also imply that the progressive decrements of antipsychotic concentration could beneficial beyond the drug is cleared from plasma. A potential explanation for the protective effect of more progressive transition to a state in which there is no pharmacological modulation of dopamine D2 receptors is that such progressive changes may mitigate abrupt increases in dopamine synthesis capacity, allowing for a more homeostatic transition to a new equilibrium in the dopaminergic system.
Searching for predictors of rebound psychosis in patients stabilized on OAPs, we performed a sensitivity analysis for relapse within the first month after treatment withdrawal, not finding any predictor for relapse, except for age, with a very small effect. Previous literature has identified a schizophrenia patients’ subgroup at increased risk for rebound psychosis following antipsychotic discontinuation, who were of older age and demonstrated tardive dyskinesia.39,40 In our pooled sample, results for the presence of at least moderate tardive dyskinesia symptoms (TD) did not converge in a multivariate model, so that we could not estimate hazard ratios for TD. We had previously highlighted the elevated relapse risk for patients with TD with potential mechanisms including dysregulation of the dopamine system due to prolonged antipsychotic exposure.34 The lack of other predictors adds to the literature that using a systematic approach has failed to identify epidemiological characteristics that would identify or distinguish a presumed rebound psychosis.6,41 Similarly, a study of binding potential to dopamine D2 receptors after treatment discontinuation did not find an elevation of this marker upon treatment discontinuation, which also questions the proposed biological mechanism for rebound psychosis.37
The results of this study need to be interpreted within its limitations. First, one limitation of this dataset derives from the compromise of smaller sample size and follow-up period, in favor of randomization and greater granularity of data. Thus, relapse rates might have been higher if patients had been followed for a longer time. Given our finding of relatively high relapse rates after antipsychotic discontinuation, this risk of bias is conservative, highlighting that the already high relapse rates are probably even higher during longer follow-up periods. Relapse rates were even higher in patients stabilized on OAPs, indicating that longer observation periods may not be necessary when conducting randomized controlled and blinded antipsychotic withdrawal trials in stabilized individuals with schizophrenia. Given that 75.1% of the patients had been stabilized with LAIs, which were associated with a lower relapse risk, the overall relapse rate and median time to relapse are an underestimate of expected usual-care relapse rates, given an 89% relapse rate in patients previously stabilized on OAPs, which is still the common treatment paradigm. Second, data derived from trials using LAIs or OAPs were unbalanced for sample size and follow-up. However, given the lower relapse rates for patients stabilized on LAIs, it seems appropriate that they were followed longer. Furthermore, groups were balanced regarding follow-up for the cohort with time to event (ie, censoring or relapse) longer than 5 half-lives of the antipsychotic, yielding consistent results. Third, we included only covariates with data in all RCTs, producing more consistent results yet for a more limited set of predictors. Future research may investigate the role of other clinical risk factors not included in our study, although given the negative results with clinical predictors of non-relapse almost across the board and given very high relapse rates, the focus should at least include neurobiological phenotypes that reflect more closely the pathophysiology of relapse in schizophrenia. Fourth, data were restricted to multi-episode patients, and relapse rates and predictors may differ in first-episode or early illness phase samples. Fifth, antipsychotics in the included studies were discontinued immediately, which does not reflect common clinical practice. This design might have influenced the results and their interpretability. Finally, our data are derived from clinical trials, which tend to underrepresent individuals with worse prognosis, indicating that our observed relapse rates may be conservative estimates. Given the inability to generate randomized data from real-world samples, this limitation reflects the trade-offs between internal and external validity.
In summary, while larger randomized studies would be necessary to reach a definitive conclusion about the risk of relapse following antipsychotic discontinuation in schizophrenia, the interpretation of these data, in addition to the previous literature, suggests that even in individuals who have reached symptom stability over time, there may be a high risk of relapse, and probably with the exception of having relapsed in the past, there are few clinical variables that can be used to predict a subsequent relapse. Adherence differences between patients stabilized on OAP vs LAI may have contributed to earlier relapse in patients stabilized on OAPs once medication is fully discontinued. Despite the high relapse rate following antipsychotic discontinuation, no clinical predictors exist that can inform safe antipsychotic discontinuation, except that patients and clinicians should consider stabilization with LAIs vs OAPs. The tradeoff of shorter follow-up time in favor of randomization limits the amount of observable data regarding covariates. However, we would argue that if any of the included covariates were “actionable” to predict relapses, we would have detected such a signal (ie, minimum sensitivity and specificity) with elevated risk of relapse over a median of 4 months of follow-up. Plus, our findings of the lack of clinical predictors of relapse after antipsychotic discontinuation aligns with the lack of consistent predictors in previous studies,17 which altogether allows us to conclude that to date there are no clinical variables, possibly with the exception of history of previous relapse, with promise to allow clinicians and patients to make an informed decision about the risk of relapse upon discontinuation. Future research should examine clinical and biomarkers of relapse upon antipsychotic discontinuation to further guide treatment.
Supplementary Material
Acknowledgments
The presented data are the in part the result of a study carried out under YODA Project #2020–4175, used data obtained from the Yale University Open Data Access Project, which has an agreement with JANSSEN RESEARCH & DEVELOPMENT, L.L.C.. The interpretation and reporting of research using this data are solely the responsibility of the authors and does not necessarily represent the official views of the Yale University Open Data Access Project or JANSSEN RESEARCH & DEVELOPMENT, L.L.C
Funding
This study was funded by Northwell Health.
Conflict of Interest
Dr Schoretsanitis declares no conflict of interest. Dr Kane has been a consultant and/or advisor for or has received honoraria from Alkermes, Allergan, LB Pharmaceuticals, H. Lundbeck, Intracellular Therapies, Janssen Pharmaceuticals, Johnson and Johnson, Merck, Minerva, Neurocrine, Newron, Otsuka, Pierre Fabre, Reviva, Roche, Sumitomo Dainippon, Sunovion, Takeda, Teva and UpToDate and is a shareholder in LB Pharmaceuticals and Vanguard Research Group. Dr Correll has been a consultant and/or advisor to or has received honoraria from: Acadia, Alkermes, Allergan, Angelini, Axsome, Gedeon Richter, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedinCell, Medscape, Merck, Mitsubshi Tanabe, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Rovi, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva. He has provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Rovi, Supernus, and Teva. He received royalties from UpToDate and grant support from Janssen and Takeda. He is also a stock option holder of LB Pharma. Dr Rubio has been a consultant or has received speaker honoraria from: Lundbeck, Teva and Medscape. He has also received royalties from UpToDate, and grant support from Alkermes.
References
- 1. Millan MJ, Andrieux A, Bartzokis G, et al. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016;15(7):485–515. [DOI] [PubMed] [Google Scholar]
- 2. Almond S, Knapp M, Francois C, Toumi M, Brugha T. Relapse in schizophrenia: costs, clinical outcomes and quality of life. Br J Psychiatry. 2004;184:346–351. [DOI] [PubMed] [Google Scholar]
- 3. Andreasen NC, Liu D, Ziebell S, Vora A, Ho BC. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emsley R, Chiliza B, Asmal L. The evidence for illness progression after relapse in schizophrenia. Schizophr Res. 2013;148(1-3):117–121. [DOI] [PubMed] [Google Scholar]
- 5. Mayoral-van Son J, de la Foz VO, Martinez-Garcia O, et al. Clinical outcome after antipsychotic treatment discontinuation in functionally recovered first-episode nonaffective psychosis individuals: a 3-year naturalistic follow-up study. J Clin Psychiatry. 2016;77(4):492–500. [DOI] [PubMed] [Google Scholar]
- 6. Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. [DOI] [PubMed] [Google Scholar]
- 7. Kishimoto T, Hagi K, Nitta M, Kane JM, Correll CU. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry. 2019;18(2):208–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taipale H, Tanskanen A, Mehtälä J, Vattulainen P, Correll CU, Tiihonen J. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry. 2020;19(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rubio JM, Lencz T, Barber A, Moyett A, Ali S, Ventura G, Germano N, Bassaw F, Malhotra A, Kane JM, Striatal functional connectivity in psychosis relapse: a comparison between antipsychotic adherent and non-adherent patients at the time of relapse. Biological Psychiatry. 2021;89(9):S86. [Google Scholar]
- 11. Wunderink L, Nieboer RM, Wiersma D, Sytema S, Nienhuis FJ. Recovery in remitted first-episode psychosis at 7 years of follow-up of an early dose reduction/discontinuation or maintenance treatment strategy: long-term follow-up of a 2-year randomized clinical trial. JAMA Psychiatry. 2013;70(9):913–920. [DOI] [PubMed] [Google Scholar]
- 12. Correll CU, Rubio JM, Kane JM. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry. 2018;17(2):149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goff DC, Falkai P, Fleischhacker WW, et al. The long-term effects of antipsychotic medication on clinical course in schizophrenia. Am J Psychiatry. 2017;174(9):840–849. [DOI] [PubMed] [Google Scholar]
- 14. Wils RS, Gotfredsen DR, Hjorthøj C, et al. Antipsychotic medication and remission of psychotic symptoms 10years after a first-episode psychosis. Schizophr Res. 2017;182:42–48. [DOI] [PubMed] [Google Scholar]
- 15. Harrow M, Jobe TH, Faull RN. Does treatment of schizophrenia with antipsychotic medications eliminate or reduce psychosis? A 20-year multi-follow-up study. Psychol Med. 2014;44(14):3007–3016. [DOI] [PubMed] [Google Scholar]
- 16. Moilanen J, Haapea M, Miettunen J, Jaaskelainen E, Veijola J, Isohanni M, Koponen H. Characteristics of subjects with schizophrenia spectrum disorder with and without antipsychotic medication - a 10-year follow-up of the Northern Finland 1966 Birth Cohort study. Eur Psychiatry. 2013;28(1):53–58. [DOI] [PubMed] [Google Scholar]
- 17. Bowtell M, Ratheesh A, McGorry P, Killackey E, O’Donoghue B. Clinical and demographic predictors of continuing remission or relapse following discontinuation of antipsychotic medication after a first episode of psychosis. A systematic review. Schizophr Res. 2018;197:9–18. [DOI] [PubMed] [Google Scholar]
- 18. Wunderink L, Nienhuis FJ, Sytema S, Slooff CJ, Knegtering R, Wiersma D. Guided discontinuation versus maintenance treatment in remitted first-episode psychosis: relapse rates and functional outcome. J Clin Psychiatry. 2007;68(5):654–661. [DOI] [PubMed] [Google Scholar]
- 19. Wunderink L, van Bebber J, Sytema S, Boonstra N, Meijer RR, Wigman JTW. Negative symptoms predict high relapse rates and both predict less favorable functional outcome in first episode psychosis, independent of treatment strategy. Schizophr Res. 2020;216:192–199. [DOI] [PubMed] [Google Scholar]
- 20. Hui CLM, Honer WG, Lee EHM, Chang WC, Chan SKW, Chen EYH. Factors associated with successful medication discontinuation after a randomized clinical trial of relapse prevention in first-episode psychosis: a 10-year follow-up. JAMA Psychiatry. 2019;76(2):217–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kane JM, Rifkin A, Quitkin F, Nayak D, Ramos-Lorenzi J. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Arch Gen Psychiatry. 1982;39(1):70–73. [DOI] [PubMed] [Google Scholar]
- 22. Hui CL, Wong GH, Tang JY, et al. Predicting 1-year risk for relapse in patients who have discontinued or continued quetiapine after remission from first-episode psychosis. Schizophr Res. 2013;150(1):297–302. [DOI] [PubMed] [Google Scholar]
- 23. Emsley R, Nuamah I, Gopal S, Hough D, Fleischhacker WW. Relapse after antipsychotic discontinuation in schizophrenia as a withdrawal phenomenon vs illness recurrence: a post hoc analysis of a randomized placebo-controlled study. J Clin Psychiatry. 2018;79(4):17m11874. [DOI] [PubMed] [Google Scholar]
- 24. Weiden PJ, Kim E, Bermak J, Turkoz I, Gopal S, Berwaerts J. Does half-life matter after antipsychotic discontinuation? A relapse comparison in schizophrenia with 3 different formulations of paliperidone. J Clin Psychiatry. 2017;78(7):e813–e820. [DOI] [PubMed] [Google Scholar]
- 25. Correll CU, Jain R, Meyer JM, et al. Relationship between the timing of relapse and plasma drug levels following discontinuation of cariprazine treatment in patients with schizophrenia: indirect comparison with other second-generation antipsychotics after treatment discontinuation. Neuropsychiatr Dis Treat. 2019;15:2537–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ravenstijn P, Remmerie B, Savitz A, et al. Pharmacokinetics, safety, and tolerability of paliperidone palmitate 3-month formulation in patients with schizophrenia: a phase-1, single-dose, randomized, open-label study. J Clin Pharmacol. 2016;56(3):330–339. [DOI] [PubMed] [Google Scholar]
- 27. Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90(8):1067–1076. [DOI] [PubMed] [Google Scholar]
- 28. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830–839. [DOI] [PubMed] [Google Scholar]
- 29. Fu DJ, Turkoz I, Simonson RB, et al. Paliperidone palmitate once-monthly reduces risk of relapse of psychotic, depressive, and manic symptoms and maintains functioning in a double-blind, randomized study of schizoaffective disorder. J Clin Psychiatry. 2015;76(3):253–262. [DOI] [PubMed] [Google Scholar]
- 30. Hough D, Gopal S, Vijapurkar U, Lim P, Morozova M, Eerdekens M. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116(2-3):107–117. [DOI] [PubMed] [Google Scholar]
- 31. Kramer M, Simpson G, Maciulis V, et al. Paliperidone extended-release tablets for prevention of symptom recurrence in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2007;27(1):6–14. [DOI] [PubMed] [Google Scholar]
- 32. Zhang H, Li H, Liu Y, et al. Safety and efficacy of paliperidone extended-release in Chinese patients with schizophrenia: a 24-week, open-label extension of a randomized, double-blind, placebo-controlled study. Neuropsychiatr Dis Treat. 2016;12:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hui CL, Tang JY, Leung CM, et al. A 3-year retrospective cohort study of predictors of relapse in first-episode psychosis in Hong Kong. Aust N Z J Psychiatry. 2013;47(8):746–753. [DOI] [PubMed] [Google Scholar]
- 34. Rubio JM, Schoretsanitis G, John M, et al. Psychosis relapse during long-acting injectable antipsychotic treatment: an individual participant data meta-analysis of 19 trials and 5,111 individuals with schizophrenia-spectrum disorders. Lancet Psychiatry. 2020;7(9):749–761. [DOI] [PubMed] [Google Scholar]
- 35. Pathak S, Jiang Y, DiPetrillo L, Todtenkopf MS, Liu Y, Correll CU. Course of psychosis in schizophrenia with alcohol use disorder: a post hoc analysis of the clinical antipsychotic trials of intervention effectiveness in schizophrenia phase 1 study. J Clin Psychiatry. 2020;81(2):19m12731. [DOI] [PubMed] [Google Scholar]
- 36. Emsley R, Asmal L, Rubio JM, Correll CU, Kane JM. Predictors of psychosis breakthrough during 24 months of long-acting antipsychotic maintenance treatment in first episode schizophrenia. Schizophr Res. 2020;225:55–62. [DOI] [PubMed] [Google Scholar]
- 37. Kim S, Shin SH, Santangelo B, et al. Dopamine dysregulation in psychotic relapse after antipsychotic discontinuation: an [(18)F]DOPA and [(11)C]raclopride PET study in first-episode psychosis. Mol Psychiatry. 2020. doi: 10.1038/s41380-020-00879-0. Published online ahead of print. [DOI] [PubMed] [Google Scholar]
- 38. Horowitz MA, Murray RM, Taylor D. Tapering antipsychotic treatment. JAMA Psychiatry. 2021;78(2):125–126. [DOI] [PubMed] [Google Scholar]
- 39. Apud JA, Egan MF, Wyatt RJ. Neuroleptic withdrawal in treatment-resistant patients with schizophrenia: tardive dyskinesia is not associated with supersensitive psychosis. Schizophr Res. 2003;63(1-2):151–160. [DOI] [PubMed] [Google Scholar]
- 40. Fallon P, Dursun SM. A naturalistic controlled study of relapsing schizophrenic patients with tardive dyskinesia and supersensitivity psychosis. J Psychopharmacol. 2011;25(6):755–762. [DOI] [PubMed] [Google Scholar]
- 41. Takeuchi H, Kantor N, Sanches M, Fervaha G, Agid O, Remington G. One-year symptom trajectories in patients with stable schizophrenia maintained on antipsychotics versus placebo: meta-analysis. Br J Psychiatry. 2017;211(3):137–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.