Abstract
Poor phonological processing has typically been considered the main cause of dyslexia. However, visuo‐attentional processing abnormalities have been described as well. The goal of the present study was to determine the involvement of visual attention during fluent reading in children with dyslexia and typical readers. Here, 75 children (8–12 years old; 36 typical readers, 39 children with dyslexia) completed cognitive and reading assessments. Neuroimaging data were acquired while children performed a fluent reading task with (a) a condition where the text remained on the screen (Still) versus (b) a condition in which the letters were being deleted (Deleted). Cognitive assessment data analysis revealed that visual attention, executive functions, and phonological awareness significantly contributed to reading comprehension in both groups. A seed‐to‐voxel functional connectivity analysis was performed on the fluency functional magnetic resonance imaging task. Typical readers showed greater functional connectivity between the dorsal attention network and the left angular gyrus while performing the Still and Deleted reading tasks versus children with dyslexia. Higher connectivity values were associated with higher reading comprehension. The control group showed increased functional connectivity between the ventral attention network and the fronto‐parietal network during the Deleted text condition (compared with the Still condition). Children with dyslexia did not display this pattern. The results suggest that the synchronized activity of executive, visual attention, and reading‐related networks is a pattern of functional integration which children with dyslexia fail to achieve. The present evidence points toward a critical role of visual attention in dyslexia.
Keywords: attention, brain, dyslexia, executive function, learning disabilities, magnetic resonance imaging, reading
Typical readers showed greater functional connectivity between the dorsal attention network and the left angular gyrus while performing the Still and Deleted reading tasks versus children with dyslexia. The control group showed increased functional connectivity between the ventral attention network and the fronto‐parietal network during the Deleted text condition (compared with the Still condition). The results suggest that the synchronized activity of executive, visual attention, and reading‐related networks is a pattern of functional integration which children with dyslexia fail to achieve.
1. INTRODUCTION
1.1. Reading, executive functions, and visual attention
Developmental dyslexia (henceforth, dyslexia) is a heritable neurodevelopmental disorder affecting 5–12% of children (Peterson & Pennington, 2015). It is characterized by an impaired reading acquisition that cannot be explained by deficient neurological or sensorial functioning or below‐average intelligence (American Psychiatric Association, 2015). Vast scientific research has been conducted aiming to clarify its etiology and provide effective treatment. However, there is a controversy regarding the most effective type of intervention (Peters, De Losa, Bavin, & Crewther, 2019; Peterson & Pennington, 2015). This controversy is partly due to several components involved in the reading process.
The Simple View of Reading model divides the reading process into decoding skills and language comprehension, both contributing to reading comprehension (Hoover & Gough, 1990). These two components are of equal importance, both being necessary for reading success and none of them being sufficient by itself (Hoover & Gough, 1990). Decoding is defined as efficient word recognition, that is, the ability to access the appropriate mental lexicon representation from a printed series of graphemes (Hoover & Gough, 1990). Language comprehension is the ability to understand language, to derive and interpret the relevant information from a complex linguistic stimulus. The Simple View of Reading was selected as the supporting framework for our analyses for two main reasons: first, other models like the Componential Model of Reading and different interactive reading models, such as Goodman's model, seem to be influenced greatly by the Simple View of Reading and their main claims included in this model (Harm & Seidenberg, 2004; Joshi, 2019). Second, the Simple View of Reading has received extensive empirical support and has been updated recently, including executive functions (EF) as a relevant factor (Spencer, Richmond, & Cutting, 2020).
An updated version of the model suggested the involvement of EF as a contributor to reading comprehension—specifically working memory and cognitive flexibility (Spencer et al., 2020). EFs are an umbrella term for a set of mental abilities allowing individuals to engage in goal‐directed behavior and respond to new situations in an adaptive manner (Cristofori, Cohen‐Zimerman, & Grafman, 2019). Reading requires a multitude of different processes including various EFs, such as the ability to retain and manipulate phonological information (i.e., working memory), the ability to repress multiple competing lexicon entries crowding in on consciousness (i.e., inhibition), and the capability to shift between multiple sources of orthographic, phonological, and semantic information (i.e., cognitive flexibility) (Spencer et al., 2020). Furthermore, the development of EF is tightly connected to reading development and reading difficulties: impairments in inhibition, working memory, shifting, planning, speed of processing and attention have been related to dyslexia (Farah, Ionta, & Horowitz‐Kraus, 2021).
Arguably, the main challenge in dyslexia is a deficient decoding skill (oral language comprehension is typically more intact), which has been traditionally considered as a phonological system dysfunction (Langerberg et al., 2000; Melby‐Lervag, Lyster, & Hulme, 2012). According to the phonological deficit hypothesis, the decoding deficit in dyslexia (compared with typical reading) stems from an impairment in phonological awareness, defined as the ability to think, reflect and manipulate the sounds in speech (Preston & Edwards, 2010). However, the multifactorial nature of the disorder has been elaborated to include additional challenges. Research has shown that deficits in phonological awareness and reading fluency, defined as fast and accurate reading, which traditionally were considered as dyslexia's core features (Peterson & Pennington, 2012), are not the exclusive impairments suffered by individuals with dyslexia. Deficits in nonverbal processing, such as impaired visual attention (Facoetti, Corradi, Ruffino, Gori, & Zorzi, 2010; Franceschini, Gori, Ruffino, Pedrolli, & Facoetti, 2012) and multiple EF (Smith‐Spark, Henry, Messer, Edvardsdottir, & Ziecik, 2016) have been reported as well.
There is a large amount of experimental data suggesting that the reading deficit in dyslexia is closely linked to EF: children with dyslexia often show reduced EF when compared to age‐matched typical readers (TR) (Barbosa, Rodrigues, Mello, Silva, & Bueno, 2019). Individuals with dyslexia show impaired EF such as inhibition, working memory (Brosnan et al., 2002), and shifting (Hari & Renvall, 2001), among others. Furthermore, EF‐related brain regions show lower connectivity indices in children with dyslexia in comparison with TR (Horowitz‐Kraus, Buck, & Dorrmann, 2016). EF rely on the executive component of the attention system of the brain (Petersen & Posner, 2012). Several brain networks (e.g., fronto‐parietal network, cingulo‐opercular network) play an extensive role in setting goals, choosing a behavioral response, and monitoring the executed plan (Petersen & Posner, 2012). Midline cortex areas (anterior cingulate cortex, medial frontal gyrus), the dorsolateral prefrontal cortex, and the posterior parietal cortex constitute the executive component of the attention system (Petersen & Posner, 2012; Smith et al., 2009).
Visual attention is defined as the process by which one item—the target—is selected for analysis from among several competing items or distractors (American Psychiatric Association, 2007). Visual attention is sustained by the orienting component of the attention system (Petersen & Posner, 2012). The orienting component, comprised of the dorsal attention network (DAN) and ventral attention networks (VANs), supports the ability to prioritize sensory input and to shift attention, and accounts for both bottom‐up reorienting processes and top‐down visuospatial functions (Petersen & Posner, 2012). Active visual reorienting is required for reading; precise gaze direction characterizes fluent reading (Biscaldi, Fischer, & Hartnegg, 2016). Visual orienting occurs when attention is directed to a given spatial location. This process can occur overtly (with eye movements) or covertly—when the facilitation toward a visual location exists, without convergent visual gaze toward this location (Petersen & Posner, 2012; Posner & Petersen, 1990). The orienting attention network includes distinct areas within the frontal and parietal lobules, such as the inferior frontal gyrus (IFG), frontal eye fields, posterior parietal lobe, and the temporoparietal junction (Corbetta & Shulman, 2002).
The literature suggests that visual attention impairment plays a key role in the reading difficulties of individuals with dyslexia: children with dyslexia show deficits in distinct visual abilities such as visual perception, visual temporal processing, and the rapid engagement of attention (Peters et al., 2019). In the pre‐reading stage, visual attention abilities are predictive of reading acquisition (Facoetti et al., 2010; Franceschini et al., 2012). For these reasons, visual attention is a basic skill for reading development, as was also previously demonstrated neurobiologically by highlighting the increased functional connections between the dorsal attention system and the putative visual word form area (VWFA) over development and in relations to reading ability (Vogel, Petersen, & Schlaggar, 2014). Several authors support the hypothesis that faulty activity of visual pathways is a fundamental cause of dyslexia and argue against the assumption that a phonological deficit per se is the cause of reading deficiencies (Lawton, 2016; Vidyasagar, 2019; Vidyasagar & Pammer, 2010). As such, a greater reliance on visual attention‐related brain regions seems to be beneficial for reading performance (Horowitz‐Kraus, DiFrancesco, Kay, Wang, & Holland, 2015).
1.2. Neurobiological correlates for EFs and visual attention difficulties in dyslexia
Dyslexia has been related to reduced activity in different areas of the reading network, comprising left ventral occipito‐temporal and perisylvian areas such as the inferior parietal, IFG, inferior and middle temporal lobules (IPL, IFG, ITL, and MTL, respectively), fusiform regions (Norton, Beach, & Gabrieli, 2015; Richlan, Kronbichler, & Wimmer, 2009), and the putative VWFA—which was found to be linked to the dorsal attention system in relations to reading skills (Vogel et al., 2014). Some of the constituent regions of the reading network are also key nodes within the VAN and DAN, namely distinct areas within the parietal and frontal lobes (Igelstrom & Graziano, 2017).
Existing literature suggests that stronger functional connections between higher‐order cognitive, visual, and language‐related areas are related to improved reading ability, both at rest (Krishnamurthy et al., 2019; Stevens, Kravitz, Peng, Tessler, & Martin, 2017) and during reading (Schurz et al., 2015). In the same vein, atypical functional connectivity (specifically lower connectivity) between these areas has been described in children and adults with dyslexia (Finn et al., 2014). The areas reported in previous research include the fusiform gyrus, the IFG, the middle and superior temporal lobe, and more. Noteworthy, as already mentioned, most of these areas are constituent regions of the DAN and VAN. Taken together, the aforementioned experimental evidence suggests that the reading deficiencies in children with dyslexia are related to brain regions involved in visuo‐attentional processes. Inefficient functioning of these areas might be provoking an abnormal serial selection of the graphemes within a word further impeding the individual to read fluently and accurately. In the current work, we aim to describe the neurobiological correlates for the visual attention deficit in individuals with dyslexia.
The traditional approach to the study of brain networks architecture is based on resting‐state functional magnetic resonance imaging (fMRI) data. A different methodology, termed task‐residual functional connectivity, has been developed in recent years (Fornito, Harrison, Zalesky, & Simons, 2012; Tran et al., 2018). This method can be defined as: functional connectivity analysis based on blood oxygenated level‐dependent (BOLD) signal registered during the performance of a task. Cognitive brain networks can be identified and analyzed by correlating the BOLD timeseries of different regions‐of‐interest (ROIs). It has been observed that task‐residuals highlight specific network patterns to a greater degree than resting‐state analyses (Tran et al., 2018). Importantly, the effect of the task blocks is removed from the signal using linear regression. Subsequent functional connectivity analysis can reveal patterns of synchronous network activity characteristic of the task that the participant is performing.
Numerous studies show that visual attention trainings are capable of enhancing reading speed and accuracy in individuals with dyslexia (Facoetti, Lorusso, Paganoni, Umilta, & Mascetti, 2003; Franceschini et al., 2013; Lorusso, Facoetti, Paganoni, Pezzani, & Molteni, 2006; Peters et al., 2019). Vidyasagar and Pammer (2010) proposed that poor visual coding and deficient attentional mechanisms are the main causes of the disorder, further giving rise to deficient phonological processing and thus, poor general reading ability. According to these authors, poor phonological awareness in dyslexia “could be the result of the poor orthographic inputs feeding into the regions mediating grapheme–phoneme correspondence” (Vidyasagar & Pammer, 2010). A consensus regarding the role of visual attention in dyslexia is lacking. Several research questions guided the present investigation: Do children with dyslexia have an impairment in visual attention? How is this impairment related to the reading difficulty? Are there connectivity differences between TR and children with dyslexia in visual attention‐related brain networks?
The goal of the present study is to characterize the role of visual attention during the reading process in typical and atypical readers and to add this component to the Simple View of Reading model. We will compare the functional connectivity patterns associated with visual and orienting attention in children with dyslexia and TR in a task that presents written materials in a way that triggers visual attention abilities. We will achieve that by presenting deleted text from the screen, a manipulation that was found to trigger visual attention (Breznitz et al., 2013; Karni & Sagi, 1993). Reading interventions based on deleted text reading were found to enhance reading speed (Horowitz‐Kraus, Vannest, et al., 2014) and comprehension (Horowitz‐Kraus, Cicchino, Amiel, Holland, & Breznitz, 2014). Neurobiologically, deleted text reading has been related to increased functional connectivity between cognitive‐control networks and visual regions (Horowitz‐Kraus et al., 2015). However, to our knowledge, functional connectivity occurring while reading deleted text has not been investigated.
We hypothesize that visual attention will play a key role in reading in both TR and children with dyslexia: the group of children with dyslexia will show impaired performance on reading and visual attention tasks. Visual attention scores will be a statistical predictor of reading scores. Furthermore, the functional connectivity between different neural systems related to visual attention and reading will be altered in children with dyslexia, when compared to TR. Specifically, we expect to find lower functional connectivity between distinct brain systems related to visual attention, that is, the DAN and VAN (the attention system) and brain regions supporting reading in children with dyslexia compared with TR. We chose the DAN and VAN defined by Power et al. (2011) to conduct our analyses.
2. METHODS
2.1. Participants
Seventy‐five native English‐speaking children (between the ages of 8 and 12 years) participated in the current study in the Cincinnati Children's Hospital Medical Center, OH. Recruitment flyers were handed out and displayed on bulletin boards in Cincinnati Children's Hospital Medical Center, surrounding clinics, libraries, and Cincinnati public schools. Initially, 86 participants were recruited. However, the neuroimaging data of nine of the participants showed excessive noise as detected via visual inspection of the images and they were discarded from all analyses, leaving us with a valid sample N = 75. Then, 36 of them were TR (mean age: 9.82, SD = 1.39 years, 11 females) and 39 were children with dyslexia (dyslexia; mean age: 10.03, SD = 1.4 years, 18 females). Participants in the latter group received the diagnosis of dyslexia by a certified educational psychologist or mental health professional, as reported by their parents. Participants were matched for age (t(73) = −.915, p > .05). All participants with dyslexia received a score lower than 1 SD below the mean on at least two of the utilized reading and/or phonological measures (see the Behavioral tasks section below), thus confirming the reading impairment (Kovelman et al., 2012). There were no significant differences between the two groups regarding parental level of education (dyslexia mean: 17.4 years, SD = 2.8; TR mean: 18.1, SD = 2; t(74) = 1.2, p > .05) or average household income (dyslexia mean scaled score: 7.8, SD = 2.1; TR mean: 8.1, SD = 1.6; t(74) = 0.7, p > .05). Only children without a history of neurological or psychiatric impairments including attention deficit hyperactivity disorder were eligible to participate in the study. Informed consents and assents were signed by the parents of the participants and the children participating in the study, respectively.
2.2. Study procedure
Following enrollment to the study, participants underwent cognitive and reading assessments that included EF, language, reading, and visual attention tasks. After completion of the behavioral assessment (approximately 2 hr), they participated in an fMRI session while performing a reading task, in which visual attention was manipulated (see Section 2.4). The study was conducted in accordance with the Declaration of Helsinki. The experimental procedure was approved by Cincinnati Children's Hospital Medical Center USA Institutional Review Board.
2.3. Cognitive testing
2.3.1. Reading measures
Distinct reading abilities were assessed in the behavioral testing session: (a) orthographical abilities were measured using the timed Test of Word Reading Efficiency (TOWRE II, specifically the Sight Word Efficiency [SWE]) and the non‐timed Letter‐Word Identification subtest of the Woodcock‐Johnson (Schrank, Mather, & McGrew, 2014); (b) decoding was assessed using the timed phonetic decoding efficiency [PDE] subtest of the TOWRE II (Torgesen, Wagner, & Rashotte, 1999) and the non‐timed Word‐attack subtest (Woodcock‐Johnson); (c) reading fluency and comprehension were tested using the Gray Reading Oral Test (GORT; Wiederholt & Bryant, 2012)—and the Test Of Silent Reading Efficiency and Comprehension (TOSREC; Wagner, Torgesen, Rashotte, & Pearson, 2010); (d) phonological processing was assessed using the Elision and Blending subtests of the Comprehensive Test of Phonological Processing (CTOPP; Wagner, Torgesen, Rashotte, & Pearson, 2013); and (e) naming abilities were measured using the Rapid Digit Naming subtest of the CTOPP battery (Wagner et al., 2013).
2.3.2. Executive functioning measures
Several domains of EF were tested: (a) cognitive flexibility, via the Trail Making Test (letter‐number sequencing) of the Delis–Kaplan Executive Function System test (D‐KEFS) (Delis, Kaplan, & Kramer, 2011); (b) inhibition was measured using the Color Word Stroop subtest of the D‐KEFS (Delis et al., 2011); and (c) working memory was assessed using with the Digit span test (overall subset scores of forward/backward subsets) of the Wechsler Intelligence Scale for Children, Fourth Version (WISC‐IV; Wechsler, 2012).
2.3.3. Visual attention
The Sky Search subtest of the Test of Everyday Attention for Children or TEA‐Ch was used in order to assess visual attention ability (Manly et al., 2001). For this task, participants are given an A3 (29.7 × 42 cm) landscape orientation sheet, which contains 128 items printed in an irregular 13 × 10 matrix‐like array. Then, 20 target items and 108 distractor items are randomly distributed in the array. Each item comprises either two identical or two different line drawings. Children are instructed to find and encircle all the target items (identical drawings), while leaving the distractor items (different drawings) unmodified.
Then, 11 (6 children with dyslexia, 5 TR) of the 75 participants did not perform the visual attention task due to a lack of time.
2.3.4. Basic verbal and nonverbal abilities
The Test of Nonverbal Intelligence was used to evaluate the intellectual ability of the participants (Brown, Sherbenou, & Johnsen, 2010). Receptive vocabulary and comprehension was assessed using the Peabody Picture Vocabulary Test, Fifth Edition, or PPVT‐5 (Dunn & Dunn, 2007).
2.3.5. Processing speed
This cognitive ability was assessed using the Coding subtest of the WISC‐IV (Wechsler, 2012).
2.4. Neuroimaging task
The fluency task was administered while acquiring fMRI data. In this task, children were instructed to attend to written stories presented on the screen and informed that questions would follow the text. After each story was presented, participants were asked to answer a yes/no question presented on the screen based on the written text that had just been read. Ten stories were presented for 44 s each. Five of them pertained to the “Still condition” and the other five stories were part of the “Deleted” condition. The third condition (a control condition presented five times) was “Fixation cross,” and the three of them were presented in an interleaved fashion throughout the task (see Figure 1). In the “Still text” condition, the whole story appeared on the screen for 44 s. In the “Deleted text” condition, the letters of the words were being erased at a constant pace (119 ms per letter on average, see Supplementary Table 2). The erasure rate was determined based on the average reading rate of children with dyslexia and TR of the same age, as reported previously (Horowitz‐Kraus, Vannest, et al., 2014). The text was completely deleted after 44 s.
FIGURE 1.
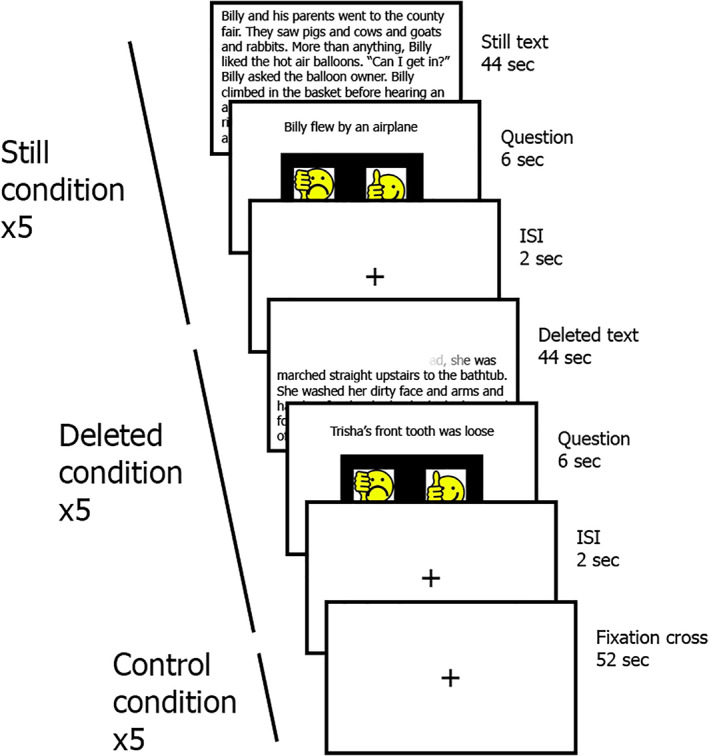
The fluency functional magnetic resonance imaging (fMRI) task. An overview of the fluency task: After reading a Still or Deleted text for 44 s, participants had 6 s to read a question and answer it. There was also a Control condition, the duration of which was 52 s. Each condition was presented five times, and none of the stories were repeated. ISI, interstimulus interval
The control condition consisted of a fixation cross presented in the center of the screen for 44 s. Following each text presentation (Deleted and Still), participants had 6 s to respond to a Yes/No question based on the text. After the Fixation condition, the message “Push any button” appeared on the screen and the subjects had 6 s to do so. Following the response screen, a 2‐s inter‐stimulus‐interval fixation cross appeared on the screen, for a total duration of 52 s per trial (see supplemental Table 2). The fMRI task was used as a measure of reading fluency (reading accuracy and speed).
2.5. Neuroimaging data acquisition
Participants were desensitized before the MRI scan through the exploration of the environment with positive reinforcement and practicing sitting on the scanner bed “as still as a statue” (Vannest et al., 2014). Motion was controlled by using foam pads on either side of the head‐coil apparatus. All participants were scanned using a 3 T Philips Ingenia MRI scanner (Philips Ingenia, Philips Healthcare, Best, Netherlands). For presentation of the stimuli comprising the neuroimaging task, we used an MRI‐compatible audio/visual system (Avotec, SS3150/SS7100). A gradient echo‐planar sequence was used for T2*‐weighted BOLD fMRI scans with the following parameters: repetition time/echo time = 1,000/30 ms; FOV = 20 × 20 × 14.4 cm; matrix = 80 × 80; and slice thickness = 3 mm. Seven hundred and eighty volumes were acquired during the fMRI experiment. The experimental run comprised 15 trials, 52 s each, for a total fMRI acquisition time of 13 min (780 s). For each participant, a 3D T1‐weighted inversion recovery gradient echo whole‐brain scan was also acquired for anatomical coregistration and use in spatial normalization of the fMRI data (TR/TE = 8.1/3.7 ms, inversion time = 940 ms, flip angle = 8°, FOV = 22.4 × 25.6 × 16 cm, matrix = 224 × 256, slice thickness = 1 mm).
2.6. Behavioral data analysis
To quantify the magnitude of the differences in reading, EF, and visual attention performance between the TR and the children with dyslexia, independent t tests were conducted on the standard scores of a variety of tasks while correcting for multiple comparisons. A 2 × 2 repeated measures analysis of variance (ANOVA) was used for the statistical analysis of the fluency task; the significance of main effects and interaction effects between the Group variable (TR, dyslexia) and the Condition variable (Still, Deleted) was tested. Data distribution was tested for normality with the Shapiro–Wilk test (Shapiro & Wilk, 1965) and visual inspection of the box plots. Homoscedasticity was tested using Levene's test (Levene, 1960). All t tests were Bonferroni‐corrected by the number of comparisons (n = 17), in order to minimize the probability of Type I error. Statistical analyses of the cognitive variables were two‐tailed with a significance alpha (α) level of .05.
Path analysis using IBM SPSS Amos 26 Graphics was performed in order to test for the indirect contributions of a set of relevant cognitive abilities on reading comprehension (Streiner, 2005). The created mediated or indirect model tested the effect of several cognitive abilities (the exogenous variables) on reading comprehension (endogenous variable) through their influence on language comprehension and decoding (mediators). The cognitive abilities selected for analysis were: phonological awareness (measured using the Blending subtest of the CTOPP), visual attention (Sky‐search subtest of the TEA‐Ch) and three different EF—inhibition (Color‐Word subtest of the D‐KEFS), working memory (Digit subtest of the WISC‐IV) and cognitive flexibility (Trail Making subtest of the D‐KEFS). The PDE subtest (TOWRE) was used as an indicator of decoding ability. Language comprehension was assessed using the PPVT test. The GORT test was used as an indicator of reading comprehension in the model. General model fit was assessed using chi‐square (χ 2), root mean square error of approximation (RMSEA), and comparative fit index (CFI). A nonsignificant chi‐square statistic, an RMSEA < 0.05, and CFI > 0.95 together indicate a strong fit of the data with the model, whereas a significant chi‐square, an RMSEA < 0.08, and a CFI > 0.90 indicate an adequate fit (Kline, 2016).
2.7. Neuroimaging data analysis
2.7.1. Data preprocessing
Preprocessing of the fMRI data included realignment to the first image of the session for motion correction using three translational and three rotational parameters, coregistration of the anatomical image to the mean aligned functional image, segmentation of the different tissue types (gray matter, white matter, and cerebrospinal fluid), normalization of all images to the Montreal Neurological Institute template, suited for children age 5 and above, and spatial smoothing with an 8‐mm full width at half‐maximum Gaussian kernel. The functional images were standardized to a resolution of 2 mm3 voxel size. Anatomical image resolution was 1 mm3. The data were band‐pass filtered in order to improve the signal‐to‐noise ratio by eliminating confounding frequencies (0.008 Hz < ƒ < 0.09 Hz). Neuroimaging data preprocessing was carried out using the CONN functional connectivity toolbox Version 19c (Whitfield‐Gabrieli & Nieto‐Castanon, 2012).
In order to assess and minimize movement‐related noise, as it is a confounding factor (especially in pediatric populations), several control measures were taken. From the original sample of 84 participants, nine were excluded from analyses due to excessive artifactual/motion‐related noise, as observed during visual inspection of the images (6 children with dyslexia, 3 TR). Additionally, motion was controlled for via regression of the six motion parameters and their first‐order derivatives. The principal component analysis‐based aCompCor approach was adopted to minimize the effects of confounding nonneuronal signal from white matter and cerebrospinal fluid tissue classes—five principal components for each irrelevant tissue type were included as temporal first‐level covariates (Behzadi, Restom, Liau, & Liu, 2007). Finally, scrubbing included flagging of all consecutive functional volumes with global signal changes above z = 3 and framewise displacement above 0.5 mm, which were discarded from further analyses. Temporal censoring techniques (scrubbing) were complemented with regression techniques (aCompCor), adopting the recommendations from a recent benchmarking study (Ciric et al., 2017).
2.7.2. Seed‐to‐voxel analysis
The preprocessed images were submitted to a seed‐to‐voxel functional connectivity analysis as defined in CONN 19c (Whitfield‐Gabrieli & Nieto‐Castanon, 2012). In a first‐level analysis, the degree of synchrony or similarity between the mean time course of a set of seed regions and the time course of the rest of the voxels in the brain was calculated using bivariate correlation. In a second‐level analysis, the mean correlation values were transformed into Fisher's z normal distribution and statistically significant differences in correlation values (z > 3, associated p‐value < .001) were explored across groups and conditions using t tests. A previously described brain parcellation atlas based on anatomical and functional evidence (see Figure 2), was used to determine the location of the networks that were used as seed regions in the functional connectivity analysis—DAN and VAN (Power et al., 2011). In our analyses, the entire defined network of interest was used as a seed region. That is, the BOLD signal timeseries of all the voxels within all the different ROIs comprising the network were averaged. The resulting timeseries was then correlated to every other voxel in the brain. For contrast analyses (Deleted > Still, TR > dyslexia and dyslexia>TR), a corrected Gaussian random field theory threshold was utilized: False discovery rate‐corrected p < .001 at the voxel level (Worsley et al., 1996). Subcortical structures were discarded from further analyses; the focus of interest of the present study was on the connectivity indices between cortical regions.
FIGURE 2.
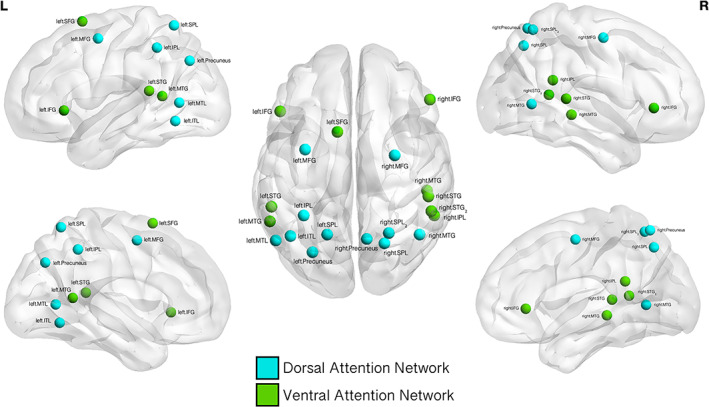
Ventral and dorsal attention networks. A graphical representation of the regions‐of‐interest (ROIs) comprising the ventral attention network and dorsal attention network as identified by Power et al. (2011). These networks were defined as the seed regions in the conducted seed‐to‐voxel analysis. L: left hemisphere: R: right hemisphere. Dorsal attention network regions and Montreal Neurological Institute (MNI) coordinates: right precuneus (10–62 61); left medial temporal lobe (MTL, −52 −63 5); right superior parietal lobe (SPL, 22–65 48); right medial temporal gyrus (MTG, 46–59 4); right SPL2 (lobe, 25–58 60); left inferior parietal lobe (IPL, −33 −46 47), left precuneus (−27 −71 37); left medial frontal gyrus (MFG, −32 −1 54); left inferior temporal lobe (ITL, −42 −60 −9); left SPL (−17 −59 64), and right MFG (29–5 54). Ventral attention network regions: left superior frontal gyrus (SFG, −10 11 67); right IPL (54–43 22); left MTG (−56 −50 10); left superior temporal gyrus (STG, −55 −40 14); right STG (52–33 8); right MTG (51 −29 −4); right STG2 (56 −46 11); right inferior frontal gyrus (IFG, 53 33 1) and left IFG (−49 25 −1)
2.8. Correlations between neuroimaging and behavioral data
To examine the association between the seed‐to‐voxel results (functional connections between DAN and VAN and voxels throughout the cerebral cortex) and the behavioral data, Pearson's r and Spearman's rho correlation coefficients were calculated between the average functional connectivity values and the scores of the fMRI task as well as the relevant behavioral measures (all visual attention, reading and EF tests described in the Behavioral tasks section).
3. RESULTS
3.1. Behavioral analysis results
As hypothesized, TR significantly outperformed the children with dyslexia in a variety of language and reading‐related tests (phonological manipulation, naming, reading efficiency, reading and oral comprehension, spelling, and vocabulary). Children with dyslexia also showed significantly lower EF scores compared to neurotypical controls (working memory, cognitive flexibility and inhibition). See Table 1 for detailed information. There was no significant difference in visual attention between children with dyslexia and TR. However, we found a significant positive correlation between visual attention and word reading in both groups combined (r(64) = .395, p < .001). See Figure 3.
TABLE 1.
Behavioral tests results. Independent t tests comparing the behavioral scores between children with dyslexia and TR. Bonferroni‐corrected p < .0025
| TR | Children with dyslexia | ||||
|---|---|---|---|---|---|
| Males | Females | Males | Females | Chi‐squared | |
| Sex a | 25 | 11 | 22 | 17 | 1.668, p > .05 |
| Mean | SD | Mean | SD | Student's t test | |
| Age | 10.06 | 1.39 | 9.77 | 1.39 | .915, p > .05 |
| Reading measures | |||||
|
Spelling WJ—Standard score |
104.36 | 11.84 | 85.21 | 15.6 | 6.049*** |
|
Oral comprehension WJ passage comprehension—Standard score |
105.53 | 11.77 | 84.47 | 14.03 | 7.141*** |
|
Phoneme deletion CTOPP elision—Scaled score |
11.17 | 2.26 | 7.84 | 2.81 | 5.722*** |
|
Phoneme blending CTOPP blending words—Scaled score |
10.94 | 2.46 | 8.19 | 2.73 | 4.68*** |
|
Word reading TOWRE SWE—Standard score |
104.25 | 10.93 | 82.4 | 14.35 | 7.48*** |
|
Pseudoword reading TOWRE PDE—Standard score |
102.97 | 10.20 | 82.65 | 12.67 | 7.745*** |
|
Word and nonword reading ability TOWRE SWEPDE—Standard score |
104.72 | 12.27 | 82.12 | 21.29 | 5.065*** |
|
Reading comprehension TOSREC—Percentile |
54.91 | 26.63 | 25.67 | 24.39 | 5.05*** |
|
Letter and word reading WJ letter word—Standard score |
113.11 | 11.12 | 87.49 | 17.05 | 7.737*** |
|
Reading comprehension GORT—Percentile |
59.46 | 30.04 | 25.42 | 19.23 | 6.063*** |
| Visual attention | |||||
|
Visual attention TEA‐Ch sky search—Scaled score |
8.83 | 2.679 | 7.36 | 2.933 | 2.148, p = .035 |
| Executive functions | |||||
|
Flexibility DKEFS—Trail Making Test (letter‐word sequencing)—Scaled score |
10.08 | 2.49 | 7.53 | 4 | 4.06*** |
|
Inhibition DKEFS color‐word—Scaled score |
10.17 | 2.65 | 8.68 | 3.02 | 2.03, p = .046 |
|
Working memory WISC digit span—Scaled score |
11.22 | 2.81 | 9.03 | 2.74 | 3.449*** |
|
Digit naming CTOPP—Scaled score |
9.81 | 2.47 | 7.37 | 3.04 | 3.85*** |
| Basic verbal and nonverbal abilities | |||||
|
Nonverbal intelligence TONI—Percentile |
72 | 20.26 | 54.18 | 21.98 | 3.612*** |
|
Receptive vocabulary PPVT—Standard score |
120.66 | 16.163 | 106.78 | 15.232 | 3.849*** |
| Processing speed | |||||
|
Processing speed WISC coding—Scaled score |
9.56 | 2.27 | 7.8 | 2.98 | 2.863, p = .015 |
Note: Standard score is mean of 100, SD of 15; scaled score is mean of 10, SD of 3. ***p < .001.
Abbreviations: CTOPP, Comprehensive Test of Phonological Processing; DKEF, Delis‐Kaplan Executive Functions; GORT, Gray Oral Reading Test; PDE, Phonemic Decoding Efficiency; PPVT, Peabody Picture‐Vocabulary Test; SD, Standard Deviation; SWE, Sight Word Efficiency; TEA‐Ch, Test of Everyday Attention for Children; TOSREC, Test Of Silent Reading Efficiency and Comprehension; TOWRE, Test of Word Reading Efficiency; TR, typical readers; WISC, Wechsler Intelligence Scale for Children; WISC, Wechsler Intelligence Scale for Children; WJ, Woodcock‐Johnson IV Test of Achievement.
For the categorical variable gender, Pearson's chi‐squared test was utilized to identify differences between the groups.
FIGURE 3.
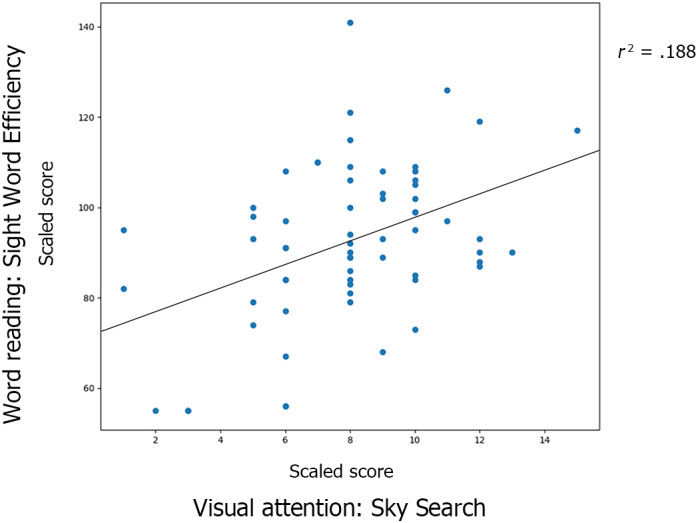
Scatterplot for the correlation between visual attention and word reading in both groups. The Y‐axis represents the scores in single word reading. The X‐axis represents the scores in the administered visual attention task. There was a significant positive correlation between visual attention score and word reading ability when examined across groups (r = .433, p < .001)
The path analysis indicated that the final model (Figure 4) had a general adequate to strong fit: chi‐square χ 2(11) = 13.854, p = .241; CFI > .95; RMSEA < .08. The model provided statistically significant results with a statistical power of 70%. Cognitive flexibility did not display a relevant contribution to the model: it was not explaining any variance in language comprehension nor decoding, so it was removed from the final model. In the final model, only two paths were not statistically significant: phonological awareness—language comprehension (β = .172, p = .136) and inhibition—language comprehension (β = −.046, p = .685). The seven remaining direct paths were significant, and the model explained 40% (R 2 = .40) of variance in reading comprehension. As expected, there was a significant direct effect of visual attention on decoding (β = .252, p < .05). The remaining observed cognitive abilities (phonological awareness and EF) were significantly contributing to decoding through direct paths.
FIGURE 4.
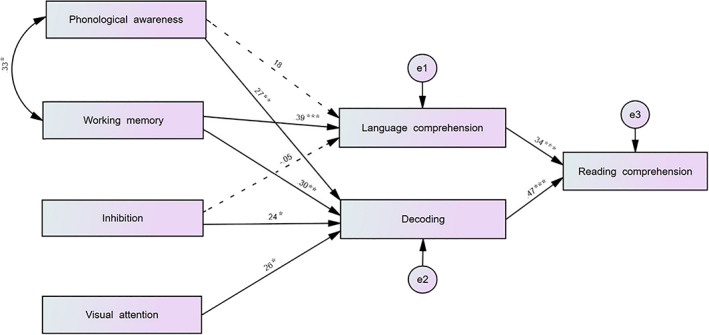
Reading comprehension model (path analysis). Graphical representation of the path analysis of the reading model, including standardized beta estimates (*p < .05, **p < .01, ***p < .001). Nonsignificant pathways are represented with dashed lines
3.2. Neuroimaging task results: Fluency task
3.2.1. Accuracy
The 2 × 2 repeated measures ANOVA revealed a Group × Condition interaction (F(1,74) = 6.094, ŋ 2 = .075, p < .01). Independent samples t tests, paired samples t tests and visual inspection of the scatterplots revealed that TR showed a significant increase in accuracy in the Deleted task condition (compared to the Still condition) while the difference between the conditions in dyslexia was smaller (see Table 2). A main effect of Condition was also found (F(1,74) = 19.53, ŋ 2 = .207, p < .001). The results indicated an overall higher accuracy for the Deleted versus the Still condition in both groups. Paired groups t tests for accuracy (Deleted vs. Still) in the fluency task results showed that only TR showed significantly higher accuracy in the Deleted condition than in the Still condition (t(35) = −4.592, p < .001), with no penalty on response time. No main effect of Group was found (F(1,74) = 2.648, ŋ 2 = .034, p > .05). When comparing the two groups using independent samples t tests, TR outperformed children with dyslexia in the Deleted condition (t(74) = −3.321, p < .001) but there were no significant differences in the Still text condition (t(74) = .027, p > .05).
TABLE 2.
Accuracy and response time differences for the Deleted and Still text conditions in children with dyslexia and TR
| Dyslexia | TR | Contrast | T(p) | |||
|---|---|---|---|---|---|---|
| Deleted text mean (SD) (A) | Still text mean (SD) (B) | Deleted text mean (SD) (C) | Still text mean (SD) (D) | |||
| Accuracy (%) | 83.9 (18) | 79 (19.9) | 96.1 (14.2) | 78.9 (24.8) | C > A | 3.321, p < .001 |
| D > B | −.027, p > .05 | |||||
| A > B | 1.463, p > .05 | |||||
| C > D | 4.592, p < .001 | |||||
| Response time (s) | 4.00 (1.01) | 4.03 (1.1) | 3.59 (0.94) | 3.77 (0.99) | C > A | −1.839, p > .05 |
| D > B | −1.033, p > .05 | |||||
| A > B | −.235, p > .05 | |||||
| C > D | −1.333, p > .05 |
Abbreviations: SD, standard deviation; TR, typical reader.
3.2.2. Response time
The 2 × 2 repeated measures ANOVA revealed no significant Group × Condition interaction (F(1,74) = .983, ŋ 2 = .013, p > .05), neither a main effect of Condition (F(1,74) = 1.59, ŋ 2 = .021, p > .05)) or Group (F(1,74) = 2.298, ŋ 2 = .030, p > .05). See Table 2. There were no significant differences between TR and children with dyslexia in their response time for any of the conditions (Still and Deleted text).
3.3. Neuroimaging results
The number of invalid volumes did not differ between groups (TR mean invalid volumes = 85.8, SD = 94.6, dyslexia: mean = 86.1, SD = 96.4; t(73) = .017, p > .05). This variable did not correlate with sex (Spearman's rho = .181, p > .05), race (Spearman's rho = .205, p > .05), handedness (Spearman's rho = .143, p > .05), age (Pearson's r = .141, p > .05), household income (Pearson's r = .116, p > .05), or maternal education (Pearson's r = .155, p > .05). The seed‐to‐voxel analysis was performed on the DAN and VAN to determine if these networks showed differential functional connectivity with other cortical areas depending on the group or experimental condition. All seed‐to‐voxel results are summarized in Supplementary Table 1. The 2 × 2 group by condition ANOVA did not reveal significant differences for Group × Condition interaction for any of the studied networks.
3.3.1. Dorsal attention network
Typical readers versus children with dyslexia
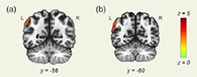
We found a greater functional connectivity between the DAN and the left angular gyrus in the TR group compared with the dyslexia group in the Still condition (see Figure 5a).
FIGURE 5.
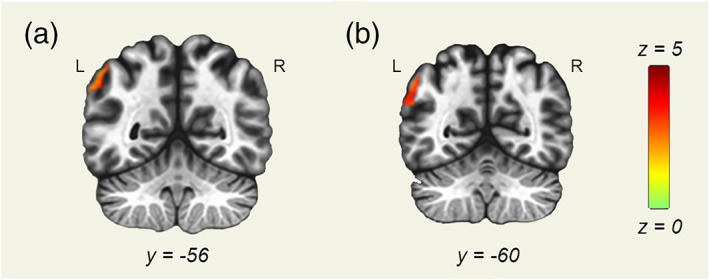
Dorsal attention network (DAN) seed‐to‐voxel analysis results. Seed‐to‐voxel analysis results with the DAN as a seed region. Coronal slices displayed in neurological orientation. (a) Still condition. Contrast: typical readers (TR) > dyslexia. y = −56. (b) Deleted condition. Contrast: TR > dyslexia. y = −60. Green color represents z scores equal to 0. Red color represents z scores equal to 5
In the Deleted condition, greater functional connectivity between the DAN and left angular gyrus was found in TR compared with the children with dyslexia (see Figure 5b). Greater functional connectivity between DAN and superior parietal and temporal clusters was found in the dyslexia group.
Typical readers
In the Still condition, significant positive functional connectivity between the DAN and several brain regions associated with reading and visual attention was found: bilateral superior parietal lobules, supramarginal gyri, angular gyri, superior temporal gyri, and precuneus. Additionally, two frontal cortical clusters (comprising a portion of the frontal eye fields) showed a significant positive functional connectivity with the DAN.
Similar to the Still condition, in the Deleted condition a significant positive functional connectivity between DAN and left and right superior parietal lobes, supramarginal gyri, left and right angular gyri, superior temporal gyri, precuneus, and frontal eye fields was found. Furthermore, the DAN displayed significant positive functional connectivity with the right dorsolateral prefrontal cortex in this condition, considered as one of the main areas underlying executive functioning.
When contrasting the two conditions, the DAN showed increased functional connectivity in the Deleted condition with regions of the right fronto‐parietal network: the right ventrolateral and dorsolateral prefrontal cortex, the right posterior parietal cortex, and the left cerebellum. The right IFG was also identified as an area showing increased functional connectivity with the DAN in the Deleted condition (compared with the Still condition).
Children with dyslexia
A significant positive functional connectivity was found in the Still condition between the DAN and bilateral lateral occipital cortex, supramarginal gyri, medial temporal gyri, precuneus, and frontal eye fields. Furthermore, the left dorsolateral prefrontal cortex, the right and left IFG and the left cerebellum displayed a significant positive functional connectivity with the DAN (Supplementary Table 1).
When analyzing the Deleted condition, we found a significant positive functional connectivity between the DAN and bilateral lateral occipital cortex, precuneus, superior parietal lobes, supramarginal gyri, medial temporal gyri, and frontal eye fields. Additionally, a significant positive functional connectivity was found with some frontal lobe regions: the dorsolateral prefrontal cortex and the inferior frontal gyri, bilaterally.
The right dorsolateral and ventrolateral prefrontal cortex, the right supramarginal gyrus, the superior parietal lobe, the right superior/medial frontal gyrus, and the left cerebellum showed increased functional connectivity with the DAN in the Deleted when compared with the Still condition.
3.3.2. Ventral attention network
Typical readers versus children with dyslexia
We did not find any region yielding significance in any of the between‐group functional connectivity analyses for the ventral attention network (see Supplementary Table 1).
Typical readers
In the Still condition, a significant positive functional connectivity between the VAN and the bilateral supramarginal gyri, angular gyri, medial and superior temporal gyri, and inferior frontal gyri was found. Additionally, two medial frontal clusters reached the significance threshold, comprising the superior frontal gyrus/anterior cingulate and the medial prefrontal cortex.
The Deleted condition analysis revealed a significant positive functional connectivity between the VAN and the bilateral supramarginal gyri, angular gyri, medial and superior temporal gyri, and inferior frontal gyri was found. Two temporo‐occipital clusters covering the left fusiform gyrus were identified as well.
The right and left dorsolateral prefrontal cortex and superior parietal lobe showed significantly positive functional connectivity in the Deleted condition when compared to the Still condition. Furthermore, the anterior cingulate cortex and the left insula displayed increased functional connectivity in this condition contrast as well. See Figure 6.
FIGURE 6.
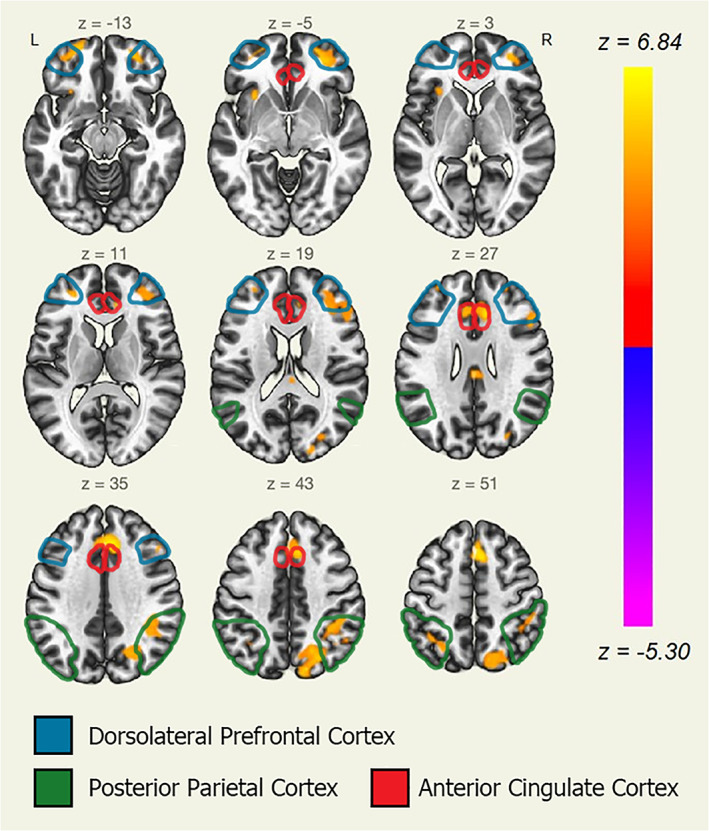
Ventral attention network (VAN) seed‐to‐voxel analysis in typical readers (Deleted > Still contrast). Seed‐to‐voxel analysis results with the VAN as a seed region. Axial slices displayed in neurological orientation. Group: typical readers. Condition: Deleted > Still contrast. Yellow color for positive z scores (yellow for z = 6.84, p < .00001). Red color for null z scores (red for z = 0, p = .5)
Children with dyslexia
A significant positive functional connectivity between VAN and bilateral supramarginal gyri, superior and medial temporal gyri, and inferior frontal gyri was observed in the Still condition. Additionally, the left fusiform gyrus and the anterior cingulate cortex were found to be significantly connected (positive functional connectivity) with the VAN.
The results for the Deleted condition were virtually identical to the results obtained in the Still condition (see Supplementary Table 1).
When contrasting both conditions, we found stronger positive functional connectivity between the VAN and the following regions in the Deleted condition: left lateral occipital cortex, the left fusiform gyrus, and the left dorsolateral prefrontal cortex.
3.4. Correlations between neuroimaging and behavioral results
A significant positive correlation was found only between the variables (a) DAN‐left angular gyrus connectivity during Deleted text reading and (b) accuracy level in Deleted text questions (Spearman's rho = .471, p < .001, see Figure 7). Furthermore, the functional connectivity index for DAN and left angular gyrus in the Deleted text condition was significantly correlated with performance in multiple language abilities (spelling, oral comprehension, phoneme deletion), and standardized reading tests (letter, word, and nonword reading; reading comprehension). See Table 3.
FIGURE 7.
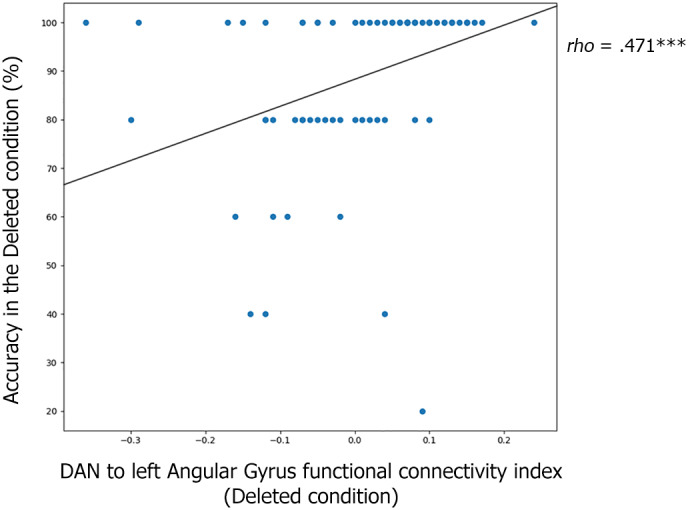
Scatterplot of the correlation between dorsal attention network (DAN) functional connectivity and reading comprehension. Graphical representation of the correlation between the variables: (a) connectivity between DAN and left angular gyrus and (b) fluent reading accuracy (Deleted condition). Both groups (typical reader [TR] and dyslexia) are represented. There was a significant positive correlation between the two variables (Spearman's rho = .471, p < .001). The X‐axis represents the connectivity index between the DAN and the left angular gyrus while performing the Deleted condition. The Y‐axis represents the accuracy level (percentage of correct responses) in the Deleted condition
TABLE 3.
DAN‐to‐left‐angular‐gyrus functional connectivity associated with language and reading scores
| Pearson's r | r 2 | F (df 1 = 1, df 2 = 73) | |
|---|---|---|---|
| Language abilities | |||
|
Spelling WJ spelling—Standard score |
.510 | .260 | 27.009*** |
|
Oral comprehension WJ passage comprehension—Standard score |
.475 | .226 | 22.479*** |
|
Phoneme deletion CTOPP elision—Scaled score |
.356 | .127 | 11.165*** |
| Reading | |||
|
Word reading TOWRE SWE—Standard score |
.567 | .322 | 36.516*** |
|
Pseudoword reading TOWRE PDE—Standard score |
.547 | .299 | 32.896*** |
|
Word and nonword reading ability TOWRE SWE PDE—Standard score |
.466 | .217 | 21.324*** |
|
Reading comprehension TOSREC percentile |
.482 | .232 | 22.99*** |
|
Letter and word reading WJ letter word—Standard score |
.578 | .334 | 38.64*** |
Note: Correlation coefficient and Linear Regression analyses for the different behavioral test scores and the average functional connectivity value of the DAN‐to‐left‐angular‐gyrus during the Deleted condition. Bonferroni Type I error correction applied. ***p < .001.
Abbreviations: CTOPP, Comprehensive Test of Phonological Processing; DAN, dorsal attention network; PDE, Phonemic Decoding Efficiency; SWE, Sight Word Efficiency; TOSREC, Test Of Silent Reading Efficiency and Comprehension; TOWRE, Test of Word Reading Efficiency; WJ, Woodcock‐Johnson IV Test of Achievement.
4. DISCUSSION
The present study aimed to determine the involvement of visual attention in fluent reading in children with dyslexia and TR, in the context of the Simple View of Reading model. In line with the a priori hypotheses, visual attention, as well as phonological awareness and EF were associated with word decoding. In line with previous evidence, Deleted text reading was associated with higher reading comprehension (Horowitz‐Kraus, Cicchino, et al., 2014). TR performed better in the Deleted than the Still condition. Children with dyslexia showed a trend toward a better performance in the Deleted condition, even though the difference did not reach the significance threshold. These results indicate the importance of convergent rapid visual and semantic processing in achieving global word recognition and reading comprehension. In the terms of the Simple View of Reading model, multiple basic cognitive processes are required for successful word decoding and, ultimately, reading comprehension. Future studies should investigate the neurobiological correlates for the putative reading improvement following an intervention based on the fluency task.
Crucially, our fMRI findings are in line with the cognitive testing‐based expansion of the SVR model described by Spencer et al. (2020). Greater functional connectivity between the left angular gyrus and the dorsal attention network was found in TR when compared with children with dyslexia in both Still and Deleted reading conditions. This functional connectivity index was found to be correlated with reading comprehension in the fluency task in both groups. The DAN plays a crucial role in visual orienting and reorienting processes (Szczepanski & Kastner, 2013). The angular gyrus is one of the brain's regions related to reading and, specifically, semantic processing (Dehaene, 2009; Humphreys & Lambon Ralph, 2015). More precisely, and according to current computational perspectives, some specific processing features of this brain area are particularly helpful for the integration of semantic information (Sporns, 2011; Vogel et al., 2013). The connectivity between the DAN and different brain regions relevant for reading (such as the angular gyrus) might be an indicator of visual attention and semantic integration features. Thus, the present results suggest a critical role for visuo‐attentional processes in the reading impairment of children with dyslexia.
The fronto‐parietal network is a bilateral large‐scale neural network associated with multiple EF, such as working memory and cognitive flexibility and is mainly involved in the initial engagement in a task and performance monitoring (Assem, Blank, Mineroff, Ademoğlu, & Fedorenko, 2020; Dosenbach et al., 2007). Reading is a very recent complex behavior in the human repertoire (in evolutionary terms) (Schlaggar & McCandliss, 2007). Complex behaviors require the synchronization/integration of multiple cognitive processes/domains which rely on distinct large‐scale neural networks (Sporns, 2011). The neuroimaging data analysis results suggest that successful integration of visual attention and EF networks is associated with fluent (fast and accurate) reading: TR showed increased functional connectivity in the Deleted condition (compared with the Still reading condition) between the ventral attention network and regions within the fronto‐parietal network, namely, the bilateral dorsolateral prefrontal cortex and posterior parietal cortex. Children with dyslexia did not display this connectivity pattern during Deleted reading, suggesting that impaired network integration might be an underlying cause for the reading deficit characterizing dyslexia. These results confirmed the a priori hypothesis and will be discussed in the context of the visual attention deficit in dyslexia and the Simple View of Reading model.
4.1. EFs, phonological awareness, and visual attention are involved in the reading impairment characterizing dyslexia
Different cognitive functions appeared impaired in children with dyslexia. TR outperformed children with dyslexia on reading tasks, phonological processing, and EF tests. However, we did not find significant differences between groups in the administered visual attention test. In the present study, only one visual attention task was administered (visual search task). Different visual attention tasks are to be utilized in future studies in order to test exhaustively the visual attention deficit previously described in children with dyslexia (Vidyasagar & Pammer, 2010). Importantly, the scores of the participants in the visual attention task were associated with their reading performance (for both typical and impaired readers), suggesting that these two skills are closely related.
Children with dyslexia performed lower than TR on various phonological and EF tasks. However, methodological issues arise when differentiating between the mentioned cognitive constructs, given that many common EF tests include linguistic stimuli, such as naming tests, word‐based and digit‐based working memory tasks, and Stroop inhibition tests (Wechsler, 2012). Furthermore, visual attention is such a basic cognitive ability that it can play a role in EF tasks. It is thus challenging to isolate EF, visuo‐attentional and phonological components, as these processes co‐occur in natural settings and show a degree of correlation (Ólafsdóttir, Kristjánsson, Gestsdóttir, Jóhannesson, & Kristjánsson, 2016).
The performed path analysis, based on the Simple View of Reading Model, indicated that phonological awareness, EF, and visual attention contribute to the decoding impairment present in children with dyslexia. In line with previous literature, the present report supports recent additions to the Simple View of Reading model suggesting that both EF and visual attention play a crucial role in reading comprehension and, specifically, in the severity of the reading difficulties characterizing dyslexia (Parkosadze, Tatishvili, Lomidze, & Kunchulia, 2019; Spencer et al., 2020). Considering the close relationship between the abovementioned psychological constructs, we suggest that the integration of phonological, executive, and visuo‐attentional skills necessary for reading is impaired in children with dyslexia. The neurobiological findings discussed in the next paragraphs support this suggestion.
4.2. Reading, visual attention, and EF brain areas show lower functional connectivity in children with dyslexia
The fMRI seed‐to‐voxel analysis informed neurological differences between groups while performing the fluency task. Significant between‐group differences were identified in the angular gyrus (Brodmann area 39) and the DAN. TR showed greater functional connectivity between these brain regions during both reading conditions when compared with children with dyslexia. The angular gyrus is a crucial reading‐related region (Dehaene, 2009) involved in semantic processing (Buchweitz, Mason, Meschyan, Keller, & Just, 2014; Chou, Chen, Wu, & Booth, 2009; Cutting et al., 2006). The abnormal function of the angular gyrus has been described in individuals with dyslexia (Norton et al., 2015). Previous research reported reduced connectivity between the left angular gyrus and other reading and semantics‐related areas (Horwitz, Rumsey, & Donohue, 1998; Sandak, Mencl, Frost, & Pugh, 2004). Our results confirm and extend this finding; we found a disconnection between the left angular gyrus and visual orienting‐related regions. The higher functional connectivity between this region and the DAN in the TR group found in the present experiment points toward the involvement of not only the left angular gyrus but also the DAN (a visual attention network) in the reading difficulties of individuals with dyslexia. Furthermore, the functional connectivity of the left angular gyrus and the DAN during Deleted text reading correlated with performance in the task for both groups, with higher functional connectivity values indicating better performance. The fact that this connectivity value was strongly associated with reading and EF performance suggests that some specific functional integration properties of the DAN underlie certain cognitive abilities necessary for reading. The initial predictions were thus confirmed: using a design combining neuroimaging and cognitive testing, it was found that the involvement of visual attention areas together with reading areas is crucial in the performance of a reading task, predicting successful or unsuccessful reading comprehension.
The neuroimaging data analysis revealed increased functional connectivity for the dyslexia group (compared to TR) between the DAN and (a) the left superior temporal gyrus and (b) supramarginal gyrus and in the Deleted condition. This hyperconnectivity involving the putative Wernicke's area supports the existence of a previously described compensation mechanism (Saralegui et al., 2014). Adopting a mechanistic perspective, children with dyslexia may rely on auditory and phonological loop areas (Papagno et al., 2017) in difficult reading conditions. This phenomenon is to be addressed in future research.
The VAN seed‐to‐voxel analysis revealed increased functional connectivity between the regions comprising the VAN and several regions within the fronto‐parietal network. This connectivity pattern was present only in TR but not in children with dyslexia when reading Deleted text (compared with Still text reading). This result suggests that the fluency task elicits visual attention and EF processes, and thus, that both cognitive domains are involved in fluent reading. This suggestion is in line with a previous report of the link between increased connectivity between visual attention‐related and EF‐related brain regions and increased reading ability (Twait & Horowitz‐Kraus, 2019).
The VAN displayed a pattern of increased functional connectivity with the anterior cingulate cortex, left insula, bilateral dorsolateral prefrontal cortex, and superior parietal lobe in the Deleted condition when compared to standard reading in TR. These areas constitute the fronto‐parietal network, which is engaged in top‐down cognitive control (Chand, Wu, Hajjar, & Qiu, 2017; Mao, Kanai, Ding, Bi, & Qiu, 2020). The results indicate that reading with an imposed time constraint enhances the connectivity between visual attention and EF brain regions in TR. This pattern of connectivity was not present in children with dyslexia. We found that impaired readers recruited left hemisphere brain areas related to orthographic processing (the putative Visual Word Form Area, see Vogel et al., 2014) and the dorsolateral prefrontal cortex. The connectivity/integration scores between the fronto‐parietal network together with the VAN were low in the group with dyslexia. This might have been the reason for their low reading comprehension scores (compared with the TR).
Basic visual processing temporo‐occipital areas were not found to have significantly different functional connectivity with the VAN and DAN, neither between‐groups nor between‐conditions. Even though it may sound counterintuitive, this is in line with our predictions given that we hypothesized a deficient integration of visual attention, EF and semantics‐related cortical networks and areas. Basic visual processing (shape, color, motion) deficit is not a characteristic trait of dyslexia and thus, visual occipital areas were not expected to show connectivity differences with any other brain regions.
Altogether, the results of the analysis suggest that visual attention is an important basic cognitive skill underlying the reading impairment in dyslexia. TR and children with dyslexia display distinct brain network connectivity patterns when reading. TR recruit the DAN together with the left angular gyrus to a higher degree than children with dyslexia and this functional connectivity index correlates with reading performance. These results are in line with previous research pointing toward the phenomenon of angular gyrus disconnection being a key factor in dyslexia (Horwitz et al., 1998; Sandak et al., 2004). The VAN is functionally connected to the fronto‐parietal network in TR but not in children with dyslexia, suggesting deficiencies in the integration of visual attention processes and executive functioning in dyslexia.
4.3. Study limitations
The present study has several limitations, which were taken into account throughout all research steps. First, the experimental fluent reading task (the fluency task) did not target visual attention exclusively. Other cognitive skills, mainly within the EF domain were putatively elicited during the Deleted condition (Breznitz et al., 2013). Future studies aimed at discerning the different cognitive abilities targeted by the Deleted condition should be conducted. Second, the observed between‐groups functional connectivity differences in the seed‐to‐voxel analysis cannot be attributed exclusively to visual attention, even if the studied networks are known to be the main neurobiological correlate for this ability. The use of phonologically meaningful stimuli prevents any kind of post hoc explanation of the results ignoring the linguistic aspect of the task. Further research utilizing distinct paradigms and pure visual attention engaging tasks with no reading involved should be carried out on children with dyslexia to confirm the predictions of the visual attention theory (Vidyasagar & Pammer, 2010). To our knowledge, no experimental data are available examining the brain activity of children with dyslexia performing a visual attention task that does not contain linguistic material. Further work should examine whether training the visual aspect of reading enhances the connectivity of visual attention regions with reading and EF regions in TR children as well as children with dyslexia and improves the reading performance of the latter. This kind of experimental research project is needed to validate our conclusions and provide a better understanding of dyslexia.
5. CONCLUSIONS
Children who are TR performed better in the Deleted text reading condition when compared to Still text reading. Our results indicate that the attention brain networks (VAN and DAN) in the TR group increased their functional connectivity with different reading and EF‐related areas during the Deleted reading condition (compared with standard reading). Children with dyslexia failed to achieve these patterns of relevant brain connections for succeeding at the task. The TR group successfully recruited a key region of the language network (the left angular gyrus), showing increased functional connectivity with the DAN, and the connectivity value between these regions was associated with improved performance on the reading task. The faulty simultaneous activation of the angular gyrus together with visual attention‐related brain areas might be one of the factors explaining the visual attention processing bias in dyslexia and, subsequently, reading impairment (Valdois, Bosse, & Tainturier, 2004; Vidyasagar & Pammer, 2010). Furthermore, we did not observe significant synchrony (integration) between EF and visual attention brain areas in children with dyslexia, whereas TR elicited this mechanism in order to achieve fluent reading.
Overall, the present study allowed us to draw a set of reading‐related neural regions whose connectivity with visual attention‐related brain areas was predicting the performance of typical and impaired readers in a simultaneous reading task. Our results are in line with existing theoretical perspectives suggesting a key role for visual attention in the reading deficit characterizing dyslexia (Vidyasagar & Pammer, 2010).
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
Tzipi Horowitz‐Kraus designed the study and participated in data collection. Tzipi Horowitz‐Kraus, Jennifer Vannest, and Scott Holland designed the fMRI task. Nikolay Taran, Rola Farah, and Tzipi Horowitz‐Kraus analyzed the data and wrote the manuscript. Tzipi Horowitz‐Kraus and Rola Farah supervised the data analysis and findings of the work. Mark DiFrancesco, Jennifer Vannest, Keri Rosch, Bradley L. Schlaggar, Mekibib Altaye, and Tzipi Horowitz‐Kraus monitored the data collection. Bradley L. Schlaggar, Keri Rosch, and Tzipi Horowitz‐Kraus revised the manuscript.
ETHICS STATEMENT
The present research project was conducted in accordance with the Declaration of Helsinki, reviewed and approved by the institutional review board of the CCHMC.
Supporting information
Supplementary Table 1 Seed‐to‐voxel analysis results for the Dorsal and Ventral Attention Networks. ACC: Anterior Cingulate Cortex; AG: Angular Gyrus; CBL: Cerebellum; dlPFC: dorsolateral Prefrontal Cortex; FEF: Frontal Eye Fields; IFG: Inferior Frontal Gyrus; ITG: Inferior Temporal Gyrus; LOC: Lateral Occipital Cortex; MFG: Medial Frontal Gyrus; mPFC: medial Prefrontal Cortex; MTG: Medial Temporal Gyrus; SFG: Superior Frontal Gyrus; SMG: Supramarginal Gyrus; SPL: Superior Parietal Lobe; vlPFC: ventrolateral Prefrontal Cortex. R/r: right, L/l: left.
Supplementary Table 2. Fluency task stimuli.
ACKNOWLEDGMENT
This study was supported by the National Institute of Child Health and Human Development (R01 HD086011; PI: T. H.‐K.).
Taran, N. , Farah, R. , DiFrancesco, M. , Altaye, M. , Vannest, J. , Holland, S. , Rosch, K. , Schlaggar, B. L. , & Horowitz‐Kraus, T. (2022). The role of visual attention in dyslexia: Behavioral and neurobiological evidence. Human Brain Mapping, 43(5), 1720–1737. 10.1002/hbm.25753
Funding information Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: R01 HD086011
DATA AVAILABILITY STATEMENT
Data are currently not publicly available.
REFERENCES
- American Psychiatric Association . (2007). APA dictionary of psychology. Washington, DC: American Psychological Association. [Google Scholar]
- American Psychiatric Association . (2015). APA dictionary of psychology. In VandenBos, G. R. (Ed.). (2nd ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Assem, M. , Blank, I. A. , Mineroff, Z. , Ademoğlu, A. , & Fedorenko, E. (2020). Activity in the fronto‐parietal multiple‐demand network is robustly associated with individual differences in working memory and fluid intelligence. Cortex, 131, 1–16. 10.1016/j.cortex.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa, T. , Rodrigues, C. C. , Mello, C. B. , Silva, M. , & Bueno, O. F. A. (2019). Executive functions in children with dyslexia. Arquivos de Neuro‐Psiquiatria, 77(4), 254–259. 10.1590/0004-282x20190033 [DOI] [PubMed] [Google Scholar]
- Behzadi, Y. , Restom, K. , Liau, J. , & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscaldi, M. , Fischer, B. , & Hartnegg, K. (2016). Voluntary saccadic control in dyslexia. Perception, 29(5), 531–542. 10.1068/p2666a [DOI] [PubMed] [Google Scholar]
- Breznitz, Z. , Shaul, S. , Horowitz‐Kraus, T. , Sela, I. , Nevat, M. , & Karni, A. (2013). Enhanced reading by training with imposed time constraint in typical and dyslexic adults. Nature Communications, 4(1), 1–6. 10.1038/ncomms2488 [DOI] [PubMed] [Google Scholar]
- Brosnan, M. , Demetre, J. , Hamill, S. , Robson, K. , Shepherd, H. , & Cody, G. (2002). Executive functioning in adults and children with developmental dyslexia. Neuropsychologia, 40(12), 2144–2155. 10.1016/s0028-3932(02)00046-5 [DOI] [PubMed] [Google Scholar]
- Brown, L. , Sherbenou, R. , & Johnsen, S. (2010). Test of nonverbal intelligence (4th ed.). Austin, TX: Pro‐Ed. [Google Scholar]
- Buchweitz, A. , Mason, R. A. , Meschyan, G. , Keller, T. A. , & Just, M. A. (2014). Modulation of cortical activity during comprehension of familiar and unfamiliar text topics in speed reading and speed listening. Brain and Language, 139, 49–57. 10.1016/j.bandl.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand, G. B. , Wu, J. , Hajjar, I. , & Qiu, D. (2017). Interactions of the salience network and its subsystems with the default‐mode and the central‐executive networks in normal aging and mild cognitive impairment. Brain Connectivity, 7(7), 401–412. 10.1089/brain.2017.0509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, T. L. , Chen, C. W. , Wu, M. Y. , & Booth, J. R. (2009). The role of inferior frontal gyrus and inferior parietal lobule in semantic processing of Chinese characters. Experimental Brain Research, 198(4), 465–475. 10.1007/s00221-009-1942-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric, R. , Wolf, D. H. , Power, J. D. , Roalf, D. R. , Baum, G. L. , Ruparel, K. , … Satterthwaite, T. D. (2017). Benchmarking of participant‐level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage, 154, 174–187. 10.1016/j.neuroimage.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. L. (2002). Control of goal‐directed and stimulus‐driven attention in the brain. Nature Reviews. Neuroscience, 3(3), 201–215. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- Cristofori, I. , Cohen‐Zimerman, S. , & Grafman, J. (2019). Executive functions. In Handbook of clinical neurology (Vol. 163, pp. 197–219). Amsterdam, Netherlands: Elsevier. 10.1016/b978-0-12-804281-6.00011-2 [DOI] [PubMed] [Google Scholar]
- Cutting, L. E. , Clements, A. M. , Courtney, S. , Rimrodt, S. L. , Schafer, J. G. B. , Bisesi, J. , … Pugh, K. R. (2006). Differential components of sentence comprehension: Beyond single word reading and memory. NeuroImage, 29(2), 429–438. 10.1016/j.neuroimage.2005.07.057 [DOI] [PubMed] [Google Scholar]
- Dehaene, S. (2009). Reading in the brain: The science and evolution of a human invention. Westminster, London: Penguin Books. [Google Scholar]
- Delis, D. C. , Kaplan, E. , & Kramer, J. H. (2011). Delis‐Kaplan Executive Function System (D–KEFS). London, England: Pearson. [Google Scholar]
- Dosenbach, N. U. , Fair, D. A. , Miezin, F. M. , Cohen, A. L. , Wenger, K. K. , Dosenbach, R. A. , … Petersen, S. E. (2007). Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America, 104(26), 11073–11078. 10.1073/pnas.0704320104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, L. M. , & Dunn, D. M. (2007). Peabody picture vocabulary test. Bloomington, MN: NCS Pearson Inc. [Google Scholar]
- Facoetti, A. , Corradi, N. , Ruffino, M. , Gori, S. , & Zorzi, M. (2010). Visual spatial attention and speech segmentation are both impaired in preschoolers at familial risk for developmental dyslexia. Dyslexia, 16(3), 226–239. 10.1002/dys.413 [DOI] [PubMed] [Google Scholar]
- Facoetti, A. , Lorusso, M. L. , Paganoni, P. , Umilta, C. , & Mascetti, G. G. (2003). The role of visuospatial attention in developmental dyslexia: Evidence from a rehabilitation study. Brain Research. Cognitive Brain Research, 15(2), 154–164. 10.1016/s0926-6410(02)00148-9 [DOI] [PubMed] [Google Scholar]
- Farah, R. , Ionta, S. , & Horowitz‐Kraus, T. (2021). Neuro‐behavioral correlates of executive dysfunctions in dyslexia over development from childhood to adulthood. Frontiers in Psychology, 12, 708863. 10.3389/fpsyg.2021.708863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, E. S. , Shen, X. , Holahan, J. M. , Scheinost, D. , Lacadie, C. , Papademetris, X. , … Constable, R. T. (2014). Disruption of functional networks in dyslexia: A whole‐brain, data‐driven analysis of connectivity. Biological Psychiatry, 76(5), 397–404. 10.1016/j.biopsych.2013.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito, A. , Harrison, B. J. , Zalesky, A. , & Simons, J. S. (2012). Competitive and cooperative dynamics of large‐scale brain functional networks supporting recollection. Proceedings of the National Academy of Sciences of the United States of America, 109(31), 12788–12793. 10.1073/pnas.1204185109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini, S. , Gori, S. , Ruffino, M. , Pedrolli, K. , & Facoetti, A. (2012). A causal link between visual spatial attention and reading acquisition. Current Biology, 22(9), 814–819. 10.1016/j.cub.2012.03.013 [DOI] [PubMed] [Google Scholar]
- Franceschini, S. , Gori, S. , Ruffino, M. , Viola, S. , Molteni, M. , & Facoetti, A. (2013). Action video games make dyslexic children read better. Current Biology, 23(6), 462–466. 10.1016/j.cub.2013.01.044 [DOI] [PubMed] [Google Scholar]
- Hari, R. , & Renvall, H. (2001). Impaired processing of rapid stimulus sequences in dyslexia. Trends in Cognitive Sciences, 5(12), 525–532. 10.1016/s1364-6613(00)01801-5 [DOI] [PubMed] [Google Scholar]
- Harm, M. W. , & Seidenberg, M. S. (2004). Computing the meanings of words in reading: Cooperative division of labor between visual and phonological processes. Psychological Review, 111(3), 662–720. 10.1037/0033-295x.111.3.662 [DOI] [PubMed] [Google Scholar]
- Hoover, W. A. , & Gough, P. B. (1990). The simple view of reading. Reading and Writing: An Interdisciplinary Journal, 2(2), 127–160. [Google Scholar]
- Horowitz‐Kraus, T. , Buck, C. , & Dorrmann, D. (2016). Altered neural circuits accompany lower performance during narrative comprehension in children with reading difficulties: An fMRI study. Annals of Dyslexia, 66(3), 301–318. 10.1007/s11881-016-0124-4 [DOI] [PubMed] [Google Scholar]
- Horowitz‐Kraus, T. , Cicchino, N. , Amiel, M. , Holland, S. K. , & Breznitz, Z. (2014). Reading improvement in English‐ and Hebrew‐speaking children with reading difficulties after reading acceleration training. Annals of Dyslexia, 64(3), 183–201. 10.1007/s11881-014-0093-4 [DOI] [PubMed] [Google Scholar]
- Horowitz‐Kraus, T. , DiFrancesco, M. , Kay, B. , Wang, Y. , & Holland, S. K. (2015). Increased resting‐state functional connectivity of visual‐ and cognitive‐control brain networks after training in children with reading difficulties. NeuroImage: Clinical, 8, 619–630. 10.1016/j.nicl.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz‐Kraus, T. , Vannest, J. J. , Kadis, D. S. , Cicchino, N. D. , Wang, Y. Y. , & Holland, S. K. (2014). Reading acceleration training changes brain circuitry in children with reading difficulties. Brain and Behavior, 4(6), 886–902. 10.1002/brb3.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, B. , Rumsey, J. M. , & Donohue, B. C. (1998). Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy of Sciences of the United States of America, 95(15), 8939–8944. 10.1073/pnas.95.15.8939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys, G. F. , & Lambon Ralph, M. A. (2015). Fusion and fission of cognitive functions in the human parietal cortex. Cerebral Cortex, 25(10), 3547–3560. 10.1093/cercor/bhu198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igelstrom, K. M. , & Graziano, M. S. A. (2017). The inferior parietal lobule and temporoparietal junction: A network perspective. Neuropsychologia, 105, 70–83. 10.1016/j.neuropsychologia.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Joshi, R. M. (2019). The componential model of reading (CMR): Implications for assessment and instruction of literacy problems. In Kilpatrick D. A., Joshi R. M., & Wagner R. K. (Eds.), Reading development and difficulties: Bridging the gap between research and practice (pp. 3–18). Cham: Springer International Publishing. [Google Scholar]
- Karni, A. , & Sagi, D. (1993). The time course of learning a visual skill. Nature, 365(6443), 250–252. 10.1038/365250a0 [DOI] [PubMed] [Google Scholar]
- Kline, R. B. (2016). Principles and practice of structural equation modeling (4th ed.). New York, NY: Guilford Press. [Google Scholar]
- Kovelman, I. , Norton, E. S. , Christodoulou, J. A. , Gaab, N. , Lieberman, D. A. , Triantafyllou, C. , … Gabrieli, J. D. (2012). Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cerebral Cortex, 22(4), 754–764. 10.1093/cercor/bhr094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy, L. C. , Krishnamurthy, V. , Crosson, B. , Rothman, D. L. , Schwam, D. M. , Greenberg, D. , … Morris, R. D. (2019). Strength of resting state functional connectivity and local GABA concentrations predict oral reading of real and pseudo‐words. Scientific Reports, 9(1), 11385. 10.1038/s41598-019-47889-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langerberg, D. L. , Correro, G. , Ehri, L. , Ferguson, G. , Garza, N. , Kamil, M. L. , … Yatvin, J. (2000). Report of the national reading panel. Washington, DC: U.S. Department of Education. [Google Scholar]
- Lawton, T. (2016). Improving dorsal stream function in dyslexics by training figure/ground motion discrimination improves attention, reading fluency, and working memory. Frontiers in Human Neuroscience, 10, 397. 10.3389/fnhum.2016.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levene, H. (1960). Robust tests for equality of variances. In Olkin I. (Ed.), Contributions to probability and statistics. Palo Alto, CA: Stanford University Press. [Google Scholar]
- Lorusso, M. L. , Facoetti, A. , Paganoni, P. , Pezzani, M. , & Molteni, M. (2006). Effects of visual hemisphere‐specific stimulation versus reading‐focused training in dyslexic children. Neuropsychological Rehabilitation, 16(2), 194–212. 10.1080/09602010500145620 [DOI] [PubMed] [Google Scholar]
- Manly, T. , Anderson, V. , Nimmo‐Smith, I. , Turner, A. , Watson, P. , & Robertson, I. H. (2001). The differential assessment of children's attention: The test of everyday Attention for children (TEA‐Ch), normative sample and ADHD performance. Journal of Child Psychology and Psychiatry, 42(8), 1065–1081. 10.1111/1469-7610.00806 [DOI] [PubMed] [Google Scholar]
- Mao, Y. , Kanai, R. , Ding, C. , Bi, T. , & Qiu, J. (2020). Temporal variability of brain networks predicts individual differences in bistable perception. Neuropsychologia, 142, 107426. 10.1016/j.neuropsychologia.2020.107426 [DOI] [PubMed] [Google Scholar]
- Melby‐Lervag, M. , Lyster, S. A. , & Hulme, C. (2012). Phonological skills and their role in learning to read: A meta‐analytic review. Psychological Bulletin, 138(2), 322–352. 10.1037/a0026744 [DOI] [PubMed] [Google Scholar]
- Norton, E. S. , Beach, S. D. , & Gabrieli, J. D. (2015). Neurobiology of dyslexia. Current Opinion in Neurobiology, 30, 73–78. 10.1016/j.conb.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ólafsdóttir, I. M. , Kristjánsson, T. , Gestsdóttir, S. , Jóhannesson, Ó. I. , & Kristjánsson, Á. (2016). Understanding visual attention in childhood: Insights from a new visual foraging task. Cognitive Research: Principles and Implications, 1(1), 18. 10.1186/s41235-016-0016-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagno, C. , Comi, A. , Riva, M. , Bizzi, A. , Vernice, M. , Casarotti, A. , … Bello, L. (2017). Mapping the brain network of the phonological loop. Human Brain Mapping, 38(6), 3011–3024. 10.1002/hbm.23569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkosadze, K. , Tatishvili, T. , Lomidze, N. , & Kunchulia, M. (2019). Issues of visual attention and executive functions in children with dyslexia. Georgian Medical News, 287, 61–66. [PubMed] [Google Scholar]
- Peters, J. L. , De Losa, L. , Bavin, E. L. , & Crewther, S. G. (2019). Efficacy of dynamic visuo‐attentional interventions for reading in dyslexic and neurotypical children: A systematic review. Neuroscience and Biobehavioral Reviews, 100, 58–76. 10.1016/j.neubiorev.2019.02.015 [DOI] [PubMed] [Google Scholar]
- Petersen, S. E. , & Posner, M. I. (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience, 35, 73–89. 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, R. L. , & Pennington, B. F. (2012). Developmental dyslexia. Lancet, 379(9830), 1997–2007. 10.1016/s0140-6736(12)60198-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, R. L. , & Pennington, B. F. (2015). Developmental dyslexia. Annual Review of Clinical Psychology, 11(1), 283–307. 10.1146/annurev-clinpsy-032814-112842 [DOI] [PubMed] [Google Scholar]
- Posner, M. , & Petersen, S. (1990). The attention system of the human brain. Annual Review of Neuroscience, 13, 25–42. 10.1146/annurev.ne.13.030190.000325 [DOI] [PubMed] [Google Scholar]
- Power, J. D. , Cohen, A. L. , Nelson, S. M. , Wig, G. S. , Barnes, K. A. , Church, J. A. , … Petersen, S. E. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston, J. , & Edwards, M. L. (2010). Phonological awareness and types of sound errors in preschoolers with speech sound disorders. Journal of Speech, Language, and Hearing Research, 53(1), 44–60. 10.1044/1092-4388(2009/09-0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan, F. , Kronbichler, M. , & Wimmer, H. (2009). Functional abnormalities in the dyslexic brain: A quantitative meta‐analysis of neuroimaging studies. Human Brain Mapping, 30(10), 3299–3308. 10.1002/hbm.20752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandak, R. , Mencl, W. E. , Frost, S. J. , & Pugh, K. R. (2004). The neurobiological basis of skilled and impaired reading: Recent findings and new directions. Scientific Studies of Reading, 8(3), 273–292. 10.1207/s1532799xssr0803_6 [DOI] [Google Scholar]
- Saralegui, I. , Ontañón, J. M. , Fernandez‐Ruanova, B. , Garcia‐Zapirain, B. , Basterra, A. , & Sanz‐Arigita, E. J. (2014). Reading networks in children with dyslexia compared to children with ocular motility disturbances revealed by fMRI. Frontiers in Human Neuroscience, 8, 936. 10.3389/fnhum.2014.00936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar, B. L. , & McCandliss, B. D. (2007). Development of neural systems for reading. Annual Review of Neuroscience, 30, 475–503. 10.1146/annurev.neuro.28.061604.135645 [DOI] [PubMed] [Google Scholar]
- Schrank, F. , Mather, N. , & McGrew, K. (2014). Woodcock‐Johnson IV tests of achievement. Rolling Meadows, IL: Riverside. [Google Scholar]
- Schurz, M. , Wimmer, H. , Richlan, F. , Ludersdorfer, P. , Klackl, J. , & Kronbichler, M. (2015). Resting‐state and task‐based functional brain connectivity in developmental dyslexia. Cerebral Cortex, 25(10), 3502–3514. 10.1093/cercor/bhu184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro, S. , & Wilk, M. (1965). An analysis of variance test for normality. Biometrika, 53(4), 591–611. [Google Scholar]
- Smith, S. , Fox, P. T. , Miller, K. L. , Glahn, D. C. , Fox, P. M. , Mackay, C. E. , … Beckmann, C. F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13040–13045. 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith‐Spark, J. H. , Henry, L. A. , Messer, D. J. , Edvardsdottir, E. , & Ziecik, A. P. (2016). Executive functions in adults with developmental dyslexia. Research in Developmental Disabilities, 53‐54, 323–341. 10.1016/j.ridd.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Spencer, M. , Richmond, M. C. , & Cutting, L. E. (2020). Considering the role of executive function in reading comprehension: A structural equation modeling approach. Scientific Studies of Reading, 24(3), 179–199. 10.1080/10888438.2019.1643868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns, O. (2011). Networks of the brain. Cambridge, MA: MIT Press. [Google Scholar]
- Stevens, W. D. , Kravitz, D. J. , Peng, C. S. , Tessler, M. H. , & Martin, A. (2017). Privileged functional connectivity between the visual word form area and the language system. The Journal of Neuroscience, 37(21), 5288–5297. 10.1523/jneurosci.0138-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streiner, D. L. (2005). Finding our way: An introduction to path analysis. The Canadian Journal of Psychiatry, 50(2), 115–122. 10.1177/070674370505000207 [DOI] [PubMed] [Google Scholar]
- Szczepanski, S. , & Kastner, S. (2013). Shifting attentional priorities: Control of spatial attention through hemispheric competition. The Journal of Neuroscience, 33, 5411–5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen, J. , Wagner, R. , & Rashotte, C. (1999). Test of word reading efficiency (TOWRE). Austin, TX: Pro‐Ed. [Google Scholar]
- Tran, S. M. , McGregor, K. M. , James, G. A. , Gopinath, K. , Krishnamurthy, V. , Krishnamurthy, L. C. , & Crosson, B. (2018). Task‐residual functional connectivity of language and attention networks. Brain and Cognition, 122, 52–58. 10.1016/j.bandc.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twait, E. , & Horowitz‐Kraus, T. (2019). Functional connectivity of cognitive control and visual regions during verb generation is related to improved reading in children. Brain Connectivity, 9(6), 500–507. 10.1089/brain.2018.0655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdois, S. , Bosse, M. L. , & Tainturier, M. J. (2004). The cognitive deficits responsible for developmental dyslexia: Review of evidence for a selective visual attentional disorder. Dyslexia, 10(4), 339–363. 10.1002/dys.284 [DOI] [PubMed] [Google Scholar]
- Vannest, J. , Rajagopal, A. , Cicchino, N. D. , Franks‐Henry, J. , Simpson, S. M. , Lee, G. , … Holland, S. K. (2014). Factors determining success of awake and asleep magnetic resonance imaging scans in nonsedated children. Neuropediatrics, 45(6), 370–377. 10.1055/s-0034-1387816 [DOI] [PubMed] [Google Scholar]
- Vidyasagar, T. R. (2019). Visual attention and neural oscillations in reading and dyslexia: Are they possible targets for remediation? Neuropsychologia, 130, 59–65. 10.1016/j.neuropsychologia.2019.02.009 [DOI] [PubMed] [Google Scholar]
- Vidyasagar, T. R. , & Pammer, K. (2010). Dyslexia: a deficit in visuo‐spatial attention, not in phonological processing. Trends in Cognitive Sciences, 14(2), 57–63. 10.1016/j.tics.2009.12.003 [DOI] [PubMed] [Google Scholar]
- Vogel, A. C. , Church, J. A. , Power, J. D. , Miezin, F. M. , Petersen, S. E. , & Schlaggar, B. L. (2013). Functional network architecture of reading‐related regions across development. Brain and Language, 125(2), 231–243. 10.1016/j.bandl.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, A. C. , Petersen, S. E. , & Schlaggar, B. L. (2014). The VWFA: It's not just for words anymore. Frontiers in Human Neuroscience, 8, 88. 10.3389/fnhum.2014.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, R. , Torgesen, J. , Rashotte, C. , & Pearson, N. (2010). Test of silent Reading efficiency and comprehension, grades 1–12 (TOSREC). Austin, TX: Pro‐Ed. [Google Scholar]
- Wagner, R. , Torgesen, J. , Rashotte, C. , & Pearson, N. (2013). The Comprehensive Test of Phonological Processing, (CTOPP‐2). Austin, TX: Pro‐Ed. [Google Scholar]
- Wechsler, D. (2012). Wechsler preschool and primary scale of intelligence (4th ed.). London, England: Pearson. [Google Scholar]
- Whitfield‐Gabrieli, S. , & Nieto‐Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wiederholt, J. L. , & Bryant, B. R. (2012). Gray Oral Reading Tests. Fifth Edition. (GORT‐5). Austin, TX: Pro‐Ed. [Google Scholar]
- Worsley, K. J. , Marrett, S. , Neelin, P. , Vandal, A. C. , Friston, K. J. , & Evans, A. C. (1996). A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping, 4(1), 58–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Seed‐to‐voxel analysis results for the Dorsal and Ventral Attention Networks. ACC: Anterior Cingulate Cortex; AG: Angular Gyrus; CBL: Cerebellum; dlPFC: dorsolateral Prefrontal Cortex; FEF: Frontal Eye Fields; IFG: Inferior Frontal Gyrus; ITG: Inferior Temporal Gyrus; LOC: Lateral Occipital Cortex; MFG: Medial Frontal Gyrus; mPFC: medial Prefrontal Cortex; MTG: Medial Temporal Gyrus; SFG: Superior Frontal Gyrus; SMG: Supramarginal Gyrus; SPL: Superior Parietal Lobe; vlPFC: ventrolateral Prefrontal Cortex. R/r: right, L/l: left.
Supplementary Table 2. Fluency task stimuli.
Data Availability Statement
Data are currently not publicly available.


