Abstract
Acute disseminated encephalomyelitis (ADEM) is an inflammatory emyelinating disease of the central nervous system that is usually considered a monophasic disease Post-vaccination ADEM has been associated with several vaccines, however, there is scarce information related to SARS-CoV-2 vaccines. We present the case of a 26- year-old female who suffered from ADEM four weeks after Gam-COVID-Vac administration.
Keywords: SARS-CoV-2 vaccination, Acute disseminated encephalomyelitis, Corticosteroid therapy
Highlights
-
●
Acute disseminated encephalomyelitis is a monophasic acute non-vasculitic inflammatory demyelinating disorder of the central nervous system.
-
●
Pathogenesis is suspected to be related to an autoimmune response to myelin triggered by infection or immunisation via molecular mimicry.
-
●
Once the SARS-CoV-2 infection was declared a global pandemic, vaccines became one the most important strategies to reduce mortality.
-
●
There is current uncertainty about the safety and efficacy of vaccines in all populations of interest.
1. Introduction
Acute disseminated encephalomyelitis (ADEM) is a monophasic acute non-vasculitic inflammatory demyelinating disorder of the central nervous system (CNS) characterized by diffuse neurologic signs and symptoms coupled with evidence of multifocal lesions of demyelination on neuroimaging (Farag and Hesham, 2013).
Pathogenesis is suspected to be related to an autoimmune response to myelin triggered by infection or immunization via molecular mimicry (Lin et al., 2007). ADEM most often occurs in childhood and it has been estimated to account for 10–15% of acute encephalitis cases in the United States (Leake et al., 2004a). Uniform diagnostic criteria did not exist until the expert-defined consensus developed by the International Pediatric Multiple Sclerosis Society Group published in 2007, which was updated in 2013 (Lauren et al., 2013). However, these criteria have only been performed for the pediatric population and we have not yet had criteria for the adult population.
Since the SARS-CoV-2 infection has been declared a global pandemic, vaccines have become one the most important strategies to reduce mortality. In response, massive vaccination campaigns have been developed around the world (Alberto et al., 2021).
Post-vaccination ADEM has been associated with several vaccines, however, there is scarce information related to SARS-CoV-2 vaccines (Li et al., 1101). We present the case of a 26-year-old female who suffered from ADEM four weeks after Gam-COVID-Vac administration.
2. Case report
The patient was admitted after 10 days of disorientation, inappropriate behavior, headache and gait imbalance. She had no personal history of infection, fever or weight loss. Her family history was negative for autoimmune diseases. Four weeks before admission she received a first dose of Gam-COVID-Vac vaccine (human adenovirus viral vector).
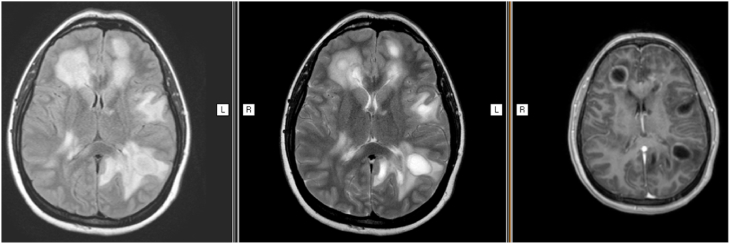
The initial examination revealed deferred memory, hypoprosexia, anosognosia, incoherent speech and visuospatial failures. Right upper limb weakness and gait ataxia were also noted. Brain Magnetic Resonance Imaging (MRI) showed nodular hyperintense lesions on T2-weighted image and fluid attenuated inversion recovery without restricted diffusion on diffusion. Marked vasogenic edema and T1-weighted image post contrast incomplete annular enhancement was observed (Fig. 1).
Fig. 1.
Brain MRI a). T2 brain MRI showing hyperintensity involving frontal, temporal and parietal lobe with perilesional edema. (b). FLAIR image with the same hyperintensity lesions with perilesional edema (c). Gadolinium T1 sequence showing multiple focal pathological ring enhancement.
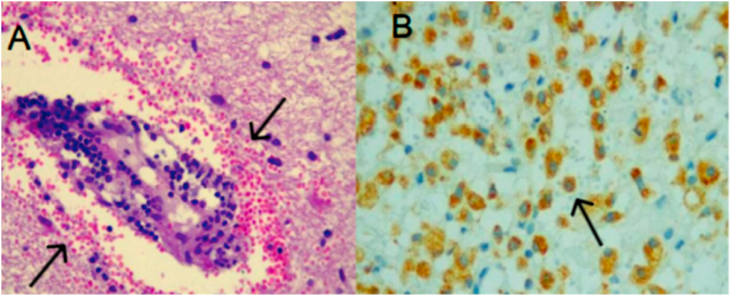
The cerebrospinal fluid (CSF) contained 3 cells, 50 g proteins/L, normal glucose. Oligoclonal bands (OCB) were positive. An extensive microbiological investigation, including CSF markers for viral and bacterial agents responsible for encephalitis showed no evidence of recent infection. Anti-myelin oligodendrocyte glycoprotein antibody (anti-MOG) IGG was negative. Chest, abdomen and pelvis computed tomography, breast and gynecological ultrasound ruled out a neoplasm (Table 1). A brain biopsy confirmed a perivascular demyelination and reactive astrocytosis (Fig. 2).
Table 1.
Csf analysis, blood tests, brain tissue biopsy.
| CSF | |
|---|---|
| White blood cell count/mm | 3–66% mononuclear |
| Proteins (mg/dl) | 50.6 |
| Glucose (mg/dl) | 78.3 |
| Lactic acid (mmol/L) | 1.74 |
| Culture (bacterial, fungal and KOCH) | Negative |
| VDRL | Negative |
| Viral PCR (Herpes simplex I/I, Varicella Zoster, Cytomegalovirus, Epstein Barr, Enterovirus, Chagas, John Cunningham) | Negative |
| Mycobacterium tuberculosis PCR | Negative |
| Oligoclonal Bands | Type 2 |
| Blood tests | |
| ESR | 21 |
| CRP | 6.1 |
| Neuronal autoantibodies: | |
| Anti-MOG IgG | Negative |
| Immunoglobulin A, E, G, M | Normal |
| Complement C3/C4 | Normal |
| Tumor markers: | |
| CA 125 | Negative |
| CA 15.3 | Negative |
| CA 19.9 | Negative |
| HIV | Negative |
| VDRL | Negative |
| Hepatitis B/C | Negative |
| Cytomegalovirus, Toxoplasmosis IgM | Negative |
| Brain tissue biopsy | |
| Culture (bacterial, fungal and KOCH) | Negative |
| Viral PCR (Cytomegalovirus, Epstein Barr, John Cunningham, Toxoplasmosis) | Negative |
ESR: erythrocyte sedimentation rate. CRP: C-reactive protein. Anti-MOG-IgG: Myelin oligodendrocyte glycoprotein antibodies; CA: carcinoembryonic antigen; HIV: Human immunodeficiency virus; VDRL: Venereal Disease.
Fig. 2.
Histopathology of a biopsied subcortical lesion of the right frontal lobe (hematoxylin and eosin staining). (a) perivascular mononuclear infiltrate and B) macrophage immunostaining 40X.
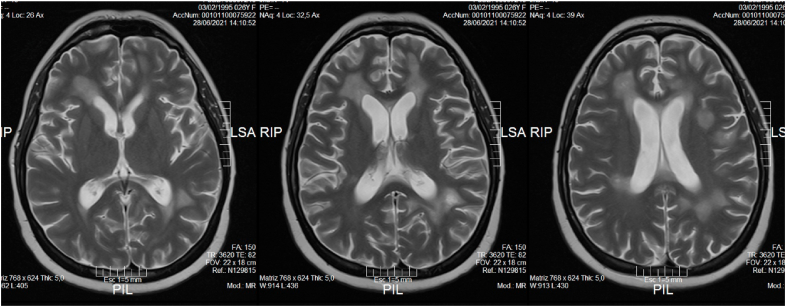
Postvaccinal ADEM was suspected and the patient was treated with intravenous methylprednisolone 1000 mg daily over 5 days. The clinical course was favourable. Her neurological examination was normal. The MRI was repeated after three months, showing clear imaging improvement of all the lesions (Fig. 3).
Fig. 3.
T2 brain control MRI showing the hyperintensity images in frontal and temporal lobes smaller in size and with less edema.
3. Discussion
Post-vaccination reactions have declined with the use of recombinant proteins compared to those based on in vivo infected animal tissue (Boziki et al., 2020). In the present, post-infectious and post-immunisation encephalomyelitis represent about three-quarters of ADEM cases (Huynh et al., 2008a). Post-vaccination ADEM is associated with several vaccines including those against rabies, diphtheria–tetanus–polio, smallpox, measles, mumps, rubella, Japanese B encephalitis, pertussis, influenza and hepatitis B (Bennetto and Scolding, 2004).
Incidence rates are as low as 0.1 to 0.2 per 100,000 vaccinated individuals (Menge et al., 2005) and it seems to occur much more frequently after the first dose rather than revaccination (Huynh et al., 2008b). Although it is an underdiagnosed pathology, the estimated incidence in children and adolescents is 0.8 per 100,000 inhabitants per year (Leake et al., 2004b). The incidence in the adult population is not exactly known. There are few case series that describe the presentation of this pathology in the adult population (Schwarz et al., 2001).
Regarding the vaccine against SARS-CoV-2 Xintong Li et al. reported population-based, age and sex specific background incidence rates of potential adverse events of special interest (AESI) in adults in eight countries using thirteen databases (Li et al., 1101). For both men and women, ADEM was a very rare entity (<1/10,000) in individuals under 64 years, and rare (1/1000 to 1/10,000) in those of 65 years and older (Li et al., 1101).
Roman et al. have described 3 acute transverse myelitis after vaccination with ChAdOx1 nCoV-19 (AZD1222), thus, this review has identified possible CNS demyelination events after the vaccine (Román et al., 2021).
ADEM lesions are typically bilateral, asymmetrical, large (>2 cm) and poorly demarcated. Both white and gray matter can be affected. Cortical as well as deep gray matter lesions have been described (Paolilo et al., 2020). Gadolinium enhancement is not a typical finding, although it has been reported in up to 30% of cases (Tenembaum et al., 2002). Our patient's MRI, with multiple lesions and anatomic areas with mass effect and ring enhancement, is compatible with ADEM according to the International Pediatric Multiple Sclerosis Study Group (Lauren et al., 2013).
Based on the imaging features and positive OCB, other CNS demyelinating diseases should be considered as differential diagnoses. Despite our patient clinical and imaging improvement after corticosteroid therapy, it can be the beginning of a tumefactive multiple sclerosis (MS).
Since the images can be similar in both diseases, the following pathological findings can generally help to differentiate between ADEM and MS. There are demyelination “sleeves” surrounding the venules associated with significant inflammatory infiltrates dominated by T lymphocytes and macrophages. Another feature is pronounced inflammation with only minor demyelination restricted to the vicinity of perivascular inflammatory infiltrates and that all lesions must be of the same age (Habek and Žarković, 2011). The biopsy of one of our patient's lesions showed this demenilization and perivascular inflammatory infiltrate.
Another differential diagnosis is myelin oligodendrocyte glycoprotein antibody-associated disorders (MOGAD) with ADEM-like presentacion. In both, ADEM and MOGAD, the clinical condition is similar. There are changes in mental status and increased frequency of seizures (Salama et al., 2019).
In adults with the positive anti-MOG test, ADEM presentation varies from a few up to 18% of cases (Armangue et al., 2020). Therefore at this low frequency, the anti-MOG IGG dosage is recommended (Jarius et al., 2018). Our patient had a negative anti-MOG antibody for cell-based assays (CBAs). This method for detection of anti-MOG antibody has a specificity close to 100% (Gastaldi et al., 2020).
The association between the SARS CoV-2 vaccination and ADEM has emerged in recent months, hence, up to now no large population studies or estimated incidence rates are known. Vaccine safety must continue to be monitored to complement what has initially been learned during clinical development. In a recently vaccinated patient, if neurological manifestations and neuroimaging features suggested ADEM, appropriate treatment with corticosteroid pulses, plasmapheresis, or immunoglobulin should be started to prevent sequelae.
4. Conclusions
Our patient has met the ADEM diagnostic criteria set by the International Pediatric MS Study Group. Other alternatives have been excluded. An association with the vaccine has been suspected. This does not mean that vaccines pose a risk that does not outweigh their benefit. Up to now, based on the positive result of the OCB, the onset of pseudotumoral MS cannot be ruled out.
Declaration of competing interest
The authors declare that there is no conflict of interest.
References
- Alberto M., Urdiales M., Xanthi A., et al. Initial impact of SARS-Cov-2 vaccination on healthcare workers in Italy– Update on the 28th of March 2021. Elsevier. 2021;39:4788–4792. doi: 10.1016/j.vaccine.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armangue T., Olivé-Cirera G., Martínez-Hernandez E., Sepulveda M., Ruiz-Garcia R., Muñoz-Batista M., Ariño H., González-Álvarez V., Felipe-Rucián A., Jesús Martínez-González M. Associations of paediatric demyelinating and encephalitic syndromes with myelin oligodendrocyte glycoprotein antibodies: a multicentre observational study. Lancet Neurol. 2020;19:234–246. doi: 10.1016/S1474-4422(19)30488-0. [DOI] [PubMed] [Google Scholar]
- Bennetto L., Scolding N. Inflammatory/post-infectious encephalomyelitis. J. Neurol. Neurosurg. Psychiatry. 2004;75(Suppl. 1):22–28. doi: 10.1136/jnnp.2003.034256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boziki M.K., Mentis A.F., Shumilina M., Makshakov G., Evdoshenko E., Grigoriadis N. COVID-19 immunopathology and the central nervous system: implications for multiple sclerosis and other autoimmune diseases with associated demyelination. Brain Sci. 2020;10:345. doi: 10.3390/brainsci10060345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag A., Hesham A. Acute demyelinating encephalomyelitis: clinical characteristics and outcome. J. Pediatr. Neurosci. 2013;8(1):26–30. doi: 10.4103/1817-1745.111418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldi M., Scaranzin S., Jarius S., Wildeman B., Zardini E., Mallucci G., Rigoni E., Vegezzi E., Foiadelli T., Savasta S., Banfi P., Versino M., Benedetti L., Novi G., Mancardi M.M., Giacomini T., Annovazzi P., Baroncini D., Ferraro D., Lampasona V., Reindl M., Waters P., Franciotta D. Cell-based assays for the detection of MOG antibodies: a comparative study. J. Neurol. 2020 Dec;267(12):3555–3564. doi: 10.1007/s00415-020-10024-0. [DOI] [PubMed] [Google Scholar]
- Habek M., Žarković K. Pathology of acute disseminated encephalomyelitis. Transl. Neurosci. 2011;2:252. doi: 10.2478/s13380-011-0030-5. [DOI] [Google Scholar]
- Huynh W., Cordato D.J., Kehdi E., Masters L.T., Dedousis C. Post-vaccination encephalomyelitis: literature review and illustrative case. J. Clin. Neurosci. 2008 Dec;15(12):1315–1322. doi: 10.1016/j.jocn.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh W., Cordato D.J., Kehdi E., Masters L.T., Dedousis C. Post-vaccination encephalomyelitis: literature review and illustrative case. J. Clin. Neurosci. 2008;15(12):1315–1322. doi: 10.1016/j.jocn.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S., Paul F., Aktas O., Asgari N., Dale R.C., de Seze J., Franciotta D., Fujihara K., Jacob A., Kim H.J., Kleiter I., Kümpfel T., Levy M., Palace J., Ruprecht K., Saiz A., Trebst C., Weinshenker B.G., Wildemann B. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J. Neuroinflammation. 2018 May 3;15(1):134. doi: 10.1186/s12974-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauren B., Marc T., Maria P., et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult. Scler. 2013;19(10) doi: 10.1177/1352458513484547. 1261-7. [DOI] [PubMed] [Google Scholar]
- Leake J.A., Albani S., Kao A.S., Senac M.O., Billman G.F., Nespeca M.P., Paulino A.D., Quintela E.R., Sawyer M.H., Bradley J.S. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr. Infect. Dis. J. 2004;23(8):756–764. doi: 10.1097/01.inf.0000133048.75452.dd. doi: 10.1097/01.inf.0000133048.75452.dd. PMID: 15295226. [DOI] [PubMed] [Google Scholar]
- Leake J.A., Albani S., Kao A.S., Senac M.O., Billman G.F., Nespeca M.P., et al. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr. Infect. Dis. J. 2004;23:756–764. doi: 10.1097/01.inf.0000133048.75452.dd. [DOI] [PubMed] [Google Scholar]
- Li X., Ostropolets A., Makadia R., Shaoibi A., Rao G., Sena A. G., et al. Characterizing the Incidence of Adverse Events of Special Interest for COVID-19 Vaccines across Eight Countries: a Multinational Network Cohort Study medRxiv. doi: https://doi.org/10.1101/2021.03.25.21254315; version posted April 17, 2021. [DOI] [PMC free article] [PubMed]
- Lin C., Jeng J., Hsieh S., Yip P., Wu R. Acute disseminated encephalomyelitis: a follow-up study in Taiwan. J. Neurol. Neurosurg. Psychiatry. 2007;78:162–167. doi: 10.1136/jnnp.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge T., Hemmer B., Nessler S., Wiendl H., Neuhaus O., Hartung H.P., Kieseier B.C., Stüve O. Acute disseminated encephalomyelitis: an update. Arch. Neurol. 2005;62(11):1673–1680. doi: 10.1001/archneur.62.11.1673. [DOI] [PubMed] [Google Scholar]
- Paolilo R.B., Deiva K., Neuteboom R., Rostásy K., Lim M. Acute disseminated encephalomyelitis: current perspectives. Children. 2020;7(11):210. doi: 10.3390/children7110210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román G.C., Gracia F., Torres A., Palacios A., Gracia K., Harris D. Acute transverse myelitis (ATM):Clinical review of 43 patients with COVID-19-associated ATM and 3 post-vaccination ATM serious adverse events with the ChAdOx1 nCoV-19 vaccine (AZD1222) Front. Immunol. 2021;12:653786. doi: 10.3389/fimmu.2021.653786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama S., Khan M., Pardo S., Izbudak I., Levy M. MOG antibody-associated encephalomyelitis/encephalitis. Mult. Scler. 2019;25(11):1427–1433. doi: 10.1177/1352458519837705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S., Mohr A., Knauth M., Wildemann B., Storch-Hagenlocher B. Acute disseminated encephalomyelitis: a follow-up study of 40 adult patients. Neurology. 2001;56:1313–1318. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- Tenembaum S., Chamoles N., Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59(8):1224–1231. doi: 10.1212/wnl.59.8.1224. [DOI] [PubMed] [Google Scholar]