Abstract
Introduction
Low muscle mass is a common condition in the critically ill population and is associated with adverse clinical outcomes. The primary aim of this study was to analyze the prognostic significance of low muscle mass using computed tomography (CT) scans in COVID-19 critically ill patients. A second objective was to determine the accuracy and agreement in low muscle mass identification using diverse markers compared to CT as the gold standard.
Methods
This was a prospective cohort study of COVID-19 critically ill patients. Skeletal muscle area at the third lumbar vertebra was measured. Clinical outcomes (intensive care unit [ICU] and hospital length of stay [LOS], tracheostomy, days on mechanical ventilation [MV], and in-hospital mortality) were assessed. Phase angle, estimated fat-free mass index, calf circumference, and mid-upper arm circumference were measured as surrogate markers of muscle mass.
Results
Eighty-six patients were included (mean age ± SD: 48.6 ± 12.9; 74% males). Patients with low muscle mass (48%) had a higher rate of tracheostomy (50 vs 20%, p = 0.01), prolonged ICU (adjusted HR 0.53, 95%CI 0.30–0.92, p = 0.024) and hospital LOS (adjusted HR 0.50, 95% CI 0.29–0.86, p = 0.014). Bedside markers of muscle mass showed poor to fair agreement and accuracy compared to CT-assessed low muscle mass.
Conclusion
Low muscle mass at admission was associated with prolonged length of ICU and hospital stays. Further studies are needed to establish targeted nutritional interventions to halt and correct the catabolic impact of COVID-19 in critically ill patients, based on standardized and reliable measurements of body composition.
Keywords: COVID-19, Mechanical ventilation, Nutritional status, Mortality, Muscle mass
1. Introduction
Over 250 million patients worldwide have been affected by Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. Studies have shown that ∼30% of the hospitalized patients require ventilatory support and admission to Intensive Care Units (ICUs) [2].
The acute phase of critical illness, immobilization, and drug administration (sedatives and neuromuscular blocking agents) results in disturbed metabolism, with a catabolic state characterized by increased protein breakdown and decreased protein synthesis, leading to a rapid wasting of skeletal muscle mass [[3], [4], [5]].
Muscle mass assessment at hospital admission can be useful to identify patients with higher nutritional risk, while its monitoring could offer important opportunities to guide nutritional therapy adjustments during ICU stay [6]. Magnetic resonance imaging and computed tomography (CT) are gold standard techniques for the assessment of body composition in clinical populations [7,8]. CT employs a beam of X-rays that produces signals, that once processed by a computer, can generate cross-sectional images of the body. Using specialized software, skeletal muscle mass can be measured at the third lumbar vertebra (L3), which correlates well with whole-body skeletal muscle mass, being the preferred landmark for the estimation of whole-body muscle mass [9,10]. Skeletal muscle cross-sectional area is then used to calculate skeletal muscle index (SMI, cm2/m2) and compared to one of the available diagnostic criteria.
Although not optimal, other landmarks and isolated muscle groups have been used in clinical settings, including pectoralis muscle area, psoas muscle area or thigh muscles [11,12]. Low muscle mass is associated with clinical outcomes in COVID-19 patients; according to Besutti et al., higher pectoralis muscle cross-sectional area, measured by chest CT images, showed a protective effect on hospitalization, mechanical ventilation (MV) and death [13]. Similar findings were reported by Ufuk et al. using chest images for pectoralis SMI assessment in 130 patients [14]. Furthermore, limited data is available regarding the clinical prognosis of low muscle mass in critically ill patients.
Despite the usefulness of imaging methods for SMI quantification, they are rarely available in hospital settings. As such, bedside techniques can be used as a marker of muscle mass; these include anthropometric measurements [mid-upper arm circumference (MUAC), and calf circumference (CC)], and bioelectrical impedance analysis (BIA). Nevertheless, they lack accuracy, especially considering IV infusion therapy and fluid overload, which are treatments commonly observed in critically ill patients [[15], [16], [17]]. Despite its limitations, BIA also allows for the assessment of phase angle (PhA), which has been proposed as an indirect marker of muscle mass and quality, and a predictor of clinical outcomes [18,19].
Considering the potential importance of body composition to COVID-19 patients, and the need for surrogate markers of low muscle mass assessment, the primary aim of the study was to assess muscle mass at the L3 using CT-assessed SMI to evaluate its prognostic significance in predicting clinical outcomes. Secondly, we aimed to assess agreement and accuracy of markers of muscle mass compared to SMI, including CC, MUAC, estimated fat-free mass index, and PhA in COVID-19 critically ill patients.
2. Methods
This was a prospective cohort of consecutive patients admitted to the ICU of the National Institute of Respiratory Diseases, in Mexico City, from November 2020 to March 2021. Adults (age ≥18 years) diagnosed with COVID-19 (confirmed by both reverse transcription-polymerase chain reaction (RT PCR) for SARS-CoV-2 and suggestive tomographic findings) under MV were included. Only patients with CT scans performed in the first 24–48 hours after admission were included. This study was reviewed and approved by the Institutional Review Board of the National Institute of Respiratory Diseases (Register #C16-21) and the University of Alberta Research Ethics Office (Pro00111147).
2.1. Nutritional assessment
Nutritional status was assessed in all patients at the first 24–48 hours after ICU admission. Nutritional risk was calculated using the modified NUTRIC-Score during the first 24 hours of initiating mechanical ventilation. This assessment tool includes age, Sequential Organ Failure Assessment (SOFA) score, Acute Physiology And Chronic Health Evaluation II (APACHE II) score at admission, the number of comorbidities, and pre-ICU hospital length of stay (LOS). High nutritional risk was established at a score ≥5 [20].
2.2. Anthropometric assessment
Anthropometric assessment included MUAC, CC, waist circumference, and half-arm span; measurements were done using a tape graduated in centimeters with 0.1 cm precision (SECA 201, Germany). Anthropometry was assessed using the standard procedures described by Lohman et al. [21]. MUAC was measured at the mid-point between the tip of acromion process and the tip of the olecranon process. CC was measured by wrapping the tape around the widest part of the calf. Body weight and height were estimated using validated equations [22]. Body mass index (BMI) was calculated and was classified using World Health Organization criteria [23].
2.3. Body composition assessment
Body composition was assessed by both BIA and CT scans. Regarding BIA assessment, a multi-frequency device was used (InBody S10®, InBody Co., Ltd., Seoul, Korea). Measurements were performed with the patient in a supine position. Eight adhesive electrodes were used: one on each wrist, one on the distal part of the third metacarpal bone of each hand, one on the central part of each ankle, and one on the distal part of the second metatarsal bone in each foot. Estimated body weight and height were inputted into the device. PhA and estimated fat free mass (FFM) were recorded from the machine output. Fat-free mass index (FFMI) was calculated as FFM/height (kg/m2).
Regarding CT images, a SIEMENS brand multidetector CT (SOMATON Sensations model) with 64 detectors was used; the studies were performed with a volumetric acquisition in the supine position during maximum inspiration in the pulmonary and mediastinal windows. The main scanning parameters were as follows: tube voltage = 100 kVp, automatic modulation of the electric current tube (70–120 mAs), pitch = 1, slice thickness = 1 mm and reconstruction matrix = 512 × 512. All images were reconstructed with a high spatial resolution algorithm and a B70 lung filter with a window amplitude of −600/1200; for the mediastinum, a B30 filter with a window width of 50/350 was applied. On each CT scan, the L3 slice was located by two experts’ radiologists and was exported as DICOM files. Specific tissue demarcation using predefined thresholds in Hounsfield Units (HU) was performed at the Human Nutrition Research Unit (University of Alberta, Canada), as previously described [24,25]. CT images were processed with the SliceOmatic v5.0 (TomoVision, Montreal, Canada) software, and manually corrected as necessary. Cross-sectional area of skeletal muscle (i.e., skeletal muscle area [SMA]), intermuscular adipose tissue (IMAT), subcutaneous adipose tissue, visceral adipose tissue and low attenuation muscle area were determined using thresholds described elsewhere [25]. SMA was adjusted for height in meters to determine SMI (cm2/m2). Skeletal muscle density was generated by the software as the mean radiation attenuation value of the whole muscle area at L3 [26]. Low muscle radiodensity was defined as an SMD lower than 35.5 HU and lower than 32.5 HU for men and women, respectively [27].
2.4. CT- assessed low muscle mass and surrogate markers
Low muscle mass was identified using previously published cut-off points based on sex and BMI categories [27]. For patients with a BMI <30 kg/m2, low muscle mass was defined as an SMI ≤52.3 cm2/m2 for men and ≤38.6 cm2/m2 for women. For those with a BMI ≥30 kg/m2, an SMI ≤54.3 cm2/m2 for men and ≤46.6 cm2/m2 for women was considered. The following surrogate markers were used for low mass identification: a) FFMI <17 kg/m2 for males or <15 kg/m2 in females [28,29], endorsed by the Global Leadership Initiative on Malnutrition (GLIM) consensus statement [30]; b) low sex-specific, BMI-adjusted CC was defined using Gonzalez et al. references for males and females using 1 SDs below each mean [31]; c) low MUAC (<5th percentile) was defined based on an Mexican-American population (25.7 cm for females, 28 cm for males) [32], d) and low PhA values (<3.85° in females and <5.25° in males), as reported for COVID-19 critically ill patients [19].
2.5. Clinical data
Days under invasive MV, ICU LOS (calculated as days from ICU admission to ICU discharge, and hospital LOS (days from hospital admission to discharge dates), tracheostomy placement, diabetes and hypertension diagnosis, acute kidney injury (AKI) diagnosis during LOS, and all-cause hospital mortality were recorded.
2.6. Nutritional therapy
Nutritional therapy was prescribed by ICU dietitians. Energy and protein requirements were calculated according to recommendations by the American Society of Parenteral and Enteral Nutrition (ASPEN) and European Society for Clinical Nutrition and Metabolism (ESPEN), with a general target of 25 kcal/kg and 1.3 g/kg, respectively [33,34]. Adjusted body weight was used for patients with obesity (BMI >30 kg/m2) [35]. Calories derived from non-nutritional sources such as propofol and glucose IV infusion were factored into the nutrition prescriptions to avoid overfeeding.
2.7. Statistical analysis
All statistical analyses were performed using Stata Intercooled (Version 14, STATA Corporation, College Station, TX, USA) and graphics were elaborated in GraphPad Prism (GraphPad Software, Inc). Normality was verified with the Shapiro Wilk test. Descriptive statistics were used to analyze categorical variables (absolute and relative frequency) and quantitative variables (mean and standard deviation [SD] or median and interquartile range [IQR]). Differences between normal and low muscle mass were compared using Student's t-test, Mann–Whitney U-test, or χ 2 test. Univariate logistic regression and Kaplan Meier survival analysis with log-rank test and the Cox proportional hazards model were performed to assess the association between low muscle mass and tracheostomy placement, MV days, and ICU and hospital LOS in survival patients. Multivariate regression models were performed and fitted to the data using backward stepwise selection. Analyzed variables (categorized age [20–30, 31–40, 41–50, 51–60, 61–70, >71 years], SOFA and APACHE II scales, NUTRIC-Score, IMAT, subcutaneous adipose tissue, visceral adipose tissue, diabetes diagnosis [yes or no], hypertension [yes or no] and acute kidney injury [yes or no]) were retained in the model if they had a p-value that was less or equal to the maximum p-value selection criteria of 0.1. Pearson's correlation (rho) and linear regression were used to assess the relationship between SMI and MUAC, CC, FFM, and PhA derived from BIA. Agreement between low muscle mass and markers of muscle mass were analyzed by the kappa (κ) statistic; values < 0.2 indicating poor, 0.2–0.4 indicating fair, 0.4–0.6 indicating moderate, 0.6–0.8 indicating substantial, and >0.8 indicating almost perfect concordance [36]. The accuracy of each marker of muscle mass to predict CT-assessed low muscle mass was analyzed by sensitivity, specificity, and area under the receiver operating characteristic curve. The area under the curve (AUC) was interpreted as follows: no discrimination AUC 0.5, fail discrimination 0.5 to 0.6, poor discrimination 0.6 to 0.7, fair discrimination 0.7 to 0.8, good discrimination 0.8 to 0.9 and excellent discrimination 0.9 [37]. Statistical significance was defined as p < 0.05.
3. Results
A total of 98 patients with available abdominal CT scans at admission were screened. Of these, 12 had CT scans with streak artifacts from metallic hardware or limited field of view. Finally, 86 critically ill patients with COVID-19 were included. Detailed clinical and body composition characteristics of all samples and by muscle mass status are summarized in Table 1 . Mean age was 48.6 ± 12.9 years, most patients were males (73%). A total of 41 patients (48%) were classified as having low muscle mass. Patients with normal muscle mass had higher muscle radiodensity (p = 0.003), lower IMAT (p = 0.02) and higher values of MUAC (p = 0.02), CC (p = 0.02), PhA (p=<0.001) and FFMI (p = 0.003).
Table 1.
Clinical characteristics and body composition data of COVID-19 critically ill patients.
| Characteristics | All patients (n = 86) | Normal muscle mass (n = 45) | Low muscle mass (n = 41) | p value |
|---|---|---|---|---|
| Age, years mean (SD) | 48.6 ± 12.9 | 46.4 ± 12.4 | 51.0 ± 13 | 0.08 |
| 20–30 y n (%) | 8 (9%) | 5 (11%) | 3 (7%) | |
| 31–40 y n (%) | 16 (18%) | 9 (20%) | 7 (17%) | |
| 41–50 y n (%) | 25 (29%) | 15 (33%) | 10 (24%) | 0.77 |
| 51–60 y n (%) | 21 (25%) | 10 (22%) | 11 (27%) | |
| 61–70 y n (%) | 13 (15%) | 5 (12%) | 8 (20%) | |
| >71 y n (%) | 3 (4%) | 1 (2%) | 2 (5%) | |
| Sex, n (%) | ||||
| Males | 63 (73%) | 37 (82%) | 26 (64%) | 0.04∗ |
| Comorbidities | ||||
| Diabetes | 33 (39%) | 11 (25%) | 22 (54%) | 0.005∗ |
| Hypertension | 33 (39%) | 14 (31%) | 19 (46%) | 0.14 |
| Acute kidney injury | 34 (39%) | 22 (49%) | 12 (29%) | 0.06 |
| BMI, kg/m2, mean (SD) | 29.2 ± 5.5 | 29.4 ± 4.3 | 29.1 ± 6.5 | 0.79 |
| 18.5–24.9, n (%) | 16 (19%) | 6 (14%) | 10 (24%) | |
| 25–29.9.9, n (%) | 33 (38%) | 20 (44%) | 13 (32%) | 0.49 |
| ≥30–34.9, n (%) | 24 (28%) | 12 (27%) | 12 (29%) | |
| ≥35, n (%) | 13 (15%) | 7 (15%) | 6 (15%) | |
| Weight, kg, mean (SD) | 83.5 ± 16.5 | 84.7 ± 14.3 | 82.1 ± 18.7 | 0.46 |
| Males | 85.1 ± 17.1 | 86.3 ± 13.4 | 83.4 ± 21.4 | 0.50 |
| Females | 78.9 ± 14.2 | 77.3 ± 16.9 | 79.8 ± 13.2 | 0.69 |
| Height, cm, mean (SD) | 167.5 ± 9.3 | 168.8 ± 7.9 | 166.1 ± 10.6 | 0.18 |
| Males | 171.5 ± 6.4 | 171.0 ± 6.5 | 172.3 ± 6.3 | 0.43 |
| Females | 156.6 ± 7.3 | 158.8 ± 6.2 | 155.5 ± 7.8 | 0.30 |
| Mid-upper arm circumference, cm, mean (SD) | 31.7 ± 3.7 | 32.6 ± 3.1 | 30.8 ± 4.2 | 0.02∗ |
| Males | 31.8 ± 4.0 | 32.6 ± 3.0 | 30.6 ± 4.9 | 0.04∗ |
| Females | 31.6 ± 3.0 | 32.3 ± 3.4 | 31.2 ± 2.8 | 0.40 |
| Calf circumferencea, cm, mean (SD) | 35.4 ± 3.6 | 36.2 ± 2.8 | 34.5 ± 3.8 | 0.02∗ |
| Males | 35.6 ± 3.4 | 36.4 ± 2.7 | 34.6 ± 4.0 | 0.04∗ |
| Females | 34.8 ± 3.6 | 35.6 ± 3.2 | 34.3 ± 3.7 | 0.42 |
| Phase angle (°), mean (SD) | 5.1 ± 1.1 | 5.6 ± 1.0 | 4.6 ± 0.9 | <0.001∗ |
| Males | 5.2 ± 1.2 | 5.7 ± 1.1 | 4.5 ± 0.9 | <0.001∗ |
| Females | 4.8 ± 0.7 | 5.3 ± 0.6 | 4.6 ± 0.7 | 0.02∗ |
| Fat free mass index (kg/m2), mean (SD) | 19.7 ± 3.1 | 20.8 ± 3.1 | 18.7 ± 2.8 | 0.001∗ |
| Males | 20.2 ± 3.4 | 21.3 ± 3.2 | 19.0 ± 3.2 | 0.008∗ |
| Females | 18.4 ± 1.8 | 18.8 ± 1.6 | 18.3 ± 1.8 | 0.49 |
| NUTRIC-Score, mean (SD) | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.60 |
| High risk (%) | 36 (41%) | 17 (38%) | 19 (46%) | 0.42 |
| Skeletal Muscle Area (cm2), mean (SD) | 144.5 ± 39.2 | 167.3 ± 33.0 | 118.6 ± 28.4 | <0.001∗ |
| Males | 159.9 ± 31.0 | 177.7 ± 25.0 | 130.8 ± 15.9 | <0.001∗ |
| Females | 100.7 ± 23.7 | 119.6 ± 21.8 | 90.6 ± 18.2 | 0.002∗ |
| Skeletal Muscle Index (cm2/m2), mean (SD) | 50.8 ± 11.0 | 58.2 ± 8.3 | 42.3 ± 6.7 | <0.001∗ |
| Males | 54.3 ± 9.7 | 60.6 ± 6.5 | 45.3 ± 5.5 | <0.001∗ |
| Females | 40.7 ± 7.5 | 47.3 ± 6.6 | 37.2 ± 5.5 | 0.001∗ |
| Muscle radiodensity (HU), mean (SD) | 30.4 ± 7.2 | 32.9 ± 6.0 | 27.5 ± 7.6 | <0.001∗ |
| Males | 32.3 ± 6.7 | 33.7 ± 6.1 | 30.4 ± 7.1 | 0.04∗ |
| Females | 25.1 ± 6.2 | 29.5 ± 4.1 | 22.7 ± 6.0 | 0.01∗ |
| Muscle Radiodensity | ||||
| Normal muscle radiodensity | 26 (30%) | 19 (42%) | 7 (17%) | 0.01 |
| Low muscle radiodensity | 60 (70%) | 26 (58%) | 34 (83%) | |
| Visceral adiposity (cm2), median (IQR) | 186 (148–249) | 143 (109–267) | 184 (154–230) | 0.81 |
| Males | 206 (161–273) | 214 (153–274) | 191 (176–268) | 0.80 |
| Females | 148 (119–184) | 120 (116–154) | 154 (132–213) | 0.052 |
| Subcutaneous adiposity (cm2), median (IQR) | 228 (166–297) | 228 (188–289) | 234 (144–307) | 0.66 |
| Males | 212 (164–274) | 214 (173–277) | 166 (130–274) | 0.12 |
| Females | 289 (235–362) | 259 (210–362) | 303 (249–459) | 0.61 |
| Intermuscular adipose tissue (cm2), median (IQR) | 11 (7–17) | 9 (7–16) | 12 (9–22) | 0.02∗ |
| Males | 10 (7–16) | 8 (7–16) | 12 (8–19) | 0.19 |
| Females | 16 (10–26) | 10 (8–19) | 17 (12–27) | 0.13 |
| Total adipose tissue (cm2), median (IQR) | 450 (362–540) | 455 (374–540) | 448 (349–539) | 0.57 |
| Males | 450 (362–540) | 469 (393–541) | 410 (336–535) | 0.17 |
| Females | 453 (402–497) | 414 (356–486) | 482 (414–625) | 0.22 |
| SOFA Score, mean (SD) | 9.7 ± 2.9 | 9.5 ± 2.7 | 9.9 ± 3.2 | 0.56 |
| APACHE II Score, mean (SD) | 19 ± 6 | 19 ± 6 | 19 ± 5 | 0.94 |
SD: Standard Deviation, IQR: Interquartile Range, BMI: Body Mass Index.
∗Significant results (P < 0.05).
After BMI-adjusted HU: Hounsfield Unit, SOFA: Sequential Organ Failure Assessment, APACHE: Acute Physiology And Chronic Health Evaluation II.
3.1. Prognostic significance of low muscle mass
No difference in mortality rate was observed between patients with low and normal muscle mass (22 vs 27%, p = 0.61). From 21 patients who died during ICU LOS, 12 had normal and 9 had low muscle mass. Patients with low muscle mass who survived during hospital stay had higher MV days (25 days vs 15 days, p = 0.06), ICU (27 vs 18 days, p = 0.02) and hospital (35 vs 23 days, p = 0.02) LOS, and higher tracheostomy requirement (50 vs 21%, p = 0.01), compared to their counterparts (Table 2 ).
Table 2.
Differences in clinical outcomes between normal and low muscle mass in survival patients.
| Characteristics | All patients | Normal muscle mass | Low muscle mass | p value |
|---|---|---|---|---|
| Mechanical ventilation, days | ||||
| Survived | 17 (11–39) | 15 (9–26) | 25 (13–41) | 0.06 |
| Tracheostomy placement | ||||
| Survived | 7 (21%) | 9 (60%) | 16 (50%) | 0.01∗ |
| Intensive care unit length of stay, days | ||||
| Survived | 21 (14–40) | 18 (12–29) | 27 (18–46) | 0.02∗ |
| Died | 18 (10–35) | 19 (11–35) | 18 (9–29) | 0.61 |
| Hospital length of stay, days | ||||
| Survived | 28 (19–47) | 23 (17–35) | 35 (20–56) | 0.02∗ |
∗Significant results (P < 0.05). median (IQR), n (%).
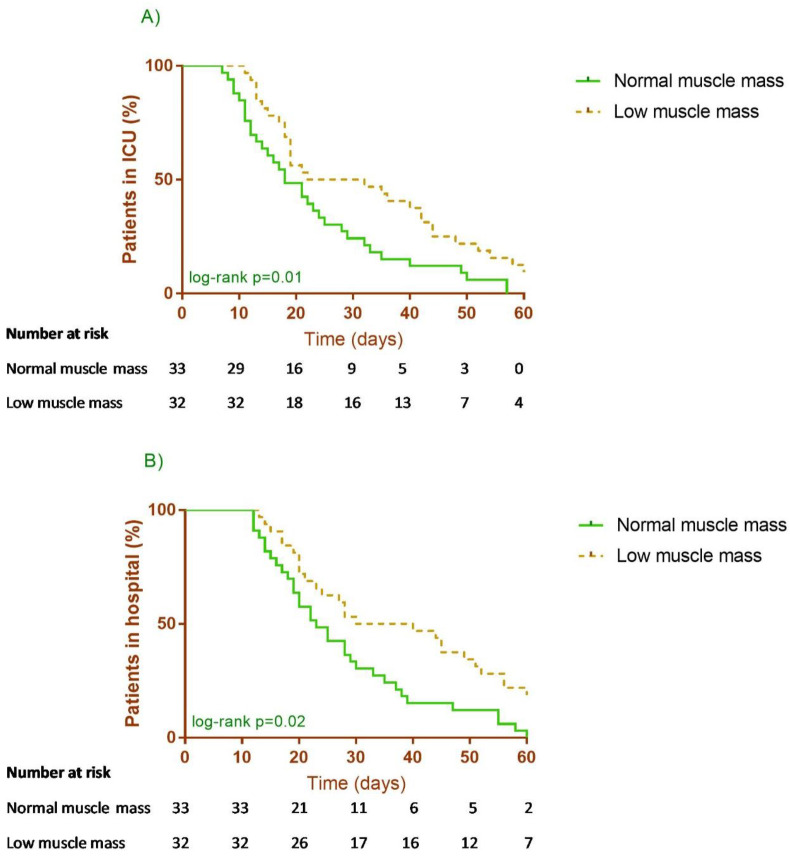
The result of the Kaplan–Meier and Cox analysis showed significant associations between low muscle mass and ICU (HR 0.56, 95% CI 0.33–0.94, p = 0.028) and hospital (HR 0.56, 95% CI 0.34–0.95, p = 0.03) LOS but not with MV days (HR 0.61, 95% CI 0.37–1.02, p = 0.06). Alternatively, we also considered age (categorized), hypertension diagnosis, APACHE II score and IMAT as covariates for adjusted HR models for ICU (adjusted HR 0.53, 95% CI 0.30–0.92, p = 0.024) and hospital (adjusted HR 0.50, 95% CI 0.29–0.86, p = 0.014) LOS (Fig. 1 ).
Fig. 1.
A) Intensive care unit (ICU) length of stay and B) Hospital length of stay in patients with normal or low muscle mass.
Univariate logistic regression showed a significant association between low muscle mass and higher tracheostomy placement events in crude (OR 4.0, 95% CI 1.35–11.7, p = 0.012) and adjusted model (OR 7.3, 95% CI 1.82–29.4, p = 0.005) (Table 3 ).
Table 3.
Cox regression analysis of low muscle mass and clinical outcomes in COVID-19 Critically ill patients.
| Variable | Univariate analysis |
Multivariate analysisa |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Mechanical ventilation, days | 0.61 | 0.37–1.02 | 0.06 | 0.60 | 0.35–1.05 | 0.07 |
| Intensive care unit length of stay, days | 0.56 | 0.33–0.94 | 0.028∗ | 0.53 | 0.30–0.92 | 0.024∗ |
| Hospital length of stay, days | 0.56 | 0.34–0.95 | 0.03∗ | 0.50 | 0.29–0.86 | 0.014∗ |
∗Significant results (P < 0.05). HR: Hazard ratio, CI: Confidence interval.
Model adjusted to age (categorized), hypertension diagnosis, APACHE II score and intermuscular adipose tissue.
3.2. Performance of markers of muscle mass
Correlations between surrogate muscle markers and SMA were performed. Statistical significance differences were observed with MUAC, CC, PhA, and FFM (Table 4 ). Poor concordance was observed for MUAC (κ 0.15, p = 0.009) and fair concordance for FFMI (κ 0.20, p < 0.001). PhA showed fair concordance (κ 0.34, p < 0.001) and poor accuracy (AUC 0.67, 95% CI 0.57–0.77) for low muscle mass identification, with a sensitivity of 56% and specificity of 78% (Table 5 ).
Table 4.
Relationships between computerized tomography-assessed skeletal muscle area (cm2) and markers of muscle mass.
| Rhoa | β (95% CI)b | R2 | |
|---|---|---|---|
| Mid-upper arm circumference, cm | r: 0.44, p < 0.001∗ | 4.5 (2.5–6.5), p 0.001∗ | 0.18 |
| Calf circumference, cm | r: 0.45, p < 0.001∗ | 5.1 (2.9–7.3), p 0.001∗ | 0.20 |
| Fat free mass (kg) | r: 0.70, p < 0.001∗ | 2.1 (1.7–2.6), p 0.001∗ | 0.49 |
| Phase angle (°) | r: 0.39, p < 0.001∗ | 13.5 (6.5–20.4), p 0.001∗ | 0.14 |
∗Significant results (P < 0.05).
Pearson test.
Univariate linear regression.
Table 5.
Concordance of markers of muscle mass for low muscle mass identification.
| Calf circumference | Mid-upper arm circumference | Fat free mass index | Phase angle | |
|---|---|---|---|---|
| Concordance | κ 0.05, p 0.27 | κ 0.15, p 0.009∗ | κ 0.20, p 0.004∗ | κ 0.34, p < 0.001∗ |
| Agreement | 51.8% | 58.8% | 60.7% | 67% |
| Sensitivity | 80.5% | 17% | 24.4% | 56% |
| Specificity | 25% | 98% | 95.3% | 78% |
| Area under the curve | 0.52 (0.43–0.61) | 0.57 (0.51–0.63) | 0.59 (0.52–0.67) | 0.67 (0.57–0.77) |
∗Significant results (P < 0.05).
4. Discussion
Low skeletal muscle mass is a common condition in the ICU population. Our observational study showed an association between low muscle mass and prolonged ICU and hospital LOS, and a higher rate of tracheostomy.
The identification of low muscle mass at an early stage of critical illness may improve risk stratification, although little is known of this association in the context of COVID-19 patients on MV. To our knowledge, this is the first study that analyzed low muscle mass as a predictor for increased risk of prolonged LOS in a Mexican cohort of critical patients with COVID-19.
We were able to identify low muscle mass using CT scans in 48% of our population, which is a lower frequency compared to the 65% reported in an Italian cohort using different cut-off values for low muscle identification (45.4 cm2/m2 for males and 34.4 cm2/m2 for females) [38]. In critically-ill septic patients, Cox Mc et al., reported a prevalence of baseline low muscle mass in 50% of patients [39]. Another study in hospitalized patients with COVID-19 showed that muscle mass was related to the need for ICU admission (17%), longer hospital LOS (mean, 10.8 days), and mortality (6.6%) [40]. Although their results are not comparable to our population of critically ill patients as muscle mass was assessed using ultrasound, their findings corroborate with ours by highlighting low muscle mass as an independent predictor of negative clinical outcomes [37], including higher rates of extubation failure, defined as reintubation within 48 hours after extubation following long-term MV for >7 days [41]. Notably, our lack of association with mortality can be simply due to our limited sample size to explore this specific question. In our sample, we observed a trend toward more MV days in patients with low muscle mass.
One important difference often observed across CT-based studies is the choice of thresholds to define low muscle mass [42,43]. Some studies in patients with COVID used references derived from healthy populations [44,45]. In this study, we used sex and BMI-adjusted thresholds proposed by Caan et al. [27] in the absence of data for Mexican patients with COVID. We acknowledge this cutpoint is not cohort-specific, as they were derived from oncology patients. Despite differences across populations, these thresholds showed a good prognosis capacity.
In our sample, 39% of patients had low muscle mass and low muscle radiodensity. The latter, also called myosteatosis is indicative of abnormal muscle “quality” (i.e., depicting fat infiltration into muscle) [46]. Although we have not explored the clinical implications of myosteatosis or a combined condition with low muscle mass in our study due to sample size limitations, this condition has been previously linked to extubation success [38], less ventilator-free and ICU-free days [47], poor survival and higher mortality in mechanically ventilated patients [[48], [49], [50], [51]]. The mechanism explaining the association of myosteatosis and worse outcomes is unclear, but insulin resistance, oxidative stress and inflammation responses may be implicated [52]. Notably, although we did not fully explore the consequences of myosteatosis in our study, IMAT was included in regression analysis, which improved model adjustment.
Additionally, we also explored the impact of high adiposity and of high adiposity with low muscle mass (sarcopenic obesity) on the studied clinical outcomes, also using the definition per Caan J et al. [27]. No differences between groups were detected for mortality, hospital or ICU LOS, likely due to the small sample size (data not shown).
Most of the studies carried out to date in patients with COVID-19 have not described the impact of muscle mass and the number of tracheostomies as a negative clinical result. In our analysis, we identified a 50% increase in the number of tracheostomies performed in the group of patients with low muscle mass prior to a successful withdrawal from mechanical ventilation, which may in turn impact LOS, morbidity, and mortality.
CT scan is considered a gold standard technique for body composition assessment, with the disadvantage that it is not available in all clinical settings, and not all critical patients had a CT scan for diagnosis purpose. Notably, body composition assessment is not an indication for CT scan due to its high radiation exposure. Therefore, the identification of bedside surrogate markers for the diagnosis of low muscle mass is important when CT scans are not available. In our study, anthropometric and FFMI/BIA-derived indicators showed insufficient accuracy and agreement with SMA by CT. Despite the evidence of CC as a marker of muscle mass, the lack of accuracy between abnormal CC and CT values may be due to how cut-off points were derived, the former using DXA data from healthy subjects, the latter using CT data from patients with cancer. Similar results were obtained using the BIA-derived FFM, as our cut-off points were not device and population specific.
PhA obtained from BIA is an indicator of cell mass and membrane integrity that is adversely affected by inflammation, disease, and immobilization due to decreased electrical properties of tissues [53]. PhA has been proposed as a surrogate marker for muscle mass in different clinical settings such as patients with cirrhosis [38,54]. In our study, cut-off values of PhA showed a fair agreement and poor accuracy. Our findings highlight the need for simple and non-invasive tools for muscle mass evaluation and monitoring.
Our study has several limitations: 1) our cohort was enrolled at a single center; 2) the number of female participants was limited; 3) we did not include a non-COVID-19 control group which would allow us to distinguish potential differences associated with COVID-19 or with low muscle mass per se; 4) thresholds for low muscle mass identification were derived from another clinical population, in absence of a Mexican references population or a stablished cut-off for critically-ill patients; 5) Long-term survivorship was not accessible due to the impact of COVID-19 on the workload of the nutrition department, and 6) analysis of additional body composition phenotypes was limited by our small sample size. However, clinical data obtained in this study supports the use of CT a safe non-invasive and reliable technique to detect low muscle mass in critical care patients with COVID-19.
5. Conclusion
Low muscle mass was associated with prolonged ICU and hospital LOS. Further studies are needed to establish nutritional interventions to ameliorate the catabolic impact of COVID-19 in critically ill patients, based on standardized and reliable measurements of body composition.
Statement of authorship
IAOP is the guarantor of the content of the manuscript. NCRM and SRL contributed substantially to the study design, data collection, analysis, and interpretation, and the writing of the first draft and subsequent revisions of the manuscript. MARA, CEO and CMP contributed substantially to data interpretation, and the writing of the manuscript. PVC and AAV contributed substantially to data collection and writing of the manuscript. LEPP, FJH contributed to the analysis and interpretation of data. CMHC contributes to writing of the first draft and subsequent revisions of the manuscript.
Conflict of interest
All authors declare that they have no conflict of interests.
Acknowledgment
The authors acknowledge InBodyCo, Ltd for providing the BIA equipment for this research. InBody Co, Ltd did not interfere in the research design or results analysis. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.World Health Organization Coronavirus Disease (COVID-19) Dashboard World Health organization. https://covid19.who.int
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kizilarslanoglu M.C., Kuyumcu M.E., Yesil Y., Halil M. Sarcopenia in critically ill patients. J Anesth. 2016;30(5):884–890. doi: 10.1007/s00540-016-2211-4. [DOI] [PubMed] [Google Scholar]
- 4.Akan B. Influence of sarcopenia focused on critically ill patients. Acute Crit Care. 2021;36(1):15–21. doi: 10.4266/acc.2020.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gualtieri P., Falcone C., Romano L., Macheda S., Correale P., Arciello P., et al. Body composition findings by computed tomography in SARS-CoV-2 patients: increased risk of muscle wasting in obesity. Int J Mol Sci. 2020;21(13):4670. doi: 10.3390/ijms21134670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Looijaard W.G.P.M., Molinger J., Weijs P.J.M. Measuring and monitoring lean body mass in critical illness. Curr Opin Crit Care. 2018;24(4):241–247. doi: 10.1097/MCC.0000000000000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyére O., Cederholm T., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tolonen A., Pakarinen T., Sassi A., Kyttä J., Cancino W., Rinta-Kiikka I., et al. Methodology, clinical applications, and future directions of body composition analysis of computed tomography (CT) images: a review. Eur J Radiol. 2021:109943. doi: 10.1016/j.ejrad.2021.109943. Published online August 30, 2021. [DOI] [PubMed] [Google Scholar]
- 9.Shen W., Punyanitya M., Wang Z., Gallagher D., St-Onge M.P., Albu J., et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 10.Mundi M.S., Patel J.J., Martindale R. Body composition technology: implications for the ICU. Nutr Clin Pract. 2019;34(1):48–58. doi: 10.1002/ncp.10230. [DOI] [PubMed] [Google Scholar]
- 11.Lee K., Shin Y., Huh J., Sung Y.S., Lee I.S., Yoon K.H., et al. Recent issues on body composition imaging for sarcopenia evaluation. Korean J Radiol. 2019;20(2):205–217. doi: 10.3348/kjr.2018.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baracos V.E. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle. 2017;8(4):527–528. doi: 10.1002/jcsm.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besutti G., Pellegrini M., Ottone M., Cantini M., Milic J., Bonelli E., et al. The impact of chest CT body composition parameters on clinical outcomes in COVID-19 patients. PLoS One. 2021;16(5) doi: 10.1371/journal.pone.0251768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ufuk F., Demirci M., Sagtas E., Akbudak I.H., Ugurlu E., Sari T. The prognostic value of pneumonia severity score and pectoralis muscle Area on chest CT in adult COVID-19 patients. Eur J Radiol. 2020;131:109271. doi: 10.1016/j.ejrad.2020.109271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuchnia A., Earthman C., Teigen L., Cole A., Mourtzakis M., Paris M., et al. Evaluation of bioelectrical impedance analysis in critically ill patients: results of a multicenter prospective study. J Parenter Enteral Nutr. 2017;41(7):1131–1138. doi: 10.1177/0148607116651063. [DOI] [PubMed] [Google Scholar]
- 16.Price K.L., Earthman C.P. Update on body composition tools in clinical settings: computed tomography, ultrasound, and bioimpedance applications for assessment and monitoring. Eur J Clin Nutr. 2019;73(2):187–193. doi: 10.1038/s41430-018-0360-2. [DOI] [PubMed] [Google Scholar]
- 17.Yap J., Rafii M., Azcue M., Pencharz P. Effect of intravenous infusion solutions on bioelectrical impedance spectroscopy. J Parenter Enteral Nutr. 2017;41(4):641–646. doi: 10.1177/0148607115619598. [DOI] [PubMed] [Google Scholar]
- 18.Cornejo-Pareja I., Vegas-Aguilar I.M., García-Almeida J.M., et al. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID-19: a longitudinal cohort study. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.02.017. Published online February 17, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osuna-Padilla I.A., Rodríguez-Moguel N.C., Rodríguez-Llamazares S., et al. Low Phase angle is associated with 60-day mortality in COVID-19 Critically ill-patients. J Parenter Enteral Nutr. 2021 doi: 10.1002/jpen.2236. Published online July 22, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman A., Hasan R.M., Agarwala R., Martin C., Day A.G., Heyland D.K. Identifying critically-ill patients who will benefit most from nutritional therapy: further validation of the “modified NUTRIC” nutritional risk assessment tool. Clin Nutr. 2016;35(1):158–162. doi: 10.1016/j.clnu.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Lohmann T.G., Roche A.F., Martorell R. Human Kinetics Books; Champaign, Ill.: 1988. Anthropometric standardization reference manual. [Google Scholar]
- 22.Rabito E.I., Mialich M.S., Martínez E.Z., García R.W.D., Jordao A.A., Marchini J.S. Validation of predictive equations for weight and height using a metric tape. Nutr Hosp. 2008;23(6):614–618. [PubMed] [Google Scholar]
- 23.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894(i-xii):1–253. [PubMed] [Google Scholar]
- 24.Prado C.M.M., Birdsell L.A., Baracos V.E. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3(4):269–275. doi: 10.1097/SPC.0b013e328331124a. [DOI] [PubMed] [Google Scholar]
- 25.Prado C.M., Cushen S.J., Orsso C.E., Ryan A.M. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75(2):188–198. doi: 10.1017/S0029665115004279. [DOI] [PubMed] [Google Scholar]
- 26.Aubrey J., Esfandiari N., Baracos V.E., Buteau F.A., Frenette J., Putman C.T., et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol. 2014;210(3):489–497. doi: 10.1111/apha.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caan B.J., Meyerhardt J.A., Kroenke C.H., Alexeeff S., Xiao J., Weltzien E., et al. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS study) Cancer Epidemiol Biomarkers Prev. 2017;26(7):1008–1015. doi: 10.1158/1055-9965.EPI-17-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schutz Y., Kyle U.U.G., Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18–98 y. Int J Obes. 2002;26(7):953–960. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 29.Cederholm T., Bosaeus I., Barazzoni R., Bauer J., Van Gossum A., Klek S., et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr. 2015;34(3):335–340. doi: 10.1016/j.clnu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Cederholm T., Jensen G.L., Correia M.I.T.D., Gonzalez M.C., Fukushima R., HIgashiguchi T., et al. GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38(1):1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez M.C., Mehrnezhad A., Razaviarab N., Barbosa-Silva T.G., Heymsfield S.B. Calf circumference: cutoff values from the NHANES 1999-2006. Am J Clin Nutr. 2021;113(6):1679–1687. doi: 10.1093/ajcn/nqab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fryar C.D., Carroll M.D., Gu Q., Afful J., Ogden C.L. Anthropometric reference data for children and adults: United States, 2015-2018. Vital Health Stat 3. 2021;(36):1–44. [PubMed] [Google Scholar]
- 33.Martindale R., Patel J.J., Taylor B., Arabi Y.M., Warren M., McClave S.A. Nutrition therapy in critically ill patients with coronavirus disease (COVID-19) J Parenter Enteral Nutr. 2020;44(7):1174–1184. doi: 10.1002/jpen.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barazzoni R., Bischoff S.C., Breda J., Wickramashinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barazzoni R., Bischoff S.C., Busetto L., Cederholm T., Chourdakis M., Cuerda C., et al. Nutritional management of individuals with obesity and COVID-19: ESPEN expert statements and practical guidance. Clin Nutr. 2021;S0261-5614(21) doi: 10.1016/j.clnu.2021.05.006. Published online May 11, 2021 00248-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 37.Li F., He H. Assessing the accuracy of diagnostic tests. Shanghai Arch Psychiatry. 2018;30(3):207–212. doi: 10.11919/j.issn.1002-0829.218052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Damanti S., Cristel G., Ramirez G.A., Bozzolo E.P., Da Prat V., Gobbi A., et al. Influence of reduced muscle mass and quality on ventilator weaning and complications during intensive care unit stay in COVID-19 patients. Clin Nutr. 2021;S0261-5614(21) doi: 10.1016/j.clnu.2021.08.004. Published online August 16, 2021 00375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox M.C., Booth M., Ghita G., Wang Z., Gardner A., Hawkins R.B., et al. The impact of sarcopenia and acute muscle mass loss on long-term outcomes in critically ill patients with intra-abdominal sepsis. J Cachexia Sarcopenia Muscle. 2021;12(5):1203–1213. doi: 10.1002/jcsm.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gil S., Jacob Filho W., Shinjo S.K., Ferriolli E., Busse A.L., Avelino-Silva T.J., et al. Muscle strength and muscle mass as predictors of hospital length of stay in patients with moderate to severe COVID-19: a prospective observational study. J Cachexia Sarcopenia Muscle. 2021 doi: 10.1002/jcsm.12789. Published online September 14, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woo H.Y., Oh S.Y., Lee H., Ryu H.G. Evaluation of the association between decreased skeletal muscle mass and extubation failure after long-term mechanical ventilation. Clin Nutr. 2020;39(9):2764–2770. doi: 10.1016/j.clnu.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Meyer H.J., Wienke A., Surov A. CT-defined low skeletal muscle mass as a prognostic marker for short-term mortality in critical ill patients. A systematic review and meta analysis. Nutrition. 2021;91–92:111417. doi: 10.1016/j.nut.2021.111417. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X.M., Chen D., Xie X.H., Zhang J.E., Zeng Y., Cheng A.S. Sarcopenia as a predictor of mortality among the critically ill in an intensive care unit: a systematic review and meta-analysis. BMC Geriatr. 2021;21(1):339. doi: 10.1186/s12877-021-02276-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Derstine B.A., Holcombe S.A., Ross B.E., Wang N.C., Su G.L., Wang S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci Rep. 2018;8(1):11369. doi: 10.1038/s41598-018-29825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahat G., Turkmen B.O., Aliyev S., Catikkas N.M., Bakir B., Karan M.A. Cut-off values of skeletal muscle index and psoas muscle index at L3 vertebra level by computerized tomography to assess low muscle mass. Clin Nutr. 2021;40(6):4360–4365. doi: 10.1016/j.clnu.2021.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Correa-de-Araujo R., Addison O., Miljkovic I., Goodpaster B.H., Bergman B.C., Clark R.V., et al. Myosteatosis in the context of skeletal muscle function deficit: an interdisciplinary workshop at the national Institute on aging. Front Physiol. 2020;11:963. doi: 10.3389/fphys.2020.00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moisey L.L., Mourtzakis M., Cotton B.A., Premji T., Heyland D.K., Wade C.E., et al. Nutrition and Rehabilitation Investigators Consortium (NUTRIC). Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17(5):R206. doi: 10.1186/cc12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Looijaard W.G.P.M., Dekker I.M., Beishuizen A., Girbes A.R.J., Oudemans-van Straaten H.M., Weijs P.J.M. Early high protein intake and mortality in critically ill ICU patients with low skeletal muscle area and -density. Clin Nutr. 2020;39(7):2192–2201. doi: 10.1016/j.clnu.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Weijs P.J., Looijaard W.G., Dekker I.M., Stapel S.N., Girbes A.R., Oudemans-van Straaten H.M., et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18(2):R12. doi: 10.1186/cc13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Looijaard W.G., Dekker I.M., Stapel S.N., Girbes A.R., Twisk J.W., Oudemans-van Straaten H.M., et al. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit Care. 2016;20(1):386. doi: 10.1186/s13054-016-1563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loosen S.H., Schulze-Hagen M., Püngel T., Bündgens L., Wirtz T., Kather J.N., et al. Skeletal muscle composition predicts outcome in critically ill patients. Crit Care Explor. 2020;2(8) doi: 10.1097/CCE.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dusseaux M.M., Antoun S., Grigioni S., Béduneau G., Carpentier D., Girault C., et al. Skeletal muscle mass and adipose tissue alteration in critically ill patients. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0216991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.da Silva B.R., Gonzalez M.C., Cereda E., Prado C.M. Exploring the potential role of phase angle as a marker of oxidative stress: a narrative review. Nutrition. 2022;93:111493. doi: 10.1016/j.nut.2021.111493. [DOI] [PubMed] [Google Scholar]
- 54.Ruiz-Margáin A., Xie J.J., Román-Calleja B.M., Pauly M., White M.G., Chapa-Ibargüengoitia M., et al. Phase Angle from bioelectrical impedance for the assessment of sarcopenia in cirrhosis with or without ascites. Clin Gastroenterol Hepatol. 2021;19(9):1941–1949. doi: 10.1016/j.cgh.2020.08.066. e2. [DOI] [PubMed] [Google Scholar]