Abstract
SARS-CoV-2 can cause diverse severe and lasting damage to the kidneys. In the latest issue of Cell Stem Cell, Jansen et al. utilized data gleaned from human kidney autopsies and human induced pluripotent stem cell-derived kidney organoids to investigate the direct effects of SARS-CoV-2 infection on kidney cells. They found that such infections resulted in renal scarring (notably, tubulointerstitial fibrosis).
SARS-CoV-2 can cause diverse severe and lasting damage to the kidneys. In the latest issue of Cell Stem Cell, Jansen et al. utilized data gleaned from human kidney autopsies and human induced pluripotent stem cell-derived kidney organoids to investigate the direct effects of SARS-CoV-2 infection on kidney cells. They found that such infections resulted in renal scarring (notably, tubulointerstitial fibrosis).
Main text
As the coronavirus disease 2019 (COVID-19) global pandemic continues for a third year, more has been unveiled about its culprit, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Aside from triggering a possibly dire innate immune response, it also causes severe respiratory illness and a myriad of acute and chronic multi-organ damage, with the kidneys being one of the most frequently targeted organs outside the lungs. Accumulating evidence suggests the presence of SARS-CoV-2 in some but not all kidneys from COVID-19 cases, yet at higher concentrations compared to other organs (Puelles et al., 2020). Renal manifestations of COVID-19 can range from acute kidney injury (AKI), hematuria, proteinuria, podocytopathy, and tubulointerstitial fibrosis (Diao et al., 2021). Importantly, a substantial number of individuals with COVID-19 experience post-acute sequelae, a syndrome now referred to as “long COVID,” many of whom suffered worse outcomes of AKI such as rapid kidney function decline and chronic kidney disease (CKD) (Bowe et al., 2021). One key to understanding these phenomena is to determine if the virus infects kidney cells directly and, if so, if this leads to short- and/or long-term renal damage.
In the latest issue of Cell Stem Cell, Jansen et al. comprehensively investigated whether direct infection of the kidneys by SARS-CoV-2 introduces a pro-fibrotic phenotype (Jansen et al., 2022). The authors compared kidneys of individuals with COVID-19 compared to matched controls. With data derived from 61 human autopsies and single-RNA nucleus sequencing, the study associated increased interstitial fibrosis with the presence of higher activity in proinflammatory and fibrosis-driving pathways (notably, tumor necrosis factor alpha [TNFα], transforming growth factor beta, nuclear factor kB, and JAK-STAT). Taking this to the next level, the study utilized human induced pluripotent stem cell (hiPSC)-derived kidney organoids to fine map cellular locations of SARS-CoV-2 infection and accompanying signaling changes. In addition to verifying upregulated activity of pro-fibrotic pathways found in SARS-CoV-2-infected autopsied kidneys, the team identified evidence of a molecular crosstalk between proximal tubular cells and PDGFRa/b+ mesenchymal cells (a key source of myofibroblasts) that further contributed to fibrosis.
The findings by Jansen and colleagues are exciting and supported by both human tissues and hiPSC-derived organoids. They can serve as strong evidence to support the notion that SARS-CoV-2 can directly drive renal fibrosis, a CKD hallmark, suggesting a likely impact on the worse AKI outcomes during COVID-19 that are often observed. These findings also bridged a knowledge gap, linking acute viral illness to kidney disease during long-COVID. hiPSC-derived organoids have the advantage of being able to assess intercellular interactions, which possibly explains the greater extent of cytopathic changes seen in organoids compared to other studies using SARS-CoV-2-infected ex vivo models comprised solely of tubular epithelial cells and that lacked cytopathic changes (Omer et al., 2021). Jansen et al. provided the first hints that SARS-CoV-2 infection in kidney organoids is amenable to a treatment with a protease inhibitor, providing a glimpse into potential future treatments for COVID-19-related kidney injury. The findings also stimulate further clinical and translational investigations for testing SARS-CoV-2 tropism in the kidneys of infected individuals to identify potential virus-free kidneys for transplantation procured from individuals who died of fulminant COVID-19 (Lee et al., 2022).
The use of hiPSC-kidney organoids is not without caveats, however. As the authors pointed out, the protocols to differentiate hiPSCs to model the adult kidney in its entirety are still suboptimal. While excluding extra-renal factors, such as intensive care treatment, the model also isolates kidney cells from the involvement of immune cells. For example, the recruitment of CD68+ macrophages, CD56+ natural killer cells, and CD8+ T cells, alongside strong C5b-9 deposition, was found with tubular necrosis in the kidneys of patients with COVID-19 (Diao et al., 2021). Jansen et al. also observed an interaction between podocytes and the mesenchymal endothelial progenitor population. The cytologic consequences were beyond the study’s scope and therefore not described, but one must recognize podocytopathy, glomerular collapsing, and sclerosis as indispensable parts of the COVID-19-related kidney disease spectrum, in which innate immunity likely plays an essential role. The massive release of immune mediators such as soluble urokinase plasminogen activator receptor (suPAR), TNFα, and interferon gamma, i.e., a cytokine storm, signifies severe COVID-19 and confers substantial risk for AKI (Azam et al., 2020; Karki et al., 2021). Biomarkers predictive of outcomes that are elevated early during disease progression, such as suPAR, have been successfully applied in clinical practice to guide immunomodulative treatment and improve prognosis (Kyriazopoulou et al., 2021). Future studies aiming to examine viral triggers, innate immune mediators, and kidney cell reactivity in concert will need to be performed. In the meantime, Jansen et al. allow us to draw the conclusion that the renal presence of SARS-CoV-2 can bring about pro-inflammatory and pro-fibrotic molecular changes that may prime the kidneys for further damage (including hits from circulating mediators such as suPAR and TNFα), ultimately leading to lasting kidney disease in long COVID.
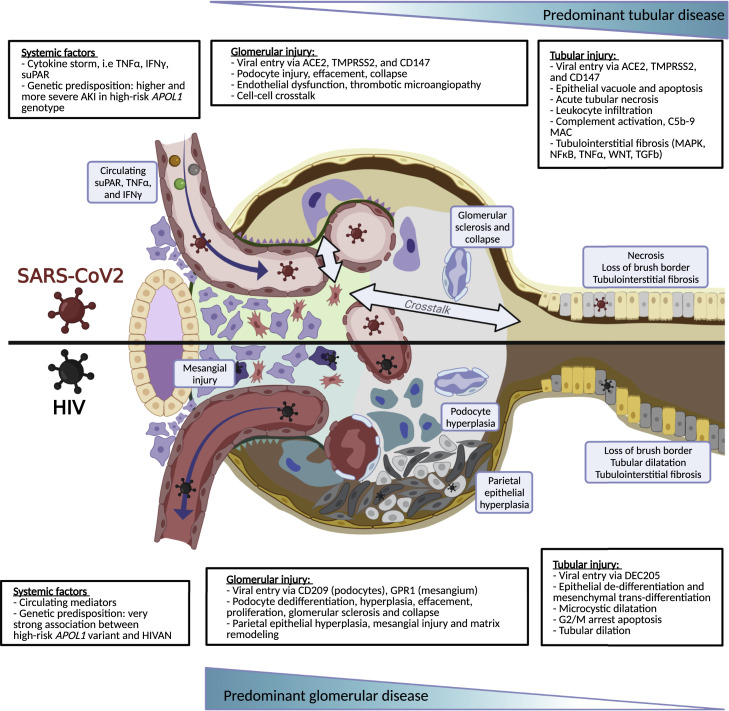
The study by Jansen et al. provides additional insights to allow for a comparison of kidneys infected by SARS-CoV-2 and other viral infections, especially human immunodeficiency virus (HIV) infection (Figure 1 ). Both SARS-CoV-2 and HIV directly infect podocytes and tubular epithelial cells. Collapsing glomerulopathy, tubulointerstitial fibrosis, and mesangial injury can be seen in both COVID-19 and HIV infection and are perhaps dependent on the amount of the innate immune activation that is mounted as an anti-infection response. Lessons learned from our experience with HIV infection may offer insight into the study of COVID-19-related kidney disease. Is there a set of COVID-19 gene products that converges pro-fibrotic and other pathways in diverse kidney cells and structures? African Americans develop COVID-19 associated with stronger AKI more often than other racial and ethnic groups (Hung et al., 2022). Are socioeconomic factors the sole drivers of these disparities, or is there a role for other genetic factors and environmental factors? It is noteworthy that untreated HIV infection caused glomerulopathy in 50% of African Americans with two APOL1 risk alleles. Data from patients with COVID-19 also suggested AKI, proteinuria, and podocytopathy were more prevalent among those with the high-risk APOL1 genotype. Will there be a need for screening and early detection of CKD in long COVID? Which antivirals and what duration of treatment will ensure clearance of SARS-CoV-2 in the kidneys? A stage is now set for these topics to be addressed.
Figure 1.
Schematic overview of the kidney infected by SARS-CoV-2 or HIV
Mechanisms and details of SARS-CoV-2 and human immunodeficiency virus (HIV) and their impact on the kidney. SARS-CoV-2, more than HIV, triggers an innate immune response that imposes systemic effects on the kidney, impacting glomerular injury and tubular injury. Both viruses can also infect kidney cells directly, which in the case of SARS-CoV-2 may contribute to fibrosis. Notably, systemic and cell-specific effects are compounded, which can increase risk further upon additional factors (e.g., suPAR, APOL1 risk variants).
Acknowledgments
The authors thank Vineet Gupta, Ph.D. for critical reading of the manuscript. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (RO1DK125858, RO1DK109720, and R01DK113761) to J.R.
Declaration of interests
J.R. is cofounder, scientific advisory board co-chair, and shareholder of Walden Biosciences, a kidney therapeutic company. Other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Azam T.U., Shadid H.R., Blakely P., O’Hayer P., Berlin H., Pan M., Zhao P., Zhao L., Pennathur S., Pop-Busui R., et al. International Study of Inflammation in COVID-19 Soluble urokinase receptor (SuPAR) in COVID-19-related AKI. J. Am. Soc. Nephrol. 2020;31:2725–2735. doi: 10.1681/ASN.2020060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe B., Xie Y., Xu E., Al-Aly Z. Kidney outcomes in long COVID. J. Am. Soc. Nephrol. 2021;32:2851–2862. doi: 10.1681/ASN.2021060734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Wang R., Feng Z., Zhang J., Yang H., Tan Y., Wang H., Wang C., Liu L., et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat. Commun. 2021;12:2506. doi: 10.1038/s41467-021-22781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung A.M., Shah S.C., Bick A.G., Yu Z., Chen H.C., Hunt C.M., Wendt F., Wilson O., Greevy R.A., Chung C.P., et al. VA Million Veteran Program COVID-19 Science Initiative APOL1 risk variants, acute kidney injury, and death in participants with African ancestry hospitalized with COVID-19 from the Million Veteran Program. JAMA Intern. Med. 2022 doi: 10.1001/jamainternmed.2021.8538. Published online January 28, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J., Reimer K.C., Nagai J.S., Varghese F.S., Overheul G.J., de Beer M., Roverts R., Daviran D., Fermin L.A.S., Willemsen B., et al. COVID Moonshot consortium SARS-CoV-2 infects the human kidney and drives fibrosis in kidney organoids. Cell Stem Cell. 2022;29:217–231.e8. doi: 10.1016/j.stem.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki R., Sharma B.R., Tuladhar S., Williams E.P., Zalduondo L., Samir P., Zheng M., Sundaram B., Banoth B., Malireddi R.K.S., et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–168.e17. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazopoulou E., Poulakou G., Milionis H., Metallidis S., Adamis G., Tsiakos K., Fragkou A., Rapti A., Damoulari C., Fantoni M., et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat. Med. 2021;27:1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Desai N.M., Resnick J., Li M., Johanson A., Pekosz A., Rabb H., Mankowski J.L. Successful kidney transplantation from a deceased donor with severe COVID-19 respiratory illness with undetectable SARS-CoV-2 in donor kidney and aorta. Am. J. Transplant. 2022 doi: 10.1111/ajt.16956. Published online January 13, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer D., Pleniceanu O., Gnatek Y., Namestnikov M., Cohen-Zontag O., Goldberg S., Friedman Y.E., Friedman N., Mandelboim M., Vitner E.B., et al. Human kidney spheroids and monolayers provide insights into SARS-CoV-2 renal interactions. J. Am. Soc. Nephrol. 2021;32:2242–2254. doi: 10.1681/ASN.2020111546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L., Chilla S., Heinemann A., Wanner N., Liu S., et al. Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]