Abstract
In guard cells of open stomata under daylight, long actin filaments are arranged at the cortex, radiating out from the stomatal pore. Abscisic acid (ABA), a signal for stomatal closure, induces rapid depolymerization of cortical actin filaments and the slower formation of a new type of actin that is randomly oriented throughout the cell. This change in actin organization has been suggested to be important in signaling pathways involved in stomatal closing movement, since actin antagonists interfere with normal stomatal closing responses to ABA. Here we present evidence that the actin changes induced by ABA in guard cells of dayflower (Commelina communis) are mediated by cytosolic calcium levels and by protein phosphatase and protein kinase activities. Treatment of guard cells with CaCl2 induced changes in actin organization similar to those induced by ABA. Removal of extracellular calcium with EGTA inhibited ABA-induced actin changes. These results suggest that Ca2+ acts as a signal mediator in actin reorganization during guard cell response to ABA. A protein kinase inhibitor, staurosporine, inhibited actin reorganization in guard cells treated with ABA or CaCl2, and also increased the population of cells with long radial cortical actin filaments in untreated control cells. A protein phosphatase inhibitor, calyculin A, induced fragmentation of actin filaments in ABA- or CaCl2-treated cells and in control cells, and inhibited the formation of randomly oriented long actin filaments induced by ABA or CaCl2. These results suggest that protein kinase(s) and phosphatase(s) participate in actin remodeling in guard cells during ABA-induced stomatal closure.
In guard cells of open stomata under daylight, long actin filaments are arranged at the cortex, radiating out from the stomatal pore (Kim et al., 1995; Eun and Lee, 1997). When the guard cells detect abscisic acid (ABA), a signal for stomatal closure, these cortical actin filaments disintegrate (Eun and Lee, 1997). This change in actin organization appears to be important in stomatal closing movement, since actin antagonists alter the normal stomatal responses to ABA (Hwang et al., 2000). Although many lines of evidence suggest that actin participates in signaling pathways involved in stomatal movement, upstream regulators and downstream targets of actin have not been well characterized.
One of the earliest responses of guard cells to stomatal closing signals is an increase in the intracellular calcium ion concentration ([Ca2+]i). Experimental elevation of [Ca2+]i induces stomatal closure and mimics several effects of ABA on ion channels in guard cells (Assmann, 1993; McAinsh et al., 1995). In addition, Ca2+ regulates cellular actin dynamics in animal cells via Ca2+-dependent actin-binding proteins including gelsolin, filamin, fimbrin, and α-actinin (Puius et al., 1998). Therefore, cytosolic free Ca2+ is a second messenger for stomatal closing signals and is a candidate mediator of actin changes during stomatal closure.
Other potential mediators of actin changes in guard cells are protein kinases and protein phosphatases, which are expressed in guard cells and are reported to play important roles in the signaling cascades involved in stomatal movement (Leung and Giraudat, 1998; Li et al., 1998, 2000). Protein kinases and phosphatases are known to act directly on actin-binding proteins in other cell types (Hartiwig et al., 1992; Smertenko et al., 1998; Guillén et al., 1999). Light and ABA activate calcium-dependent and calcium-independent protein kinases, respectively, in guard cells (Shimazaki et al., 1992; Li and Assmann, 1996; Mori and Muto, 1997). Arabidopsis mutants with defects in protein phosphatase 2C are insensitive to ABA (Leung et al., 1994, 1997), and protein phosphatase 2C activity is necessary for normal increases in [Ca2+]i, activation of calcium-dependent anion channels, and inactivation of inward K+ channels in guard cells in response to ABA (Armstrong et al., 1995; Grabov et al., 1997; Pei et al., 1997; Allen et al., 1999). In addition, protein phosphatases that are sensitive to okadaic acid and calyculin A (inhibitors of type 1 and type 2A phosphatases) are active in guard cells (Li et al., 1994; Kinoshita and Shimazaki, 1999), and guard cell K+ and slow anion currents are affected by these inhibitors (Li et al., 1994; Thiel and Blatt, 1994; Schmidt et al., 1995; Pei et al., 1997).
Here we present evidence that cytosolic calcium, protein kinase(s), and protein phosphatase(s) are involved in ABA-induced actin reorganization in guard cells of dayflower (Commelina communis). Changes in actin organization similar to those induced by ABA were observed when guard cells were exposed to CaCl2, and EGTA slowed down ABA-induced actin reorganization. Inhibitors of protein kinases or phosphatases also altered ABA- and CaCl2-induced actin remodeling in guard cells.
RESULTS
Actin Organization in Guard Cells Labeled with Rhodamine-Phalloidin: Response to ABA
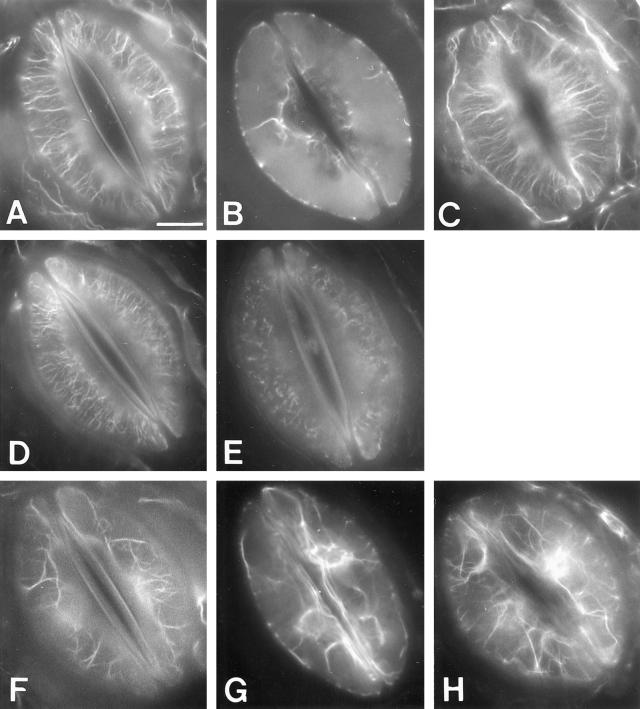
In previous studies we labeled actin filaments in mature guard cells with an actin antibody that permeated the cells through cracks made by freeze-cracking (Kim et al., 1995; Eun and Lee, 1997; Eun and Lee, 2000). To improve the visibility of actin and increase the number of labeled cells we modified the labeling method and used rhodamine-phalloidin for actin staining, eliminating the cracking and extraction procedures. Most guard cells of stomata that were open under white light during h 6 to 7 of the photoperiod had long cortical actin filaments radiating out from the stomatal pore (type 1, Fig. 1, A and C); this observation is consistent with a previous report concerning guard cells of open stomata (Eun and Lee, 1997). Treatment with ABA caused striking changes in the actin organization of these guard cells (Fig. 1; Table I). Ten minutes after the addition of 10 μm ABA, the stomatal aperture had decreased and actin filaments had begun to break down, forming fragmented filaments in a radial pattern (type 2, Fig. 1D) and short fragmented filaments in a spotted pattern (type 3, Fig. 1E). The proportion of cells showing type 1 actin decreased from 72% to 48% during this period, whereas the proportions of cells with type 2 and type 3 actin increased from 27% to 32% and from 1% to 18%, respectively. By 30 min, stomata closed further and actin disintegration had progressed to a greater extent: cells with types 1 and 2 actin had decreased to 4% and 12%, respectively, whereas cells with type 3 actin had increased to 65% of the cell population. By 60 min, stomatal closing movement had slowed and the aperture had begun to stabilize, and cells with types 2 and 3 actin had decreased to 5% and 22%, respectively, and cells with sparse, randomly organized long filaments (type 4, Fig. 1, F and H) made up 71% of the cell population. In cells with type 4 actin, bright fluorescence was also associated with the nucleus (Fig. 1, B and G) on staining with rhodamine-phalloidin. The proportion of cells containing type 4 actin continued to increase with further ABA treatment and reached 90% after 2 h (data not shown).
Figure 1.
Actin organization in mature guard cells of dayflower. A through C, Typical pattern of actin organization in guard cells of open stomata (type 1); D and E, patterns of actin organization dominating during stomatal closing (types 2 and 3); F through H, typical pattern of actin organization in guard cells of stomata following closure in response to ABA or CaCl2 (type 4). A and D through F show actin filaments at the cortex near the outer periclinal wall (note the outer ledges that outline the stomatal pore). B and G are images focused on the nucleus. C and H show the cortex near the inner periclinal wall. Guard cells were fixed for actin visualization in a fixative including detergent, as described in “Materials and Methods.” As reported previously (Eun and Lee, 1997), fixed guard cells lost their turgor and even the previously open stomata (A–C) looked closed when actin was observed. The convexity of guard cells made it difficult to obtain good photographic images at all three focal levels from a single stoma, so representative pictures from different stomata are shown. Scale bar = 10 μm.
Table I.
Actin reorganization in guard cells following 10-μm ABA treatment
| Time | Actin
Patterna
|
No. of Cellsb | Stomatal Aperturec | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| min | % of total | μm | ||||
| 0 | 72 | 27 | 1 | 0 | 680 | 9.6 ± 0.16 (145) |
| 10 | 48 | 32 | 18 | 2 | 704 | 6.2 ± 0.27 (105) |
| 30 | 4 | 12 | 65 | 19 | 744 | 2.8 ± 0.27 (105) |
| 60 | 2 | 5 | 22 | 71 | 462 | 1.1 ± 0.14 (215) |
Epidermal fragments were fixed for actin staining after measurement of stomatal apertures. Actin organization in guard cells was classified into four types (Fig. 1). Between 40 and 80 guard cells were analyzed from each epidermal fragment and the results from six independent experiments were combined for statistical analysis.
Actin patterns were classified into four types: 1, long filaments in a radial pattern; 2, filaments fragmented, but still in a radial pattern; 3, short and spot-like filaments lacking any regular orientation; and 4, sparse, randomly distributed long filaments.
No. of cells examined for changes in actin pattern.
Average ± se (n).
This time-dependent actin change in guard cells consisted of an initial disintegration followed by reorganization of actin filaments into a different pattern in response to ABA. Type 1, the “open” pattern, was most prevalent in guard cells of open stomata, as reported previously (Eun and Lee, 1997); type 4 is the “closed” pattern and was predominant in guard cells of stomata that were stably closed in the presence of ABA. Types 2 and 3 were observed primarily during stomatal closing movement and appear to represent “transitional patterns.” As suggested previously (Kim et al., 1995; Hwang et al., 1997), long cortical actin filaments may play a negative regulatory role in stomatal movement, and disintegration of actin filaments may be important for rapid stomatal closure. Further studies are necessary to clarify the role of formation of random long filaments in the guard cells of closed stomata.
Treatment of Guard Cells with CaCl2 Induced Actin Reorganization Similar to That Induced by ABA
To test whether cytosolic Ca2+ increase mediates the actin reorganization induced by ABA we treated epidermal fragments with 2 mm CaCl2, which has been reported to increase [Ca2+]i in guard cells (McAinsh et al., 1995; Allen et al., 1999). The actin reorganization observed after treatment with 2 mm CaCl2 resembled that induced by ABA (Table II). Actin filaments of guard cells first disintegrated and then reorganized into the closed (type 4) pattern. Stomatal aperture decreased rapidly between 10 and 30 min after the addition of 2 mm CaCl2, during which period the actin filaments in guard cells disintegrated significantly. Cells with types 1 and 2 actin decreased from 55% to 29% and from 34% to 21%, respectively, of the cell population. In contrast, cells with type 3 actin increased from 11% to 37%. As stomata closed still further during the following 30 min, the proportions of cells containing types 1, 2, and 3 actin decreased to 13%, 8%, and 31%, whereas cells with the closed pattern (type 4 actin) increased from 13% to 48%. The proportion of cells containing type 4 actin continued to increase during a further 60 min of CaCl2 treatment (data not shown). The close similarity between the effects of CaCl2 and ABA indicates that cytosolic calcium increase may act as a signal mediator for actin reorganization induced by ABA in guard cells of dayflower.
Table II.
CaCl2-induced actin reorganization in guard cells
| Time | Actin
Pattern
|
No. of Cellsa | Stomatal Aperturec | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| min | % of total | μm | ||||
| 0 | 45 | 44 | 11 | 0 | 480 | 12.8 ± 0.38 (60) |
| 10 | 55 | 34 | 11 | 0 | 460 | 11.9 ± 0.38 (60) |
| 30 | 29 | 21 | 37 | 13 | 480 | 3.4 ± 0.42 (60) |
| 60 | 13 | 8 | 31 | 48 | 500 | 1.5 ± 0.36 (60) |
Epidermal fragments were treated with 2 mm CaCl2 prior to measurement of stomatal apertures and actin labeling. Actin organizations in guard cells observed in six epidermal fragments from three independent experiments were classified into four types as in Figure 1 and Table I.
No. of cells examined for changes in actin pattern.
Average ± se (n).
Guard cell responses to 2 mm CaCl2 were not as rapid as those induced by 10 μm ABA; no decrease in stomatal aperture was apparent until 10 min after CaCl2 treatment began, and actin disintegration was likewise slower (Table II). In fact, in some epidermal fragments, a slight increase in the fraction of cells containing type 1 actin was observed after 10 min of CaCl2 treatment. The response of guard cells to 5 mm CaCl2 was similarly slow, suggesting that rapid stomatal and actin responses to ABA may require activation of other signal components, as well as cytosolic Ca2+ increase.
EGTA Inhibited ABA-Induced Actin Changes
To further test whether an increase in [Ca2+]i mediates ABA-induced actin reorganization we removed external Ca2+ from the cell environment by the addition of 5 mm EGTA. The response of actin to ABA was slowed by EGTA (Table III). In the absence of EGTA, disintegration of actin filaments was apparent in 10 min and the majority of cells had type 4 actin in 60 min of treatment with ABA (Table I). In contrast, cells treated with 5 mm EGTA showed slow disintegration of actin filaments compared with their control untreated cells until 30 min after the addition of ABA, and actin in the majority of EGTA-treated cells remained disintegrated and did not form the closed pattern even after 60 min of ABA treatment (Table III). This result indicates that an increase in [Ca2+]i is necessary for normal actin reorganization in response to ABA.
Table III.
Treatment with 5 mm EGTA slowed down the actin changes induced by 10 μm ABA
| Time | Actin
Pattern
|
No. of Cellsa | Stomatal Apertureb | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| min | % of total | μm | ||||
| 0 | 80 | 20 | 0 | 0 | 680 | 10.7 ± 0.16 (145) |
| 10 | 61 | 38 | 1 | 0 | 704 | 10.3 ± 0.18 (105) |
| 30 | 22 | 45 | 25 | 8 | 744 | 7.7 ± 0.23 (105) |
| 60 | 8 | 6 | 46 | 40 | 461 | 5.7 ± 0.20 (215) |
EGTA was added 30 min prior to treatment with ABA and was maintained at 5 mm throughout the experiment. Time-dependent changes in actin organization in guard cells and in stomatal apertures were measured in the presence of EGTA. The results from 10 to 16 epidermal fragments observed in six independent experiments were combined for analysis. The control time course in the absence of EGTA is shown in Table I.
No. of cells examined for changes in actin pattern.
Average ± se (n).
Although removal of external calcium significantly slowed down the progress of actin reorganization, it did not completely abolish the change. This may be due to the existence of alternative, calcium-independent ABA signaling pathways, or it may simply be that a small increase in [Ca2+]i, which might occur in response to ABA even in the presence of EGTA, is sufficient for initiation of actin reorganization.
Protein Kinase and Protein Phosphatase Inhibitors Altered Actin Organization in Guard Cells of Open Stomata
We also investigated whether protein phosphorylation participates in actin reorganization in guard cells by treating epidermal fragments with staurosporine, a broad-range protein kinase inhibitor, and calyculin A, a type 1/2A phosphatase inhibitor. Cells containing type 1 actin made up 63% of the untreated control cell population, whereas treatment with 10 μm staurosporine for 90 min increased the proportion of cells with type 1 actin to 98% and also significantly increased the stomatal aperture (P < 0.05; Table IV). The same inhibitory effect of staurosporine on fragmentation of actin filaments was observed at concentrations from 2 to 20 μm (data not shown). Calyculin A had the opposite effect, inducing stomatal closure and depolymerization of actin filaments (Table IV). We observed short, fragmented actin filaments (Fig. 1, D and E) in guard cells treated with 1 μm calyculin A for 90 min. The proportion of cells containing type 1 actin decreased to 3%, whereas cells with type 2 or type 3 actin increased to 90% of the cell population. Okadaic acid, a phosphatase inhibitor that is chemically unrelated to calyculin A, had effects similar to those of calyculin A on actin organization and stomatal aperture (data not shown). These alterations of actin organization in the presence of protein kinase or phosphatase inhibitors suggest that protein phosphorylation is important in depolymerization of actin filaments in guard cells.
Table IV.
Effects of kinase and phosphatase inhibitors on actin organization in guard cells of open stomata
| Treatment | Actin
Pattern
|
No. of Cellsa | Stomatal Apertureb | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| % of total | μm | |||||
| Control | 63 | 26 | 11 | 1 | 740 | 8.4 ± 0.29 (100) |
| Staurosporine (10 μm) | 98 | 2 | 0 | 0 | 800 | 10.2 ± 0.28c (90) |
| Control | 72 | 22 | 5 | 0 | 1,876 | 11.9 ± 0.18 (265) |
| Calyculin A (1 μm) | 3 | 46 | 44 | 7 | 1,680 | 6.2 ± 0.18c (265) |
After treatment with inhibitors for 90 min, stomatal apertures were measured and actin was visualized with rhodamine-phalloidin. The protein kinase inhibitor staurosporine suppressed the depolymerization of actin filaments, whereas the protein phosphatase inhibitor calyculin A induced actin depolymerization. The results of eight epidermal fragments from three independent experiments (staurosporine) or 28 epidermal fragments from 14 independent experiments (calyculin A) were combined for analysis.
No. of cells examined for changes in actin pattern.
Average ± se (n).
Significantly different from control at P < 0.05 (Student's t test).
Protein Kinase and Phosphatase Inhibitors Altered ABA-Induced Actin Reorganization in Guard Cells
In the presence of 20 μm staurosporine, the majority (76%) of cells retained long radial actin filaments even after treatment with 10 μm ABA for 1 h (Table V). Actin reorganization into type 4 was remarkably inhibited with 18% compared with 93% in the control samples treated with ABA only. Similar effects were observed at 5 μm staurosporine (data not shown). The effects of staurosporine indicate that activation of staurosporine-sensitive protein kinase(s) is necessary to trigger reorganization of guard cell actin in response to ABA. Staurosporine might block the disintegration of radial cortical actin filaments and thereby indirectly inhibit the appearance of type 4 actin organization.
Table V.
Effects of protein kinase and phosphatase inhibitors on 10-μm ABA-induced actin reorganization in guard cells
| Treatment | Actin
Pattern
|
No. of Cellsa | Stomatal Apertureb | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| % of total | μm | |||||
| ABA | 1 | 0 | 6 | 93 | 740 | 1.1 ± 0.09 (120) |
| Staurosporine + ABA | 76 | 4 | 2 | 18 | 820 | 10.6 ± 0.29 (130) |
| ABA | 3 | 1 | 20 | 76 | 844 | 1.2 ± 0.17 (165) |
| Calyculin A + ABA | 2 | 28 | 53 | 17 | 868 | 4.8 ± 0.22 (165) |
Guard cells were treated with staurosporine (20 μm) or calyculin A (1 μm) for 30 min, then with 10 μm ABA for 1 h. Immediately after measurement of stomatal apertures, epidermal fragments were fixed and stained with rhodamine-phalloidin. Actin patterns were observed in 40 to 80 guard cells from each fragment. The results of 12 epidermal fragments from three independent experiments (staurosporine) or 13 epidermal fragments from seven independent experiments (calyculin A) were combined for analysis.
No. of cells examined for changes in actin pattern.
Average ± se (n).
Calyculin A (1 μm) promoted actin disintegration in ABA-treated (Table V) and control (Table IV) guard cells, and suppressed ABA-induced reorganization of actin into type 4 (Table V). These two effects of calyculin A resulted in an increase in the population of guard cells with fragmented or spot-like actin filaments. The effects of calyculin A indicate that activation of calyculin A-sensitive protein phosphatase(s) is necessary for formation and/or maintenance of long actin filaments in guard cells of open and closed stomata.
Protein Kinase and Phosphatase Inhibitors Altered CaCl2-Induced Actin Reorganization
The effects of staurosporine and calyculin A on actin reorganization induced by 2 mm CaCl2 were similar to their respective effects on ABA-induced actin changes. Staurosporine at 5 μm inhibited the stomatal closure and the actin reorganization induced by 2 mm CaCl2 (data not shown), and 10 μm staurosporine blocked these responses completely (Table VI). Calyculin A at 1 μm inhibited formation of random long actin filaments in the closed pattern and increased the proportion of cells with fragmented actin filaments. Taken together, our results indicate that protein kinase(s) sensitive to staurosporine and/or protein phosphatase(s) sensitive to calyculin A may play roles downstream of Ca2+ in the signaling pathway that leads to actin reorganization in guard cells.
Table VI.
Effects of protein kinase and phosphatase inhibitors on the actin reorganization induced in guard cells by 2 mm CaCl2
| Treatment | Actin
Pattern
|
No. of Cellsa | Stomatal Apertureb | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| % of total | μm | |||||
| CaCl2 | 32 | 20 | 31 | 17 | 674 | 1.1 ± 0.15 (80) |
| Staurosporine + CaCl2 | 91 | 8 | 1 | 0 | 820 | 10.7 ± 0.22 (80) |
| CaCl2 | 38 | 8 | 31 | 23 | 642 | 5.0 ± 0.27 (205) |
| Calyculin A + CaCl2 | 0 | 2 | 94 | 4 | 566 | 3.4 ± 0.20 (215) |
Cells were treated with staurosporine (10 μm) or calyculin A (1 μm) for 30 min, then with 2 mm CaCl2 for 1 h. Immediately after measurement of stomatal apertures, epidermal fragments were fixed and stained with rhodamine-phalloidin. Actin patterns were observed in 40 to 80 guard cells from each fragment. The results of eight epidermal fragments from three independent experiments (staurosporine) or 15 epidermal fragments from seven independent experiments (calyculin A) were combined for analysis.
No. of cells examined for changes in actin pattern.
Average ± se (n).
DISCUSSION
Actin in mature guard cells changes rapidly in response to physiological stimuli that induce stomatal movement (Hwang et al., 2000). To investigate the mechanism of changes in actin structure we looked at cellular factors likely to be involved in actin reorganization during stomatal closure in response to ABA. We tested the possible involvement of intracellular calcium levels and protein kinase and protein phosphatase activities because calcium increase and protein phosphorylation are early responses of guard cells to ABA that induces rapid stomatal closure (Leung and Giraudat, 1998), as well as changes in guard cell actin (Eun and Lee, 1997), and also because these factors are important regulators of cytoskeletal actin in other cell types (Hartiwig et al., 1992; Menzel et al., 1995; Smertenko et al., 1998).
In the present study, we visualized actin by staining with rhodamine-phalloidin, unlike our earlier studies in which actin was immunolocalized (Kim et al., 1995; Eun and Lee, 1997). The overall structure of actin visualized with rhodamine-phalloidin was generally the same as that shown in Eun and Lee (1997), although there were a few differences. With rhodamine-phalloidin, nuclei were rarely stained until the latter stages of stomatal closure and were strongly stained in cells showing the closed actin pattern (Fig. 1, F–H). However, when actin was immunostained, nuclei were stained in the guard cells of open and closed stomata (Eun and Lee, 1997). In addition, the diffuse staining by actin antibody on the ventral side of guard cells treated with ABA was never apparent with rhodamine-phalloidin. These differences may be due to differences in the affinities of the molecules used for staining actin: rhodamine-phalloidin preferentially labels filamentous actin, whereas the anti-actin antibody also recognizes globular actin. Another difference between the present study and our earlier work was apparent in guard cells of stomata stably closed in response to ABA. During stomatal closing, actin disintegrated as reported previously (Table I; Eun and Lee, 1997). After stomatal apertures stabilized at low level, however, guard cells reorganized actin into a distinct pattern: long actin filaments in a sparse and random distribution in cortical and subcortical areas (Figs. 1 and 2; Table I). With rhodamine-phalloidin staining, this closed pattern was found in the majority of guard cells of closed stomata, whereas with the previous method, it was not so frequently observed. This difference may be due to differences in experimental conditions. Even after 1 h of treatment with ABA, type 4 was not the major pattern observed in incubation buffer containing 5 mm EGTA in which the stomata did not close as much as in their control cells untreated with EGTA (compare Tables I and III). In a similar manner, incubation medium containing 50 mm KCl, which was used in the experiments reported in Eun and Lee (1997), does not allow such rapid stomatal closure as the medium containing 30 mm KCl used in the present paper. Formation of the closed actin pattern may therefore have been hindered in our earlier experiments (Eun and Lee, 1997).
Figure 2.
A model of the pathways of actin reorganization in mature guard cells activated by ABA treatment and the signal mediators involved therein. Cortical actin filaments are broken down during stomatal closure induced by ABA. Randomly distributed actin filaments subsequently form in a process mediated by cytosolic calcium. Staurosporine-sensitive protein kinase activity plays a positive role in the disintegration of cortical actin filaments, whereas calyculin A-sensitive protein phosphatase activity inhibits disintegration of radial cortical actin filaments and/or promotes the formation of randomly oriented long actin filaments.
It has been suggested that long actin filaments may play a negative regulatory role in stomatal movements (Kim et al., 1995; Hwang et al., 1997), and we have previously suggested that the initial disintegration of long actin filaments may be necessary for rapid stomatal closure (Hwang et al., 2000). In a similar manner, long actin filaments in the guard cells of closed stomata might help to maintain the closed state, and filament disintegration in response to opening stimuli might allow stomata to open readily and reorganize actin into the open pattern. To elucidate the roles of different actin organizations in guard cells it will be necessary to study actin changes in response to other closing or opening stimuli and to monitor daily changes in guard cell actin organization.
In the present study, treatment with CaCl2 caused actin reorganization in guard cells in a similar manner to ABA (Tables I and II). Furthermore, removal of external Ca2+ with 5 mm EGTA slowed down ABA-induced actin changes. It is therefore likely that an increase in intracellular calcium level acts as a mediator of the ABA signal for actin reorganization in guard cells of dayflower. However, the fact that 5 mm EGTA did not abolish ABA-induced actin reorganization suggests that a Ca2+-independent signal transducing pathway may also contribute to the ABA-induced actin reorganization. This latter pathway may include regulators of cytosolic pH, which has been shown to mediate ABA signal transduction in parallel with Ca2+ (Blatt and Armstrong, 1993; MacRobbie, 1998), and ABA-activated Ca2+-independent protein kinase activities (Li and Assmann, 1996; Mori and Muto, 1997).
There are experimental data to suggest that Ca2+ plays a role in the opening and closing responses of stomata (Irving et al., 1992; Assmann, 1993; Cousson and Vavasseur, 1998). How then does the ABA-induced increase in [Ca2+]i initiate changes in actin structure that are specific for stomatal closing? Since each stimulus is thought to induce a unique spatial and temporal pattern of Ca2+ response in guard cells (McAinsh and Hetherington, 1998), it is possible that stimulus-specific characteristics of changes in Ca2+ levels may encode the final pattern of actin organization. Alternatively, induction of the appropriate actin responses may require coordination of calcium flux with other stimulus-specific signals.
It has been suggested that kinases and phosphatases with positive and negative regulatory effects participate in the complex network of signaling processes involved in stomatal closing movement (Allen et al., 1999). It therefore seems likely that regulation of actin organization in guard cells may involve a variety of kinase and phosphatase activities. Treatment with specific inhibitors produced consistent effects on actin changes in guard cells while stomata were open and during stomatal closure induced by ABA or CaCl2. Staurosporine increased the proportion of cells with radial actin filaments, whereas calyculin A had the opposite effect (Tables IV–VI). These data suggest that the critical step for actin depolymerization involves activation of staurosporine-sensitive kinase(s), whereas the critical step for formation of long actin filaments involves the activity of phosphatase(s) sensitive to calyculin A (Fig. 2).
In summary, we present here evidence for the reg-ulation of actin organization in guard cells by Ca2+, protein kinase(s), and protein phosphatase(s), although the detailed mechanism of regulation remains unknown. In the complex signaling network involved in stomatal movement, these three signal mediators appear to function as early regulators of actin organization, which in turn regulates ion channel activity (Hwang et al., 1997; Liu and Luan, 1998) as found in animal systems (Rosenmund and Westbrook, 1993; Cantiello, 1996; Henson, 1999), and thereby coordinates the timely responses of stomata to environmental and internal stimuli.
MATERIALS AND METHODS
Plant Materials and Treatment with Kinase or Phosphatase Inhibitors
Dayflower (Commelina communis) plants were grown as described by Kim et al. (1995) and tissue samples were taken from fully expanded second leaves. All measurements were made between h 6 and 10 of the usual 16-h photoperiod. After the leaves were harvested, the epidermis was peeled off and transferred to a buffer containing 30 mm KCl and 10 mm K+-MES (2-(N-morpholino)-ethanesulfonic acid; pH 6.1). Kinase or phosphatase inhibitors were added 30 min prior to treatment with ABA or CaCl2 and were maintained at the same concentration throughout the experiment.
Visualization of Actin
Immediately after measurement of stomatal apertures (with the method described in Hwang et al., 1997), epidermal fragments were fixed for 3 to 5 h at 37°C in PM5E buffer (50 mm PIPES [ 1,4-piperazinediethanesulfonic acid], 2 mm MgSO4, 5 mm EGTA, 0.25% [v/v] dimethyl sulfoxide, and 0.05% [v/v] Triton X-100) containing 0.2 mm m-maleimidobenzoyl N-hydroxysuccinimide ester. Actin filaments in guard cells were then stained by overnight incubation at room temperature in phosphate-buffered saline containing rhodamine-phalloidin (1 unit/100 μL), 0.05% (v/v) Triton X-100, and 0.1% (v/v) p-phenylenediamine. Actin was observed using a fluorescence microscope (Optiphot-2, Nikon, Tokyo) equipped with narrow band pass filter blocks (excitation 540/25 nm, barrier BA605/55). Images were recorded on T-Max 100/400 film using a Microflex UFX-DX photographic attachment (Nikon).
At least two epidermal fragments per sample were observed in each experiment, and the actin patterns observed in 40 to 80 guard cells per epidermal fragment were categorized into four different types of actin organization. Results from three to 14 experiments were used for statistical analysis.
ACKNOWLEDGMENT
We thank Dr. Soon-Ok Eun for critical reading of the manuscript.
Footnotes
This work was supported by the Science and Engineering Foundation of Korea (grant no. 98–0401–07–3 to Y.L.).
LITERATURE CITED
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI. Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell. 1999;11:1785–1798. doi: 10.1105/tpc.11.9.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Kwak JM, Chu SP, Llopis J, Tsien RY, Harper JF, Schroeder JI. Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 1999;19:735–747. doi: 10.1046/j.1365-313x.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- Armstrong F, Leung J, Grabov A, Brearley J, Giraudat J, Blatt MR. Sensitivity to abscisic acid of guard cell K+ channels is suppressed by abi1-1, a mutant Arabidopsis gene encoding a putative protein phosphatase. Proc Natl Acad Sci USA. 1995;92:9520–9524. doi: 10.1073/pnas.92.21.9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM. Signal transduction in guard cells. Annu Rev Cell Biol. 1993;9:345–375. doi: 10.1146/annurev.cb.09.110193.002021. [DOI] [PubMed] [Google Scholar]
- Blatt MR, Armstrong F. K+ channels of stomatal guard cells: abscisic acid-evoked control of the outward rectifier mediated by cytoplasmic pH. Planta. 1993;191:330–341. [Google Scholar]
- Cantiello HF. Role of actin cytoskeleton on epithelial Na+ channel regulation. Kidney Int. 1996;48:907–984. doi: 10.1038/ki.1995.379. [DOI] [PubMed] [Google Scholar]
- Cousson A, Vavasseur A. Putative involvement of cytosolic Ca2+ and GTP-binding proteins in cyclic-GMP-mediated induction of stomatal opening by auxin in Commelina communis L. Planta. 1998;206:308–314. [Google Scholar]
- Eun S-O, Lee Y. Actin filaments of guard cells are reorganized in response to light and abscisic acid. Plant Physiol. 1997;115:1491–1498. doi: 10.1104/pp.115.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun S-O, Lee Y. Stomatal opening by fusicoccin is accompanied by depolymerization of actin filaments in guard cells. Planta. 2000;210:1014–1017. doi: 10.1007/s004250050711. [DOI] [PubMed] [Google Scholar]
- Grabov A, Leung J, Giraudat J, Blatt MR. Alteration of anion channel kinetics in wild-type and abi1-1 transgenic Nicotiana benthamiana guard cells by abscisic acid. Plant J. 1997;12:203–213. doi: 10.1046/j.1365-313x.1997.12010203.x. [DOI] [PubMed] [Google Scholar]
- Guillén G, Valdés-López V, Noguez R, Olivares J, Rodríguez-Zapata LC, Pérez H, Vidali L, Villanueva MA, Sánchez F. Profilin in Phaseolus vulgaris is encoded by two genes (only one expressed in root nodules) but multiple isoforms are generated in vivo by phosphorylation on tyrosine residues. Plant J. 1999;19:497–508. doi: 10.1046/j.1365-313x.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- Hartiwig JH, Thelen M, Rosen A, Janmey PA, Nairn AC, Aderem A. MARCKS is an actin filaments cross-linking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992;16:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Henson JH. Relationships between the actin cytoskeleton and cell volume regulation. Microsc Res Technol. 1999;47:155–162. doi: 10.1002/(SICI)1097-0029(19991015)47:2<155::AID-JEMT7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Hwang J-U, Eun S-O, Lee Y. Structure and function of actin filaments in mature guard cells. In: Staiger CJ, Baluska F, Volkmann D, Barlow PW, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 427–436. [Google Scholar]
- Hwang J-U, Suh S, Yi H, Kim J, Lee Y. Actin filaments modulate both stomatal opening and inward K+-channel activities in guard cells of Vicia faba L. Plant Physiol. 1997;115:335–342. doi: 10.1104/pp.115.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving HR, Gehring CA, Parish RW. Changes in cytosolic pH and calcium of guard cells precede stomatal movements. Proc Natl Acad Sci USA. 1992;89:1790–1794. doi: 10.1073/pnas.89.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Hepler PK, Eun S-O, Ha KS, Lee Y. Actin filaments in mature guard cells are radially distributed and involved in stomatal movement. Plant Physiol. 1995;109:1077–1084. doi: 10.1104/pp.109.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K. Characterization of cytosolic cyclophilin from guard cells of Vicia faba L. Plant Cell Physiol. 1999;40:53–59. doi: 10.1093/oxfordjournals.pcp.a029474. [DOI] [PubMed] [Google Scholar]
- Leung J, Bouvier-Durand M, Morris P-C, Guerrier D, Chefdor F, Giraudat J. Arabidopsis ABA-response gene ABI1: features of a calcium-modulated protein phosphatase. Science. 1994;264:1448–1452. doi: 10.1126/science.7910981. [DOI] [PubMed] [Google Scholar]
- Leung J, Giraudat J. Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J. The Arabidopsis ABSCISIC ACID-INSENSITIVE 2 (ABI2) and ABI1 genes encode redundant protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell. 1997;9:759–771. doi: 10.1105/tpc.9.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Assmann SM. An abscisic acid-activated and calcium-independent protein kinase from guard cells of fava bean. Plant Cell. 1996;8:2359–2368. doi: 10.1105/tpc.8.12.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lee Y-RJ, Assmann SM. Guard cells possess a calcium-dependent protein kinase that phosphorylates the KAT1 potassium channel. Plant Physiol. 1998;116:785–795. doi: 10.1104/pp.116.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang X-Q, Waston MB, Assmann SM. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science. 2000;287:300–303. doi: 10.1126/science.287.5451.300. [DOI] [PubMed] [Google Scholar]
- Li W, Luan S, Schreiber SL, Assmann SM. Evidence for protein phosphatase 1 and 2A regulation of K+ channels in two types of leaf cells. Plant Physiol. 1994;106:963–970. doi: 10.1104/pp.106.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Luan S. Voltage-dependent K+ channels as targets of osmosensing in guard cells. Plant Cell. 1998;10:1957–1970. doi: 10.1105/tpc.10.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EAC. Signal transduction and ion channels in guard cells. Philos Trans R Soc Lond B Biol Sci. 1998;353:1475–1488. doi: 10.1098/rstb.1998.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Hetherington AM. Encoding specificity in Ca2+signalling systems. Trends Plant Sci. 1998;3:32–36. [Google Scholar]
- McAinsh MR, Webb AAR, Taylor JE, Hetherington AM. Stimulus-induced oscillations in guard cell cytoplasmic free calcium. Plant Cell. 1995;7:1207–1219. doi: 10.1105/tpc.7.8.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel D, Vugrek O, Frank S, Elsner-Menzel C. Protein phosphatase 2A, a potential regulator of actin dynamics and actin-based organelle motility in the green alga Acetabularia. Eur J Cell Biol. 1995;67:179–187. [PubMed] [Google Scholar]
- Mori IC, Muto S. Abscisic acid activates a 48-kilodalton protein kinase in guard cell protoplasts. Plant Physiol. 1997;113:833–839. doi: 10.1104/pp.113.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z-M, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puius YA, Mahoney NM, Almo SC. The modular structure of actin-regulatory proteins. Curr Opin Cell Biol. 1998;10:23–34. doi: 10.1016/s0955-0674(98)80083-5. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Schelle I, Liao Y-J, Schroeder JI. Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc Natl Acad Sci USA. 1995;92:9535–9539. doi: 10.1073/pnas.92.21.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K, Kinoshita T, Nishimura M. Involvement of calmodulin and calmodulin-dependent myosin light chain kinase in blue light-dependent H+ pumping by guard cell protoplasts from Vicia faba L. Plant Physiol. 1992;99:1416–1421. doi: 10.1104/pp.99.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko AP, Jiang C-J, Simmons NJ, Weeds AG, Davies DR, Hussey PJ. Ser6 in the maize actin-depolymerizing factor, ZmADF3, is phosphorylated by a calcium-stimulated protein kinase and is essential for the control of functional activity. Plant J. 1998;14:187–193. doi: 10.1046/j.1365-313x.1998.00107.x. [DOI] [PubMed] [Google Scholar]
- Thiel G, Blatt MR. Phosphatase antagonist okadaic acid inhibits steady-state K+ currents in guard cells of Vicia faba. Plant J. 1994;5:727–733. [Google Scholar]