Abstract
Objectives
To assess clinical and cardiac magnetic resonance (CMR) imaging features of patients with peri-myocarditis following Coronavirus Disease 2019 (COVID-19) vaccination.
Methods
We retrospectively collected a case series of 27 patients who underwent CMR in the clinical suspect of heart inflammation following COVID-19 vaccination, from 16 large tertiary centers. Our patient’s cohort was relatively young (36.6 ± 16.8 years), predominately included males (n = 25/27) with few comorbidities and covered a catchment area of approximately 8 million vaccinated patients.
Results
CMR revealed typical mid-subepicardial non-ischemic late gadolinium enhancement (LGE) in 23 cases and matched positively with CMR T2 criteria of myocarditis. In 7 cases, typical hallmarks of acute pericarditis were present. Short-term follow-up (median = 20 days) from presentation was uneventful for 25/27 patients and unavailable in two cases.
Conclusions
While establishing a causal relationship between peri-myocardial inflammation and vaccine administration can be challenging, our clinical experience suggests that CMR should be performed for diagnosis confirmation and to drive clinical decision-making and follow-up.
Key Points
• Acute onset of dyspnea, palpitations, or acute and persisting chest pain after COVID-19 vaccination should raise the suspicion of possible myocarditis or pericarditis, and patients should seek immediate medical attention and treatment to help recovery and avoid complications.
• In case of elevated troponin levels and/or relevant ECG changes, cardiac magnetic resonance should be considered as the best non-invasive diagnostic option to confirm the diagnosis of myocarditis or pericarditis and to drive clinical decision-making and follow-up.
Keywords: Magnetic resonance imaging, COVID-19, Vaccination, Myocarditis, Pericarditis
Since the beginning of the global severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, an unprecedented massive effort has been carried out worldwide to rapidly provide acquired immunity against the development of the coronavirus disease 2019 (COVID-19) [1].
As of December 2021, over 8.2 billion doses of a range of different COVID-19 vaccines have been administered, prioritizing distribution to categories that are at highest risk of complications and/or transmission, such as the elderly and the healthcare workers.
While reported side effects following these vaccines have been mild and short-lasting in the overwhelming majority of cases, some series of rare but more significant complications have been collected in various international registries and databases [2].
Myocardial and/or pericardial inflammation is a rare yet known adverse event that has been described in relation to several vaccines (from influenza to smallpox) and also, in recent reports, following SARS-CoV-2 vaccine administration [3, 4].
In the USA, as of November 10, 2021, the Vaccine Adverse Event Reporting System (VAERS) has received 1793 reports of myocarditis or pericarditis happening after COVID-19 vaccination [2]. Of these, the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) did confirm 1049 reports of myocarditis or pericarditis, particularly among male adolescents and young adults aged below 30 after messenger ribonucleic acid (mRNA) COVID-19 vaccination [2].
The underlying pathogenesis is reasonably considered to be multifactorial and likely dependent on the activation of an uncontrolled autoimmune response to the vaccine triggered by molecular mimicry and cross-reaction mechanisms occurring in genetically susceptible individuals [4].
While establishing a causal relationship between myocardial and/or pericardial inflammation and vaccine administration can be challenging, recognition of such a clinical entity can be relevant, not only for epidemiological purposes but also to define the appropriate clinical management and follow-up.
The diagnostic contribution of cardiac magnetic resonance (CMR) to non-invasively depict COVID-19–associated myocarditis and pericarditis has been already extensively described in the acute/active and chronic setting of the disease [5].
We retrospectively collected data from a series of 23 cases observed by 16 large tertiary centers in the period from March to July 2021, representing patients in which CMR was performed between 1 and 25 days after vaccination in the clinical setting of a suspected cardiac involvement. Four patients were scanned between 32 and 82 days after vaccination, due to clinical relapse of a previously documented acute myocarditis.
Diagnosis of acute myocarditis was established according to the updated Lake-Louise criteria [6].
Detailed clinical and imaging features of our patient cohort, composed of a total of 27 patients, are summarized in Table 1.
Table 1.
Summary of clinical and CMRI features of the 27 cases. LVEF_cmr: LVEF estimated by CMR; LVEDVI_cmr: LVEDVI estimated by CMR
| Case | No. of doses | Vaccine | Days from injection to presentation | Age | Sex | BMI | Autoimmunities | Fever (> 37.5 °C) | Chest pain | Palpitations | Myalgia | Dyspnea | Troponin (hs-cTnT/cTnl) level baseline | Troponin lab cutoff value | Elevated troponins | ECG baseline anomalies | CMR date | LVEF_cmr | LVEDVI_cmr | LGE | LGE segments (AHA) | LGE pattern | T1 mapping global | ECV_cmr | T2 mapping global | Pericarditis_cmr | FU days | At FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 1 | Vaxzevria (AstraZeneca) | 19 | 20 | M | 24.07 | 0 | 0 | 1 | 0 | 0 | 0 | 593 | cTnT < 14 ng/L | 1 | 1 | 27/06/2021 | 47 | 89 | 1 | 11, 12, 16 | Mid-epicardial | 1026 | 26 | 48 | 0 | 44 | 0 |
| Case 2 | 1 | Comirnaty (Pfizer/BioNTech) | 1 | 43 | M | 25.95 | 0 | 0 | 1 | 1 | 1 | 0 | 706 | cTnT < 14 ng/L | 11 | 1 | 17/06/2021 | 50 | 73 | 1 | 5, 6, 11, 12, 13, 14, 15, 16 | Mid-epicardial | 1201 | 42 | 65 | 0 | 49 | 0 |
| Case 3 | 1 | Comirnaty (Pfizer/BioNTech) | 8 | 41 | F | 31.22 | 1 | 0 | 1 | 1 | 0 | 1 | 676 | cTnT < 14 ng/L | 1 | 1 | 21/05/2021 | 62 | 54 | 1 | 2, 3, 8, 9, 10 | Mid-epicardial | Not performed | Not performed | Not performed | 1 | 13 | 0 |
| Case 4 | 2 | Comirnaty (Pfizer/BioNTech) | 3 | 44 | M | 28.4 | 0 | 0 | 1 | 0 | 1 | 1 | 7400 | cTnT < 34.2 ng/L | 1 | 1 | 25/07/2021 | 69 | 67 | 1 | 4, 5, 10, 15 | Mid-epicardial | 1280 (3T) | 27 (3T) | 56 (3T) | 1 | 4 | 0 |
| Case 5 | 2 | Comirnaty (Pfizer/BioNTech) | 4 | 26 | M | 23.7 | 0 | 1 | 1 | 1 | 0 | 0 | 2500 | cTnT < 57 ng/L | 1 | 1 | 18/03/2021 | 70 | 90 | 1 | 4, 5, 6, 10, 11, 12, 15 | Mid-epicardial | Not performed | Not performed | Not performed | 0 | 82 | 0 |
| Case 6 | 2 | Comirnaty (Pfizer/BioNTech) | 9 | 41 | M | 27.6 | 0 | 1 | 1 | 1 | 0 | 0 | 5533 | cTnT < 57 ng/L | 1 | 1 | 05/08/2021 | 57 | 121 (dilated) | 1 | 4, 5, 10, 11, 16 | Mid-epicardial | 1075 | 33 | 53 | 1 | 6 | 0 |
| Case 7 | 2 | Spikevax (Moderna) | 6 | 27 | M | 22.5 | 0 | 1 | 1 | 0 | 1 | 0 | 119 | cTnT < 14 ng/L | 1 | 0 | 15/06/2021 | 60 | 94 | 1 | 1, 4 | Mid-epicardial | Not performed | Not performed | Not performed | 0 | 4 | 0 |
| Case 8 | 1 | Spikevax (Moderna) | 1 | 57 | M | 23.63 | 0 | 1 | 1 | 0 | 0 | 0 | 715 | cTnT < 14 ng/L | 1 | 0 | 17/06/2021 | 70 | 76 | 1 | 6, 5, 11, 12 | Mid-wall | Not performed | Not performed | Not performed | 0 | 35 | 0 |
| Case 9 | 1 | Comirnaty (Pfizer/BioNTech) | 2 | 12 | M | 17.2 | 0 | 0 | 1 | 0 | 0 | 0 | 695 | cTnT < 14 ng/L | 1 | 1 | 14/07/2021 | 80 | 95 | 0 | Pericardial | 980 | 25 | 51 | 1 | 2 | 0 | |
| Case 10 | 1 | Comirnaty (Pfizer/BioNTech) | 6 | 20 | M | 20.43 | 0 | 0 | 1 | 0 | 0 | 0 | 1406 | cTnT < 14 ng/L | 1 | 0 | 07/07/2021 | 58 | 93.4 | 1 | 6 | Mid-epicardial | Not performed | Not performed | Not performed | 0 | 25 | 0 |
| Case 11 | 2 | Comirnaty (Pfizer/BioNTech) | 14 | 18 | M | 22.09 | 0 | 1 | 1 | 0 | 0 | 0 | 427 | cTnT < 14 ng/L | 1 | 1 | 18/06/2021 | 62 | 63 | 1 | 7 | Mid-epicardial | 1076 | 29 | 54 | 0 | 43 | 0 |
| Case 12 | 1 | Comirnaty (Pfizer/BioNTech) | 3 | 33 | M | 28.3 | 0 | 1 | 1 | 0 | 0 | 0 | 27 | cTnT < 19,8 ng/L | 1 | 1 | 01/04/2021 | 54 | 84 | 1 | 3, 4, 13, 16 | Mid-epicardial | 1110 | Not performed | 58 | 0 | 30 | 0 |
| Case 13 | 2 | Vaxzevria (AstraZeneca) | 7 | 26 | M | 41.5 | 0 | 0 | 1 | 0 | 0 | 0 | 2500 | cTnT < 14 ng/L | 1 | 0 | 16/06/2021 | 56 | 89.7 | 1 | 2, 3, 4, 5, 8, 9, 10, 11 | Epicardial | 1157 | 35 | 47 | 0 | Unknown | Unknown |
| Case 14 | 2 | Vaxzevria (AstraZeneca) | 6 | 21 | M | 32 | 0 | 1 | 1 | 1 | 0 | 1 | 657 | cTnT < 14 ng/L | 1 | 1 | 23/06/2021 | 58 | 83 | 1 | 4, 5, 6, 11, 12 | Epicardial | 961 | 28 | 46 | 0 | Unknown | Unknown |
| Case 15 | 1 | Spikevax (Moderna) | 2 | 49 | M | 24.62 | 1 | 0 | 1 | 0 | 0 | 0 | 524 | cTnT < 14 ng/L | 1 | 1 | 18/05/2021 | 65 | 50 | 0 | 1045 | Not performed | 61 | 0 | 70 | 0 | ||
| Case 16 | 2 | Comirnaty (Pfizer/BioNTech) | 3 | 57 | M | 25.6 | 0 | 0 | 1 | 0 | 0 | 1 | 218 | cTnT < 14 ng/L | 1 | 1 | 21/06/2021 | 59 | 95.2 | 0 | 1037 | 25.32 | 50 | 1 | 26 | 0 | ||
| Case 17 | 2 | Comirnaty (Pfizer/BioNTech) | 7 | 26 | M | 27.4 | 0 | 0 | 1 | 1 | 0 | 0 | 382 | cTnT < 14 ng/L | 1 | 1 | 30/06/2021 | 61 | 81.3 | 0 | 987 | 24.28 | 48 | 1 | 9 | 0 | ||
| Case 18 | 2 | Comirnaty (Pfizer/BioNTech) | 5 | 55 | M | 33.8 | 0 | 1 | 1 | 1 | 1 | 1 | 1790 | cTnT < 14 ng/L | 1 | 1 | 07/07/2021 | 64 | 60.9 | 1 | 10, 11, 15, 16 | Mid-epicardial | 1043 | 25.61 | 48 | 0 | 14 | 0 |
| Case 19 | 2 | Spikevax (Moderna) | 4 | 29 | M | 28.1 | 0 | 1 | 1 | 0 | 1 | 0 | 516 | cTnT < 14 ng/L | 1 | 1 | 23/07/2021 | 52 | 76.8 | 1 | 4, 5, 10, 11, 12, 15, 16, 17 | Mid-epicardial | 1021 | 31.13 | 55 | 0 | 25 | 0 |
| Case 20 | 2 | Comirnaty (Pfizer/BioNTech) | 3 | 51 | M | 26.22 | 0 | 1 | 1 | 0 | 0 | 0 | 270 | cTnT < 14 ng/L | 1 | 1 | 10/07/2021 | 61 | 67 | 1 | 5 | Mid-epicardial | 1022 | Not performed | 43 | 0 | 20 | 0 |
| Case 21 | 2 | Comirnaty (Pfizer/BioNTech) | 2 | 31 | M | 23.67 | 0 | 1 | 1 | 0 | 0 | 0 | 378 | cTnT < 14 ng/L | 1 | 1 | 25/05/2021 | 75 | 79 | 1 | 3 | Mid-epicardial | 1030 | Not performed | 38 | 0 | 62 | 0 |
| Case 22 | 1 | Comirnaty (Pfizer/BioNTech) | 10 | 32 | M | 21.39 | 0 | 1 | 1 | 1 | 1 | 0 | 639 | cTnT < 14 ng/L | 1 | 1 | 14/07/2021 | 61 | 75 | 1 | 8 | Mid-epicardial | 1075 | Not performed | 59 | 0 | 15 | 0 |
| Case 23 | 1 | Comirnaty (Pfizer/BioNTech) | 23 | 19 | M | 24.62 | 0 | 1 | 1 | 1 | 1 | 0 | 587 | cTnT < 14 ng/L | 1 | 0 | 19/07/2021 | 49 | 88 | 1 | 5 | Mid-epicardial | 1010 | Not performed | 54 | 0 | 13 | 0 |
| Case 24 | 1 | Spikevax (Moderna) | 4 | 20 | M | 20.76 | 0 | 0 | 1 | 0 | 0 | 1 | 1494 | cTnT < 14 ng/L | 1 | 1 | 14/07/2021 | 62 | 86 | 1 | 10, 11, 15, 16 | Mid-epicardial | Not performed | Not performed | Not performed | 0 | 18 | 0 |
| Case 25 | 1 | Comirnaty (Pfizer/BioNTech) | 4 | 44 | M | 24 | 0 | 1 | 0 | 0 | 0 | 0 | 216 | cTnT < 34.2 ng/L | 1 | 1 | 14/07/2021 | 59 | 89.8 | 1 | 4.5 | Mid-wall | 1020 | 27 | 52 | 1 | 6 | 0 |
| Case 26 | 2 | Spikevax (Moderna) | 46 | 66 | F | 26 | 0 | 1 | 1 | 1 | 1 | 0 | 4209 | cTnT < 14 ng/L | 1 | 1 | 28/07/2021 | 51 | 73 | 1 | 4, 5, 10, 11 | Mid-epicardial | 1175 | 38 | 62 | 0 | 14 | 0 |
| Case 27 | 2 | Comirnaty (Pfizer/BioNTech) | 20 | 80 | M | 26.4 | 1 | 1 | 0 | 0 | 1 | 1 | 562 | cTnT < 14 ng/L | 1 | 0 | 15/06/2021 | 62 | 111 (dilated) | 1 | 3 | Mid-wall | 1060 | 31 | 55 | 0 | 76 | 0 |
| Mean | 8.222222222 | 36.5926 | 25.96926 | 0.111111111 | 0.592592593 | 0.92592593 | 0.37037037 | 0.33333333 | 0.25925926 | 1 | 0.777777778 | 60.5185185 | 78.924 | 0.851852 | 1055.55 | 30.02428571 | 52.35 | 0.259259259 | Median = 20 | 0 | ||||||||
| Standard deviation | 9.540735874 | 16.8163 | 4.792807 | 0.320256308 | 0.500711744 | 0.26688026 | 0.492102878 | 0.48038446 | 0.44657608 | 0 | 0.423659273 | 7.75772751 | 12.93980551 | 0.362014 | 63.66811563 | 5.357679914 | 6.690724137 | 0.446576085 | Range = 2-82 | 0 | ||||||||
1 = true ; 0 = false
Briefly, our patient population was relatively young (average age 36.6 ± 16.8 years), mostly included males (n = 25/27) and with few comorbidities; notably, autoimmune disorders were observed in 3/27 cases. In addition to suspected post-vaccine forms of myocardial injury, all recruiting centers were also asked to collect data for all patients who received a CMR diagnosis of acute peri-myocarditis in the same observational period, for comparative purposes. With this regard, our consortium has observed overall 238 cases of myocarditis, including 27 cases in vaccinated patients and 211 in unvaccinated individuals (n = 14 cases with history of COVID-19 disease ; n = 197 unvaccinated without history of COVID-19 disease); a descriptive summary of patients’ risk factors and comorbidities among these different groups is displayed in Table 2.
Table 2.
Descriptive table reporting prevalence of cardiovascular risk factors and main comorbidities among consecutive patients with a CMR diagnosis of myocarditis and/or pericarditis, observed in the period March–July 2021. Our cohort is categorized into 3 groups: vaccinated: n = 27 vaccinated patients, COVID-19+ (unvaccinated): n = 14 unvaccinated patients with diagnosis of acute or healed COVID-19 disease (based on clinical presentation and PCR confirmation), and COVID-19− (unvaccinated): n = 197 patients, unvaccinated and without history of COVID-19 disease. Definitions of listed risk factors and comorbidities: hypertension = systolic blood pressure ≥ 130 mmHg or a diastolic blood pressure ≥ 80 mmHg or current medical treatment for hypertension; diabetes = fasting glucose > 126 mg/dL or current treatment; smoking = current smoker or ex-smoker with suspension less than 5 years before observation; hyperlipidemia = LDL > 130 mg/dL or current treatment; moderate/high physical activity = at least 150 min per week of moderate-intensity aerobic activity or 75 min per week of vigorous aerobic activity, or a combination of both; autoimmunities = history of autoimmune diseases
| Age years, (mean) | Gender (%male) | BMI (kg/m2) (mean) | Hypertension (%) | Diabetes (%) | Smoking (%) | Moderate/high physical activity (%) | Hyperlipidemia (%) | Autoimmunities (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Vaccinated | 36.6 | 92.6 | 25.9 | 22.8 | 6.2 | 20.4 | 35.7 | 22.6 | 11.1 |
| COVID-19+ (unvaccinated) | 46.2 | 84.8 | 26.2 | 25.5 | 13 | 29.7 | 22.4 | 44.8 | 9.8 |
| COVID-19− (unvaccinated) | 38.2 | 82.5 | 24.3 | 20 | 10.1 | 26.5 | 33.8 | 29.1 | 7.2 |
In vaccinated patients, CMR diagnosis of myocarditis and/or pericarditis more commonly followed immunization with mRNA vaccines (n = 24/27), after the second jab (n = 15/27), and within 10 days from administration (n = 22/27; average 8 ± 9 days). Clinical presentations included chest pain (n = 25/27), palpitations (n = 10/27), arthralgias and myalgias (n = 9/27), and dyspnea (n = 7/27). High-sensitivity cardiac troponin T (hs-cTnT) or high-sensitivity cardiac troponin I (hs-cTnI) levels were systematically elevated in 27/27 cases and associated with a variable spectrum of electrocardiogram (ECG) abnormalities including ST–segment elevation and T-wave inversion (n = 21/27).
CMR revealed typical mid-subepicardial non-ischemic late gadolinium enhancement (LGE) in 23 cases and matched positively with CMR T2 criteria of myocarditis (Fig. 1). In 7 cases, CMR showed typical hallmarks of acute pericarditis (effusion with thickening and/or enhancement of pericardial layers).
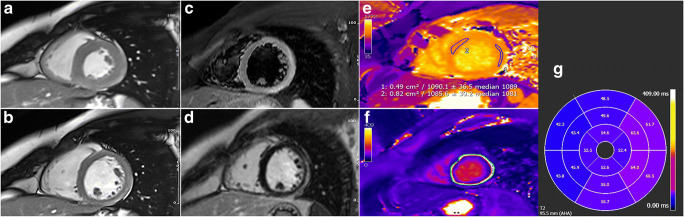
Fig. 1.
Acute myocarditis 4 days after Spikevax (Moderna) vaccine administration in a 29-year-old patient (images refer to patient n. 19 from Table 1) presenting with infarct-like symptoms of acute chest pain, with ECG ST-elevation changes and troponin rise. End-systolic and end-diastolic cine-SSFP frames (a and b) show a non-dilated and functionally preserved left ventricular cavity (EF 61%; LVEDVI: 76.8 mL/m2). Typical CMR hallmarks of an acute myocarditis can be observed in “edema-weighted” T2w-STIR short axis plane (c), consisting of the presence of a non-ischemic epicardial stria of high signal intensity involving the anterior- and infero-lateral mid-basal wall (arrows) and closely matching with LGE findings (d) (mid-ventricular level shown). Acute inflammation was also confirmed at myocardial mapping images showing focally increased native T1 mapping (1090 ms of a ROI on the middle-apical lateral wall; n.v. 950–1000 ms; e) and T2 mapping values (avg. 55 ms; n.v. < 50 ms; f) (AHA segments T2 mapping values shown in g). The patient’s clinical course was benign and uneventful at 25 days follow-up
Left ventricular (LV) systolic function was mildly reduced in 3/27 cases and normal in the remaining population (average ejection fraction: 60.5 ± 7.7%); indexed LV end-diastolic volume (LVEDVI) was normal in all cases (79 ± 13 mL/m2), except for an 80-year-old male and a 41-year-old male presenting with a mildly dilated LV cavity (111 and 121 mL/m2, respectively).
Short-term follow-up from presentation was uneventful for 25/27 patients (median = 20 days; range = 2–82 days) and unavailable in two cases.
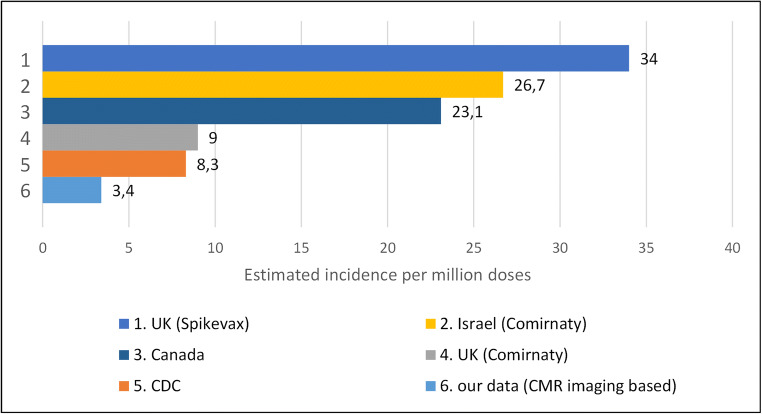
We collected a case series from the joint efforts of 16 tertiary referral centers, roughly covering a catchment area of approximately 8 million patients vaccinated with at least one dose in the period from March to July. We could therefore estimate an incidence of approximately 3.4 observed cases of myocarditis per million administered doses. Our incidence is significantly lower as compared to most international registries, in which a range of 8.3–34 cases per million was reported (see Fig. 2) [2, 7–9].
Fig. 2.
Estimated incidences of myocarditis after COVID-19 vaccine administration derived from our data and as reported in the following government registries or studies: UK (Spikevax) = reported incidence of myocarditis (34 per million doses) after Spikevax (Moderna) vaccine administration in the UK (government report) [7]; Israel (Comirnaty) = reported incidence of myocarditis (26.7 per million doses) after Comirnaty (Pfizer-BioNTech) vaccine administration in Israel (observational retrospective study based on Ministry of Health database) [8]; Canada = reported incidence of myocarditis (23.1 per million doses) after COVID-19 vaccine administration in Canada (government report) [9]; UK (Comirnaty) = reported incidence of myocarditis (9 per million doses) after Comirnaty (Pfizer-BioNTech) vaccine administration in the UK (government report) [7]; CDC = reported incidence of myocarditis (8.3 per million doses) after COVID-19 vaccines administration in the USA (CDC report) [2]; current research = estimated incidence (3.4 per million doses) from CMR data reported in the present study
This reflects an intrinsic selection difference of our study, in which diagnosis was established with a non-invasive gold standard technique as CMR instead of using clinical diagnostic criteria, like in the Vaccine Adverse Event Reporting System (VAERS), for the CDC, which is a passive reporting system that relies on individuals to send in reports of their experiences [2].
Our findings need to be cautiously contextualized and commented on, because of their potential implications on the perception of vaccine safety by the general population.
A clear causative relationship cannot be established as we only referred to a post-vaccination temporal criterion; moreover, the background prevalence of myocarditis remains uncertain but is likely to be ~ 22 per 100,000 [10]. Finally, myocarditis and pericarditis are also both recognised complications of SARS-CoV-2 and it is entirely plausible that there are overlapping mechanisms involved in both natural infection and vaccine-mediated autoimmunity [11].
Even though we discussed about suspected cardiac side effects of the vaccine, the benefits of the immunization in preventing severe morbidity and mortality from SARS-CoV-2 infection still outweigh the risks of complications after vaccine administration [12].
Further work is required to establish whether there are any adverse sequelae associated with the cases of acute myocarditis observed in this case series; however, the largely preserved LV function and pattern of late enhancement may portend a good prognosis, although the presence of LGE highlights the need for careful surveillance.
Acute onset of dyspnea, palpitations, or acute and persisting chest pain after vaccination should raise the suspicion of possible myocarditis or pericarditis, and patients should seek immediate medical attention and treatment to help recovery and avoid complications. In case of elevated troponin levels and/or relevant ECG changes, CMR should be considered as the best non-invasive diagnostic option to confirm the diagnosis and to drive clinical decision-making and follow-up.
Abbreviations
- AHA
American Heart Association
- CDC
Centers for Disease Control and Prevention
- Cine-SSFP
Cine steady-state free precession
- CMR
Cardiac magnetic resonance
- CMRI
Cardiac magnetic resonance imaging
- COVID-19
Coronavirus disease 2019
- ECG
Electrocardiogram
- ECV_cmr
Myocardial extracellular volume fraction estimated by CMR
- EF
Ejection fraction
- FU days
Follow-up days from presentation
- hs-cTnI
High-sensitivity cardiac troponin I
- hs-cTnT
High-sensitivity cardiac troponin T
- LGE
Late gadolinium enhancement
- LGE segments (AHA)
LGE left ventricular distribution based on the “17 segments cardiac segmentation model” by the American Heart Association
- LV
Left ventricular
- LVEDVI
Indexed left ventricular end-diastolic volume
- LVEDVI_cmr
LVEDVI estimated by CMR
- LVEF
Left ventricular ejection fraction
- LVEF_cmr
LVEF estimated by CMR
- mRNA
Messenger ribonucleic acid
- n.v.
Normal values
- Pericarditis_cmr
Pericarditis detected by CMR
- ROI
Region of interest
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- T2w-STIR
T2-weighted short-tau inversion recovery
- VAERS
Vaccine Adverse Event Reporting System
Funding
The authors declare this study received no funding.
Declarations
Guarantor
The scientific guarantor of this publication is Prof. Marco Francone, MD, PhD.
IRCCS Humanitas Research Hospital, Department of Biomedical Sciences of Humanitas University ViaRita Levi Montalcini 4, 20072, Pieve Emanuele, Milan, Italy
E-mail: marco.francone@hunimed.eu; phone: +39 0282243076; fax +39 0282242299
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and Biometry
No complex statistical methods were necessary for this paper.
Informed Consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical Approval
Ethical approval was obtained from IRB on 25th May 2021, number 2551.
Methodology
retrospective
observational
multicenter study
Footnotes
Position statement on COVID-19 vaccines: The authors are firm supporters of the COVID-19 vaccination campaign and vaccinated themselves as well.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Selected adverse events reported after COVID-19 vaccination. USA: Centers for Disease Control and Prevention; 2021. [Google Scholar]
- 3.Engler RJ, Nelson MR, Collins LC, Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi: 10.1371/journal.pone.0118283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montgomery J, Ryan M, Engler R et al (2021) Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 10.1001/jamacardio.2021.2833 [DOI] [PMC free article] [PubMed]
- 5.Catapano F, Marchitelli L, Cundari G, et al. Role of advanced imaging in COVID-19 cardiovascular complications. Insights Imaging. 2021;24:12–28. doi: 10.1186/s13244-021-00973-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 7.Medicines and Healthcare products Regulatory Agency (MHRA, Government of the United Kingdom) Summary of yellow card reporting updated to include figures up to and including 17 nov. 2021. [Google Scholar]
- 8.Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Public Health Agency of Canada (2021) Canadian COVID-19 vaccination safety report. November 26, 2021. Available via https://health-infobase.canada.ca/covid-19/vaccine-safety/
- 10.Heymans S, Eriksson U, Lehtonen J, Cooper LT., Jr The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol. 2016;68:2348–2364. doi: 10.1016/j.jacc.2016.09.937. [DOI] [PubMed] [Google Scholar]
- 11.Galea N, Marchitelli L, Pambianchi G, et al. T2-mapping increase is the prevalent imaging biomarker of myocardial involvement in active COVID-19: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2021;23(1):68. doi: 10.1186/s12968-021-00764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pormohammad A, Zarei M, Ghorbani S et al (2021) Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel) 9:467 [DOI] [PMC free article] [PubMed]