Abstract
Vitamin B12, widely known as cobalamin, is a water-soluble vitamin crucial for human metabolism. It is synthesized only by prokaryotic organisms and since humans do not have the ability to synthesize it, they rely on its exogenous dietary intake. After its consumption, vitamin B12 undergoes a complicated procedure of absorption and assimilation and it is essential for cellular function, especially for nervous system, red blood cell production and DNA synthesis. Deficiency of vitamin B12 is considered as an important public health issue worldwide, while it is common in the elderly. Deficiency of this vitamin, as well as high levels, indicate a risk factor for morbidity with various clinical manifestations. Frailty is an age-related syndrome, which affects the elderly and is characterized by decreased function in many physiological systems, accompanied by vulnerability to stressors. A narrative non-systematic mini review of the literature was conducted and highlighted that vitamin B12 levels may have an impact on frailty and vice versa. As shown in several studies, vitamin B12 levels may be related to sarcopenia, cognitive and musculoskeletal disorders, neurological or psychiatric symptoms, which are closely linked to frailty. Furthermore, it is suggested that the extensions of frailty may affect the bioavailability of vitamin B12.
Keywords: Bioavailability, Cognitive disorders, Frailty syndrome, Sarcopenia, Vitamin B12
Introduction
Vitamin B12, also known as cobalamin, is a water-soluble vitamin, essential for human metabolism[1,2]. It is synthesized by prokaryotic organisms, since only bacteria and archaea have the enzymes required for its synthesis[1,3]. The process of its synthesis is complex and involves around 30 steps -including the form of a corrin ring-, while both aerobic and anaerobic biosynthetic pathways exist[1,4]. Humans are unable to synthesize vitamin B12 and thus they rely on exogenous sources, such as dietary intakes. The main source of B12 is predominantly animal protein, however, many foods contain vitamin B12 due to bacterial symbiosis[2,3,5]. After its consumption, B12 undergoes a complicated procedure of absorption and assimilation[2]. Vitamin B12 is essential for cellular function, especially for nervous system, red blood cell production and DNA synthesis[5,6].
Dietary deficiency of vitamin Β12, appears to be the most common cause of deficiency worldwide[1]. Deficiency and low levels of vitamin B12 are common in the elderly[2,7]. Since frailty syndrome is an age-related clinical condition and deficiency of vitamin B12 appears mostly in the elderly, it is of great importance to investigate the possible correlation between these two conditions.
This paper reviews the interplay between frailty syndrome and vitamin B12 levels.
B12 vitamin profile
Vitamin B12 as a member of the corrinoids, a group of molecules with a corrin ring structure and central cobalt atom. It is found in many forms such as hydroxocobalamin, methylcobalamin, and 50-deoxyadenosylcobalamin, which are chemically more unstable in chemical terms, than cyanocobalamin[4,8].
Vitamin B12 is available only through dietary intakes. According to the European Food Safety Authority, the recommended sufficient intake of vitamin B12 is considered to be 4.0 µg per day for adults, while in some cases such as pregnancy and lactation the requirements are higher[7].
Meat is considered to be the richest source of vitamin B12, while milk is reported as a remarkable source for increasing serum B12 levels. Generally, meat derived from ruminant animals, such as fish, shellfish, eggs and other dairy products are the most important dietary sources of vitamin B12[2,4,7]. Different types of animal meats such as beef, veal, mutton, contain higher levels of B12, than meats derived from omnivorous animals, such as pork and poultry[4]. It has been calculated that meat contains approximately over 10 µg/100 g wet weight of B12, while fish, milk products and egg yolks contain about 1 to 10 µg/100 g wet weight[2].
In general, vitamin B12 is not present in plant foods, however it can be found at edible plants, mushrooms, and algae, due to their microbial interactions. Even though most plants (except from some algae), neither produce nor require B12, their interactions with bacteria leads to the presence of vitamin B12[2,4]. Additionally, fortified breakfast cereals and some nutritional yeast products are good bioavailability source of B12[7].
It has been reported that the unabsorbed vitamin B12 affects the intestinal microbiome, while it is considered that its absorption in healthy humans is at about 50%[4].
Dietary originated cobalamin is usually found in the form of coenzyme deoxyadenosylcobalamin or methylcobalamin and is bounded to animal protein. In stomach, proteolysis (due to HCl and pepsin) is the first initial step concerning the release of B12 originating from food proteins[2,7]. After cobalamin is released, it binds to the glycoprotein haptocorrin, also known as transcobalamin-I or TCI[7,8].
In order to be absorbed, a specific factor named intrinsic factor (IF) is required. This factor is produced by the parietal cells of the stomach[2]. An important step in the absorption of vitamin B12, is when vitamin B12 - IF complex reaches the distal ileum, where it binds to the specific cubilin receptor. There through an active and saturable mechanism, B12 is absorbed through receptor-induced endocytosis[3,7]. The presence of transcobalamin is crucial for the transport of B12 into blood circulation and tissue and hepatic uptake[3]. More than 80% of serum vitamin B12 is stored bound to haptocorrin (TCI) and is available only to storage liver cells. The remaining of serum B12 (less than 20%) is stored bound to transcobalamin-II (TC-II) and is available to all cells that undergo DNA synthesis[8]. Through enterohepatic recirculation, vitamin B12 is released into the bile from the liver and is reabsorbed at the distal ileum. While its reabsorption, vitamin B12 binds to TCII, in order to be redistributed to other tissues[8].
Vitamin B12 is essential for cellular function, especially for nervous system, DNA synthesis and haemopoiesis, while it is involved in many key metabolic pathways such as lipids, carbohydrate and protein metabolism[2,6]. Methylcobalamin (MeCbl) and adenosylcobalamin (AdoCbl) are co-enzyme forms of vitamin B12[7]. Methylcobalamin is important for the homocysteine to methionine conversion, as it is a coenzyme in the cytosolic methionine-synthase dependent remethylation. Adenosylcobalamin is essential for methylmalonic acid to succinyl coenzyme A conversion, as it is a coenzyme for the mitochondrial methylmalonyl-CoA mutase[4,5,7].
Vitamin B12 is also essential for other body processes, such as the isomerization of d-leucine to leucine, the conversion of 5-methyltetrahydrofolate to tetrahydrofolate and for monoamine neurotransmitter brain synthesis[2,6,9]. Additionally vitamin B12 is crucial for cellular respiration and energy, due to its impact on Krebs cycle, which leads to the production of cellular energy in the form of adenosine triphosphate (ATP)[2].
Deficiency of vitamin B12 is considered as a common public health issue worldwide[5]. Vitamin B12 deficiency is well known for its direct effects, such as megaloblastic anemia and subacute combined degeneration affecting spinal cord and peripheral nerves, dementia, etc[1]. Moreover, deficiency of B12 or low levels, may be a risk factor for other clinical manifestations such as fatigue, weakness, constipation, balance disorder, mental fogginess, peripheral tingling, depression, cognitive disorders, hematological, neurological and psychiatric disorders, coronary artery disease, or even autoimmune damage that may lead to hypothyroidism, type I diabetes mellitus, vitiligo[1,2,9]. Vitamin B12 deficiency is also responsible for causing many cases of mental disorders[9]. While clinical vitamin B12 deficiency could lead to death, if left untreated, subclinical vitamin B12 deficiency (usually defined as a total serum B12 of <200 pmol/L) presents asymptomatically or mild general symptoms often mistaken for other disorders[5].
Some results of vitamin B12 deficiency may be explained by the cause of subacute combined degeneration (also known as combined systems disease), due to the demyelination of the cervical and thoracic dorsal and lateral columns of the spinal cord and demyelination of the white matter of the brain, or cranial and peripheral nerves[2]. In addition, vitamin B12 deficiency leads to increase of methylmalonic acid and homocysteine[10].
Deficiency of B12 may affect a large amount of people. Those who do not eat animal products are at high risk of deficiency[1]. Moreover, certain long-term medication such as metformin (for more than four months), proton pump inhibitors or histamine H2 blockers (for more than 12 months) and angiotensin converting enzyme inhibitors could contribute to vitamin B12 deficiency1,2,5,6. Besides those factors, H. pylori infection, inflammatory bowel disease, or chronic gastritis are also important causes of B12 deficiency, most likely through gastric acid and intrinsic factor production changes[1,6]. The elderly seem to be a high risk group due to malabsorption, as it is estimated that more than 15% patients over 65 are B12 deficient[2,5].
High levels of vitamin B12 are also associated with negative effects, such as inflammation and poor outcome for critically ill patients[2]. The etiological profile of high vitamin B12 levels, mainly includes severe cases of solid neoplasms, hematologic malignancies and liver diseases[3].
In general, a combination of at least two biomarkers is recommended for the diagnosis of vitamin B12 deficiency due to low specificity of available biomarkers[7]. Functional biomarkers that could be used instead of serum vitamin B12 levels, are methylmalonic acid and homocysteine, while the most used biomarker is total vitamin B12[7]. Another assay, suggests the holotranscobalamin, as a more sensitive biomarker than serum vitamin B12 levels[10].
Frailty Syndrome
Human life expectancy has risen dramatically worldwide. In fact, from the beginning of this century till 2016, life expectancy has increased by 5.5 years and in 2016 it was reported at 72 years[11]. Aging is a gradual heterogenous and multifactorial phenomenon, in which plenty biopsychosocial changes occur[11,12]. Biological features of aging include genomic instability, attrition of telomeres, proteostasis loss, epigenetic changes, mitochondrial dysfunction, deregulated nutrient-sensing, cellular senescence, changes in intercellular communication and last but not least stem cell exhaustion[11]. The rise in population life expectancy is associated with vulnerability[13].
Frailty is an age-related syndrome characterized by decreased function in many physiological systems, accompanied by vulnerability to stressors, both endogenous and exogenous[14,15]. The number of scientific publications on frailty has been increasing during the past 15 years, leading to a multitude of operational definitions of frailty in the literature[16]. It has been estimated that this condition has a prevalence of 10 % in community-dwellers and higher in other settings, such as hospitals where it may range from 18 % to 40 % of patients[17]. Frailty, as a multidimensional syndrome, leads to different negative health associated outcomes, such as reduction in physical activity, disability, slowness, weakness, exhaustion, cognitive dysfunctions and hospitalizations due to the impact on many systems, such as the inflammatory system, the endocrine system, the respiratory system and the skeletal muscle[13,15,18]. Frail people are at higher risk of cardiovascular disease, depression, reduced quality of life and increased hospitalization as a result of postoperative complications[13]. The “tentacles” of frailty are summarized in Figure 1.
Figure 1.
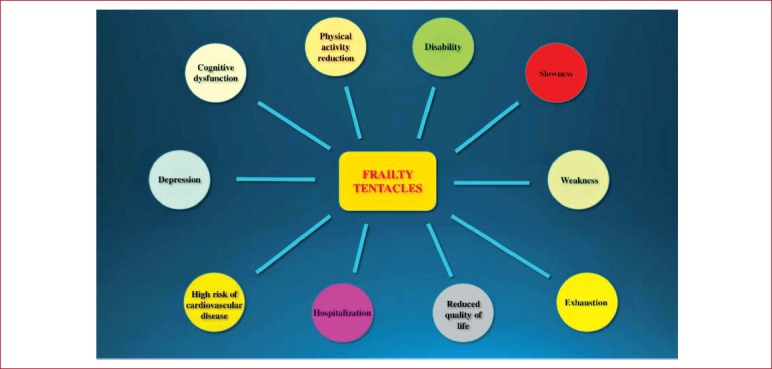
The frailty “tentacles”: The multidimensional aspects of frailty syndrome.
Till now, the multidimensional aspects of frailty condition could be captured by geriatric assessment scores and indexes. The most common, applied to multiply studies is “Fried’s frailty phenotype”, also known as the “Cardiovascular Health Study (CHS) index”, which considers frailty by its phenotype, measuring the presence of three or more of shrinking, weakness, exhaustion, slowness and low physical activity. Additional scores and indexes of frailty used, are the “Frailty Index of Accumulative Deficits” (FI-CD), the “Geriatric 8 score” (G8 score), the “simplified Five-item Index” (sFI), the “American Society of Anesthesiologists (ASA) physical status classification”, the “Mini-Cognitive (mini-COG) assessment”, the “Eastern Cooperative Oncology Group (ECOG) performance status”, the “Cumulative Illness Score Rating-Geriatrics (CISR-G)”, the “Charlson Comorbidity Index (CCI)”, the “Study of Osteoporotic Fractures (SOF) index”, the “Fatigue, Resistance, Ambulation, Illness, Loss of Weight (FRAIL) index”, the “Clinical Frailty Scale” (CFS), the “Comprehensive Geriatric Assessment (CGA)” and its derived “Multidimensional Prognostic Index (MPI)”[13,17,19].
Investigating the B12-frailty interplay
Frailty is an age-related clinical condition, which affects mostly the elderly, associated with various conditions and outcomes, as it is already mentioned. Alongside, the elderly are a high-risk group of vitamin B12 deficiency, due to frequency of multidrug use, pernicious anemia and B12 malabsorption[10].
A non-systematic review from PUBMED database was conducted, in order to investigate the B12-frailty correlation, using the terms “B12 and frailty”, “B12 and frailty syndrome”, “B12 and frail”, “association between B12 and frailty”, as key words to detect relative studies published the last five years until March 2021. As a result, 10 relevant original studies emerged from this search and the number of articles concerning this correlation, using the abovementioned key words at this specific period of time and they were all selected as appropriate to capture and demonstrate the potential interplay between B12 levels and frailty syndrome. Nevertheless, limitations of this study should be stated such as small number of available relevant research, inconsistency in the variables examined and associations made.
Sarcopenia is an important component of frailty, sharing similarities in their etiology and is also possibly caused by similar mechanisms, such as aging, impaired endocrine function, chronic diseases, inflammation, insulin resistance, oxidative stress, malnutrition and other causes. Skeletal muscle mass decrease, concerning both strength and function due to sarcopenia, may reduce self-sufficiency of elderly, leading to repeated falls, mobility restriction, nursing home admissions and increased morbidity and when this process progresses to chronic, it could lead to frailty. The European Working Group on Sarcopenia in Older People (EWGSOP) has developed an algorithm to aid the screening and diagnosis of sarcopenia[20-23]. In a case control study of 66 sarcopenic and 66 non-sarcopenic patients, Verlaan et al. (2017) showed that with similar energy intakes, the sarcopenic group consumed less protein/kg (-6%) along with less vitamin B12 (-22%) than the non-sarcopenic group. The serum levels of vitamin B12 were 15% lower in the sarcopenic group[22]. The sarcopenic group was also characterized by less physical activity, whilst the muscle mass between the two groups was significantly different. On the other hand, vitamin B12 and other nutrients may have an impact on sarcopenia, due to reduction of serum levels of homocysteine. High levels of homocysteine are related to sarcopenia, because of the reduction of muscle strength and the speed of gait[22]. Ates Bulut et al. (2017) concluded that sarcopenia and dynapenia (decreased muscle strength without concomitant loss of muscle mass and functionality), might be related to vitamin B12 deficiency. In this study, 403 geriatric patients recorded a frequency of sarcopenia and dynapenia between 31.6% and 35.4%, respectively and lower lean body mass, total skeletal mass and skeletal muscle mass index, in patients with vitamin B12 levels less than 400 pg/mL[23].
B12 deficiency is also associated with muscular and skeletal disorders witch are linked to frailty syndrome[24]. Pannérec et al. (2018) demonstrated that aging and frailty are related to a higher probability of functional vitamin B12 deficiency, that can be recognized by increased levels of methylmalonic acid (MMA) in blood[24]. Another conclusion was that both aging and frailty cause intrinsic vitamin B12 deficiency regardless the dietary intake, while the amnionless protein levels seem to affect vitamin B12 bioavailability, during aging and physical frailty. A possible explanation is the physiological reduction of both intestinal intake and renal reabsorption concerning vitamin B12[24].
Macêdo et al. (2017) reviewed several studies, in which three of them demonstrated an association between vitamin B12 and fracture risk and/or bone mineral density (BMD reduction), while in fourteen studies no association was recorded[25]. In another study that Macêdo et al. reviewed, it was concluded that supplementation of vitamin B12 and folic acid is beneficial for the prevention of osteoporotic fractures. However further studies are required in order to determine the association between B12 and bone metabolism[25].
Vitamin B12 is reported to have an important role in the synthesis of myelin in the central system[26]. Therefore, many studies investigate the association of vitamin B12 and cognitive decline. Soh et al. (2020) evaluated data of 2991 people with a median age of 76.4±3.9 years who were divided into two groups, B12 insufficient and B12 sufficient. The results of this study, suggests that B12 levels could be a contributing factor to cognition along with sociological and demographic factors, given the fact that the sufficient B12 group performed better in the cognitive tests[26]. It is considered that, the development of cognitive impairment in dementia is a multifactorial process involving genetic and environmental factors[27]. In a multiple linear regression analysis, Mizrahi et al. (2017) examined 91 elderly hip fracture patients and they linked serum vitamin B12 levels ≤350 pg/ml to a higher risk of cognitive decline. It was also suggested, that serum vitamin B12 may indicate patients in the early stages of cognitive impairment[27]. Moore et al. (2012) reviewed 43 studies and revealed that B12 subclinical levels (<250 pmol/L) are associated with Alzheimer’s disease, vascular dementia and Parkinson’s disease, whilst there is a small subset of dementias that are reversible with vitamin B12 administration, however this therapy does not improve cognition in patients without preexisting deficiency[8]. Finally, due to the role of vitamin B12 in the nervous system, Sangle et al. (2020) suggested that the lower levels of vitamin B12 are associated with a higher risk of developing depression[9]. All these, suggest a strong correlation between low B12 levels and frailty.
Zik et al. (2019) examined different studies and demonstrated that a significant percentage of elderly and elderly hospitalized patients were vitamin B12 deficient. Furthermore, they emerged through many studies, that vitamin B12 deficiency may lead to neurologic or psychiatric symptoms, without concomitant hematological abnormalities[10]. It was suggested that these results were due to defective methylation of myelin basic protein and a loss of white matter of the brain and spinal cord[10]. In another study 326 women were monitored, using methylmalonic acid as a biomarker for vitamin B12 deficiency and it was suggested that specific genes (such as TCN2) may lead to reduction of available vitamin B12 and as a result decreased energy metabolism contributing to frailty syndrome[28,29]. Wolffenbuttel et al. (2020) in a study of 9645 participants, linked the occurrence of increased methylmalonic acid with poor functional status and physical performance[30]. Behrouzi et al. (2020) combining data of 4 studies in older Dutch people, using copula graphical models, determined that vegetable protein, vitamin B-6, folate, and vitamin B-12 intakes are partly associated with improved functional outcome measurements[31].
Conzade et al. (2017) examining data among older adults, aged 65 to 92, from the KORA-Age study in Augsburg, Germany (1079 participants), using serum nutritional biomarkers and multiple logistic regression, concluded that more than a quarter of individuals had low vitamin B12 levels. Furthermore, they revealed that conditions such as aging, lack of physical activity, frailty, no use or irregular use of micronutrient-containing supplements consist common predictors of subclinical micronutrient deficiencies[32]. Soh et al. (2020), in another study among 2938 participants (1400 men and 1538 women) from the Korean Frailty and Aging Cohort Study with mean age of 77.8 years for the frail group and 76.7 for the non-frail group, concluded that low B12 levels increased the incidence of frailty and also affected physical performance[33].
Finally, Couderc et al. (2020) studied the effects of hypercobalaminemia in 621 older cancer adults in outpatient care with a median age of 81 years. Their study demonstrated that hypercobalaminemia and vitamin B12-C-reactive protein index may constitute important markers for older cancer frail patients with poor prognosis[34].
Conclusion
Literature review revealed that frailty is linked to vitamin B12 levels. Deficiency or low levels of vitamin B12 seems to have a potential negative effect on conditions such as sarcopenia, dynapenia and further musculoskeletal disorders. Additionally, it is highlighted a probable correlation between decreased vitamin B12 levels and cognitive decline, as well as aggravation of neurologic or psychiatric symptoms. Generally, it is shown that abnormal vitamin B12 levels lead to manifestations of frailty, while at the same time it is suggested that aging and frailty lead to vitamin B12 deficiency. Alongside, hypercobalaminemia constitutes an important prognostic factor for older cancer frail patients.
Therefore, it seems necessary to maintain normal levels of vitamin B12. Patients with severe deficiency or malabsorption syndromes could be treated with intramuscular injections of cyanocobalamin, while those with asymptomatic mild disease without absorption or compliance problems could be treated with oral vitamin B12 therapy, either through dietary intake (food fortified with vitamin B12) or through dietary supplementation[6].
It is suggested that the use of vitamin B12 supplements, fortified breakfast cereals and dairy products, as milk, may lead to a higher absorption of vitamin B12, than in the case of meat, poultry and fish sources, due to the fact that in these cases vitamin B12 is less likely to suffer heat degradation[35]. Especially with regard to fortified cereals a strong relation to plasma vitamin B12 concentrations, with evidence of a dose response, is demonstrated[35].
Due to the growing geriatric population, it is important for physicians to consider the correlation between B12 levels and frailty, in order to avoid the adverse effects of their interaction. However, more research is needed in this direction and possibly a closer follow-up of geriatric patients in this regard, while the measurement of B12 could be common practice in the elderly and be restored, if deficiency is observed.
Footnotes
Edited by: George Lyritis
References
- 1.Chittaranjan Y. Vitamin B12:An Intergenerational Story. Nestle Nutr Inst Workshop Ser. 2020;93:91–102. doi: 10.1159/000503358. [DOI] [PubMed] [Google Scholar]
- 2.Romain M, Sviri S, Linton DM, Stav I, van Heerden PV. The role of Vitamin B12 in the critically ill--a review. Anaesth Intensive Care. 2016;44(4):447–52. doi: 10.1177/0310057X1604400410. [DOI] [PubMed] [Google Scholar]
- 3.Andrès E, Serraj K, Zhu J, Vermorken AJM. The pathophysiology of elevated vitamin B12 in clinical practice. QJM Mon J Assoc Physicians. 2013;106(6):505–15. doi: 10.1093/qjmed/hct051. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe F, Bito T. Vitamin B12 sources and microbial interaction. Exp Biol Med Maywood NJ. 2018;243(2):148–58. doi: 10.1177/1535370217746612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hannibal L, Lysne V, Bjørke-Monsen A-L, Behringer S, Grünert SC, Spiekerkoetter U, et al. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front Mol Biosci. 2016;3:27. doi: 10.3389/fmolb.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langan RC, Goodbred AJ. Vitamin B12 Deficiency:Recognition and Management. Am Fam Physician. 2017;96(6):384–9. [PubMed] [Google Scholar]
- 7.Obeid R, Heil SG, Verhoeven MMA, van den Heuvel EGHM, de Groot LCPGM, Eussen SJPM. Vitamin B12 Intake From Animal Foods, Biomarkers, and Health Aspects. Front Nutr. 2019;6:93. doi: 10.3389/fnut.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore E, Mander A, Ames D, Carne R, Sanders K, Watters D. Cognitive impairment and vitamin B12:a review. Int Psychogeriatr. 2012;24(4):541–56. doi: 10.1017/S1041610211002511. [DOI] [PubMed] [Google Scholar]
- 9.Sangle P, Sandhu O, Aftab Z, Anthony AT, Khan S. Vitamin B12 Supplementation:Preventing Onset and Improving Prognosis of Depression. Cureus. 2020;12(10):e11169. doi: 10.7759/cureus.11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zik C. Late Life Vitamin B12 Deficiency. Clin Geriatr Med. 2019;35(3):319–25. doi: 10.1016/j.cger.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Sathyan S, Verghese J. Genetics of frailty:A longevity perspective. Transl Res. 2020;221:83–96. doi: 10.1016/j.trsl.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribeiro IA, Lima LR de, Volpe CRG, Funghetto SS, Rehem TCMSB, Stival MM. Frailty syndrome in the elderly in elderly with chronic diseases in Primary Care. Rev Esc Enferm USP. 2019;53:e03449. doi: 10.1590/S1980-220X2018002603449. [DOI] [PubMed] [Google Scholar]
- 13.Kostakopoulos NA, Karakousis ND. Frailty assessment and postoperative complications in urologic oncology operations. J Frailty Sarcopenia Falls. 2020;05(03):57–61. doi: 10.22540/JFSF-05-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty:implications for clinical practice and public health. Lancet Lond Engl. 2019;394(10206):1365–75. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 15.Kostakopoulos NA, Karakousis ND, Moschotzopoulos D. Frailty associated urinary tract infections (FaUTIs) J Frailty Sarcopenia Falls. 2021;06(01):9–13. doi: 10.22540/JFSF-06-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cesari M, Calvani R, Marzetti E. Frailty in Older Persons. Clin Geriatr Med. 2017;33(3):293–303. doi: 10.1016/j.cger.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Pilotto A, Custodero C, Maggi S, Polidori MC, Veronese N, Ferrucci L. A multidimensional approach to frailty in older people. Ageing Res Rev. 2020;60:101047. doi: 10.1016/j.arr.2020.101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammami S, Zarrouk A, Piron C, Almas I, Sakly N, Latteur V. Prevalence and factors associated with frailty in hospitalized older patients. BMC Geriatr. 2020;20(1):144. doi: 10.1186/s12877-020-01545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice:A review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Marques A, Queirós C. Frailty, Sarcopenia and Falls. In: Hertz K, Santy-Tomlinson J, editors. Fragility Fracture Nursing. Cham: Springer International Publishing; 2018. pp. 15–26. (Perspectives in Nursing Management and Care for Older Adults) [PubMed] [Google Scholar]
- 21.Nascimento CM, Ingles M, Salvador-Pascual A, Cominetti MR, Gomez-Cabrera MC, Viña J. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. 2019;132:42–9. doi: 10.1016/j.freeradbiomed.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 22.Verlaan S, Aspray TJ, Bauer JM, Cederholm T, Hemsworth J, Hill TR, et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults:A case-control study. Clin Nutr. 2017;36(1):267–74. doi: 10.1016/j.clnu.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Ates Bulut E, Soysal P, Aydin AE, Dokuzlar O, Kocyigit SE, Isik AT. Vitamin B12 deficiency might be related to sarcopenia in older adults. Exp Gerontol. 2017;95:136–40. doi: 10.1016/j.exger.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Pannérec A, Migliavacca E, De Castro A, Michaud J, Karaz S, Goulet L, et al. Vitamin B12 deficiency and impaired expression of amnionless during aging:Aging and frailty associate with vitamin B12 deficiency. J Cachexia Sarcopenia Muscle. 2018;9(1):41–52. doi: 10.1002/jcsm.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macêdo de LLG, Carvalho de CMRG, Cavalcanti JC, Freitas B, deJ e S de A. Vitamin B12, bone mineral density and fracture risk in adults:A systematic review. Rev Assoc Médica Bras. 2017;63(9):801–9. doi: 10.1590/1806-9282.63.09.801. [DOI] [PubMed] [Google Scholar]
- 26.Soh Y, Lee DH, Won CW. Association between Vitamin B12 levels and cognitive function in the elderly Korean population. Medicine (Baltimore) 2020;99(30):e21371. doi: 10.1097/MD.0000000000021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizrahi EH, Lubart E, Leibovitz A. Low Borderline Levels of Serum Vitamin B12 May Predict Cognitive Decline in Elderly Hip Fracture Patients. Isr Med Assoc J IMAJ. 2017;19(5):305–8. [PubMed] [Google Scholar]
- 28.Matteini AM, Walston JD, Bandeen-Roche K, Arking DE, Allen RH, Fried LP, et al. Transcobalamin-II variants, decreased vitamin B12 availability and increased risk of frailty. J Nutr Health Aging. 2010;14(1):73–7. doi: 10.1007/s12603-010-0013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matteini AM, Walston JD, Fallin MD, Bandeen-Roche K, Kao WHL, Semba RD, et al. Markers of B-vitamin deficiency and frailty in older women. J Nutr Health Aging. 2008;12(5):303–8. doi: 10.1007/BF02982659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolffenbuttel BHR, Wouters HJCM, de Jong WHA, Huls G, van der Klauw MM. Association of vitamin B12, methylmalonic acid, and functional parameters. Neth J Med. 2020;78(1):10–24. [PubMed] [Google Scholar]
- 31.Behrouzi P, Grootswagers P, Keizer PLC, Smeets ETHC, Feskens EJM, de Groot LCPGM, et al. Dietary Intakes of Vegetable Protein, Folate, and Vitamins B-6 and B-12 Are Partially Correlated with Physical Functioning of Dutch Older Adults Using Copula Graphical Models. J Nutr. 2020;150(3):634–43. doi: 10.1093/jn/nxz269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conzade R, Koenig W, Heier M, Schneider A, Grill E, Peters A, et al. Prevalence and Predictors of Subclinical Micronutrient Deficiency in German Older Adults:Results from the Population-Based KORA-Age Study. Nutrients. 2017;9(12):1276. doi: 10.3390/nu9121276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soh Y, Won CW. Association between frailty and vitamin B12 in the older Korean population. Medicine (Baltimore) 2020;99(43):e22327. doi: 10.1097/MD.0000000000022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couderc A-L, Puchades E, Villani P, Arcani R, Farnault L, Daumas A, et al. High Serum Vitamin B12 Levels Associated with C-Reactive Protein in Older Patients with Cancer. The Oncologist. 2020;25(12):e1980–9. doi: 10.1634/theoncologist.2019-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tucker KL, Rich S, Rosenberg I, Jacques P, Dallal G, Wilson PW, et al. Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring Study. Am J Clin Nutr. 2000;71(2):514–22. doi: 10.1093/ajcn/71.2.514. [DOI] [PubMed] [Google Scholar]


