Abstract
The NAD+ metabolite cADP-Rib (cADPR) elevates cytosolic free Ca2+ in plants and thereby plays a central role in signal transduction pathways evoked by the drought and stress hormone abscisic acid. cADPR is known to mobilize Ca2+ from the large vacuole of mature cells. To determine whether additional sites for cADPR-gated Ca2+ release reside in plant cells, microsomes from cauliflower (Brassica oleracea) inflorescences were subfractionated on sucrose density gradients, and the distribution of cADPR-elicited Ca2+ release was monitored. cADPR-gated Ca2+ release was detected in the heavy-density fractions associated with rough endoplasmic reticulum (ER). cADPR-dependent Ca2+ release co-migrated with two ER markers, calnexin and antimycin A-insensitive NADH-cytochrome c reductase activity. To investigate the possibility that contaminating plasma membrane in the ER-rich fractions was responsible for the observed release, plasma membrane vesicles were purified by aqueous two-phase partitioning, everted with Brij-58, and loaded with Ca2+: These vesicles failed to respond to cADPR. Ca2+ release evoked by cADPR at the ER was fully inhibited by ruthenium red and 8-NH2-cADPR, a specific antagonist of cADPR-gated Ca2+ release in animal cells. The presence of a Ca2+ release pathway activated by cADPR at higher plant ER reinforces the notion that, alongside the vacuole, the ER participates in Ca2+ signaling.
Multiple and variable environmental signals are sensed by plants and lead to coordination of their growth and development. Stimulus-response coupling for many of these signals is widely accepted to be mediated (at least in the early steps) by modulation of cytosolic free Ca2+ ([Ca2+]c; Sanders et al., 1999). Intracellular Ca2+ stores are integral components of Ca2+-based signal transduction pathways, operating both as efficient sites for Ca2+ sequestration and as sources for rapid and localized release of the ion in response to a stimulus (Malhó et al., 1998; Sanders et al., 1999). The imposing presence of a large vacuole, containing millimolar levels of Ca2+ (Felle, 1988) and occupying up to 90% of the total volume in most mature cell types, has led to the view that the vacuole is quantitatively the most significant intracellular Ca2+ pool. Identification of several active Ca2+ transporters and Ca2+ release channels at the vacuolar membrane (Allen and Sanders, 1997; Evans and Williams, 1998) has further reinforced the appreciation of the vacuole as a major Ca2+ store with the potential to participate in signaling-related Ca2+ mobilization. Thus, the vacuolar membrane possesses release pathways for Ca2+ that are gated by voltage (Johannes et al., 1992; Allen and Sanders, 1994a; Ward and Schroeder, 1994) by inositol 1,4,5-trisphosphate (InsP3: Alexandre et al., 1990; Allen and Sanders, 1994b) and by the NAD+ metabolite cADP-Rib (cADPR: Allen et al., 1995).
In the context of Ca2+ signaling in plants, limited attention has been paid to the endoplasmic reticulum (ER), despite the clear prominence of the ER in both Ca2+ homeostasis and signaling in animal cells (Pozzan et al., 1994). Nevertheless, in the last few years, the biochemical characterization and molecular cloning from plants of the ER luminal Ca2+ buffering protein calreticulin (Chen et al., 1994; Navazio et al., 1995) and of P-type Ca2+-ATPase pumps located at ER membranes (Thomson et al., 1993; Liang et al., 1997; Harper et al., 1998; Hong et al., 1999), have confirmed the potential importance of the ER in cellular Ca2+ relations. The discovery of a voltage-gated Ca2+ channel that mediates Ca2+ release from the ER of tendrils of Bryonia dioica (Klüsener et al., 1995) has served to reinforce the possibility of a role for the ER in higher plant Ca2+ signaling. Furthermore, microinjection studies in pollen tubes have revealed the presence of a non-vacuolar InsP3-releasable Ca2+ store that is possibly associated with the ER (Franklin-Tong et al., 1996), and membrane fractionation studies on cauliflower (Brassica oleracea) florets have led tentatively to similar conclusions (Muir and Sanders, 1997). An additional class of Ca2+ release pathway residing exclusively on the ER is activated by the NADP metabolite nicotinic acid adenine dinucleotide phosphate (NAADP: Navazio et al., 2000). The ER has also been hypothesized to be responsible, through Ca2+ release, for the generation of the Charophyte action potential (Plieth et al., 1998) and of repetitive Ca2+ spikes in the freshwater green alga Eremosphaera viridis (Bauer et al., 1998). In this latter case, the pharmacology of Ca2+ release suggested the involvement of a ryanodine receptor-like Ca2+ channel, possibly activated by cADPR.
cADP-Rib mobilizes Ca2+ in a range of plant and animal cell-types (Lee, 1997). Firm evidence for a second messenger role for cADPR has been established through measurement of changes in cADPR concentration, which parallel those in [Ca2+ ]c (Guse et al., 1999). In plants, a physiological role for cADPR in signaling by the drought and stress hormone abscisic acid has been demonstrated (Wu et al., 1997; Leckie et al., 1998). cADPR-mediated induction of abscisic acid-responsive gene expression was shown to be exerted by means of mobilization of internal Ca2+ stores, although the cytological identity of the Ca2+ pools on which cADPR acts still remains to be fully defined (Wu et al., 1997).
In this study we have investigated the localization of cADPR-sensitive Ca2+ stores in cauliflower inflorescences. This plant tissue represents an ideal system to investigate the nature of Ca2+ channels in the ER since the rapidly dividing cells of the inflorescence possess an extensive ER network but relatively small vacuoles (Muir and Sanders, 1997). By cell fractionation and subsequent Ca2+ transport analysis of the microsomal subfractions we show here that cADPR is effective at releasing Ca2+ not only from the vacuole but also from the ER membrane pool. The occurrence of agonist-mobilizable Ca2+ discharge from the ER suggests that the role of this compartment in origination of cytosolic Ca2+ signals in plant cell stimulus-response coupling is more crucial than has hitherto been envisaged.
RESULTS
Distribution of ER and Vacuolar Membrane Markers after Continuous Gradient Centrifugation of Cauliflower Microsomes
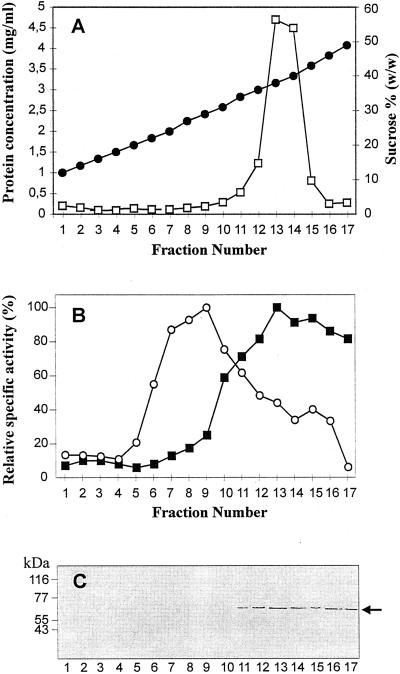
Figure 1A shows the distribution of total protein in the subfractions obtained by isopycnic centrifugation of cauliflower microsomes on a linear 10% to 45% (w/w) Suc density gradient made up over a 50% Suc cushion. To facilitate the separation of the ER from the vacuolar membrane vesicles, 3 mm MgSO4 was included in both homogenization medium and Suc solutions (Liang and Sze, 1998). Most of the protein was recovered in fractions 13 to 14 collected at high buoyant density (38%–40% [w/w] Suc), resembling the pattern previously reported by Muir and Sanders (1997) in the same experimental system, although we observed a narrower peak here.
Figure 1.
Subfractionation of cauliflower microsomes by Suc gradient centrifugation. A, Suc concentrations (●) determined by refractometry, and distribution of protein (□) among microsomal subfractions. B, Distribution of ER and vacuolar marker enzyme activities. ○, Bafilomycin A1-sensitive H+-ATPase (100% = 99.4 nmol mg−1 min−1); ▪, antimycin A-insensitive NADH-Cyt c reductase (100% = 59.5 nmol mg−1 min−1). C, Western analysis of microsomal subfractions (10 μg protein per lane) decorated with antibodies against calnexin (1:1,000 diluted). The arrow indicates 65 kD cauliflower calnexin.
The distribution of the ER marker enzyme antimycin A-insensitive NADH-Cyt c reductase among the microsomal subfractions is illustrated in Figure 1B. The presence of Mg2+ in all preparative media resulted in ER residing exclusively at densities higher than 30% (w/w) Suc, as anticipated for ER membrane vesicles bearing attached ribosomes (rough ER) (Robinson et al., 1994).
Vacuolar membranes, identified by bafilomycin-sensitive H+-ATPase activity, peaked in the low-density region of the gradient (Fig. 1B, fractions 7–9, 24%–29% [w/w] Suc), in agreement with previous results obtained by Askerlund (1997) in cauliflower.
Antibodies against ER marker proteins were also used to study the distribution of ER membranes after continuous Suc gradient centrifugation. Figure 1C shows western blots of cauliflower microsomal subfractions decorated with antibodies against the integral ER membrane protein calnexin from Arabidopsis (Huang et al., 1993). A polypeptide of around 65 kD was detected in agreement with the Mr of plant calnexin. The presence of calnexin only in the heavier Suc fractions (fractions 11–17) gives further support to the results obtained by enzyme assay, confirming the distribution of ER membranes in this region of the gradient.
Antibodies against the intraluminal ER Ca2+-binding proteins calreticulin (Navazio et al., 1995) and BiP (Denecke et al., 1991) were also used, but both of these reticuloplasmins were found to be spread throughout the gradient (data not shown). This wide distribution is likely to be due to the release of soluble proteins from the ER lumen during homogenization.
Distribution of cADPR-Dependent Ca2+ Release among Microsomal Subfractions
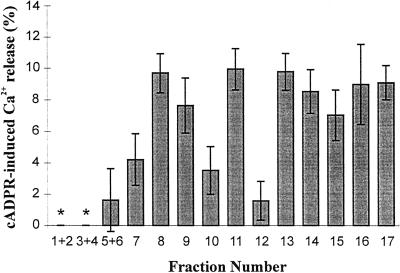
Membrane vesicles separated on linear Suc gradients were subsequently used for Ca2+ transport studies. With the exception of fractions 1 to 4, collected at 12% to 18% (w/w) Suc, all other fractions exhibited ATP-dependent Ca2+ accumulation (data not shown). The transport-incompetence of the lightest fractions is likely to be due to the non-membranous origin of these samples, containing only soluble proteins released during cell disruption and fractionation. Vesicles from each fraction were incubated for 60 min in the uptake medium to obtain steady-state Ca2+ loading and then analyzed with respect to their ability to release Ca2+ in response to cADPR. Figure 2 shows that 1 μm cADPR released Ca2+ from two populations of membrane vesicles in cauliflower. The first peak of cADPR-sensitive membranes was found at 27% to 29% (w/w) Suc (fractions 8 and 9), coinciding with the peak of vacuolar membrane marker enzyme activity. The second population of vesicles responsive to cADPR was located at 34% to 49% (w/w) Suc (fractions 11–17), where most of the ER marker enzyme activity is recovered. It has to be noted that membrane vesicles from fraction 12, collected at 36% (w/w) Suc, although exhibiting a fairly high ER marker enzyme activity could not be discharged by addition of cADPR. Failure of fraction 12 to release Ca2+ in response to this agonist was apparent in two different membrane preparations, suggesting the occurrence of ER subcompartments where the density of the receptor is too low to be detected. However, given the low signal-to-noise ratio, we cannot be confident that fraction 12 is a cADPR-insensitive fraction.
Figure 2.
Distribution of cADPR-induced Ca2+ release among cauliflower microsomal subfractions. Vesicles from each fraction were preloaded with Ca2+ and potential further uptake following Ca2+ release subsequently inhibited as detailed in “Materials and Methods.” An aliquot was removed for radioactivity counting and cADPR (1 μm) was then added. Three aliquots were removed successively over the ensuing 2 min to measure changes in accumulated Ca2+. Results are the means ± se of three to six replicates from two different membrane preparations. Stars indicate non-detectable uptake.
The magnitude of Ca2+ release was found to be similar in both vacuolar and rough ER membrane populations, ranging from 8% to 10% of the total accumulated A23187-sensitive Ca2+ (Fig. 2).
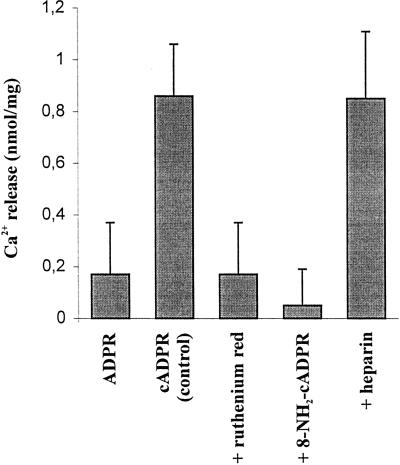
The cADPR-elicited Ca2+ release properties of fraction 13, collected in the middle of the ER marker enzyme activity (38% [w/w] Suc) were further investigated. Figure 3 shows that when 1 μm ADPR (the hydrolytic product of cADPR) was administrated to the Ca2+-loaded vesicles instead of 1 μm cADPR, no Ca2+ release was detected, demonstrating specificity of release for the cyclic isomer only. A higher concentration of ADPR (10 μm) was also ineffective in releasing Ca2+ from a microsomal preparation (data not shown). The cADPR-induced Ca2+ release was found to be effectively blocked by ruthenium red (83% inhibition at 30 μm, Fig. 3), an antagonist of the cADPR-dependent Ca2+ release mechanism in both animal (Galione et al., 1991) and plant vacuolar membranes (Allen et al., 1995). However, since ruthenium red has also been shown to inhibit other classes (non-ligand gated) of plant Ca2+ channel (Marshall et al., 1994), we tested the effect of a more specific antagonist of cADPR action: 8-NH2-cADPR (Walseth et al., 1993). This 8-substituted analog of cADPR was found to block Ca2+ release by cADPR almost completely (92% inhibition at 2.5 μm, Fig. 3). Heparin, a specific inhibitor of the InsP3-mediated pathway in cauliflower microsomes (Muir et al., 1997), was found to be completely ineffective at blocking the Ca2+ mobilizing action of cADPR on the same ER-enriched membrane vesicles (Fig. 3).
Figure 3.
Specificity and inhibitor sensitivity of cADPR-induced Ca2+ release from rough ER-enriched fractions. Cauliflower membrane vesicles collected at 38% (w/w) Suc were allowed to accumulate Ca2+ for 60 min before addition of either 1 μm ADPR (first bar) or 1 μm cADPR (remaining bars). Potential inhibitors of Ca2+ release (30 μm ruthenium red, 2.5 μm 8-NH2-cADPR, and 10 μm heparin [Mr = 4k–6k]) were added 1 min prior to the addition of cADPR. Results are the means ± se of three experiments.
Ca2+ Transport Properties of Ribosome-Denuded ER Membrane Vesicles
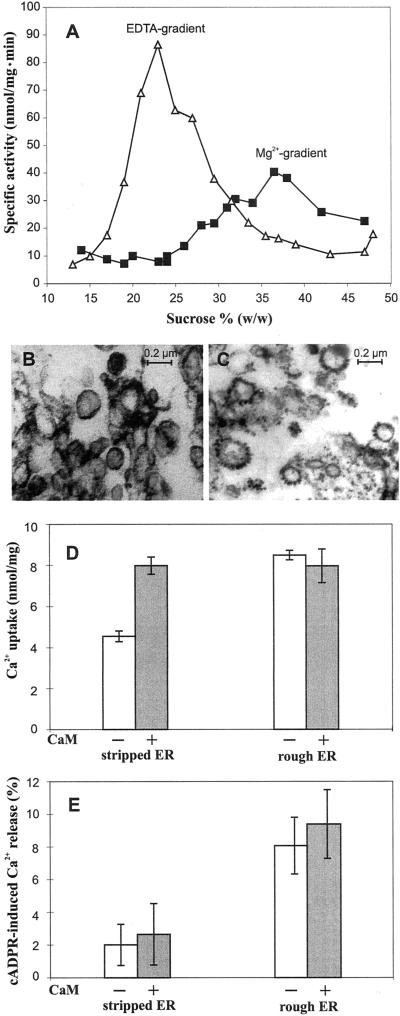
In an attempt to purify further the ER membranes responsive to cADPR, the Mg2+-containing Suc gradient fractions corresponding to the peak activity of antimycin-insensitive NADH-Cyt c reductase (37%–38% [w/w] Suc: Fig. 4A) were treated with EDTA to dissociate ribosomes from the ER. The membranes were then recentrifuged isopycnically onto a second Suc gradient containing EDTA. As a result of stripping, the ribosome-denuded ER membranes shifted to a region of the gradient free of contaminating membranes (21%–27% [w/w] Suc), causing a 2-fold increase in the specific activity of NADH-Cyt c reductase (Fig. 4A). This attests to the good degree of purity of ER membranes obtained by this procedure. Electron microscopy of the membrane material collected at the peak of the ER marker enzyme activity before and after the density-shift further supported the biochemical evidence for the removal of ribosomes after EDTA treatment. ER vesicles collected from the Mg2+-containing gradient consisted of vesicles heavily studded with ribosomes (Fig. 4C), whereas those collected from the EDTA-containing gradient showed mainly smooth vesicles, suggestive of stripped ER (Fig. 4B).
Figure 4.
Comparison of the Ca2+ transport properties of cauliflower ER membranes before and after ribosome stripping. A, Distribution of antimycin A-insensitive NADH-Cyt c reductase activity among cauliflower microsomal subfractions separated on two subsequent Suc gradients, the first containing 3 mm MgSO4 (▪), the second 3 mm EDTA (▵). Fractions comprising the 37% to 38% (w/w) Suc zone of the Mg2+-containing gradient were pooled, treated with EDTA, and recentrifuged isopycnically into an EDTA-containing gradient. B and C, Electron micrographs of the Suc fractions collected at the peak of NADH-Cyt c activity on the EDTA-containing gradient (ribosome-stripped ER) and the Mg2+-containing gradient (rough ER), respectively. D and E, Ca2+ uptake and Ca2+ release induced by 1 μm cADPR from the same samples analyzed by electron microscopy. Vesicles were incubated for 60 min in Ca2+ transport medium in the absence (white bars) or presence (gray bars) of spinach calmodulin (6 μg mL−1; 150 units mL−1). Results are the means ± se of three experiments.
In 45Ca2+ uptake experiments, the stripped ER membranes exhibited a reduced ability to accumulate Ca2+ in comparison with the rough ER membranes from which they have derived. The level of Ca2+ uptake was found to drop from 8.5 to 4.5 nmol mg−1 (Fig. 4D). However, Ca2+ uptake by artificially smooth ER vesicles could be restored to levels comparable with those of rough ER vesicles by addition of 6 μg mL−1 spinach calmodulin. Inclusion in the Ca2+ transport medium of the same dose of exogenous calmodulin did not have any significant effect on the Ca2+ accumulation capacity of the rough ER-derived vesicles (Fig. 4C). These results suggest that endogenous calmodulin, which is very tightly associated with plant Ca2+ pumps in vivo (Evans and Williams, 1998) and still present in membrane fractions separated on Mg2+-containing gradients, is at least partially lost after continuous gradient centrifugation in the presence of EDTA. In summary, substantial enrichment of these fractions by ER is suggested by the visualization of ribosomes on the vast majority of vesicles, by the strong EDTA-dependent density shift of marker enzyme activity, and by the presence of both calmodulin-independent and -dependent components to Ca2+ uptake (compare with Hong et al., 1999; Hwang et al., 2000).
It is surprising that the ribosome-denuded ER membrane vesicles were found not to release Ca2+ in response to cADPR: When fractions corresponding to the NADH-Cyt c reductase peak on the EDTA-containing gradient were pooled and challenged with 1 μm cADPR, no significant cADPR-dependent Ca2+ release was observed (Fig. 4E). On the other hand, no cADPR-elicited Ca2+ release was detected in any other region of the gradient (data not shown), ruling out the obvious possibility that the responsiveness to cADPR observed in the high-density zone of the Mg2+-containing gradient could be due to contaminating vesicles of different membrane origin. Inclusion of calmodulin in the Ca2+ transport assay was not sufficient to re-establish the cADPR-elicited Ca2+ release previously observed in the rough ER preparation (Fig. 4E).
Treatment with 15 mm pyrophosphate, an alternative Mg2+ chelator that detaches ribosomes from rough ER fractions as effectively as EDTA (Amar-Costesec et al., 1974) resulted in the same loss of sensitivity of the uncoated ER vesicles to the Ca2+-mobilizing effect of cADPR (data not shown).
The Ca2+-Mobilizing Action of cADPR Is Exerted on Internal Ca2+ Stores Only
Plant cell plasma membranes exhibit equilibrium densities (30%–40%) on Suc gradients that overlap significantly with those of rough ER membranes (Robinson et al., 1994). To investigate whether the cADPR-responsiveness of the high density Suc fractions and in particular that of fraction 11, collected at 34% (w/w) Suc (Fig. 2), could be due to contamination of rough ER with plasma membrane vesicles, cauliflower microsomes were separated by aqueous two phase partitioning and the resulting upper and lower phases analyzed with the 45Ca2+ filtration assay. Plasma membrane fractions obtained by phase partitioning are superior in terms of purity to those obtained by Suc density centrifugation (Robinson et al., 1994). This was confirmed by a glucan synthase II enzyme assay, which demonstrated a higher specific activity of this plasma membrane marker in the upper phase obtained by two phase partitioning than in any of the Suc gradient fractions (Table I).
Table I.
Analysis of glucan synthase II activity in plasma membrane-enriched fractions obtained by two-phase partitioning and Suc density centrifugation
| Membrane Fraction | Specific Activity | Enrichment |
|---|---|---|
| nmol mg−1 min−1 | ||
| Microsomes | 242.5 | 1 |
| Upper phase | 760.6 | 3.1 |
| Gradient fraction (34% [w/w] Suc) | 288.5 | 1.2 |
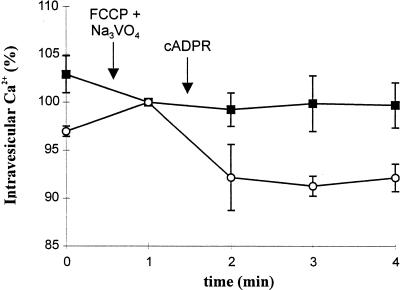
Vesicles from the upper phase were treated with Brij 58 to produce a uniform population of cytosol-side-out plasma membrane vesicles (Johansson et al., 1995) and were then Ca2+-loaded. Challenge with saturating doses (1 μm) of cADPR failed to evoke Ca2+ release (Fig. 5). By contrast, administration of the same dose of cADPR resulted in significant Ca2+ release from the lower phase (Fig. 5), which contains the bulk of endomembranes. These data demonstrate that Ca2+ influx across the plasma membrane is not involved in cADPR-elicited Ca2+ release in cauliflower.
Figure 5.
cADPR-sensitivity of the upper and lower phases obtained by aqueous two phase partitioning of cauliflower microsomes. Loading of Ca2+ into vesicles from the upper (▪) and lower (○) phases was terminated by addition of FCCP (10 μm) and Na3VO4 (200 μm). Curves are standardized to this point (100% Ca2+ accumulation = 31.9 ± 1.2 [▪] and 14.4 ± 0.9 nmol mg−1 [○]). cADPR (1 μm) was subsequently added and three aliquots removed to estimate Ca2+ remaining in the vesicles by measuring radioactivity content. Data are the means ± se of three experiments.
DISCUSSION
The ability of cADPR to mobilize intracellular Ca2+ from storage pools has long been known in animal cells (Clapper et al., 1987; Lee et al., 1989) and has more recently been documented in plant cells (Allen et al., 1995; Leckie et al., 1998). Plant Ca2+ release channels gated open by cADPR have been reported at the vacuolar membrane of plants (Allen et al., 1995; Leckie et al., 1998), where their biochemical and pharmacological properties are very similar to those of animal ryanodine receptors (Muir and Sanders, 1996; Muir et al., 1997). However, the occurrence of a cADPR-gated Ca2+ release pathway at the vacuolar membrane does not preclude, in principle, the possibility that channels belonging to the same class could also be located at plant cell compartments other than the vacuole.
In this study we have investigated in more detail the distribution of plant cADPR-sensitive Ca2+ stores, using membrane vesicles prepared from inflorescences of cauliflower. We show here that, in addition to the vacuole, the ER can be regarded as a bona fide cADPR-sensitive intracellular Ca2+ store, at least in cauliflower. cADPR-elicited Ca2+ release at the ER membrane was found to share the same pharmacological features as the cADPR-gated mechanism located at the vacuolar membrane (Muir and Sanders, 1996; Leckie et al., 1998), i.e. insensitivity to the InsP3-receptor antagonist heparin and effective inhibition by the cADPR-receptor blockers ruthenium red and 8-NH2-cADPR. Moreover, the release pathway at the ER is selectively activated by cADPR, ADPR being ineffective in triggering Ca2+ release.
The magnitude of the Ca2+ release induced by cADPR did not exceed 10% of the total Ca2+ accumulated by vesicles from both intracellular membrane locations (ER and vacuole), suggesting that in cauliflower the cADPR-gated channels are present at a lower density than in red beet vesicles (Allen et al., 1995). This could be attributed to species-specific variations (Muir et al., 1997) or, alternatively, to a level of Ca2+ channel expression that depends on the state of cell differentiation (Gollasch et al., 1998).
The use of high Mg2+ conditions for the isolation and separation of membrane vesicles on Suc gradients will have reduced to a minimum the risk of contamination of rough ER by vacuolar membranes. Indeed, bafilomycin-sensitive H+-ATPase (vacuolar marker) and antimycin-insensitive NADH-Cyt c reductase (ER marker) showed good separation between the two membrane populations. The ER location of cADPR-sensitive Ca2+ release detected in the high density region of the gradients was further confirmed by immunoblot analysis with antibodies against the ER integral membrane protein calnexin. Antibodies against the ER luminal reticuloplasmins calreticulin and BiP were also used in this study, but the leakage of soluble proteins from the ER lumen during homogenization and the possibility of their subsequent trapping in the forming vesicles, even of different origin, warn against the use of non-membrane-anchored proteins as reliable ER markers in cell fractionation studies.
The possibility that cADPR-induced Ca2+ mobilization in the ER-containing fraction of the Suc gradient was actually mediated by contaminating plasma membrane vesicles was tested with highly purified plasma membrane vesicles obtained by two-phase partitioning of cauliflower microsomes. Failure of inside-out (cytosol-side-out) plasma membrane vesicles to release the accumulated Ca2+ in response to cADPR indicates that the plasma membrane is not involved in cADPR-dependent Ca2+ release, in contrast to the two-phase fraction that was enriched in endomembranes. A similar conclusion has emerged from studies on sea urchin eggs, which established that external Ca2+ was not required for the increase of cytosolic Ca2+ generated by cADPR (Lee et al., 1994).
Further purification of ER membranes on Suc gradients in the presence of EDTA or pyrophosphate was found to affect the functional integrity of the cADPR-gated Ca2+ channels located on these membranes. The “artificially smooth” ER vesicles produced in this way lost their ability to be discharged by cADPR and were severely impaired in their Ca2+ accumulation properties as well. The effect of both of these Mg2+-chelating agents is suspected not to be restricted to the detachment of ribosomes from ER membranes but to involve the removal of some soluble proteins adsorbed on microsomes (such as calmodulin; see “Results”) and peripheral membrane proteins as well (Amar-Costesec et al., 1974; Fujiki et al., 1982). Photoaffinity-labeling experiments of cADPR-binding sites in sea urchin egg microsomes have led to the proposal that cADPR does not interact directly with the channel itself but may rather exert its effect through intermediate proteins of 140 and 100 kD (Walseth et al., 1993). We can speculate that these, or some other accessory proteins, required for functional cADPR-gated Ca2+ channels, are lost during the EDTA/pyrophosphate-dependent density shift of rough ER membranes.
Our finding that higher plant ER is mobilized by the endogenous activator of ryanodine receptors, cADPR, reinforces and extends the recent results of Bauer et al. (1998), who postulated the existence of ryanodine-like receptors at an algal ER on the basis of the inhibitory effect of ruthenium red and ryanodine on Ca2+-spiking in these cells.
The cADPR-dependent mechanism of Ca2+ mobilization from the ER appears to be widespread and well conserved throughout evolution, being present with the same characteristics in species that are phylogenetically very distant, including plant, invertebrate, and mammalian cells. Furthermore, the signaling role of this molecule seems to have an ancient origin, the early branching photosynthetic protist Euglena gracilis already possessing the cADPR-release pathway (Masuda et al., 1997). Since the same organism has also been demonstrated to express the ER Ca2+ buffering protein calreticulin (Navazio et al., 1998), it seems that both these components of the internal Ca2+ homeostat appeared very early during eukaryote evolution.
At least two classes of Ca2+-permeable channel are present at plant ER membranes, i.e. voltage-gated (Klüsener et al., 1995) and ligand-gated by NAADP (Navazio et al., 2000). Our present findings demonstrate the presence of an additional class of ligand-gated channel at the ER. In animals, NAADP and cADPR are regarded a sibling messengers, both being produced by a single enzyme from different pyridine nucleotide substrates (Lee, 1999).
The presence of a variety of Ca2+ channel types on different plant membranes might give clues to a long-standing problem in Ca2+ signaling, which is how (given the wide array of stimulus-response pathways in which Ca2+ is involved) stimulus specificity can be encoded. The specificity of the Ca2+ signal (the so-called “Ca2+ signature”; Webb et al., 1996) is likely to rely on the identity of the particular intracellular Ca2+ pool from which Ca2+ release is triggered, thereby encoding spatial information, as well as on the dynamic properties of the channels through which release occurs (McAinsh and Hetherington, 1998). Further work will be required to establish whether cADPR and NAADP are differentially used in alternative signaling pathways, or whether they participate jointly in individual Ca2+ signaling events.
MATERIALS AND METHODS
Preparation of Cauliflower Microsomal Subfractions
Cauliflowers (Brassica oleracea) were purchased from a local market, and the outermost part (top 0.5 cm) of inflorescences was used. A crude microsomal fraction was isolated as previously described (Muir and Sanders, 1997) except that EDTA was replaced by 3 mm MgSO4 in all buffers and NaCl was omitted from the buffer used to wash the microsomal pellet. The final pellet was resuspended in gradient basal medium A {25 mm Tris-MES [2-(N-morpholino)ethanesulfonic acid], pH 7.5, 3 mm MgSO4, 2 μg mL−1 leupeptin, and 0.5 mm phenylmethylsulfonyl fluoride} containing 8% (w/w) Suc, at a protein concentration of 10 to 15 mg mL−1.
Subfractionation of crude microsomes (2 mL) was carried out on a linear 10% to 45% (w/w) Suc gradient (32 mL) over a 50% (w/w) Suc cushion (2 mL). This was centrifuged at 100,000g for 3 h and fractions (2 mL) collected from the top of the centrifuge tube. Centrifugations for 6 and 16 h showed the same distribution of protein and marker enzymes as for 3 h, so the gradient is known to be isopycnic at that time.
Stripping of ribosomes was done by pooling fractions corresponding to the peak of rough ER membranes from six Mg2+-gradients prepared as above and diluting with twice their volume of gradient basal medium B (25 mm Tris-MES, pH 7.5, 3 mm EDTA, 2 μg mL−1 leupeptin, 0.5 mm phenylmethylsulfonyl fluoride). After centrifugation at 150,000g for 1 h, the pellet was resuspended in 2 mL of the same buffer containing 8% (w/w) Suc and layered over a second Suc gradient identical to the first one but made up in gradient medium B. The EDTA-containing gradient was centrifuged at 100,000g for 3 h and fractions collected as above. All operations were performed at 4°C. Suc concentration was measured with a refractometer.
Suc fractions were either used directly for marker enzyme assays and immunoblot analysis, or diluted 3-fold, pelletted (at 100,000g for 1 h), and resuspended in 400 mm glycerol, 5 mm bis-tris propane (BTP)-MES, pH 7.4, 25 mm KCl, 3 mm MgSO4, 0.3 mm NaN3 for Ca2+ transport assays. Samples were frozen in liquid nitrogen and stored at −80°C until use.
Aqueous two-phase partitioned plasma membrane was prepared from cauliflower microsomes exactly as described by Thomson et al. (1993), using 6.5% (w/w) Dextran T500 and 6.5% (w/w) polyethyleneglycol 3350, and performing three successive steps of partitioning. For Ca2+ transport assays, upper phase membrane vesicles were treated with 0.05% (w/v) Brij 58 according to Johansson et al. (1995).
Protein concentration was determined using an assay kit (Bio-Rad Laboratories, Hercules, CA) based on the method of Bradford (1976); bovine serum albumin was used as a standard.
Marker Enzyme Assays
Antimycin A-insensitive NADH-Cyt c reductase (ER marker) activity was measured as described by Hodges and Leonard (1974). Bafilomycin A1-sensitive H+-ATPase (vacuolar marker) determinations were carried out according to Muir and Sanders (1997). Glucan synthase II (plasma membrane marker) was determined as described previously (Navazio et al., 2000).
Marker enzyme analyses were carried out on at least two different membrane preparations and the assays were conducted in duplicates. The results shown are from representative experiments.
SDS-PAGE and Immunoblot Analysis
SDS-slab gel electrophoresis was carried out according to Laemmli (1970), using 12% (w/v) polyacrylamide mini-gels. For immunoblot analysis, proteins were electrophoretically transferred onto nitrocellulose membranes and blots were incubated with antibodies against the following: (a) Arabidopsis calnexin (Huang et al., 1993; a gift from N.E. Hoffman, Stanford, CA), (b) spinach calreticulin (Navazio et al., 1995), and (c) tobacco binding protein (BiP; Denecke et al., 1991; a gift from J. Denecke, Leeds, UK). Antibody binding was detected with alkaline phosphatase-conjugated anti-rabbit secondary antibodies (Boehringer Mannheim, Basel) and color development by reaction with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium tablets (Sigma, St. Louis).
Ca2+ Transport Assay
Ca2+ transport by membrane vesicles was measured by a 45Ca2+ filtration method (Muir and Sanders, 1996). The assay was carried out at room temperature in 500 μL of a medium containing 400 mm glycerol, 5 mm BTP-MES, pH 7.4, 25 mm KCl, 3 mm MgSO4, 0.3 mm NaN3, 3 mm ATP-BTP, 50 μg of protein, 10 μm CaCl2, together with 5.92 kBq 45Ca2+(Amersham, Buckinghamshire, UK, original specific activity 63 GBq mmol−1). Vesicles were incubated in the Ca2+ transport medium for 60 min to allow steady-state intravesicular levels of Ca2+ to be obtained. Potential reloading following Ca2+ release was abolished by addition of 10 μm carbonyl cyanide p-(trifluoromethoxy) phenyl-hydrazone (FCCP) and 200 μm Na3VO4, inhibitors of Ca2+/H+ antiport and Ca2+-pumping ATPases, respectively. Subsequently, 1 μm cADPR (Molecular Probes, Eugene, OR) was added. Potential inhibitors of Ca2+ release (ruthenium red, 8-NH2-cADPR, heparin) were added 1 min prior to the addition of cADPR. Finally, administration of the Ca2+ ionophore A23187 (10 μm) was used to collapse the remaining Ca2+ gradient. No additions contained more than 1% of the total assay volume. At defined time intervals, 50-μL aliquots were removed, filtered on prewetted nitrocellulose filters (0.45-μm pore size, type WCN, Whatman, Clifton, NJ), and rapidly washed once with 5 mL of ice-cold wash medium (400 mm glycerol, 5 mm BTP-MES, pH 7.4, 0.2 mm CaCl2), using a filtration unit under vacuum (Amicon, Beverly, MA). Intravesicular content of 45Ca2+was determined by liquid scintillation counting. Radioactivity remaining on the filters after the addition of A23187 is defined as nonaccumulated Ca2+ and was subtracted from all the data points; typically, this correction amounted to approximately 25% of the overall maximum Ca2+ accumulation.
Cauliflower membrane vesicles do not show any significant “leak” of Ca2+ in the absence of effectors over the time course of the release experiment (6 min).
Electron Microscopy
Membrane samples were fixed in 1% (w/v) cacodylate-buffered glutaraldehyde and post-fixed in 1% (w/v) OsO4 in 0.1 m cacodylate buffer. After dehydration in an ethanol series, samples were embedded in Araldite resin. After lead citrate staining, thin sections were observed with a 300 electron microscope (Hitachi, Tokyo) operating at 75 kV.
ACKNOWLEDGMENTS
We thank Tim Walseth for the kind gift of 8-NH2-cADPR, and Neil E. Hoffman and Jurgen Denecke for the anti-calnexin and anti-BiP antibodies, respectively. We are grateful to Michael Bewell, Lorraine Williams, and Barbara Baldan for helpful advice concerning vesicular 45Ca2+ flux assay, phase partitioning, and electron microscopy, respectively.
Footnotes
This work was supported by the European Molecular Biology Organization (award of a long-term fellowship to L.N.), by the Ministero Università Ricerca Scientifica e Tecnologica (to P.M.), and by the Biotechnology and Biological Sciences Research Council (to D.S.).
LITERATURE CITED
- Alexandre J, Lassalles JP, Kado RT. Opening of Ca2+ channels in isolated red beet root vacuole membrane by inositol 1,4,5-trisphosphate. Nature. 1990;343:567–570. [Google Scholar]
- Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;268:735–737. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- Allen GJ, Sanders D. Two voltage-gated calcium release channels co-reside in the vacuolar membrane of broad bean guard cells. Plant Cell. 1994a;6:685–694. doi: 10.1105/tpc.6.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Sanders D. Osmotic stress enhances the competence of Beta vulgaris vacuoles to respond to inositol 1,4,5-trisphosphate. Plant J. 1994b;6:687–695. [Google Scholar]
- Allen GJ, Sanders D. Vacuolar ion channels of higher plants. Adv Bot Res. 1997;25:217–252. [Google Scholar]
- Amar-Costesec A, Wibo M, Thinès-Sempoux D, Beaufay H, Berthet J. Analytical study of microsomes andisolated subcellular membranes from rat liver. J Cell Biol. 1974;62:717–745. doi: 10.1083/jcb.62.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askerlund P. Calmodulin-stimulated Ca2+-ATPases in the vacuolar and plasma membranes in cauliflower. Plant Physiol. 1997;114:999–1007. doi: 10.1104/pp.114.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CS, Plieth C, Bethmann B, Popescu O, Hansen UP, Simonis W, Schönknecht G. Strontium-induced repetitive calcium spikes in a unicellular green alga. Plant Physiol. 1998;117:545–557. doi: 10.1104/pp.117.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen F, Hayes PM, Mulrooney DM, Pan A. Identification and characterization of cDNA clones encoding plant calreticulin in barley. Plant Cell. 1994;6:835–843. doi: 10.1105/tpc.6.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper DL, Walseth TF, Dargie PJ, Lee HC. Pyridine-nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem. 1987;262:9561–9568. [PubMed] [Google Scholar]
- Denecke J, Goldman MHS, Demolder J, Seurinck J, Botterman J. The tobacco luminal binding protein is encoded by a multigene family. Plant Cell. 1991;3:1025–1035. doi: 10.1105/tpc.3.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DE, Williams LE. P-type calcium ATPases in higher plants: biochemical, molecular and functional properties. Biochim Biophys Acta. 1998;1376:1–25. doi: 10.1016/s0304-4157(97)00009-9. [DOI] [PubMed] [Google Scholar]
- Felle H. Auxin causes oscillations of cytosolic free calcium and pH in Zea mays coleoptiles. Planta. 1988;176:248–255. doi: 10.1007/BF00634478. [DOI] [PubMed] [Google Scholar]
- Franklin-Tong VE, Drobak BK, Allan AC, Watkins PAC, Trewavas AJ. Growth of pollen tubes of Papaver rhoeas is regulated by a slow-moving calcium wave propagated by inositol 1,4,5-trisphosphate. Plant Cell. 1996;8:1305–1321. doi: 10.1105/tpc.8.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y, Hubbard AL, Fowler S, Lazarow PB. Isolation of intracellular membranes by means of sodium-carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982;93:97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione A, Lee HC, Busa WB. Ca2+-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science. 1991;253:1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- Gollasch M, Haase H, Ried C, Lindschau C, Morano I, Luft FC, Haller H. l-Type calcium channel expression depends on the differentiated state of vascular smooth muscle cells. FASEB J. 1998;12:593–601. doi: 10.1096/fasebj.12.7.593. [DOI] [PubMed] [Google Scholar]
- Guse AH, daSilva CP, Berg I, Skapenko AL, Weber K, Heyer P, Hohenegger M, Ashamu GA, Schulze-Koops H, Potter BVL. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–73. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- Harper JF, Hong B, Hwang I, Guo HQ, Stoddard R, Huang JF, Palmgren MG, Sze H. A novel calmodulin-regulated Ca2+-ATPases (ACA2) from Arabidopsis with an N-terminal autoinhibitory domain. J Biol Chem. 1998;273:1099–1106. doi: 10.1074/jbc.273.2.1099. [DOI] [PubMed] [Google Scholar]
- Hodges TK, Leonard RT. Plasma membrane ATPases. Methods Enzymol. 1974;32:397–398. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Hong BA, Ichida S, Wang Y, Gens JS, Pickard BG, Harper JF. Identification of a calmodulin-regulated Ca-ATPase in the ER. Plant Physiol. 1999;119:1165–1176. doi: 10.1104/pp.119.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Franklin AE, Hoffman NE. Primary structure and characterization of an Arabidopsis thaliana calnexin-like protein. J Biol Chem. 1993;268:6560–6566. [PubMed] [Google Scholar]
- Hwang I, Sze H, Harper JF. A calcium-dependent protein kinase can inhibit a calmodulin-stimulated Ca2+ pump (ACA2) located in the endoplasmic reticulum of Arabidopsis. Proc Natl Acad Sci USA. 2000;97:6224–6229. doi: 10.1073/pnas.97.11.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes E, Brosnan JM, Sanders D. Parallel pathways for intracellular Ca2+ release from the vacuole of higher plants. Plant J. 1992;2:97–102. [Google Scholar]
- Johansson F, Olbe M, Sommarin M, Larsson C. Brij-58, a polyoxyethylene acyl ether, creates membrane vesicles of uniform sidedness: a new tool to obtain inside-out (cytoplasmic side-out) plasma membrane vesicles. Plant J. 1995;7:165–173. doi: 10.1046/j.1365-313x.1995.07010165.x. [DOI] [PubMed] [Google Scholar]
- Klüsener B, Boheim G, Liss H, Engelberth J, Weiler EW. Gadolinium-sensitive, voltage-dependent calcium release channels in the endoplasmic reticulum of a higher plant mechanoreceptor organ. EMBO J. 1995;14:2708–2714. doi: 10.1002/j.1460-2075.1995.tb07271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leckie CP, McAinsh MR, Allen GJ, Sanders D, Hetherington AM. Abscissic acid induced stomatal closure mediated by cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:15837–15842. doi: 10.1073/pnas.95.26.15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol Rev. 1997;77:1133–1164. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]
- Lee HC. A unified mechanism of enzymatic synthesis of two calcium messengers: cyclic ADP-ribose and NAADP. Biol Chem. 1999;380:785–793. doi: 10.1515/BC.1999.098. [DOI] [PubMed] [Google Scholar]
- Lee HC, Galione A, Walseth TF. Cyclic ADP-ribose: metabolism and calcium-mobilizing function. Vitam Horm. 1994;48:199–257. doi: 10.1016/s0083-6729(08)60499-9. [DOI] [PubMed] [Google Scholar]
- Lee HC, Walseth TF, Bratt GT, Hayes RN, Clapper DL. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+ mobilizing activity. J Biol Chem. 1989;264:1608–1615. [PubMed] [Google Scholar]
- Liang F, Cunningham KW, Harper JF, Sze H. ECA1 complements yeast mutants defective in Ca2+ pumps and encodes an endoplasmic reticulum-type Ca2+-ATPase in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:8579–8584. doi: 10.1073/pnas.94.16.8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang F, Sze H. A high affinity Ca2+ pump, ECA1, from the endoplasmic reticulum is inhibited by cyclopiazonic acid but not by thapsigargin. Plant Physiol. 1998;118:817–825. doi: 10.1104/pp.118.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhó R, Moutinho A, van der Luit A, Trewavas AJ. Spatial characteristics of calcium signalling: the calcium wave as a basic unit in plant cell calcium signalling. Philos Trans R Soc Lond B. 1998;353:1463–1473. [Google Scholar]
- Marshall J, Corzo A, Leigh RA, Sanders D. Membrane potential-dependent calcium transport in right-side-out plasma membrane vesicles from Zea mays L. roots. Plant J. 1994;5:683–694. [Google Scholar]
- Masuda W, Takenaka S, Tsuyama S, Tokunaga M, Yamaji R, Inui H, Miyatake K, Nakano Y. Inositol 1,4,5-trisphosphate and cyclic ADP-ribose mobilize Ca2+ in a protist, Euglena gracilis. Comp Biochem Physiol. 1997;118C:279–283. doi: 10.1016/s0742-8413(97)00173-4. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Hetherington AM. Encoding specificity in Ca2+ signalling systems. Trends Plant Sci. 1998;3:32–36. [Google Scholar]
- Muir SR, Bewell MA, Sanders D, Allen GJ. Ligand-gated Ca2+ channels and Ca2+ signalling in higher plants. J Exp Bot. 1997;48:589–597. doi: 10.1093/jxb/48.Special_Issue.589. [DOI] [PubMed] [Google Scholar]
- Muir SR, Sanders D. Pharmacology of Ca2+ release from red beet microsomes suggests the presence of ryanodine receptor homologs in higher plants. FEBS Lett. 1996;395:39–42. doi: 10.1016/0014-5793(96)01000-9. [DOI] [PubMed] [Google Scholar]
- Muir SR, Sanders D. Inositol 1,4,5-trisphosphate-sensitive Ca2+ release across nonvacuolar membranes in cauliflower. Plant Physiol. 1997;11:1511–1521. doi: 10.1104/pp.114.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Baldan B, Dainese P, James P, Damiani E, Margreth A, Mariani P. Evidence that spinach leaves express calreticulin but not calsequestrin. Plant Physiol. 1995;109:983–990. doi: 10.1104/pp.109.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Bewell MA, Siddiqua A, Dickinson GD, Galione A, Sanders D. Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc Natl Acad Sci USA. 2000;97:8693–8698. doi: 10.1073/pnas.140217897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Nardi MC, Pancaldi S, Dainese P, Baldan B, Fitchette-Lainé A-C, Faye L, Meggio F, Martin W, Mariani P. Functional conservation of calreticulin in Euglena gracilis. J Eukaryot Microbiol. 1998;45:307–313. doi: 10.1111/j.1550-7408.1998.tb04541.x. [DOI] [PubMed] [Google Scholar]
- Plieth C, Sattelmacher B, Hansen U-P, Thiel G. The action potential in Chara: Ca2+ release from internal stores visualised by Mn2+-induced quenching of furadextran. Plant J. 1998;13:167–175. [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Robinson DG, Hinz G, Oberbeck K. Isolation of endo- and plasma membranes. In: Harris N, Oparka KJ, editors. Plant Cell Biology, A Practical Approach. Oxford: IRL Press; 1994. pp. 245–272. [Google Scholar]
- Sanders D, Brownlee C, Harper JF. Communicating with calcium. Plant Cell. 1999;11:691–706. doi: 10.1105/tpc.11.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson LJ, Xing T, Hall JL, Williams LE. Investigation of the calcium-transporting ATPases at the endoplasmic reticulum and plasma membrane of red beet. Plant Physiol. 1993;102:553–564. doi: 10.1104/pp.102.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walseth TF, Aarhus R, Kerr JA, Lee HC. Identification of cyclic ADP-ribose-binding proteins by photoaffinity labeling. J Biol Chem. 1993;268:26686–26691. [PubMed] [Google Scholar]
- Ward JM, Schroeder JI. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cells vacuoles implicated in the control of stomatal closure. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AAR, McAinsh MR, Taylor JE, Hetherington AM. Calcium ions as intracellular second messenger in higher plants. Adv Bot Res. 1996;22:45–96. [Google Scholar]
- Wu Y, Kuzma J, Maréchal E, Graeff R, Lee HC, Foster R, Chua NH. Abscissic acid signaling through cyclic ADP-ribose in plants. Science. 1997;278:2126–2130. doi: 10.1126/science.278.5346.2126. [DOI] [PubMed] [Google Scholar]