Abstract
Background
The epidemiology and treatment of pneumococcal meningitis has changed with the implementation of conjugate vaccines and the introduction of adjunctive dexamethasone therapy.
Methods
We analyzed episodes of community-acquired pneumococcal meningitis in adults (≥16 years) in the Netherlands, identified by the National Reference Laboratory for Bacterial Meningitis or treating physician between October 1, 1998, and April 1, 2002, and between January 1, 2006, and July 1, 2018. We studied incidence, pneumococcal serotypes, and clinical features. Predictors for unfavorable outcome (Glasgow Outcome Scale score 1–4) were identified in a multivariable logistic regression model. Two physicians independently categorized causes of death as neurological or systemic.
Results
There were 1816 episodes in 1783 patients. The incidence of 7- and 10−7-valent pneumococcal conjugate vaccine serotypes decreased (from 0.42 to 0.06, P = .001; from 0.12 to 0.03 episodes per 100 000 population per year, P = .014). Incidence of nonvaccine serotypes increased (from 0.45 to 0.68, P = .005). The use of adjunctive treatment with dexamethasone increased and was administered in 85% of patients in 2018. In-hospital death occurred in 363 episodes (20%) and unfavorable outcome in 772 episodes (43%). Delayed cerebral thrombosis occurred in 29 patients (2%), of whom 15 patients (52%) died. Adjunctive dexamethasone therapy was associated with favorable outcome (adjusted odds ratio 2.27, P < .001), individual pneumococcal serotypes were not.
Conclusion
Implementation of conjugate vaccines and adjunctive dexamethasone therapy have changed the incidence and outcome of pneumococcal meningitis in adults over the last two decades. Despite recent advances pneumococcal meningitis remains associated with a residual high rate of mortality and morbidity.
Keywords: bacterial meningitis, pneumococcal disease, conjugate vaccines, dexamethasone, epidemiology
Progress has been made in the prevention and treatment of pneumococcal meningitis. The rate of morbidity and mortality is however still high, warranting continuous efforts in identifying new adjunctive treatments and pushing them forward into clinical trials.
A pneumococcal conjugate vaccine including 7 of the most common and virulent pneumococcal serotypes causing invasive disease at that time (4, 6B, 9V, 14, 18C, 19F, 23F) was recommended for routine use in the United States in 2000 [1]. This has been followed by introduction of new vaccines including 9, 10, 11, or 13 serotypes (additional serotypes 1, 3, 5, 6A, 7F, 19A) [2, 3]. Routine use of these vaccines resulted in decreased nasopharyngeal carriage in children and a decreased incidence of invasive pneumococcal disease [4]. The incidence of meningitis in the (nonvaccinated) adult population has also decreased as result of herd protection [5, 6]. Serotype replacement by nonvaccine serotypes has been reported following the introduction of vaccines and has abolished the overall effect of vaccination on pneumococcal meningitis incidence in some regions [4].
In 2002, a randomized controlled study showed that early treatment with dexamethasone improved outcome in adults with bacterial meningitis [7]. A post hoc analysis showed that dexamethasone decreased death in this subgroup because of a beneficial effect on systemic complications [8]. A meta-analysis of individual patient data, however, showed that dexamethasone did not seem to reduce death or neurological disability overall [9]. A prospective cohort study including 357 episodes with pneumococcal meningitis in the period 2006–2009 showed that dexamethasone therapy has been implemented on a large scale as adjunctive treatment of adults with pneumococcal meningitis in the Netherlands [10].
Case series on adults with pneumococcal meningitis presented a limited number of patients precluding stratification of pneumococcal serotypes and age groups [11–13]. We studied clinical features, prognostic factors, and pneumococcal serotypes of 2 nationwide perspective cohort studies on adult pneumococcal meningitis over a 20-year period.
METHODS
Patient Inclusion and Data Collection
We identified adult patients (≥16 years of age) with pneumococcal meningitis from 2 nationwide prospective cohort studies in the Netherlands, between October 1, 1998, and April 1, 2002, and between January 1, 2006, and July 1, 2018. Methods of patient identification and inclusion have been published previously [6, 14]. Patients were identified by the treating physician or the Netherlands Reference Laboratory for Bacterial Meningitis, which receives an estimated 85%–90% of clinical cerebrospinal fluid (CSF) isolates of patients in the Netherlands (17.3 million population). Extensive data on the patients’ clinical presentation and admission were collected in an online case record form.
Inclusion and Exclusion Criteria
Episodes of bacterial meningitis were included if a bacterial pathogen was cultured from the CSF, or if the CSF chemistry results were indicative of bacterial meningitis according to the Spanos criteria in combination with a positive blood culture, CSF polymerase chain reaction, or CSF antigen test [15]. Episodes in patients with a neurosurgical device, episodes <1 month after complicated head trauma or neurosurgery, and episodes that occurred in hospital or within 1 week after discharge were excluded. Episodes for which the clinical outcome was unknown or without information on the initial clinical presentation were also excluded.
Procedures and Definitions
Clinical outcome at discharge was scored on the Glasgow Outcome Scale by the treating physician: 1 = death, 2 = vegetative state (unable to interact with environment), 3 = severe disability (unable to live independently), 4 = moderate disability (unable to return to work or school), and 5 = mild to no disability. A score ≤4 was considered as unfavorable outcome. Cause of death was categorized independently by 2 physicians (D. L. H. K. and L. t. H.), unaware of the use of adjunctive dexamethasone, as neurological (brain herniation, cerebral infarction and/or hemorrhage, intractable seizures, withdrawal of care because of poor neurologic prognosis, or other neurological complications) or systemic (septic shock, cardiorespiratory failure, multiorgan dysfunction syndrome, myocardial infarction, or other systemic complications), as described previously [8, 12]. Inconsistencies were resolved after discussion. The interrater agreement was assessed by calculation of the kappa coefficient. The effect of per protocol use of dexamethasone (10 mg 4dd for 4 days, administered before or together with the first dose of antibiotics [16]) was assessed according the intention to treat principle: patients who did not complete the full course because of death, withdrawal of care, or other complications/contraindications were analyzed as having received dexamethasone per protocol.
Pneumococcal isolates were serotyped by coagglutination and capsular swelling (Quellung reaction) using specific antisera (Statens Serum Institute, Denmark). Pneumococcal isolates were screened for penicillin susceptibility by culture on blood agar plates with 1-µg oxacillin discs. Isolates with low susceptibility were assessed by E-test to determine the minimum inhibitory concentration (MIC) for penicillin (susceptible if MIC ≤0.06 mg/L, otherwise resistant) and ceftriaxone (susceptible if MIC ≤0.05 mg/L, intermediate susceptible if MIC >0.05 mg/L and ≤2.0 mg/L, and resistant if MIC >2.0 mg/L; European Committee on Antimicrobial Susceptibility Testing norm for meningitis).
Statistical Analysis
The cohort was summarized using counts and proportions for categorical variables, and medians with the interquartile range (IQR) describing their 25th to 75th percentile for continuous variables. Differences were tested using χ 2 and Mann-Whitney U tests as appropriate. Incidences were calculated per epidemiological year (July 1 to June 30) and compared using incidence rate ratios (epitools package). Trends were analyzed using linear regression. The value of prognostic factors previously identified in bacterial meningitis or in pneumococcal meningitis was assessed in univariable and multivariable logistic regression models [6, 14, 17–19]. For the multivariable model, missing values in the selected prognostic factors (median 4.6% per prognostic factor [IQR 0.8–8.9]) were imputed (mice package), subsequently pooling the results of each dataset (n = 8) according to Rubin’s rule [20]. We performed an exploratory analysis of the association between the identified prognostic factors with death from neurological and systemic causes. We used Kaplan-Meier estimates to assess the effect of dexamethasone on the timing of death from any cause, and separately for neurological and systemic causes. All statistical analyses were performed using R statistical programming language (version 3.6.1).
Role of Funding Source
The funding source has had no involvement in study design, collection analysis, or interpretation of data, writing the report, or in the decision to submit the paper for publication.
RESULTS
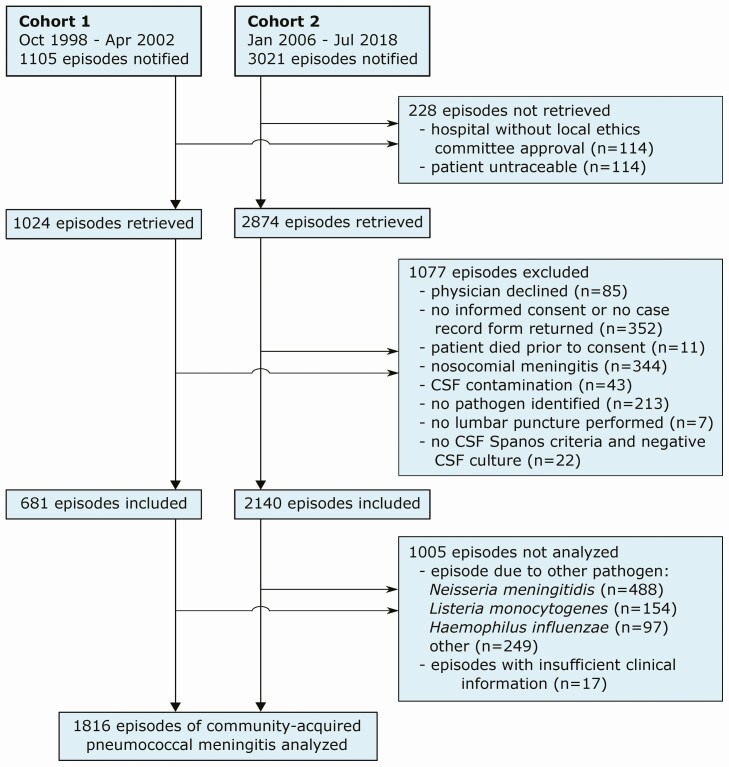
A total of 4126 episodes of bacterial meningitis were reported, 3518 by the reference laboratory and 508 by the treating physician (Figure 1). A total of 228 episodes could not be traced, 1077 episodes were excluded from the cohort, 988 episodes were due to other pathogens, and 17 episodes had insufficient clinical information, leaving 1816 episodes of community-acquired pneumococcal meningitis for the analysis. These episodes occurred in 1783 patients: 27 patients (2%) had 2 episodes and 3 patients had 3 episodes during the study periods.
Figure 1.
Selection of patients. Patients were identified through surveillance of the Netherlands Reference Laboratory for Bacterial Meningitis (n = 3518) or by the treating physician (n = 508). Streptococcus pneumoniae was identified by CSF culture in 1739 episodes, and in the remaining 77 episodes by both blood culture and CSF PCR (n = 7), blood culture and CSF antigen (n = 7), or only by blood culture (n = 42), CSF PCR (n = 15), or CSF antigen (n = 6). Of the 1480 analyzed episodes of community-acquired pneumococcal meningitis in cohort 2, 1314 episodes were identified by the reference laboratory (mortality rate 18%) and 166 episodes by the treating physician (mortality rate 10%). Abbreviations: CSF, cerebrospinal fluid; PCR, polymerase chain reaction.
Median age at presentation was 62 years [IQR 51–70] and 902 episodes (50%) occurred in males (Table 1). Median age increased from 60 years [IQR 47–71] in 1999–2002 to 62 years [IQR 54–70] in 2015–2018 (linear regression β = 0.19, P = .002). The triad of fever, neck stiffness, and altered consciousness was present in 842 of the 1661 episodes (51%). At least 2 of the 4 signs (triad plus headache) were present in 1335 episodes (96%), 3 of 4 in 1127 episodes (81%), and all 4 in 621 episodes (45%). Focal neurological abnormalities were present upon admission in 665 of 1804 patients (37%). Patients were comatose in 296 of 1800 episodes (16%).
Table 1.
Clinical Presentation and Ancillary Investigations on Admission
| Features | 1816 Patients |
|---|---|
| Age at presentation (years) | 62 [51–70] |
| Male | 902/1816 (50) |
| Medical history | |
| History of meningitis | 120/1809 (7) |
| Cerebrospinal fluid leak | 53/1768 (3) |
| Immunocompromising condition | 462/1812 (25) |
| History of cancer | 192/1475 (13) |
| Extrameningeal focus of infection | 959/1811 (53) |
| Otitis or sinusitis | 771/1737 (44) |
| Pneumonia | 213/1738 (12) |
| Endocarditis | 13/1712 (1) |
| Symptoms <24 hours | 877/1722 (51) |
| Pretreatment with antibiotics | 194/1768 (11) |
| Clinical presentation | |
| Headache | 1242/1535 (81) |
| Nausea | 862/1444 (60) |
| Seizures before or on admission | 150/1704 (9) |
| Fever before or on admission (≥38°C) | 1508/1743 (87) |
| Heart rate (bpm) | 100 [85–115] |
| Systolic blood pressure (mm Hg) | 147 [130–167] |
| Rash | 50/1639 (3) |
| Score on Glasgow Coma Scale | 10 [8–13] |
| <14 (indicating altered consciousness) | 1446/1800 (80) |
| <8 (indicating coma) | 296/1800 (16) |
| Neck stiffness | 1279/1680 (76) |
| Aphasia | 299/929 (32) |
| Ataxia | 32/808 (4) |
| Cranial nerve palsy | 168/1567 (11) |
| Paresis | 184/1568 (12) |
| Babinski sign | 290/1553 (19) |
| Blood and CSF findings | |
| C-reactive protein (mg/dL) | 20 [9.2–31.7] |
| Thrombocyte count (units per µL) | 199,000 [151,000–255,750] |
| Positive blood culture | 1288/1590 (81) |
| CSF opening pressure (cm H2O) | 44 [31–50] |
| ≥50 cm H2O | 284/674 (42) |
| CSF white cell count (cells per µL) | 2.570 [561–7.063] |
| <1000 cells per µL | 561/1709 (33) |
| CSF protein concentration (g/dL) | 0.43 [0.25–0.65] |
| CSF:blood glucose ratio | 0.03 [0.01–0.22] |
| Spanos criteriaa | 1626/1756 (93) |
| Positive CSF culture | 1739/1816 (96) |
| Neuroimaging on admission | 1615/1816 (89) |
| Mastoid or sinus opacification | 554/1615 (34) |
| Generalized brain oedema | 151/1615 (9) |
| Recent brain infarction | 84/1615 (5) |
| Hydrocephalus | 73/1615 (5) |
Data are median [IQR] or n/N (%). Heart rate was obtained in 1727 episodes, systolic blood pressure in 1750 episodes, CSF protein in 1688 episodes, CSF:blood glucose ratio in 1631 episodes, C-reactive protein in 1606 episodes, and thrombocyte count in 1718 episodes.
Abbreviation: CSF, cerebrospinal fluid.
a1549 of 1679 episodes with positive CSF culture (92%) met the Spanos criteria (at least 1 of the following: CSF glucose concentration <34.23 mg/dL (or 1.9 mmol/L; to convert glucose to mmol/L, multiply values by 0.0555), CSF:blood glucose ratio <0.23, CSF protein concentration >0.22 g/dL, CSF white cell count >2000 cells per µL, or >1180 CSF polymorphonuclear leukocytes per µL).
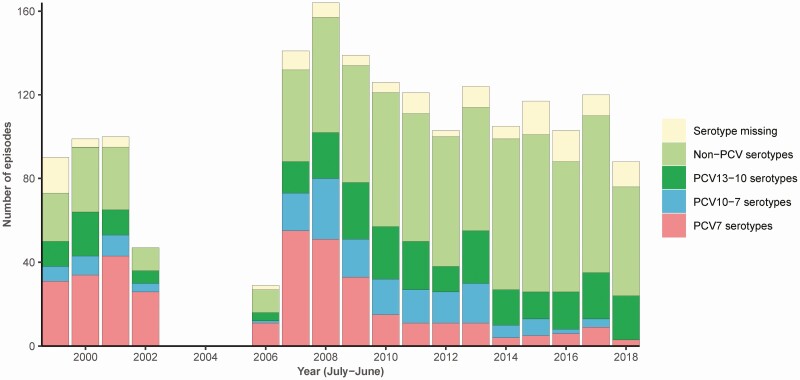
The mean annual incidence was 0.87 per 100 000 population per year. The reference laboratory received and performed serotyping on isolates of 1711 episodes (94%). After introduction of PCV7 vaccination in June 2006, the incidence decreased from 1.07 to 0.73 (2007 vs 2017–2018, incidence rate ratio 0.69 (95% CI: 0.56–0.85), P < .001), with a decrease in incidence of PCV7 serotypes from 0.42 to 0.06 between 2007 and 2017 (linear regression β = −0.04, P = .001; Figure 2). The incidence of PCV10-7 serotypes (serotypes targeted by PCV10 [introduced May 2011] but not by PCV7) decreased from 0.12 to 0.03 between 2011 and 2017 (linear regression β = −0.02, P = .014). Concomitantly, there was an increase in the incidence of non-PCV serotypes from 0.45 to 0.68 between 2007 and 2017 (linear regression β = 0.01, P = .005). PCV13-10 serotypes remained stable (P = .39). Of the 1697 isolates tested for penicillin susceptibility, 36 isolates (2%) were resistant to penicillin. Of the 34 isolates subsequently tested for ceftriaxone susceptibility, 31 isolates were susceptible and 3 isolates showed intermediate susceptibility.
Figure 2.
Pneumococcal serotype of episodes included in the Netherlands, 1998–2018. Serotype distribution according to pneumococcal conjugate vaccine (PCV) group of included episodes of pneumococcal meningitis in adults per epidemiological year (July–June, represented as year on January 1). Included episodes were due to serotype (number of episodes): 3a (187), 8 (158), 7Fb (148), 22F (108), 19Aa (78), 19Fc (75), 10A (68), 12F (67), 23B (62), 23Fc (60), 14c (56), 4c (47), 9Vc (46), 9N (45), 6Bc (39), 11A (37), 18Cc (36), 23A (35), 33F (32), 1b (31), 6Aa (30), 24F (26), 16F (25), 6C (23), 15B (22), 35F (20), 15A (17), 17F (16), 38 (14), 31 (11), 15C (10), 18B (8), 34 (7), 20 (6), 35B (6), 22A (5), 37 (4), 5b (4), 10B (2), 27 (2), 13 (1), 21 (1), 24B (1), 25A (1), 28F (1), 7A (1), 7B (1). Serotyping was not performed or subtyping incomplete in 136 episodes. aPCV13-10, bPCV10-7, cPCV7.
Initial antibiotic treatment was known for 1714 patients (94%) and consisted of third-generation cephalosporins in combination with amoxicillin in 752 patients (44%) and in combination with penicillin or ampicillin in 57 patients (3%). Dexamethasone was administered per protocol in 1188 of 1774 episodes (67%), and after the antibiotics or clinical deterioration in 144 episodes, and in a lower dose in 37 episodes. A total of 498 of the 1216 patients (41%) who underwent neuroimaging before lumbar puncture received antibiotics before neuroimaging. The proportion of patients who underwent neuroimaging before lumbar puncture increased over time (192 of 279 [69%] in 1998–2002 to 283 of 315 [90%] in 2015–2018 [P < .001]), as did the proportion of these patients receiving antibiotics before neuroimaging (52 of 192 [27%] in 1998–2002 to 140 of 273 [51%] in 2015–2018 [P < .001]).
Neuroimaging was performed on and/or during admission in 1679 of 1816 episodes (92%) and revealed abnormalities in 1094 episodes: mastoid and/or sinus opacification in 639 of 1816 episodes (35%), hypodensities suspect for recent cerebral infarction in 279 (15%), generalized brain edema in 202 (11%), hydrocephalus in 105 (6%), cerebral hemorrhage (excluding microbleeds) in 24 (1%), subdural empyema in 23 (1%), venous sinus thrombosis in 22 (1%), brain abscesses in 14 (1%), or other abnormalities in 142 patients (8%).
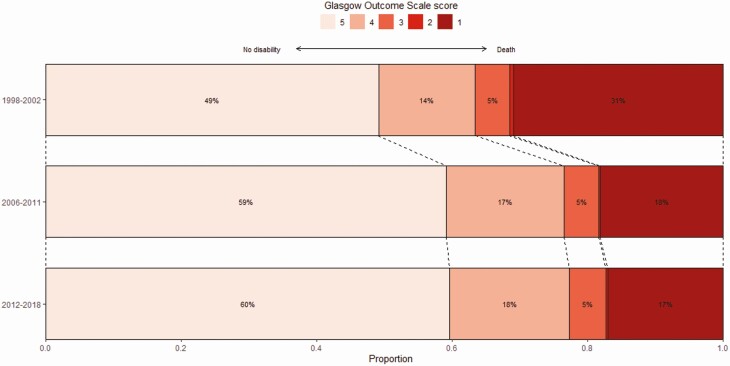
Cardiorespiratory failure (circulatory shock or respiratory failure) developed in 578 of 1770 (33%) patients, of whom 469 (74%) required mechanical ventilation. Neurological complications occurred in 1149 of 1797 patients (64%), including deterioration of consciousness in 971 of 1693 patients (57%), in-hospital seizures in 315 of 1744 patients (18%), and focal neurological abnormalities in 364 of 1690 patients (22%). Clinical outcome at discharge was unfavorable in 772 of 1816 episodes (43%), with in-hospital death occurring in 363 episodes (20%), vegetative survival occurring in 6 episodes, severe disability in 95 episodes (5%), and moderate disability in 308 episodes (17%) (Figure 3).
Figure 3.
Change in clinical outcome over a 20-year period. Histogram showing the clinical outcome as scored on the Glasgow Outcome Scale (GOS) in 1998–2002, 2006–2011, and 2012–2018. Score 5 indicates mild to no deficits (left), score 1 indicates death (right). Adjunctive dexamethasone was administered between 1992 and 2002 in 1/104 patients (1%), 0/2 patients (0%), 0/17 patients (0%), 1/48 patients (2%), and 9/165 patients (5%) from GOS 1 to 5, respectively, between 2006 and 2011 in 77/108 patients (71%), 1/1 patients (100%), 22/29 patients (76%), 87/104 patients (84%), and 305/352 patients (87%) from GOS 1 to 5 respectively, and between 2012 and 2018 in 100/141 patients (71%), 2/3 patients (67%), 33/44 patients (75%), 118/148 patients (80%), and 432/508 (85%) from GOS 1 to 5, respectively.
Deaths were attributed to neurological complications in 192 patients and from systemic complications in 166 patients, with a kappa coefficient for interrater agreement of 0.60 in cohort 1, and 0.90 in cohort 2 (cause of death missing in 5 episodes). Among the 1044 patients with a favorable outcome (mild to no disability), hearing loss was identified in 310 of 971 episodes (32%) (153/351 [44%] of cases with severe to moderate disability had hearing loss), and cognitive impairment in 50 of 692 episodes (7%, only scored in 2006–2018 cohort). Among the 29 patients with delayed cerebral thrombosis, 15 patients died (52%). Two of these patients (8%) had a favorable outcome (of whom 1 was restarted on steroids).
Male sex, age, presence of an immunocompromising condition, heart rate, low Glasgow Coma Scale score, cranial nerve palsy, low CSF leukocyte count, CSF protein, low CSF:blood glucose ratio, low thrombocyte count, C-reactive protein, and not using dexamethasone were associated with unfavorable outcome in a multivariable model (Table 2; Table 3). The clinical outcome has improved over the past 2 decades but the epidemiological year of infection was not associated with unfavorable outcome when added to this multivariable model. The use of dexamethasone, a strong predictor for favorable outcome, increased significantly from 11 of 336 (3%) in 1998–2002 to 1177 of 1438 (82%) between 2006 and 2018 (P < .001). In 2018, 73 of 86 patients (85%) received dexamethasone.
Table 2.
Baseline Variables Associated With Unfavorable Outcome
| Prognostic Factors | Favorable (n = 1044) | Unfavorable (n = 772) | Univariable OR | Multivariable aOR | P Value |
|---|---|---|---|---|---|
| Age (years) | |||||
| 16–39 | 141/1044 (14) | 61/772 (8) | 0.77 [0.55–1.09] | 0.88 [0.59–1.32] | 0.55 |
| 40–59 | 372/1044 (36) | 208/772 (27) | (reference) | (reference) | … |
| 60–74 | 458/1044 (44) | 316/772 (41) | 1.23 [0.99–1.54] | 1.06 [0.81–1.38] | 0.67 |
| 75+ | 73/1044 (7) | 187/772 (24) | 4.58 [3.33–6.30] | 4.03 [2.75–5.89] | <0.001 |
| Male | 502/1044 (48) | 400/772 (52) | 1.16 [0.96–1.40] | 1.51 [1.20–1.90] | <0.001 |
| Immunocompromised | 223/1041 (21) | 239/771 (31) | 1.65 [1.33–2.04] | 1.50 [1.13–1.99] | 0.005 |
| Alcoholism | 37/1036 (4) | 63/776 (8) | 2.42 [1.59–3.67] | 1.42 [0.83–2.45] | 0.20 |
| Otitis or sinusitis | 512/1017 (50) | 259/720 (36) | 0.55 [0.46–0.67] | 0.87 [0.68–1.10] | 0.24 |
| Pneumonia | 86/1017 (8) | 127/721 (18) | 2.31 [1.73–3.10] | 1.29 [0.91–1.82] | 0.15 |
| Heart rate (beats/min) | 100 [84–111] | 103 [88–120] | 1.14 [1.09–1.19] | 1.08 [1.02–1.13] | 0.005 |
| Systolic BP (mm Hg) | 146 [130–165] | 150 [130–170] | 1.01 [0.98–1.05] | 0.99 [0.95–1.03] | 0.66 |
| Rash | 29/951 (3) | 21/688 (3) | 1.00 [0.57–1.77] | 1.07 [0.54–2.12] | 0.85 |
| Glasgow Coma Scale score | 11 [9–13] | 10 [8–12] | 0.86 [0.83–0.89] | 0.89 [0.86–0.93] | <0.001 |
| Cranial nerve palsy | 49/927 (5) | 88/640 (14) | 2.86 [1.98–4.12] | 2.39 [1.56–3.68] | <0.001 |
| Seizures (baseline) | 74/1012 (7) | 76/692 (11) | 1.56 [1.12–2.19] | 1.27 [0.83–1.94] | 0.27 |
| CSF white cellsa | |||||
| <100 cells/μL | 65/989 (7) | 125/720 (17) | 3.60 [2.59–5.02] | 2.09 [1.38–3.15] | <0.001 |
| 100–999 cells/μL | 549/989 (56) | 197/720 (27) | 2.09 [1.63–2.67] | 1.75 [1.27–2.41] | <0.001 |
| 1000–10,000 cells/μL | 177/989 (36) | 293/720 (41) | (reference) | (reference) | … |
| >10,000 cells/μL | 198/989 20) | 105/720 (15) | 0.99 [0.75–1.31] | 0.77 [0.55–1.06] | 0.11 |
| CSF protein (g/dL) | 0.37 [0.22–0.59] | 0.51 [0.33–0.75] | 1.11 [1.08–1.14] | 1.05 [1.01–1.08] | 0.007 |
| CSF:blood glucose ratio | |||||
| <0.02 | 346/959 (36) | 363/672 (54) | 3.70 [2.80–4.90] | 2.49 [1.76–3.52] | <0.001 |
| 0.02–0.23 | 306/959 (32) | 222/672 (33) | 2.56 [1.91–3.44] | 1.83 [1.26–2.65] | 0.002 |
| >0.23 | 307/959 (32) | 87/672 (13) | (reference) | (reference) | … |
| Positive blood culture | 744/924 (81) | 544/666 (82) | 1.08 [0.84–1.39] | 0.87 [0.64–1.18] | 0.37 |
| C-reactive protein (mg/dL)a | 15.6 [7.2–26.0] | 26.1 [14.3–37.0] | 1.05 [1.04–1.05] | 1.03 [1.02–1.04] | <0.001 |
| Thrombocyte count | |||||
| <75,000 | 19/993 (2) | 49/725 (7) | 4.20 [2.44–7.22] | 2.34 [1.23–4.47] | 0.01 |
| 75,000–149,000 | 163/993 (16) | 178/725 (25) | 1.78 [1.40–2.26] | 1.17 [0.87–1.55] | 0.29 |
| ≥150,000 | 811/993 (82) | 498/725 (69) | (reference) | (reference) | … |
| Dexamethasone | 746/1044 (73) | 442/772 (59) | 0.54 [0.44–0.66] | 0.58 [0.46–0.74] | <0.001 |
The study included 1816 episodes of community-acquired pneumococcal meningitis in 1783 patients; data are median [IQR] or n/N (%), and results of the univariable and multivariable model are represented as the (adjusted) odds ratios ((a)OR) [95% CI] for unfavorable outcome. P values are specified for the multivariable model. The multivariable analysis used an imputed dataset with 8 imputation rounds; all variables in the table were entered in the multivariable logistic regression model simultaneously. Odds ratios are calculated per increment of 10 beats/min heart rate, 10 mm Hg systolic blood pressure, 0.1 g/dL CSF protein, and 1 mg/dL C-reactive protein. Heart rate was obtained in 1727 episodes, systolic blood pressure in 1750 episodes, Glasgow Coma Scale score in 1800 episodes, CSF protein in 1688 episodes, and C-reactive protein in 1606 episodes.
Abbreviations: BP, blood pressure; CSF, cerebrospinal fluid.
aThere was a negative correlation between CSF cell count and blood C-reactive protein, in particular in the subgroup of patients with CSF cell counts <1000 cells per µL: for every additional mg/dL of C-reactive protein, there were 100 fewer white cells in the CSF (linear regression, P < .001).
Table 3.
Prognostic Multivariable Model for Systemic and Neurological Cause of Death
| Features | Systemic Death (n = 166) |
P Value | Neurological Death (n = 192) |
P Value |
|---|---|---|---|---|
| Age, years | ||||
| 16–39 | 0.97 [0.37–2.55] | 0.94 | 1.66 [0.94–2.95] | .08 |
| 40–59 | (reference) | … | (reference) | … |
| 60–74 | 2.49 [1.46–4.24] | <0.001 | 0.93 [0.62–1.39] | .73 |
| 75+ | 10.30 [5.79–18.34] | <0.001 | 1.28 [0.78–2.09] | .33 |
| Male | 1.84 [1.25–2.70] | 0.002 | 1.45 [1.04–2.03] | .03 |
| Immunocompromising condition | 1.28 [0.86–1.89] | 0.22 | 1.45 [1.01–2.08] | .04 |
| Heart rate | 1.14 [1.05–1.24] | 0.001 | 1.01 [0.94–1.08] | .81 |
| Glasgow Coma Scale score | 0.96 [0.90–1.02] | 0.21 | 0.84 [0.80–0.89] | <.001 |
| Cranial nerve palsy | 0.92 [0.49–1.73] | 0.80 | 1.69 [0.99–2.88] | .05 |
| CSF white cell count | ||||
| <100 cells | 1.57 [0.91–2.71] | 0.10 | 2.38 [1.41–4.02] | .001 |
| 100–999 cells | 1.23 [0.76–2.01] | 0.40 | 1.73 [1.08–2.79] | .02 |
| 1,000–10,000 | (reference) | … | (reference) | … |
| >10,000 | 0.96 [0.53–1.73] | 0.89 | 0.76 [0.45–1.28] | .30 |
| CSF protein (g/dL) | 1.01 [0.97–1.06] | 0.54 | 1.07 [1.03–1.11] | <.001 |
| CSF:blood glucose ratio | ||||
| <0.02 | 1.87 [0.94–3.71] | 0.08 | 2.87 [1.52–5.42] | .01 |
| 0.02–0.23 | 2.02 [1.02–4.01] | 0.04 | 1.73 [0.86–3.50] | .13 |
| >0.23 | (reference) | … | (reference) | … |
| C-reactive protein (mg/dL) | 1.03 [1.02–1.05] | <0.001 | 1.02 [1.01–1.04] | .002 |
| Thrombocytes | ||||
| <75 | 5.88 [3.11–11.12] | <0.001 | 1.14 [0.57–2.30] | .71 |
| 75–149 | 1.10 [0.70–1.72] | 0.69 | 1.02 [0.69–1.52] | .91 |
| 150+ | (reference) | … | (reference) | … |
| Dexamethasone | 0.43 [0.30–0.63] | <0.001 | 0.59 [0.42–0.83] | .003 |
The study included 1816 episodes of community-acquired pneumococcal meningitis in 1783 patients; data are median [IQR] or n/N (%), and results of the multivariable model are represented as the adjusted odds ratios [95% confidence interval] for systemic and neurological deaths. The multivariable analysis used an imputed dataset with 8 imputation rounds, all variables in the table were entered in the multivariable logistic regression model simultaneously. Odds ratios are calculated per increment of 10 beats/min heart rate, 0.1 g/dL CSF protein, and 1 mg/dL C-reactive protein.
Abbreviation: CSF, cerebrospinal fluid.
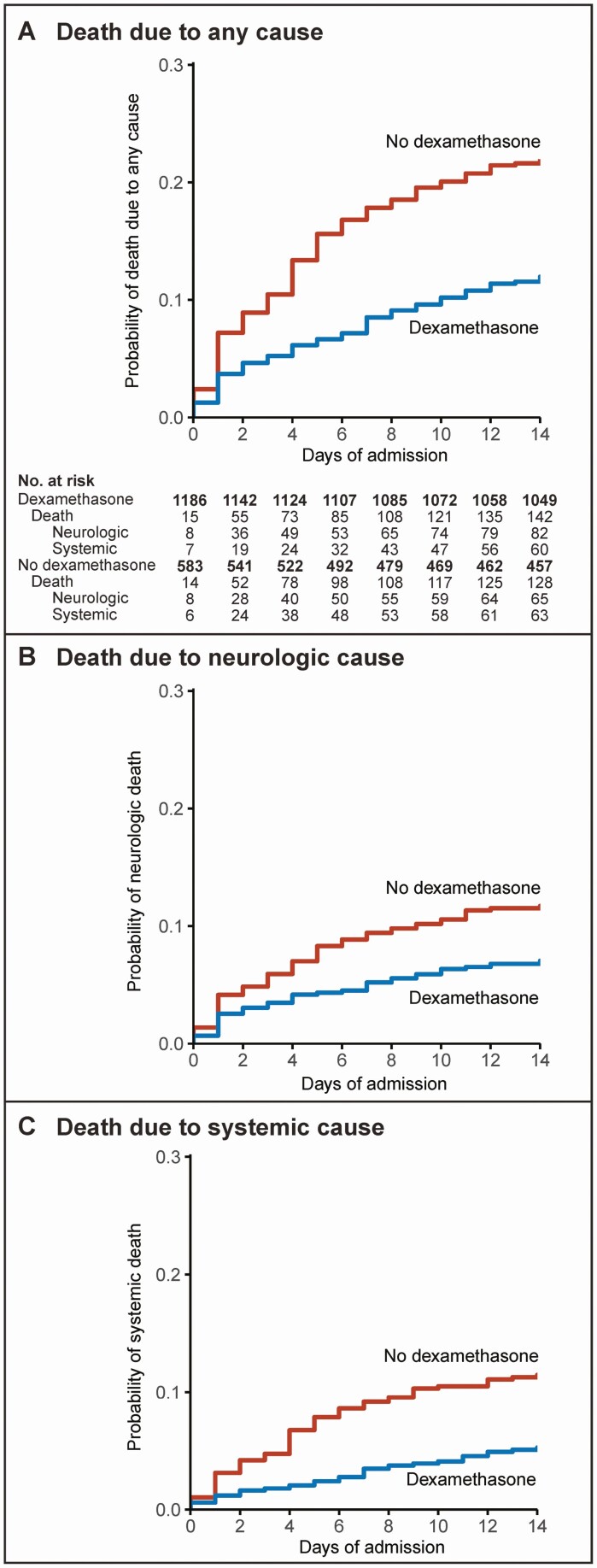
Dexamethasone use was associated with a lower rate of cardiorespiratory failure (323 of 1156 [28%] vs 237 of 575 [41%], P < .001), lower rate of in-hospital seizures (174 of 1139 [15%] vs 132 of 569 [23%], P < .001), and a lower rate of focal neurological abnormalities (214 of 1099 [19%] vs 142 of 558 [25%], P = .005), leading to a lower rate of unfavorable outcome (442 of 1188 [37%] vs 307 of 586 [52%], P < .001). Dexamethasone was associated with lower mortality rate (178 of 1188 [15%] vs 175 of 586 [30%], P < .001), both from neurological complications (9% vs 15%, P < .001) and systemic complications (6% vs 15%, P < .001; Figure 4). Dexamethasone did not alter the median time to death which was 6 days (IQR 2–13). The mortality rate among patients who did not receive dexamethasone according to the protocol did not differ between patients in cohort 1 and cohort 2 (103 of 325 (32%) vs 72 of 261 (28%); P = .294). Delayed cerebral thrombosis occurred in 22 of 1188 episodes (2%) with dexamethasone treatment and in 7 of 586 episodes (1%) without dexamethasone treatment (P = .30). Patients with delayed cerebral thrombosis more often had symptom onset within 24 hours of presentation (71% vs 51%, P = .03) and a low leukocyte count (<1000 cells/µL) in the diagnostic lumbar puncture (58% vs 32%, P = .007).
Figure 4.
Effect of dexamethasone on survival in pneumococcal meningitis. (A) Kaplan-Meier estimates stratified for the use of dexamethasone for overall mortality, and death from (B) systemic and (C) neurological complications. P values of the respective log-rank tests were all < 0.0001. Patients discharged within 14 days of admission were not censored but considered to survive. Cause of death was assigned by 2 independent reviewers. Death from systemic complications: cardiorespiratory failure (n = 72), septic shock (n = 42), multiorgan dysfunction (n = 21), or myocardial infarction (n = 6), or other (n = 25); death from neurologic complications: brain herniation (n = 78), brain infarction and/or hemorrhage (n = 56), withdrawal of care because of poor neurological prognosis (n = 44), intractable seizures (n = 7), or other (n = 7).
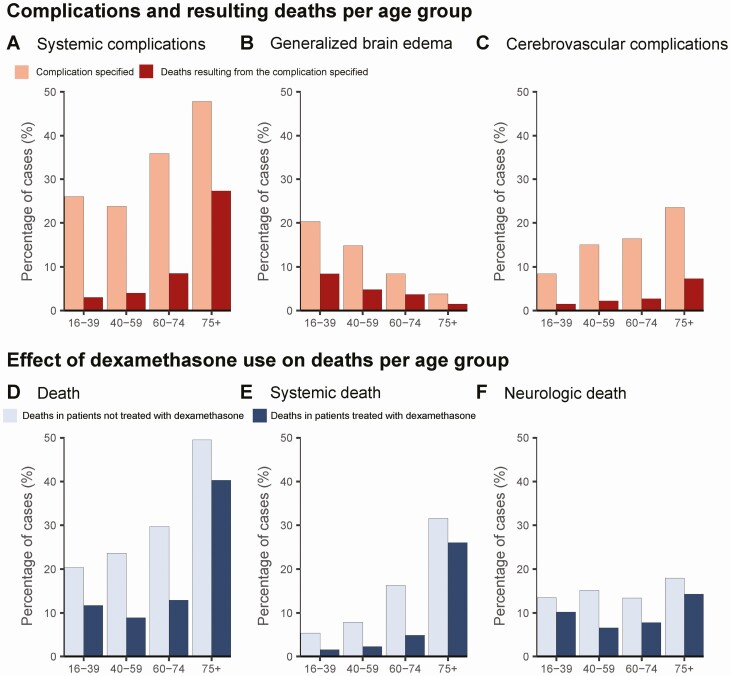
Older patients more often suffered from cardiorespiratory failure and more often died from these systemic complications (Figure 5A). For neurological deaths, older patients more often died from cerebrovascular complications, whereas younger patients more often died from brain herniation (Figure 5B-C). Time to death was 7 days (IQR 3–12) in the 75+ year age group and 3 days (IQR 1–9) in the 16–39 age group (P = .03). There was an interaction between dexamethasone and age ≥75 years and their association with unfavorable outcome (interaction term P = .03 in the multivariable model). Dexamethasone reduced deaths in all age groups (Figure 5D), but was less effective in reducing deaths because of systemic complications in patients ≥75 years of age (relative change −18% in 75+ age group [from 32% to 26%] compared with −70% in other age groups [from 12% to 3%], Figure 5E).
Figure 5.
Age-related differences in pneumococcal meningitis. (A) Rate of cardiorespiratory complications and death from systemic complications, (B) generalized brain edema and death from brain herniation, and (C) rate of cerebral infarction and/or hemorrhage and resulting death per age group. (D) The effect of dexamethasone use on overall mortality, and death (E) from systemic and (F) neurological complications per age group.
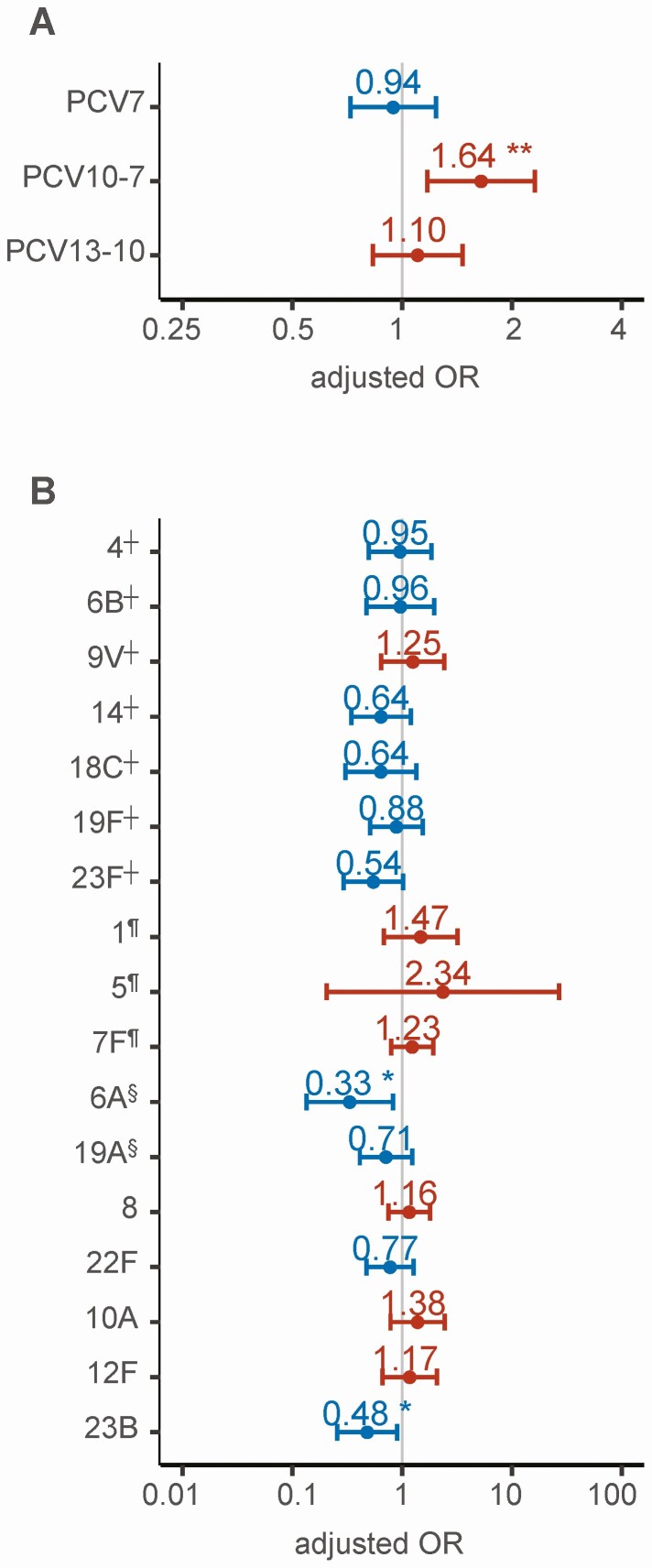
PCV10-7 serotypes, but not PCV7 serotypes, were associated with unfavorable outcome using non-PCV serotypes as a reference group in a multivariable model corrected for age group, dexamethasone use, and multiple testing (adjusted odds ratio 1.64 [IQR 1.17–2.31], P = .004; Figure 6A). None of the individual serotypes were associated with unfavorable outcome in this analysis (Figure 6B).
Figure 6.
Effect of pneumococcal serotype on unfavorable outcome. Regression coefficients and confidence intervals of a multivariable regression analysis of PCV group (using non-PCV serotypes as reference group) and use of dexamethasone, corrected for age, on unfavorable outcome (A), and the regression coefficients and confidence intervals of a multivariable regression analysis of the 20 most common pneumococcal serotypes (using serotype 3§ as reference group), corrected for dexamethasone use and age, on unfavorable outcome (B). Red dots and confidence intervals represent categories associated with an unfavorable outcome (Glasgow Outcome Scale score ≤4); blue represents categories associated with favorable outcome (Glasgow Outcome Scale score = 5). Dexamethasone was significantly associated with favorable outcome in both models (P < .001) and remained significant when corrected for multiple testing. *Indicates significant results (*P < .05, **P < .01, ***P < .001). ┼PCV7, ¶PCV10-7, §PCV13-10. Abbreviation: PCV, pneumococcal conjugate vaccine.
DISCUSSION
Our study shows that, despite advances in vaccination and treatment, pneumococcal meningitis is still a severe disease and associated with a residual high rate of mortality and morbidity. The use of pneumococcal conjugate vaccines has led to a decrease in the incidence of pneumococcal meningitis by diminishing the incidence of PCV serotypes causing meningitis. The extent of this decrease has been partly limited by serotype replacement, with a subsequent increase in meningitis caused by non-PCV serotypes. We did not find evidence that this shift toward nonvaccine serotypes has improved clinical outcome following pneumococcal meningitis. Though PCV10 serotypes were significantly associated with unfavorable outcomes, the effect size of the association and proportion of cases caused by PCV10 was relatively small, limiting the overall effect. The PCV10 serotypes 1, 5, and 7F have been reported in the literature previously as invasive serotypes, but whether this is a spurious association, or related to the polysaccharide capsule itself, clustering of virulence genes, or other bacterial factors remains unknown [21, 22]. We did not identify individual serotypes to be associated with clinical outcome.
Adjunctive use of dexamethasone was associated with a decreased mortality rate from 30% to 15% and unfavorable outcome from 52% to 37%. This decline in mortality is in accordance with results of the randomized clinical trial, where 14% of patients with pneumococcal meningitis in the dexamethasone group died, compared with 34% in the placebo group [7]. The mortality rate among patients not treated with dexamethasone was similar before 2002 and from 2006 onwards (32% vs 28%, respectively), suggesting only a modest positive effect on mortality from improvements of intensive care unit care, more timely clinical presentation and/or administration of antibiotics, or other factors. Of note, in our study, mortality rate was substantially higher than the 5% reported in a German tertiary referral single-center experience following dexamethasone introduction, likely because of the use of prospective surveillance by the reference laboratory [13]. Moribund patients are less likely to be transferred to a tertiary referral center. Also, physicians are hesitant to include moribund patients in clinical studies (demonstrated by the low mortality in patients identified by the treating physician), whereas 30% of deaths occurred within the first 3 days [23].
Our findings illustrate that age modulates the disease course and effectiveness of adjunctive treatment. For neurological cause of death, young adults mainly died of brain herniation, whereas older patients died from cerebrovascular complications or withdrawal of care because of poor prognosis. We found that dexamethasone mainly reduced the rate of systemic deaths from 15% to 6%. This is concordant with the post hoc analysis in the randomized clinical trial [8]. Dexamethasone also lowered the rate of neurological deaths from 15% to 9%. Dexamethasone, however, was not as effective in reducing systemic deaths in the older population. The role of corticosteroids in patients with sepsis has been investigated in several randomized clinical trials with conflicting results and its use in clinical practice is variable. The most recent Cochrane review, including 61 studies involving 12 192 patients, concluded that corticosteroids probably reduce mortality in patients with sepsis and result in large reductions of intensive care unit and in-hospital stay duration [24]. The lower mortality in critically ill coronavirus disease 2019 patients treated with adjunctive corticosteroids has generated renewed interest in the use of corticosteroids in other infectious diseases [25]. Clinical trials in promising new treatments for pneumococcal meningitis such as adjuvant complement system intervention could consider post hoc analyses for systemic and neurological complications and for different age groups [26].
Delayed cerebral thrombosis is a devastating complication that has been associated with poor outcome in 93% and death in 52% in our cohort. This complication, defined as the occurrence of multiple cerebral infarctions in patients who initially recover, was not recognized until the implementation of dexamethasone [27]. Although the rate of delayed cerebral thrombosis was higher in patients treated with dexamethasone (2%), this complication also does occur in patients without adjunctive dexamethasone therapy. We hypothesize that the use of dexamethasone may suppress initial cerebral vasculopathy in many patients. However, in some patients vasculopathy may still occur after the 4-day course of adjunctive therapy, stressing the need for evaluation of prolonged or intensified immunosuppressive treatment in bacterial meningitis.
Our study has several limitations. The methods of patient identification and data collection differed to some extent between cohorts 1 and 2. The categorization of cause of death was based on more elaborate clinical documentation in cohort 2, resulting in improved interrater agreement compared with cohort 1, which could have introduced heterogeneity between the time periods. Most importantly, only a small proportion of pneumococcal meningitis cases included in our cohort had negative CSF cultures (4%) because the Netherlands Reference Laboratory for Bacterial Meningitis identifies patients with a positive CSF culture. Though not specific to pneumococcal meningitis, a recent study found that 22% of patients with a clinical diagnosis of bacterial meningitis aided by CSF chemistry results had a negative CSF culture [28, 29]. Patients in whom a lumbar puncture is postponed (eg, anticoagulant therapy, risk of brain herniation), which increased the rate of negative CSF cultures in our cohort, or not undergo lumber puncture at all, are thus underrepresented in our cohort. A substantial proportion of identified patients with bacterial meningitis were not included in our cohort.
In conclusion, progress has been made in the prevention and treatment of pneumococcal meningitis over the past 2 decades. Pneumococcal conjugate vaccines have reduced the incidence of pneumococcal meningitis, but serotype replacement highlights that new approaches to prevention are needed. The shift in the pneumococcal population has not significantly altered the clinical course of pneumococcal meningitis. The improved clinical outcome over the past 2 decades is mainly attributable to the implementation of adjunctive dexamethasone. The rate of morbidity and mortality is still high, however, especially in the elderly in whom the rate of death from systemic complications is still high in spite of dexamethasone therapy. This warrants continuous efforts in identifying new adjunctive treatments and pushing them forward into clinical trials.
Notes
Acknowledgments. We thank the many physicians in the Netherlands who have contributed to the study.
Financial support. The work was supported by the Netherlands Organization for Health Research and Development (ZonMw; NWO-Vidi- Grant [grant number 917.17.308] to M. C. B.; NWO-Vici-Grant [grant number 918.19.627] to D. v. d. B.); and Academic Medical Center (AMC PhD Scholarship to D. L. H. K.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. ACIP. Preventing pneumococcal disease among infants and young children. Recommendations of the advisory committee on immunization practices (ACIP). MMWR Morb Mortal Wkly Rep 2000; 49:1–35. [PubMed] [Google Scholar]
- 2. McIntyre PB, O’Brien KL, Greenwood B, van de Beek D. Effect of vaccines on bacterial meningitis worldwide. Lancet 2012; 380:1703–11. [DOI] [PubMed] [Google Scholar]
- 3. van de Beek D, Brouwer M, Hasbun R, Koedel U, Whitney CG, Wijdicks E. Community-acquired bacterial meningitis. Nat Rev Dis Primers 2016; 2:16074. [DOI] [PubMed] [Google Scholar]
- 4. Koelman DLH, Brouwer MC, van de Beek D. Resurgence of pneumococcal meningitis in Europe and Northern America. Clin Microbiol Infect 2020; 26:199–204. [DOI] [PubMed] [Google Scholar]
- 5. Fine P, Eames K, Heymann DL. “Herd immunity”: a rough guide. Clin Infect Dis 2011; 52:911–6. [DOI] [PubMed] [Google Scholar]
- 6. Bijlsma MW, Brouwer MC, Kasanmoentalib ES, et al. . Community-acquired bacterial meningitis in adults in the Netherlands, 2006–14: a prospective cohort study. Lancet Infect Dis 2016; 16: 339–47. [DOI] [PubMed] [Google Scholar]
- 7. de Gans J, van de Beek D; European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators . Dexamethasone in adults with bacterial meningitis. N Engl J Med 2002; 347:1549–56. [DOI] [PubMed] [Google Scholar]
- 8. van de Beek D, de Gans J. Dexamethasone and pneumococcal meningitis. Ann Intern Med 2004; 141:327. [DOI] [PubMed] [Google Scholar]
- 9. van de Beek D, Farrar JJ, de Gans J, et al. . Adjunctive dexamethasone in bacterial meningitis: a meta-analysis of individual patient data. Lancet Neurol 2010; 9:254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brouwer MC, Heckenberg SG, de Gans J, Spanjaard L, Reitsma JB, van de Beek D. Nationwide implementation of adjunctive dexamethasone therapy for pneumococcal meningitis. Neurology 2010; 75:1533–9. [DOI] [PubMed] [Google Scholar]
- 11. Ubukata K, Takata M, Morozumi M, et al. . Effects of pneumococcal conjugate vaccine on genotypic penicillin resistance and serotype changes, Japan, 2010–2017. Emerg Infect Dis 2018; 24:2010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J. Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol 2006; 5:123–9. [DOI] [PubMed] [Google Scholar]
- 13. Buchholz G, Koedel U, Pfister HW, Kastenbauer S, Klein M. Dramatic reduction of mortality in pneumococcal meningitis. Crit Care 2016; 20:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004; 351:1849–59. [DOI] [PubMed] [Google Scholar]
- 15. Spanos A, Harrell FE Jr, Durack DT. Differential diagnosis of acute meningitis. an analysis of the predictive value of initial observations. JAMA 1989; 262:2700–7. [PubMed] [Google Scholar]
- 16. van de Beek D, Brouwer MC, Thwaites GE, Tunkel AR. Advances in treatment of bacterial meningitis. Lancet 2012; 380:1693–702. [DOI] [PubMed] [Google Scholar]
- 17. Bijlsma MW, Brouwer MC, Bossuyt PM, et al. . Risk scores for outcome in bacterial meningitis: systematic review and external validation study. J Infect 2016; 73:393–401. [DOI] [PubMed] [Google Scholar]
- 18. Schut ES, Lucas MJ, Brouwer MC, Vergouwen MD, van der Ende A, van de Beek D. Cerebral infarction in adults with bacterial meningitis. Neurocrit Care 2012; 16:421–7. [DOI] [PubMed] [Google Scholar]
- 19. Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J. A risk score for unfavorable outcome in adults with bacterial meningitis. Ann Neurol 2008; 63:90–7. [DOI] [PubMed] [Google Scholar]
- 20. Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons Inc., 1987. [Google Scholar]
- 21. Alanee SR, McGee L, Jackson D, et al. ; International Pneumococcal Study Group . Association of serotypes of streptococcus pneumoniae with disease severity and outcome in adults: an international study. Clin Infect Dis 2007; 45:46–51. [DOI] [PubMed] [Google Scholar]
- 22. Brueggemann AB, Peto TE, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis 2004; 190:1203–11. [DOI] [PubMed] [Google Scholar]
- 23. De Backer D, Schortgen F. Physicians declining patient enrollment in clinical trials: what are the implications? Intensive Care Med 2014; 40:117–9. [DOI] [PubMed] [Google Scholar]
- 24. Annane D, Bellissant E, Bollaert PE, et al. . Corticosteroids for treating sepsis in children and adults. Cochrane Database Syst Rev 2019; 12:Cd002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020; 324:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koelman DLH, Brouwer MC, van de Beek D. Targeting the complement system in bacterial meningitis. Brain 2019; 142:3325–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schut ES, Brouwer MC, de Gans J, Florquin S, Troost D, van de Beek D. Delayed cerebral thrombosis after initial good recovery from pneumococcal meningitis. Neurology 2009; 73:1988–95. [DOI] [PubMed] [Google Scholar]
- 28. Khatib U, van de Beek D, Lees JA, Brouwer MC. Adults with suspected central nervous system infection: a prospective study of diagnostic accuracy. J Infect 2017; 74:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010; 23:467–92. [DOI] [PMC free article] [PubMed] [Google Scholar]