Abstract
Background
Evidence that opioid agonist therapy (OAT) is associated with increased odds of hepatitis C virus (HCV) treatment initiation among people who use drugs (PWUD) is emerging. The objective of this study was to determine the association between current OAT and HCV treatment initiation among PWUD in a population-level linked administrative dataset.
Methods
The British Columbia Hepatitis Testers Cohort was used for this study, which includes all people tested for or diagnosed with HCV in British Columbia, linked to medical visits, hospitalizations, laboratory, prescription drug, and mortality data from 1992 until 2019. PWUD with injecting drug use or opioid use disorder and chronic HCV infection were identified for inclusion in this study. HCV treatment initiation was the main outcome, and subdistribution proportional hazards modeling was used to assess the relationship with current OAT.
Results
In total, 13 803 PWUD with chronic HCV were included in this study. Among those currently on OAT at the end of the study period, 47% (2704/5770) had started HCV treatment, whereas 22% (1778/8033) of those not currently on OAT had started HCV treatment. Among PWUD with chronic HCV infection, current OAT was associated with higher likelihood of HCV treatment initiation in time to event analysis (adjusted hazard ratio 1.84 [95% confidence interval {CI}, 1.50, 2.26]).
Conclusions
Current OAT was associated with a higher likelihood of HCV treatment initiation. However, many PWUD with HCV currently receiving OAT have yet to receive HCV treatment. Enhanced integration between substance use care and HCV treatment is needed to improve the overall health of PWUD.
Keywords: hepatitis C virus, people who inject drugs, opioid agonist therapy, care cascade, linked data
In this retrospective longitudinal cohort study among 13 803 people who use drugs who were confirmed to have chronic hepatitis C virus (HCV) infection in British Columbia, Canada; current opioid agonist therapy was associated with higher likelihood of HCV treatment initiation.
People who use drugs (PWUD), including people who have current or previous history of injecting drug use (IDU), are disproportionately represented among those living with hepatitis C virus (HCV) infection. In British Columbia, Canada, the population prevalence of HCV infection is estimated to be 1.2% [1], whereas prevalence among people who inject drugs (PWID) is estimated to be 32%[2], with similar patterns mirrored globally[3]. Despite the disproportionate burden of HCV infection among PWID and PWUD, HCV treatment initiation among these groups remains lower than non-PWUD [4, 5].
Mortality from drug related causes among PWUD has also increased substantially in recent years, largely driven by opioid overdoses [6]. The impact of interventions such as opioid agonist therapy (OAT) on preventing or reducing opioid-related harms is well established. OAT reduces the risk of opioid overdose death [7, 8], decreases incidence of human immunodeficiency virus (HIV) [9, 10] and HCV acquisition [11, 12] and reduces HCV reinfection risk [13, 14] as well as increases antiretroviral treatment (ART) adherence among PWID living withHIV [15, 16]. Although impact of OAT is well known, access to and initiation of OAT remains suboptimal [17].
Retention in OAT is also generally low; among people who begin methadone treatment, 46–65% discontinue in the first year [18, 19], and among those receiving buprenorphine/naloxone, 40–70% discontinue treatment in the first 6 months [20, 21]. It remains unclear if currently receiving OAT at time of HCV treatment assessment, or length of retention on OAT, is associated with HCV treatment initiation, completion, or sustained virologic response (SVR) [22, 23]. Although OAT has been found to increase likelihood of linkage to HCV care, longitudinal studies with large sample sizes are lacking [24]. Previous studies have enhanced understanding of barriers and facilitators of HCV treatment among PWUD; however, there are limited data on impact of integration of HCV treatment and OAT on HCV treatment initiation. The assessment of the intersection between HCV treatment, drug use, and OAT could identify more holistic approaches toward health and wellness among PWUD.
The objective of this study was to describe patterns of HCV treatment initiation in relation to differing lengths of retention on OAT, and to determine the association between current OAT and HCV treatment initiation among PWUD diagnosed with chronic HCV infection in a population-level linked administrative data set.
MATERIALS AND METHODS
Study Population
The BC Hepatitis Testers Cohort (BC-HTC) was used for this analysis, which includes all individuals tested for or diagnosed with HCV in British Columbia since 1992, linked to various province-wide registries including data on medical visits, hospitalizations, and ambulatory care visits until 31 December 2015, and all prescription drugs (PharmaNet includes all prescriptions dispensed in the province), public health laboratory testing, and mortality data until 30 June 2019 [25, 26] (Supplementary Table 1). The BC-HTC construction and data linkage was reviewed and approved by the research ethics board at the University of British Columbia (H14-01649). For this analysis, we selected all people diagnosed with chronic HCV infection (RNA positive), and either identified as: “ever PWID” from 1 January 1996 to 30 June 2019, as indicated by a previously validated algorithm (Supplementary Table 2; “IDU-2M” algorithm used has 78% sensitivity and 83% specificity) [2], or "non-IDU PWUD,” based on not being identified as a PWID but having ever received OAT, as indicated by an OAT dispensation record in the BC PharmaNet database from 1 January 1996 to 30 June 2019. OAT dispensations were identified by selecting drug identification numbers (DINs) that are specific to OAT and not pain, which was verified by subject matter experts (Supplementary Table 2). Participants with no evidence of HCV treatment, whose last HCV RNA result was negative, after a previous positive HCV RNA result, were excluded due to likely spontaneous or natural HCV clearance.
Study Measures
HCV treatment initiation was assessed using PharmaNet HCV treatment dispensation data from 1992 up to 30 June 2019, with date of treatment initiation assigned as the dispensation date. Treatment initiation was defined as at least one dispensation; completion of the regimen was not required. OAT status was assessed using PharmaNet OAT dispensation data from 1 January 1996 to 30 June 2019. We created 2 different variables for OAT; one was a time-varying variable for current OAT, and the other was an anchored variable for recent, past, or never OAT. The time-varying variable for current OAT allowed a gap of less than or equal to 7 days between subsequent OAT dispensation records before a participant was counted as being “off OAT” (Supplementary Figure 1). If a subsequent OAT dispensation record did not occur after 7 days, participants were counted as “off OAT” from the date last covered by a dispensation, based on BC OAT Prescribing Guidelines [27]. Participants continued to be counted as “off OAT” either until a new OAT dispensation occurred or end of study follow-up. This 7-day margin between dispensation records was used to account for gaps in dispensation resulting from short periods of missed doses, or receiving OAT while hospitalized or incarcerated, as medications may not be recorded in PharmaNet in those situations. The second anchored variable for OAT, classified participants as never receiving OAT at all in the data set, recently having OAT (<6 months before HCV treatment initiation, or <6 months before 30 June 2019 if untreated), or having past OAT (last dose of OAT at least >6 months before HCV treatment initiation, or 6 months before 30 June 2019 if untreated). All other covariates were considered as “ever” during the study period or anchored at the end of the study period (as specified in Table 1).
Table 1.
Comparison of Characteristics Between Recent, Past, and Non-PWID Receiving Opioid Agonist Therapy Diagnosed With Chronic Hepatitis C Virus (HCV) Infection, in the BC-HTC from 2013 to 2019
| Characteristics | Overall N = 13 803 | |
|---|---|---|
| N (%) | N | (%) |
| Male sex | 9204 | 67 |
| Birth cohort | ||
| <1945 | 110 | 1 |
| 1945–1964 | 6198 | 45 |
| 1965–1974 | 3935 | 29 |
| >1974 | 3560 | 26 |
| Ethnicity | ||
| Other | 13353 | 97 |
| East Asian | 119 | 1 |
| South Asian | 218 | 2 |
| Unknown | 113 | 1 |
| Material deprivation quintilea | ||
| Q1 (most privileged) | 1962 | 14 |
| Q2 | 1695 | 12 |
| Q3 | 2085 | 15 |
| Q4 | 2944 | 21 |
| Q5 (most deprived) | 4830 | 35 |
| Unknown | 287 | 2 |
| Social deprivation quintilea | ||
| Q1 (most privileged) | 997 | 7 |
| Q2 | 1264 | 9 |
| Q3 | 1607 | 12 |
| Q4 | 2577 | 19 |
| Q5 (most deprived) | 7071 | 51 |
| Unknown | 287 | 2 |
| Urbanicitya | ||
| Rural | 1246 | 9 |
| Urban | 12468 | 90 |
| Unknown | 89 | 1 |
| HIV/AIDS coinfectionb | 1409 | 10 |
| HBV coinfectionb | 1056 | 8 |
| Active TB coinfectionb | 118 | 1 |
| Liver cirrhosisb | 842 | 6 |
| Harmful alcohol useb | 6680 | 48 |
| Major mental illness diagnosisb | 7459 | 54 |
| Stimulant useb | 6739 | 49 |
| Opioid useb | 7144 | 52 |
| Opioid agonist therapyc | ||
| Never | 4594 | 33 |
| Recent | 6897 | 50 |
| Past | 2312 | 17 |
| Injecting drug use | ||
| Non-IDU | 2159 | 15 |
| PWID | 11644 | 85 |
| Past | 6164 | 45 |
| HCV treatment initiation | ||
| Untreated | 9321 | 68 |
| Treated with interferon-free DAA regimen | 4360 | 32 |
| Treated with interferon-containing regimen | 122 | 1 |
| Died during study follow-up (all causes) | 2164 | 16 |
Abbreviations: BC-HTC, British Columbia Hepatitis Testers Cohort; DAA, direct acting antiviral; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; PWID, people who inject drugs; Q, quintile; TB, tuberculosis.
aAnchored at end of study period.
bEver during study period.
cNever opioid agonist therapy (OAT); during study period, recent OAT; <6 months before HCV treatment initiation, or 30 June 2019 if untreated, or past OAT; 6 months before HCV treatment initiation, or 30 June 2019 if untreated.
OAT Retention Cascade Construction
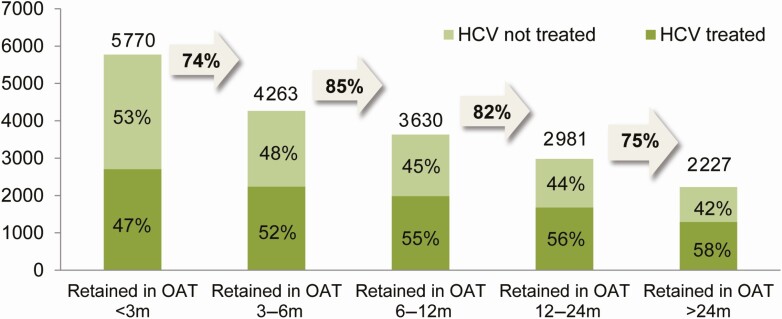
To create a cascade of retention in OAT, all those participants who had a record of OAT dispensation on or covering 30 June 2019 were identified, and their length of retention in OAT was characterized by assessing the preceding length of time they had continuously received OAT dispensations [28]. We used the current OAT study measure, then looked at how many days prior to 30 June 2019 had been covered continuously by OAT. We then created the OAT care cascade by grouping participants by the length of the preceding time they had received OAT continuously for: <3 months, 3–6 months, 6–12 months, 12–24 months, or >24 months. We assessed the proportion of people in each cascade stage who had received HCV treatment as of 30 June 2019, and the proportion of people in the previous cascade stage who were retained in the next cascade stage.
Statistical Analysis
To model the relationship of a time-varying covariate such as current OAT status on HCV treatment initiation, time to event analysis was chosen. To account for significant changes over time in the HCV treatment paradigm, the analysis period to determine the impact of OAT on HCV treatment initiation was restricted to the DAA era, which was deemed to begin on 27 October 2013, when the first DAA containing regimen was approved by Health Canada for treatment of chronic HCV infection. Participants were followed from 27 October 2013 if they were already determined to be HCV RNA positive prior to this date (including treatment naive, as well as previously treated but no SVR or reinfected). Participants who were not HCV RNA positive on 27 October 2013 were followed from the date they were confirmed HCV RNA positive (see Supplementary Table 2 for case definition). All participants were followed until HCV treatment initiation, death, or 30 June 2019, whichever came first. The analysis was also performed with an unrestricted observation period (eg, from 1 January 1996 to 30 June 2019) to determine if the DAA period-only analysis introduced any bias. Adjusted and unadjusted models were fit with current OAT as the time-dependent exposure covariate, and HCV treatment initiation as the outcome of interest using Fine-Gray subdistribution proportional hazards modelling to account for the presence of competing mortality risk. Confounding factors included in the adjusted models were, identified through a directed acyclic graph (Supplementary Figure 2). Confounding factors adjusted for in multivariable models were sex, age, ethnicity, material and social deprivation, urban versus rural location, HIV coinfection, cirrhosis, major mental health disorder, harmful alcohol use, stimulant use, and opioid use.
RESULTS
Characteristics of Study Cohort
There were 19 108 participants identified in the BC-HTC who were diagnosed with chronic HCV infection and history of either IDU or OAT, up to 30 June 2019 (Figure 1). From 27 October 2013 up to 30 June 2019 in the DAA era, there were 13 803 people identified with chronic HCV infection and history of either IDU or OAT (Table 1). Among these, 84% (11 644/13 803) were PWID, and 15% (2159/13 803) had no history of IDU but had ever received OAT (“non-IDU PWUD”). This was similar to the proportions observed in the overall cohort, with 86% (16 399/19 108) identified as PWID, and 14% (2709/19108) non-IDU PWUD (Supplementary Table 3). In the cohort followed during the DAA era, people living with HIV/AIDS or HBV coinfection made up 10% (1409/13803) and 8% (1056/13 803) of the study population, respectively (Table 1). Harmful alcohol use and major mental illness diagnosis was identified among 48% (6680/13 803) and 54% (7459/13 803) of participants and were both highest among past PWID. Among recent and past PWID, 52% (2865/5480) and 69% (4297/6164) had a history of opioid use, respectively, whereas 56% (3076/5480) and 59% (3663/6164) had a history of stimulant use.
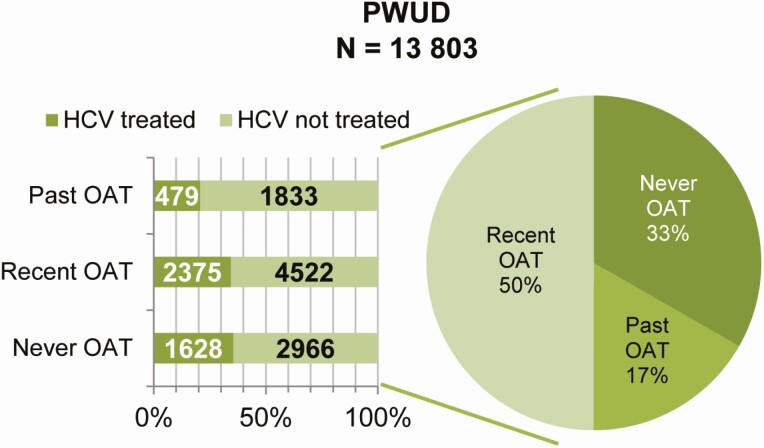
Figure 1.
OAT history and HCV treatment initiation among PWUD diagnosed with chronic HCV infection in the BC-HTC from 27 October 2013 to 30 June 2019. Abbreviations: BC-HTC, British Columbia Hepatitis Testers Cohort; HCV, hepatitis C virus; OAT, opioid agonist therapy; PWUD, people who use drugs.
Exposure to OAT and HCV Treatment Initiation Overall
Overall, 39% (7390/19 108) of participants had never received OAT (Figure 1, Supplementary Table 3). This was lower among participants followed during the DAA era, with only 33% (4594/13 803) never receiving OAT (Table 1). The majority of participants overall never started treatment for HCV (60%; 11 547/19 108). Among participants followed during the DAA era, 68% (9321/13 803) were untreated, with almost all those treated receiving an interferon-free DAA regimen (4360/4482), as opposed to an interferon-containing DAA regimen (Table 1).
OAT Retention Cascade and HCV Treatment Initiation
Overall, there were 5770 participants on OAT as of 30 June 2019 (Figure 2). Among all those currently on OAT as at this date, 47% (2704/5770) had started HCV treatment at any time up until then. As length of time retained in OAT increased, the proportion of people who had received HCV treatment increased. By the final stage of the OAT retention cascade, where those remaining had been retained continuously in OAT >24 months, 58% (1290/2227) had started HCV treatment. This contrasted with PWUD not currently on OAT at the end of the study period, with only 22% (1778/8033) having started HCV treatment The number of people in each subsequent OAT retention cascade stage decreased as the length of time continuously retained in OAT increased.
Figure 2.
OAT retention cascade for PWUD with chronic HCV as of 31 December 2019 (gray arrows are proportion of previous bar who were retained in next cascade stage) in the BC-HTC. Abbreviations: BC-HTC, British Columbia Hepatitis Testers Cohort; HCV, hepatitis C virus; OAT, opioid agonist therapy; PWUD, people who use drugs.
Association Between Current OAT and HCV Treatment Initiation
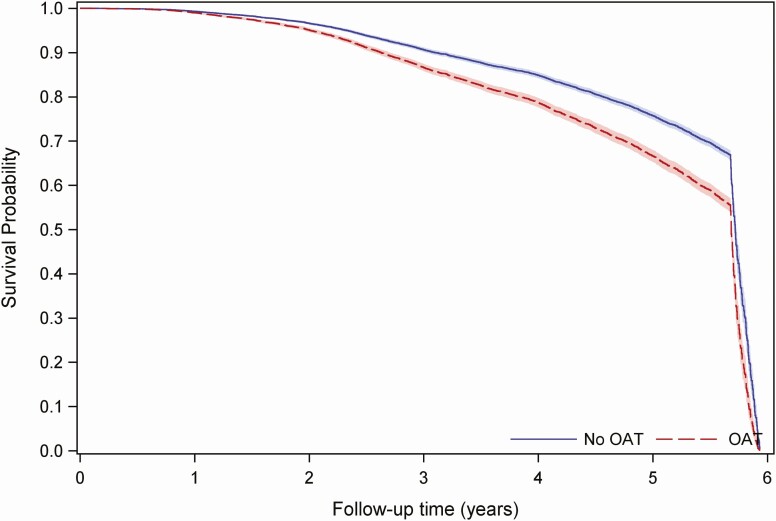
In the DAA era in univariate analysis, current OAT was significantly associated with HCV treatment initiation (hazards ratio [HR] 1.50 [95% confidence interval {CI)} 1.41, 1.59], Table 2), which remained significant in multivariate model (adjusted HR [aHR] 1.50 [95% CI 1.40, 1.61], Table 2). In the overall population, OAT was also significantly associated with HCV treatment initiation in both unadjusted and adjusted analyses (Supplementary Table 4). From 1 year post HCV RNA diagnosis onward in both the DAA era and in the overall study period, those currently on OAT have a higher probability of starting HCV treatment to those not on OAT, and the probability of HCV treatment initiation remains proportional between both groups (Figure 3, Supplementary Figure 3).
Table 2.
Hazard Ratios (HR) for Association Between Hepatitis C Virus (HCV) Treatment Initiation and Current Opioid Agonist Therapy (OAT) From Fine-Gray Subdistribution Models Among People Who Use Drugs (N = 13 803) in the BC-HTC from 2013 to 2019
| Covariates | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|
| Current OATa | ||
| No | 1.00 (Ref.) | 1.00 (Ref.) |
| Yes | 1.50 (1.41, 1.59) | 1.50 (1.40, 1.61) |
| Sex | ||
| Male | 1.00 (Ref.) | 1.00 (Ref.) |
| Female | 0.99 (.93,1.05) | 0.99 (.93, 1.07) |
| Birth cohort | ||
| >1974 | 1.00 (Ref.) | 1.00 (Ref.) |
| 1965–1974 | 0.97 (.89, 1.06) | 0.95 (.86, 1.04) |
| 1945–1964 | 1.42 (1.31, 1.53) | 1.4 (1.28, 1.53) |
| <1945 | 1.15 (.80, 1.63) | 1.25 (.84, 1.86) |
| Ethnicity | ||
| Other | 1.00 (Ref.) | 1.00 (Ref.) |
| East Asian | 1.17 (.87, 1.58) | 1.29 (.89, 1.87) |
| South Asian | 1.04 (.82, 1.33) | 0.99 (.74, 1.34) |
| Unknown | 1.13 (.82, 1.56) | 1.43 (1.01, 2.03) |
| Material deprivation quintileb | ||
| Q1 (most privileged) | 1.00 (Ref.) | 1.00 (Ref.) |
| Q2 | 0.96 (.86, 1.07) | 0.97 (.86, 1.10) |
| Q3 | 1.03 (.93, 1.15) | 1.04 (.93, 1.17) |
| Q4 | 1.03 (.93, 1.13) | 1.05 (.95, 1.17) |
| Q5 (most deprived) | 0.83 (.76, .90) | 0.88 (.79, .97) |
| Unknown | … | … |
| Social deprivation quintileb | ||
| Q1 (most privileged) | 1.00 (Ref.) | 1.00 (Ref.) |
| Q2 | 0.88 (.77, 1.02) | 0.93 (.80, 1.09) |
| Q3 | 0.94 (.82, 1.07) | 0.98 (.85, 1.13) |
| Q4 | 0.94 (.84, 1.07) | 0.93 (.81, 1.07) |
| Q5 (most deprived) | 0.85 (.76, .95) | 0.87 (.76, .98) |
| Unknown | … | … |
| Urbanicityb | ||
| Urban | 1.00 (Ref.) | 1.00 (Ref.) |
| Rural | 0.86 (.79, .95) | 0.93 (.84, 1.04) |
| Unknown | … | … |
| HIV/AIDS coinfectionc | ||
| No | 1.00 (Ref.) | 1.00 (Ref.) |
| Yes | 1.66 (1.53, 1.80) | 1.79 (1.64, 1.96) |
| Liver cirrhosisc | ||
| No | 1.00 (Ref.) | 1.00 (Ref.) |
| Yes | 1.39 (1.22, 1.58) | 1.23 (1.06, 1.41) |
| Harmful alcohol usec | ||
| No | 1.00 (Ref.) | 1.00 (Ref.) |
| Yes | 1.09 (1.03, 1.15) | 1.03 (.97, 1.11) |
| Major mental illness diagnosisc | ||
| No | 1.00 (Ref.) | 1.00 (Ref.) |
| Yes | 1.14 (1.08, 1.21) | 1.16 (1.09, 1.24) |
| Stimulant usec | ||
| No | 1.00 (Ref.) | 1.00 (Ref.) |
| Yes | 0.91 (0.85, 0.96) | 0.88 (.82, .94) |
| Opioid usec | ||
| No | 1.00 (Ref.) | 1.00 (Ref.) |
| Yes | 0.98 (.92, 1.04) | 0.89 (.83, .95) |
Abbreviations: BC-HTC, British Columbia Hepatitis Testers Cohort; CI, confidence interval; DAA, direct acting antivirals; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HR, hazard ratio; OAT, opioid agonist therapy; PWUD, people who use drugs; Q, quintile; TB, tuberculosis.
aOAT variable used in models is dynamic, time-varying categorical covariate, with OAT yes or no updated daily based on whether or not this day was covered by a dispensation record.
bAnchored at end of study period.
cEver during entire study period. Excluded due to collinearity.
Figure 3.
Predicted HCV treated (survival) proportion from Cox proportional hazards model with 95% confidence intervals for those without OAT compared to those with current OAT (time-varying covariate). Model was fit among 13 803 PWUD contributing 61 305.87 person years of follow-up time in the BC-HTC from 27 October 2013 to 30 June 2019. X axis shows time from HCV RNA positive result (or from 27 October 2013 if diagnosed prior to then) to HCV treatment initiation or death or censoring (30 June 2019) from 27 October 2013 to 30 June 2019. Y axis shows probability of not receiving HCV treatment at any time point during the observation period, since HCV RNA diagnosis or beginning of the observation period. Abbreviations: BC-HTC, British Columbia Hepatitis Testers Cohort; HCV, hepatitis C virus; OAT, opioid agonist therapy; PWUD, people who use drugs.
DISCUSSION
Our study used an innovative approach to examine the impact of engagement in OAT programs on HCV treatment initiation among PWUD. This approach includes incorporation of the temporal effects of engagement in OAT (eg, recent, past, or current OAT), as well as the length of retention in OAT. Our study shows that currently being on OAT has a clear association with HCV treatment initiation both in the DAA era and historically prior to DAAs, and these findings provide further rationale to increase integration of HCV treatment with substance use services, in an effort to intensify HCV treatment initiation.
HCV treatment initiation was associated with current OAT in the proportional hazards modelling, which may be due to multiple factors. The association may be present because current OAT has a direct causal effect on HCV treatment initiation, or because the group of people on OAT are different from the group of people off OAT, due to systematic bias. The difference between the 2 groups may be related to people currently on OAT having more regular contact with healthcare providers (HCPs), compared to those not on OAT, which would afford greater opportunity to be assessed for and offered HCV treatment. Healthcare utilization patterns were not investigated in this study. However, previous studies have found regular healthcare utilization is still common among PWUD not engaged in OAT [28]; therefore, increased contact with HCPs may not completely explain this. People currently on OAT may have greater financial and social stability due to decreased illicit drug use and increased disposable income. This could reduce competing priorities and afford more personal resources to dedicate toward health and wellbeing, such as engaging in HCV treatment. These explanations are not mutually exclusive though, and may both play a role in the association between higher HCV treatment initiation and current OAT.
Attitudes have been observed in qualitative studies among HCV and addiction treatment providers suggesting they believe PWUD not on OAT are unstable, and unable to adhere to HCV treatment, and therefore they do not offer them HCV treatment [29–32]. While this belief among treatment providers could contribute to the higher HCV treatment initiation associated with current OAT, it does not fully explain it, as we observed that even among people never on OAT, the proportional HCV treatment initiation was similar to those with recent OAT (Figure 1). Treatment gatekeeping contributes to stigma and discrimination experienced by PWUD, and may hamper efforts to achieve HCV elimination goals. At the same time, there are clearly multiple HCV treatment providers who are treating PWUD who have never been engaged in OAT, and more research on the strategies that these providers use to engage patients could help to provide better training and support to other HCV treatment providers. Patient-centered care that restores the agency of PWUD, granting them more control over decisions related to their healthcare, has been proposed as a way to improve addiction treatment and OAT [33]. This could also be considered as a potential solution to increase HCV treatment initiation for all PWUD, regardless of OAT status. Additional strategies to increase HCV treatment initiation among PWUD not engaged in OAT should also be explored, such as experiential worker (peer)-based outreach strategies, and expanding testing and treatment in prisons.
The proportion of people who received HCV treatment increased as the length of retention in OAT increased. This suggests that improved OAT retention may facilitate HCV treatment initiation and other improved health outcomes, although also highlighting that survival analysis approaches that take into account variable lengths of follow-up time are important for these investigations. PWUD who are yet to receive HCV treatment are frequently labeled “hard to reach,” yet many of them are currently receiving OAT. In this study, “hardly reached” by HCV treatment providers may be a more apt description, providing an area for healthcare systems to focus on to adapt and enhance integration between substance use care, primary care, and HCV treatment. These findings are consistent with previous studies focused on OAT retention [28] and suggests the need to continue improving access and retention on OAT. Improving access to and retention on OAT may also reduce the impact of opioid overdose, protect against HCV infection or reinfection among PWID, and improve access to and retention in the HCV care cascade.
Although we attempted to adjust for events that could hinder or accelerate the chance that HCV treatment would happen, due to the nature of administrative health data, some of the measurements obtained and used in this study may not represent the full extent of what occurs at the patient level. The introduction of DAAs in more recent years, and subsequent removal of treatment restrictions means that the likelihood of PWID and PWUD starting HCV treatment could have changed over time. Our study restricted the observation period to just the DAA era, as well as the entire observable period to account for these changes, finding little difference in the impact of OAT on HCV treatment initiation in the 2 time periods. This study included people with both opioid and stimulant use, which was determined based on diagnostic codes in administrative data, not self-reported drug use; therefore, it is likely an underreport of both stimulant and opioid drug use and is an inherent limitation in using administrative data. We assessed the impact of current OAT on HCV treatment initiation among all PWUD, which may have included some people who only use stimulant drugs, potentially reducing the observed impact of current OAT. Although people who exclusively use stimulants would not benefit from OAT, 67% of PWID in British Columbia reported concurrent opioid and stimulant drug use in the last 7 days in a recent survey of harm reduction site clients [34]. Therefore, there may still be benefit from the integration of HCV treatment with OAT for people who use stimulant drugs if they also use opioid drugs. Further characterization of stimulant use among PWUD diagnosed with HCV in British Columbia is warranted to better understand the needs of this group.
Overall, these findings suggest that increased integration of HCV treatment with OAT—in addition to lowering barriers to OAT access, increasing coverage of OAT services, and improving retention on OAT—may improve HCV treatment initiation among PWID and PWUD. These strategies could enable achievement of HCV elimination targets and reduce the impact of harms associated with opioid drug use. To maximize the effectiveness of HCV elimination strategies and interventions to increase HCV treatment initiation, PWUD must be involved in designing public health policies, particularly those to support engagement or re-engagement with harm reduction (including OAT) and HCV treatment. Codesign and open policy approaches to HCV elimination may result in more people-centered strategies, fostering greater overall wellness and achievement of health equity for PWUD.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. N. Z. J., S. W., A. Y., M. A., and M. K. participated in the data acquisition. S. R. B. and N. Z. J. conceived the analysis presented in this paper. S. R. B. and N. Z. J. designed the study. S. R. B., S. W., and A. Y. performed analyses guided by N. Z. J. S. R. B. wrote the first draft of the paper. S. R. B. incorporated revisions. All authors contributed to the interpretation of results, manuscript preparation, and revisions. All authors read and approved the final manuscript.
Acknowledgments. The authors gratefully acknowledge the residents of British Columbia whose data are integrated in the BC Hepatitis Testers Cohort, and for who this work is intended to benefit. They acknowledge and thank all of the BC Hepatitis Testers Cohort Team members. They acknowledge the assistance of British Columbia Centre for Disease Control (BCCDC), Provincial Health Services Authority Performance Measurement and Reporting, Information Analysts, Ministry of Health Data Access, Research and Stewardship, Medical Service Plan, Discharge Abstract Database, Medical Beneficiary and Pharmaceutical Services program areas, BC Ministry of Health, BC Cancer Agency, and their staff involved in data access, procurement, and data management.
Disclaimer. All inferences, opinions, and conclusions drawn in this manuscript are those of the authors and do not reflect the opinions or policies of the Data Steward(s).
Financial support. This work was supported by British Columbia Centre for Disease Control and the Canadian Institutes of Health Research (grant numbers NHC-142832, PJT-156066, and PHE-141773).
Potential conflict of interest. M. K. has received research grant funding/contracts via his institution from Roche Molecular Systems, Boehringer Ingelheim, Merck, Siemens Healthcare Diagnostics and Hologic Inc. S. R. B. has advised/consulted and spoken for Gilead Sciences and AbbVie (all personal payments given as unrestricted donations to BC Centre for Disease Control Foundation for Public Health); reports investigator-initiated grant, payment made directly to institution from Canadian Institute for Health Research, and Michael Smith Foundation for Health Research, during the conduct of the study; reports investigator-initiated grant, payment made directly to institution from Public Health Agency of Canada and Gilead Sciences Canada, outside the submitted work; reports travel support (payment made directly to institution) from Michael Smith Foundation for Health Research; reports unpaid service on advisory board from AbbVie Canada; has served as unpaid nonexecutive director for HepCBC, unpaid executive director for Grass Skirt Project Ltd, and unpaid steering for Action Hepatitis Canada committee member, outside the submitted work. J. M. has advised and spoken for Gilead Sciences and Merck (unpaid). N. Z. J. reports leadership/fiduciary roles with Canadian Hepatitis C Network and Canadian HIV Trials Network, outside the submitted work. S. N. reports serving on Gilead Ad Board, April 2019 (paid personally), outside the submitted work.
All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bartlett SR, Yu A, Chapinal N, et al. The population level care cascade for hepatitis C in British Columbia, Canada, as of 2018: impact of direct acting antivirals. Liver Int 2019. doi:10.1111/liv.14227 [DOI] [PubMed] [Google Scholar]
- 2. Janjua NZ, Islam N, Kuo M, et al. ; BC Hepatitis Testers Cohort Team . Identifying injection drug use and estimating population size of people who inject drugs using healthcare administrative datasets. Int J Drug Policy 2018; 55:31–9. [DOI] [PubMed] [Google Scholar]
- 3. Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health 2017; 5:e1192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Socías ME, Ti L, Wood E, et al. Disparities in uptake of direct-acting antiviral therapy for hepatitis C among people who inject drugs in a Canadian setting. Liver Int 2019; 39:1400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bajis S, Grebely J, Hajarizadeh B, et al. ; LiveRLife Study Group . Hepatitis C virus testing, liver disease assessment and treatment uptake among people who inject drugs pre- and post-universal access to direct-acting antiviral treatment in Australia: the LiveRLife study. J Viral Hepat 2020; 27:281–93. [DOI] [PubMed] [Google Scholar]
- 6. Krajden M, Cook DA, Wong S, et al. What is killing people with hepatitis C virus infection? Analysis of a population-based cohort in Canada. Int J Drug Policy 2019; 72:114–22. [DOI] [PubMed] [Google Scholar]
- 7. Degenhardt L, Grebely J, Stone J, et al. Global patterns of opioid use and dependence: harms to populations, interventions, and future action. Lancet 2019; 394:1560–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 2017; 357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Degenhardt L, Mathers BM, Wirtz AL, et al. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Policy 2014; 25:53–60. [DOI] [PubMed] [Google Scholar]
- 10. MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 2012; 345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nolan S, Dias Lima V, Fairbairn N, et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction 2014; 109:2053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. White B, Dore GJ, Lloyd AR, Rawlinson WD, Maher L. Opioid substitution therapy protects against hepatitis C virus acquisition in people who inject drugs: the HITS-c study. Med J Aust 2014; 201:326–9. [DOI] [PubMed] [Google Scholar]
- 13. Rossi C, Butt ZA, Wong S, et al. ; BC Hepatitis Testers Cohort Team . Hepatitis C virus reinfection after successful treatment with direct-acting antiviral therapy in a large population-based cohort. J Hepatol 2018; 69:1007–14. [DOI] [PubMed] [Google Scholar]
- 14. Islam N, Krajden M, Shoveller J, et al. ; British Columbia Hepatitis Testers Cohort (BC-HTC) team . Incidence, risk factors, and prevention of hepatitis C reinfection: a population-based cohort study. Lancet Gastroenterol Hepatol 2017; 2:200–10. [DOI] [PubMed] [Google Scholar]
- 15. Nosyk B, Min JE, Colley G, et al. The causal effect of opioid substitution treatment on HAART medication refill adherence. AIDS 2015; 29:965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Low AJ, Mburu G, Welton NJ, et al. Impact of opioid substitution therapy on antiretroviral therapy outcomes: a systematic review and meta-analysis. Clin Infect Dis 2016; 63:1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacka B, Larney S, Degenhardt L, et al. Prevalence of injecting drug use and coverage of interventions to prevent HIV and hepatitis C virus infection among people who inject drugs in Canada. Am J Public Health 2020; 110:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nosyk B, Marsh DC, Sun H, Schechter MT, Anis AH. Trends in methadone maintenance treatment participation, retention, and compliance to dosing guidelines in British Columbia, Canada: 1996–2006. J Subst Abuse Treat 2010; 39:22–31. [DOI] [PubMed] [Google Scholar]
- 19. Socias ME, Wood E, Kerr T, et al. Trends in engagement in the cascade of care for opioid use disorder, Vancouver, Canada, 2006–2016. Drug Alcohol Depend 2018; 189: 90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenthal RN, Lofwall MR, Kim S, Chen M, Beebe KL, Vocci FJ; PRO-814 Study Group . Effect of buprenorphine implants on illicit opioid use among abstinent adults with opioid dependence treated with sublingual buprenorphine: a randomized clinical trial. JAMA 2016; 316:282–90. [DOI] [PubMed] [Google Scholar]
- 21. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev 2014:CD002207. doi:10.1002/14651858.CD002207 [DOI] [PubMed] [Google Scholar]
- 22. Grebely J, Bruneau J, Lazarus JV, et al. Research priorities to achieve universal access to hepatitis C prevention, management and direct-acting antiviral treatment among people who inject drugs. Int J Drug Policy 2017. doi:10.1016/j.drugpo.2017.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bajis S, Dore GJ, Hajarizadeh B, Cunningham EB, Maher L, Grebely J. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: a systematic review. Int J Drug Policy 2017; 47:34–46. [DOI] [PubMed] [Google Scholar]
- 24. Heo M, Pericot-Valverde I, Rennert L, et al. Hepatitis C virus DAA treatment adherence patterns and SVR among people who inject drugs treated in opioid agonist therapy programs. Clin Infect Dis 2021; 73:2093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Janjua NZ, Kuo M, Chong M, et al. Assessing hepatitis C burden and treatment effectiveness through the British Columbia hepatitis testers cohort (BC-HTC): design and characteristics of linked and unlinked participants. PLoS One 2016; 11:e0150176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janjua NZ, Kuo M, Yu A, et al. The population level cascade of care for hepatitis C in British Columbia, Canada: the BC Hepatitis Testers Cohort (BC-HTC). EBioMedicine 2016; 12:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. British Columbia Centre on Substance Use and B.C. Ministry of Health. A Guideline for the Clinical Management of Opioid Use Disorder. Available at: http://www.bccsu.ca/care-guidance-publications/. Accessed 5 June 2017. [Google Scholar]
- 28. Piske M, Zhou H, Min JE, et al. The cascade of care for opioid use disorder: a retrospective study in British Columbia, Canada. Addiction 2020; 115:1482–93. [DOI] [PubMed] [Google Scholar]
- 29. Marshall AD, Grebely J, Dore GJ, Treloar C. Barriers and facilitators to engaging in hepatitis C management and DAA therapy among general practitioners and drug and alcohol specialists: the practitioner experience. Drug Alcohol Depend 2020; 206:107705. [DOI] [PubMed] [Google Scholar]
- 30. Myles A, Mugford GJ, Zhao J, Krahn M, Wang PP. Physicians’ attitudes and practice toward treating injection drug users with hepatitis C: results from a national specialist survey in Canada. Can J Gastroenterol 2011; 25: 135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan J, Young J, Cox J, Nitulescu R, Klein MB. Patterns of practice and barriers to care for hepatitis C in the direct-acting antiviral (DAA) era: a national survey of Canadian infectious diseases physicians. Can Liver J 2018; 1: 231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Litwin AH, Drolet M, Nwankwo C, et al. Perceived barriers related to testing, management and treatment of HCV infection among physicians prescribing opioid agonist therapy: the C-SCOPE study. J Viral Hepat 2019; 26:1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brothers TD, Bonn M. Patient-centred care in opioid agonist treatment could improve outcomes. CMAJ 2019; 191:E460–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karamouzian M, Papamihali K, Graham B, et al. Known fentanyl use among clients of harm reduction sites in British Columbia, Canada. Int J Drug Policy 2020; 77:102665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.