Abstract
Rationale
Indoor particulate matter is associated with worse chronic obstructive pulmonary disease (COPD) outcomes. It remains unknown whether reductions of indoor pollutants improve respiratory morbidity.
Objectives
To determine whether placement of active portable high-efficiency particulate air cleaners can improve respiratory morbidity in former smokers.
Methods
Eligible former smokers with moderate-to-severe COPD received active or sham portable high-efficiency particulate absolute air cleaners and were followed for 6 months in this blinded randomized controlled trial. The primary outcome was 6-month change in St. George’s Respiratory Questionnaire (SGRQ). Secondary outcomes were exacerbation risk, respiratory symptoms, rescue medication use, and 6-minute-walk distance (6MWD). Intention-to-treat analysis included all subjects, and per-protocol analysis included adherent participants (greater than 80% use of air cleaner).
Measurements and Main Results
A total of 116 participants were randomized, of which 84.5% completed the study. There was no statistically significant difference in total SGRQ score, but the active filter group had greater reduction in SGRQ symptom subscale (β, −7.7 [95% confidence interval (CI), −15.0 to −0.37]) and respiratory symptoms (Breathlessness, Cough, and Sputum Scale, β, −0.8 [95% CI, −1.5 to −0.1]); and lower rate of moderate exacerbations (incidence rate ratio, 0.32 [95% CI, 0.12–0.91]) and rescue medication use (incidence rate ratio, 0.54 [95% CI, 0.33–0.86]) compared with sham group (all P < 0.05). In per-protocol analysis, there was a statistically significant difference in primary outcome between the active filter versus sham group (SGRQ, β −4.76 [95% CI, −9.2 to −0.34]) and in moderate exacerbation risk, Breathlessness, Cough, and Sputum Scale, and 6MWD. Participants spending more time indoors were more likely to have treatment benefit.
Conclusions
This is the first environmental intervention study conducted among former smokers with COPD showing potential health benefits of portable high-efficiency particulate absolute air cleaners, particularly among those with greater adherence and spending a greater time indoors.
Keywords: COPD, particulate matter, environment, air filters, clinical trial
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic obstructive pulmonary disease (COPD) is a progressive disease, and beyond smoking, indoor air particulate matter and NO2 concentrations in homes of former smokers with COPD have been associated with respiratory morbidity. Portable air cleaner intervention strategies are easily implemented; however, it is unknown whether portable air cleaner use can reduce pollutants in homes of individuals with COPD and improve respiratory outcomes.
What This Study Adds to the Field
We conducted a randomized controlled trial of high-efficiency particulate air and carbon filter air cleaners among former smokers with COPD showing that use of active compared with sham air cleaners was associated with reduction in indoor particulate matter and NO2 concentrations. Though the study did not reach statistical significance for the primary outcome in intention to treat analysis, at 6 months, the active air cleaner arm had fewer respiratory symptoms, lower rescue medication use, and moderate exacerbation risk compared to the sham arm, particularly among those with greater than 80% compliance with the air cleaner, and those that spent more time in their home. Interventions that improve air quality represent a potentially novel approach to reducing respiratory morbidity in patients with COPD.
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterized by lung injury and inflammation secondary to particulate and gaseous exposures. Treatment options are limited, and the current treatments focus on control of symptoms and prevention of exacerbations using medications and avoidance of noxious exposures. Smoking cessation is associated with reduced incidence and slower progression of COPD; however, former smokers continue to suffer significant respiratory morbidity.
Although outdoor air pollution has known adverse respiratory effects (1), the indoor environment is of particular concern as most individuals with COPD spend the majority of their time indoors and indoor air particulate matter (PM) concentrations in homes of former smokers with COPD have been associated with worse respiratory symptoms, worse quality of life, and increased respiratory exacerbations (2). Unlike outdoor air, the indoor air environment may be modified at the individual level (3). Portable air-cleaner intervention strategies are practical and easily implemented by individuals at the household level, and improve respiratory symptoms in other chronic respiratory diseases, including in children with asthma (4–7); however, such intervention studies have not been conducted in COPD and it is unknown whether use of portable air cleaners in homes of individuals with COPD can reduce indoor pollutants and improve COPD outcomes. Accordingly, we conducted a randomized controlled trial of high-efficiency particulate absolute (HEPA) and carbon filter air cleaners among former smokers with COPD.
Methods
Participant Enrollment
Inclusion criteria were age ⩾40 years, former smoker (self-report and exhaled carbon monoxide ⩽ 6 ppm [8]), physician diagnosis of COPD, FEV1/FVC ⩽ 70%, FEV1 < 80% predicted (9), and ⩾10 pack-years smoking. Participants residing in homes with PM values above 10 μg/m3, measured over 2–7 days using a non–size-selective direct reading nephelometer (pDR1200s, Thermo Fisher Scientific), were included. This was based on World Health Organization (WHO) recommendations for indoor air quality of PM2.5 < 10 μg/m3 (10).
Randomization and Blinding
A randomized, controlled clinical trial was conducted with 1:1 randomization (clinicaltrials.gov #NCT02236858). Participants received either two portable air cleaners (Austin HealthMate HM400) with HEPA and carbon filters for the reduction of PM and NO2 or two sham air cleaners and were followed for 6 months. Sham air cleaners had internal HEPA and carbon filters removed, but had similar noise, airflow, and overall appearance compared with active air cleaners. Investigators, research staff performing clinical assessments, and participants were masked to treatment arm. Air cleaners were placed in the bedroom and room the participant reported spending the most time. AC current sensors with data-logging capabilities (Split-core AC current sensor model CTV-A connected to HOBO analog data logger model UX120-006M) were used to provide objective evidence of adherence and total time air purifiers were in use. Subjects were assessed before randomization and at 1 week, 3 months, and 6 months after intervention for clinical assessments, preceded by 1 week of home air monitoring. Participants provided written informed consent and the Johns Hopkins Medical Institutional Review Board approved the protocol (NA_00085617).
Outcomes
The primary outcome was health-related quality of life determined using St. George’s Respiratory Questionnaire (SGRQ) (11). Respiratory status was assessed by modified Medical Research Council Dyspnea Scale and COPD Assessment Test (CAT) (12). The Breathlessness, Cough, and Sputum Scale (BCSS) was asked daily and averaged over the 1-week monitoring period (13). Information on exacerbations was collected by monthly telephone calls: moderate exacerbations were defined as those requiring use of systemic steroids and/or antibiotics or urgent health care visit, and severe exacerbations were those requiring emergency department (ED) visit or hospitalization. Functional capacity was determined by 6-minute-walk distance (6MWD) (14).
Clinical and Exposure Assessments
Demographics, smoking history, comorbid diseases, medication use, and body mass index (BMI) were assessed. Pulmonary function was measured as FEV1 and FEV1% predicted (15) according to American Thoracic Society guidelines (16). Baseline serum was assessed for sensitization to five common indoor aeroallergens (cat, dog, cockroach, mouse, and dust mite) using ImmunoCAP (Phadia, ThermoFisher) and complete blood count with differential. Participants were asked to keep a simple time activity diary (TAD) during each week of sampling determining proportion of their time spent indoors in the home. Indoor air quality monitoring was conducted over a 1-week period, at each monitoring period, in the room the participant reported spending the most time. Measurements included particulate matter with aerodynamic diameter less than or equal to 2.5 μm (PM2.5) or 10 μm (PM10), NO2, and airborne nicotine (2). Detailed home assessment was conducted by a trained home inspector. Residential addresses were geocoded and linked to their respective area deprivation index (17), national ranking score at the census block group level, divided into quintiles (18).
Statistical Analysis
Descriptive statistics to characterize patient sample and baseline imbalance of patient characteristics were assessed using t test or chi-square test. Primary analysis was an intention-to-treat analysis including all subjects randomized. Per-protocol analysis included only adherent participants (greater than 80% use of at least one air cleaner). A priori prespecified subgroup analyses included subgroups by time spent indoors and clinical characteristics of baseline lung function (FEV1), atopic status, eosinophil count, and BMI (19, 20). For continuous outcomes, treatment group difference in change in score was assessed, using analysis of covariance, with the score change between baseline and 6 months as the dependent variable and the baseline score a covariate. For count outcomes (frequency of short-acting β-agonist use and exacerbations), negative binomial regression was used, estimating the incidence rate ratio (IRR) for treatment difference in frequency rate. For exacerbation models only, an offset for duration of follow-up included all monthly data until dropout or study completion. All analyses were adjusted for baseline characteristics with treatment imbalance and baseline prognostic factors associated with the primary outcome based on backward selection with P < 0.2 as the criteria. The final models presented in results included race, comorbidity count (21), controller medication use, season, and area deprivation index quintile as covariates. Secondary analysis explored group differences 3 months after randomization. Exploratory analysis of the association of reduction in PM and NO2 concentrations with improvements in respiratory morbidity was assessed using random-effects models, relating longitudinal changes in pollutant concentrations to health outcomes. For these random-effects models, data were used from all available visits and adjusted for covariates as above. Two-sided P values less than 0.05 were considered significant. All analyses were performed using STATA version 15.1 (Stata Corp).
Pre-specified power calculations
Based on prior observational studies (2), the trial was designed to have a sample size of 120 participants, for 80% power to detect a group difference of 4.27 in change of SGRQ score, assuming an SD of 16.1 and residual SD of 8.35, based on within person correlation of 0.855 and an α of 0.05 (two-sided).
Results
Participant Characteristics
Participants were recruited from April 2014 to January 2019. Of 375 screened participants, 207 passed clinical screen, of whom 30.9% (n = 68) had indoor PM levels below 10 μg/m3 and were not randomized. Of the remaining 126 participants, 10 participants dropped during the run-in period and 116 participants were randomized (58 into each treatment group) and included in primary intention-to-treat analysis, with 94 (81%) (51 [87.9%] in active and 43 [74.1%] in sham group) completing 6-month visits (Figure 1).
Figure 1.
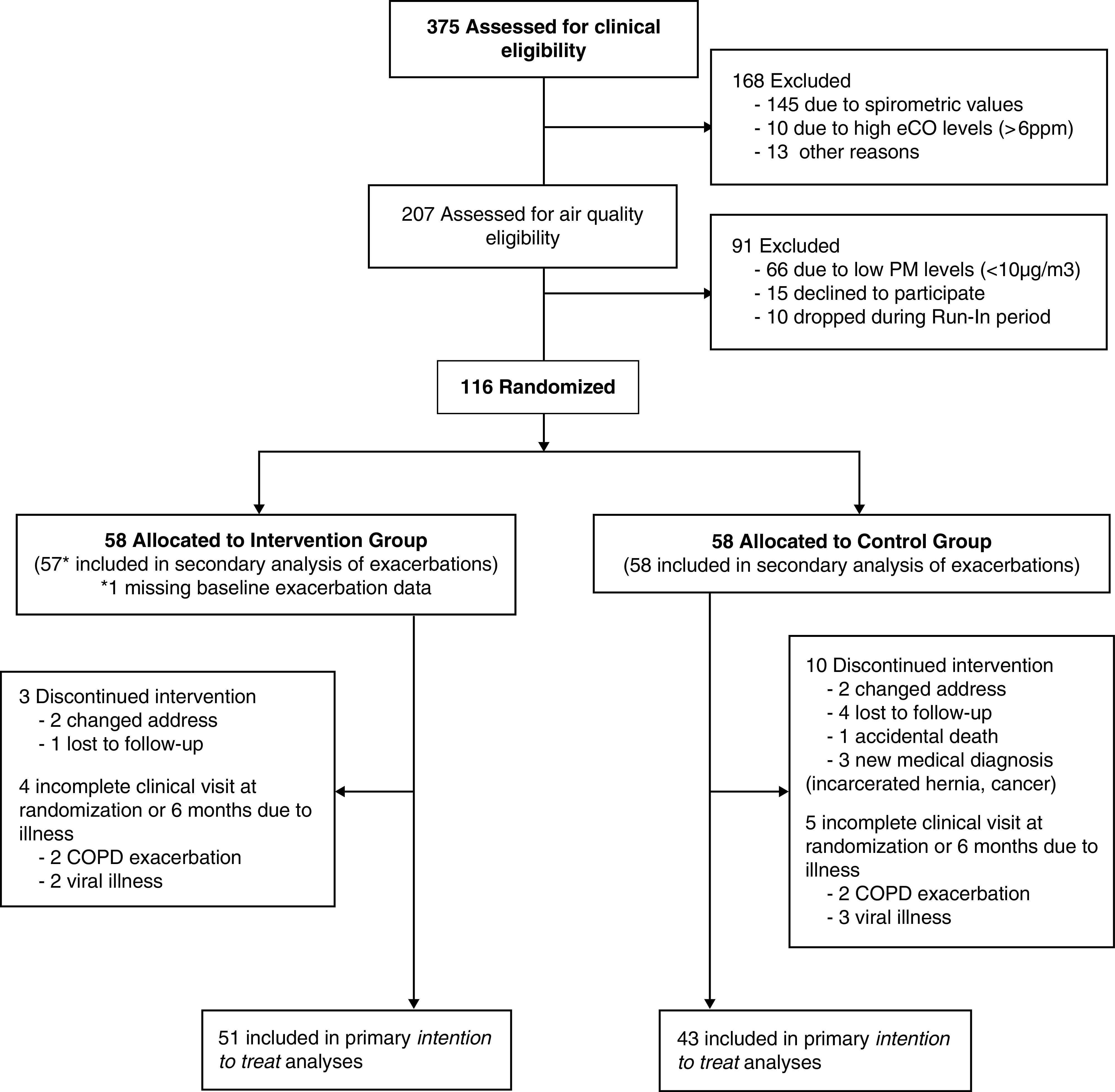
Consolidated Standards of Reporting Trials flow diagram—CLEAN AIR Study. CLEAN AIR = Clinical Trial of Air Cleaners to Improve Indoor Air Quality and COPD Health; COPD = chronic obstructive pulmonary disease; eCO = exhaled carbon monoxide; PM = particulate matter.
Of the 116 randomized participants, mean age was 65.7 years (SD, 8.3), mean pack-years smoked was 52.3 years and mean FEV1 was 53.9 (17.5) % predicted. The active filter group had a greater proportion of White participants compared with sham group; otherwise, the groups were well balanced by other demographic factors, season of recruitment, and COPD severity measures (Table 1).
Table 1.
Baseline Participant Characteristics
| All (N = 116) | Active (n = 58) | Sham (n = 58) | |
|---|---|---|---|
| Demographic | |||
| Age | 65.74 ± 8.28 | 66.62 ± 8.04 | 64.86 ± 8.50 |
| Female | 60 (51.7%) | 32 (55.2%) | 28 (48.3%) |
| Race | |||
| White | 75 (64.7%) | 43 (74.1%) | 32 (55.2%) |
| Black | 37 (31.9%) | 15 (25.9%) | 22 (37.9%) |
| Multiracial/other | 4 (3.5%) | 0 (0.0%) | 4 (6.9%) |
| Some college or above | 69 (59.5%) | 36 (62.1%) | 33 (56.9%) |
| Income | |||
| <$30,000 | 62 (53.4%) | 29 (50.0%) | 33 (56.9%) |
| ⩾$30,000 | 43 (37.1%) | 25 (43.1%) | 18 (31.0%) |
| Refuse or missing | 11 (9.5%) | 4 (6.9%) | 7 (12.1%) |
| BMI | 32.22 ± 8.52 | 31.55 ± 9.00 | 32.89 ± 8.04 |
| Pack-years | 52.30 ± 33.37 | 49.30 ± 29.30 | 55.31 ± 37.02 |
| Time since quit smoking, mo | 125 ± 113 | 116 ± 105 | 133 ± 120 |
| Comorbidity burden | 3.53 ± 2.16 | 3.79 ± 2.32 | 3.28 ± 1.98 |
| Atopic | 36 (39.6%) | 14 (31.8%) | 22 (46.8%) |
| Eosinophil count, cells/ml | 215.3 ± 166.6 | 230.6 ± 196.5 | 200.5 ± 131.9 |
| Controller medication use (ICS, LABA, or LAMA) | 93 (80.2%) | 43 (74.1%) | 50 (86.2%) |
| Any ICS | 88 (75.9%) | 40 (69.0%) | 48 (82.3%) |
| Any LABA | 81 (69.8%) | 33 (56.9%) | 48 (82.8%) |
| Any LAMA | 52 (44.8%) | 24 (41.4%) | 28 (48.3%) |
| Season of baseline visit | |||
| Winter | 24 (20.7%) | 12 (20.7%) | 12 (20.7%) |
| Spring | 29 (25.0%) | 16 (27.6%) | 13 (22.4%) |
| Summer | 28 (24.1%) | 13 (22.4%) | 15 (25.9%) |
| Fall | 35 (30.2%) | 17 (29.3%) | 18 (31.0%) |
| Living with a smoker | 30 (25.9%) | 17 (29.3%) | 13 (22.4%) |
| Neighborhood ADI | 51.22 ± 27.02 | 50.98 ± 27.02 | 51.45 ± 27.25 |
| Time spent indoors, h | 17.63 ± 4.12 | 18.13 ± 3.48 | 17.12 ± 4.66 |
| Pollutant levels | |||
| PM2.5, geometric mean ± GSD, μg/m3 | 13.02 ± 2.44 | 13.30 ± 2.79 | 12.73 ± 2.08 |
| PM10, geometric mean ± GSD, μg/m3 | 19.80 ± 2.20 | 20.22 ± 2.52 | 19.38 ± 1.86 |
| NO2, geometric mean ± GSD, ppb | 7.25 ± 2.64 | 7.41 ± 2.73 | 7.09 ± 2.57 |
| Nicotine, detectable | 30 (25.9%) | 17 (29.3%) | 13 (22.4%) |
| Clinical status | |||
| SGRQ | 44.99 ± 16.45 | 43.07 ± 16.38 | 46.91 ± 16.45 |
| CAT | 17.00 ± 7.87 | 16.32 ± 7.97 | 17.69 ± 7.78 |
| mMRC | 1.62 ± 0.93 | 1.57 ± 0.95 | 1.66 ± 0.92 |
| BCSS | 3.31 ± 2.06 | 3.12 ± 2.11 | 3.51 ± 2.00 |
| 6MWD, m (n = 75) | 236.6 ± 106.7 | 240.7 ± 102.8 | 231.5 ± 112.9 |
| Post FEV1% predicted | 54.28 ± 17.15 | 55.70 ± 17.03 | 52.87 ± 17.30 |
| Exacerbations (prior 6 mo) | |||
| Moderate | 29 (25.2%) | 16 (28.1%) | 13 (22.4%) |
| Severe | 18 (15.7%) | 9 (15.8%) | 9 (15.5%) |
Definition of abbreviations: 6MWD = 6-minute-walk distance; ADI = area deprivation index; BCSS = Breathlessness Cough and Sputum Scale (on a 5-point scale from 0 [no symptoms] to 4 [severe symptoms] rating breathlessness, cough and sputum); BMI = body mass index; CAT = COPD Assessment Test (on a scale of 0 to 40, in which higher score denotes more severe impact of COPD on patient’s life); COPD = chronic obstructive pulmonary disease; GSD = geometric standard deviation; ICS = inhaled corticosteroid; LABA = long-acting β2-agonist; LAMA = long-acting muscarinic antagonist; mMRC = modified Medical Research Council (on a categorical scale of 1 to 5; higher scores indicate more limitation on daily activities due to breathlessness); PM2.5 = particulate matter with an aerodynamic diameter ⩽2.5 μm; PM10 = particulate matter with an aerodynamic diameter ⩽10 μm; SGRQ = St. George’s Respiratory Questionnaire (on a scale of 0 to 100, in which 0 is the best quality-of-life score and 100 is the worst).
Data are shown as mean ± SD or n (%) unless otherwise indicated.
The geometric mean (geometric standard deviation) baseline pollutant concentrations were 13.02 (2.44) μg/m3 for PM2.5, 19.80 (2.20) μg/m3 for PM10, and 7.05 (2.47) ppb for NO2. Approximately a quarter (25.9%) of homes had detectable air nicotine. There were no differences in pollutant levels between treatment groups.
Intention-to-treat analysis
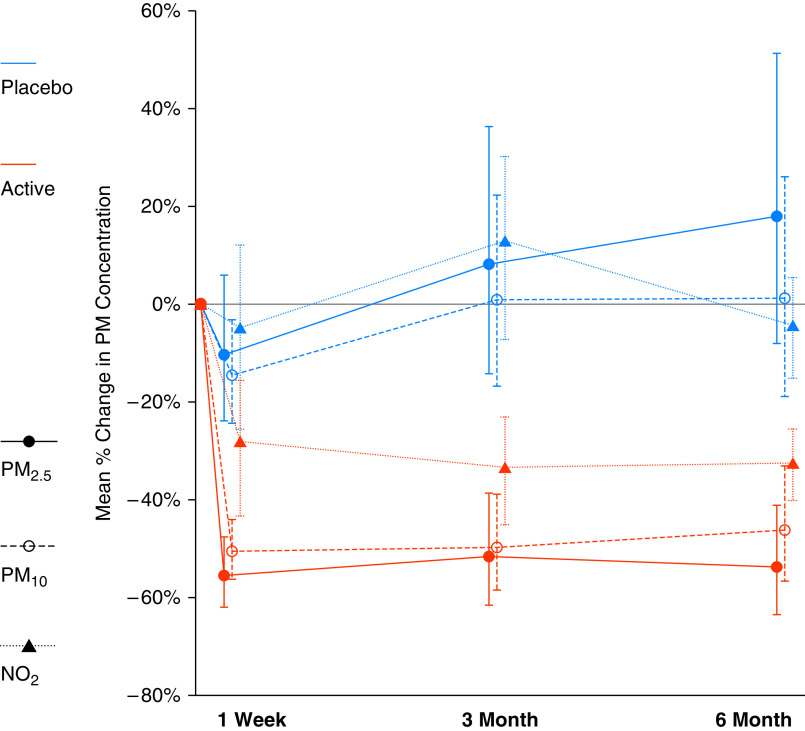
At 6 months, the active treatment group had significant reduction in PM (PM2.5: −53.5% [95% confidence interval (CI), −63.4% to −41.0%]; PM10: −46.0% [95% CI, −56.5% to −33.1%], both P < 0.001) and NO2 (−28.0% [95% CI, −40.4% to −12.9%], P = 0.001); while no change in sham group (PM2.5: 17.9% [95% CI, −8.1–51.2%], P = 0.19; PM10: 1.1% [95% CI, −18.8–26.0%], P = 0.92; NO2: −4.8% [95% CI, −21.8–16.0%], P = 0.62). This resulted in significant group difference in PM and NO2 reduction (geometric mean ratio, PM2.5: 0.39 [95% CI, 0.28–0.56], P < 0.001; PM10: 0.53 [95% CI, 0.39–0.73], P < 0.001; NO2: 0.76 [95% CI, 0.58–0.996], P = 0.046). This treatment difference in pollutant reduction was obtained within 1 week of randomization and sustained at 3 and 6 months (Figure 2).
Figure 2.
PM2.5, PM10, and NO2 reduction by treatment arm at 1 week, 3 months, and 6 months after randomization. PM2.5 = particulate matter with an aerodynamic diameter ⩽2.5 μm; PM10 = particulate matter with an aerodynamic diameter ⩽10 μm.
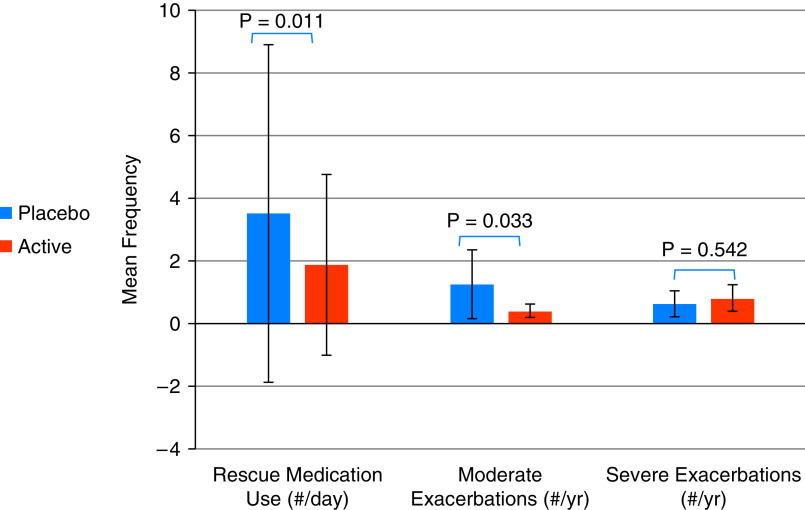
At 6 months, in adjusted analyses, there was no statistically significant difference in score change in primary outcome between treatment groups (SGRQ, −1.55 [95% CI, −5.75 to 2.65]; P = 0.465) (Table 2). However, those in the active filter arm, had significantly greater improvement in symptom subscale of the SGRQ (−7.67 [95% CI, −14.97 to −0.37]; P = 0.040) and respiratory symptoms (BCSS, −0.81 [95% CI, −1.53 to −0.09]; P = 0.029) compared with sham group. Individuals in the active filter arm also reported significantly lower rate of moderate exacerbations (IRR, 0.32 [95% CI, 0.12–0.91]; P = 0.033) and less frequent rescue medication use, in terms of mean puffs/d (IRR, 0.54 [95% CI, 0.33–0.86]; P = 0.011), compared with sham group (Figure 3). There was no significant difference in CAT, modified Medical Research Council Dyspnea Scale score, or 6MWD change though the directions of effect favored the treatment arm. In secondary analysis, there was no significant difference in outcome changes at 3 months (Table E1 in the online supplement).
Table 2.
Intention-to-Treat and Per-Protocol Analyses
| Intention-to-Treat |
Per-Protocol (>80% Air Cleaner Use) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean Change (SE) |
Group Difference [β (95% CI)] | P Value | Mean Change (SE) |
Group Difference [β (95% CI)] | P Value | |||
| Outcome | Active (n = 51) | Sham (n = 43) | Active (n = 39) | Sham (n = 31) | ||||
| SGRQ total | −0.60 (1.34) | 0.95 (1.51) | −1.55 (−5.75 to 2.65) | 0.465 | −2.78 (1.36) | 1.98 (1.58) | −4.76 (−9.17 to −0.34) | 0.035 |
| Symptom | −7.42 (2.34) | 0.25 (2.63) | −7.67 (−14.97 to −0.37) | 0.040 | −10.48 (2.59) | 1.91 (3.00) | −12.39 (−20.75 to −4.02) | 0.004 |
| Impact | −0.17 (1.54) | 0.60 (1.69) | −0.77 (−5.55 to 4.00) | 0.748 | −2.85 (1.65) | 1.23 (1.88) | −4.08 (−9.37 to 1.21) | 0.128 |
| Activity | 1.69 (1.76) | 1.73 (1.94) | −0.04 (−5.49 to 5.42) | 0.989 | 1.56 (1.94) | 1.89 (2.21) | −0.33 (−6.55 to 5.88) | 0.915 |
| mMRC | 0.11 (0.12) | 0.22 (0.13) | −0.11 (−0.48 to 0.25) | 0.549 | 0.14 (0.14) | 0.21 (0.16) | −0.08 (−0.52 to 0.37) | 0.734 |
| CAT | −1.09 (0.81) | −0.58 (0.90) | −0.51 (−3.03 to 2.00) | 0.686 | −1.61 (0.94) | −0.37 (1.07) | −1.24 (−4.24 to 1.76) | 0.413 |
| BCSS | −0.89 (0.23) | −0.09 (0.26) | −0.81 (−1.53 to −0.09) | 0.029 | −1.12 (0.23) | −0.27 (0.26) | −0.86 (−1.61 to −0.11) | 0.026 |
| 6MWD*, m | 12.6 (22.3) | −42.0 (25.5) | 54.5 (−16.8 to 125.9) | 0.130 | 25.2 (25.9) | −62.3 (31.5) | 87.5 (0.06 to 174.9) | 0.0498 |
| Intention-to-Treat |
Per-Protocol (>80% Air Cleaner Use) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Mean Count (SE) |
Group Difference [IRR (95% CI)] | P Value | Mean Count (SE) |
Group Difference [IRR (95% CI)] | P Value | |||
| Outcome | Active (n = 57) | Sham (n = 58) | Active (n = 42) | Sham (n = 40) | ||||
| Rescue inhaler use | 1.88 (1.47) | 3.51 (2.75) | 0.54 (0.33 to 0.86) | 0.011 | 1.25 (0.64) | 1.96 (0.92) | 0.63 (0.34 to 1.19) | 0.157 |
| Moderate exacerbations | 0.40 (0.11) | 1.25 (0.56) | 0.32 (0.12 to 0.91) | 0.033 | 0.43 (0.15) | 2.61 (2.62) | 0.17 (0.03 to 0.98) | 0.047 |
| Severe exacerbations | 0.81 (0.22) | 0.64 (0.21) | 1.26 (0.60 to 2.61) | 0.542 | 0.82 (0.31) | 0.69 (0.24) | 1.18 (0.44 to 3.12) | 0.743 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; BCSS = Breathlessness, Cough, and Sputum Scale (on a 5-point scale from 0 [no symptoms] to 4 [severe symptoms] rating breathlessness, cough, and sputum); CAT = COPD Assessment Test (on a scale of 0 to 40, in which higher score denotes more severe impact of COPD on patient’s life); CI = confidence interval; COPD = chronic obstructive pulmonary disease; IRR = incidence rate ratio; mMRC = modified Medical Research Council (on a categorical scale of 1 to 5; higher scores indicate more limitation on daily activities due to breathlessness); SGRQ = St. George’s Respiratory Questionnaire (on a scale of 0 to 100, in which 0 is the best quality-of-life score and 100 is the worst).
All analyses were adjusted for race, comorbidity count, controller medication use, season, and area deprivation index quintile.
6MWD was completed in 48 participants included in intention-to-treat analyses and 39 participants included in per-protocol analysis.
Figure 3.
Intention-to-treat analysis.
Per-protocol analysis
Seventy-six percent of participants used at least one air cleaner for more than 80% of the time over the 6-month period. There was no significant difference in compliance by treatment arm. Among those who had 80% adherence with air cleaners, at 6 months, there was a statistically significant difference in the improvement in primary outcome between those in the active filter versus sham group (SGRQ; −4.76 [95% CI, −9.12 to −0.34]; P = 0.035) with the largest difference in the symptom-subscale (−12.39 [95% CI, −20.75 to −4.02]; P = 0.004) in adjusted analyses. Per-protocol analysis of secondary outcomes showed significant improvement in moderate exacerbations, BCSS, and 6MWD (Table 2).
Almost one-third (32%; n = 35) of participants used at least one air cleaner for the entire 6-month period (e.g., 100% compliance). Among the subgroup with continuous air cleaner use, there were larger between-group differences in primary outcome improvement (SGRQ; −10.54 [95% CI, −17.32 to −3.74]; P = 0.005) and the symptom subscale (−26.27 [95% CI, −40.21 to −12.33]; P = 0.001) showing a dose–response in primary outcome with air cleaner compliance (Figure 4).
Figure 4.
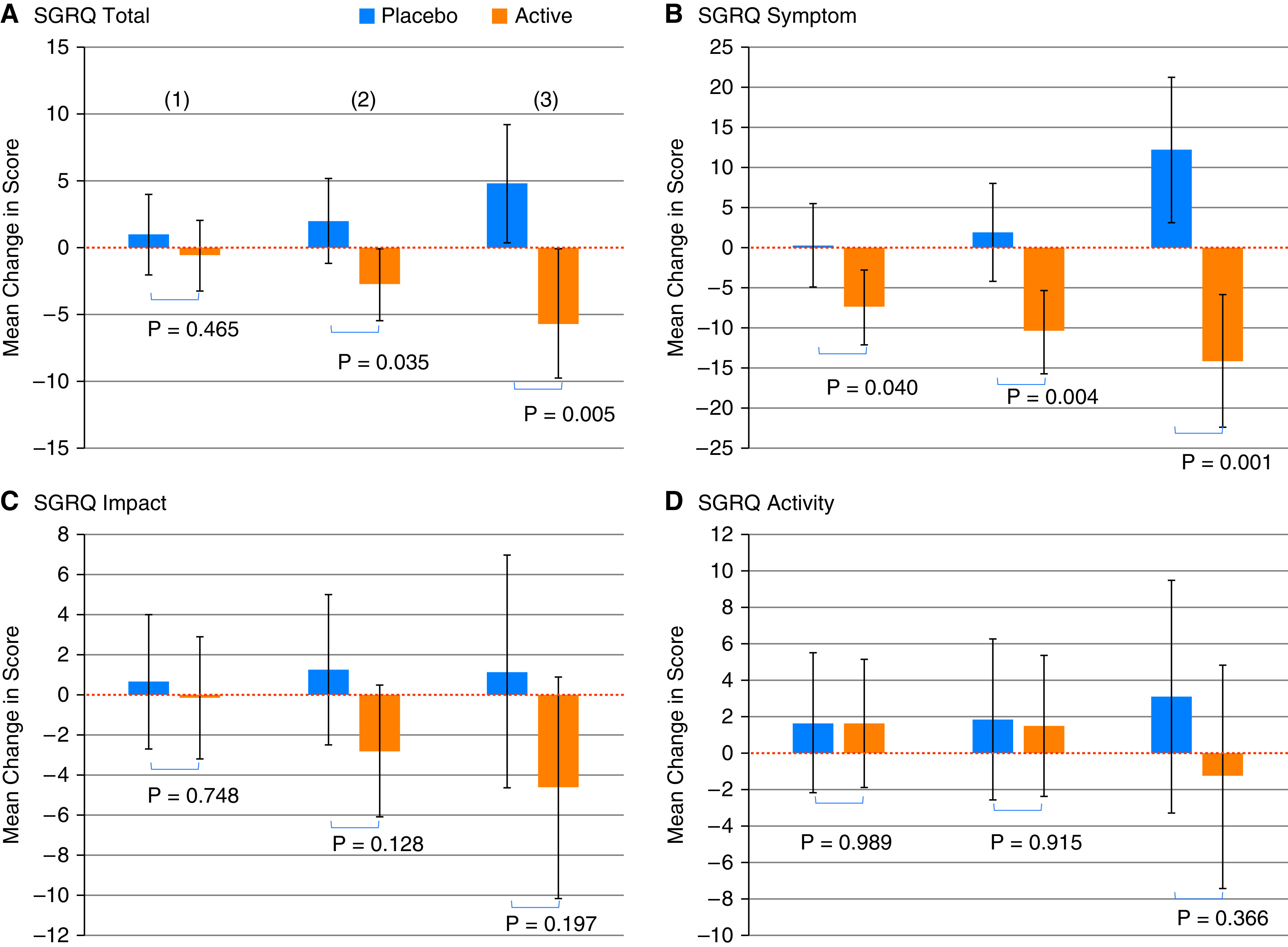
Dose–response in primary outcome by degree of air cleaner use. (A) SGRQ total. (B) SGRQ symptom. (C) SGRQ impact. (D) SGRQ activity. (1) All participants; (2) participants who used air cleaner(s) more than 80% of the time; (3) participants who used air cleaner(s) continuously throughout the study (100% of the time). SGRQ = St. George’s Respiratory Questionnaire.
Pre-specified subgroup analysis
Participants spent a median of 18.3 (interquartile range, 15.9–20.8) h/d inside their home. There was a statistically significant interaction for SGRQ, CAT, and BCSS (all Pinteraction [Pint] < 0.05), such that those spending more time indoors were more likely to have treatment benefit (Figure E1). If dichotomized above and below the median, individuals who spent more time indoors had greater statistically significant improvement in SGRQ (−6.80 [95% CI, −12.55 to −1.06]; P = 0.021), CAT (−3.92 [95% CI, −7.22 to −0.49]; P = 0.023), and BCSS (−1.86 [95% CI, −2.82 to −0.90]; P < 0.001) score in the active compared with sham treatment arm, whereas individuals who spent less time at home had no statistically significant treatment group differences. There was no statistically significant interaction for other outcomes.
There was interaction between treatment effect and FEV1 (Pint = 0.041) for the primary outcome of SGRQ, such that score improvement was greater and statistically significant among those with lower (1 SD below the mean predicted) baseline FEV1 (SGRQ; −5.62 [95% CI, P = 0.021) 11.10 to −0.13]; P = 0.045) but not among those with higher FEV1. There was also a trend for differences by treatment effect for CAT score change by FEV1 (Pint = 0.059), such that those with lower baseline FEV1 had tendency for greater treatment effect. There was no evidence that treatment effect differed in other prespecified clinical subgroups (i.e., eosinophil count, atopic status, or BMI; all Pint > 0.05).
Effects of pollution reduction on respiratory outcomes
Exploratory analyses examining the effect of reducing PM concentrations on respiratory outcomes showed that reduction in PM2.5 or PM10 was associated with statistically or nominally significant lower CAT and BCSS scores; and decreased rescue medication use and moderate or severe exacerbations. Reduction in NO2 was associated with lower BCSS score (Table E2).
Discussion
Use of air cleaners with HEPA and carbon filters in the homes of former smokers with COPD was associated with 61% greater reduction in indoor PM2.5 concentrations and a 24% reduction in NO2 concentrations at 6 months compared with homes with sham air cleaners. While the study did not meet statistical significance for the primary outcome of respiratory specific quality of life in intention-to-treat analysis, former smokers with COPD residing in homes with active air cleaners experienced clinically meaningful benefits, including significantly lower rate of moderate exacerbations, fewer respiratory symptoms, and less frequent rescue medication use and at 6 months. In addition, per-protocol analysis suggested an increasing treatment response with increasing adherence to air cleaner use; and among participants who used at least one air cleaner greater than 80% of the study period met the primary endpoint of treatment difference in SGRQ. Further, those who spent more time in their homes, and those with lower FEV1, were also more likely to benefit from air cleaner use.
To our knowledge, this is the first study to demonstrate a potential benefit of environmental interventions, beyond clean cookstove use in biomass homes showing reduced incidence of COPD (22), in improving respiratory morbidity in adults with COPD. In a small (n = 35) randomized crossover intervention trial among nonsmoking senior participants, of whom only 20 had COPD, a 2-week deployment of portable air filtration units was associated with reduction in systemic IL-8 concentrations but no change in lung function (23). Though our study did not meet statistical significance for the primary outcome of respiratory specific quality of life in intention-to-treat analysis, former smokers with COPD residing in homes with active air cleaners experienced significantly greater improvement in respiratory symptoms, as measured by greater reduction in symptom subscale score of the SGRQ and a 0.9 greater reduction in total BCSS score, supporting a clinically meaningful and substantial symptom difference between groups, given the MCID of BCSS is as low as 0.3 (13). Further, the impact on respiratory symptoms is supported by a concomitant lower rate of moderate exacerbations and lower frequency of rescue medication use in the active filter group compared with the sham group, though there was no difference in severe exacerbations. This reflects an annualized moderate exacerbation rate of 0.40 in the active filter group compared with 1.24 in the sham group; a difference an exacerbation rate comparable or greater than seen in large scale clinical trials (24–26). Differences in respiratory outcomes between treatment groups were noted at 6 but not 3 months. This is in line with several environmental studies conducted in children with asthma, showing reduction in asthma symptoms only 6 or 9 months after HEPA filter placement, suggesting that several months of pollutant reduction may be required to achieve health benefit (6, 27). Overall, taken together these study results suggest that the placement of two portable air cleaners in the home of former smokers with COPD has the potential to have a moderate impact on respiratory symptoms and exacerbation risk.
Our study assessed air purifier adherence with an electronic sensor technology that provides objective evidence of air purifier use throughout the duration of the study, with approximately three-quarters of participants using at least one air cleaner greater than 80% of the time. In per-protocol analysis, increased adherence to air cleaner use was associated with larger difference in clinical outcomes between groups. Among those that were 80% compliant with air cleaner use, the estimated difference in SGRQ score gain of 4.9 between groups is larger than the minimally clinical important difference (MCID) for SGRQ (28) and larger than group difference seen in several large scale clinical therapeutic studies of COPD. For instance, mean between-group difference in SGRQ total score was 2.7 favoring tiotropium compared with placebo in the UPLIFT (Understanding the Potential Long-Term Impacts on Function with Tiotropium) trial (24). Similarly, there was a mean difference of 3.1 in SGRQ score with salmeterol/fluticasone combination therapy compared with placebo in patients with COPD from the TORCH (Towards a Revolution in COPD Health) study (25) and there was a mean difference of 1.8 between triple therapy (inhaled corticosteroid/long-acting β2-agonist/long-acting muscarinic antagonist) and dual therapy combinations (inhaled corticosteroid/long-acting β2-agonist or long-acting β2-agonist/long-acting muscarinic antagonist) in the IMPACT (Informing the Pathway of COPD Treatment) trial (26). These therapies are currently recommended as first line therapy for COPD based on the Global Initiative for Chronic Obstructive Lung Disease report because they are deemed to have a clinically meaningful impact on disease outcomes. Accordingly, findings from the present study suggest an environmental intervention has the potential to have a similarly significant and clinically meaningful impact on COPD but without potential side effects of medications for the treatment of disease.
In addition, though the participants spent most of their time indoors, they still spent some time outdoors or other indoor locations which might have reduced the protective effects from indoor air filtration. In a priori, secondary analyses, study results suggest that the effectiveness of air cleaner use is greater among individuals who spend more time indoors. In particular, among those who spent more time indoors, those in the active filter arm had substantial greater reduction in SGRQ, CAT, and BCSS scores and less rescue medication use, with effect sizes that reflect moderate to substantial treatment effects. Thus together, these results suggest that individuals who spend more time indoors, and who have lower FEV1 and who use the air cleaners greater than 80% of the time are most likely to benefit from portable air cleaner placement.
Our study has a number of limitations. The trial was designed to have a sample size of 120 participants; however, only 94 participants completed the study and were included in primary intention-to-treat analyses of outcomes measured at 6 months. Accordingly, reduced sample size limited our ability to detect statistical significance in our primary outcome; nonetheless, several trends were observed in the expected direction for multiple secondary outcomes, and the dose dependent increase in treatment effect in the primary outcome in per-protocol analysis supports the hypothesis that improvement in home air quality in non-biomass and largely nonsmoking homes may lead to improved respiratory health in patients with COPD. Further, almost one-third (30.9%) of screened participants had indoor PM levels below 10 μg/m3 and were not randomized and though differences in pollutant concentrations at low levels are thought to be associated with respiratory morbidity (2), the benefit of home air quality improvement in homes with lower pollutant burden is unclear. In our study, relative reductions in PM2.5 concentrations were similar to those of other studies of inner-city homes of children with asthma (5, 6). In addition, the air cleaners used included a charcoal filter (29), in addition to the HEPA filter, which are capable of removing gaseous species (e.g., NO2). To our knowledge, this is the first study showing the ability of charcoal fitted air cleaners to reduce in-home NO2 concentrations in a real-world setting, which has implications for future environmental studies targeting NO2 reduction. Relative reduction in PM concentrations was greater than the relative reduction in NO2 concentrations however, it remains unclear whether improvement in respiratory morbidity may be more attributable to the PM rather than NO2 reduction. Future principal stratification analyses may further estimate the extent to which changes in the indoor air pollution concentration explain the observed health effects of the environmental intervention (30, 31). Adherence to air cleaner use was moderate, similar to other intervention trials in asthma (5, 6); thus, strategies to understand facilitators and barriers to air cleaner use are needed to further maximize use of air cleaners and enhance effectiveness of future interventions. The use of two air cleaners was chosen based on previous environmental studies of asthma conducted in the Baltimore–Washington area (5, 6) which showed reduction in indoor PM and improvement in respiratory outcomes. Ambient pollutants may penetrate indoors; therefore, indoor filtration approaches may reduce ambient exposures, including in areas that are affected by wildfires (32), and thus, quantifying the health benefits of use during such episodic events is of interest. Future, larger studies that have geographic diversity and are adaptive to variable home characteristics are needed. Further, the 6-month follow-up does not allow long-term evaluation of reduction in indoor PM concentrations or the sustainability of air cleaner use. Lastly, the current study included only former smokers; thus, the study results do not address whether an air cleaner intervention can improve COPD outcomes among those who continue to smoke. To our knowledge, there are no environmental intervention studies targeting indoor air quality to improve respiratory health of smokers; however, several studies suggest that adverse environmental effects are not obscured by active smoking (18–20, 33). Given the evidence suggesting that smokers with COPD are also susceptible to indoor air pollution (34), future studies addressing whether indoor pollutant reduction strategies among smokers with COPD may be a potential target for harm reduction even among those who are unable to quit smoking is warranted.
Conclusions
Although the study did not reach statistical significance for the primary outcome in intention-to-treat analysis, portable HEPA air cleaner use improved several respiratory outcomes, particularly among those with greater than 80% compliance with the air cleaner, and those that spent a larger portion of their time in their home. Interventions that improve air quality represent a potentially novel approach to reducing respiratory morbidity in patients with COPD and persistent respiratory symptoms and exacerbation risk despite smoking cessation. Further, given that environmental exposures contribute to a large proportion of COPD burden worldwide (35), the study may have broad implications. A larger study may be needed to confirm health benefit across a larger geographic area, and future efforts should be focused on improving adherence to HEPA filtration to maximize benefit.
Acknowledgments
Acknowledgment
The authors thank Austin Air Cleaners for the donation of air cleaners used in this trial. The company did not have any input on study design, analysis, or manuscript preparation.
Footnotes
The Clinical Trial of Air Cleaners to Improve Indoor Air Quality and COPD Health (CLEAN AIR) was supported by National Institute of Environmental Health Sciences grant R01ES022607. A.F. was supported by NHLBI grant T32HL007534. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Author Contributions: N.N.H. wrote the original draft of the manuscript with input from all coauthors. H.W. contributed to the analysis, interpretation of data, and manuscript writing. R.P. contributed to data analysis and manuscript writing. G.B.D. and P.N.B. made substantial contributions to the conception or design and implantation of the work and contributed to revising the manuscript providing critically important intellectual content. A.F. curated data and revised and approved of the final version of the manuscript. R.A.W. participated in drafting and reviewing the manuscript. K.R. contributed to data cleaning and quality control, oversaw clinical trial, and drafted some sections of the manuscript. M.F.D. assisted with study design and data interpretation, and edited and reviewed the manuscript. A.M.R. managed the exposure assessment team, collected data, conducted data analysis and quality control, and reviewed the manuscript. M.N.E. contributed to the data analysis plan, interpretation of the data, and editing and revising the manuscript. N.P. and M.C.M. contributed to the design, conduct, interpretation of results, and writing of the manuscript. K.K. collected data and reviewed the manuscript. N.N.H., N.P., H.W., K.R., A.F., A.M.R., and K.K. all verified the data in this manuscript. All authors had full access to the full data in the study and accept responsibility to submit for publication. The corresponding author had final responsibility for the decision to submit for publication.
Data Sharing Statement: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) beginning 9 months and ending 5 years after article publication, will be available to researchers who provide a methodologically sound proposal. Study protocol and statistical analysis plan will also be available upon request. Proposals should be directed to nhansel@jhmi.edu. To gain access, data requestors will need to sign a data access agreement.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202103-0604OC on August 27, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Hansel NN, McCormack MC, Kim V. The effects of air pollution and temperature on COPD. COPD . 2016;13:372–379. doi: 10.3109/15412555.2015.1089846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hansel NN, McCormack MC, Belli AJ, Matsui EC, Peng RD, Aloe C, et al. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2013;187:1085–1090. doi: 10.1164/rccm.201211-1987OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, et al. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol . 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 4. Cui X, Li Z, Teng Y, Barkjohn KK, Norris CL, Fang L, et al. Association between bedroom particulate matter filtration and changes in airway pathophysiology in children with asthma. JAMA Pediatr . 2020;174:533–542. doi: 10.1001/jamapediatrics.2020.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butz AM, Matsui EC, Breysse P, Curtin-Brosnan J, Eggleston P, Diette G, et al. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch Pediatr Adolesc Med . 2011;165:741–748. doi: 10.1001/archpediatrics.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eggleston PA, Butz A, Rand C, Curtin-Brosnan J, Kanchanaraksa S, Swartz L, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. Ann Allergy Asthma Immunol . 2005;95:518–524. doi: 10.1016/S1081-1206(10)61012-5. [DOI] [PubMed] [Google Scholar]
- 7. James C, Bernstein DI, Cox J, Ryan P, Wolfe C, Jandarov R, et al. HEPA filtration improves asthma control in children exposed to traffic-related airborne particles. Indoor Air . 2020;30:235–243. doi: 10.1111/ina.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest . 2000;117:758–763. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- 9.GOLD. Guide to COPD diagnosis, management, and prevention: a guide for healthcare professionals. 2020 [Google Scholar]
- 10.World Health Organization. WHO guidelines for indoor air quality: selected pollutants. 2010 [PubMed] [Google Scholar]
- 11. Barr JT, Schumacher GE, Freeman S, LeMoine M, Bakst AW, Jones PW. American translation, modification, and validation of the St. George’s Respiratory Questionnaire. Clin Ther . 2000;22:1121–1145. doi: 10.1016/S0149-2918(00)80089-2. [DOI] [PubMed] [Google Scholar]
- 12. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J . 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 13. Leidy NK, Rennard SI, Schmier J, Jones MK, Goldman M. The breathlessness, cough, and sputum scale: the development of empirically based guidelines for interpretation. Chest . 2003;124:2182–2191. doi: 10.1378/chest.124.6.2182. [DOI] [PubMed] [Google Scholar]
- 14. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med . 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 15. Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis . 1981;123:659–664. doi: 10.1164/arrd.1981.123.6.659. [DOI] [PubMed] [Google Scholar]
- 16. American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med . 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 17. Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Ann Intern Med . 2014;161:765–774. doi: 10.7326/M13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galiatsatos P, Woo H, Paulin LM, Kind A, Putcha N, Gassett AJ, et al. The association between neighborhood socioeconomic disadvantage and chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis . 2020;15:981–993. doi: 10.2147/COPD.S238933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaji DA, Belli AJ, McCormack MC, Matsui EC, Williams DL, Paulin L, et al. Indoor pollutant exposure is associated with heightened respiratory symptoms in atopic compared to non-atopic individuals with COPD. BMC Pulm Med . 2014;14:147. doi: 10.1186/1471-2466-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCormack MC, Paulin LM, Gummerson CE, Peng RD, Diette GB, Hansel NN. Colder temperature is associated with increased COPD morbidity. Eur Respir J . 2017;49:1601501. doi: 10.1183/13993003.01501-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Putcha N, Puhan MA, Drummond MB, Han MK, Regan EA, Hanania NA, et al. A simplified score to quantify comorbidity in COPD. PLoS One . 2014;9:e114438. doi: 10.1371/journal.pone.0114438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou Y, Zou Y, Li X, Chen S, Zhao Z, He F, et al. Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: a 9-year prospective cohort study. PLoS Med . 2014;11:e1001621. doi: 10.1371/journal.pmed.1001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shao D, Du Y, Liu S, Brunekreef B, Meliefste K, Zhao Q, et al. Cardiorespiratory responses of air filtration: A randomized crossover intervention trial in seniors living in Beijing: Beijing Indoor Air Purifier StudY, BIAPSY. Sci Total Environ . 2017;603-604:541–549. doi: 10.1016/j.scitotenv.2017.06.095. [DOI] [PubMed] [Google Scholar]
- 24. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. UPLIFT Study Investigators A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med . 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 25. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. TORCH investigators Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med . 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 26. Lipson DA, Barnhart F, Brealey N, Brooks J, Criner GJ, Day NC, et al. IMPACT Investigators Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med . 2018;378:1671–1680. doi: 10.1056/NEJMoa1713901. [DOI] [PubMed] [Google Scholar]
- 27. Lanphear BP, Hornung RW, Khoury J, Yolton K, Lierl M, Kalkbrenner A. Effects of HEPA air cleaners on unscheduled asthma visits and asthma symptoms for children exposed to secondhand tobacco smoke. Pediatrics . 2011;127:93–101. doi: 10.1542/peds.2009-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J . 2002;19:398–404. doi: 10.1183/09031936.02.00063702. [DOI] [PubMed] [Google Scholar]
- 29. Rubel AM, Stewart ML, Stencel JM. Activated carbon for control of nitrogen oxide emissions. J Mater Res . 1995;10:562–567. [Google Scholar]
- 30. Peng RD, Butz AM, Hackstadt AJ, Williams DL, Diette GB, Breysse PN, et al. Estimating the health benefit of reducing indoor air pollution in a randomized environmental intervention. J R Stat Soc Ser A Stat Soc . 2015;178:425–443. doi: 10.1111/rssa.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hackstadt AJ, Matsui EC, Williams DL, Diette GB, Breysse PN, Butz AM, et al. Inference for environmental intervention studies using principal stratification. Stat Med . 2014;33:4919–4933. doi: 10.1002/sim.6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stauffer DA, Autenrieth DA, Hart JF, Capoccia S. Control of wildfire-sourced PM2.5 in an office setting using a commercially available portable air cleaner. J Occup Environ Hyg . 2020;17:109–120. doi: 10.1080/15459624.2020.1722314. [DOI] [PubMed] [Google Scholar]
- 33. Paulin LM, Gassett AJ, Alexis NE, Kirwa K, Kanner RE, Peters S, et al. Association of long-term ambient ozone exposure with respiratory morbidity in smokers. JAMA Intern Med . 2020;180:106–115. doi: 10.1001/jamainternmed.2019.5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Osman LM, Douglas JG, Garden C, Reglitz K, Lyon J, Gordon S, et al. Indoor air quality in homes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2007;176:465–472. doi: 10.1164/rccm.200605-589OC. [DOI] [PubMed] [Google Scholar]
- 35. Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet . 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]