Abstract
Rationale
Environmental exposures have been associated with adverse outcomes in chronic obstructive pulmonary disease (COPD). Approximately one-third of individuals with COPD have allergic sensitization, but it is unknown whether exposure to allergens in the home is associated with outcomes.
Objectives
To determine the prevalence and associations of allergen sensitization with exposure to common indoor allergens with symptoms and exacerbation risk in COPD.
Methods
Allergen sensitization to five common indoor allergens was assessed in former smokers with COPD. Home settled dust was assessed for presence of corresponding allergens. Sensitization and exposure status was determined and associations evaluated in adjusted models with longitudinal outcomes including symptoms, lung function, and exacerbations. Interactions were assessed between sensitization/exposure status and lung function.
Measurements and Main Results
One hundred eighty-three individuals studied were on average 67.3 years of age (SD, 8.22) with average FEV1 of 53.2% (SD, 17.6%). Seventy-seven percent of participants were exposed to at least one tested allergen, and 17% had sensitization with corresponding allergen exposure. After adjustment, sensitization with exposure was associated with lower lung function (β, −8.29; 95% confidence interval [CI], −14.80 to −1.77), higher St. George’s Respiratory Questionnaire Total Score (β, 6.71; 95% CI, 0.17 to 13.25), and higher exacerbation risk (odds ratio, 2.31; 95% CI, 1.11 to 4.79). Associations appeared to be more pronounced among individuals with lower lung function.
Conclusions
Allergen exposures are common in COPD and associated with adverse outcomes among those with concomitant allergen sensitization. This study establishes allergens as an important home exposure that potentially could be addressed with comprehensive home environmental modification strategies to improve COPD outcomes.
Keywords: COPD, allergen sensitization, allergen exposure
At a Glance Commentary
Scientific Knowledge on the Subject
In-home exposures such as particulate matter air pollution as well as nitrogen dioxide have been shown to be associated with adverse outcomes in chronic obstructive pulmonary disease (COPD). Although allergen sensitization is prevalent in individuals with COPD, little is known about the implication of allergen exposures among sensitized individuals with COPD.
What This Study Adds to the Field
This study demonstrates that home dust allergen exposure among those with corresponding sensitization is associated with adverse COPD outcomes including exacerbation events, and these associations are more pronounced among individuals with lower lung function. These findings highlight another home-based exposure that may contribute to COPD morbidity and have potential implications for future strategies for home environmental modifications that have the goal of improving outcomes in COPD.
Recent studies of chronic obstructive pulmonary disease (COPD) have explored subtypes of disease including asthmatic features, eosinophilia, and concomitant allergic disease (1–5). The overarching goal of such studies is to identify subgroups of individuals with COPD more likely to respond to existing treatments or to identify novel targets for treatment. One important example of the utility of such studies is the demonstration of efficacy of inhaled corticosteroids for individuals with COPD having eosinophilia as well as frequent exacerbations (6, 7).
Allergic sensitization has been studied extensively among individuals with asthma and is recognized to be an important phenotypic characteristic to help guide disease management (8). In addition, it is recognized that allergen exposure among sensitized individuals with asthma is linked with higher disease morbidity, and therefore, environmental modification strategies have been studied at length (9–14). Previous studies have demonstrated that nearly one-third or more of individuals with COPD have concomitant allergic sensitization (5, 15–17). Despite this, the clinical significance of allergic sensitization in COPD remains unclear. Some studies demonstrated that individuals with allergic sensitization and COPD have higher risk for adverse outcomes (5, 16). However, a recently published study of individuals in the COPDGene (Genetic Epidemiology of COPD Study) and SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) cohorts demonstrated that allergic sensitization was not associated with risk for exacerbations and adverse outcomes in COPD, but instead possibly was associated with preserved lung function (15).
It is likely that the home environment is an important determinant of respiratory outcomes among individuals with COPD (18). Environmental pollutant exposures (19–21), particularly indoor particulate matter exposures (22), have been associated with adverse outcomes in COPD, particularly among those with allergic sensitization (18). However, to date, the association of allergen exposures with outcomes in COPD is not clearly known. This study sought to understand the prevalence of allergic sensitization and exposure and associated outcomes among a well-characterized population of former smokers with COPD. We hypothesized that among individuals with allergic sensitization, exposure to corresponding allergens would be associated with exacerbation risk and symptom burden.
Methods
Former smokers with moderate to severe COPD according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometry class (23) were recruited for three environmental studies (22) in Baltimore, Maryland, with similar clinical and environmental assessments and inclusion criteria (age ⩾40 yr, >10 pack-year smoking, post-bronchodilator spirometry showing FEV1/FVC <0.7 and FEV1 <80% predicted, and residing in the same home for study duration). Two studies were observational, and participants had a baseline visit and two additional visits at 3 and 6 months. The third study included air cleaner intervention; however, only data before randomization were used (two visits, 3 months apart). Written, informed consent was obtained from participants.
Assessments
Baseline blood was analyzed for sensitization to five common indoor aeroallergens (cat, dog, cockroach, mouse, and dust mite) using ImmunoCAP (Phadia, ThermoFisher) and complete blood count with differential (see the Methods section in the online supplement). Within 2 weeks, home visits were conducted to collect settled dust from the main living area with a standardized protocol (24, 25) using the Duststream collector (Indoor Biotechnologies). Dust was assessed for allergens by extraction of protein and quantification using ELISA for Fel d 1, Can f 1, Mus m 1, Bla g 1, and Der f 1 (26). For dust mite, Der f 1 was measured given evidence from previous studies in childhood asthma that demonstrated that Der f 1 was slightly higher among homes of participants in Baltimore compared with Der p 1 (27).
Spirometry was performed with the American Thoracic Society protocol (28) (Koko spirometer; nSpire). Standard questionnaires included the modified Medical Research Council Questionnaire (mMRC [29]), St. George’s Respiratory Questionnaire (SGRQ; higher scores indicate more morbidity [30]), American Thoracic Society-Division of Lung Disease questionnaire (multiitem questionnaire about exposures, respiratory-related health, and family history and symptoms [31]), and assessment of exacerbation events at baseline and prospectively over follow-up. Exacerbations were defined as events leading to a change in medications or an unscheduled visit to a physician, emergency department, or hospital in the last 3 months assessed at each visit. Severe exacerbations included events leading to an emergency department visit or hospitalization only.
Statistical Analysis
Sensitization status was determined as positive for a threshold of ⩾0.1 kU/L for each allergen. Positive sensitization to dust mite was determined by positive sensitization to either Dermatophagoides farinae or D. pteronyssinus. Exposure to allergen from settled dust was defined using cutoffs from previous studies (1 U/g for cockroach, 1 μg/g for mouse, 2 μg/g for dust mite [Der f 1], and 8 μg/g for cat and dog) (24). The primary trait of interest was combined sensitization and exposure to any allergen tested (dichotomous), similar to strategies of categorizing exposure and sensitization status used in studies of asthma (12, 24).
Participant characteristics were analyzed using t tests and chi-square tests. To assess the association between COPD outcomes and sensitization/exposure, linear and logistic regressions of repeated outcomes on baseline sensitization/exposure were run, using generalized estimating equations with exchangeable correlation structure. Mean differences in continuous outcomes and odds ratios (ORs) for exacerbation outcomes were estimated between those with sensitization and exposure to any allergens and comparison group. Models were analyzed unadjusted and then adjusted for age, sex, race (African American vs. others), educational attainment (high school or less vs. more than high school), pack-years, and baseline season. Secondary analyses were conducted including baseline blood eosinophil count and reported history of doctor-diagnosed asthma before age 12 (separately), and separately for sensitization alone without exposure, and sensitization and exposure to individual subgroups of allergens, including pet (dog and/or cat), cockroach, and dust mite. Given differences in FEV1% predicted between sensitized and exposed individuals and the comparison group, the possibility of mediation of adverse outcome measures by lung function was explored. Mediation analysis was performed to estimate the total, direct, and mediated (indirect) effect estimates between any sensitization/exposure and COPD outcomes, with FEV1% predicted as the mediator. The mediation analysis was performed in the traditional framework of Baron and Kenny (32), using the difference-method, with point estimates and 95% confidence intervals (CIs) of mediated (indirect) effect being obtained using the recommended nonparametric bootstrap approach (33) (see Methods section in the online supplement).
Effect modification by severity of disease was explored by modeling two-way interaction between sensitization/exposure status and continuous baseline FEV1% predicted. Significant interactions were followed with stratified analysis by GOLD spirometry category (8). Analyses were conducted with StataMP, version 15.1. Statistical significance was defined as P < 0.05 for main effects and for interaction terms.
Results
Participant Characteristics
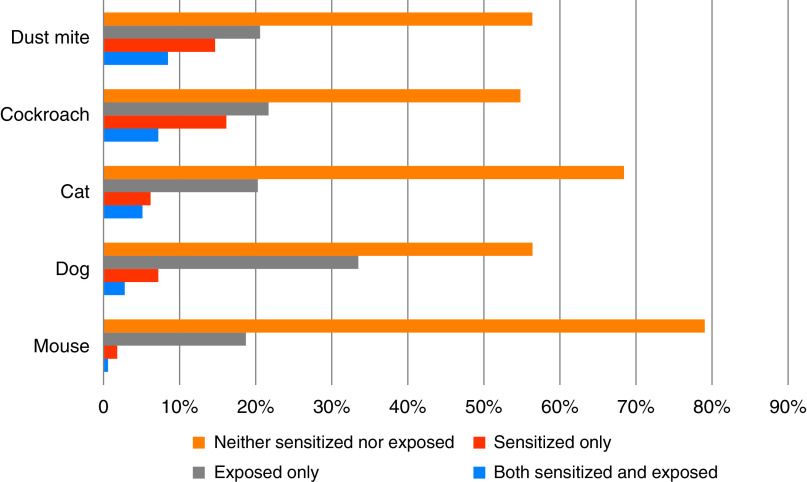
In the final sample, 183 former smokers were included. Average pack-years smoked was 54.7 (SD, 31.9), 44% were female, and 21% were African American, with an average FEV1% predicted of 53.2% (SD, 17.6%). One-third of individuals (33%) were sensitized to at least one of the allergens tested, with 22% sensitized to dust mite, 21% to cockroach, 11% to cat, 9.8% to dog, and 2% to mouse (see Table E1 and Figure E1 in the online supplement). Seventy-seven percent of participants had at least one allergen exposure, with 36% exposed to dog, 29% to roach, 29% to dust mite, 25% to cat, and 19% to mouse allergen (Figure E2).
Thirty-one individuals (17%) had sensitization with simultaneous exposure to at least one of the tested allergens. The most common sensitization/exposure combination was for dust mite (14 individuals, or 8.5% of sample), and the least common was for mouse (1 individual, or 0.6% of the sample) (Figure 1). Participant characteristics were compared between individuals sensitized and exposed to any allergen (n = 31) compared with those without any combined sensitization and exposure (n = 152; Table 1). A higher proportion of sensitized and exposed individuals were African American (39% vs. 18%; P = 0.009). Sensitized and exposed individuals had higher total IgE (mean 492.9 kU/L compared with 91.3 kU/L; P < 0.001), lower FEV1% predicted (mean 46.9% predicted vs. 54.4%; P = 0.033), and a nonsignificant trend toward higher SGRQ (49.5 vs. 42.9; P = 0.063) compared with the group without combined sensitization and exposure. Other characteristics were comparable (i.e., age, educational attainment, and burden of smoking history) between groups. Racial differences in sensitized and exposed status were likely driven by differences in sensitization, with 31.7% of sensitized participants being African American (compared with 16.3% of nonsensitized participants being African American; P = 0.017).
Figure 1.
Groups of neither sensitized nor exposed, exposed only, sensitized only, and both sensitized and exposed by allergen.
Table 1.
Baseline Cohort Characteristics
| Sensitized and Exposed to Any Allergen | No Sensitization/Exposure Combinations | P Value | |
|---|---|---|---|
| n | 31 | 152 | |
| Age, yr | 65.8 (9.4) | 67.6 (7.9) | 0.3 |
| Female, n (%) | 16 (51.6) | 64 (42.1) | 0.3 |
| African American, n (%) | 12 (38.7) | 27 (17.8) | 0.009 |
| More than high school degree, n (%) | 25 (80.6) | 131 (86.2) | 0.4 |
| Pack-years smoked | 56.7 (39.1) | 54.3 (30.4) | 0.7 |
| Eosinophil count, cells/μl | 215.9 (127.7) | 210.4 (160.5) | 0.9 |
| Total IgE, kU/L | 492.9 (699.5) | 91.3 (248.6) | <0.001 |
| Season of dust assessment, n (%) | |||
| Winter | 10 (32.3) | 36 (23.7) | 0.3 |
| Spring | 4 (12.9) | 38 (25.0) | 0.1 |
| Summer | 10 (32.3) | 30 (19.7) | 0.1 |
| Fall | 7 (22.6) | 48 (31.6) | 0.3 |
| FEV1% predicted | 46.9 (18.5) | 54.4 (17.2) | 0.03 |
| SGRQ* | 49.5 (20.23) | 42.9 (17.5) | 0.06 |
| mMRC | 2.2 (1.2) | 2.1 (1.1) | 0.5 |
| Moderate or severe exacerbations in the last 3 mo, n (%) | 7 (24.1) | 27 (17.8) | 0.4 |
Definition of abbreviations: mMRC = modified Medical Research Council dyspnea score; SGRQ = St. George’s Respiratory Questionnaire.
Data are shown as mean (SD) unless otherwise indicated. Bold values are statistically significant.
Higher scores indicate higher morbidity.
Association of Sensitization and Exposure with COPD Outcomes
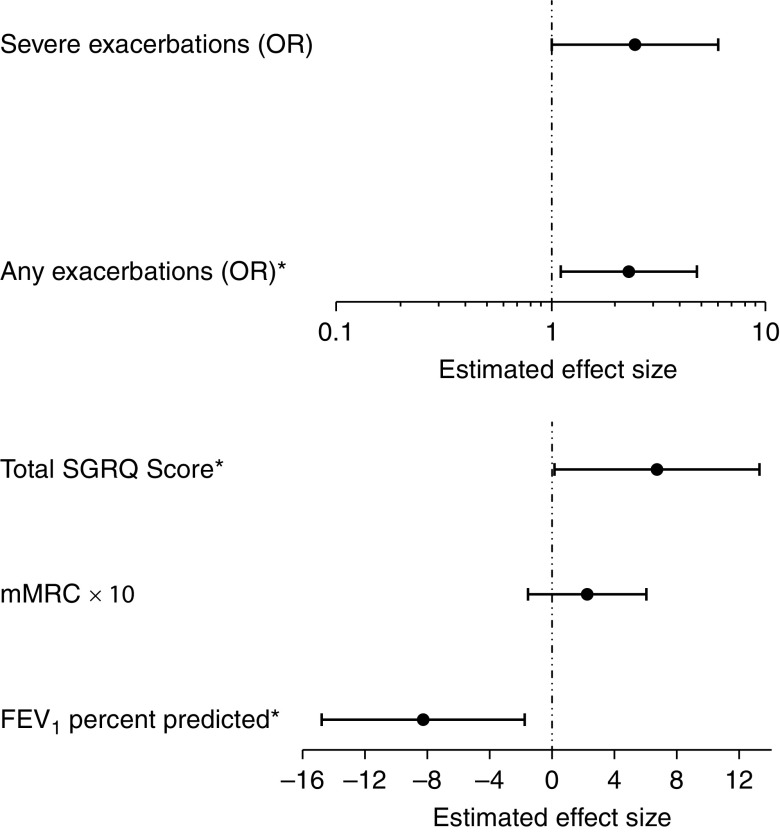
After adjusting for covariates, sensitization and exposure to any of the allergens tested continued to be significantly associated with lower lung function, with sensitization and exposure being associated with 8.3% lower FEV1% predicted (95% CI, 1.8–14.8%). Sensitization and exposure was also associated with significantly worse respiratory-specific quality of life as measured by higher SGRQ total score (β, 6.71; 95% CI, 0.17–13.25) compared with other individuals with COPD. In addition, individuals with sensitization and exposure to any allergen had higher risk for exacerbation events than individuals without sensitization and exposure (Table 2 and Figure 2), with more than twofold higher risk for any exacerbation reported (OR, 2.31; 95% CI, 1.11–4.79), with similar trend with increased risk of severe exacerbations reported, although this association did not meet statistical significance (OR, 2.44; 95% CI, 0.99–6.03). Sensitization and exposure was not significantly associated with dyspnea, as measured by mMRC.
Table 2.
Associations of Sensitization and Exposure to Any of the Tested Allergens with Outcomes, Adjusted (Models 1–3)
| Main Model (Model 1) |
Model 2: Model 1 Adjustments + Baseline Eosinophil Count |
Model 3: Model 1 Adjustments + Childhood Asthma Diagnosis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coeff | 95% CI | P Value | Coeff | 95% CI | P Value | Coeff | 95% CI | P Value | |
| SGRQ total score* | 6.71 | 0.17 to 13.25 | 0.044 | 7.44 | 0.72 to 14.15 | 0.030 | 6.19 | −0.25 to 12.64 | 0.060 |
| mMRC | 0.22 | −0.16 to 0.60 | 0.266 | 0.24 | −0.15 to 0.64 | 0.226 | 0.19 | −0.21 to 0.58 | 0.358 |
| FEV1% predicted | −8.29 | −14.80 to −1.77 | 0.013 | −9.64 | −15.90 to −3.37 | 0.003 | −8.95 | −15.57 to −2.32 | 0.008 |
| Severe exacerbations (OR) | 2.44 | 0.99 to 6.03 | 0.054 | 2.57 | 1.05 to 6.27 | 0.038 | 2.52 | 0.98 to 6.49 | 0.055 |
| Moderate or severe exacerbations (OR) | 2.31 | 1.11 to 4.79 | 0.025 | 2.36 | 1.15 to 4.88 | 0.020 | 2.27 | 1.08 to 4.76 | 0.030 |
Definition of abbreviations: CI = confidence interval; Coeff = coefficient; mMRC = modified Medical Research Council dyspnea score; OR = odds ratio; SGRQ = St. George’s Respiratory Questionnaire.
Model 1 adjusted for age, sex, race (African American vs. other), educational attainment (more than high school education vs. others), pack-years smoked, and season of baseline assessment. Model 2 includes model 1 adjustments as well as baseline eosinophil count. Model 3 includes model 1 adjustments as well as report of childhood asthma diagnosis. Bold values are statistically significant.
Higher scores indicate higher morbidity.
Figure 2.
Associations of sensitization/exposure status with adverse outcomes in chronic obstructive pulmonary disease. *Statistically significant association (P < 0.05). mMRC = modified Medical Research Council dyspnea score; OR = odds ratio; SGRQ = St. George’s Respiratory Questionnaire.
Additional adjustments for eosinophil level and reported doctor diagnosis of childhood asthma (in separate models) did not attenuate results (Table 2). Sensitization alone, compared with those without sensitization to any allergen regardless of exposure status, showed trends for association with adverse outcomes as previously described (5), but no associations with outcomes were statistically significant (Table E2), as opposed to results that take exposure into consideration as well. Sensitivity analyses with additional adjustment for use of inhaled corticosteroids to the main models (models 1–3) were minimally different from main results, but overall observations were similar (Table E3).
When examining specific allergen subgroups (Table 3), sensitization with exposure to pets (cat and/or dog) was associated with higher SGRQ score (β, 10.33; 95% CI, 1.66 to 18.99) and lower FEV1% predicted (β, −11.08; 95% CI, −18.72 to −3.44). Sensitization and exposure to cockroach was associated with a higher SGRQ score (β, 10.99; 95% CI, 1.97 to 20.02) and stronger association with any (OR, 5.04; 95% CI, 1.45 to 17.51) or severe exacerbations (OR, 11.53; 95% CI, 2.73 to 48.73). Dust mite sensitization with exposure was not associated with outcomes (Table 3), and low prevalence of mouse sensitization/exposure (n = 1) precluded analysis of this subgroup.
Table 3.
Adjusted Associations of Sensitization and Exposure to Subgroups of Allergens (Pet, Pest, and Dust Mite) with Outcomes
| Pet Sensitization and Exposure (Cat and/or Dog, n = 13) |
Cockroach Sensitization and Exposure (n = 9) |
Dust Mite Sensitization and Exposure (n = 14) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coeff | 95% CI | P Value | Coeff | 95% CI | P Value | Coeff | 95% CI | P Value | |
| SGRQ total score* | 10.33 | 1.66 to 18.99 | 0.019 | 10.99 | 1.97 to 20.02 | 0.017 | 1.18 | −8.60 to 10.97 | 0.813 |
| mMRC | 0.31 | −0.25 to 0.88 | 0.275 | 0.70 | −0.04 to 1.44 | 0.065 | −0.16 | −0.60 to 0.28 | 0.466 |
| FEV1% predicted | −11.08 | −18.72 to −3.44 | 0.004 | −14.45 | −27.99 to −0.91 | 0.036 | 0.09 | −10.84 to 11.02 | 0.987 |
| Severe exacerbations (OR) | 1.54 | 0.48 to 4.96 | 0.472 | 11.53 | 2.73 to 48.73 | 0.001 | 0.82 | 0.23 to 2.95 | 0.763 |
| Moderate or severe exacerbations (OR) | 2.43 | 0.85 to 6.98 | 0.099 | 5.04 | 1.45 to 17.51 | 0.011 | 0.65 | 0.28 to 1.50 | 0.310 |
For definition of abbreviations, see Table 2.
Models adjusted for age, sex, race (African American vs. other), educational attainment (more than high school education vs. others), pack-years smoked, and season of baseline assessment. Mouse was not included because of low prevalence of sensitization and exposure. Bold values are statistically significant.
Higher scores indicate higher morbidity.
Given the sensitized/exposed group had significantly lower FEV1, possible mediation of effects of sensitization/exposure on COPD outcomes through lung function was explored (Table E4) and demonstrated significant mediated effects with attenuation of direct effects for SGRQ and severe and moderate/severe exacerbations.
Effect Modification by Disease Severity
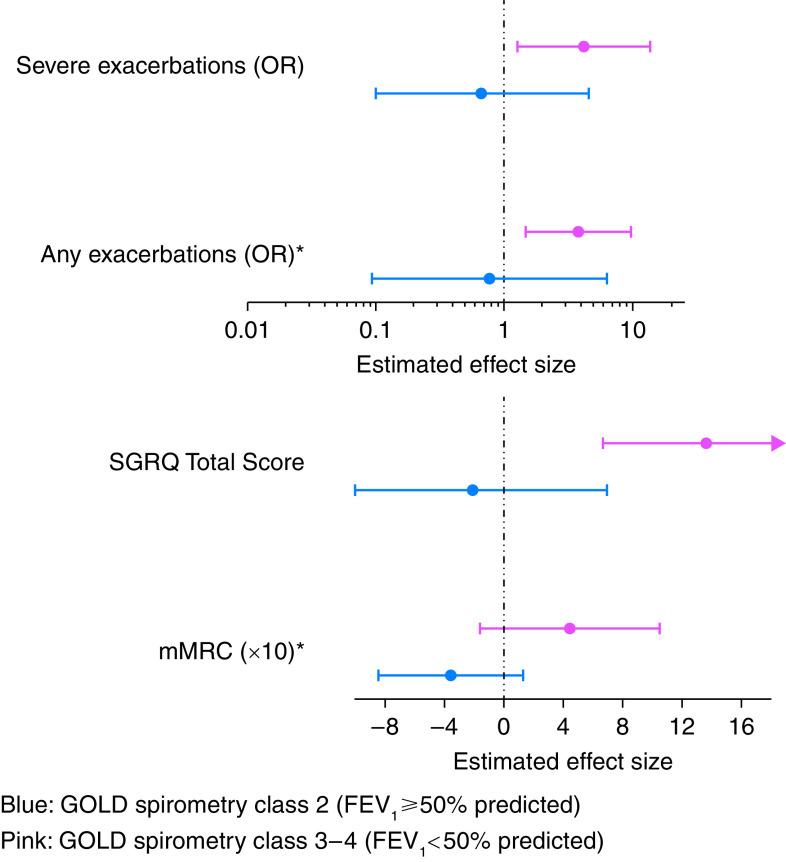
Interaction analyses were constructed to determine whether adjusted associations between sensitization and exposure differed based on FEV1% predicted. Interactions were significant for mMRC and prospectively reported exacerbations such that individuals with lower FEV1% predicted had stronger and more significant associations between sensitization and exposure to allergens with specified symptoms and exacerbations. For example, individuals with GOLD spirometry class 3–4 disease (FEV1% predicted <50% predicted) had substantially higher association of sensitization and exposure to allergens with risk for any exacerbation (OR, 3.77; 95% CI, 1.45–9.77) than those with GOLD spirometry class 2 disease (FEV1 ⩾50% predicted). Although the interaction term for mMRC was significant, subgroup effects for GOLD spirometry class 3–4 disease compared with GOLD spirometry class 2 did not meet the threshold for statistical significance (Figure 3).
Figure 3.
Associations of sensitization/exposure status with adverse outcomes in chronic obstructive pulmonary disease; subgroup analysis based on GOLD spirometry classes. *Statistically significant interaction (P < 0.05). GOLD spirometry class 2 includes individuals with FEV1 ⩾50% predicted. GOLD spirometry class 3–4 includes individuals with FEV1 <50% predicted. GOLD = Global Initiative for Chronic Obstructive Lung Disease; mMRC = modified Medical Research Council dyspnea score; OR = odds ratio; SGRQ = St. George’s Respiratory Questionnaire.
Discussion
This analysis of former smokers with COPD demonstrates the critical importance of the indoor environment for respiratory morbidity among individuals with COPD. First, allergen exposures, which have not been studied to any large extent in COPD, are common in this population. Next, and most importantly, the combination of sensitization and exposure to indoor allergens is associated with risk for adverse outcomes including increased symptoms, worse disease status, and higher risk for exacerbation events. Finally, although these effects appear to be independent of eosinophil level and asthma diagnosis, they were more pronounced among individuals with worse lung function. Overall, these results are suggestive that sensitization and exposure to common indoor allergens may represent a novel treatable trait in COPD (34).
Allergen sensitization with exposure has been well studied in asthma, particularly in the pediatric population, with prior studies conducted for more than a decade showing strong associations between allergen sensitization with exposure and heightened symptoms and healthcare utilization in asthma (10, 11, 35). In the COPD population, environmental exposures—including indoor fine particulate matter exposure, secondhand smoke, and ambient pollutant exposures—have been associated with disease morbidity (19, 20, 22, 36). However, other than one study of 10 individuals with COPD (16), there have not been larger studies assessing the importance of indoor allergen exposure.
One study demonstrated exposure to indoor fine particulate matter, which may carry allergens (37), was associated with worse respiratory morbidity among individuals with allergic sensitization (18). Understanding the intersection of allergic sensitization with environmental exposures is important given that it is estimated that between one-fourth and one-third of individuals with COPD have allergic sensitization (5, 15, 17), consistent with findings from this group of former smokers with COPD showing 34% sensitized to any allergen. In addition, there is increasing recognition and study of allergic and eosinophilic pathways in COPD in recent years (1, 7), with the inclusion of blood eosinophils as a biomarker of interest when determining appropriate treatments in individuals with COPD having exacerbations (8).
Sensitization and exposure to allergens was associated with adverse outcomes including increased exacerbation risk as well as worse respiratory-specific quality of life in this population of former smokers with COPD. Furthermore, the differences in morbidity estimates based on sensitization-and-exposure status were substantial, with those with both sensitization and exposure having a 6.7-point increase in SGRQ score, which is well above the minimal clinically important difference for SGRQ (38). Furthermore, those with sensitization and exposure had more than twice the risk for future exacerbations requiring hospitalization (OR, 2.44) compared with those without sensitization and exposure. In addition, these results are independent of eosinophil level, which has already been identified as an important clinical marker used to guide pharmacologic treatment in individuals with frequent exacerbations (8), as well as history of overlapping asthma diagnosis, which also signifies a high-risk subgroup (4, 39). Furthermore, previous studies in COPD populations have been conflicting in demonstrating the association of allergic sensitization alone with respiratory morbidity (15). Analysis of this cohort demonstrated lack of significant association of allergen sensitization alone with adverse outcomes but highlights the importance of the added value of considering environmental exposures to better understand risk. Although only 17% of the cohort ultimately had exposure/sensitization combinations, there are likely implications for treatment strategies including allergen avoidance and environmental modifications in this susceptible subgroup to help reduce risk for exacerbations and poor outcomes. COPD has also been recognized as a contributor to hospital admissions and readmissions and accordingly is associated with a tremendous public health burden (40, 41). Accordingly, the presence of targets for potential strategies to reduce healthcare utilization in COPD is of great interest. Interestingly, there was no significant difference in blood eosinophils between the group with sensitization with exposure and comparison group, although total IgE was significantly higher in the sensitized/exposed group. These findings, which are seemingly contradictory, are consistent with a previous analysis of COPDGene in which eosinophils were not significantly different between COPD groups with concomitant atopy or asthma, whereas IgE levels were significantly higher in the atopic groups (15). These findings support others that suggest that serum total IgE and blood eosinophils incompletely overlap and may represent differing pathways of inflammation in asthma and COPD.
An important finding of this study is the common nature of dust allergen exposures among individuals with COPD. When combined with allergen sensitization, the most common sensitization/exposure combinations were dust mite and cockroach; however, some individuals also had cat and dog sensitization with corresponding allergen exposure. This study represents one of the first studies to assess the role of allergen exposure in COPD. One small study of 10 individuals in Singapore assessed allergen exposures in COPD and demonstrated that allergens were associated with adverse COPD outcomes. Although that study did not correlate exposures with sensitization to corresponding allergens (16), it does further support the current study results in demonstrating the importance of allergen exposures in determining respiratory outcomes in COPD. Notably, in our U.S.-based study, both pet (cat and dog) and cockroach sensitization/exposure appeared to be associated separately with symptom outcomes, whereas cockroach sensitization/exposure was associated with significantly higher exacerbation risk (OR, 11.53; P < 0.001). These findings argue for the importance of cockroach sensitization/exposures in determining exacerbation outcomes in COPD, consistent with previous studies of asthma that demonstrate cockroach sensitization with exposure to be associated with significant asthma morbidity, including healthcare utilization (12, 42), in both childhood and early life.
Another key finding of this work is the potential for effect modification, such that individuals with COPD who had lower lung function appear to have higher susceptibility to the negative impacts of sensitization and exposure to common indoor allergens. It is possible that among individuals who have lower lung function due to ongoing inflammation including allergic inflammation, the threshold for susceptibility to exacerbations in the face of the added insult of allergen exposures may be lower. In addition, it is possible there are other unmeasured factors or exposures that covary with low lung function in COPD and are synergistic with allergen exposure to lead to adverse outcomes. It is also interesting that the group with sensitization and exposure to corresponding allergens appeared to have lower baseline lung function, a finding that may be suggestive of the lasting impact of the chronicity of the exposures. In addition, the results of mediation analysis were suggestive that the adverse impacts of sensitization and exposure may lead to worsened lung function, which in turn could lead to risk for adverse outcomes including symptoms and exacerbation risk. Previous studies in children and adults with asthma have demonstrated that allergic sensitization, allergen exposures, or the combination of allergic sensitization with exposure are associated with lower lung function and spirometric measures in asthma (43–46). Although comparable studies about sensitization with exposure in COPD do not exist, the body of evidence in asthma is supportive of the conceptual model of lung function mediating the association between allergen sensitization and exposure with exacerbations and symptom outcomes in COPD.
This analysis is subject to limitations. First, this is a single-site study, and accordingly, observations about environmental exposures may not be fully generalizable to different geographic locations where allergen prevalence and climatic factors may differ. Next, this analysis was conducted in former smokers only. The impacts of allergen exposures with sensitization among current smokers with COPD may differ. In addition, the results for association of sensitization and exposure with outcomes were attenuated with adjustment for lung function, which was hypothesized to be due to lung function mediating the observed associations. However, an alternate explanation would be potential confounding, and it is a limitation that the current study is not able to establish causality for the current hypothesized pathway for observed associations. Finally, although this represents the largest study of allergen exposure in individuals with COPD, the study sample was still too small to definitively determine individual sensitization/exposure combinations that are associated with outcomes in COPD, and outdoor allergens were not specifically analyzed in this study. However, the study results suggest that pet and cockroach allergens may be of particular importance. Although mouse allergen exposure was present in 20% of the cohort, sensitization was uncommon, with only one individual having both sensitization and exposure to mouse allergen, and this prevented determination of whether mouse sensitization and exposure may contribute to the risk of morbidity. Previous work in childhood asthma studies (35), including those in the same study area (Baltimore) (24, 47), has suggested mouse allergen sensitization and exposure to be a significant contributor to adverse asthma outcomes. Furthermore, dust mite sensitization/exposure was not separately associated with risk for symptoms or outcomes, contrary to results in asthma (48). Previous studies have suggested significant variation in dust mite exposures based on geographic location, climate, and humidity (49). Accordingly, future studies with more geographic variability may be able to further elucidate potential associations between dust mite and respiratory outcomes in COPD. Ultimately, larger studies are required to help better guide future environmental modification strategies in individuals with COPD.
To our knowledge, this is the first study demonstrating that individuals with COPD having allergic sensitization with exposure to common indoor allergens have higher risk for exacerbations and worse respiratory-specific qualify of life than individuals without such sensitization and exposure. Furthermore, these large, clinically significant associations appear to be independent of eosinophil count or asthma diagnosis and are more pronounced in individuals with lower lung function. This study highlights the potential value of environmental modification strategies (50) that have the potential to reduce morbidity and healthcare utilization in patients with COPD and allergic sensitization. Further studies are still required to better understand the specific allergens that, when targeted and mitigated, have the highest potential to improve outcomes. Finally, allergen sensitization with exposure could be studied as a novel treatable trait (34) that would guide selection of existing treatments including inhaled corticosteroids or biologics such as anti-IgE therapy, both of which at present are used less commonly in COPD.
Acknowledgments
Acknowledgment
The authors thank the Bridging Research, Lung Health and the Environment (BREATHE) center staff and investigators from Johns Hopkins University Schools of Medicine and Public Health as well as the participants of the studies that were included in the manuscript.
Footnotes
Supported by NHLBI grant K23HL123594 (N.P.); National Institute of Environmental Health Sciences grants R21ES015781 (N.N.H.), R01ES022607 (N.N.H.), R01ES023447 (E.C.M.), R01ES026170 (E.C.M.), and R21ES025840 (M.C.M.); National Institute on Minority Health and Health Disparities grant P50MD010431 (N.N.H.); Environmental Protection Agency grant EPA83615001 (N.N.H.); and National Institute of Allergy and Infectious Diseases grant K24AI114769 (E.C.M.).
Author Contributions: N.P. and N.N.H. contributed to conception, design, and acquisition, analysis, and interpretation of data. A.F., K.R., and K.K. contributed to conception and acquisition, analysis, and interpretation of data. M.C.M., G.B.D., M.F.D., R.A.W., and E.C.M. contributed to conception, design, and analysis and interpretation of data. H.W. contributed to conception and analysis and interpretation of data. All authors contributed to drafting and revising the work for intellectual content and approved the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202103-0583OC on November 9, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Christenson SA, Steiling K, van den Berge M, Hijazi K, Hiemstra PS, Postma DS, et al. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2015;191:758–766. doi: 10.1164/rccm.201408-1458OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med . 2015;373:1241–1249. doi: 10.1056/NEJMra1411863. [DOI] [PubMed] [Google Scholar]
- 3. Lange P, Çolak Y, Ingebrigtsen TS, Vestbo J, Marott JL. Long-term prognosis of asthma, chronic obstructive pulmonary disease, and asthma-chronic obstructive pulmonary disease overlap in the Copenhagen City Heart study: a prospective population-based analysis. Lancet Respir Med . 2016;4:454–462. doi: 10.1016/S2213-2600(16)00098-9. [DOI] [PubMed] [Google Scholar]
- 4. Hardin M, Cho M, McDonald ML, Beaty T, Ramsdell J, Bhatt S, et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J . 2014;44:341–350. doi: 10.1183/09031936.00216013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jamieson DB, Matsui EC, Belli A, McCormack MC, Peng E, Pierre-Louis S, et al. Effects of allergic phenotype on respiratory symptoms and exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2013;188:187–192. doi: 10.1164/rccm.201211-2103OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: a population-based cohort study. Lancet Respir Med . 2018;6:855–862. doi: 10.1016/S2213-2600(18)30368-0. [DOI] [PubMed] [Google Scholar]
- 7. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med . 2015;3:435–442. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 8.Global Initiative for Chronic Obstructive Lung Disease. 2019.
- 9. Matsui EC, Perzanowski M, Peng RD, Wise RA, Balcer-Whaley S, Newman M, et al. Effect of an integrated pest management intervention on asthma symptoms among mouse-sensitized children and adolescents with asthma: a randomized clinical trial. JAMA . 2017;317:1027–1036. doi: 10.1001/jama.2016.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grant T, Aloe C, Perzanowski M, Phipatanakul W, Bollinger ME, Miller R, et al. Mouse sensitization and exposure are associated with asthma severity in urban children. J Allergy Clin Immunol Pract . 2017;5:1008–1014.e1. doi: 10.1016/j.jaip.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gold DR, Adamkiewicz G, Arshad SH, Celedón JC, Chapman MD, Chew GL, et al. NIAID, NIEHS, NHLBI, and MCAN Workshop Report: the indoor environment and childhood asthma-implications for home environmental intervention in asthma prevention and management. J Allergy Clin Immunol . 2017;140:933–949. doi: 10.1016/j.jaci.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med . 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 13.Global Initiative for Asthma. Global strategy for asthma management and prevention. 2019. www.ginasthma.org
- 14.National Asthma Education and Prevention Program. Bethesda, MD: National Heart, Lung and Blood Institute; 2007. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. [Google Scholar]
- 15. Putcha N, Fawzy A, Matsui EC, Liu MC, Bowler RP, Woodruff PG, et al. Clinical phenotypes of atopy and asthma in COPD: a meta-analysis of SPIROMICS and COPDGene. Chest . 2020;158:2333–2345. doi: 10.1016/j.chest.2020.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tiew PY, Ko FWS, Pang SL, Matta SA, Sio YY, Poh ME, et al. Environmental fungal sensitisation associates with poorer clinical outcomes in COPD. Eur Respir J . 2020;56:2000418. doi: 10.1183/13993003.00418-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fattahi F, ten Hacken NH, Löfdahl CG, Hylkema MN, Timens W, Postma DS, et al. Atopy is a risk factor for respiratory symptoms in COPD patients: results from the EUROSCOP study. Respir Res . 2013;14:10. doi: 10.1186/1465-9921-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaji DA, Belli AJ, McCormack MC, Matsui EC, Williams DL, Paulin L, et al. Indoor pollutant exposure is associated with heightened respiratory symptoms in atopic compared to non-atopic individuals with COPD. BMC Pulm Med . 2014;14:147. doi: 10.1186/1471-2466-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paulin LM, Gassett AJ, Alexis NE, Kirwa K, Kanner RE, Peters SP, et al. Association of long-term ambient ozone exposure with respiratory morbidity in smokers. JAMA Intern Med . 2020;180:106–115. doi: 10.1001/jamainternmed.2019.5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Paulin LM, Diette GB, Blanc PD, Putcha N, Eisner MD, Kanner RE, et al. SPIROMICS Research Group Occupational exposures are associated with worse morbidity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2015;191:557–565. doi: 10.1164/rccm.201408-1407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li MH, Fan LC, Mao B, Yang JW, Choi AMK, Cao WJ, et al. Short-term exposure to ambient fine particulate matter increases hospitalizations and mortality in COPD: a systematic review and meta-analysis. Chest . 2016;149:447–458. doi: 10.1378/chest.15-0513. [DOI] [PubMed] [Google Scholar]
- 22. Hansel NN, McCormack MC, Belli AJ, Matsui EC, Peng RD, Aloe C, et al. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2013;187:1085–1090. doi: 10.1164/rccm.201211-1987OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Criner GJ, Celli BR, Brightling CE, Agusti A, Papi A, Singh D, et al. GALATHEA Study Investigators TERRANOVA Study Investigators. Benralizumab for the prevention of COPD exacerbations. N Engl J Med . 2019;381:1023–1034. doi: 10.1056/NEJMoa1905248. [DOI] [PubMed] [Google Scholar]
- 24. Ahluwalia SK, Peng RD, Breysse PN, Diette GB, Curtin-Brosnan J, Aloe C, et al. Mouse allergen is the major allergen of public health relevance in Baltimore City. J Allergy Clin Immunol . 2013;132:830–835.e831–832. doi: 10.1016/j.jaci.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diette GB, Hansel NN, Buckley TJ, Curtin-Brosnan J, Eggleston PA, Matsui EC, et al. Home indoor pollutant exposures among inner-city children with and without asthma. Environ Health Perspect . 2007;115:1665–1669. doi: 10.1289/ehp.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamilton RG, Chapman MD, Platts-Mills TAE, Adkinson NF., Jr House dust aeroallergen measurements in clinical practice: a guide to allergen free home and work environments. Immunol Allergy Pract . 1992;14:9–25. [Google Scholar]
- 27. Huss K, Adkinson NF, Jr, Eggleston PA, Dawson C, Van Natta ML, Hamilton RG. House dust mite and cockroach exposure are strong risk factors for positive allergy skin test responses in the Childhood Asthma Management Program. J Allergy Clin Immunol . 2001;107:48–54. doi: 10.1067/mai.2001.111146. [DOI] [PubMed] [Google Scholar]
- 28. American Thoracic Society. Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med . 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 29. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax . 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis . 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 31. Comstock GW, Tockman MS, Helsing KJ, Hennesy KM. Standardized respiratory questionnaires: comparison of the old with the new. Am Rev Respir Dis . 1979;119:45–53. doi: 10.1164/arrd.1979.119.1.45. [DOI] [PubMed] [Google Scholar]
- 32. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol . 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 33. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods . 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 34. Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med . 2019;381:1257–1266. doi: 10.1056/NEJMra1900500. [DOI] [PubMed] [Google Scholar]
- 35. Grant T, Phipatanakul W, Perzanowski M, Balcer-Whaley S, Peng RD, Curtin-Brosnan J, et al. Reduction in mouse allergen exposure is associated with greater lung function growth. J Allergy Clin Immunol . 2020;145:646–653.e1. doi: 10.1016/j.jaci.2019.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Putcha N, Barr RG, Han MK, Woodruff PG, Bleecker ER, Kanner RE, et al. SPIROMICS Investigators Understanding the impact of second-hand smoke exposure on clinical outcomes in participants with COPD in the SPIROMICS cohort. Thorax . 2016;71:411–420. doi: 10.1136/thoraxjnl-2015-207487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Matsui EC, Simons E, Rand C, Butz A, Buckley TJ, Breysse P, et al. Airborne mouse allergen in the homes of inner-city children with asthma. J Allergy Clin Immunol . 2005;115:358–363. doi: 10.1016/j.jaci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 38. Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD . 2005;2:75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 39. Menezes AMB, Montes de Oca M, Pérez-Padilla R, Nadeau G, Wehrmeister FC, Lopez-Varela MV, et al. PLATINO Team Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest . 2014;145:297–304. doi: 10.1378/chest.13-0622. [DOI] [PubMed] [Google Scholar]
- 40. Shah T, Churpek MM, Coca Perraillon M, Konetzka RT. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest . 2015;147:1219–1226. doi: 10.1378/chest.14-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wier LM, Elixhauser A, Pfuntner A, Au DH. Healthcare Cost and Utilization Project (HCUP) statistical briefs [Internet]. Rockville, MD: Agency for Healthcare Research and Quality (US); 2011. Overview of hospitalizations among patients with COPD, 2008: Statistical Brief #106.https://www.ncbi.nlm.nih.gov/books/NBK53969/#sb106.s1 [PubMed] [Google Scholar]
- 42. Arruda LK, Vailes LD, Ferriani VPL, Santos ABR, Pomés A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol . 2001;107:419–428. doi: 10.1067/mai.2001.112854. [DOI] [PubMed] [Google Scholar]
- 43. Marinho S, Simpson A, Marsden P, Smith JA, Custovic A. Quantification of atopy, lung function and airway hypersensitivity in adults. Clin Transl Allergy . 2011;1:16. doi: 10.1186/2045-7022-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hallberg J, Anderson M, Wickman M, Svartengren M. Factors in infancy and childhood related to reduced lung function in asthmatic children: a birth cohort study (BAMSE) Pediatr Pulmonol . 2010;45:341–348. doi: 10.1002/ppul.21190. [DOI] [PubMed] [Google Scholar]
- 45. Sheehan WJ, Permaul P, Petty CR, Coull BA, Baxi SN, Gaffin JM, et al. Association between allergen exposure in inner-city schools and asthma morbidity among students. JAMA Pediatr . 2017;171:31–38. doi: 10.1001/jamapediatrics.2016.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anthracopoulos MB, Mantzouranis E, Paliatsos AG, Tzavelas G, Lagona E, Nicolaidou P, et al. Different effects of sensitization to mites and pollens on asthma symptoms and spirometric indices in children: a population-based cohort study. Ann Allergy Asthma Immunol . 2007;99:122–129. doi: 10.1016/S1081-1206(10)60635-7. [DOI] [PubMed] [Google Scholar]
- 47. Matsui EC, Eggleston PA, Buckley TJ, Krishnan JA, Breysse PN, Rand CS, et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol . 2006;97:514–520. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 48. Platts-Mills TAE, de Weck AL, Aalberse RC, Bessot JC, Bjorksten B, Bischoff E, et al. Dust mite allergens and asthma—a worldwide problem. J Allergy Clin Immunol . 1989;83:416–427. doi: 10.1016/0091-6749(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 49. Zock JP, Heinrich J, Jarvis D, Verlato G, Norbäck D, Plana E, et al. Indoor Working Group of the European Community Respiratory Health Survey II Distribution and determinants of house dust mite allergens in Europe: the European Community Respiratory Health Survey II. J Allergy Clin Immunol . 2006;118:682–690. doi: 10.1016/j.jaci.2006.04.060. [DOI] [PubMed] [Google Scholar]
- 50. Crocker DD, Kinyota S, Dumitru GG, Ligon CB, Herman EJ, Ferdinands JM, et al. Task Force on Community Preventive Services Effectiveness of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity: a community guide systematic review. Am J Prev Med . 2011;41(2) Suppl 1:S5–S32. doi: 10.1016/j.amepre.2011.05.012. [DOI] [PubMed] [Google Scholar]