Abstract
Depression and related mood disorders constitute an enormous burden on health, quality of life, and the global economy, and women have roughly twice the lifetime risk of men for experiencing depression. Here, we review sex differences in human brain physiology that may be connected to the increased susceptibility of women to major depressive disorder (MDD). Moreover, we summarize decades of preclinical research using animal models for the study of mood dysfunction that uncover some of the potential molecular, cellular, and circuit-level mechanisms that may underlie sex differences and disease etiology. We place particular emphasis on a series of recent studies demonstrating the central contribution of the circuit projecting from ventral hippocampus to nucleus accumbens and how inherent sex differences in the excitability of this circuit may predict and drive depression-related behaviors. The findings covered in this review underscore the continued need for studies using preclinical models and circuit-specific strategies for uncovering molecular and physiological mechanisms that could lead to potential sex-specific diagnosis, prognosis, prevention, and/or treatments for MDD and other mood disorders.
The World Health Organization recently named depression as the number one cause of disability globally (World Health Organization [WHO] 2017), highlighting its significant social and financial burden. Surprisingly, women have roughly twice the lifetime risk of men for experiencing depression (Kessler 2003), but the reasons for this remain unknown. Many genetic and environmental factors, such as inherited traits, early life adversity, and societal inequities, intersect at the level of the individual to produce depressive disorders. One of the most important of these factors is neurophysiology, which itself is influenced by countless external and internal forces, such as hormone status, stress exposure, age, and many others. The neurophysiology of depression is difficult to study in humans, as direct study of the structure and function of neurons and circuits requires invasive or terminal procedures. Accordingly, the field has relied on animal models for the study of depression, which often use stress and behavioral assays to assess depressive-like symptoms. Whereas previous work in animals has been instrumental in defining some basic physiological mechanisms important for depression, the bulk of research has been done in males and as such has only just begun to explore the etiology of sex differences in depression incidence. As our current body of knowledge regarding sex differences in depression is lacking, preclinical studies are needed to investigate neurophysiology, hormonal signaling, and other factors that intersect to produce these differences.
MAJOR DEPRESSIVE DISORDER (MDD) IN MEN AND WOMEN
One of the most striking statistics of major depressive disorder (MDD) is that women are twice as likely to develop the disorder as men (Seedat et al. 2009). Interestingly, when considering sex differences in MDD before puberty, boys are more likely to meet diagnostic criteria than girls (Douglas and Scott 2014). This begins to reverse at puberty, with female prevalence increasing to adulthood levels with each year of age post-puberty (Avenevoli et al. 2015). Depression symptom profiles also differ between men and women: women are more likely to report “atypical” symptoms of increased appetite and sleep, and to experience more fatigue and pain (Marcus et al. 2008).
Sex differences in depression rates may arise in part due to specific biological and environmental risk factors experienced by men and women (Kuehner 2017). Twin studies suggest that MDD carries ∼37% heritability (Sullivan et al. 2000), and genome-wide association studies (GWAS) have uncovered a wide variety of potential heritable genetic loci that can contribute to depression pathogenesis (Flint and Kendler 2014). Hormonal influences can also affect individual susceptibility to MDD, with evidence supporting hormone fluctuation as one factor that increases MDD risk in women (Martel 2013). Differential activation of the HPA axis in men and women has also been implicated in the pathogenesis of MDD, as women experience hypoactivation of hypothalamic-pituitary-adrenal (HPA) responses that, evolutionarily, may protect a fetus from the effects of maternal stress (Kajantie and Phillips 2006). This hypoactivation, however, may deprive women of the possible protective effects of cortisol on depression-related changes to emotional brain circuitry (Het et al. 2012). Many environmental factors such as gender-based violence and early life stress can also affect gender disparities in MDD. While not an exhaustive list, these biological and environmental risk factors, along with many others, underscore the complexity and heterogeneity of mood disorder etiology. Recognition of these factors, taken together with the neurophysiological bases for depression, inform our understanding of individual, sex- and gender-based variations in MDD experience.
PRECLINICAL ANIMAL MODELS OF DEPRESSION
Stress, along with other environmental factors, can be very influential on the highly plastic circuitry of the brain. Changes in activity or connectivity in the brain in response to an initial stressful scenario can alter many functional processes (e.g., learning, memory), resulting in an adaptation that better prepares the animal for future stressful events (McEwen 2008). However, when stress is chronic or traumatic, these changes can also coalesce to cause maladaptation to stress with accompanying susceptibility to depression or anxiety. Vulnerability to stress and subsequent maladaptation vary from individual to individual, with some individuals maintaining physiological and psychological function in the face of stress, while others suffer any number of pathologies in response to the same insult. Those that do not experience maladaptive stress response are considered resilient, and those that develop depression or other conditions are susceptible. Critically, differences in stress resilience can be modeled in rodents, and gene expression profiling of resilient and susceptible rodents has revealed that resilience may be an active mechanism (i.e., not simply the “absence” of susceptibility): more genes are differentially regulated in resilient mice compared to nonstressed controls than are regulated in susceptible mice (Krishnan et al. 2007). Two major stress paradigms for the study of depression, chronic social defeat stress (CSDS) and subchronic variable stress (SCVS), are discussed below.
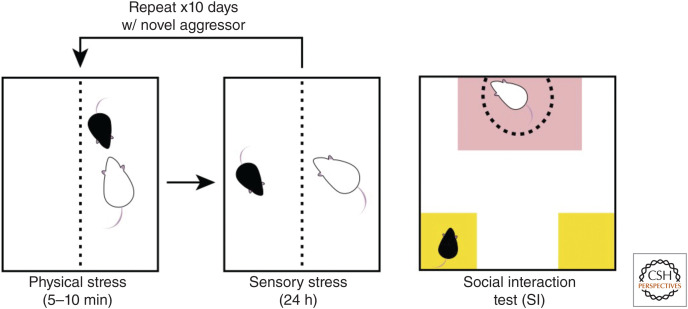
Many life events (e.g., divorce, moving to a new city, illness) represent significant stressors and can precipitate the onset or recurrence of depression (Brown 1993). Life stressors in humans can lead to loss of social rank or loss of control over one's life circumstances (e.g., job loss leading to loss of income). To mimic human social stressors, rodent models have used social subordination as an ethologically relevant stressor that a rodent might face in its natural life. CSDS (Fig. 1, left) uses a resident-intruder paradigm in which the experimental male mouse (intruder) is placed into the home cage of an aggressive male mouse (resident), usually of a different strain (e.g., a smaller C57BL/6 mouse is placed into the home cage of a larger, sexually experienced CD-1 mouse; Fig. 1). Evaluation of susceptibility to CSDS is typically achieved through the social interaction (SI) test (Fig. 1, right), in which the experimental mouse is placed into an arena containing a novel aggressor mouse (social target) in a caged enclosure. Social withdrawal behavior is quantified by calculation of a social interaction ratio: the time spent in the interaction zone when the social target is present over the time spent in the interaction zone when the social target is absent. The time spent in the corners by the experimental mouse is also indicative of social withdrawal, as susceptible mice are more likely to “hide” in the corners; resilient mice are eager to interact with the social target, as this interaction is rewarding.
Figure 1.
Chronic social defeat stress and social interaction test. Resident aggressor mice (e.g., white CD-1) are singly housed in cages for several days prior to testing to establish territory. The experimental intruder mouse (e.g., C57BL/6) is placed into the home cage of the aggressor and the two mice interact for 5–10 min (physical stress, left). Typically, the experimental mouse is quickly attacked by the aggressor mouse, and these attacks continue throughout the duration of the physical stress period. The experimental mouse is then moved to the other side of a perforated divider where it is safe from further attack, but can still see, hear, and smell the aggressor (sensory stress, middle). The experimental mouse is housed here for 24 h. The cycle then repeats for 10 or more days with the experimental mouse exposed to a novel aggressor mouse each day. Social interaction (SI) testing (right) is used following the chronic stress to evaluate social withdrawal as a measure of stress susceptibility. Susceptible mice exhibit social withdrawal and spend less time in the interaction zone (red) with the social target, and more time in the corners (yellow), withdrawn and isolated.
In addition to exhibiting social withdrawal, susceptible mice also show other depression-like behaviors (Krishnan et al. 2007). Compared to nonstressed controls, susceptible mice show a significant decrease in body weight. They also demonstrate a reduction in sucrose preference, which is a measure of anhedonia (Willner et al. 1992). Both resilient and susceptible mice exhibit anxiety-like behavior, such as elevated corticosterone responses to forced swim test (FST) and decreased open arm time in the elevated plus maze (EPM).
The pharmacological validity of CSDS has been verified by experiments using standard antidepressant treatments (Tsankova et al. 2006; Zachariou et al. 2006). Treatment of susceptible animals with the antidepressants imipramine and fluoxetine reduced social withdrawal behavior caused by CSDS. Importantly, chronic administration was required, as an acute injection with these drugs did not reverse the withdrawal behavior (Zachariou et al. 2006). More recently, the fast-acting antidepressant ketamine has also been shown to reverse CSDS-induced phenotypes, and in this case a single injection was sufficient to reverse susceptibility (Donahue et al. 2014; Brachman et al. 2016).
SUBCHRONIC VARIABLE STRESS (SCVS)
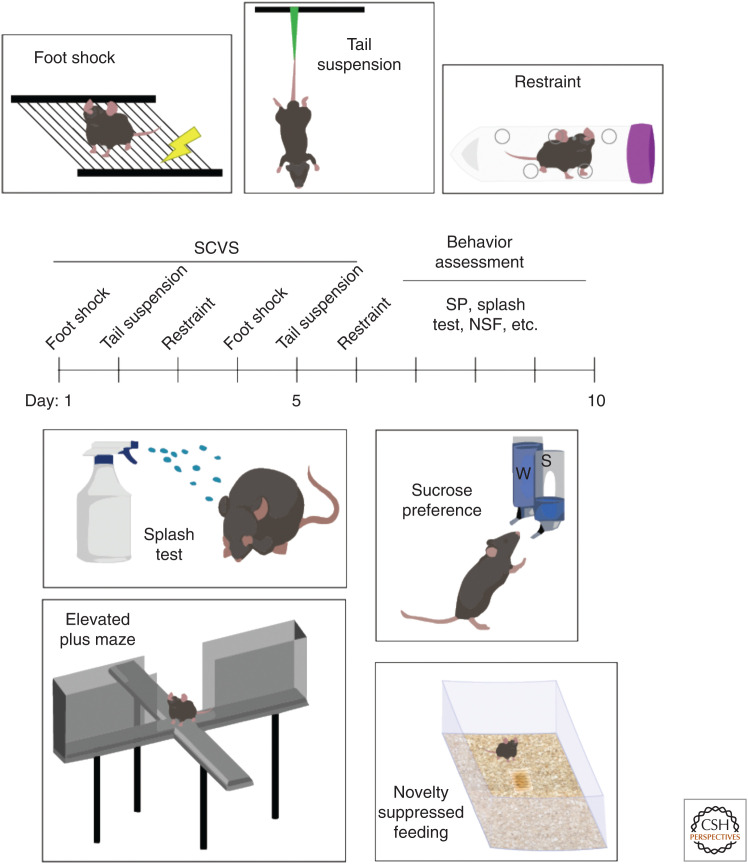
One of the major limitations of CSDS is that it relies on territorial aggression between conspecific adult males and has not classically worked in females (Warren et al. 2020; although see Newman et al. 2019). In contrast, SCVS can be used with both sexes, with only female subjects subsequently susceptible to depression-related behaviors (Hodes et al. 2015; Williams et al. 2020). SCVS comprises 6 d of alternating stressors: foot shock, tail suspension, and restraint (Fig. 2, top). Following stress, mice are then assessed using a variety of assays: sucrose preference, SI, EPM, novelty-suppressed feeding (NSF) test, and splash test (Fig. 2, bottom). Following SCVS, female mice spend less time grooming in the splash test, display increased latency to eat in a novel environment, and have reduced sucrose preference (Hodes et al. 2015). Corticosterone is also only increased in stressed female mice, indicating dysregulation of the HPA axis (Hodes et al. 2015). These behavioral responses to SCVS do not occur in males, making SCVS useful for modeling the increased prevalence of MDD in women.
Figure 2.
Subchronic variable stress (SCVS) and associated behavioral assays. SCVS comprises a 6-d battery of repeated stressors (top): foot shock, tail suspension, and restraint stress. Each stress session typically lasts 1 h per day. Following the stress period, a variety of behavioral assays are used to assess stress susceptibility (bottom): splash test, sucrose preference (SP), elevated plus maze (EPM), novelty suppressed feeding (NSF), and social interaction ([SI], not pictured). Splash test measures grooming behavior, with susceptible animals showing reduced grooming time when sprayed with a sticky solution. Sucrose preference indicates anhedonic response with two-bottle choice task, with susceptible animals showing no preference for sucrose drinking solution. EPM measures anxiety response, with “anxious” animals spending less time in open arms and more time in closed arms of the apparatus. Novelty suppressed feeding represents a more subtle measure of anxiety, with “anxious” animals exhibiting increased latency to eat (following overnight food deprivation) in a novel environment.
SEX DIFFERENCES IN STRESS RESPONSES
It is clear from the female-specific susceptibility in the SCVS paradigm that sex differences in stress response exist. These differences may account for the disproportionate number of women diagnosed with depression compared to men. Studies of stress responses in animal models of depression have revealed myriad sex differences, including differences in cellular and hormonal responses, brain circuits, synaptic and intrinsic plasticity, cognition, and emotional processing.
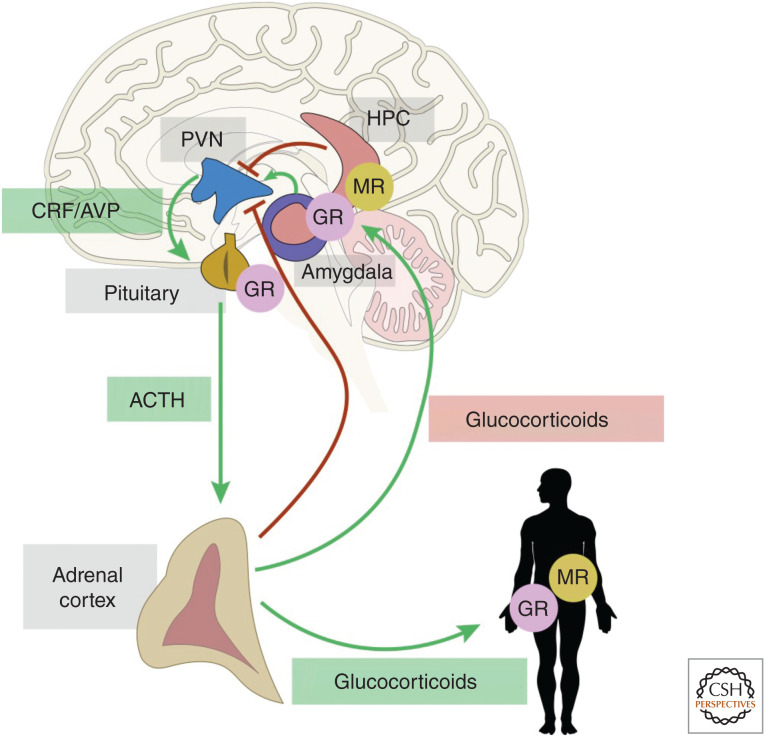
The HPA axis is a central component of stress responses and a key player in the pathophysiology of anxiety and depression. Corticotropin-releasing factor (CRF) initiates the HPA response. Neurons in the paraventricular nucleus of the hypothalamus (PVN) release CRF in response to stress, which reaches the pituitary and stimulates the release of adrenocorticotropic hormone (ACTH) into the systemic circulation, stimulating glucocorticoid release from the adrenal glands (Fig. 3); the PVN receives negative feedback regulation from the hippocampus (HPC) and other limbic structures (Herman et al. 2005). CRF also acts at the level of individual neurons via CRF receptors (Owens and Nemeroff 1991) and is released from the emotion-regulating limbic regions such as the bed nucleus of the stria terminalis (BNST) and central nucleus of the amygdala (CeA) (Walker et al. 2003) and can act throughout the brain as a neuromodulator. Indeed, CRF is elevated in cerebrospinal fluid of depressed humans (Nemeroff et al. 1984) and antidepressants decrease these levels (De Bellis et al. 1993; Heuser et al. 1998). CRF1 receptor expression is also disturbed in many areas of the brain in depressed patients, including an increase in PVN (Wang et al. 2008) and possible compensatory decreases in cortical regions (Merali et al. 2004). Generally, HPA axis hormones (e.g., corticosterone) are elevated in female rodents compared to males at baseline (Kitay 1961). Female rodents, when stressed, release more ACTH and corticosterone than males and elevated levels also persist longer (Heinsbroek et al. 1991; Seale et al. 2004). These sex differences in rodents appear to reflect increased activation of the HPA system by CRF in females. PVN expression of CRF is elevated in females at baseline, particularly when estrogen levels are high (i.e., proestrus stage). Furthermore, stress increases CRF in female rodents only (Desbonnet et al. 2008; Iwasaki-Sekino et al. 2009). Epigenetic regulation of CRF is sexually dimorphic, with elevated methylation of Crf globally in the brain of female rats, but only in BNST and CeA in male rats (Sterrenburg et al. 2011). Particularly interesting is the differential expression of CRF1 receptors in the CA1 region of the hippocampus, an area of the brain central to emotional integration and regulation of behavioral responses to stress. Female rats, especially with elevated estrogen levels, have elevated CRF1 receptor expression on pyramidal cells of the CA1 region (McAlinn et al. 2018); however, males have higher CRF1 receptor expression on inhibitory interneurons in the dentate gyrus (DG). The same study demonstrated that CRF1 receptor expression increases in CA1 pyramidal cells in male and female rodents following chronic immobilization stress, but male levels do not even reach the female baseline expression level. Collectively, these studies indicate that increased CRF signaling in females can differentially influence the HPA axis and central neuromodulation, contributing to observed sex differences in stress outcomes (Bangasser and Valentino 2012).
Figure 3.
Hypothalamic-pituitary-adrenal (HPA) axis and hippocampal negative feedback regulation. The HPA axis is activated by stress and integrates stress responses. Anxiety responses via activation of the amygdala can exacerbate the stress response through its projections to the paraventricular nucleus of the hypothalamus (PVN). The hippocampus (HPC) is important for the evaluation of stress and is a site of glucocorticoid receptor (GR)- and mineralocorticoid (MR)-mediated negative feedback to the PVN. Release of the neuropeptides corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) from the hypothalamus promotes the release of adrenocorticotropin (ACTH) from the pituitary. ACTH then stimulates the release of glucocorticoids from the adrenal cortices. These hormones circulate to the body and brain and bind to intracellular steroid hormone receptors. (CRF) Corticotropin-releasing factor, (MR) mineralocorticoid receptor.
A variety of brain circuits are implicated in mediating sex differences in stress responses. The connection between the medial prefrontal cortex (mPFC) and amygdala is of particular interest, as the amygdala regulates responses to negative and traumatic stimuli (Hendler et al. 2003) and the mPFC regulates a wide range of functions including cognition and affect (Lieberman et al. 2019). Together, these brain regions play a significant role in behavioral responses to emotionally relevant contexts (Maren and Quirk 2004). Indeed, mood disorders including MDD correlate with overactivity in the amygdala (Drevets 2003) as well as decreased mPFC activity (Pizzagalli et al. 2004). Moreover, chronic restraint stress in rodents causes restructuring and reorganization of dendrites in pyramidal cells of mPFC, which is posited to decrease the region's ability to suppress HPA activation (Radley et al. 2004). Chronic stress also causes hyperexcitability (Rosenkranz et al. 2010) and increased dendritic arborization (Vyas et al. 2002) in rat basolateral amygdala (BLA) neurons. Finally, BLA-projecting mPFC neurons increase in dendritic arborization in response to stress only in estrogen-treated, ovariectomized female rats (Shansky et al. 2010), suggesting that the response of this circuit to stress may be dependent on sex hormones.
Animal models for the study of depression have also highlighted sex differences in locus coeruleus (LC) norepinephrine (NE) circuitry (Bangasser and Valentino 2014). Dendritic architecture of LC neurons in female rats is more complex than in males, with female LC dendrites receiving significantly more synaptic input (Bangasser et al. 2011). Moreover, tonic and phasic firing patterns are linked to changes in arousal, and, while not significantly different at baseline, female LC neuron firing increases to a greater degree than male following swim stress in rats (Curtis et al. 2006). Taken together, these findings identify the LC NE circuitry as a key substrate in the mediation of sex differences in arousal, which may contribute to differences in male and female stress response and development of psychiatric disorders such as MDD. Together, data from humans and rodent models suggest widespread sex differences in stress-responsiveness throughout the brain that could contribute to the sex disparity in MDD. However, some of the most striking differences have been observed in the limbic structures, including HPC and nucleus accumbens (NAc).
HIPPOCAMPUS SEX DIFFERENCES IN STRESS AND DEPRESSION
The HPC is a central substrate in the pathophysiology of a wide range of mood disorders including MDD (Mervaala et al. 2000; Frodl et al. 2002; MacQueen et al. 2003), bipolar disorder (Beyer and Krishnan 2002), and posttraumatic stress disorder (PTSD) (Bremner et al. 1995). Many studies have identified varying degrees of hippocampal atrophy in patients with MDD (Sheline et al. 1996), with an inverse correlation between depression duration and hippocampus volume (Sheline et al. 1999). This atrophy is likely due to decreased neurogenesis in the DG (Malberg et al. 2000), as well as shrinking and retraction of dendrites and possible loss of glial support (Rajkowska 2000), and these stress-induced processes are often reversed by antidepressant treatment (Czeh et al. 2001). HPC abnormalities in MDD also extend to sex differences. For instance, women that do not respond to the antidepressant fluoxetine have smaller hippocampal volumes than those that do respond, an effect not observed in men (Vakili et al. 2000).
Preclinical models have revealed sex differences in female hippocampal function. CA1 dendritic spine density fluctuates with changes in estrogen levels: spine density decreases in late proestrus to estrus, then returns to early proestrus levels over a period of days (Woolley et al. 1990). These effects of estradiol on CA1 spine density are dependent upon glutamate neurotransmission via NMDA receptors (NMDARs), as density did not fluctuate when rats were treated with competitive NMDAR antagonists (Woolley and McEwen 1994). CA1 spine dynamics in response to stress are sexually dimorphic as male rat CA1 spine density increased following tail shock stress while female CA1 spine density decreased following the same stressor (Shors et al. 2001), and these opposite effects of stress on CA1 spine density are also NMDAR-dependent (Shors et al. 2004).
NUCLEUS ACCUMBENS SEX DIFFERENCES IN STRESS AND DEPRESSION
The NAc is a key substrate in the expression of depression-related behaviors in mouse models (Perrotti et al. 2004; Zachariou et al. 2006; Wilkinson et al. 2009; Vialou et al. 2010), and structural sex differences are apparent in the NAc. For example, distal dendritic spine density in NAc medium spiny neurons (MSNs) is higher in female rats (Forlano and Woolley 2010), indicating that excitatory synaptic connections in the NAc likely differ between the sexes. Indeed, there is sex-dependent variability in the morphology and distribution of dendritic spine synapses in NAc (Wissman et al. 2012).
Stress has sex-specific effects on glutamatergic inputs onto NAc (Brancato et al. 2017), which are implicated in the development of mood disorders (Russo and Nestler 2013; Thompson et al. 2015). Glutamatergic nerve terminals were reduced in the NAc of female but not male mice exposed to SCVS, but there were no post-stress sex differences in PSD95, nor sex differences in MSN spine density or morphology. This suggests sex differences in presynaptic glutamate neurotransmission in the NAc shell, supporting aberrations in excitatory signaling in this brain region as potential mediators of disparities in MDD prevalence in men and women. Excitatory neurotransmission to NAc comes from many regions, including amygdala, PFC, and ventral HPC (vHPC).
HPC To NAc CONNECTIVITY
The NAc is a key mediator of motivated behaviors and integrates its dopaminergic and glutamatergic inputs to drive motivated behaviors. Ventral tegmental area (VTA) dopaminergic input to the NAc mediates reinforcement of incentive salience (i.e., “wanting”) of a reward (Berridge 2007; Day et al. 2007), while the glutamatergic input to NAc and midbrain dopamine neurons encodes information regarding reward-related environmental stimuli (Stuber et al. 2008). The excitatory input into the NAc allows integration of context and cues with goal-directed behavioral output (Pennartz et al. 2011). Dopamine signaling in the NAc modulates glutamatergic synaptic plasticity to integrate environmental information and prioritize the salience of some cues over others (Sun et al. 2008; Wolf and Ferrario 2010). Dysfunction of this integration leads to perturbations in reward processing that can result in aberrant motivated behavior. For example, an increase in the NAc's drive of motivated behavior may lead to addiction, and a weakening of this drive may lead to anhedonic behaviors such as those found in MDD and other mood disorders.
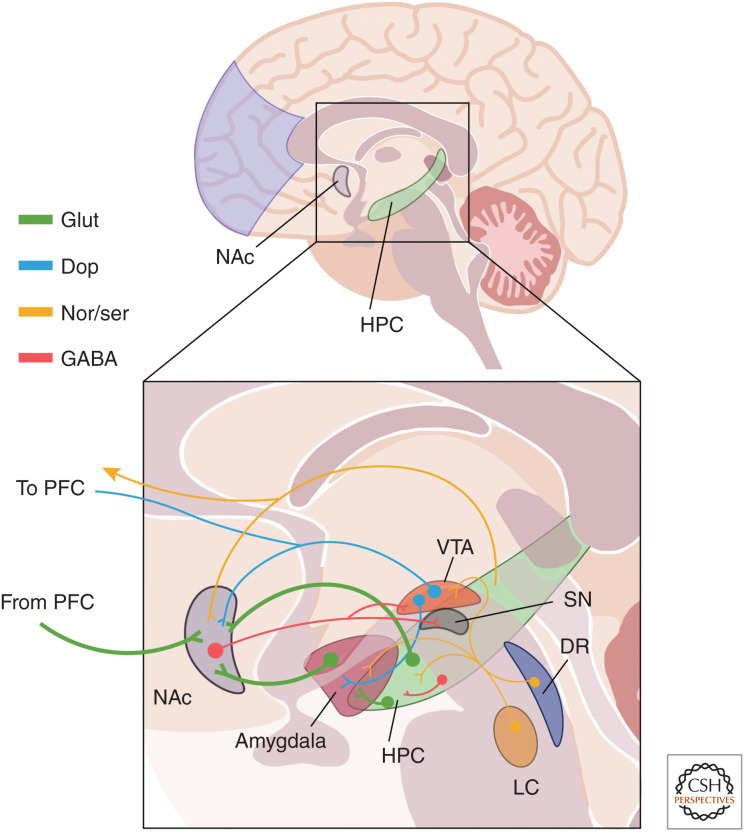
In rodents, three major glutamatergic projections to the NAc have been clearly demonstrated (Fig. 4): those from BLA, PFC, and vHPC (Britt et al. 2012). Moreover, functional magnetic resonance imaging (fMRI) has uncovered resting-state functional connectivity between the vHPC and NAc in humans (Kahn and Shohamy 2013). Projections from the vHPC are concentrated mainly at the medial NAc shell while projections from the BLA and PFC are mixed between the shell and the core (Britt et al. 2012). Furthermore, retrograde labeling studies indicate that the majority of all cells projecting to the medial shell are located in the vHPC, with fewer also coming from the BLA and PFC and the strength of vHPC inputs is strongest in NAc (Britt et al. 2012). The vHPC-NAc are uniquely able induce a stable depolarized state in NAc MSNs, increasing their likelihood of activity (O'Donnell and Grace 1995). This vHPC-mediated depolarization is necessary for the induction of spike firing by inputs from the PFC, suggesting that vHPC inputs are capable of “gating” the response of the NAc to other excitatory inputs (O'Donnell and Grace 1995).
Figure 4.
Mood and reward circuitry. A simplified depiction of neural circuits that have been implicated in the pathophysiology of depression. The glutamatergic (green) projections to the nucleus accumbens (NAc) from hippocampus (HPC), amygdala, and prefrontal cortex (PFC) are prominently featured, and many subcortical regions that mediate reward, fear, and motivation are also depicted. This diagram also shows the innervation of many regions by monoaminergic projections from the ventral tegmental area ([VTA], blue dopaminergic projections to NAc, amygdala, PFC), as well as yellow noradrenergic projections from the locus coeruleus (LC) and serotonergic projections from the dorsal raphe (DR). The NAc also sends GABAergic (red) projections to the hypothalamus (not pictured), VTA, and substantia nigra (SN), and GABAergic interneurons are found within the HPC (and other structures). This diagram, while not exhaustive, highlights some of the major circuits involved in depression and referenced throughout this work.
Bagot et al. made crucial contributions to our understanding of the function of these projections with respect to stress outcomes, demonstrating opposite regulation of vHPC-NAc and mPFC-NAc activity by CSDS (Bagot et al. 2015). Using optogenetics to activate glutamatergic terminals in NAc arising from vHPC or mPFC to NAc, they found that glutamate release from vHPC-NAc projections is reduced in resilient mice, but oppositely, glutamate release from mPFC-NAc projections is increased in resilient mice. They then optically induced long-term depression (LTD) at vHPC-NAc synapses and found that weakening of vHPC-NAc connectivity resulted in an increase in social interaction to levels observed in resilient mice. Critically, LTD induced at mPFC-NAc inputs did not affect social interaction. Conversely, acute optical stimulation of vHPC-NAc projections reduced SI ratio following CSDS and increased immobility in the FST, thus promoting susceptibility to stress. The opposite was found in acute stimulation of mPFC-NAc projections, which increased SI ratio following CSDS in a pro-resilient fashion. This seminal study, while only examining male mice, highlights the importance of glutamatergic inputs to the NAc in regulating susceptibility to stress, and demonstrates that increasing vHPC-NAc activity is pro-depressive in male mice.
More recently, LeGates et al. (2018) demonstrated that vHPC-NAc synapses display activity-dependent plasticity and that their strength directly regulates reward behavior. Optical stimulation of vHPC-NAc synapses was sufficient to induce preference of a stimulation-paired chamber, and interruption of vHPC-NAc plasticity using optical inhibition was sufficient to abolish the preference of a chamber containing the natural reward of a social target. Furthermore, chronic multimodal stress (CMS) was sufficient to weaken vHPC synapses at D1R-MSNs, accompanied by a deficit in long-term potentiation (LTP) induction at these synapses, as well as an abolishment of the preference for a reward-paired chamber mentioned above. The link between stress-induced alterations at vHPC-NAc synapses and MDD was further strengthened by the mitigation of these alterations by the antidepressant fluoxetine. Chronic fluoxetine was sufficient to restore pre-stress sucrose preference levels, as well as the optically induced preference of the light stimulation-paired chamber. Furthermore, chronic fluoxetine rescued the decrease in synaptic strength caused by chronic stress in D1R-MSNs and ameliorated the accompanying deficit in LTP induction. These findings highlight the key role of the connection between HPC and NAc in mediating reward- and stress-related behaviors.
We recently demonstrated using whole-cell patch-clamp electrophysiology and retrograde labeling that vHPC-NAc neurons in female mice are more excitable than those neurons in male mice, and that this excitability difference correlated with the unique female susceptibility to SCVS discussed above (Williams et al. 2020). Furthermore, we found that orchiectomy of male mice elevated vHPC-NAc excitability and that this change was accompanied by an elevated susceptibility SCVS-induced reduction in sucrose preference. Female mice did not have a change in vHPC-NAc activity level when ovariectomized but, when exposed to chronic testosterone, did have reduction in excitability in this circuit and resilience to SCVS-induced reduction in sucrose preference. We were then able to show that the excitability of the circuit directly mediates the observed change in behavioral response to stress using designer receptors exclusively activated by designer drugs (DREADDs). Artificially increasing excitability of vHPC-NAc neurons in male mice caused susceptibility to SCVS, while using the same method to decrease excitability of vHPC-NAc neurons in female mice promoted resilience, as measured by sucrose preference. Most recently, Muir et al. showed that increased vHPC-NAc activity is predictive of anxiety-like behavior in the open field in both sexes and may predict some aspects of stress-induced decreases in social interaction, supporting the causal connection between vHPC-NAc activity and susceptibility to stress-induced behaviors associated with mood disorders (Muir et al. 2020).
All of these studies underscore the importance of the vHPC-NAc circuit as a critical component of behavioral response to stress, and identifies this circuit as uniquely suited to mediate sex-dependent differences in stress responses. Below, hormone signaling in the brain and its effects on stress and depression will be further explored.
HORMONE SIGNALING IN THE BRAIN—EFFECTS ON STRESS RESPONSE AND DEPRESSION
Estrogen signaling in the brain is primarily accomplished via intracellular estrogen receptors (ERs), which, when bound to estrogen, can translocate to the cell nucleus and bind to hormone response elements in target genes to affect their transcription; these events typically occur over hours (Woolley and Schwartzkroin 1998). Intracellular ERs are most densely expressed in the BNST, ventromedial nucleus of the hypothalamus, amygdala, various midbrain structures, and the pituitary (Pfaff and Keiner 1973), and more sparsely expressed in the HPC and cortex (Loy et al. 1988). Progesterone receptors (PRs) are generally expressed in the same pattern as ERs (Kato 1985). In situ hybridization and immunocytochemical studies suggest that most cells in the HPC that express intracellular ERs are interneurons (Pelletier et al. 1988; DonCarlos et al. 1991).
Progesterone, estrogen, and their derivatives can also have nongenomic, traditional cell-signaling effects on neuronal physiology, including on cellular excitability (Schumacher 1990). Progesterone metabolites allosterically bind GABAA receptors in the HPC, causing potentiated chloride conductance and dampened excitability (Majewska 1992). Excitatory postsynaptic potentials (EPSPs) in CA1 pyramidal cells of ovariectomized female rats are prolonged in rats that were “primed” with estrogen injections for 2 d prior to acute slice preparation (Wong and Moss 1992). This group also demonstrated that excitatory postsynaptic currents (EPSCs) were potentiated via the actions of second messenger cascades (e.g., cAMP signaling) (Gu and Moss 1996; Moss et al. 1997). These effects, mediated by cell signaling from hormone receptors on the cell membrane, make ovarian-derived hormones, especially estradiol, interesting potential mediators of sex differences in stress outcomes, particularly in the context of circuit-level excitability.
Estrogens also modulate synaptic plasticity in the hippocampus. LTP is enhanced during proestrus in rats (Warren et al. 1995), and LTP induction in CA1 is facilitated by estradiol treatment in ovariectomized rats (Córdoba Montoya and Carrer 1997). Furthermore, priming of ovariectomized rats with a combined treatment of estrogen and progesterone enhances voltage-gated calcium channel conductances in CA1 pyramidal neurons (Joels and Karst 1995). These studies complement the finding that estradiol has a potent effect on CA1 dendritic spine density (Gould et al. 1990), and, taken together, highlight important effects of estrogens on CA1 excitatory neurotransmission. Whether through transcriptional or cell-signaling mechanisms, ovarian hormones clearly play a role in regulating HPC physiology through modulation of excitability and synaptic transmission.
Estrous cycle may directly modulate behavioral responses to stress, including those dependent on the hippocampus. Male and female rodents react differently to stress in HPC-dependent learning tasks such as Morris water maze, with the most robust differences being identified when females are in proestrus (Shors and Leuner 2003). Furthermore, studies of ovariectomized rats have shown that stress enhances performance in the radial arm maze (another HPC-dependent measurement of spatial memory and learning) in animals with estrogen replacement, but not in those without (Bowman et al. 2002). Stress effects on CA1 dendritic spine density also appear to be estrogen-dependent: a single stressor can induce an increase in spine density in male rats but a decrease in female rats in proestrus (Shors et al. 2001), when spine density is usually the highest for females (Woolley et al. 1990).
Estrogen and stress can interact to cause sex differences in other regions as well. For example, the amygdala-dependent process of extinction of a conditioned fear response is impaired in estrogen-treated female rats (Toufexis et al. 2007). There is also evidence that estrogen can enhance stress effects in the mPFC, as proestrus or estrogen-replaced ovariectomized female rats demonstrated more deficiencies in mPFC-dependent working memory tasks (Shansky et al. 2004). Overall, estrogens have a clear effect on stress outcomes and brain function and are likely key mediators of sex differences in depression.
Testosterone has also been implicated in mediating stress outcomes and depression. In humans, hypogonadism leading to lower testosterone levels in men is associated with a higher prevalence of MDD (Shores et al. 2004; Zarrouf et al. 2009), and treatment with testosterone has been shown to alleviate depression symptoms in hypogonadal (Zarrouf et al. 2009) and eugonadal men (Kanayama et al. 2007; Walther et al. 2019). There is also evidence that low-dose testosterone treatment can improve depression scores and augment the effectiveness of traditional depression pharmacotherapies in treatment-resistant women (Miller et al. 2009).
Testosterone is metabolized to a variety of different effectors. For example, 5α-reductase converts testosterone into dihydrotestosterone (DHT), which is a more potent agonist at androgen receptors (ARs) and has a longer half-life than testosterone. DHT can then be converted by aldo-keto reductases to 3α-diol or 3β-diol. The former of these metabolites has a low affinity for AR but acts in the brain as an agonist at the GABAA receptor (Frye et al. 1996), and the latter acts primarily through ERβ in the brain to affect gene transcription (Pak et al. 2005). Like estrogens, androgens can also act through transcriptional and cell-signaling mechanisms. Androgens interact with intracellular receptors and diffuse into the nucleus to affect gene transcription via androgen receptor binding to DNA at hormone response elements (Nestler et al. 2009). Androgens and estrogens, as alluded to above, can also cause more rapid, nongenomic effects through activation of cell membrane-bound receptors (Cato et al. 2002). The rapid effects of androgens in the brain include modulation of neuronal excitability and plasticity. There is evidence that androgens acutely decrease neuronal excitability (Kubli-Garfias et al. 1982), but some studies indicate increased excitability via measurement of field potentials (Smith et al. 2002).
Testosterone has potent effects on anxiety- and depression-related behaviors in animal models. Orchiectomy of adult male rodents, for example, causes an increase in anxiety-like behaviors as assessed by marble burying, EPM and open field (OF) tests (Slob et al. 1981; Frye and Seliga 2001; Fernandez-Guasti and Martinez-Mota 2005), as well as behavioral despair (via FST) following CMS, which was accompanied by a decrease in hippocampal cell proliferation and neurogenesis (Wainwright et al. 2011). Testosterone relieves these anxiety-like behaviors in intact adult male rodents (Bitran et al. 1993) as well as in the orchidectomized adult males of studies cited above. Particularly relevant to the current work, testosterone prevents anhedonia in aged adult male rats exposed to CMS if administered weeks before stress exposure (Herrera-Pérez et al. 2012). Interestingly, this effect was not observed if testosterone was administered following stress exposure, suggesting the necessity of testosterone before and during stress in male resilience. Further, prevention of anhedonia and behavioral despair by testosterone was likely mediated by aromatization to estradiol, as gonadectomized rats treated with DHT (insensitive to aromatase) still exhibited these depressive behaviors, but those treated with estradiol did not (Carrier and Kabbaj 2012a). However, other studies did implicate androgen metabolites in the mediation of stress resilience, as treatment with DHT or 3α-diol in intact adult male rodents decreases behavioral despair in FST (Frye and Walf 2009).
The effects of testosterone treatment in female rodents with respect to stress outcomes is even less clear. Testosterone, DHT, or 3α-diol treatment in intact female mice reduces anxiety-like behaviors as assessed by OF and EPM tests (Frye and Lacey 2001) and behavioral despair (FST) (Frye and Walf 2009). In contrast, however, other groups have observed no amelioration of anxiety (EPM) or depression (sucrose preference, NSF) behaviors following administration of testosterone in ovariectomized female rats (Carrier and Kabbaj 2012b). The difference between these observations may lie in the ovariectomy, as the studies demonstrating benefits of testosterone treatment in female animals did not include gonad removal. This could suggest that higher doses of testosterone are needed to induce anxiolytic or antidepressant effects in females, as maintaining the ovaries would accomplish this.
As alluded to above, the protective effects of androgens against stress-induced changes in behavior may in part be mediated through the HPC. Indeed, the volume of the hippocampal formation, its neuronal soma sizes, and extent of dendritic branching increase with androgen treatment in the perinatal period (Isgor and Sengelaub 1998, 2003). Additionally, testosterone enhances hippocampal neurogenesis induced by the antidepressant imipramine (Carrier and Kabbaj 2012b). The protective effects of neonatal androgens are associated with HPC neurogenesis and spine density, as male pups treated with the AR antagonist flutamide demonstrated depressive behaviors in FST and sucrose preference tests that correlated with decreased microtubule-associated protein-2 (MAP-2) labeling in the CA1 and DG and decreased spine density in CA1 (Zhang et al. 2010). Additionally, testosterone in orchidectomized adult male rodents may offer protection against oxidative damage and consequent changes in morphology (Meydan et al. 2010), and its metabolites in intact female adults appear to reduce HPC cell death induced by adrenalectomy (Frye and McCormick 2000). Testosterone and DHT also reverse orchidectomy-induced reduction of spine synapse density in males (independently of ER-mediated effects), while offering partial protection to females with contribution of the aromatization to estrogens (MacLusky et al. 2006). These morphological changes in response to steroid hormones may relate to HPC neuronal excitability. Teyler et al. (1980) observed field potential differences in HPC slices from rat brains in response to treatment with testosterone and estradiol: male rat slices showed increased excitability in response to estradiol but not testosterone, and female rat slices showed increased excitability in response to testosterone in diestrus but decreased excitability in proestrus.
CONCLUDING REMARKS
As described herein, genetics, neural circuits, hormones, and environment likely intersect at the level of the individual to shape behavioral responses to stress and dictate development of mood disorders. The past two decades of preclinical studies suggest that definition of the stress-responsive circuitry may guide future treatments for mood disorders such as depression and anxiety. To achieve this, we must elucidate regulatory mechanisms of these circuits, especially those mechanisms that differ between men and women. However, the mechanisms for differences in circuit physiology cannot yet be fully investigated in humans due to the invasiveness of existing methods; this underscores the continued need for studies using preclinical models and circuit-specific strategies for uncovering molecular and physiological mechanisms that could lead to potential treatments. The studies and findings summarized in this review begin to address the nature of circuit physiology in the context of stress and depression in male and female mice and may open the doors for the investigation of many new avenues for treatment or prevention of mood disorders.
ACKNOWLEDGMENTS
A.J.R. acknowledges support from the National Institutes of Mental Health (MH111604), the National Institutes of Neurological Disease and Stroke (NS085171), the National Institutes of Drug Abuse (DA040621), the National Institutes of Childhood Health and Disease (HD072968), and the Avielle Foundation. M.M.-R. acknowledges support from the National Institutes of Drug Abuse (DA039895).
Footnotes
Editors: Cynthia L. Jordan and S. Marc Breedlove
Additional Perspectives on Sex Differences in Brain and Behavior available at www.cshperspectives.org
REFERENCES
- Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. 2015. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry 54: 37–44.e2. 10.1016/j.jaac.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Parise EM, Peña CJ, Zhang HX, Maze I, Chaudhury D, Persaud B, Cachope R, Bolaños-Guzmán CA, Cheer JF, et al. 2015. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun 6: 7062. 10.1038/ncomms8062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. 2012. Sex differences in molecular and cellular substrates of stress. Cell Mol Neurobiol 32: 709–723. 10.1007/s10571-012-9824-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Valentino RJ. 2014. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol 35: 303–319. 10.1016/j.yfrne.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ. 2011. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav 103: 342–351. 10.1016/j.physbeh.2011.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. 2007. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 191: 391–431. 10.1007/s00213-006-0578-x [DOI] [PubMed] [Google Scholar]
- Beyer JL, Krishnan KR. 2002. Volumetric brain imaging findings in mood disorders. Bipolar Disord 4: 89–104. 10.1034/j.1399-5618.2002.01157.x [DOI] [PubMed] [Google Scholar]
- Bitran D, Kellogg CK, Hilvers RJ. 1993. Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav 27: 568–583. 10.1006/hbeh.1993.1041 [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. 2002. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience 113: 401–410. 10.1016/S0306-4522(02)00156-2 [DOI] [PubMed] [Google Scholar]
- Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, Gardier AM, Mendez-David I, David DJ, Hen R, et al. 2016. Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol Psychiatry 79: 776–786. 10.1016/j.biopsych.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancato A, Bregman D, Ahn HF, Pfau ML, Menard C, Cannizzaro C, Russo SJ, Hodes GE. 2017. Sub-chronic variable stress induces sex-specific effects on glutamatergic synapses in the nucleus accumbens. Neuroscience 350: 180–189. 10.1016/j.neuroscience.2017.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. 1995. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 152: 973–981. 10.1176/ajp.152.7.973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. 2012. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76: 790–803. 10.1016/j.neuron.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW. 1993. Stress: from synapse to syndrome. In Life events and illness (ed. Stanford S, Blanchard DC), pp. 20–40. Academic, Cambridge, MA. [Google Scholar]
- Carrier N, Kabbaj M. 2012a. Extracellular signal-regulated kinase 2 signaling in the hippocampal dentate gyrus mediates the antidepressant effects of testosterone. Biol Psychiatry 71: 642–651. 10.1016/j.biopsych.2011.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. 2012b. Testosterone and imipramine have antidepressant effects in socially isolated male but not female rats. Horm Behav 61: 678–685. 10.1016/j.yhbeh.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato AC, Nestl A, Mink S. 2002. Rapid actions of steroid receptors in cellular signaling pathways. Sci STKE 2002: re9. [DOI] [PubMed] [Google Scholar]
- Córdoba Montoya DA, Carrer HF. 1997. Estrogen facilitates induction of long term potentiation in the hippocampus of awake rats. Brain Res 778: 430–438. 10.1016/S0006-8993(97)01206-7 [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. 2006. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31: 544–554. 10.1038/sj.npp.1300875 [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. 2001. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci 98: 12796–12801. 10.1073/pnas.211427898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. 2007. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci 10: 1020–1028. 10.1038/nn1923 [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Gold PW, Geracioti TD Jr, Listwak SJ, Kling MA. 1993. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry 150: 656–657. 10.1176/ajp.150.4.656 [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG. 2008. Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. Int J Dev Neurosci 26: 259–268. 10.1016/j.ijdevneu.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Donahue RJ, Muschamp JW, Russo SJ, Nestler EJ, Carlezon WA Jr. 2014. Effects of striatal ΔFosB overexpression and ketamine on social defeat stress–induced anhedonia in mice. Biol Psychiatry 76: 550-558. 10.1016/j.biopsych.2013.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DonCarlos LL, Monroy E, Morrell JI. 1991. Distribution of estrogen receptor-immunoreactive cells in the forebrain of the female Guinea pig. J Comp Neurol 305: 591–612. 10.1002/cne.903050406 [DOI] [PubMed] [Google Scholar]
- Douglas J, Scott J. 2014. A systematic review of gender-specific rates of unipolar and bipolar disorders in community studies of pre-pubertal children. Bipolar Disord 16: 5–15. 10.1111/bdi.12155 [DOI] [PubMed] [Google Scholar]
- Drevets WC. 2003. Neuroimaging abnormalities in the amygdala in mood disorders. Ann NY Acad Sci 985: 420–444. 10.1111/j.1749-6632.2003.tb07098.x [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Martinez-Mota L. 2005. Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA-benzodiazepine receptors. Psychoneuroendocrinology 30: 762–770. 10.1016/j.psyneuen.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Flint J, Kendler KS. 2014. The genetics of major depression. Neuron 81: 484–503. 10.1016/j.neuron.2014.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlano PM, Woolley CS. 2010. Quantitative analysis of pre- and postsynaptic sex differences in the nucleus accumbens. J Comp Neurol 518: 1330–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jäger M, Leinsinger G, Bottlender R, Hahn K, Möller HJ. 2002. Hippocampal changes in patients with a first episode of major depression. Am J Psychiatry 159: 1112–1118. 10.1176/appi.ajp.159.7.1112 [DOI] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. 2001. Posttraining androgens’ enhancement of cognitive performance is temporally distinct from androgens’ increases in affective behavior. Cogn Affect Behav Neurosci 1: 172–182. 10.3758/CABN.1.2.172 [DOI] [PubMed] [Google Scholar]
- Frye CA, McCormick CM. 2000. The neurosteroid, 3α-androstanediol, prevents inhibitory avoidance deficits and pyknotic cells in the granule layer of the dentate gyrus induced by adrenalectomy in rats. Brain Res 855: 166–170. 10.1016/S0006-8993(99)02208-8 [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. 2001. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci 1: 371–381. 10.3758/CABN.1.4.371 [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. 2009. Depression-like behavior of aged male and female mice is ameliorated with administration of testosterone or its metabolites. Physiol Behav 97: 266–269. 10.1016/j.physbeh.2009.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duncan JE, Basham M, Erskine MS. 1996. Behavioral effects of 3a-androstanediol II: hypothalamic and preoptic area actions via a GABAergic mechanism. Behav Brain Res 79: 119–130. 10.1016/0166-4328(96)00005-8 [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. 1990. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci 10: 1286–1291. 10.1523/JNEUROSCI.10-04-01286.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Moss RL. 1996. 17β-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci 16: 3620–3629. 10.1523/JNEUROSCI.16-11-03620.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek RP, Van Haaren F, Feenstra MG, Endert E, Van de Poll NE. 1991. Sex- and time-dependent changes in neurochemical and hormonal variables induced by predictable and unpredictable footshock. Physiol Behav 49: 1251–1256. 10.1016/0031-9384(91)90359-V [DOI] [PubMed] [Google Scholar]
- Hendler T, Rotshtein P, Yeshurun Y, Weizmann T, Kahn I, Ben-Bashat D, Malach R, Bleich A. 2003. Sensing the invisible: differential sensitivity of visual cortex and amygdala to traumatic context. Neuroimage 19: 587–600. 10.1016/S1053-8119(03)00141-1 [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. 2005. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry 29: 1201–1213. 10.1016/j.pnpbp.2005.08.006 [DOI] [PubMed] [Google Scholar]
- Herrera-Pérez JJ, Martínez-Mota L, Chavira R, Fernández-Guasti A. 2012. Testosterone prevents but not reverses anhedonia in middle-aged males and lacks an effect on stress vulnerability in young adults. Horm Behav 61: 623–630. 10.1016/j.yhbeh.2012.02.015 [DOI] [PubMed] [Google Scholar]
- Het S, Schoofs D, Rohleder N, Wolf OT. 2012. Stress-induced cortisol level elevations are associated with reduced negative affect after stress: indications for a mood-buffering cortisol effect. Psychosom Med 74: 23–32. 10.1097/PSY.0b013e31823a4a25 [DOI] [PubMed] [Google Scholar]
- Heuser I, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Nemeroff CB, Holsboer F. 1998. Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety 8: 71–79. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Purushothaman I, Ahn HF, Golden SA, Christoffel DJ, Magida J, Brancato A, Takahashi A, Flanigan ME, et al. 2015. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci 35: 16362–16376. 10.1523/JNEUROSCI.1392-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. 1998. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 pyramidal cell morphology in rats. Horm Behav 34: 183–198. 10.1006/hbeh.1998.1477 [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. 2003. Effects of neonatal gonadal steroids on adult CA3 pyramidal neuron dendritic morphology and spatial memory in rats. J Neurobiol 55: 179–190. 10.1002/neu.10200 [DOI] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. 2009. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology 34: 226–237. 10.1016/j.psyneuen.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H. 1995. Effects of estradiol and progesterone on voltage-gated calcium and potassium conductances in rat CA1 hippocampal neurons. J Neurosci 15: 4289–4297. 10.1523/JNEUROSCI.15-06-04289.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Shohamy D. 2013. Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus 23: 187–192. 10.1002/hipo.22077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. 2006. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31: 151–178. 10.1016/j.psyneuen.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Kanayama G, Amiaz R, Seidman S, Pope HG Jr. 2007. Testosterone supplementation for depressed men: current research and suggested treatment guidelines. Exp Clin Psychopharmacol 15: 529-538. 10.1037/1064-1297.15.6.529 [DOI] [PubMed] [Google Scholar]
- Kato J. 1985. Progesterone receptors in brain and hypophysis. In Actions of progesterone on the brain current topics in neuroendocrinology (ed. Ganten D, Pfaff D). Springer, Berlin. [Google Scholar]
- Kessler RC. 2003. Epidemiology of women and depression. J Affect Disord 74: 5–13. 10.1016/S0165-0327(02)00426-3 [DOI] [PubMed] [Google Scholar]
- Kitay JI. 1961. Sex differences in adrenal cortical secretion in the rat. Endocrinology 68: 818–824. 10.1210/endo-68-5-818 [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, et al. 2007. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131: 391–404. 10.1016/j.cell.2007.09.018 [DOI] [PubMed] [Google Scholar]
- Kubli-Garfias C, Canchola E, Arauz-Contreras J, Feria-Velasco A. 1982. Depressant effect of androgens on the cat brain electrical activity and its antagonism by ruthenium red. Neuroscience 7: 2777–2782. 10.1016/0306-4522(82)90100-2 [DOI] [PubMed] [Google Scholar]
- Kuehner K. 2017. Why is depression more common among women than among men? The Lancet Psychiatry 4: 146–158. 10.1016/S2215-0366(16)30263-2 [DOI] [PubMed] [Google Scholar]
- LeGates TA, Kvarta MD, Tooley JR, Francis TC, Lobo MK, Creed MC, Thompson SM. 2018. Reward behaviour is regulated by the strength of hippocampus–nucleus accumbens synapses. Nature 564: 258–262. 10.1038/s41586-018-0740-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Straccia MA, Meyer ML, Du M, Tan KM. 2019. Social, self, (situational), and affective processes in medial prefrontal cortex (MPFC): causal, multivariate, and reverse inference evidence. Neurosci Biobehav Rev 99: 311–328. 10.1016/j.neubiorev.2018.12.021 [DOI] [PubMed] [Google Scholar]
- Loy R, Gerlach JL, McEwen BS. 1988. Autoradiographic localization of estradiol-binding neurons in the rat hippocampal formation and entorhinal cortex. Brain Res 467: 245–251. 10.1016/0165-3806(88)90028-4 [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. 2006. Androgen modulation of hippocampal synaptic plasticity. Neuroscience 138: 957–965. 10.1016/j.neuroscience.2005.12.054 [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. 2003. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci 100: 1387–1392. 10.1073/pnas.0337481100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD. 1992. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol 38: 379–395. 10.1016/0301-0082(92)90025-A [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. 2000. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20: 9104–9110. 10.1523/JNEUROSCI.20-24-09104.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SM, Kerber KB, Rush AJ, Wisniewski SR, Nierenberg A, Balasubramani GK, Ritz L, Kornstein S, Young EA, Trivedi MH. 2008. Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the sequenced treatment alternatives to relieve depression study. Compr Psychiatry 49: 238–246. 10.1016/j.comppsych.2007.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. 2004. Neuronal signalling of fear memory. Nat Rev Neurosci 5: 844–852. 10.1038/nrn1535 [DOI] [PubMed] [Google Scholar]
- Martel MM. 2013. Sexual selection and sex differences in the prevalence of childhood externalizing and adolescent internalizing disorders. Psychol Bull 139: 1221–1259. 10.1037/a0032247 [DOI] [PubMed] [Google Scholar]
- McAlinn HR, Reich B, Contoreggi NH, Kamakura RP, Dyer AG, McEwen BS, Waters EM, Milner TA. 2018. Sex differences in the subcellular distribution of corticotropin-releasing factor receptor 1 in the rat hippocampus following chronic immobilization stress. Neuroscience 383: 98–113. 10.1016/j.neuroscience.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. 2008. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583: 174–185. 10.1016/j.ejphar.2007.11.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H. 2004. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABAA receptor subunits in frontal cortical brain region. J Neurosci 24: 1478–1485. 10.1523/JNEUROSCI.4734-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamaki H, Karjalainen AK, et al. 2000. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 30: 117–125. 10.1017/S0033291799001567 [DOI] [PubMed] [Google Scholar]
- Meydan S, Kus I, Tas U, Ogeturk M, Sancakdar E, Dabak DO, Zararsiz I, Sarsilmaz M. 2010. Effects of testosterone on orchiectomy-induced oxidative damage in the rat hippocampus. J Chem Neuroanat 40: 281–285. 10.1016/j.jchemneu.2010.07.006 [DOI] [PubMed] [Google Scholar]
- Miller KK, Perlis RH, Papakostas GI, Mischoulon D, Losifescu DV, Brick DJ, Fava M. 2009. Low-dose transdermal testosterone augmentation therapy improves depression severity in women. CNS Spectr 14: 688–694. 10.1017/S1092852900023944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss RL, Gu Q, Wong M. 1997. Estrogen: nontranscriptional signaling pathway. Recent Prog Horm Res 52: 33–68; discussion 68-39. [PubMed] [Google Scholar]
- Muir J, Tse Y, Iyer E, Biris J, Cvetkovska V, Lopez J, Bagot R. 2020. Ventral-hippocampal afferents to nucleus accumbens encode both latent vulnerability and stress-induced susceptibility. Biol Psychiatry 88: 843–854. 10.1016/j.biopsych.2020.05.021 [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. 1984. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226: 1342–1344. 10.1126/science.6334362 [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE, Malenka RC. 2009. Molecular neuropharmacology: A foundation for clinical neuroscience. McGraw-Hill Medical, New York. [Google Scholar]
- Newman EL, Covington HE III, Suh J, Bicakci MB, Ressler KJ, DeBold JF, Miczek KA. 2019. Fighting females: neural and behavioral consequences of social defeat stress in female mice. Biol Psychiatry 86: 657–668. 10.1016/j.biopsych.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Grace AA. 1995. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 15: 3622–3639. 10.1523/JNEUROSCI.15-05-03622.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. 1991. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev 43: 425–473. [PubMed] [Google Scholar]
- Pak TR, Chung WCJ, Lund TD, Hinds LR, Clay CM, Handa RJ. 2005. The androgen metabolite, 5α-androstane-3β, 17β-diol, is a potent modulator of estrogen receptor-β1-mediated gene transcription in neuronal cells. Endocrinology 146: 147–155. 10.1210/en.2004-0871 [DOI] [PubMed] [Google Scholar]
- Pelletier G, Liao N, Follea N, Govindan MV. 1988. Mapping of estrogen receptor-producing cells in the rat brain by in situ hybridization. Neurosci Lett 94: 23–28. 10.1016/0304-3940(88)90264-9 [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Ito R, Verschure PF, Battaglia FP, Robbins TW. 2011. The hippocampal-striatal axis in learning, prediction and goal-directed behavior. Trends Neurosci 34: 548–559. 10.1016/j.tins.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. 2004. Induction of ΔFosB in reward-related brain structures after chronic stress. J Neurosci 24: 10594–10602. 10.1523/JNEUROSCI.2542-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. 1973. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol 151: 121–157. 10.1002/cne.901510204 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, Schaefer SM, Benca RM, Davidson RJ. 2004. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry 9: 393–405. 10.1038/sj.mp.4001469 [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. 2004. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 125: 1–6. 10.1016/j.neuroscience.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Rajkowska G. 2000. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry 48: 766–777. 10.1016/S0006-3223(00)00950-1 [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. 2010. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry 67: 1128–1136. 10.1016/j.biopsych.2010.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. 2013. The brain reward circuitry in mood disorders. Nat Rev Neurosci 14: 609–625. 10.1038/nrn3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M. 1990. Rapid membrane effects of steroid hormones: an emerging concept in neuroendocrinology. Trends Neurosci 13: 359–362. 10.1016/0166-2236(90)90016-4 [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. 2004. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol 16: 989–998. 10.1111/j.1365-2826.2004.01258.x [DOI] [PubMed] [Google Scholar]
- Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, Demyttenaere K, de Girolamo G, Haro JM, Jin R, et al. 2009. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiat 66: 785–795. 10.1001/archgenpsychiatry.2009.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AF. 2004. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiatry 9: 531–538. 10.1038/sj.mp.4001435 [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. 2010. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex 20: 2560–2567. 10.1093/cercor/bhq003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. 1996. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci 93: 3908–3913. 10.1073/pnas.93.9.3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. 1999. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 19: 5034–5043. 10.1523/JNEUROSCI.19-12-05034.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shores MM, Sloan KL, Matsumoto AM, Moceri VM, Felker B, Kivlahan DR. 2004. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 61: 162–167. 10.1001/archpsyc.61.2.162 [DOI] [PubMed] [Google Scholar]
- Shors TJ, Leuner B. 2003. Estrogen-mediated effects on depression and memory formation in females. J Affect Disord 74: 85–96. 10.1016/S0165-0327(02)00428-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. 2001. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci 21: 6292–6297. 10.1523/JNEUROSCI.21-16-06292.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Falduto J, Leuner B. 2004. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur J Neurosci 19: 145–150. 10.1046/j.1460-9568.2003.03065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slob AK, Bogers H, van Stolk MA. 1981. Effects of gonadectomy and exogenous gonadal steroids on sex differences in open field behaviour of adult rats. Behav Brain Res 2: 347–362. 10.1016/0166-4328(81)90017-6 [DOI] [PubMed] [Google Scholar]
- Smith MD, Jones LS, Wilson MA. 2002. Sex differences in hippocampal slice excitability: role of testosterone. Neuroscience 109: 517–530. 10.1016/S0306-4522(01)00490-0 [DOI] [PubMed] [Google Scholar]
- Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, Chen A, Peeters BW, Roubos EW, Kozicz T. 2011. Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS ONE 6: e28128. 10.1371/journal.pone.0028128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. 2008. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science 321: 1690–1692. 10.1126/science.1160873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. 2000. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 157: 1552–1562. 10.1176/appi.ajp.157.10.1552 [DOI] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. 2008. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci 28: 4216–4230. 10.1523/JNEUROSCI.0258-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, Vardaris RM, Lewis D, Rawitch AB. 1980. Gonadal steroids: effects on excitability of hippocampal pyramidal cells. Science 209: 1017–1018. 10.1126/science.7190730 [DOI] [PubMed] [Google Scholar]
- Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. 2015. An excitatory synapse hypothesis of depression. Trends Neurosci 38: 279–294. 10.1016/j.tins.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Bowser ME, Davis M. 2007. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor α (ERα) and ERβ. J Neurosci 27: 9729–9735. 10.1523/JNEUROSCI.2529-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. 2006. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci 9: 519–525. 10.1038/nn1659 [DOI] [PubMed] [Google Scholar]
- Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, Yurgelun-Todd DA. 2000. Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry 47: 1087–1090. 10.1016/S0006-3223(99)00296-6 [DOI] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE III, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ III, Watts EL, Wallace DL, et al. 2010. ΔFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci 13: 745–752. 10.1038/nn.2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. 2002. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22: 6810–6818. 10.1523/JNEUROSCI.22-15-06810.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright SR, Lieblich SE, Galea LA. 2011. Hypogonadism predisposes males to the development of behavioural and neuroplastic depressive phenotypes. Psychoneuroendocrinology 36: 1327–1341. 10.1016/j.psyneuen.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. 2003. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463: 199–216. 10.1016/S0014-2999(03)01282-2 [DOI] [PubMed] [Google Scholar]
- Walther A, Breidenstein J, Miller R. 2019. Association of testosterone treatment with alleviation of depressive symptoms in men: a systematic review and meta-analysis. JAMA Psychiatry 76: 31–40. 10.1001/jamapsychiatry.2018.2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF. 2008. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry 13: 786–799, 741. 10.1038/mp.2008.38 [DOI] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. 1995. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res 703: 26–30. 10.1016/0006-8993(95)01059-9 [DOI] [PubMed] [Google Scholar]
- Warren BL, Mazei-Robison MS, Robison AJ, Iniguez SD. 2020. Can I get a witness? Using vicarious defeat stress to study mood-related illnesses in traditionally understudied populations. Biol Psychiatry 88: 381–391. 10.1016/j.biopsych.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, Kodadek TJ, Nestler EJ. 2009. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci 29: 7820–7832. 10.1523/JNEUROSCI.0932-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ES, Manning CE, Eagle AL, Swift-Gallant A, Duque-Wilckens N, Chinnusamy S, Moeser A, Jordan C, Leinninger G, Robison AJ. 2020. Androgen-dependent excitability of mouse ventral hippocampal afferents to nucleus accumbens underlies sex-specific susceptibility to stress. Biol Psychiatry 87: 492–501. 10.1016/j.biopsych.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. 1992. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 16: 525–534. 10.1016/S0149-7634(05)80194-0 [DOI] [PubMed] [Google Scholar]
- Wissman AM, May RM, Woolley CS. 2012. Ultrastructural analysis of sex differences in nucleus accumbens synaptic connectivity. Brain Struct Funct 217: 181–190. 10.1007/s00429-011-0353-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. 2010. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev 35: 185–211. 10.1016/j.neubiorev.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Moss RL. 1992. Long-term and short-term electrophysiological effects of estrogen on the synaptic properties of hippocampal CA1 neurons. J Neurosci 12: 3217–3225. 10.1523/JNEUROSCI.12-08-03217.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. 1994. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci 14: 7680–7687. 10.1523/JNEUROSCI.14-12-07680.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Schwartzkroin PA. 1998. Hormonal effects on the brain. Epilepsia 39: S2–S8. 10.1111/j.1528-1157.1998.tb02601.x [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. 1990. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci 10: 4035–4039. 10.1523/JNEUROSCI.10-12-04035.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO). 2017. Depression and other common mental disorders: global health estimates. World Health Organization, Geneva, Switzerland. [Google Scholar]
- Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, et al. 2006. An essential role for ΔFosB in the nucleus accumbens in morphine action. Nat Neurosci 9: 205–211. 10.1038/nn1636 [DOI] [PubMed] [Google Scholar]
- Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. 2009. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract 15: 289–305. 10.1097/01.pra.0000358315.88931.fc [DOI] [PubMed] [Google Scholar]
- Zhang JM, Tonelli L, Regenold WT, McCarthy MM. 2010. Effects of neonatal flutamide treatment on hippocampal neurogenesis and synaptogenesis correlate with depression-like behaviors in preadolescent male rats. Neuroscience 169: 544–554. 10.1016/j.neuroscience.2010.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]