Abstract
Chronic obstructive pulmonary disease (COPD) is the end result of a series of dynamic and cumulative gene–environment interactions over a lifetime. The evolving understanding of COPD biology provides novel opportunities for prevention, early diagnosis, and intervention. To advance these concepts, we propose therapeutic trials in two major groups of subjects: “young” individuals with COPD and those with pre-COPD. Given that lungs grow to about 20 years of age and begin to age at approximately 50 years, we consider “young” patients with COPD those patients in the age range of 20–50 years. Pre-COPD relates to individuals of any age who have respiratory symptoms with or without structural and/or functional abnormalities, in the absence of airflow limitation, and who may develop persistent airflow limitation over time. We exclude from the current discussion infants and adolescents because of their unique physiological context and COPD in older adults given their representation in prior randomized controlled trials (RCTs). We highlight the need of RCTs focused on COPD in young patients or pre-COPD to reduce disease progression, providing innovative approaches to identifying and engaging potential study subjects. We detail approaches to RCT design, including potential outcomes such as lung function, patient-reported outcomes, exacerbations, lung imaging, mortality, and composite endpoints. We critically review study design components such as statistical powering and analysis, duration of study treatment, and formats to trial structure, including platform, basket, and umbrella trials. We provide a call to action for treatment RCTs in 1) young adults with COPD and 2) those with pre-COPD at any age.
Keywords: COPD, clinical trials, early, pre-COPD, young age
Chronic obstructive pulmonary disease (COPD) is a major global public health problem. Conventionally believed to be a self-inflicted disease owing to tobacco smoking that affects the elderly (1), recent research has shown that COPD is the end result of a series of dynamic and cumulative gene–environment interactions over the lifetime that go beyond smoking (2), can begin early in life (in utero, infancy, and/or adolescence) (3–6), and result in varying lung function trajectories (trajectome), several of which lead to COPD in adulthood (7–9) (Figure 1). This new understanding of COPD provides novel opportunities for prevention, early diagnosis, and intervention (10). This state-of-the-art review seeks to launch a call to action to investigators, funding agencies, industry, and regulators to initiate treatment trials in 1) young adults with COPD and 2) those with pre-COPD, which includes those with respiratory symptoms, abnormal imaging, and/or lung function without evidence of airflow limitation who may (or may not) develop COPD with time (11, 12).
Figure 1.
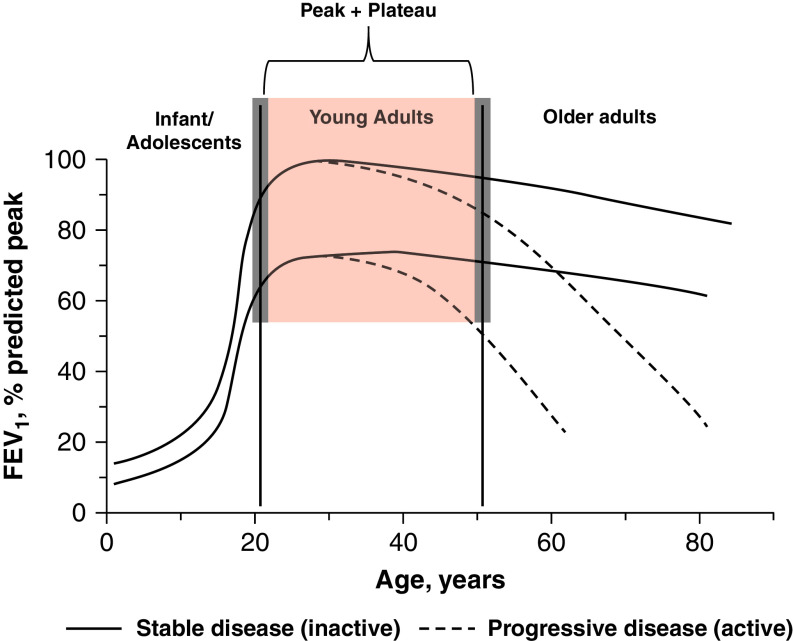
Examples of lung function trajectories from birth to death. The red shaded area highlights the population of young adults with chronic obstructive pulmonary disease to be included in treatment trials. Gray shaded areas indicate that these age limits are somewhat arbitrary (based on normal peak + plateau lung function), and therefore some age variability may be acceptable. Note also that this age range includes trajectories with normal peak lung function (100% reference) as well as those with reduced peak lung function (<80% reference) and that both can have a normal or an enhanced decline with time. For further explanations, see the main text.
Nosology
A first key step to this end is to avoid nosological confusion. Accordingly, we propose to adopt the following terminology (Table 1).
Table 1.
Nosology Used in This Review
| Term | Definition |
|---|---|
| Early COPD | Biological term that indicates that the disease is near its beginning (at any age); requires validated biomarkers to be identified/quantified in clinical practice |
| Mild COPD | Functional term that indicates that the disease is associated with mild airflow limitation (at any age) |
| COPD in Young Patients | Age-dependent term that identifies a subpopulation of patients with COPD (FEV1/FVC < 0.7) between 20 and 50 years of age (independent of the severity of airflow limitation present) |
| Pre-COPD | Individuals (of any age) who present chronic respiratory symptoms, with or without structural and/or functional abnormalities, in the absence of airflow limitation (FEV1/FVC > 0.7) who may (or may not) develop persistent airflow limitation (i.e., COPD) over time |
| Disease activity | Biological term that relates to the level of activation of the pathobiological processes (endotypes) that cause the disease; it can ideally be identified and quantified by validated biomarkers (currently lacking in COPD) |
| Disease progression | Clinical term that refers to a progressive deterioration in an objective marker of pathology or lung function |
| Primary prevention | Aimed at preventing the disease before it occurs by eliminating exposures to risk factors and/or increasing resistance to disease should exposure occur |
| Secondary prevention | Aimed at reducing the impact of a disease that has already occurred by diagnosing and treating it as soon as possible to halt or slow its progress |
| Tertiary prevention | Aimed at mitigating the impact of an ongoing illness |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
For further explanations, see the main text.
COPD
As defined by the Global Initiative for Chronic Obstructive Lung Disease, COPD is a disease “characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases and influenced by host factors including abnormal lung development” (13).
Early COPD
According to the Merriam-Webster dictionary, “early” means “near the beginning of a process.” Because COPD can start early in life (3–5) and generally takes a long time to manifest clinically (8), defining whether someone suffers “early” COPD is difficult (14). Some studies have used “mild” airflow limitation as a surrogate for “early” disease (15). This assumption would be correct if all patients started their journey from a normal peak lung function in early adulthood, and COPD would have progressed similarly in all of them. Alas, this assumption is incorrect (Figure 1) (8). “Mild” (like moderate or severe) airflow limitation can occur at any age (Figure 1) and only describes the “severity” of airflow limitation. Therefore, we propose that “mild” should not be used to identify “early” COPD. Likewise, we recognize that mild airflow limitation may not always be COPD, at least according to the current definition of airway diseases. Finally, a biological “early,” related to the initial mechanisms that eventually lead to COPD, should be differentiated from a clinical “early,” which reflects the initial perception of symptoms, functional limitation, and/or structural abnormalities noted (16). Accordingly, we propose to use the term “early COPD” only to discuss “biological early.”
COPD in Young Patients
The term “young” directly relates to the age of the subject and may seem straightforward and less confusing. However, to some extent, “young” is also a value judgment that depends on the age of the observer and the part of the globe in which the individual lives, as life expectancy varies greatly in different parts of the world. For the discussion that follows, given that lung growth and development reach their peak at around 20–25 years of age and begin to decline at 45–50 years (17), we propose to operationally consider “young” patients with COPD as those included in an age range of 20–50 years (Figure 1). In population-based studies, these younger individuals have a higher prevalence of prior asthma diagnosis (18). It is anticipated that in young patients with COPD, preventive measures and pharmacological interventions may result in better outcomes than in older patients (10, 19, 20) and may slow down disease progression (10). Importantly, this age range can include patients who never achieved normal peak lung function in early adulthood and/or those with early accelerated lung function decline (Figure 1), which may have different underlying mechanisms (i.e., endotypes [21]) and may therefore require different therapeutic interventions (5, 22, 23).
Pre-COPD
The term pre-COPD has been recently proposed to identify individuals of any age who have respiratory symptoms with or without structural and/or functional abnormalities, in the absence of airflow limitation (FEV1/FVC ⩾0.7), and who may (or may not) develop persistent airflow limitation (i.e., COPD) over time (11, 12). This term includes a heterogeneous population of patients. So far, several subtypes of pre-COPD have been reported. The best studied one includes patients with nonobstructive chronic bronchitis, in whom symptoms are associated with significant morbidity regardless of whether they ultimately progress to airflow limitation (11). Other, less well-studied subtypes of pre-COPD are subjects without airflow limitation who have emphysema detected with computed tomography (CT) (24), individuals with preserved ratio impaired spirometry (postbronchodilator FEV1 <80% predicted and FEV1/FVC ⩾0.70) (25), and subjects with low DlCO (26) or rapid FEV1 decline (27). The natural history and potential response to treatment for these heterogenous conditions is unknown.
Disease Activity versus Disease Progression
These two terms are related but not synonymous. Disease “activity” relates to the level of activation of the pathobiological processes that cause the disease (14), whereas disease “progression” refers to a deterioration over time in an objective marker of pathology, such as by CT, or function. The relationship between disease “activity” and disease “progression” in COPD remains unclear. Disease activity is probably a necessary but insufficient condition for disease progression. For instance, a given patient may suffer frequent exacerbations (a clinical surrogate marker of an “active” disease”) without a clear deterioration in lung function (a marker of disease “progression”). We currently lack validated biomarkers to identify whether or not specific endotypes are “active” in COPD (28) and, as a result, disease “activity” in COPD is often estimated post hoc by evidence of disease “progression” (29). Of note, however, in older patients with moderate to severe COPD, persistent systemic inflammation has been associated with increased all-cause mortality and exacerbation frequency (30), and the use of inflammometry and multidimensional assessment to guide treatment improved several patient-related outcomes (PROs) at 3 months in a small pilot randomized controlled trial (RCT) (31). Whether these limited preliminary data apply to younger patients is unknown. Likewise, patients with milder airflow limitation appear to progress (in terms of FEV1 decline) faster than those with more severe airflow limitation (32), so identifying biomarkers of disease activity associated with different lung function trajectories (Figure 1) would be of great value (33, 34). In any case, future treatment trials in young patients should ideally target individuals at risk of disease progression based on validated biomarkers of disease activity.
Primary, Secondary, and Tertiary Prevention
Primary prevention aims at preventing a disease before it occurs by eliminating exposures to risk factors and/or increasing resistance to it should exposure occur. Primary prevention of COPD is key in children and adolescents (Figure 1) but likely relates more to public health measures than to therapeutic interventions, although we acknowledge that boosting “catch-up” of impaired lung function in early life may deserve specific investigation (8, 24, 35, 36). Secondary prevention aims to reduce progression once disease has already manifested. This is, precisely, the goal of this call for action for treatment trials in young patients. Finally, tertiary prevention aims at reducing the impact of an ongoing illness, has been more frequently considered in the setting of COPD in older patients, and has been extensively investigated in previous RCTs.
Treatment Trials in Young Patients with COPD and Pre-COPD Patients
The discussion that follows focuses on treatment trials in young (20–50 years of age) adults with COPD or pre-COPD (any age). These trials are needed, as young patients with COPD or pre-COPD may already suffer a significant burden of disease (37, 38). In these patients, treatment cannot be neglected, although the scientific evidence supporting the best therapeutic alternatives has not been generated. In addition, it is likely that a therapeutic intervention in younger individuals with the drugs currently available, before advanced tissue destruction, multimorbidity, and effects of aging become clinically relevant, may be more effective (10) and may reduce/arrest disease progression. Finally, the combination of primary and secondary preventive measures aimed at avoiding all of those factors associated with low lung function in different age bins with appropriate, evidence-based treatment of younger patients with COPD has the potential to reduce the societal burden of disease, promote respiratory health, and eventually the development of COPD (39).
Although the results of large multicenter RCTs have driven our current understanding and management of COPD, they have historically faced a number of limitations (Table 2) (40–43). These and other considerations may also apply to future treatment trials in young patients and are discussed below.
Table 2.
Historical Factors Complicating Randomized Controlled Trials in COPD
| 1. Definition of the disease and its severity has been primarily focused on a single parameter (spirometry) |
| 2. The paucity of regulatory accepted “qualified” intermediate efficacy endpoints and validated biomarkers |
| 3. The nonnormal distribution of important trial outcomes |
| 4. Differing patterns of disease progression |
| 5. Slow FEV1 decline, which is further compounded by dropout or death among the sickest |
| 6. Disease heterogeneity: described as different phenotypes and endotypes (e.g., emphysema, airways disease, lung microbiome, neutrophilic vs. eosinophilic inflammation, aberrant tissue repair) |
| 7. Variability of endpoints and their confounders (e.g., washout of background medications, diurnal variation, seasonal effect) |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
Case Finding/Recruitment
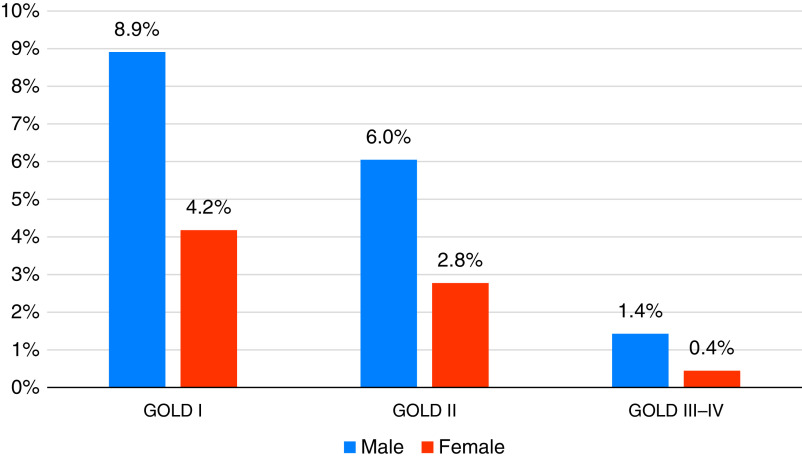
In a recent large epidemiological study in China, the prevalence of COPD in adults aged 20–49 years was 16.4% in males and 7.4% in females (Figure 2) (44), so finding and recruiting these patients into an RCT may require a combined strategy. Although widespread population spirometric screening has not been traditionally advocated (45), targeted case finding approaches are promising (46). Indeed, there has been a recent proposal supporting the use of forced spirometry in the general population (even in children and adolescents) as a marker of not only respiratory diseases but also global health (47). Symptom-based instruments (e.g., COPD Population Screener [48], International Primary Care Airways Guideline [49], COPD Questionnaire [50], and Lung Function Questionnaire [51]) and PROs questionnaires (e.g., COPD Assessment Test [52], COPD Assessment in Primary Care to Identify Undiagnosed Respiratory Disease and Exacerbation Risk [53, 54], or COPD Assessment Test/Chronic Airways Assessment Test [55]) have been developed or adapted for case findings in patients with established COPD. Their utility in young patients is unclear, as most were developed and tested in those over 40 years of age. Mucus hypersecretion can be better identified using the symptoms component of the St. George’s Respiratory Questionnaire (SGRQ) or the phlegm question in the CAT rather than the Medical Research Council definition for chronic bronchitis (11, 56–59). Physical activity, measured by daily accelerometer recordings, is reduced in patients with COPD detected by spirometry screening (60), so PROs measuring physical activity (e.g., Clinical visit-PROactive Physical Activity in COPD [61]) could potentially be used to identify these patients. Primary care networks may be particularly crucial in identifying potential young patients from electronic medical records (62), either by identifying patterns of risk factors or biomarkers or in identifying symptoms before COPD is diagnosed (63). Finally, public advertising campaigns via traditional print or electronic media, social media campaigns, and collaboration with nonprofit advocacy organizations can be useful aids to boost patient recruitment. The power of the patient-led collaborative “Venture Philanthropy” effort by the Cystic Fibrosis Foundation (64) transformed Cystic Fibrosis from a highly mortal disease in early life to a disease that can be treated with precision (65). The COPD Foundation has recently launched a multidisciplinary collaborative initiative, COPD360Net, with the mission to support the development and adoption of novel digital health tools, medical devices, and therapeutics that treat COPD, prevent its progression, and improve the lives of patients with COPD and related chronic lung conditions at all stages of disease (Figure E1 in the online supplement). COPD360Net is currently seeking partners to conduct a collaborative platform trial in young patients with pre-COPD and nonobstructive chronic bronchitis.
Figure 2.
Prevalence of chronic obstructive pulmonary disease in young individuals (20–49 years) in the general population in China. Data are from Reference 44. GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Smoking Exposure/Status
Previous RCTs in COPD have studied (older) current or former smokers. As prior studies suggested that variation in smoking status impacted the range of lung function decline (66) and mortality (67), previous history and current active smoking should be carefully monitored and adjusted for in future therapeutic trials. This is particularly relevant, as smoking cessation should be encouraged given its beneficial effects (66, 68) and is facilitated by numerous interventions (69). Notably, about one-third of patients with COPD around the world are never-smokers (70), and many other environmental risk factors are associated with low lung function through life. For example, early studies of electronic cigarette exposure have suggested that it results in altered pulmonary function and structure (71, 72). Similarly, occupational exposure and air pollution (73, 74) have been associated with respiratory disease and should be considered in study design, conduct, and analyses. It is imperative to study young never-smokers with COPD (5, 35, 75–79) exposed or not to other known (indoor pollution) or unknown environmental factors as well as those impacted by abnormal lung development before the age of 20.
Nonrespiratory Medications
It is possible that nonrespiratory medications may influence the development of COPD or its complications. For example, it remains unclear if statin therapy can favorably influence noncardiovascular complications of COPD (80). Preclinical data with metformin have suggested improvement in the development of emphysema, with cohort studies providing variable clinical correlates (81, 82). It will be important during therapeutic trials to carefully record nonrespiratory medications.
Outcome Measures
The outcome measure of any trial should be reproducible over time, have known biological variability, be responsive to treatment, and be relevant to the targeted trait. In young patients, the following outcomes could be considered.
Lung function
FEV1 is a simple, relatively inexpensive, reproducible measure recognized as an outcome measure by regulatory authorities, including the U.S. Food and Drug Administration and the European Medicines Agency. Furthermore, FEV1 decline has been traditionally used as a measure of disease progression in COPD (83). However, in young patients, there are important additional considerations. First, in these patients, a reduced FEV1 value may result from abnormal lung development and/or early enhanced decline (Figure 1) (7), and, in the absence of historic data, these two trajectories are difficult to define (7). Second, absolute decline in FEV1 is faster in patients with milder airflow limitation (32) who might be younger. If so, this may facilitate studying the impact of interventions on FEV1 decline in younger patients. Absolute FEV1 decline is also subject to bias from starting lung size, which is influenced by factors such as height and sex. Table 3 enumerates the change in FEV1 among RCTs targeting patients with mild to severe COPD with average ages of 50–65. A systematic review of RCTs suggests that a 5.0 ml/yr reduction (95% confidence interval, 0.8–9.1 ml/yr) has been suggested in the rate of FEV1 decline in active treatment arms compared with placebo (84). Arguably, younger individuals show faster FEV1 decline (32) because they have more lung function left to lose, and this might make it easier to study the impact of interventions on FEV1 decline. However, a single FEV1 measurement is not a good predictor of the future FEV1 trajectory, and rapid FEV1 decliners can only be identified in retrospect (85, 86). Thus, it may be more pragmatic to enrich the study population using surrogate markers of accelerated FEV1 decline (87), including chronic mucus hypersecretion (88), prior frequent exacerbations (32, 89), or imaging features as described below.
Table 3.
Rate of FEV1 Decline (ml/yr) Study Results
| Study | Length (yr) | n | Mean FEV1 (%) | Mean Age | Active | Placebo | Difference (95% CI) | Estimated Effective SD | Implied n/Group for 90% Power to Detect (2) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| 12 ml/yr Difference | 15 ml/yr Difference | |||||||||
| SUMMIT (138) | 1–4* | 16,485 | 60 | 65 | 38 | 46 | −8 (−15, −1) | 154 | 3,462 | 2,216 |
| Zhou and colleagues (15) | 2 | 841 | 78 | 64 | 29 | 51 | −22 (−37, −6) | 110 | 1,766 | 1,131 |
| Copenhagen CHS (166) | 3 | 290 | 86 | 59 | 45 | 42 | 3 (−13, 19) | 69 | 695 | 445 |
| EUROSCOPE (167) | 3 | 1,277 | 77 | 52 | 57 | 69 | −12 NS | UNK | NS | NS |
| TORCH (168) | 3 | 6,112 | 44 | 65 | 42/43/39 | 55 | −16 (−25, −8) | 113 | 1,864 | 1,193 |
| BRONCUS (169) | 3 | 523 | 57 | 62 | 56 | 47 | 8 (−10, 25) | 97 | 1,374 | 879 |
| ISOLDE (170) | 3 | 751 | 50 | 64 | 50 | 59 | −9 (−3, 20) | 76 | 843 | 540 |
| Lung Health Study II (171) | 3.5–4.5* | 1,116 | 68 | 56 | 44 | 47 | −3 (−11, 5) | 70 | 716 | 458 |
| UPLIFT (40, 172) | 4 | 5,993 | 48 | 65 | 40 | 42 | −2 (−6, 2) | 72 | 757 | 485 |
| Lung Health Study I (68) | 5 | 5,887 | 78 | 48 | 30 | 66 | −31 UNK | 57 | 475 | 304 |
Definition of abbreviations: BRONCUS = Bronchitis Randomized on NAC Cost-Utility Study; CI = confidence interval; COPD = chronic obstructive pulmonary disease; Copenhagen CHS = Copenhagen City Heart Study; EUROSCOPE = European Respiratory Society Study on Chronic Obstructive Pulmonary Disease; ISOLDE = Inhaled Steroids in Obstructive Lung Disease in Europe; NS = not stated; SUMMIT = Study to Understand Mortality and Morbidity in COPD; TORCH = Towards a Revolution in COPD Health; UNK = unknown; UPLIFT = Understanding Potential Long-Term Impact on Function with Tiotropium.
Variable length follow up.
Longer trials extending beyond 3 years (the minimum currently required by regulators) reduce FEV1 decline variability and, accordingly, the sample size required. Table 3 highlights that trials to evaluate rate of decline have generally been long in duration and that longer trials reduce variability. The common approach to conduct a 3-year trial still requires a relatively large sample. For example, if we assume an SD of 100 ml/yr and wish to detect a difference of 12 ml/yr, approximately 1,500/group are required to be 90% powered for α = 0.05. If instead an SD of 80 ml/yr and effect size of 15 ml/yr are assumed, then this requirement for 90% power drops to approximately 600/arm. The advantage of longer trials needs to be counterbalanced by the challenge of retaining sufficient subjects and avoiding issues with biases introduced by differential withdrawal. As such, it will be key to carefully and prospectively define the estimand (90) that we are trying to estimate with consideration of how subjects who prematurely discontinue the intervention and/or leave the trial will be handled. In any case, FEV1 decline should be measured in studies of young patients to characterize the population and to evaluate potential disease modification. Other lung function measures, such as novel spirometric parameters (91–93), inspiratory capacity, body plethysmography (to quantify hyperinflation), oscillometry, and DlCO, may also deserve investigation in this population.
Symptoms/health status/PROs
The CAT is likely to be the preferred option in most cases, irrespective of whether participants have been recruited on the basis of mucus hypersecretion, breathlessness, or exercise limitation, as its multidimensional nature will capture changes in each of these features. Unless study participants are symptomatic, symptom scores, PROs, and health-related quality of life measures will not be able to detect improvement with interventions, as CAT score values <10 are not associated with a noticeable effect on daily life (94). Yet, available evidence shows that young patients are not asymptomatic (37) and that patients with COPD with mild airflow obstruction (not necessarily young) do have marginally elevated SGRQ and CAT scores (57, 58, 95, 96), suggesting that there is potential for improvement. In fact, a trial in patients at an average age of 65 years old with COPD and mild to moderate airflow limitation showed an effect on CAT scores (15), and a subgroup analysis of the Early MAXimisation of bronchodilation for improving COPD stability (EMAX) study (mean age, 65 years) showed that the magnitude of symptomatic benefit of dual bronchodilator compared with monotherapy was similar in patients with CAT scores of ∼10 or ∼20 (97). Measures of physical activity (e.g., Daily-PROactive Physical Activity in COPD [61]) may also be considered. Bronchodilator trials in Global Initiative for Chronic Obstructive Lung Disease grade 1 patients showed variable results (98, 99). What constitutes a minimal clinically important difference (MCID) in patients with a low level of symptoms (100, 101), and whether therapeutic interventions in these patients would have a large enough effect to be detected, are uncertain. In summary, the optimum symptom score, PRO, or health-related quality of life measure to use in a particular trial will depend on the inclusion criteria. Lastly, measures of healthcare use should also be considered as relevant endpoints given their impact on patients and the healthcare system (102, 103).
Exacerbations
Exacerbations of COPD (ECOPD) remain a central, valid, and important tenet for the adequate assessment of clinical disease and therapeutic needs, and for RCTs, they represent an important outcome measure (18, 102, 104, 105). Their prevalence and severity in young patients are still not well defined, but they do indeed occur (37). ECOPD in young individuals may be influenced by symptom reporting. This, in turn, may be subject to individual variability in perception (106) and further prejudiced by societal and cultural norms for interpreting and reporting respiratory symptoms as well as by local primary care setup and its interface with tertiary hospitals. We do not know if frequent “exacerbator” phenotypes exist in young patients, so more epidemiological work on this group would assist in developing interventional studies.
Lung imaging
Imaging biomarkers can be used to identify both individuals at high risk for disease progression and endpoints in treatment trials in young patients. In particular, CT metrics of small airway abnormality may be most helpful to enrich the study population with young patients at higher risk for disease progression. Density-based metrics have the strongest supportive data for reproducibility, making them attractive as clinical endpoints, although they may be relevant only for specific therapeutic interventions.
Several airway abnormalities on chest CT scans correlate with dyspnea, quality of life, and functional capacity and predict lung function decline (Table E1) (57, 107). Pi10, a measure of the thickness of medium-size airways, relates to incident COPD over 3–5 years (108, 109) and is sensitive to change over time (110), even over short follow-up periods (111). Parametric response mapping (PRM) matches inspiratory and expiratory images to estimate nonemphysematous gas trapping or functional small airways disease and also predicts lung function decline (112, 113). The normal density E to I Ratio, another measure of gas trapping owing to small airway disease, is also associated with FEV1 decline (114). Total airway count correlates with the number of terminal bronchioles on micro-CT (115) and is associated with FEV1 decline, especially in those with mild to moderate disease (116). Airway fractal dimension, a measure of the complexity of airway branching, is lower in patients with more severe airflow limitation and is also associated with FEV1 decline (117). Finally, the airway surface area–to–volume ratio reflects a combination of airway loss and airway narrowing, is associated with FEV1 decline, and can be used to phenotype individuals into those with predominant loss versus narrowing of airways (118, 119).
Density-based measures of emphysema are also associated with lung function decline (120, 121) and mortality (117, 119, 122–125). CT emphysema progresses over time, particularly in current smokers (126, 127). The lung density metric is already in use in α-1 antitrypsin deficiency, a known cause of COPD in the young, as the primary endpoint to assess the impact of interventions targeting attenuation of disease progression (128, 129). The correlation between emphysema and lung function is weaker compared with metrics of small airway abnormality (130, 131), but the reproducibility for PRM emphysema is higher (132). This can allow a smaller sample size in RCTs (133). Finally, both PRM emphysema and PRM for functional small airways disease have histologic validation with human lung tissue (134). Studies using CT measures of lung biomechanics suggest that once emphysema is initiated, mechanotransduction can accelerate further development of emphysema; therefore, CT indices that assess alterations in lung biomechanics have been associated with FEV1 decline and BODE (125, 135). Qualitative assessment of mucus plugs on chest CT has been associated with ECOPD (136).
Other imaging techniques also hold promise. For instance, polarized gas magnetic resonance imaging can identify abnormalities in diffusion and ventilation, which may precede the development of clinically overt disease (137).
Mortality
Several issues need consideration in relation to mortality as a potential outcome in future studies of young patients. First, the comparison of death rates in major COPD trials in the 2000s (40, 43) and 2010s (138) shows that, fortunately, the risk of mortality in COPD is decreasing and may hopefully continue to decrease in the near future. Second, life expectancy varies widely across the world, so geographical variations will have to be considered in any future study in young patients. Finally, death rates are substantially lower in younger (20–50 years) than older patients with COPD included in previous studies (40, 43, 138), and this may have a direct impact on sample size estimation. For instance, in the United States in 2017, the death rate in the 35–54 age group was about 300 per 100,000 persons (139). If we hypothesize that COPD may increase this risk two- or threefold, estimated deaths in a population of young patients would be in the range of 600 to 900 deaths per 100,000 in a given year. Then, if a given therapeutic intervention was to reduce mortality in these patients by 30% (likely an optimistic estimation), an RCT with mortality as a primary endpoint would require recruiting about 80,000 patients, significantly more than those recruited in the Towards a Revolution in COPD Health (TORCH), Understanding Potential Long-Term Impact on Function with Tiotropium (UPLIFT), and Study to Understand Mortality and Morbidity in COPD (SUMMIT) trials, which randomized from 6,000 to 16,000 patients (40, 43, 138). These considerations make mortality an unlikely useful outcome measure in future treatment trials in young patients.
Composite endpoints
A composite endpoint combines different individual endpoints to increase the frequency of events, allowing RCTs to be conducted with fewer participants and/or to be shorter. A composite endpoint also enables different aspects of a disease to be evaluated together, potentially providing a broader view of the impact of a therapeutic intervention, and can be used to reduce bias caused by subjects who prematurely discontinue. The components of a composite endpoint need to be carefully selected to be sufficiently independent and of similar clinical relevance. The frequency of each component should ideally be similar so that more frequently occurring components do not dominate. Alternatively, each component can be weighted differently. For instance, in COPD trials in older patients with severe COPD, although ECOPD is likely more frequent than death, both could be components of a potential composite endpoint. In contrast, the lesser anticipated mortality of young patients suggests that such a composite endpoint may be even more dominated by ECOPD, even if these events are less prevalent than in older patients with COPD. A post hoc analysis of data from the UPLIFT trial of 5,992 patients with COPD randomized to receive tiotropium versus usual care studied over 4 years showed that a composite index consisting of death, respiratory failure, hospitalized exacerbations, and trial dropout owing to COPD worsening could reduce the number of patients needed to achieve a significant outcome by half (140).
The clinically important deterioration (CID) composite endpoint, which combines worsening of PROs and FEV1 with ECOPD, was designed for short COPD clinical trials (141), and several studies showed significant treatment differences (142–144). Furthermore, longer studies demonstrated that CID events during the first 6 months of the study predict later mortality (145–147), suggesting that pharmacological interventions that modify CID frequency in short-term trials may have longer term benefits. The use of CID in studies in young patients needs to consider several aspects. It is unclear if the MCID threshold values determined in older patients (4-unit worsening for SGRQ and 100-ml decrease for FEV1) are valid in younger patients (141). Likewise, the frequency and variability of CID components varies between cohorts with different characteristics, so they need to be established in a cohort of young patients, as they dictate the sample size calculations of future studies. In summary, CID provides a framework for a potential composite endpoint in young patients, but methodology work is needed to identify the most appropriate components and define appropriate MCID thresholds. Other potential composite endpoints to consider in this population include the Early Clinically Important Improvement (148) and COPDCompEx (see above) (149). An alternative to using a composite endpoint would be to jointly model the relevant endpoints that indicate deterioration (150), an approach that has been used in oncology trials (151).
Treatable Traits, Endotypes, and Biomarkers
As COPD is complex and heterogeneous, its clinical management requires a personalized approach. To this end, a management strategy based on treatable traits (TTs) has been proposed (152). TTs can be recognized based on their clinical characteristics (i.e., phenotypes) and/or through validated biomarkers of specific pathobiological mechanisms (i.e., endotypes) in the pulmonary, extrapulmonary, and behavioral/environmental domains (152). TTs can coexist, interact, and change with time (spontaneously or as a result of treatment) in the same patient (152). Because management guided by TTs can improve clinical outcomes (31), the design of future treatment trials in young patients needs to consider their presence or absence. Likewise, it should be noted that endotypes may vary with age, so they may differ in young and older patients with COPD, and improved understanding derived from ongoing research in young individuals may inform future treatment guidelines.
A promising biological marker is circulating eosinophils. RCTs in patients with moderate to very severe COPD have shown that higher blood eosinophil counts at baseline are associated with greater benefits from inhaled corticosteroids (ICS) (153). This biomarker is now used in clinical practice to guide ICS use in patients with a history of exacerbations (154). Bronchoscopy and sputum studies in patients with COPD have demonstrated that higher blood eosinophil counts are associated with increased lung eosinophil numbers and a profile of T2 inflammation, providing an explanation for the differential ICS effect (155, 156). Furthermore, lower blood and sputum eosinophils are associated with greater presence of proteobacteria (157, 158), with increased bacterial infections and pneumonia observed in these individuals (159). Clinical trials in younger patients with COPD may be able to use blood eosinophil counts to select subgroups with distinct inflammation and microbiome profiles, and there may be considerable potential for ICS or other interventions targeting T2 inflammation in younger patients with COPD with higher eosinophil counts. It is hoped that with improved understanding of the biological underpinnings of COPD in young individuals or those with pre-COPD, therapeutic approaches to be tested will be targeted to specific TTs.
Type of Intervention
Pharmacological interventions are likely to be central in treatment trials of young adults with COPD, but other types of intervention may also be considered, alone or in combination with drug interventions. For instance, smoking cessation measures will have to be incorporated in any study design, even to get the approval of institutional review boards. Likewise, the promotion of healthy a lifestyle (exercise, diet, sleep, and inhalational substance avoidance) will have to be considered as well.
Placebo or Comparator
Approved treatments for COPD do not have a lower age limit, but the scientific evidence supporting them has been generated in older populations. Thus, young patients are often treated without evidence for their effectiveness in COPD. As there are no specific approved treatments for COPD in young patients, there is no age-specific standard of care comparator. On the other hand, many currently available treatments are used in younger patients with asthma, and the use of a placebo may prove challenging depending on the agent being tested.
Statistical Power
Because of the relative lack of data on outcome measures in young patients, statistical power calculations will rely on information from observational cohorts (such as Early COPD in the United Kingdom; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints [ECLIPSE], COPD Genetic Epidemiology [COPDGene], or SPIROMICS Study of Early COPD Progression [SOURCE] in the United States; Chronic Airways Diseases Early Stratification [CADSET] in Europe [160]; and TRAIT in Japan [161]) and consortia that include a proportion of young patients (Table 4), electronic medical records. Blinded sample size reassessment/adaptive approaches and better define clinical trials duration. Likewise, the expected treatment effect sizes are not well established, although the expectation is that treatment differences might be greater in younger patients with (presumably) milder airflow limitation.
Table 4.
Partnerships That May Enable Treatment Trials in Young Patients
| Organization | Contribution |
|---|---|
| Professional organizations | Individuals at risk based on occupational exposure (e.g., firefighters, veterans, farm workers) |
| Primary care providers | Identify individuals at risk based on symptoms and risk (or early life events) |
| Birth cohorts (population based) with long longitudinal follow up | Risk predictors, biomarkers, participants |
| Population-based cohorts with longitudinal follow up | Risk predictors, biomarkers |
| Pharmaceutical industry | Partner on platform trials, shared risk |
| International scientific multidisciplinary team | Collaborate on trial design and implementation |
| Patient advocacy groups | Coordinate platform trials |
Platform Trials
There is increasing interest in developing innovative approaches to enhance efficiency of clinical trials while testing numerous questions at the same time; master protocols such as umbrella (multiple targeted therapies in the context of a single disease), basket (study a single targeted therapy in the context of multiple diseases or disease subtypes), and platform (multiple targeted therapies in the context of a single disease in a perpetual manner) trials are such an approach (162). Given potential heterogeneity in patient populations, platform trials may reflect a potential alternative to consider, as they can invoke adaptive designs in which progress is periodically reassessed, and participants are reallocated from ineffective treatments to contribute to the overall outcome (163). Master protocol approaches, including platform trials, have been principally used in oncology (64) but have seen a tremendous increase in the setting of the coronavirus disease (COVID-19) pandemic (164). An initial exploratory study in young patients could be done using a master protocol design, which offers the advantage of evaluating multiple therapies (162). This approach would benefit from collaborations among multiple stakeholders successfully used in the COVID-19 era (165), including industry partners and regulatory agencies.
Maintaining Participant Commitment in Long Clinical Trials
Younger patients with COPD may be less motivated to participate in RCTs, as they are likely to be employed and caring for a young family, unlikely to be symptom limited, and more likely to relocate. Hence, developing a strong relationship with participants will be key, as will be conducting trials through mechanisms that have broad geographic reaches (Table 4). Although the primary outcome assessments are likely to be clinic based, the use of digital health technology for interim assessments, monitoring trial medication adherence, and digital trial communication may aid. Regular participant contact to review symptoms and provide updates on trial progress and appropriate subject compensation will be important to reduce dropouts during follow up. Many outcomes can be followed using appropriately anonymized electronic medical records that enhance the quality of the data and assess an intervention’s cost-effectiveness.
Future Steps
It is clear that earlier intervention in younger patients with COPD or those at risk with pre-COPD will be a crucial next paradigm in the management of this impactful disorder. The most critical next steps now involve the design and development of specific RCTs in individuals with young COPD and pre-COPD. Table 5 enumerates potential issues and approaches to consider in their design.
Table 5.
Future Steps in the Design and Conduct of Intervention Studies of Young Patients with COPD or Those at Risk with Pre-COPD
| Young Patients with COPD | Pre-COPD | |
|---|---|---|
| Potential outcomes to explore | • Rate of FEV1 decline • Time to first COPD exacerbation |
• Time to onset of COPD • Time to worsening in CAT (1 point) or SGRQ (4 points) |
| Study duration | • 3 yr | • 3–5 yr |
| Interim analysis at 6–12 mo (to assess dropping therapy arms and/or extending trial duration/increase sample size) | • Rate of FEV1 decline • Time to first COPD exacerbation • CAT change • Composite outcomes* |
• Rate of FEV1 decline • CAT change • E-RS: COPD • Others (impulse oscillometry and/or lung imaging: airways disease parameters; HCRU events; CompEx COPD) • Composite outcomes* |
| Potential intervention arms | Currently approved medications for COPD | Currently approved medications for COPD as well as novel agents capable of modifying disease progression |
| Placebo control | No (as these are currently approved medications for airflow limitation with no age limits) | Yes (as these medications are not approved for this indication) |
| Study population as per the definition in the text (plus some other potential characteristics to consider in the study design to enrich the population studied) | • CAT score >10 • A respiratory HCRU event in 2 of the past 3 yr • Biomarker enrichment† |
• Individuals with NOCB symptoms as defined using the CAT or SGRQ • A respiratory HCRU event in the past 24 mo • Subjects with rapid FEV1 decline • Biomarker enrichment† |
Definition of abbreviations: CAT = COPD Assessment Test; COPD = chronic obstructive pulmonary disease; E-RS = Evaluating Respiratory Symptoms in COPD tool; HCRU = health care resource utilization; NOCB = nonobstructive chronic bronchitis; SGRQ = St. George’s Respiratory Questionnaire.
Such as clinically important deterioration examining time to FEV1 decline, exacerbation, or symptom worsening.
Circulating eosinophils and microbial assessments (see the main text).
Conclusions
Designing treatment trials in young patients and patients with pre-COPD is complex. However, the barriers mentioned herein can be overcome, and the potential rewards in terms of knowledge and improved health by conducting successful trials are likely substantial. Now is the time to refine an approach to a collaborative initiative to modify the course of the third leading cause of death in the world and a significant cause of morbidity globally. This requires commitment from industry and government funders and partnerships among diverse international stakeholders to implement platform trials using harmonized methodology and standard outcome measures that will generate robust data. These can be integrated to develop evidence-based personalized preventive and therapeutic interventions that modify disease progression based on risk factors and/or TTs (152). Working together and acting earlier in young patients and patients with pre-COPD (10) can reduce the global burden of COPD (39).
Footnotes
Supported by NIH/NHLBI grants 1R01 HL136682, U01 HL137880, R01 HL 182622, and P01 HL114501 (F.J.M.).
Author Contributions: F.J.M., A.A., B.R.C., R.T.-S., and J.A.W. prepared the first version of manuscript and finalized the last version. F.J.M., A.A., B.R.C., M.K.H., J.P.A., S.P.B., P.C., S.H.C., B.C., P. Darken, C.A.D.S., G.D., P. Dorinsky, M.D., R.F., D.M.H., P.J., J.A.K., N.L., F.D.M., H.M., D.P., K.F.R., C.R., D.S., J.V., C.F.V., R.A.W., and R.T.-S. wrote versions of key sections, reviewed, and approved the final version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202107-1663SO on October 21, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ . 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Breyer-Kohansal R, Faner R, Breyer MK, Ofenheimer A, Schrott A, Studnicka M, et al. Factors associated with low lung function in different age bins in the general population. Am J Respir Crit Care Med . 2020;202:292–296. doi: 10.1164/rccm.202001-0172LE. [DOI] [PubMed] [Google Scholar]
- 3. Martinez FD. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc . 2009;6:272–277. doi: 10.1513/pats.200808-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martinez FD. Early-life origins of chronic obstructive pulmonary disease. N Engl J Med . 2016;375:871–878. doi: 10.1056/NEJMra1603287. [DOI] [PubMed] [Google Scholar]
- 5. Agusti A, Faner R. How to define early chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2018;198:973. doi: 10.1164/rccm.201805-0880LE. [DOI] [PubMed] [Google Scholar]
- 6. Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med . 2019;381:1257–1266. doi: 10.1056/NEJMra1900500. [DOI] [PubMed] [Google Scholar]
- 7. Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med . 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 8. Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med . 2019;7:358–364. doi: 10.1016/S2213-2600(18)30529-0. [DOI] [PubMed] [Google Scholar]
- 9. Ramírez-Venegas A, Sansores RH, Quintana-Carrillo RH, Velázquez-Uncal M, Hernandez-Zenteno RJ, Sánchez-Romero C, et al. FEV1 decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am J Respir Crit Care Med . 2014;190:996–1002. doi: 10.1164/rccm.201404-0720OC. [DOI] [PubMed] [Google Scholar]
- 10. Agusti A, Alcazar B, Cosio B, Echave JM, Faner R, Izquierdo JL, et al. Scientific Committee of the ANTES programme Time for a change: anticipating the diagnosis and treatment of COPD. Eur Respir J . 2020;56:2002104. doi: 10.1183/13993003.02104-2020. [DOI] [PubMed] [Google Scholar]
- 11. Han MK, Agusti A, Celli BR, Criner GJ, Halpin DMG, Roche N, et al. From GOLD 0 to pre-COPD. Am J Respir Crit Care Med . 2021;203:414–423. doi: 10.1164/rccm.202008-3328PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Celli BR, Agustí A. COPD: time to improve its taxonomy? ERJ Open Res . 2018;4:00132-2017. doi: 10.1183/23120541.00132-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Global Initiative for Chronic Obstructive Lung Disease. 2021. www.goldcopd.org
- 14. Agustí A, Celli B. Avoiding confusion in COPD: from risk factors to phenotypes to measures of disease characterisation. Eur Respir J . 2011;38:749–751. doi: 10.1183/09031936.00062211. [DOI] [PubMed] [Google Scholar]
- 15. Zhou Y, Zhong NS, Li X, Chen S, Zheng J, Zhao D, et al. Tiotropium in early-stage chronic obstructive pulmonary disease. N Engl J Med . 2017;377:923–935. doi: 10.1056/NEJMoa1700228. [DOI] [PubMed] [Google Scholar]
- 16. Agustí A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med . 2017;5:935–945. doi: 10.1016/S2213-2600(17)30434-4. [DOI] [PubMed] [Google Scholar]
- 17. Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med . 2009;180:3–10. doi: 10.1164/rccm.200901-0047OC. [DOI] [PubMed] [Google Scholar]
- 18. Çolak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prevalence, characteristics, and prognosis of early chronic obstructive pulmonary disease. The Copenhagen general population study. Am J Respir Crit Care Med . 2020;201:671–680. doi: 10.1164/rccm.201908-1644OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morice AH, Celli B, Kesten S, Lystig T, Tashkin D, Decramer M. COPD in young patients: a pre-specified analysis of the four-year trial of tiotropium (UPLIFT) Respir Med . 2010;104:1659–1667. doi: 10.1016/j.rmed.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 20. Decramer M, Cooper CB. Treatment of COPD: the sooner the better? Thorax . 2010;65:837–841. doi: 10.1136/thx.2009.133355. [DOI] [PubMed] [Google Scholar]
- 21. Woodruff PG, Agusti A, Roche N, Singh D, Martinez FJ. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: making progress towards personalised management. Lancet . 2015;385:1789–1798. doi: 10.1016/S0140-6736(15)60693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silverman EK, Chapman HA, Drazen JM, Weiss ST, Rosner B, Campbell EJ, et al. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis. Am J Respir Crit Care Med . 1998;157:1770–1778. doi: 10.1164/ajrccm.157.6.9706014. [DOI] [PubMed] [Google Scholar]
- 23. Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. Combined impact of smoking and early-life exposures on adult lung function trajectories. Am J Respir Crit Care Med . 2017;196:1021–1030. doi: 10.1164/rccm.201703-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agustí A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med . 2019;381:1248–1256. doi: 10.1056/NEJMra1900475. [DOI] [PubMed] [Google Scholar]
- 25. Wan ES, Castaldi PJ, Cho MH, Hokanson JE, Regan EA, Make BJ, et al. COPDGene Investigators Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res . 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harvey BG, Strulovici-Barel Y, Kaner RJ, Sanders A, Vincent TL, Mezey JG, et al. Risk of COPD with obstruction in active smokers with normal spirometry and reduced diffusion capacity. Eur Respir J . 2015;46:1589–1597. doi: 10.1183/13993003.02377-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petersen H, Sood A, Polverino F, Owen CA, Pinto-Plata V, Celli BR, et al. The course of lung function in middle-aged heavy smokers: incidence and time to early onset of chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2018;198:1449–1451. doi: 10.1164/rccm.201805-0861LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vestbo J, Rennard S. Chronic obstructive pulmonary disease biomarker(s) for disease activity needed--urgently. Am J Respir Crit Care Med . 2010;182:863–864. doi: 10.1164/rccm.201004-0602ED. [DOI] [PubMed] [Google Scholar]
- 29. Celli B, Locantore N, Yates JC, Bakke P, Calverley PMA, Crim C, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Markers of disease activity in COPD: an 8-year mortality study in the ECLIPSE cohort Eur Respir J 2021. 57 2001339 33303557 [Google Scholar]
- 30. Agustí A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One . 2012;7:e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McDonald VM, Higgins I, Wood LG, Gibson PG. Multidimensional assessment and tailored interventions for COPD: respiratory utopia or common sense? Thorax . 2013;68:691–694. doi: 10.1136/thoraxjnl-2012-202646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dransfield MT, Kunisaki KM, Strand MJ, Anzueto A, Bhatt SP, Bowler RP, et al. COPDGene Investigators Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2017;195:324–330. doi: 10.1164/rccm.201605-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guerra S, Halonen M, Vasquez MM, Spangenberg A, Stern DA, Morgan WJ, et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med . 2015;3:613–620. doi: 10.1016/S2213-2600(15)00196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhai J, Stern DA, Sherrill DL, Spangenberg AL, Wright AL, Morgan WJ, et al. Trajectories and early determinants of circulating CC16 from birth to age 32 years. Am J Respir Crit Care Med . 2018;198:267–270. doi: 10.1164/rccm.201712-2398LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Melén E, Guerra S. Recent advances in understanding lung function development. F1000 Res . 2017;6:726. doi: 10.12688/f1000research.11185.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Melén E, Koppelman GH, Guerra S. On genetics, lung developmental biology, and adult lung function. Am J Respir Crit Care Med . 2020;202:791–793. doi: 10.1164/rccm.202006-2123ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cosío BG, Pascual-Guardia S, Borras-Santos A, Peces-Barba G, Santos S, Vigil L, et al. Phenotypic characterisation of early COPD: a prospective case-control study. ERJ Open Res . 2020;6:00047-2020. doi: 10.1183/23120541.00047-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sanchez-Salcedo P, Divo M, Casanova C, Pinto-Plata V, de-Torres JP, Cote C, et al. Disease progression in young patients with COPD: rethinking the Fletcher and Peto model. Eur Respir J . 2014;44:324–331. doi: 10.1183/09031936.00208613. [DOI] [PubMed] [Google Scholar]
- 39. Dransfield M, Stolz D, Kleinert S, Lancet COPD Commissioners Towards eradication of chronic obstructive pulmonary disease: a Lancet Commission. Lancet . 2019;393:1786–1788. doi: 10.1016/S0140-6736(19)30950-X. [DOI] [PubMed] [Google Scholar]
- 40. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. UPLIFT Study Investigators A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med . 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 41. Rabe KF, Martinez FJ, Ferguson GT, Wang C, Singh D, Wedzicha JA, et al. ETHOS Investigators Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med . 2020;383:35–48. doi: 10.1056/NEJMoa1916046. [DOI] [PubMed] [Google Scholar]
- 42. Lipson DA, Crim C, Criner GJ, Day NC, Dransfield MT, Halpin DMG, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2020;201:1508–1516. doi: 10.1164/rccm.201911-2207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. TORCH investigators Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med . 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 44. Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. China Pulmonary Health Study Group Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet . 2018;391:1706–1717. doi: 10.1016/S0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 45. Siu AL, Bibbins-Domingo K, Grossman DC, Davidson KW, Epling JW, Jr, García FA, et al. US Preventive Services Task Force (USPSTF) Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA . 2016;315:1372–1377. doi: 10.1001/jama.2016.2638. [DOI] [PubMed] [Google Scholar]
- 46. Martinez FJ, O’Connor GT. Screening, case-finding, and outcomes for adults with unrecognized COPD. JAMA . 2016;315:1343–1344. doi: 10.1001/jama.2016.3274. [DOI] [PubMed] [Google Scholar]
- 47. Agusti A, Fabbri LM, Baraldi E, Celli B, Corradi M, Faner R, et al. Spirometry: a practical lifespan predictor of global health and chronic respiratory and non-respiratory diseases. Eur J Intern Med . 2021;89:3–9. doi: 10.1016/j.ejim.2021.04.027. [DOI] [PubMed] [Google Scholar]
- 48. Martinez FJ, Raczek AE, Seifer FD, Conoscenti CS, Curtice TG, D’Eletto T, et al. COPD-PS Clinician Working Group Development and initial validation of a self-scored COPD Population Screener Questionnaire (COPD-PS) COPD . 2008;5:85–95. doi: 10.1080/15412550801940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Price DB, Tinkelman DG, Halbert RJ, Nordyke RJ, Isonaka S, Nonikov D, et al. Symptom-based questionnaire for identifying COPD in smokers. Respiration . 2006;73:285–295. doi: 10.1159/000090142. [DOI] [PubMed] [Google Scholar]
- 50. Maples P, Franks A, Ray S, Stevens AB, Wallace LS. Development and validation of a low-literacy Chronic Obstructive Pulmonary Disease knowledge Questionnaire (COPD-Q) Patient Educ Couns . 2010;81:19–22. doi: 10.1016/j.pec.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 51. Yawn BP, Mapel DW, Mannino DM, Martinez FJ, Donohue JF, Hanania NA, et al. Lung Function Questionnaire Working Group Development of the Lung Function Questionnaire (LFQ) to identify airflow obstruction. Int J Chron Obstruct Pulmon Dis . 2010;5:1–10. [PMC free article] [PubMed] [Google Scholar]
- 52. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J . 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 53. Martinez FJ, Mannino D, Leidy NK, Malley KG, Bacci ED, Barr RG, et al. High-Risk-COPD Screening Study Group * A new approach for identifying patients with undiagnosed chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2017;195:748–756. doi: 10.1164/rccm.201603-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Leidy NK, Martinez FJ, Malley KG, Mannino DM, Han MK, Bacci ED, et al. Can CAPTURE be used to identify undiagnosed patients with mild-to-moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis . 2018;13:1901–1912. doi: 10.2147/COPD.S152226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Han MK, Steenrod AW, Bacci ED, Leidy NK, Mannino DM, Thomashow BM, et al. Identifying patients with undiagnosed COPD in primary care settings: insight from screening tools and epidemiologic studies. Chronic Obstr Pulm Dis (Miami) . 2015;2:103–121. doi: 10.15326/jcopdf.2.2.2014.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim V, Crapo J, Zhao H, Jones PW, Silverman EK, Comellas A, et al. COPDGene Investigators Comparison between an alternative and the classic definition of chronic bronchitis in COPDGene. Ann Am Thorac Soc . 2015;12:332–339. doi: 10.1513/AnnalsATS.201411-518OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med . 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Miravitlles M, Soriano JB, García-Río F, Muñoz L, Duran-Tauleria E, Sanchez G, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax . 2009;64:863–868. doi: 10.1136/thx.2009.115725. [DOI] [PubMed] [Google Scholar]
- 59. Martinez FJ, Rabe KF, Calverley PMA, Fabbri LM, Sethi S, Pizzichini E, et al. Determinants of response to roflumilast in severe chronic obstructive pulmonary disease. Pooled analysis of two randomized trials. Am J Respir Crit Care Med . 2018;198:1268–1278. doi: 10.1164/rccm.201712-2493OC. [DOI] [PubMed] [Google Scholar]
- 60. Van Remoortel H, Hornikx M, Langer D, Burtin C, Everaerts S, Verhamme P, et al. Risk factors and comorbidities in the preclinical stages of chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2014;189:30–38. doi: 10.1164/rccm.201307-1240OC. [DOI] [PubMed] [Google Scholar]
- 61. Garcia-Aymerich J, Puhan MA, Corriol-Rohou S, de Jong C, Demeyer H, Dobbels F, et al. PROactive consortium Validity and responsiveness of the Daily- and Clinical visit-PROactive Physical Activity in COPD (D-PPAC and C-PPAC) instruments. Thorax . 2021;76:228–238. doi: 10.1136/thoraxjnl-2020-214554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ray E, Culliford D, Kruk H, Gillett K, North M, Astles CM, et al. Specialist respiratory outreach: a case-finding initiative for identifying undiagnosed COPD in primary care. NPJ Prim Care Respir Med . 2021;31:7. doi: 10.1038/s41533-021-00219-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jones RC, Price D, Ryan D, Sims EJ, von Ziegenweidt J, Mascarenhas L, et al. Respiratory Effectiveness Group Opportunities to diagnose chronic obstructive pulmonary disease in routine care in the UK: a retrospective study of a clinical cohort. Lancet Respir Med . 2014;2:267–276. doi: 10.1016/S2213-2600(14)70008-6. [DOI] [PubMed] [Google Scholar]
- 64. Park JJH, Siden E, Zoratti MJ, Dron L, Harari O, Singer J, et al. Systematic review of basket trials, umbrella trials, and platform trials: a landscape analysis of master protocols. Trials . 2019;20:572. doi: 10.1186/s13063-019-3664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Marshall E. BIOTECHNOLOGY:Disease group invests in do-it-yourself drugs. Science . 2000;288:1715b–1717b. doi: 10.1126/science.288.5472.1715b. [DOI] [PubMed] [Google Scholar]
- 66. Simmons MS, Connett JE, Nides MA, Lindgren PG, Kleerup EC, Murray RP, et al. Smoking reduction and the rate of decline in FEV(1): results from the Lung Health Study. Eur Respir J . 2005;25:1011–1017. doi: 10.1183/09031936.05.00086804. [DOI] [PubMed] [Google Scholar]
- 67. Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE, Lung Health Study Research Group The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med . 2005;142:233–239. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 68. Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA . 1994;272:1497–1505. [PubMed] [Google Scholar]
- 69. Choi HK, Ataucuri-Vargas J, Lin C, Singrey A. The current state of tobacco cessation treatment. Cleve Clin J Med . 2021;88:393–404. doi: 10.3949/ccjm.88a.20099. [DOI] [PubMed] [Google Scholar]
- 70. Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet . 2009;374:733–743. doi: 10.1016/S0140-6736(09)61303-9. [DOI] [PubMed] [Google Scholar]
- 71. Hamberger ES, Halpern-Felsher B. Vaping in adolescents: epidemiology and respiratory harm. Curr Opin Pediatr . 2020;32:378–383. doi: 10.1097/MOP.0000000000000896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Alter P, Baker JR, Dauletbaev N, Donnelly LE, Pistenmaa C, Schmeck B, et al. Update in chronic obstructive pulmonary disease 2019. Am J Respir Crit Care Med . 2020;202:348–355. doi: 10.1164/rccm.202002-0370UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schraufnagel DE, Balmes JR, Cowl CT, De Matteis S, Jung SH, Mortimer K, et al. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, part 1: the damaging effects of air pollution. Chest . 2019;155:409–416. doi: 10.1016/j.chest.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thurston GD, Balmes JR, Garcia E, Gilliland FD, Rice MB, Schikowski T, et al. Outdoor air pollution and new-onset airway disease. An official American Thoracic Society workshop report. Ann Am Thorac Soc . 2020;17:387–398. doi: 10.1513/AnnalsATS.202001-046ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pérez-Padilla R, Regalado J, Vedal S, Paré P, Chapela R, Sansores R, et al. Exposure to biomass smoke and chronic airway disease in Mexican women. A case-control study. Am J Respir Crit Care Med . 1996;154:701–706. doi: 10.1164/ajrccm.154.3.8810608. [DOI] [PubMed] [Google Scholar]
- 76. Bruce N, Perez-Padilla R, Albalak R. Indoor air pollution in developing countries: a major environmental and public health challenge. Bull World Health Organ . 2000;78:1078–1092. [PMC free article] [PubMed] [Google Scholar]
- 77. Ramírez-Venegas A, Sansores RH, Pérez-Padilla R, Regalado J, Velázquez A, Sánchez C, et al. Survival of patients with chronic obstructive pulmonary disease due to biomass smoke and tobacco. Am J Respir Crit Care Med . 2006;173:393–397. doi: 10.1164/rccm.200504-568OC. [DOI] [PubMed] [Google Scholar]
- 78. Regalado J, Pérez-Padilla R, Sansores R, Páramo Ramirez JI, Brauer M, Paré P, et al. The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am J Respir Crit Care Med . 2006;174:901–905. doi: 10.1164/rccm.200503-479OC. [DOI] [PubMed] [Google Scholar]
- 79. Olloquequi J, Jaime S, Parra V, Cornejo-Córdova E, Valdivia G, Agustí À, et al. Comparative analysis of COPD associated with tobacco smoking, biomass smoke exposure or both. Respir Res . 2018;19:13. doi: 10.1186/s12931-018-0718-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Walsh A, Perrem L, Khashan AS, Henry MT, Ni Chroinin M. Statins versus placebo for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2019;7:CD011959. doi: 10.1002/14651858.CD011959.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Polverino F, Wu TD, Rojas-Quintero J, Wang X, Mayo J, Tomchaney M, et al. Metformin: experimental and clinical evidence for a potential role in emphysema treatment. Am J Respir Crit Care Med . 2021;204:651–666. doi: 10.1164/rccm.202012-4510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wu TD, Fawzy A, Kinney GL, Bon J, Neupane M, Tejwani V, et al. Metformin use and respiratory outcomes in asthma-COPD overlap. Respir Res . 2021;22:70. doi: 10.1186/s12931-021-01658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vestbo J, Lange P. Natural history of COPD: focusing on change in FEV1. Respirology . 2016;21:34–43. doi: 10.1111/resp.12589. [DOI] [PubMed] [Google Scholar]
- 84. Celli BR, Anderson JA, Cowans NJ, Crim C, Hartley BF, Martinez FJ, et al. Pharmacotherapy and lung function decline in patients with chronic obstructive pulmonary disease. A systematic review. Am J Respir Crit Care Med . 2021;203:689–698. doi: 10.1164/rccm.202005-1854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Burrows B. An overview of obstructive lung diseases. Med Clin North Am . 1981;65:455–471. doi: 10.1016/s0025-7125(16)31509-7. [DOI] [PubMed] [Google Scholar]
- 86. Kerstjens HA, Rijcken B, Schouten JP, Postma DS. Decline of FEV1 by age and smoking status: facts, figures, and fallacies. Thorax . 1997;52:820–827. doi: 10.1136/thx.52.9.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zafari Z, Sin DD, Postma DS, Löfdahl CG, Vonk J, Bryan S, et al. Individualized prediction of lung-function decline in chronic obstructive pulmonary disease. CMAJ . 2016;188:1004–1011. doi: 10.1503/cmaj.151483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. The presence of chronic mucus hypersecretion across adult life in relation to chronic obstructive pulmonary disease development. Am J Respir Crit Care Med . 2016;193:662–672. doi: 10.1164/rccm.201511-2210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax . 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.European Medicines Agency. 2020.
- 91. Bhatt SP, Bhakta NR, Wilson CG, Cooper CB, Barjaktarevic I, Bodduluri S, et al. New spirometry indices for detecting mild airflow obstruction. Sci Rep . 2018;8:17484. doi: 10.1038/s41598-018-35930-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Dominelli PB, Foster GE, Guenette JA, Haverkamp HC, Eves ND, Dominelli GS, et al. Quantifying the shape of the maximal expiratory flow-volume curve in mild COPD. Respir Physiol Neurobiol . 2015;219:30–35. doi: 10.1016/j.resp.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 93. Topalovic M, Exadaktylos V, Decramer M, Troosters T, Berckmans D, Janssens W. Modelling the dynamics of expiratory airflow to describe chronic obstructive pulmonary disease. Med Biol Eng Comput . 2014;52:997–1006. doi: 10.1007/s11517-014-1202-6. [DOI] [PubMed] [Google Scholar]
- 94. Jones PW, Tabberer M, Chen WH. Creating scenarios of the impact of COPD and their relationship to COPD Assessment Test (CAT™) scores. BMC Pulm Med . 2011;11:42. doi: 10.1186/1471-2466-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Raghavan N, Lam YM, Webb KA, Guenette JA, Amornputtisathaporn N, Raghavan R, et al. Components of the COPD Assessment Test (CAT) associated with a diagnosis of COPD in a random population sample. COPD . 2012;9:175–183. doi: 10.3109/15412555.2011.650802. [DOI] [PubMed] [Google Scholar]
- 96. Nishimura K, Mitsuma S, Kobayashi A, Yanagida M, Nakayasu K, Hasegawa Y, et al. COPD and disease-specific health status in a working population. Respir Res . 2013;14:61. doi: 10.1186/1465-9921-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vogelmeier CF, Kerwin EM, Bjermer LH, Tombs L, Jones PW, Boucot IH, et al. Impact of baseline COPD symptom severity on the benefit from dual versus mono-bronchodilators: an analysis of the EMAX randomised controlled trial. Ther Adv Respir Dis . 2020;14:1753466620968500. doi: 10.1177/1753466620968500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gagnon P, Saey D, Provencher S, Milot J, Bourbeau J, Tan WC, et al. Walking exercise response to bronchodilation in mild COPD: a randomized trial. Respir Med . 2012;106:1695–1705. doi: 10.1016/j.rmed.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 99. O’Donnell DE, Laveneziana P, Ora J, Webb KA, Lam YM, Ofir D. Evaluation of acute bronchodilator reversibility in patients with symptoms of GOLD stage I COPD. Thorax . 2009;64:216–223. doi: 10.1136/thx.2008.103598. [DOI] [PubMed] [Google Scholar]
- 100. Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med . 2014;189:250–255. doi: 10.1164/rccm.201310-1863PP. [DOI] [PubMed] [Google Scholar]
- 101. Jones PW, Gelhorn H, Wilson H, Karlsson N, Menjoge S, Müllerova H, et al. Responder analyses for treatment effects in COPD using the St George’s Respiratory Questionnaire. Chronic Obstr Pulm Dis (Miami) . 2017;4:124–131. doi: 10.15326/jcopdf.4.2.2017.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Çolak Y, Afzal S, Nordestgaard BG, Lange P, Vestbo J. Importance of early COPD in young adults for development of clinical COPD: findings from the Copenhagen General Population Study. Am J Respir Crit Care Med . 2021;203:1245–1256. doi: 10.1164/rccm.202003-0532OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bouza E, Alvar A, Almagro P, Alonso T, Ancochea J, Barbé F, et al. Chronic obstructive pulmonary disease (COPD) in Spain and the different aspects of its social impact: a multidisciplinary opinion document. Rev Esp Quimioter . 2020;33:49–67. doi: 10.37201/req/2064.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Çolak Y, Nordestgaard BG, Vestbo J, Lange P, Afzal S. Outcomes consequent to “early” COPD for interventional studies. Eur Respir J . 2020;55:2000073. doi: 10.1183/13993003.00073-2020. [DOI] [PubMed] [Google Scholar]
- 105. Çolak Y, Nordestgaard BG, Vestbo J, Lange P, Afzal S. Comparison of five major airflow limitation criteria to identify high-risk individuals with COPD: a contemporary population-based cohort. Thorax . 2020;75:944–954. doi: 10.1136/thoraxjnl-2020-214559. [DOI] [PubMed] [Google Scholar]
- 106. Scioscia G, Blanco I, Arismendi E, Burgos F, Gistau C, Foschino Barbaro MP, et al. Different dyspnoea perception in COPD patients with frequent and infrequent exacerbations. Thorax . 2017;72:117–121. doi: 10.1136/thoraxjnl-2016-208332. [DOI] [PubMed] [Google Scholar]
- 107. Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JH, Grenier PA, et al. Genetic Epidemiology of COPD (COPDGene) Investigators Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med . 2015;175:1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mohamed Hoesein FA, de Jong PA, Lammers JW, Mali WP, Schmidt M, de Koning HJ, et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J . 2015;45:644–651. doi: 10.1183/09031936.00020714. [DOI] [PubMed] [Google Scholar]
- 109. Oelsner EC, Smith BM, Hoffman EA, Kalhan R, Donohue KM, Kaufman JD, et al. Prognostic significance of large airway dimensions on computed tomography in the general population. The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Ann Am Thorac Soc . 2018;15:718–727. doi: 10.1513/AnnalsATS.201710-820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Charbonnier JP, Pompe E, Moore C, Humphries S, van Ginneken B, Make B, et al. COPDGene investigators Airway wall thickening on CT: relation to smoking status and severity of COPD. Respir Med . 2019;146:36–41. doi: 10.1016/j.rmed.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pompe E, van Rikxoort EM, Mets OM, Charbonnier JP, Kuhnigk JM, de Koning HJ, et al. Follow-up of CT-derived airway wall thickness: correcting for changes in inspiration level improves reliability. Eur J Radiol . 2016;85:2008–2013. doi: 10.1016/j.ejrad.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 112. Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med . 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, et al. COPDGene Investigators Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2016;194:178–184. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bodduluri S, Reinhardt JM, Hoffman EA, Newell JD, Jr, Nath H, Dransfield MT, et al. COPDGene Investigators Signs of gas trapping in normal lung density regions in smokers. Am J Respir Crit Care Med . 2017;196:1404–1410. doi: 10.1164/rccm.201705-0855OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kirby M, Tanabe N, Vasilescu DM, Cooper JD, McDonough JE, Verleden SE, et al. Computed tomography total airway count is associated with the number of micro-computed tomography terminal bronchioles. Am J Respir Crit Care Med . 2020;201:613–615. doi: 10.1164/rccm.201910-1948LE. [DOI] [PubMed] [Google Scholar]
- 116. Kirby M, Tanabe N, Tan WC, Zhou G, Obeidat M, Hague CJ, et al. CanCOLD Collaborative Research Group Canadian Respiratory Research Network; CanCOLD Collaborative Research Group, the Canadian Respiratory Research Network. Total airway count on computed tomography and the risk of chronic obstructive pulmonary disease progression. Findings from a population-based study. Am J Respir Crit Care Med . 2018;197:56–65. doi: 10.1164/rccm.201704-0692OC. [DOI] [PubMed] [Google Scholar]
- 117. Bodduluri S, Puliyakote ASK, Gerard SE, Reinhardt JM, Hoffman EA, Newell JD, Jr, et al. COPDGene Investigators Airway fractal dimension predicts respiratory morbidity and mortality in COPD. J Clin Invest . 2018;128:5374–5382. doi: 10.1172/JCI120693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bodduluri S, Kizhakke Puliyakote A, Nakhmani A, Charbonnier JP, Reinhardt JM, Bhatt SP. Computed tomography-based airway surface area-to-volume ratio for phenotyping airway remodeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2021;203:185–191. doi: 10.1164/rccm.202004-0951OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bodduluri S, Nakhmani A, Reinhardt JM, Wilson CG, McDonald ML, Rudraraju R, et al. Deep neural network analyses of spirometry for structural phenotyping of chronic obstructive pulmonary disease. JCI Insight . 2020;5:e132781. doi: 10.1172/jci.insight.132781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mohamed Hoesein FA, de Hoop B, Zanen P, Gietema H, Kruitwagen CL, van Ginneken B, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax . 2011;66:782–787. doi: 10.1136/thx.2010.145995. [DOI] [PubMed] [Google Scholar]
- 121. Washko GR, Kinney GL, Ross JC, San José Estépar R, Han MK, Dransfield MT, et al. COPDGene Investigators Lung mass in smokers. Acad Radiol . 2017;24:386–392. doi: 10.1016/j.acra.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Haruna A, Muro S, Nakano Y, Ohara T, Hoshino Y, Ogawa E, et al. CT scan findings of emphysema predict mortality in COPD. Chest . 2010;138:635–640. doi: 10.1378/chest.09-2836. [DOI] [PubMed] [Google Scholar]
- 123. Johannessen A, Skorge TD, Bottai M, Grydeland TB, Nilsen RM, Coxson H, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med . 2013;187:602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 124. Lynch DA, Moore CM, Wilson C, Nevrekar D, Jennermann T, Humphries SM, et al. Genetic Epidemiology of COPD (COPDGene) Investigators CT-based visual classification of emphysema: association with mortality in the COPDGene Study. Radiology . 2018;288:859–866. doi: 10.1148/radiol.2018172294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bodduluri S, Bhatt SP, Hoffman EA, Newell JD, Jr, Martinez CH, Dransfield MT, et al. COPDGene Investigators Biomechanical CT metrics are associated with patient outcomes in COPD. Thorax . 2017;72:409–414. doi: 10.1136/thoraxjnl-2016-209544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mohamed Hoesein FA, Zanen P, de Jong PA, van Ginneken B, Boezen HM, Groen HJ, et al. Rate of progression of CT-quantified emphysema in male current and ex-smokers: a follow-up study. Respir Res . 2013;14:55. doi: 10.1186/1465-9921-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pompe E, Moore CM, Mohamed Hoesein FAA, de Jong PA, Charbonnier JP, Han MK, et al. COPDGene investigators Progression of emphysema and small airways disease in cigarette smokers. Chronic Obstr Pulm Dis (Miami) . 2021;8:198–212. doi: 10.15326/jcopdf.2020.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Stockley RA, Parr DG, Piitulainen E, Stolk J, Stoel BC, Dirksen A. Therapeutic efficacy of α-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res . 2010;11:136. doi: 10.1186/1465-9921-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chapman KR, Burdon JG, Piitulainen E, Sandhaus RA, Seersholm N, Stocks JM, et al. RAPID Trial Study Group Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet . 2015;386:360–368. doi: 10.1016/S0140-6736(15)60860-1. [DOI] [PubMed] [Google Scholar]
- 130. Thomsen LH, Shaker SB, Dirksen A, Pedersen JH, Tal-Singer R, Bakke P, et al. Correlation between emphysema and lung function in healthy smokers and smokers with COPD. Chronic Obstr Pulm Dis (Miami) . 2015;2:204–213. doi: 10.15326/jcopdf.2.3.2014.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Coxson HO, Dirksen A, Edwards LD, Yates JC, Agusti A, Bakke P, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med . 2013;1:129–136. doi: 10.1016/S2213-2600(13)70006-7. [DOI] [PubMed] [Google Scholar]
- 132. Boes JL, Hoff BA, Bule M, Johnson TD, Rehemtulla A, Chamberlain R, et al. Parametric response mapping monitors temporal changes on lung CT scans in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS) Acad Radiol . 2015;22:186–194. doi: 10.1016/j.acra.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bastarrika G, Wisnivesky JP, Pueyo JC, Díaz L, Arraiza M, Villanueva A, et al. Low-dose volumetric computed tomography for quantification of emphysema in asymptomatic smokers participating in an early lung cancer detection trial. J Thorac Imaging . 2009;24:206–211. doi: 10.1097/RTI.0b013e3181a65263. [DOI] [PubMed] [Google Scholar]
- 134. Vasilescu DM, Martinez FJ, Marchetti N, Galbán CJ, Hatt C, Meldrum CA, et al. Noninvasive imaging biomarker identifies small airway damage in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2019;200:575–581. doi: 10.1164/rccm.201811-2083OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bhatt SP, Bodduluri S, Hoffman EA, Newell JD, Jr, Sieren JC, Dransfield MT, et al. COPDGene Investigators Computed tomography measure of lung at risk and lung function decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2017;196:569–576. doi: 10.1164/rccm.201701-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Dunican EM, Elicker BM, Henry T, Gierada DS, Schiebler ML, Anderson W, et al. Mucus plugs and emphysema in the pathophysiology of airflow obstruction and hypoxemia in smokers. Am J Respir Crit Care Med . 2021;203:957–968. doi: 10.1164/rccm.202006-2248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Stewart NJ, Smith LJ, Chan HF, Eaden JA, Rajaram S, Swift AJ, et al. Lung MRI with hyperpolarised gases: current & future clinical perspectives. Br J Radiol . 2021 doi: 10.1259/bjr.20210207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, Crim C, et al. SUMMIT Investigators Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet . 2016;387:1817–1826. doi: 10.1016/S0140-6736(16)30069-1. [DOI] [PubMed] [Google Scholar]
- 139. Kochanek KD, Murphy SL, Xu J, Arias E. Deaths: final data for 2017. Natl Vital Stat Rep . 2019;68:1–77. [PubMed] [Google Scholar]
- 140. Celli BR, Decramer M, Liu D, Metzdorf N, Asijee GM, Tashkin DP. Defining a COPD composite safety endpoint for demonstrating efficacy in clinical trials: results from the randomized, placebo-controlled UPLIFT® trial. Respir Res . 2016;17:48. doi: 10.1186/s12931-016-0361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Singh D, Criner GJ, Naya I, Jones PW, Tombs L, Lipson DA, et al. Measuring disease activity in COPD: is clinically important deterioration the answer? Respir Res . 2020;21:134. doi: 10.1186/s12931-020-01387-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Naya I, Compton C, Ismaila AS, Birk R, Brealey N, Tabberer M, et al. Preventing clinically important deterioration with single-inhaler triple therapy in COPD. ERJ Open Res . 2018;4:00047-02018. doi: 10.1183/23120541.00047-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Naya IP, Tombs L, Lipson DA, Compton C. Preventing clinically important deterioration of COPD with addition of umeclidinium to inhaled corticosteroid/long-acting β2-agonist therapy: an integrated post hoc analysis. Adv Ther . 2018;35:1626–1638. doi: 10.1007/s12325-018-0771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Singh D, Maleki-Yazdi MR, Tombs L, Iqbal A, Fahy WA, Naya I. Prevention of clinically important deteriorations in COPD with umeclidinium/vilanterol. Int J Chron Obstruct Pulmon Dis . 2016;11:1413–1424. doi: 10.2147/COPD.S101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rabe KF, Halpin DMG, Han MK, Miravitlles M, Singh D, Grönke L, et al. Composite endpoints in COPD: clinically important deterioration in the UPLIFT trial. Respir Res . 2020;21:177. doi: 10.1186/s12931-020-01431-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Naya IP, Tombs L, Muellerova H, Compton C, Jones PW. Long-term outcomes following first short-term clinically important deterioration in COPD. Respir Res . 2018;19:222. doi: 10.1186/s12931-018-0928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Han MK, Criner GJ, Dransfield MT, Halpin DMG, Jones CE, Kilbride S, et al. Prognostic value of clinically important deterioration in COPD: IMPACT trial analysis. ERJ Open Res . 2021;7:00663-2020. doi: 10.1183/23120541.00663-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kostikas K, Mackay AJ, Vogelmeier CF, Frent SM, Gupta P, Banerji D, et al. Early clinically important improvement (ECII) and exacerbation outcomes in COPD patients. Int J Chron Obstruct Pulmon Dis . 2020;15:1831–1838. doi: 10.2147/COPD.S247966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Vogelmeier CF, Fuhlbrigge A, Jauhiainen A, Scheepers LEJM, Bengtsson T, Peterson S, et al. COPDCompEx: a novel composite endpoint for COPD exacerbations to enable faster clinical development. Respir Med . 2020;173:106175. doi: 10.1016/j.rmed.2020.106175. [DOI] [PubMed] [Google Scholar]
- 150. Lawrence Gould A, Boye ME, Crowther MJ, Ibrahim JG, Quartey G, Micallef S, et al. Joint modeling of survival and longitudinal non-survival data: current methods and issues. Report of the DIA Bayesian joint modeling working group. Stat Med . 2015;34:2181–2195. doi: 10.1002/sim.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol . 2010;28:2796–2801. doi: 10.1200/JCO.2009.25.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J . 2016;47:410–419. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 153. Singh D, Bafadhel M, Brightling CE, Sciurba FC, Curtis JL, Martinez FJ, et al. Blood eosinophil counts in clinical trials for chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2020;202:660–671. doi: 10.1164/rccm.201912-2384PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J . 2019;53:1900164. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 155. Higham A, Beech A, Wolosianka S, Jackson N, Long G, Kolsum U, et al. Type 2 inflammation in eosinophilic chronic obstructive pulmonary disease. Allergy . 2021;76:1861–1864. doi: 10.1111/all.14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. George L, Taylor AR, Esteve-Codina A, Soler Artigas M, Thun GA, Bates S, et al. U-BIOPRED and the EvA study teams Blood eosinophil count and airway epithelial transcriptome relationships in COPD versus asthma. Allergy . 2020;75:370–380. doi: 10.1111/all.14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Beech AS, Lea S, Kolsum U, Wang Z, Miller BE, Donaldson GC, et al. Bacteria and sputum inflammatory cell counts; a COPD cohort analysis. Respir Res . 2020;21:289. doi: 10.1186/s12931-020-01552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wang Z, Locantore N, Haldar K, Ramsheh MY, Beech AS, Ma W, et al. Inflammatory endotype-associated airway microbiome in chronic obstructive pulmonary disease clinical stability and exacerbations: a multicohort longitudinal analysis. Am J Respir Crit Care Med . 2021;203:1488–1502. doi: 10.1164/rccm.202009-3448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Martinez-Garcia MA, Faner R, Oscullo G, de la Rosa D, Soler-Cataluña JJ, Ballester M, et al. Inhaled steroids, circulating eosinophils, chronic airway infection, and pneumonia risk in chronic obstructive pulmonary disease. A network analysis. Am J Respir Crit Care Med . 2020;201:1078–1085. doi: 10.1164/rccm.201908-1550OC. [DOI] [PubMed] [Google Scholar]
- 160. Agusti A, Faner R, Donaldson G, Heuvelin E, Breyer-Kohansal R, Melén E, et al. on behalf of the CADSET Clinical Research Collaboration Current members of the CADSET Clinical Research Collaboration. Chronic Airway Diseases Early Stratification (CADSET): a new ERS Clinical Research Collaboration. Eur Respir J . 2019;53:1900217. doi: 10.1183/13993003.00217-2019. [DOI] [PubMed] [Google Scholar]
- 161. Hizawa N, Fukunaga K, Sugiura H, Nakano Y, Kato M, Sugiyama Y, et al. A prospective cohort study to assess obstructive respiratory disease phenotypes and endotypes in Japan: the TRAIT study design. Int J Chron Obstruct Pulmon Dis . 2021;16:1813–1822. doi: 10.2147/COPD.S308327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med . 2017;377:62–70. doi: 10.1056/NEJMra1510062. [DOI] [PubMed] [Google Scholar]
- 163. Saville BR, Berry SM. Efficiencies of platform clinical trials: a vision of the future. Clin Trials . 2016;13:358–366. doi: 10.1177/1740774515626362. [DOI] [PubMed] [Google Scholar]
- 164. LaVange L, Adam SJ, Currier JS, Higgs ES, Reineck LA, Hughes EA, et al. ACTIV Therapeutics-Clinical Working Group Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV): designing master protocols for evaluation of candidate COVID-19 therapeutics. Ann Intern Med . 2021;174:1293–1300. doi: 10.7326/M21-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Zhang W, Lv Y, Yang J, Chen Y, He Y, Huang J. Study design characteristics and pharmacological mechanisms in international clinical trials registry platform: registered clinical trials on antiviral drugs for COVID-19. Drug Des Devel Ther . 2020;14:3803–3813. doi: 10.2147/DDDT.S272442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Vestbo J, Sørensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet . 1999;353:1819–1823. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- 167. Pauwels RA, Löfdahl CG, Laitinen LA, Schouten JP, Postma DS, Pride NB, et al. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. N Engl J Med . 1999;340:1948–1953. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 168. Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med . 2008;178:332–338. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 169. Decramer M, Rutten-van Mölken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet . 2005;365:1552–1560. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 170. Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ . 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wise R, Connett J, Weinmann G, Scanlon P, Skeans M, Lung Health Study Research Group Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med . 2000;343:1902–1909. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 172. Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP, UPLIFT investigators Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet . 2009;374:1171–1178. doi: 10.1016/S0140-6736(09)61298-8. [DOI] [PubMed] [Google Scholar]