Abstract
Rationale
The long-term effects of vigorous physical activity (PA) on lung function in cystic fibrosis are unclear.
Objectives
To evaluate effects of a 12-month partially supervised PA intervention using motivational feedback.
Methods
In a parallel-arm multicenter randomized controlled trial (ACTIVATE-CF), relatively inactive patients aged at least 12 years were randomly assigned (1:1 ratio) to an intervention group or control group. The intervention group consented to add 3 hours of vigorous PA per week, whereas the control group was asked not to change their PA behavior. Primary endpoint was change in percent predicted FEV1 (ΔFEV1) at 6 months. Secondary endpoints included PA, exercise capacity, exercise motives, time to first exacerbation and exacerbation rates, quality of life, anxiety, depression, stress, and blood glucose control. Data were analyzed using mixed linear models.
Measurements and Main Results
A total of 117 patients (40% of target sample size) were randomized to an intervention (n = 60) or control group (n = 57). After 6 months, ΔFEV1 was significantly higher in the control group compared with the intervention group (2.70% predicted [95% confidence interval, 0.13–5.26]; P = 0.04). The intervention group reported increased vigorous PA compared with the control group at each study visit, had higher exercise capacity at 6 and 12 months, and higher PA at 12 months. No effects were seen in other secondary outcomes.
Conclusions
ACTIVATE-CF increased vigorous PA and exercise capacity, with effects carried over for the subsequent 6 months, but resulted in better FEV1 in the control group.
Keywords: cystic fibrosis, exercise program, physical activity, exercise capacity, randomized controlled trial
At a Glance Commentary
Scientific Knowledge on the Subject
Regular physical activity and exercise training are integral components of cystic fibrosis care. The effects of vigorous exercise training on lung function from randomized controlled trials in cystic fibrosis are still unclear. Large-sized and well-designed randomized controlled trials are currently lacking.
What This Study Adds to the Field
Unexpectedly, the ACTIVATE-CF study showed better FEV1 in the control group compared to the intervention group after 6 months. Future intervention programs should aim for a stepwise individualized increase in training volume to avoid overreaching/overtraining. As the multicomponent intervention increased self-reported vigorous physical activity and exercise capacity, the tools developed in the project may be used to increase physical activity.
Physical activity (PA) and exercise have become an accepted and valued component of cystic fibrosis (CF) care (1, 2). Evidence from the most recent Cochrane Review (3) on exercise training in CF suggests unclear effects of aerobic, anaerobic, or a combination of both training modalities on lung function, (i.e., FEV1). Two of the included long-term supervised exercise intervention studies have shown that regular vigorous PA might positively impact FEV1 (4, 5), which remains the most widely used and, along with the results of exercise tests, among the best prognostic factors for CF (6, 7).
Supervised exercise interventions are expensive and not easily implemented in regular patient care, and long-term adherence to supervised programs is often low (8). On the other hand, unsupervised or partially supervised programs are less costly and more flexible, but adherence to the training program can be more challenging. A small German multicenter randomized controlled trial showed that a partially supervised exercise intervention can improve FVC, aerobic exercise capacity, and quality of life in individuals with CF even 12 to 18 months after the end of the intervention (9). If the benefits of such a partially supervised intervention could be generalized by applying it to a wider population of people with CF with different health beliefs and cared for by different healthcare systems, it would have significant implications for long-term outcomes.
Changing PA behavior in the general population or people with CF is difficult due to a plethora of perceived barriers, such as lack of time, tiredness, stigma, and demoralization (10–12). In general, a multicomponent intervention has the potential to elicit beneficial effects (13). There is evidence that motivational interviewing, counseling, a written activity plan, and regular feedback from pedometers (14) or diaries are beneficial. In CF, very little evidence exists on the most promising strategies to promote PA (15).
We hypothesized that a partially supervised exercise program paired with motivational feedback aimed at increasing vigorous PA by at least 3 h/wk can improve FEV1 in CF and that this can be sustained over the subsequent 6-month timeframe. The objective of the ACTIVATE-CF trial was to test this hypothesis and to assess further effects of such a program on patient-relevant health-related outcomes in a randomly selected population of adolescents and adults from different countries across Europe and North America.
Methods
ACTIVATE-CF was a parallel-arm randomized controlled trial (Clinicaltrials.gov identifier: NCT01744561) conducted in 27 CF centers across Austria, Canada, France, Germany, Switzerland, the Netherlands, the UK, and the United States. A detailed description of the methodology is available in the online supplement and elsewhere (16).
Participants
Patients with CF aged 12 years and older with an FEV1 of at least 35% predicted, performing 4 hours or less of vigorous PA per week up to 3 months before baseline, and access to the internet were assessed for eligibility (Figure 1). Details on inclusion and exclusion criteria are given in the online supplement. Ethical approval was obtained at all sites, and patients provided written informed consent before participation in the study.
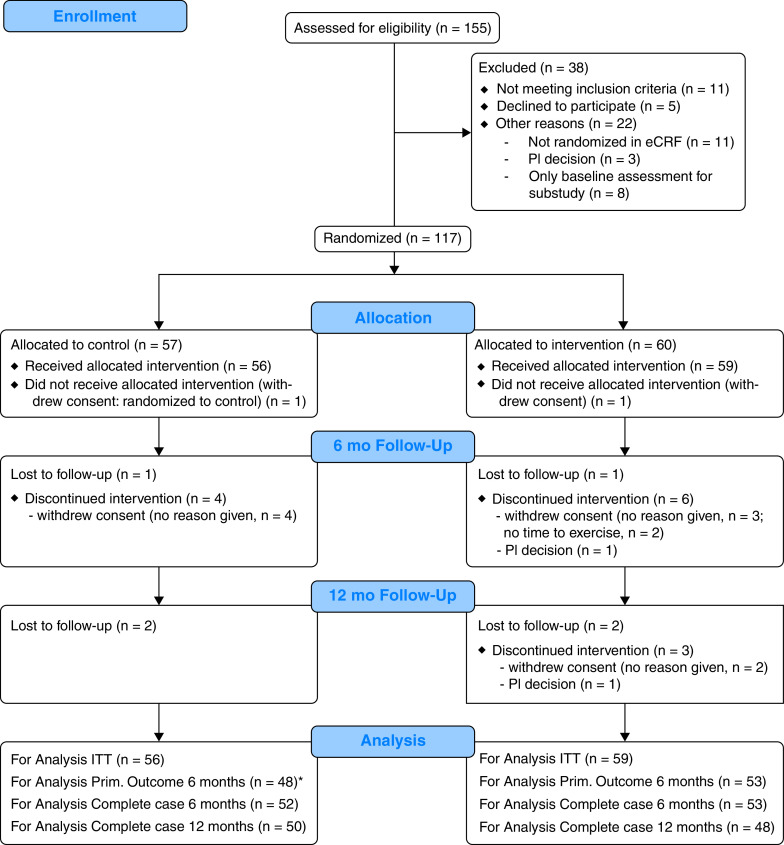
Figure 1.
The consolidated standards of reporting trials flow diagram through the ACTIVATE-CF trial. *In four participants of the control group, no valid FEV1 measurement was available at 6 months. eCRF = electronic case report form; ITT = intention to treat; PI = principal investigator; Prim. = primary.
Study Visits and Measurements
Patients were seen twice within 4 weeks for baseline assessments and every 3 months for 1 year thereafter. At each study visit, exacerbations, upper respiratory tract infections, antibiotic use, and adverse events (AEs) were documented. PA was assessed using the 7-day recall physical activity questionnaire (17) and pedometry. Height, weight, and skinfold thickness (at four sites) were measured and percent body fat calculated (18), and spirometry plus body plethysmography were performed (19). At baseline and 6 and 12 months after randomization, quality of life was assessed using the cystic fibrosis questionnaire revised (20), depression, anxiety, and stress scales (21, 22), and a modified exercise motivations inventory version 2 (23) was administered. Cardiopulmonary exercise testing was performed at baseline and after 6 and 12 months using established testing protocols (24), whereas an oral glucose tolerance test was done at baseline and after 9 months in participants not diagnosed with CF-related diabetes at baseline (25).
Randomization and Blinding
Participants were randomly allocated to the intervention or control group in a 1:1 ratio using block-randomization (block sizes of 4) and stratified by country and lung disease severity (i.e., moderate to severe lung disease [FEV1 < 70% predicted] or mild lung disease (⩾70% predicted]) (26). A statistician (C.S.) created a computer-generated list of random numbers using the statistical software, STATA. The list was implemented into the database (REDCap, Research Electronic Data Capture) by a person from the Clinical Trials Unit Bern, Switzerland, offering administrative database support. Study investigators had no access to the list. After all baseline data were collected at visits 1 and 2, randomization was done at each study site within the database, allowing complete allocation concealment. Outcome assessors were not blinded to group assignment.
Intervention
Participants in the intervention group were asked to add at least 3 hours of vigorous PA per week to their baseline activity. Including the activities already present at baseline assessment, this should have included at least 30 minutes of strength building exercises and 2 hours of aerobic exercise per week. The remaining time could be attributed according to the participants’ preferences. Further details about the exercise intervention, structured motivational interviews, exercise intensity prescription, activity counseling, and monitoring of individual training in an online diary are provided in the online supplement.
The control group participants were instructed to keep their PA level constant over the 12-month study period. They were not informed about their fitness level assessed with the exercise tests, nor did they receive any interpretation of the test results unless a finding was detected that required medical attention. They were also not given an evaluation of their answers to the activity questionnaires, nor their individual pedometry results.
Outcomes
Primary outcome was change in percent predicted FEV1 from baseline (average of the two baseline assessments) to the 6-month assessment. Secondary outcomes included changes in PA, exercise capacity, pulmonary function, body composition, quality of life, depression, anxiety and stress scales, exercise motives as well as glycemic control and time to first exacerbation. Number of upper respiratory tract infections, days on additional oral/intravenous antibiotics, and changes in body mass index and composition were monitored as explorative endpoints, whereas AEs at least possibly related to exercise (e.g., sprains, fractures, etc.) and serious AEs were documented as safety endpoints. Adherence with the intervention reported by participants and adherence assumed from changes in exercise capacity was assessed as additional endpoint. Results from substudies (16) will be reported elsewhere.
Statistical Analysis and Sample Size Calculation
Baseline data are presented as means ± SDs. Mixed models with multiple imputations for missing data were used to assess changes from baseline in intention-to-treat (ITT) analyses. A description of the imputation procedures is given in the online supplement. Models included random intercepts of countries and centers and fixed effects of group, sex, age, the interaction between sex and age, and the value of the outcome at baseline. Group-specific changes from baseline are given as means ± SEs, and estimated effects of the intervention (given by the regression coefficients for the intervention group) are reported with their 95% confidence intervals (CIs). In the online supplement, we report details on models used for per-protocol (PP) analyses limited to participants with reported adherence (control group: not more or less than 30 min/wk of reported vigorous PA compared with baseline; intervention group: at least 2 h/wk of additional reported vigorous PA) and assumed adherence (control group: increase in peak < 5% compared with baseline; intervention group: increase in peak ⩾ 5%), the number of respiratory tract infections, time to first exacerbation, and days on additional antibiotics. All analyses were performed using STATA version 15. Statistical significance was assumed at P < 0.05.
Based on our previous findings (9), we hypothesized that the intervention would induce an increase in FEV1 of at least 3% predicted from baseline to the 6-month follow-up visit in the intervention over the control group. Assuming an SD of that change of 8% predicted, 112 patients per group with complete data were required to detect the effect of the intervention with a power of 0.8 and a probability P of a type 1 error α of 0.05. Based on an estimated 15% dropout rate during the first 6 months of the study and 10% of participants with missing data, 146 participants were planned to be randomized into each group.
Our assumption of an SD of the change in FEV1 of 8% or less from baseline to 6 months was confirmed by the data (i.e., 6.9% in the control group and 7.3% in the intervention group). However, the limited sample size only allowed us to detect a difference in the change of FEV1 between the groups of 4.0% (power 0.8, P < 0.05). In the actual study, however, we used a more complex model for statistical analysis so that power is much more difficult to estimate. This would likely lead to slightly higher required effect sizes.
Results
Despite intense efforts by the study team and site investigators, and extension of the anticipated 2-year recruitment period for additional 21 months, we did not reach the target sample size of 292 participants. In total, 155 patients from 27 centers across Europe and North America were recruited, of whom 117 patients were randomized to the two study groups (Figure 1 and Table E1 in the online supplement). Table 1 summarizes the characteristics of randomized participants by group at baseline.
Table 1.
Patients’ Characteristics at Baseline
| N | Intervention (n = 60) | N | Control (n = 57) | |
|---|---|---|---|---|
| Age, yr | 60 | 25.3 ± 11.4 | 57 | 22.8 ± 10.8 |
| Sex, female n (%) | 60 | 33 (55) | 57 | 32 (56) |
| Height, cm | 60 | 166.0 ± 10.4 | 57 | 166.0 ± 10.8 |
| Weight, kg | 60 | 61.0 ± 14.5 | 57 | 57.7 ± 11.7 |
| BMI, kg/m2 | 60 | 22.0 ± 4.1 | 57 | 20.8 ± 3.5 |
| Body fat % | 59 | 23.7 ± 11.3 | 56 | 22.7 ± 10.9 |
| Lean body mass, kg | 59 | 52.9 ± 12.2 | 56 | 50.8 ± 9.5 |
| Lung function | ||||
| FVC, % predicted | 60 | 87.0 ± 18.5 | 57 | 85.6 ± 18.8 |
| FEV1, % predicted | 60 | 73.5 ± 22.4 | 57 | 73.7 ± 20.8 |
| RV/TLC % | 55 | 38.6 ± 12.9 | 52 | 36.5 ± 14.1 |
| Physical activity and exercise capacity | ||||
| Reported VPA, h/wk | 60 | 1.20 ± 1.04 | 57 | 1.63 ± 1.11 |
| Total daily steps | 50 | 5755 ± 2934 | 51 | 5660 ± 2674 |
| Aerobic daily steps | 50 | 1145 ± 1716 | 51 | 1170 ± 1723 |
| peak, % predicted | 55 | 70.7 ± 16.5 | 50 | 68.7 ± 15.4 |
| Wpeak, % predicted | 57 | 88.2 ± 17.1 | 53 | 91.4 ± 19.4 |
| Clinical characteristics | ||||
| Pseudomonas aeruginosa, n (%) | 60 | 14 (23.3) | 57 | 9 (15.8) |
| CF-related diabetes, n (%) | 60 | 13 (21.7) | 57 | 9 (15.8) |
| On ivacaftor, n (%) | 60 | 2 (3.3) | 57 | 3 (5.3) |
| On lumacaftor and ivacaftor, n (%) | 60 | 4 (6.7) | 57 | 2 (3.5) |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; RV/TLC = residual volume over total lung capacity; peak = peak oxygen uptake; VPA = vigorous physical activity; Wpeak = maximum work rate.
Data are means ± SD or n (%).
Adherence with the Exercise Program
Self-reported PA in the control group assessed at study visits suggested adherence with the activity goals in 58% (31/53) of participants during the first 6 months after randomization and in 50% (27/54) of participants during the entire 12-month period. Interestingly, 15 of the 22 control participants not reporting adherence with the activity goals during the first 6 months of the study reported an increase in vigorous PA of more than 30 min/wk, whereas only 7 control group participants provided information consistent with a reduction in vigorous PA by more than 30 min/wk after randomization. In the intervention group, reported adherence was 65% (36/55) during the first 6 months and 58% (32/55) for the entire 12-month study period. Based on the entries in the web-based activity diary, adherence in the intervention group was 56% (33/59) after both 6 and 12 months (Figures E1 and E2).
Primary endpoint
After 6 months, change in FEV1 (% predicted, ΔFEV1) from baseline was significantly higher in the control compared with the intervention group (Figure 2). The estimated mean difference (95% CIs) in FEV1 percent predicted from the ITT analysis with and without data imputation was −2.70% (−5.26 to −0.13; P = 0.039) and −2.58% (−5.35 to 0.19; P = 0.068) (see Tables E2 and E3). At other visits, no significant between-group differences in ΔFEV1 were observed in the ITT analysis (Figure 2C). The PP analyses based on reported adherence and assumed adherence showed similar effects to ITT analyses (Table E4). Mean raw values and their 95% confidence intervals for FEV1 percent predicted at all study visits are provided in Figure E3.
Figure 2.
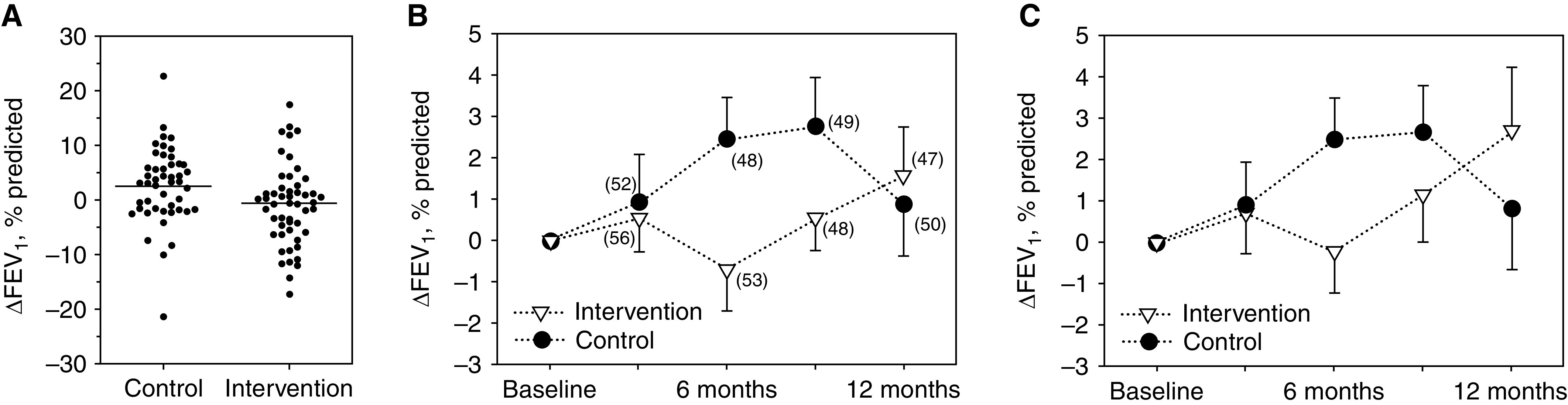
Change in FEV1 (ΔFEV1) in percent predicted from baseline. (A) Individual changes from baseline to 6 months (primary outcome). There was a significant difference between groups in favor of the control group (mean difference, 3.18% predicted [95% confidence interval, 0.39–5.97]; P = 0.026). (B) Changes in FEV1 from baseline to visits at 3, 6, 9, and 12 months. Only the actually measured data are shown. The numbers in parentheses are the respective sample sizes. (C) Intention-to-treat analysis including imputed data. Error bars in (B) and (C) are standard errors.
Secondary endpoints
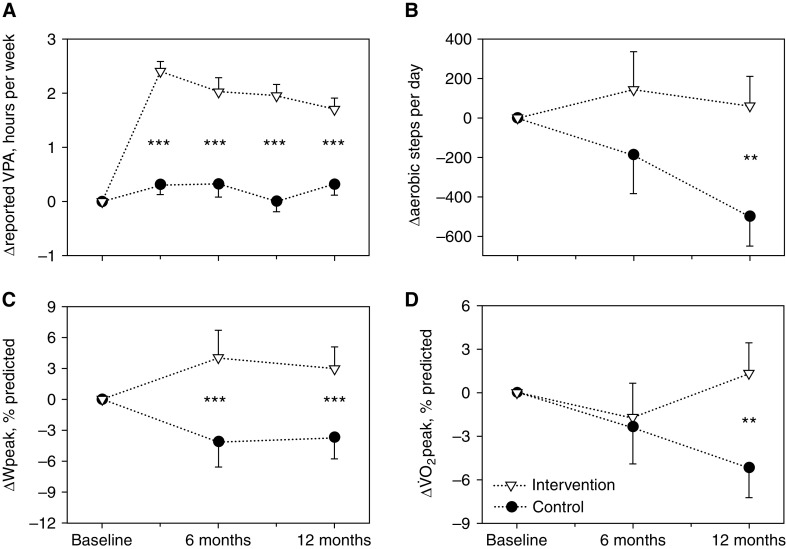
Compared with controls, the intervention was associated with an increase in self-reported vigorous PA at all study visits, increased exercise capacity (Wpeak) at 6 and 12 months, a higher peak, and a higher number of aerobic steps at 12 months (Figure 3). The effects of the intervention on further secondary outcomes, based on ITT analyses, are shown in Table 2. Results for all secondary outcomes from the ITT and PP analyses (i.e., reported and assumed adherence with the intervention) are given in the online supplement (Tables E2–E4).
Figure 3.
Changes in (A) reported vigorous physical activity (VPA) (control group: n = 57; intervention group: n = 60); (B) aerobic steps (control group: n = 50; intervention group: n = 51); (C) peak work rate (ΔWpeak) (control group: n = 50; intervention group: n = 53), and (D) peak oxygen uptake (Δ peak) (control group: n = 50; intervention group: n = 53). The graphs are based on full datasets with imputed data (intention-to-treat analyses). **P < 0.01 and ***P < 0.001. Error bars are standard errors.
Table 2.
Effect Sizes of Further Secondary Outcomes in Intention-to-Treat Analysis of the Exercise Intervention at 6 and 12 Months According to Group Allocation
| Variable | Time Period, mo | N | Change Scores Control Group means ± SE |
N | Change Scores Intervention Group means ± SE |
Effect Estimate Coefficient (95% CI) |
P Value |
|---|---|---|---|---|---|---|---|
| Lung function | |||||||
| FVC % predicted | 0–6 | 57 | 3.42 ± 1.04 | 60 | 0.32 ± 1.01 | −3.10 (−5.81 to −0.39) | 0.025 |
| 0–12 | 57 | 1.72 ± 1.24 | 60 | 3.24 ± 1.25 | 1.52 (−1.08 to 4.13) | 0.25 | |
| RV/TLC % | 0–6 | 52 | 0.01 ± 0.01 | 55 | 0.02 ± 0.01 | 0.01 (−0.02 to 0.04) | 0.58 |
| 0–12 | 52 | 0.01 ± 0.01 | 55 | 0.00 ± 0.01 | −0.01 (−0.03 to 0.02) | 0.63 | |
| Physical activity | |||||||
| Total steps, n | 0–6 | 50 | 438 ± 1210 | 55 | 1022 ± 1206 | 584 (−429 to 1,597) | 0.26 |
| 0–12 | 50 | −251 ± 912 | 55 | 555 ± 903 | 806 (−37 to 1,649) | 0.06 | |
| Body composition | |||||||
| BMI, kg/m2 | 0–6 | 57 | 0.22 ± 0.16 | 60 | 0.23 ± 0.16 | 0.01 (−0.28 to 0.29) | 0.96 |
| 0–12 | 57 | 0.04 ± 0.28 | 60 | 0.27 ± 0.29 | 0.23 (−0.23 to 0.69) | 0.33 | |
| Body fat % | 0–6 | 56 | 0.37 ± 0.76 | 59 | 0.38 ± 0.73 | 0.01 (−2.05 to 2.07) | 0.99 |
| 0–12 | 56 | −0.22 ± 0.72 | 59 | 1.77 ± 0.71 | 1.99 (0.12 to 3.87) | 0.04 | |
| Lean body mass, kg | 0–6 | 56 | 0.44 ± 0.48 | 59 | 0.93 ± 0.46 | 0.49 (−0.73 to 1.71) | 0.43 |
| 0–12 | 56 | 1.08 ± 0.56 | 59 | 1.36 ± 0.57 | 0.28 (−1.05 to 1.61) | 0.68 | |
| Glucose tolerance* | |||||||
| Fasting glucose, mmol/L | 0–9 | 40 | 0.14 ± 0.11 | 41 | −0.02 ± 0.11 | −0.16 (−0.44 to 0.13) | 0.28 |
| 1 h glucose OGTT, mmol/L | 0–9 | 31 | −0.26 ± 0.43 | 36 | −0.30 ± 0.43 | −0.04 (−1.14 to 1.05) | 0.94 |
| 2 h glucose OGTT, mmol/L | 0–9 | 40 | 0.57 ± 0.40 | 41 | 0.14 ± 0.37 | −0.44 (−1.44 to 0.57) | 0.39 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; OGTT = oral glucose tolerance test; RV/TLC = residual volume/total lung capacity ratio.
β coefficients were calculated based on a mixed model regression analysis with baseline adjustment and imputation of missing values.
The oral glucose tolerance test was only performed in participants without a diagnosis of cystic fibrosis–related diabetes at baseline.
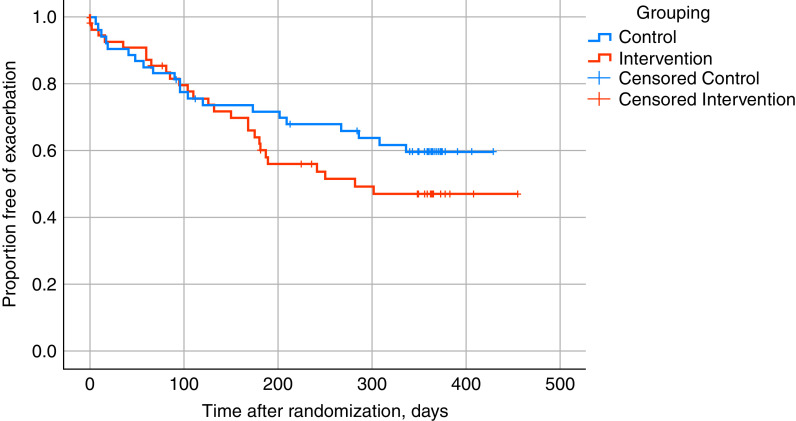
The intervention did not impact the time to first exacerbation: hazard ratio, 1.34 (95% CI, 0.65–2.80; P = 0.43) (Figure 4). Likewise, the number of pulmonary exacerbations, the number of participants reporting an upper respiratory tract infection, receiving oral or intravenous antibiotics or being hospitalized, was not affected by the intervention (Tables E5–E13). However, study visits close to a physician-diagnosed pulmonary exacerbation tended to occur more frequently in the intervention group (34 of 212 visits) compared with the control group (21 of 210 visits; P = 0.07) (Table E13).
Figure 4.
Time to first exacerbation in the intervention and the control group.
There were no differences between groups in the change of medication (e.g., CF transmembrane conductance regulator [CFTR] modulator therapy, and no evidence that the control or intervention group made changes to their airway clearance techniques other than exercise [results not shown]). Table 3 summarizes the AEs and serious AEs reported during the trial based on Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (27). These were rare, all participants recovered, and none dropped out because of (serious) adverse events.
Table 3.
Reported Adverse Events in the Intervention and Control Groups during the 12-Month Intervention Period
| Adverse Events | Control |
Intervention |
||
|---|---|---|---|---|
| AE | SAE | AE | SAE | |
| Abdominal infection | 1 | 0 | 0 | 0 |
| Upper respiratory tract infection | 0 | 0 | 2 | 0 |
| Bronchial infection | 3 | 2 | 6 | 1 |
| Other infection* | 0 | 0 | 3 | 0 |
| Acute injury | 2 | 1 | 4 (2) | 2 (1) |
| Back pain | 0 | 0 | 1 | 0 |
| Arthralgia | 0 | 0 | 3 (3) | 0 |
| Bone pain | 0 | 0 | 2 (2) | 0 |
| Bronchopulmonary hemorrhage (hemoptysis) | 0 | 0 | 2 | 1 |
| Bronchial obstruction | 0 | 0 | 1 | 0 |
| Urticaria | 0 | 0 | 2 (1) | 0 |
| DIOS | 0 | 1 | 0 | 2 |
| Abdominal pain | 0 | 0 | 0 | 1 |
| Depression | 0 | 0 | 1 | 0 |
| Surgical or medical procedures (planned intravenous therapy, wisdom teeth extraction) | 4 | 0 | 5 | 0 |
Definition of abbreviations: AE = adverse event; DIOS = distal intestinal obstructive syndrome; SAE = serious adverse event.
Numbers shown are total of reported events. Numbers in parentheses are the number of events assessed as possibly or definitively related to the intervention by investigator and/or sponsor.
Involving gastrointestinal and respiratory systems.
Discussion
Primary Endpoint: Change in FEV1 after 6 Months
This international multicenter study, ACTIVATE-CF, was designed to assess the effects of a 12-month vigorous PA intervention with motivational feedback on changes in FEV1 at 6 months as primary endpoint in people with CF and a low baseline activity level. The most counterintuitive observation was an improvement in FEV1 in the control over the intervention group at 6 months. Thus, our initial hypothesis may be wrong.
The observed mean difference of 2.70% predicted (95% CI, 0.13–5.26) in FEV1 is considered clinically relevant given the fact that the U.S. Food and Drug Administration and the European Medicines Agency approved ivacaftor–lumacaftor treatment based on an improvement of 2.6–4.0% predicted FEV1 in the active treatment arm compared with placebo arm, in addition to beneficial effects on exacerbation and hospitalization rates (28).
Based on previous long-term randomized controlled trials using exercise as intervention, we had hypothesized that there would be an increase in FEV1 in the intervention compared with the control group (5, 9). The reason for the gain in FEV1 in the control group compared with the intervention group after 6 months of intervention is unclear and completely unexpected based on the experience of expected changes in FEV1 in control group participants in drug trials of similar duration (29, 30).
In general, FEV1 in CF can be affected by many conditions and interventions, such as infections and exacerbations, bronchial asthma, diabetes, medical treatment, etc. (31, 32). There was no difference between groups in the time to first exacerbation, number of respiratory tract infections, use of oral/intravenous antibiotics, or start of CFTR modulator therapy. However, there was a trend toward more physician-diagnosed exacerbations in a temporal context to study visits in the intervention group. Pulmonary exacerbations are associated with an acute reduction in FEV1 and a higher rate of decline in FEV1 thereafter (33). Less than 30% of control participants experienced an exacerbation during the first 6 months after randomization (Figure 4). Possibly, the relatively low rate of exacerbations in the control group contributed to the gain in FEV1 during the first 6 months after randomization.
Potential Contamination of Controls and Adherence to the Intervention
Inherent to all exercise trials is the problem of adherence of the intervention group and contamination of the control group. When recruited, all participants in the project consented and were motivated to increase their vigorous PA by at least 3 hours per week if randomized to the intervention group. Although controls were instructed to keep their activity levels constant, 15 participants of the control group reported an increase in their vigorous PA during the first 6 months after randomization of more than 30 minutes per week, whereas only 7 participants reported a decrease by more than 30 minutes per week. Improvements in PA behavior are not uncommon in control group participants in intervention studies (34). Therefore, we cannot exclude that even more controls somewhat increased their activity levels without reporting. This moderate increase in vigorous PA in the control group may have contributed to the significantly higher FEV1 observed at 6 months in the control group.
In contrast, participants in the intervention group did not improve their FEV1 during the first 6 months. It could be argued that the intervention group did not increase their vigorous PA sufficiently. However, we did observe an increase in vigorous PA of, on average, about 2 hours per week reported by the participants of the intervention group and confirmed by the respective study site investigators. In line with this observation, there was also a significant effect of the intervention on the number of “aerobic steps” as measured objectively by pedometry and on exercise capacity. Thus, the multicomponent intervention used in ACTIVATE-CF to increase vigorous PA was successful. This is a major and clinically important observation as many unsupervised or partially supervised activity intervention programs have failed to induce behavioral changes. For example, Cox and colleagues (15) concluded in a systematic review that their review “provides very limited evidence that activity counselling and exercise advice, undertaken over at least 6 months, to engage in a home exercise program may result in improved PA participation in people with cystic fibrosis.”
One inclusion criterion for the study was a relatively low baseline level of vigorous PA. Before randomization, the participants of the intervention group reported on average 1.2 hours of vigorous PA per week. They were then requested to add 3 additional hours of vigorous PA per week. The fast increase in training volume with possibly inadequate recovery, especially if additional non–exercise-related stressors may have existed in symphony (35), may have resulted in unfavorable effects of the intervention that counterbalanced potential positive effects.
A specific concern associated with vigorous PA is the provocation of immune dysfunction (36), but occurrence of such a phenomenon has been questioned recently (37). Nevertheless, there was no significant effect on the number of upper respiratory tract infections, days on antibiotics, number of antibiotic treatment cycles, or exacerbations in the entire group. Finally, exercise studies (4, 5) using a higher level of supervision of the intervention than the current project observed significant beneficial effects of the intervention but did not report detrimental consequences on lung health.
Possibly, a period longer than 6 months of increased vigorous PA is more effective in terms of positively impacting FEV1. Although the effect estimates and 95% CIs for ΔFEV1 at 12 months (1.87; 95% CI, –1.00 to 4.74) were not statistically significant, some beneficial effects might have occurred in the longer term. Other studies using an exercise intervention over 12 months also saw (mostly nonsignificant) effects of the intervention on FEV1 (5, 9, 38).
Secondary Endpoints: Change in Exercise Capacity and Other Health-related Outcomes
After 6 and 12 months, the intervention group had a significantly higher exercise capacity (i.e., Wpeak at 6 and 12 months; peak at 12 months) compared with the control group (Figure 3 and Tables E2–E4). Objectively measured maximal aerobic exercise capacity using standardized testing protocols is strongly associated with survival in health (39) and CF lung disease (6, 40, 41). Based on ITT analysis, the magnitude of improvement in Wpeak (8.1% predicted [95% CI, 3.6–12.6] at 6 months, and 6.6% predicted [95% CI, 3.0–10.2] at 12 months) and peak (4.5% predicted [95% CI, 1.0–8.0]) at 12 months in the intervention group compared with the control group) may be considered clinically relevant (42). However, whether improvements in exercise capacity translate into better survival is currently unknown. No consistent differences between groups were observed in any other secondary endpoints, including quality of life, blood sugar control, and time to first exacerbation. These findings may be related, at least in part, to the lower-than-intended sample size and thus statistical power of the trial. Nevertheless, very little evidence exists on the effects of exercise training on quality of life and blood sugar control (3), and there is no information on time to first exacerbation and AEs of an exercise intervention (3). Thus, ACTIVATE-CF extends our knowledge in these respects.
There are several limitations of the trial. First, despite great efforts, we were only able to randomize 40% of the target sample size. Nevertheless, ACTIVATE-CF is the largest PA intervention trial in CF so far and addressed several new and important outcomes. For example, for the first time, AEs were systematically captured during an exercise intervention trial in CF. In line with previous reports on the risks of exercise in CF, few possible adverse reactions related to the intervention were reported (43).
Participating centers gave several reasons for the low recruitment into the study, which included the need of skilled staff and time to perform all measurements and the counseling as well as competing attractive trials (e.g., CFTR modulator therapies with financial incentives). Reported participant barriers were not wishing to be randomized to an intense PA program owing to other priorities (e.g., school exams), and the 50% chance of being randomized to the control group despite their knowledge of the importance of regular PA from the study information. The difficulties in recruitment into exercise trials have been well recognized (44) and also experienced by other investigators in CF research (45). A double-blind study design is impossible in exercise intervention trials. Further, owing to limited personnel at study centers, we were not able to consistently blind outcome assessors for allocation of participants to one of the treatment arms; participants themselves and activity specialists providing counseling could not be blinded. Thus, information may have been leaked to other team members and assessors. However, as many outcomes were objectively measured and virtual as well as on-site monitoring was used, a bias in outcomes in favor or against benefits of the intervention seems unlikely.
Potential Implications of Study Results for Medical Care
To maintain and promote health in an adult population with chronic health conditions, at least 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity PA per week are recommended (46). A supervised exercise intervention in CF (5) demonstrated that 3 × 30 minutes of either aerobic training or weight training per week could significantly improve FEV1 within 6 months of intervention. Two hours of additional vigorous PA per week in our intervention group participants should, thus, be sufficient to improve FEV1. Our findings indicate that a steep increase in vigorous PA (most likely) represents the wrong approach to improve lung health for the majority of people with CF who are relatively sedentary. In contrast, the significant but counterintuitive improvement in FEV1 in the control group possibly induced by a moderate increase in PA suggests a less stringent “motivational approach” may effectively modulate an increase in PA. In line with recommendations for the healthy population and athletes, we would now aim for a weekly increase in training volume of no more that 10% (35). The tools developed in this project to foster an increase in vigorous PA would then be introduced successively to maintain or even further increase the activity level. In view of the multiple benefits of PA established in various populations, healthcare providers are advised to motivate people with CF to be physically active, without pressure, but appropriate supervision including individual goal setting aiming to build positive long-term activity behavior (47).
Conclusions
This international multicenter study found an increase in self-reported vigorous physical activity and exercise capacity with a partially supervised exercise intervention with motivational feedback. However, the control group experienced beneficial effects compared with the intervention group for the primary endpoint (FEV1) potentially related to a moderate increase in physical activity in that group and a too-steep increase in physical activity in some intervention group participants.
Acknowledgments
Acknowledgment
The authors thank the members of the data safety monitoring board: Brenda Button, Melbourne, Australia; Christiane de Boeck, Leuven, Belgium; and John Mark, Palo Alto, California. They also thank all participants for their effort and time dedicated to this study, and they thank all study personnel at the different study sites for their hard work and contribution to this study.
Members of the ACTIVATE-CF Study Working Group: Ernst Eber, Medical University of Graz, Graz, Austria; Marlies Wagner, Medical University of Graz, Graz, Austria; Helmut Ellemunter, University Hospital Innsbruck, Innsbruck, Austria; Larry C. Lands, McGill University Health Centre, Montreal, Quebec, Canada; Nancy Alarie, McGill University Health Centre, Montreal, Quebec, Canada; Chantal Karila, Université Paris Descartes, Paris, France; Clotilde Simon, Unité Recherche Clinique Cochin Necker, Paris, France; Anne Faucou, Unité de Recherche Clinique Cochin Necker, Paris, France; Laurent Mely, Hôpital Renée Sabran, Giens, Hyères, France; Bruno Ravaninjatovo, CHR, Reims, France; Anne Prevotat, CHRU, Hôpital Calmette, Lille, France; Helge Hebestreit, University Hospitals Würzburg, Würzburg, Germany; Jonathan Schaeff, University Hospitals Würzburg, Würzburg, Germany; Lothar Stein, Medical School Hannover, Hannover, Germany; Cordula Koerner-Rettberg, University Children's Hospital of Ruhr University Bochum at St Josef-Hospital, Bochum, Germany; Jutta Hammermann, University Hospital of Dresden, Dresden, Germany; Christina Smaczny, Johann Wolfgang Goethe University, Frankfurt/Main, Germany; Inka Held, CF Zentrum Altona, Hamburg, Germany; Sibylle Junge, Hannover Medical School, Pediatric Pneumology, Allergology and Neonatology, Hannover, Germany; Oliver Nitsche, Johannes Gutenberg-Universität Mainz, Mainz, Germany; Rainald Fischer, Praxis für Lungenheilkunde, München-Pasing, Germany; Jörg Große-Onnebrink, University Hospital Münster, Münster, Germany; Anne Wesner, Klinikum Stuttgart, Olgahospital, Stuttgart, Germany; Andreas Hector, University of Tübingen, Tübingen, Germany; Alexandra Hebestreit, Christiane Herzog-Zentrum, Universitätsklinikum Würzburg, Germany; Susi Kriemler, University of Zurich, Zurich, Switzerland; Christian Schindler, University of Basel, Basel, Switzerland; Thomas Radtke, University of Zurich, Zurich, Switzerland; Christian Benden, University Hospital Zurich, Zurich, Switzerland; Carmen Casaulta, University Children’s Hospital Bern, Bern, Switzerland; Reta Fischer, Quartier Bleu, Lindenhospital Bern, Switzerland; Alexander Möller, University Children’s Hospital Zurich, Zurich, Switzerland; Erik Hulzebos, University Medical Center Utrecht, Utrecht, the Netherlands; Marcella Burghard, University Medical Center Utrecht, Utrecht, the Netherlands; Don S. Urquhart, Royal Hospital for Sick Children, Edinburgh, United Kingdom; Sarah Blacklock, Royal Hospital for Sick Children, Edinburgh, United Kingdom; Debbie Miller, Royal Hospital for Sick Children, Edinburgh, United Kingdom; Zoe Johnstone, Royal Hospital for Children and Young People, Edinburgh, United Kingdom; David M. Orenstein, Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, Pennsylvania; John D. Lowman, University of Alabama at Birmingham, Birmingham, Alabama.
Footnotes
A complete list of ACTIVATE-CF Study Working Group members may be found before the beginning of the References.
Supported by the Mukovizisose e.V. (1402); the Swiss Society for Cystic Fibrosis; Cystic Fibrosis Canada; Vaincre la Mucoviscidose; Nederlandse Cystic Fibrosis Stichting; Edinburgh Children’s Hospital Charity; the Cystic Fibrosis Foundation (ORENST14K0); Stiftung Telethon Aktion Schweiz; Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (320030_144175); and Mylan Healthcare GmbH. The international coordination of the project was funded through a Vertex Innovation Award, which is an unconditional research grant provided by Vertex Pharmaceuticals (Vertex Innovation Award ACTIVATE-CF). The funding bodies had no role in the design of the study, data collection, or writing of this manuscript.
Author Contributions: Conception and design: H.H., S.K., D.M.O., L.C.L., J.S., and T.R. Acquisition of data: C.K., D.M.O., E.E., H.H., L.C.L., L.S., D.S.U., and T.R. Statistical analysis: C.S. and H.H. Development of online diary: J.S. and H.H. Database management: T.R. and H.H. Study site initiation and data monitoring: H.H., L.S., and T.R.. Data interpretation: H.H., S.K., T.R., C.K., C.S., D.M.O., D.S.U., E.E., L.C.L., L.S., and J.S. First draft: H.H., S.K., and T.R. All authors edited, reviewed, and approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202106-1419OC on November 4, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
on behalf of the ACTIVATE-CF Study Working Group:
Ernst Eber, Marlies Wagner, Helmut Ellemunter, Nancy Alarie, Clotilde Simon, Anne Faucou, Laurent Mely, Bruno Ravaninjatovo, Anne Prevotat, Cordula Koerner-Rettberg, Jutta Hammermann, Christina Smaczny, Inka Held, Sibylle Junge, Oliver Nitsche, Rainald Fischer, Jörg Große-Onnebrink, Anne Wesner, Andreas Hector, Alexandra Hebestreit, Christian Benden, Carmen Casaulta, Reta Fischer, Alexander Möller, Erik Hulzebos, Marcella Burghard, Sarah Blacklock, Debbie Miller, Zoe Johnstone, and John D. Lowman
References
- 1. Button BM, Wilson C, Dentice R, Cox NS, Middleton A, Tannenbaum E, et al. Physiotherapy for cystic fibrosis in Australia and New Zealand: a clinical practice guideline. Respirology . 2016;21:656–667. doi: 10.1111/resp.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barker M, Hebestreit A, Gruber W, Hebestreit H. Exercise testing and training in German CF centers. Pediatr Pulmonol . 2004;37:351–355. doi: 10.1002/ppul.10430. [DOI] [PubMed] [Google Scholar]
- 3. Radtke T, Nevitt SJ, Hebestreit H, Kriemler S. Physical exercise training for cystic fibrosis. Cochrane Database Syst Rev . 2017;11:CD002768. doi: 10.1002/14651858.CD002768.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Selvadurai HC, Blimkie CJ, Meyers N, Mellis CM, Cooper PJ, Van Asperen PP. Randomized controlled study of in-hospital exercise training programs in children with cystic fibrosis. Pediatr Pulmonol . 2002;33:194–200. doi: 10.1002/ppul.10015. [DOI] [PubMed] [Google Scholar]
- 5. Kriemler S, Kieser S, Junge S, Ballmann M, Hebestreit A, Schindler C, et al. Effect of supervised training on FEV1 in cystic fibrosis: a randomised controlled trial. J Cyst Fibros . 2013;12:714–720. doi: 10.1016/j.jcf.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 6. Hebestreit H, Hulzebos EHJ, Schneiderman JE, Karila C, Boas SR, Kriemler S, et al. Prognostic Value of CPET in CF Study Group Cardiopulmonary exercise testing provides additional prognostic information in cystic fibrosis. Am J Respir Crit Care Med . 2019;199:987–995. doi: 10.1164/rccm.201806-1110OC. [DOI] [PubMed] [Google Scholar]
- 7. Ramos KJ. Cardiopulmonary exercise testing: another tool in the prognostication tool kit for cystic fibrosis. Am J Respir Crit Care Med . 2019;199:938–940. doi: 10.1164/rccm.201810-2053ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gulmans VA, de Meer K, Brackel HJ, Faber JA, Berger R, Helders PJ. Outpatient exercise training in children with cystic fibrosis: physiological effects, perceived competence, and acceptability. Pediatr Pulmonol . 1999;28:39–46. doi: 10.1002/(sici)1099-0496(199907)28:1<39::aid-ppul7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9. Hebestreit H, Kieser S, Junge S, Ballmann M, Hebestreit A, Schindler C, et al. Long-term effects of a partially supervised conditioning programme in cystic fibrosis. Eur Respir J . 2010;35:578–583. doi: 10.1183/09031936.00062409. [DOI] [PubMed] [Google Scholar]
- 10. Denford S, Mackintosh KA, McNarry MA, Barker AR, Williams CA, Youth Activity Unlimited – A Strategic Research Centre of the UK Cystic Fibrosis Trust Enhancing intrinsic motivation for physical activity among adolescents with cystic fibrosis: a qualitative study of the views of healthcare professionals. BMJ Open . 2019;9:e028996. doi: 10.1136/bmjopen-2019-028996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Denford S, Cox NS, Mackintosh KA, McNarry MA, O’Halloran P, Holland AE, et al. Physical activity for cystic fibrosis: perceptions of people with cystic fibrosis, parents and healthcare professionals. ERJ Open Res . 2020;6:00294-2019. doi: 10.1183/23120541.00294-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwartz J, Rhodes R, Bredin SSD, Oh P, Warburton DER. Effectiveness of approaches to increase physical activity behavior to prevent chronic disease in adults: a brief commentary. J Clin Med . 2019;8:E295. doi: 10.3390/jcm8030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demeyer H, Louvaris Z, Frei A, Rabinovich RA, de Jong C, Gimeno-Santos E, et al. Mr Papp PROactive study group and the PROactive consortium Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax . 2017;72:415–423. doi: 10.1136/thoraxjnl-2016-209026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA . 2007;298:2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 15. Cox NS, Alison JA, Holland AE. Interventions for promoting physical activity in people with cystic fibrosis. Cochrane Database Syst Rev . 2013;12:CD009448. doi: 10.1002/14651858.CD009448.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hebestreit H, Lands LC, Alarie N, Schaeff J, Karila C, Orenstein DM, et al. ACTIVATE-CF Study Working Group Effects of a partially supervised conditioning programme in cystic fibrosis: an international multi-centre randomised controlled trial (ACTIVATE-CF): study protocol. BMC Pulm Med . 2018;18:31. doi: 10.1186/s12890-018-0596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruf KC, Fehn S, Bachmann M, Moeller A, Roth K, Kriemler S, et al. Validation of activity questionnaires in patients with cystic fibrosis by accelerometry and cycle ergometry. BMC Med Res Methodol . 2012;12:43. doi: 10.1186/1471-2288-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slaughter MH, Lohman TG, Boileau RA, Horswill CA, Stillman RJ, Van Loan MD, et al. Skinfold equations for estimation of body fatness in children and youth. Hum Biol . 1988;60:709–723. [PubMed] [Google Scholar]
- 19. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J . 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20. Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of the cystic fibrosis questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest . 2005;128:2347–2354. doi: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- 21. Brown TA, Chorpita BF, Korotitsch W, Barlow DH. Psychometric properties of the depression anxiety stress scales (DASS) in clinical samples. Behav Res Ther . 1997;35:79–89. doi: 10.1016/s0005-7967(96)00068-x. [DOI] [PubMed] [Google Scholar]
- 22. Crawford JR, Henry JD. The depression anxiety stress scales (DASS): normative data and latent structure in a large non-clinical sample. Br J Clin Psychol . 2003;42:111–131. doi: 10.1348/014466503321903544. [DOI] [PubMed] [Google Scholar]
- 23. Ingledew DK, Markland D, Medley AR. Exercise motives and stages of change. J Health Psychol . 1998;3:477–489. doi: 10.1177/135910539800300403. [DOI] [PubMed] [Google Scholar]
- 24. Hebestreit H, Arets HG, Aurora P, Boas S, Cerny F, Hulzebos EH, et al. European Cystic Fibrosis Exercise Working Group Statement on exercise testing in cystic fibrosis. Respiration . 2015;90:332–351. doi: 10.1159/000439057. [DOI] [PubMed] [Google Scholar]
- 25. Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, et al. CFRD Guidelines Committee Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care . 2010;33:2697–2708. doi: 10.2337/dc10-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services 2017https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. [DOI] [PubMed]
- 28. Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. TRAFFIC Study Group TRANSPORT Study Group. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med . 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, Waltz D, et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med . 2017;5:107–118. doi: 10.1016/S2213-2600(16)30427-1. [DOI] [PubMed] [Google Scholar]
- 30. Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. VX17-445-102 Study Group Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med . 2019;381:1809–1819. doi: 10.1056/NEJMoa1908639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kerem E, Viviani L, Zolin A, MacNeill S, Hatziagorou E, Ellemunter H, et al. ECFS Patient Registry Steering Group Factors associated with FEV1 decline in cystic fibrosis: analysis of the ECFS patient registry. Eur Respir J . 2014;43:125–133. doi: 10.1183/09031936.00166412. [DOI] [PubMed] [Google Scholar]
- 32. Zemanick ET, Accurso FJ. Entering the era of highly effective CFTR modulator therapy. Lancet . 2019;394:1886–1888. doi: 10.1016/S0140-6736(19)32676-5. [DOI] [PubMed] [Google Scholar]
- 33. Sanders DB, Bittner RCL, Rosenfeld M, Redding GJ, Goss CH. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol . 2011;46:393–400. doi: 10.1002/ppul.21374. [DOI] [PubMed] [Google Scholar]
- 34. Waters L, Reeves M, Fjeldsoe B, Eakin E. Control group improvements in physical activity intervention trials and possible explanatory factors: a systematic review. J Phys Act Health . 2012;9:884–895. doi: 10.1123/jpah.9.6.884. [DOI] [PubMed] [Google Scholar]
- 35. Schwellnus M, Soligard T, Alonso J-M, Bahr R, Clarsen B, Dijkstra HP, et al. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br J Sports Med . 2016;50:1043–1052. doi: 10.1136/bjsports-2016-096572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walsh NP, Gleeson M, Shephard RJ, Gleeson M, Woods JA, Bishop NC, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev . 2011;17:6–63. [PubMed] [Google Scholar]
- 37. Campbell JP, Turner JE. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol . 2018;9:648. doi: 10.3389/fimmu.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moorcroft AJ, Dodd ME, Morris J, Webb AK. Individualised unsupervised exercise training in adults with cystic fibrosis: a 1 year randomised controlled trial. Thorax . 2004;59:1074–1080. doi: 10.1136/thx.2003.015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med . 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 40. Pianosi P, Leblanc J, Almudevar A. Peak oxygen uptake and mortality in children with cystic fibrosis. Thorax . 2005;60:50–54. doi: 10.1136/thx.2003.008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nixon PA, Orenstein DM, Kelsey SF, Doershuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. N Engl J Med . 1992;327:1785–1788. doi: 10.1056/NEJM199212173272504. [DOI] [PubMed] [Google Scholar]
- 42. Wilkinson TJ, Watson EL, Xenophontos S, Gould DW, Smith AC. The “minimum clinically important difference” in frequently reported objective physical function tests after a 12-week renal rehabilitation exercise intervention in nondialysis chronic kidney disease. Am J Phys Med Rehabil . 2019;98:431–437. doi: 10.1097/PHM.0000000000001080. [DOI] [PubMed] [Google Scholar]
- 43. Ruf K, Winkler B, Hebestreit A, Gruber W, Hebestreit H. Risks associated with exercise testing and sports participation in cystic fibrosis. J Cyst Fibros . 2010;9:339–345. doi: 10.1016/j.jcf.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 44. Fernandez La Puente de Battre MD, Neumeier LM, Ensslin C, Loidl M, Gräni C, Schmied C, et al. What it takes to recruit 77 subjects for a one-year study on active commuting. Scand J Med Sci Sports . 2020;30:1090–1095. doi: 10.1111/sms.13682. [DOI] [PubMed] [Google Scholar]
- 45. Sawyer A, Cavalheri V, Jenkins S, Wood J, Cecins N, Bear N, et al. High-intensity interval training is effective at increasing exercise endurance capacity and is well tolerated by adults with cystic fibrosis. J Clin Med . 2020;9:3098. doi: 10.3390/jcm9103098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med . 2020;54:1451–1462. doi: 10.1136/10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gruet M, Saynor ZL, Urquhart DS, Radtke T. Rethinking physical exercise training in the modern era of cystic fibrosis: A step towards optimising short-term efficacy and long-term engagement. J Cyst Fibros . 2021 doi: 10.1016/j.jcf.2021.08.004. [DOI] [PubMed] [Google Scholar]