To the Editor:
Recently, we published the results of a randomized trial (HENIVOT) comparing helmet noninvasive ventilation followed by high-flow nasal oxygen versus high-flow nasal oxygen alone in patients with coronavirus disease (COVID-19) and moderate to severe respiratory failure (PaO2/FiO2 < 200 mm Hg and PaCO2 ⩾ 45 mm Hg). Results showed no significant intergroup difference in the primary outcome (28-day respiratory support-free days), but lower intubation rate and increased 28-day invasive ventilation-free days in the helmet group (1). The accompanying editorial addressed the relevant issue of personalizing treatments by identifying subphenotypes of patients who may best benefit from each technique (2).
We performed post hoc analyses to establish whether any bedside available parameter before randomization (PaO2/FiO2, PaCO2, respiratory rate, visual analog scale [VAS] dyspnea, PaO2/[FiO2 × respiratory rate], SpO2/[FiO2 × respiratory rate] (3), PaO2/[FiO2 × VAS dyspnea]) could help identify subgroups of patients who could most benefit from the interventions of the trial.
The parameters that were found to identify subgroups of patients with different response to treatments were presence of hypocapnia and PaO2/(FiO2 × VAS dyspnea) < 30 before randomization. In these post hoc analyses, we report study outcomes in the two groups after classifying patients according to 1) whether they were normo- or hypocapnic; and 2) whether their PaO2/(FiO2 × VAS dyspnea) was less than 30 or at 30 or more.
Methods
A total of 109 patients admitted to four ICUs in Italy with COVID-19 and moderate to severe hypoxemic respiratory failure (PaO2/FiO2 ⩽ 200) were randomized to receive 48-hour continuous treatment with helmet noninvasive ventilation (positive end-expiratory pressure 10–12 cm H2O and pressure support 10–12 cm H2O) eventually followed by high-flow nasal oxygen, or high-flow nasal oxygen alone (flow, 60 L/min). Full details of study protocol are provided elsewhere (clinicaltrials.gov NCT04502576) (1). The study was approved by the ethics committee of all centers.
In these post hoc analyses, intergroup differences in study outcomes were analyzed in the subgroups of patients exhibiting 1) PaCO2 less than 35mm Hg or 35mm Hg or more; and 2) PaO2/(FiO2 × VAS dyspnea) < 30 or ⩾30 (median of the cohort). PaO2/FiO2, PaCO2, and VAS dyspnea were measured while patients were receiving Venturimask oxygen before randomization. VAS dyspnea was assessed by visual analog scale, ranging from 0 to 10, with 10 representing the worst symptom (4, 5). For patients with VAS dyspnea = 0, PaO2/(FiO2 × VAS dyspnea) was considered equal to PaO2/FiO2.
The number of days free of respiratory support (high-flow nasal oxygen, noninvasive, and invasive ventilation) within 28 days after enrollment was the primary endpoint. The rate of endotracheal intubation within 28 days, the number of days free of invasive mechanical ventilation at Days 28 and 60, in-ICU and in-hospital mortality, mortality at Days 28 and 60, and ICU and hospital length of stay were secondary outcomes.
Data are expressed as number of events (percentage) or median (interquartile range [IQR]). Ordinal quantitative variables were compared with the Mann-Whitney U test, after the nonnormal distribution was determined with the Shapiro-Wilk test. Comparisons between groups regarding qualitative variables were performed with the Fisher’s exact or the chi-square test, as appropriate. Multivariate analyses adjusting for simplified acute physiology score II, sequential organ failure assessment, PaO2/FiO2 at inclusion, and site of enrollment and time of randomization as random effects were conducted through linear or logistic regression models. Kaplan-Meier curves are displayed for results concerning intubation. All results with two-sided P ⩽ 0.05 are considered statistically significant. Statistical analysis was performed with IBM SPSS 26.
Results
Demographic study endpoints are displayed in Table 1. Kaplan-Meier tables are displayed in Figure 1.
Table 1.
Characteristics at Inclusion and Study Outcomes, according to Study Group*
| PaCO2 < 35mm Hg (n = 59) |
PaCO2 ⩾ 35 mm Hg (n = 50) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Helmet Noninvasive Ventilation (n = 28) | High-Flow Nasal Oxygen (n = 31) | Absolute or Mean Difference (95% CI) | OR (95% CI) | P Value | Helmet Noninvasive Ventilation (n = 26) | High-Flow Nasal Oxygen (n = 24) | Absolute or Mean Difference (95% CI) | OR (95% CI) | P Value | |
| Characteristics at study inclusion | ||||||||||
| Age, yr | 66 (53 to 73) | 64 (55 to 71) | −1 (−8 to 4) | — | 0.93 | 66 (60 to 72) | 61 (53 to 68) | 8 (1 to 14) | — | 0.056 |
| Sex, F, n (%) | 4 (14) | 5 (16) | −2 (−20 to 18) | 0.87 (0.21 to 3.6) | >0.99 | 18 (69) | 20 (83) | −14 (−36 to 10) | 2.22 (0.57 to 8.65) | 0.33 |
| Sex, M, n (%) | 24 (86) | 26 (84) | 2 (−18 to 20) | 1.15 (0.28 to 4.81) | >0.99 | 8 (31) | 4 (17) | 14 (−10 to 36) | 0.45 (0.12 to 1.75) | 0.33 |
| Body mass index† | 26 (26 to 29) | 27 (23 to 32) | 0 (−1 to 1) | — | 0.97 | 28 (26 to 31) | 30 (23 to 33) | −2 (−5 to 1) | — | 0.18 |
| Respiratory rate at enrollment, breaths/min | 28 (24 to 35) | 26 (23 to 32) | 1 (−3 to 5) | — | 0.61 | 30 (24 to 31) | 28 (23 to 31) | 1 (−2 to 3) | — | 0.69 |
| Device-related discomfort at enrollment‡ | 1 (0 to 3) | 0 (0 to 1) | 1 (0 to 2) | — | 0.13 | 0 (0 to 5) | 0 (0 to 2) | 0 (−1 to 2) | — | 0.96 |
| VAS dyspnea at enrollment‡ | 3 (2 to 6) | 4 (0 to 6) | 1 (−1 to 2) | — | 0.41 | 4 (1 to 7) | 3 (1 to 7) | 2 (−1 to 2) | — | 0.98 |
| VAS dyspnea change after 1 h of treatment‡ | 1 (0 to 3) | 0 (−1 to 1) | 2 (0 to 3) | — | 0.006 | 1 (0 to 3) | 0 (−1 to 3) | 1 (−1 to 2) | — | 0.10 |
| Arterial blood gases at enrollment | ||||||||||
| PaO2/FiO2 ratio, mm Hg | 103 (84 to 126) | 93 (80 to 122) | 10 (−4 to 17) | — | 0.30 | 106 (82 to 126) | 109 (84 to 130) | −4 (−21 to 13) | — | 0.68 |
| PaCO2, mm Hg | 31 (28 to 33) | 32 (30 to 34) | −1 (−3 to 0) | — | 0.046 | 37 (36 to 39) | 37 (36 to 40) | 0 (−1 to 2) | — | 0.85 |
| SAPS II | 32 (25 to 35) | 29 (24 to 37) | 0 (−4 to 3) | — | 0.98 | 32 (28 to 37) | 24 (28 to 32) | 4 (0 to 9) | — | 0.024 |
| Outcomes | ||||||||||
| Respiratory support–free days | 21 (11 to 25) | 14 (0 to 21) | 5 (0 to 11) | — | 0.07 | 16 (0 to 24) | 20 (1 to 23) | −2 (−8 to 4) | — | 0.80 |
| Intubation within 28 d from enrollment | 5 (18) | 19 (61) | −43 (−61 to −19) | 0.14 (0.04 to 0.46) | 0.001 | 11 (42) | 9 (38) | 9 (−17 to 33) | 1.22 (0.39 to 3.80) | 0.78 |
| 28-d invasive ventilation-free days | 28 (28 to 28) | 19 (3 to 28) | 8 (2 to 14) | — | 0.003 | 28 (4 to 28) | 28 (9 to 28) | −2 (−8 to 5) | — | 0.81 |
| 60-d invasive ventilation-free days | 60 (60 to 60) | 50 (5 to 60) | 17 (5 to 30) | — | 0.002 | 60 (8 to 60) | 60 (33 to 60) | −8 (−20 to 5) | — | 0.36 |
| 28-d mortality | 3 (11) | 8 (26) | −15 (−34 to 5) | 0.34 (0.08 to 1.46) | 0.19 | 5 (19) | 2 (8) | 11 (−10 to 30) | 2.62 (0.46 to 15) | 0.42 |
| 60-d mortality | 5 (18) | 10 (32) | −14 (−35 to 8) | 0.46 (0.13 to 1.55) | 0.24 | 8 (31) | 2 (8) | 22 (0 to 43) | 4.89 (0.92 to 25.97) | 0.78 |
| In-ICU mortality | 3 (11) | 12 (39) | −28 (−47 to −6) | 0.19 (0.05 to 0.77) | 0.018 | 8 (31) | 2 (8) | 22 (0 to 43) | 4.89 (0.92 to 25.97) | 0.78 |
| In-hospital mortality§ | 5 (18) | 12 (39) | −21 (−41 to 2) | 0.34 (0.10 to 1.15) | 0.092 | 8 (31) | 2 (8) | 22 (0 to 43) | 4.89 (0.92 to 25.97) | 0.78 |
| Duration of stay in the ICU, d | 8 (4 to 17) | 12 (6 to 23) | −9 (−18 to 0) | — | 0.12 | 9 (4 to 22) | 8 (5 to 17) | −3 (−13 to 8) | — | 0.88 |
| Duration of stay in the hospital, d | 22 (14 to 33) | 23 (13 to 47) | −8 (−20 to 3) | — | 0.37 | 20 (13 to 28) | 18 (13 to 32) | −4 (−15 to 6) | — | 0.91 |
| PaO2/(FiO2 × VAS Dyspnea) ⩾ 30 (n = 55) |
PaO2/(FiO2 × VAS Dyspnea) < 30 (n = 54) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Helmet Noninvasive Ventilation (n = 27) | High-Flow Nasal Oxygen (n = 28) | Absolute or Mean Difference (95% CI) | OR (95% CI) | P Value | Helmet Noninvasive Ventilation (n = 27) | High-Flow Nasal Oxygen (n = 27) | Absolute or Mean Difference (95% CI) | OR (95% CI) | P Value | |
| Characteristics at study inclusion | ||||||||||
| Age, yr | 65 (59 to 72) | 64 (57 to 69) | 3 (−3 to 9) | — | 0.36 | 67 (53 to 73) | 59 (53 to 70) | 2 (−4 to 8) | — | 0.44 |
| Sex, F, n (%) | 5 (18) | 6 (21) | −3 (−24 to 18) | 0.83 (0.22 to 3.14) | >0.99 | 7 (26) | 3 (11) | 15 (−6 to 35) | 2.8 (0.6 to 12.2) | 0.29 |
| Sex, M, n (%) | 22 (82) | 22 (79) | 3 (−18 to 24) | 1.2 (0.32 to 4.52) | >0.99 | 20 (74) | 24 (89) | −15 (−35 to 6) | 0.36 (0.08 to 1.56) | 0.29 |
| Body mass index† | 26 (26 to 29) | 28 (25 to 31) | −1.2 (−3.8 to 1.3) | — | 0.62 | 28 (26 to 30) | 28 (27 to 32) | −0.5 (−2.5 to 1.5) | — | 0.72 |
| Respiratory rate at enrollment, breaths/min | 29 (24 to 31) | 25 (22 to 29) | 2 (0 to 5) | — | 0.11 | 28 (24 to 32) | 30 (25 to 34) | −1 (−5 to 3) | — | 0.42 |
| Device-related discomfort at enrollment‡ | 0 (0 to 2) | 0 (0 to 0) | 0 (0 to 1) | — | 0.67 | 2 (0 to 5) | 1 (0 to 5) | 0 (−1 to 2) | — | 0.47 |
| VAS dyspnea at enrollment‡ | 2 (0 to 3) | 1 (0 to 2) | 0 (0 to 1) | — | 0.37 | 7 (5 to 7) | 6 (4 to 7) | 1 (0 to 1) | — | 0.15 |
| VAS dyspnea change after 1 h of treatment‡ | 0 (0 to 2) | 0 (−2 to 0) | 1 (0 to 2) | — | 0.005 | 2 (1 to 4) | 1 (0 to 3) | 1 (0 to 3) | — | 0.04 |
| Arterial blood gases at enrollment | ||||||||||
| PaO2/FiO2 ratio, mm Hg | 114 (83 to 133) | 115 (92 to 136) | 1 (−17 to 19) | — | 0.98 | 97 (82 to 115) | 90 (72 to 115) | 7 (−6 to 20) | — | 0.34 |
| PaCO2, mm Hg | 34 (31 to 37) | 34 (32 to 38) | −1 (−3 to 1) | — | 0.51 | 34 (31 to 38) | 34 (32 to 37) | 0 (−2 to 2) | — | 0.93 |
| SAPS II | 32 (27 to 35) | 29 (24 to 36) | 1 (−3 to 5) | — | 0.56 | 32 (29 to 35) | 29 (24 to 32) | 2 (−2 to 6) | — | 0.10 |
| Outcomes | ||||||||||
| Respiratory support-free days | 22 (13 to 25) | 21 (10 to 23) | 1 (5 to 6) | — | 0.49 | 13 (0 to 24) | 1 (0 to 19) | 3 (−2 to 9) | — | 0.29 |
| Intubation within 28 d from enrollment | 6 (22) | 9 (32) | −10 (−32 to 13) | 0.6 (0.18 to 2.01) | 0.55 | 10 (37) | 19 (70) | −33 (−54 to −7) | 0.25 (0.08 to 0.77) | 0.03 |
| 28-d invasive ventilation-free days | 28 (28 to 28) | 28 (18 to 28) | 1 (−5 to 6) | — | 0.46 | 28 (5 to 28) | 9 (2 to 28) | 6 (0 to 13) | — | 0.04 |
| 60-d invasive ventilation-free days | 60 (60 to 60) | 60 (50 to 60) | 2 (−10 to 13) | — | 0.45 | 60 (9 to 60) | 30 (9 to 60) | 10 (−3 to 23) | — | 0.09 |
| 28-d mortality | 3 (11) | 4 (14) | −3 (−22 to 16) | 0.75 (0.15 to 3-72) | >0.99 | 5 (18) | 6 (22) | −4 (−25 to 18) | 0.80 (0.21 to 3) | >0.99 |
| 60-d mortality | 5 (19) | 5 (18) | 1 (−20 to 21) | 1.05 (0.27 to 4.12) | >0.99 | 8 (30) | 7 (26) | 4 (−20 to 26) | 1.2 (0.36 to 4) | >0.99 |
| In-ICU mortality | 4 (15) | 5 (18) | −3 (−23 to 17) | 0.8 (0.19 to 3.36) | >0.99 | 7 (26) | 9 (33) | −7 (−30 to 16) | 0.7 (0.22 to 2.27) | 0.76 |
| In-hospital mortality§ | 4 (15) | 5 (18) | −3 (−23 to 17) | 0.8 (0.19 to 3.36) | >0.99 | 9 (33) | 9 (33) | 0 (−24 to 24) | 1 (0.32 to 3.1) | >0.99 |
| Duration of stay in the ICU, d | 5 (3 to 10) | 8 (5 to 11) | −1 (−8 to 6) | — | 0.21 | 13 (6 to 26) | 14 (5 to 57) | −11 (−23 to 0) | — | 0.42 |
| Duration of stay in the hospital, d | 17 (11 to 26) | 19 (13 to 30) | −1 (−9 to 6) | — | 0.50 | 24 (16 to 36) | 23 (14 to 70) | −12 (−26 to 1) | — | 0.50 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; SAPS II = Simplified Acute Physiology Score II; VAS = visual analog scale.
There were no missing data among the two groups. For calculations, PaO2 was expressed in mm Hg and FiO2 as fraction of the unity (0.21–1). For nonnormal quantitative variables, comparison between groups was performed with Mann-Whitney test. Comparisons between groups for qualitative variables were performed with the chi-square test or the Fisher’s exact test, as appropriate in agreement with test’s assumptions. Mean differences and odds ratios are unadjusted. For adjusted results, see the main text. Respiratory support: invasive or noninvasive mechanical ventilation, high-flow nasal cannula.
Values are displayed as median (interquartile range) if not otherwise specified.
The body mass index is the weight in kilograms divided by the square of the height in meters.
Dyspnea and discomfort were assessed through visual analog scales adapted for patients in the ICU ranging from 0 to 10.
One patient was discharged from hospital but died upon readmission.
Figure 1.
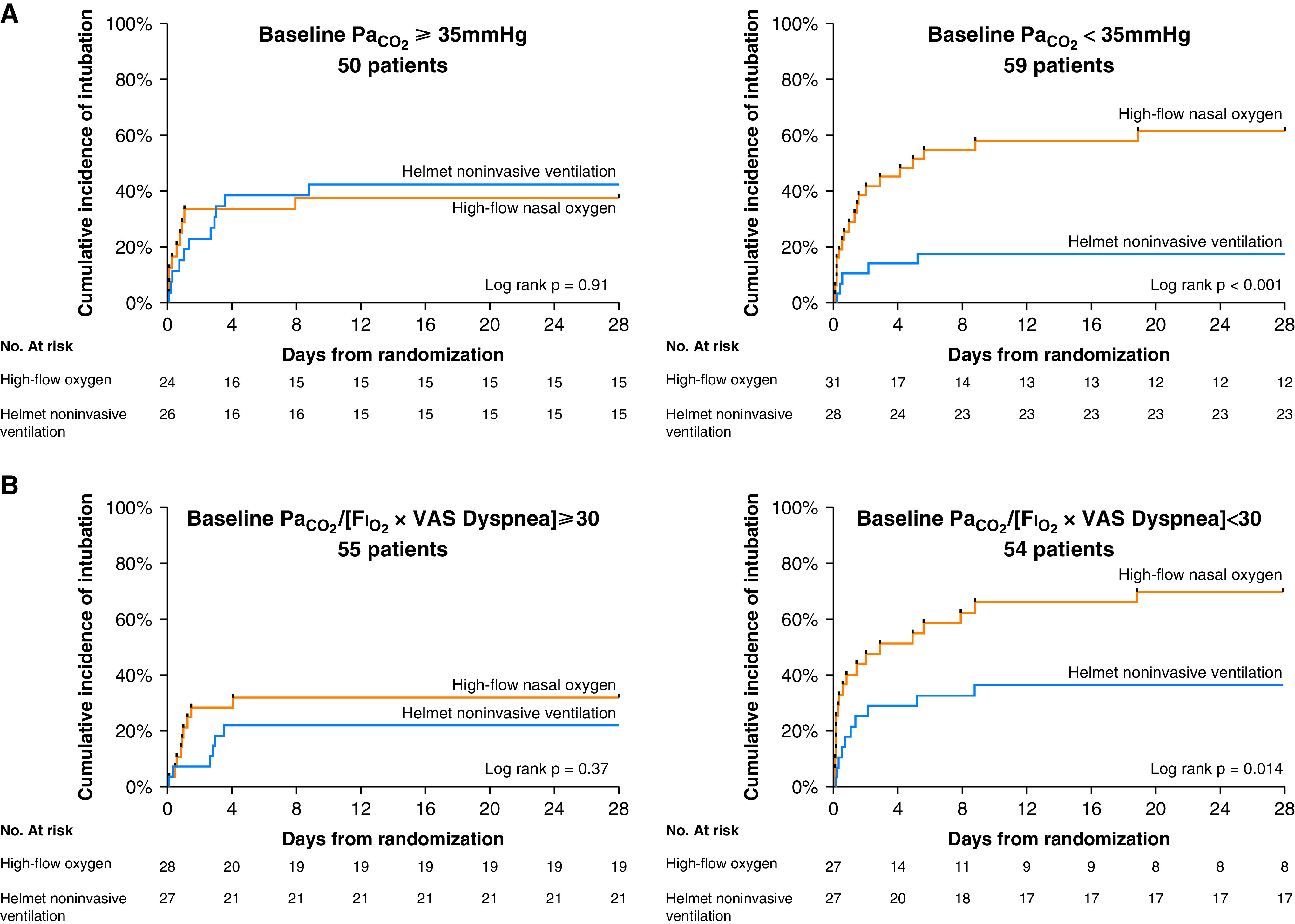
(A) Kaplan-Meier plots of the cumulative incidence of intubation from randomization to Day 28 in the subgroup of patients with PaCO2 of less than 35 mm Hg (n = 59 patients) and 35 mm Hg or more (n = 50 patients) at study enrollment. The hazard ratio for endotracheal intubation in the helmet noninvasive ventilation group in patients with PaCO2 of less than 35 mm Hg was 0.25 (95% CI, 0.11–0.57). The hazard ratio for endotracheal intubation in the helmet noninvasive ventilation group in patients with PaCO2 of at least 35 mm Hg was 1.05 (95% CI, 0.44–2.53). (B) Kaplan-Meier plots of the cumulative incidence of intubation from randomization to Day 28 in the subgroup of patients with PaO2/(FiO2 × dyspnea) lower (n = 54 patients) and equal to or higher than (n = 55 patients) 30 at study enrollment. The hazard ratio for endotracheal intubation in the helmet noninvasive ventilation group in patients with PaO2/(FiO2 × VAS dyspnea) < 30 was 0.39 (95% CI, 0.19–0.82). The hazard ratio for endotracheal intubation in the helmet noninvasive ventilation group in patients with PaO2/(FiO2 × VAS dyspnea) ⩾ 30 was 0.63 (95% CI, 0.23–1.73). CI = confidence interval; VAS = visual analog scale.
PaCO2 before treatment start
Among 109 analyzed patients, 59 patients had PaCO2 of less than 35 mm Hg and 50 had PaCO2 of 35 mm Hg or more.
In patients with PaCO2 of less than 35 mm Hg, the median (IQR) days free of respiratory support within 28 days after randomization were 21 (11–25) in the helmet group and 14 (0–21) in the high-flow group, a difference that was not significant before or after adjustment for covariates (P = 0.07).
The rate of endotracheal intubation was significantly lower in the helmet group than in the high-flow group: 18% versus 61%, with an absolute risk reduction of −43% (95% confidence interval [CI], −61% to −19%) and an adjusted odds ratio of 0.10 (95% CI, 0.22 to 0.42; P = 0.002) (Figure 1C).
In-ICU mortality was significantly lower in the helmet group than in the high-flow group: 11% versus 39%, with an absolute risk reduction of −28% (95% CI, −47% to −6%) and an adjusted odds ratio of 0.15 (95% CI, 0.03 to 0.69; P = 0.015).
In patients with PaCO2 of 35 mm Hg or less, there were no significant differences between the helmet and the high-flow group for any analyzed outcome.
PaO2/(FiO2 × VAS dyspnea) before treatment start
Among 109 analyzed patients, 55 patients had PaO2/(FiO2 × VAS dyspnea) ⩾ 30 and 54 had PaO2/(FiO2 × VAS dyspnea) ⩽ 30.
In patients with PaO2/(FiO2 × VAS dyspnea] < 30, the median (IQR) days free of respiratory support within 28 days after randomization was 13 (0–24) in the helmet group and 1 (0–19) in the high-flow group, a difference that was not statistically significant (P = 0.29). At the adjusted analysis, the number of days free of respiratory support at 28 days was significantly higher in the helmet group, with an adjusted mean difference of 5 (95% CI, 0–10; P = 0.04).
The rate of endotracheal intubation was significantly lower in the helmet group than in the high-flow group: 37% versus 70%, with an absolute risk reduction of 33% (95% CI, −7% to 54%) and an adjusted odds ratio of 0.11 (95% CI, 0.02 to 0.55; P = 0.008) (Figure 1B).
In patients with PaO2/(FiO2 × VAS dyspnea) ⩾ 30, there were no significant differences between the helmet and the high-flow group for any analyzed outcome.
Discussion
The results of these post hoc analyses of the HENIVOT trial indicate that the beneficial effects of helmet noninvasive ventilation over high-flow nasal oxygen in patients with COVID-19 with moderate to severe hypoxemia are magnified and limited to the subgroup of patients with PaO2/(FiO2 × VAS dyspnea) < 30 and/or PaCO2 of less than 35mm Hg before treatment start.
PaO2/FiO2 and VAS dyspnea are markers of disease severity (5); hypocapnia may reflect dysregulation of brain homeostasis toward a lower level of PaCO2, resulting in increased inspiratory effort, high Vt, and tachypnea (6).
Results from this post hoc analysis are consistent with data indicating that the physiologic benefit of helmet noninvasive ventilation over high-flow nasal oxygen is prominent among patients with more severe oxygenation impairment and intense inspiratory effort (7).
These results may aid bedside patient phenotyping for clinical decision making and personalizing treatments. High-flow nasal oxygen is a simple, easy-to-use tool applied worldwide (8). Conversely, helmet noninvasive ventilation is a less diffuse technique (9) and requires a mechanical ventilator and personnel expertise, whose shortage in the context of the COVID-19 pandemic may limit the number of patients who may have access to this kind of support. PaO2/(FiO2 × VAS dyspnea) and PaCO2 are bedside-available parameters that may help identify patients in whom helmet noninvasive ventilation as applied in the HENIVOT trial may improve clinical outcome (7, 10).
Our study has limitations: The post hoc nature of these analyses and the small sample make the results hypothesis generating, warranting further confirmatory investigations; the thresholds proposed should be taken cautiously; and VAS dyspnea is mainly used to compare dyspnea within a subject before and after a stimulus is applied, but it has been recently used to compare subjects undergoing noninvasive support (4, 5). We believe that its application in the present investigation is legitimate.
In patients with COVID-19 and moderate to severe hypoxemic respiratory failure, these analyses suggest that high-flow oxygen is as effective as helmet noninvasive ventilation in patients who show PaO2/(FiO2 × VAS dyspnea) ⩾ 30 and/or PaCO2 of 35 mm Hg or more under conventional oxygen, whereas helmet noninvasive ventilation as applied in the HENIVOT trial may improve clinical outcome among subjects exhibiting PaO2/(FiO2 × VAS dyspnea) < 30 and/or PaCO2 of less than 35 mm Hg.
Acknowledgments
Acknowledgments
The authors thank all ICU doctors, residents, nurses, and personnel from the participating centers, whose sacrifice, efforts, devotion to patients, and passion have made possible this timely report. They also thank Dr. Cristina Cacciagrano and Dr. Emiliano Tizi for their contribution to study organization.
Members of the COVID-ICU Gemelli Study Group: Jonathan Montomoli, Giulia Falò, Tommaso Tonetti, Salvatore L. Cutuli, Gabriele Pintaudi, Eloisa S. Tanzarella, Edoardo Piervincenzi, Antonio M. Dell’Anna, Luca Delle Cese, Simone Carelli, Maria Grazia Bocci, Luca Montini, Giuseppe Bello, Daniele Natalini, Gennaro De Pascale, Matteo Velardo, Carlo Alberto Volta, V. Marco Ranieri, Giorgio Conti, Riccardo Maviglia, Giovanna Mercurio, Paolo De Santis, Mariano Alberto Pennisi, Gian Marco Anzellotti, Flavia Torrini, Carlotta Rubino, Tony C. Morena, Veronica Gennenzi, Stefania Postorino, Joel Vargas, Nicoletta Filetici, Donatella Settanni, Miriana Durante, Laura Cascarano, Mariangela Di Muro, Roberta Scarascia, Martina Murdolo, Alessandro Mele, Serena Silva, Carmelina Zaccone, Francesca Pozzana, Alessio Maccaglia, Martina Savino, Antonella Potalivo, Francesca Ceccaroni, Angela Scavone, Gianmarco Lombardi, and Teresa Michi.
Footnotes
Supported by the Italian Society of Anesthesia, Analgesia, and Intensive Care Medicine 2017 MSD award. The funder had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. D.L.G. and L.S.M. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The study was endorsed by the “Insufficienza respiratoria acuta e assistenza respiratoria-IRAAR” study group of the Italian Society of Anesthesia, Analgesia and Intensive Care Medicine.
Author Contributions: D.L.G. and M.A. conceived the study. All authors contributed to data acquisition. L.S.M. conducted statistical analysis. D.L.G. interpreted the data and wrote the first draft of the manuscript. S.M.M. and M.A. critically revised the manuscript. M.A. organized the study as an overall supervisor. All authors reviewed the final draft of the manuscript and agreed on submitting it to the Journal.
Originally Published in Press at DOI: 10.1164/rccm.202105-1212LE on November 17, 2017
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
the COVID-ICU Gemelli Study Group:
Jonathan Montomoli, Giulia Falò, Tommaso Tonetti, Salvatore L. Cutuli, Gabriele Pintaudi, Eloisa S. Tanzarella, Edoardo Piervincenzi, Antonio M. Dell’Anna, Luca Delle Cese, Simone Carelli, Maria Grazia Bocci, Luca Montini, Giuseppe Bello, Daniele Natalini, Gennaro De Pascale, Matteo Velardo, Carlo Alberto Volta, V. Marco Ranieri, Giorgio Conti, Riccardo Maviglia, Giovanna Mercurio, Paolo De Santis, Mariano Alberto Pennisi, Gian Marco Anzellotti, Flavia Torrini, Carlotta Rubino, Tony C. Morena, Veronica Gennenzi, Stefania Postorino, Joel Vargas, Nicoletta Filetici, Donatella Settanni, Miriana Durante, Laura Cascarano, Mariangela Di Muro, Roberta Scarascia, Martina Murdolo, Alessandro Mele, Serena Silva, Carmelina Zaccone, Francesca Pozzana, Alessio Maccaglia, Martina Savino, Antonella Potalivo, Francesca Ceccaroni, Angela Scavone, Gianmarco Lombardi, and Teresa Michi
References
- 1. Grieco DL, Menga LS, Cesarano M, Rosà T, Spadaro S, Bitondo MM, et al. COVID-ICU Gemelli Study Group Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA . 2021;325:1731–1743. doi: 10.1001/jama.2021.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Munshi L, Hall JB. Respiratory support during the COVID-19 pandemic: is it time to consider using a helmet? JAMA . 2021;325:1723–1725. doi: 10.1001/jama.2021.4975. [DOI] [PubMed] [Google Scholar]
- 3. Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med . 2019;199:1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 4. Dres M, Similowski T, Goligher EC, Pham T, Sergenyuk L, Telias I, et al. Dyspnoea and respiratory muscle ultrasound to predict extubation failure. Eur Respir J . 2021;58:2100002. doi: 10.1183/13993003.00002-2021. [DOI] [PubMed] [Google Scholar]
- 5. Dangers L, Montlahuc C, Kouatchet A, Jaber S, Meziani F, Perbet S, et al. REVA Network (Research Network in Mechanical Ventilation) and the Groupe de Recherche en Réanimation Respiratoire en Onco-Hématologie (GrrrOH) List of contributors who included study patients: Angers University Hospital, Angers, France. Dyspnoea in patients receiving noninvasive ventilation for acute respiratory failure: prevalence, risk factors and prognostic impact: a prospective observational study. Eur Respir J . 2018;52:1702637. doi: 10.1183/13993003.02637-2017. [DOI] [PubMed] [Google Scholar]
- 6. Vaporidi K, Akoumianaki E, Telias I, Goligher EC, Brochard L, Georgopoulos D. Respiratory drive in critically ill patients. Pathophysiology and clinical implications. Am J Respir Crit Care Med . 2020;201:20–32. doi: 10.1164/rccm.201903-0596SO. [DOI] [PubMed] [Google Scholar]
- 7. Grieco DL, Menga LS, Raggi V, Bongiovanni F, Anzellotti GM, Tanzarella ES, et al. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med . 2020;201:303–312. doi: 10.1164/rccm.201904-0841OC. [DOI] [PubMed] [Google Scholar]
- 8. Rochwerg B, Einav S, Chaudhuri D, Mancebo J, Mauri T, Helviz Y, et al. The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Intensive Care Med . 2020;46:2226–2237. doi: 10.1007/s00134-020-06312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grieco DL, Maggiore SM, Roca O, Spinelli E, Patel BK, Thille AW, et al. Noninvasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med . 2021;47:851–866. doi: 10.1007/s00134-021-06459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tonelli R, Busani S, Tabbì L, Fantini R, Castaniere I, Biagioni E, et al. Inspiratory effort and lung mechanics in spontaneously breathing patients with acute respiratory failure due to COVID-19: a matched control study. Am J Respir Crit Care Med . 2021;204:725–728. doi: 10.1164/rccm.202104-1029LE. [DOI] [PMC free article] [PubMed] [Google Scholar]


