Abstract
Of troops returning from Iraq and Afghanistan, approximately 5–20% have PTSD, and another 11–23% have traumatic brain injury (TBI). Cognitive-behavioral therapies (CBTs) are empirically validated treatment strategies for PTSD. However, cognitive limitations may interfere with the ability to adhere to, and benefit from, CBTs. Co-morbid TBI has not been systematically taken into consideration in PTSD outcome research or in treatment planning guidance. We hypothesized that poorer pre-treatment cognitive abilities would be associated with poorer treatment outcomes from CBTs for PTSD. The present study was a naturalistic examination of “treatment as usual” in an outpatient clinic that provides manualized CBTs for PTSD to military service members and veterans. Participants were 23 veterans aged 18–50 years with combat-related PTSD and symptom duration more than 1 year; 16 of whom had mild TBI. Our predictor variables were well-normed objective tests of cognitive ability measured at baseline; our outcome variables were: a) individual slopes of change of the PTSD Checklist 5 (PCL-5) and the Clinician Assessment of PTSD Scale (CAPS-5) over weeks of treatment; and b) pre- to post-treatment change (Δ) in PCL-5 and CAPS-5. Contrary to our prediction, neither pre-treatment cognitive performance, nor the presence of co-morbid mild TBI, predicted poorer response to CBTs for PTSD. Our results discourage any notion of excluding PTSD patients with poorer cognitive ability from CBTs. Study limitations include a naturalistic treatment design, which did not allow for control of confounders, and an inability to completely rule out type II error because of small sample size.
Keywords: posttraumatic stress disorder, evidence-based intervention, cognitive behavioral therapy, veteran, cognition
Introduction
Approximately 5–20% of non-treatment samples of troops returning from Iraq and Afghanistan have posttraumatic stress disorder (PTSD) {1; 2}. Over 400,000 service members have been treated for PTSD at Veterans Administration (VA) facilities between 2002 and 2015 {3}. A signature weapon of the present conflicts in the Middle East has been the improvised explosive device, which can cause concussive injuries. Consequently, in addition to the psychological consequences of combat exposure, an estimated 11–23% of combatants return with traumatic brain injury (TBI) {4}. From 2001 to 2013 over 200,000 cases of TBI were recorded in the VA Health Registry; of these, 80% were service related and associated with ongoing symptomatology; 80% were of mild severity {5}.
Both PTSD and mild TBI are associated with some degree of neurocognitive limitation {6}, although with somewhat differing profiles. PTSD has been associated with limitations in initial stimulus encoding, sustained attention, acquisition, retrieval of new verbal information, and executive functions {7–9}. However, in the absence of prospective studies, it may be impossible to rule out the possibility that at least some of these limitations may have pre-dated both the trauma and PTSD {10}. There are also a wide range of potential confounding factors, including: direct CNS effects associated with the trauma, e.g. torture, toxic exposures {11}; patterns of post-trauma substance use {12}; the presence of co-morbid psychiatric conditions {13}; and incentive to perform poorly on testing for financial and other reasons {14–16}.
Acute mild TBI (mTBI) is associated with slowed cognition, attentional limitations, defects in new verbal and visual learning, impaired oral fluency, and executive function limitations {17; 18}. Research suggests that objective indices of cognitive limitation following mTBI tend to normalize by about three months postinjury {19}, although that view has been recently challenged {20}.
An additive effect of co-morbid PTSD and TBI is suggested by findings of more extensive self-reported and objective cognitive limitations (viz., processing speed and executive functioning) in veterans afflicted with both conditions compared to those with mild TBI alone {21}. Given the high rates of PTSD and mTBI in returning service members, as well as the high rate of their co-occurrence, it is important to understand how mTBI may affect the treatment of PTSD.
At present, cognitive behavioral therapies (CBTs), specifically prolonged exposure (PE) {22} and cognitive processing therapy (CPT) {23}, are the first choices of evidence-based treatments for PTSD {24}. Yet it is unclear whether individuals with cognitive limitations related to either PTSD or mild TBI are able to benefit from treatments that rely, at least in part, on cognition. Some investigators have found that poorer cognitive performance (viz., verbal memory) is associated with poorer response to CBT for PTSD {25–27}. Poorer verbal learning has also been associated with poorer response to treatment for PTSD nightmares {28}. Alternatively, cognitive limitations may affect adherence to but not benefit from treatment in those who complete treatment. For example, in women who have PTSD as a result of sexual assault, lower intelligence scores (although within the normal range), and lower education have been associated with higher CBT dropout, but not with less treatment efficacy among those who complete treatment {29}. Similarly, in patients with PTSD and co-morbid schizophrenia, schizoaffective disorder, major depression, or bipolar disorder, poorer cognitive performance (viz., on a composite measure including attention, information processing speed, verbal learning and memory, and executive functioning) predicted poorer learning of information about posttraumatic stress symptoms but not clinical benefit from CBT {30}.
The influence of TBI on the efficacy of CBT in individuals with PTSD has not been systematically examined. Two studies examined the effectiveness of CPT-Cognitive in veterans with PTSD and co-morbid TBI in a residential treatment setting. After 7 weeks of treatment, veterans had pre- to post-treatment reductions in both PTSD scores {31; 32} and postconcussive symptoms {32}; these reductions were positively correlated {32}. However, the intervention included cognitive rehabilitation, which might have confounded the association between CBT and change in PTSD symptoms. Also, cognitive limitations were measured by self-report rather than objective tests, which diminishes the validity of the results. A third small study including veterans with PTSD and TBI of mild (n = 6) and moderate (n = 4) severity found nearly 50% reduction in PTSD symptoms following a course of PE. Both the mild and moderate TBI severity groups improved pre- to post-treatment {33}. This study included objective cognitive limitation measurements, but they were not part of the published analyses. However, it cannot be assumed that TBI necessarily equates with cognitive limitations. None of the above studies included the latter as an independent (predictor) variable.
In summary, the literature on cognitive predictors of treatment response to CBT for PTSD remains insufficient and inconclusive, because studies are scant, study populations differ, treatment models are difficult to compare, and few studies include objective measures of cognition. The current study examined whether objectively measured pre-treatment cognitive performance would predict PTSD treatment outcome in veterans and active duty military service members receiving PE or CPT in a naturalistic outpatient setting. We hypothesized that poorer pre-treatment cognitive ability (new learning, memory, processing speed, complex attention, inhibition, and flexibility) would be associated with poorer treatment response to CBT for PTSD.
Method
Participants
Participants were recruited from the Massachusetts General Hospital (MGH) Home Base Program (HBP), a private-public partnership between MGH and the Red Sox Foundation whose mission is to serve the clinical needs of Operation Enduring Freedom, Operation Iraqi Freedom and Operation New Dawn (OEF/OIF/OND) active duty service members, reservists, and veterans. As part of its treatment as usual, the HBP provides manualized individual CBT (PE or CPT) for patients with PTSD. Participants (N = 23; 20 male, 3 female) were a convenience sample of OEF/OIF/OND veterans and active duty service members between the ages of 18–50 years who met DSM-5 criteria for chronic (more than 1 year post-trauma) combat-related PTSD. They were classified in one of 2 groups: 1) the PTSD only group (i.e., no history of TBI); 2) the PTSD and mTBI group. In addition to the inclusion criteria for the PTSD only group, the PTSD and mTBI group had to have had a) Diagnosis of mTBI as recommended by the VA/DoD Clinical Practice Guidelines for Management of Concussion/Mild Traumatic Brain Injury {34}; b) mTBI that occurred 12 or more months prior to study entry (to minimize natural recovery from TBI that could confound treatment outcome). c) Current post-concussive symptoms with a score of at least 20 on the neurobehavioral symptoms inventory (NSI); d) Current cognitive complaints with a score of at least 3 (severe) on at least one the following NSI items: d1) “Poor concentration, can’t pay attention, easily distracted,” d2) “Forgetfulness, can’t remember things,” or d3) “Slowed thinking, difficulty getting organized, can’t finish things.” Because mTBI patients frequently recover to their baseline cognitive function, this additional requirement was designed to capture TBI subjects who continued to experience cognitive symptoms at the time of the treatment intervention, which we hypothesized may be a pivotal factor interfering with their ability to successfully utilize CBT. Patients identified as eligible were approached by their therapist about their interest in participating.
Exclusion criteria were: 1) greater than mild TBI (as defined in the VA/DoD Clinical Practice Guideline, Table A1) {34}; 2) a history of neurological disorder (e.g., stroke, epilepsy, multiple sclerosis, HIV, neurodegenerative disorder); 3) an acute or unstable medical condition likely to impair cognition and/or interfere with participation; 4) current risk of suicide (by CHRT-SR) {35}; 5) current psychotic disorder or melancholia; 6) current or lifetime history of bipolar disorder; 7) inability to attend regular appointments; 8) prior intolerance or failure of an adequate trial of CBT; 9) use of a psychostimulant (including Modafinil); 10) presence of skeletal muscle relaxants, narcotics, anticonvulsants, neuroleptics, or any other medication that could impair cognition or interfere with the assessments, as determined by history or urine testing; and 11) a medication dosage that was likely to change during the study time period. The PTSD only group included seven male veterans; the PTSD and mild TBI group included 13 male and three female veterans. Other characteristics appeared to be similar between the two groups.
Procedure
Patients were screened for eligibility during their regular evaluation process at the MGH HBP. Those who appeared to satisfy the inclusion and exclusion criteria were approached for potential participation. They provided written consent after a full explanation of the procedures. Participants received a baseline screening that included the CAPS-5 and the Structured Clinical interview for DSM-IV – Axis I Disorders (SCID I/P). They completed cognitive measures at the baseline assessment, at CBT completion, and 6 months post-treatment completion. Participants completed the PCL-5 at baseline, at each weekly CBT session, 1 month after completing CBT, and 6 months after completing CBT. In addition to baseline, the CAPS-5 was administered after every four sessions of CBT, 1 month after completing CBT, and 6 months after completing CBT. Participants received a small remuneration for their time participating in study assessments. There were 12 planned manualized sessions of either PE or CPT, according to therapist judgment and patient choice. Participants who received PE were instructed about the nature of PTSD with an emphasis on the role of avoidance behaviors that serve to maintain the disorder. PE treatment consisted of breathing retraining, in vivo exposure to feared situations and places, and imaginal exposure to the trauma memory. Participants who received CPT were instructed about the nature of PTSD with an emphasis on the role of maladaptive cognitions (i.e., “stuck points”) that serve to maintain the disorder. During CPT, participants learned about the connection between thoughts and feelings, to identify problematic patterns of thinking, and to effectively question assimilated (e.g., “It’s my fault”) and accommodated (e.g., “Authority cannot be trusted”) assumptions regarding safety, trust, intimacy, power, control, and self-esteem. Therapists were blind to the baseline neurocognitive assessment. The study was approved by the Partners Health Care System IRB.
Measures
PCL-5 total scores served as the primary treatment outcome measure {36}. The PCL-5 is a validated 20-item self-report assessment of PTSD severity with good internal consistency (alpha = .96), good test–retest reliability (r = .84), and good convergent and discriminant validity {37}. Internal consistency for the current study was very good (Standardized Cronbach’s alpha = .77). Additionally, an experienced psychometrician administered the “gold standard” for evaluating PTSD, the Clinician Assessment of PTSD Scale for DSM-5 (CAPS-5) {38}. The CAPS-5 internal consistency for the current study was very good (Standardized Cronbach’s alpha = .81).
Our predictor measures, assessed at pre-treatment baseline, were selected to assess several cognitive domains important to PTSD. They consisted of the following measures:
Rey Auditory Verbal Learning test (RAVLT) {39}, a measure of new learning and verbal memory. We calculated the sum of scores for trials 1–5 (RAVLT1–5) and the delayed recall score after distraction score (trial 7). Z-scores from published normative samples (age adjusted) {40} were used.
Wechsler Adult Intelligence Scale – Fourth Edition (WAIS-IV) {41} letter-number sequencing subtest (scaled score, age adjusted), a measure of complex attention and working memory.
WAIS-IV coding subtest (scaled score, age adjusted), a measure of cognitive processing speed.
Delis-Kaplan Executive Function System (D-KEFS) {42}, a measure of inhibition and cognitive flexibility. The color-word inhibition, inhibition switching, and total switching accuracy subtests of the D-KEFS were used (scaled score, age adjusted).
D-KEFS letter fluency and category fluency subtests (scaled score, age adjusted), which measure verbal fluency.
Advanced Clinical Solutions Test of Premorbid Functioning (ACS ToPF) {41} standard score (age adjusted), a measure of premorbid intellectual function.
In addition, the Test of Memory Malingering (TOMM) is a visual recognition test sensitive to reduced effort or malingering, which is insensitive to neurological impairments {43}. A TOMM Trial 1 score of 41 or greater has been shown to be a useful indicator of adequacy of effort in veterans {44}, so that administration of Trials 2 and 3 is unnecessary in this population.
Participants were classified as mTBI if A) there was a history of TBI identified through review of the clinical record or through a structured interview {45} and B) they scored 3 (severe) or more on at least one of the following neurobehavioral symptom inventory questions, a) “Poor concentration, can’t pay attention, easily distracted,” b) “Forgetfulness, can’t remember things,” or c) “Slowed thinking, difficulty getting organized, can’t finish things.” Because some mTBI patients recover to their baseline cognitive function, the additional requirement (B) was designed to capture TBI participants who continue to experience cognitive symptoms at the time of the treatment intervention, which we hypothesized may be necessary to interfere with their ability to successfully utilize CBT.
Data Analysis
Linear regression was used to regress the slope of PCL-5 scores on weeks after informed consent separately for each individual. Next, individual PCL-5 slopes were correlated with cognitive scores measured at the baseline assessment, separately. The same 2-step analysis was used for CAPS-5 scores. Because a negative slope indicates a progressive reduction of PTSD symptoms, i.e., improvement, and because higher baseline cognitive testing scores indicate better cognitive functioning, the hypothesis that poorer cognitive functioning will be associated with poorer treatment response predicts a negative correlation. Additionally, individual PCL-5 and CAPS-5 change scores (Δ, i.e., post- minus pre-) were correlated with baseline cognitive scores. Because a poorer treatment response means a lower change score, again the hypothesis predicts a negative correlation. No data imputation was performed. SAS 9.4 software package (Cary, NC) was used for all analyses.
Results
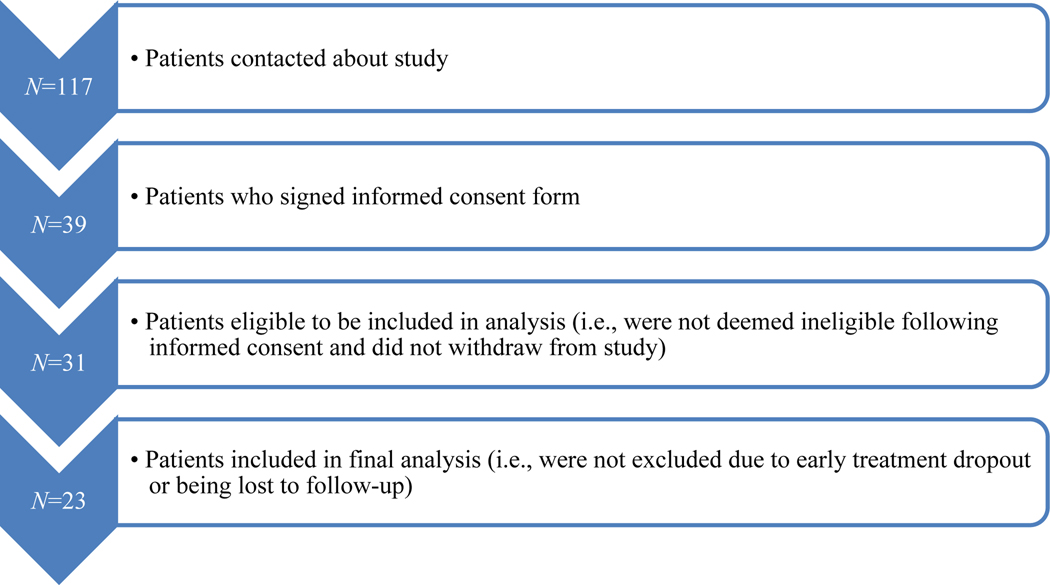
Participant recruitment within our naturalistic design is presented in Figure 1. Demographic information is shown in Table 1. Briefly, the majority of participants were male (87.0%), white (82.6%), single (47.8%) and of mean age 32.4 years (SD = 6.0). Seventy percent (69.6%) had comorbid PTSD and mTBI. Most (73.9%) received PE treatment. Means and standard deviations for our baseline predictor measures and for our pre-treatment and post-treatment outcome measures are shown in Table 2. It will be noted that PCL score decreased by an average of 26 points and CAPS by 14 points, indicating substantial but incomplete improvement. Cognitive score mean were generally within the normal range., but there was a good variability in the cognitive test scores and the clinical outcome measures. Table 3 displays the correlations between the baseline cognitive scores and PCL-5, CAPS-5 individual slopes (top half), ΔPCL-5, and ΔCAPS-5 (bottom half). D-KEFS color-word inhibition positively correlated with PCL-5 individual slope but not with CAPS-5 slope, ΔPCL-5, or ΔCAPS-5. No other significant correlations were found between baseline cognitive scores and individual PCL or CAPS-5 slopes or ΔPCL-5, or ΔCAPS-5. Accounting for the effect of age, education, ethnicity, and type of therapy (viz., CPT vs. PE) by using each of them separately in partial correlations did not change the above correlations. All but one subject showed good effort on our validity measure, the TOMM Trial 1: of 23 veterans, only one fell below 41 (score of 29). Removing that subject’s data did not substantially change the results. Therefore, we included all 23 subjects in final analyses.
Figure 1.
Recruitment Flow Chart
Table 1.
Participants’ Demographic Characteristics
| Study Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| PTSD only | PTSD+mTBI | Combined | |||||||
|
|
|
|
|||||||
| Variable | Total N | M | SD | Total N | M | SD | Total N | M | SD |
|
| |||||||||
| Age (years) | 7 | 30.43 | 6.88 | 16 | 33.25 | 5.64 | 23 | 32.39 | 6.03 |
|
| |||||||||
| PTSD only | PTSD+mTBI | Combined | |||||||
|
|
|
|
|||||||
| Total N | % | n | Total N | % | n | Total N | % | n | |
|
| |||||||||
| Sex | 7 | 16 | 23 | ||||||
| Male | 100.0 | 7 | 81.3 | 13 | 87.0 | 20 | |||
| Female | 0.0 | 0 | 18.8 | 3 | 13.0 | 3 | |||
| Marital Status | 7 | 16 | 23 | ||||||
| Single | 57.1 | 4 | 43.8 | 7 | 47.8 | 11 | |||
| Married | 14.3 | 1 | 37.5 | 6 | 30.4 | 7 | |||
| Divorced | 14.3 | 1 | 12.5 | 2 | 13.0 | 3 | |||
| Separated | 14.3 | 1 | 6.3 | 1 | 8.7 | 2 | |||
| Handedness | 7 | 16 | 23 | ||||||
| Right | 100.0 | 7 | 93.8 | 15 | 95.7 | 22 | |||
| Left | 0.0 | 0 | 6.3 | 1 | 4.4 | 1 | |||
| Race | 7 | 16 | 23 | ||||||
| White | 71.4 | 5 | 87.5 | 14 | 82.6 | 19 | |||
| Black or African | |||||||||
| American | 14.3 | 1 | 6.3 | 1 | 8.7 | 2 | |||
| Other | 14.3 | 1 | 6.3 | 1 | 8.7 | 2 | |||
| Ethnicity | 7 | 16 | 23 | ||||||
| Hispanic | 85.7 | 6 | 81.3 | 13 | 82.6 | 19 | |||
| Non-Hispanic | 14.3 | 1 | 18.8 | 3 | 17.4 | 4 | |||
| Education | 7 | 16 | 23 | ||||||
| High school diploma | 14.3 | 1 | 6.3 | 1 | 8.7 | 2 | |||
| 1 year of college | 14.3 | 1 | 18.8 | 3 | 17.4 | 4 | |||
| Associate’s degree or 2 years of college | 14.3 | 1 | 25.0 | 4 | 21.7 | 5 | |||
| 3–5 years of college but no degree | 0.0 | 0 | 18.8 | 3 | 13.0 | 3 | |||
| Bachelor’s degree | 28.6 | 2 | 18.8 | 3 | 21.7 | 5 | |||
| Post-bachelor’s or master’s degree | 14.3 | 1 | 6.3 | 1 | 8.7 | 2 | |||
| Post-master’s, no doctorate | 0.0 | 0 | 6.3 | 1 | 4.4 | 1 | |||
| Doctorate (PhD, MD, JD) | 14.3 | 1 | 0.0 | 0 | 4.4 | 1 | |||
| Therapy Type | 7 | 16 | 23 | ||||||
| CPT | 28.6 | 2 | 25.0 | 4 | 26.1 | 6 | |||
| PE | 71.4 | 5 | 75.0 | 12 | 73.9 | 17 | |||
Table 2.
Cognitive Scores Before Treatment, and Clinical Measures Before and After Treatment
| Variable | Study Group: PTSD Only | Study Group: PTSD + mTBI | Study Groups Combined | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Pre-treatment Cognitive Predictors | n | M | SD | n | M | SD | n | M | SD |
|
| |||||||||
| TOMM Trial 1 | 7 | 47.71 | 2.63 | 16 | 46.19 | 5.14 | 23 | 46.65 | 4.52 |
| RAVLT1-5 Z-Score* | 7 | 0.13 | 0.71 | 16 | −0.78 | 1.13 | 23 | −0.51 | 1.09 |
| RAVLT Trial 7 Z-Score* | 7 | 0.08 | 0.80 | 16 | −0.54 | 1.05 | 23 | −0.35 | 1.00 |
| WAIS Coding Normative Score* | 7 | 10.43 | 4.61 | 16 | 9.75 | 2.35 | 23 | 9.96 | 3.11 |
| WAIS Letter-Number Normative Score* | 7 | 12.00 | 4.65 | 16 | 9.81 | 2.32 | 23 | 10.48 | 3.26 |
| D-KEFS Color-Word Inhibition Scaled Score* | 7 | 10.57 | 4.54 | 16 | 10.13 | 3.46 | 23 | 10.26 | 3.72 |
| D-KEFS Inhibition/Switching Scaled Score* | 7 | 10.14 | 3.39 | 16 | 9.13 | 2.99 | 23 | 9.43 | 3.07 |
| D-KEFS Letter Fluency Total Correct Scaled Score* | 7 | 10.71 | 4.79 | 16 | 10.06 | 4.54 | 23 | 10.26 | 4.51 |
| D-KEFS Category Fluency Total Correct Scaled Score* | 7 | 13.00 | 4.55 | 16 | 10.00 | 4.07 | 23 | 10.91 | 4.35 |
| ACS Test of Premorbid Functioning Standard Score* | 7 | 109.71 | 14.08 | 16 | 104.31 | 10.09 | 23 | 105.96 | 11.40 |
|
| |||||||||
| Pre-Treatment Outcomes | |||||||||
|
| |||||||||
| PCL Total Score | 7 | 47.00 | 12.41 | 16 | 45.75 | 9.46 | 23 | 46.13 | 10.17 |
| CAPS Total Score | 7 | 29.71 | 10.21 | 15 | 43.20 | 7.80 | 22 | 38.91 | 10.57 |
| NSI Total Score | 7 | 33.71 | 16.92 | 15 | 40.07 | 11.94 | 22 | 38.05 | 13.64 |
|
| |||||||||
| Post-Treatment Outcomes | |||||||||
|
| |||||||||
| PCL Total Score | 7 | 21.14 | 21.40 | 15 | 29.20 | 14.64 | 22 | 26.64 | 16.99 |
| CAPS Total Score | 4 | 11.50 | 4.80 | 11 | 29.36 | 12.62 | 15 | 24.60 | 13.62 |
|
| |||||||||
| Differences Between Post-and Pre-Treatment Outcomes | |||||||||
|
| |||||||||
| ΔPCL | 7 | −25.86 | 22.33 | 15 | −17.60 | 15.80 | 22 | −20.23 | 18.01 |
| ΔCAPS | 4 | −13.75 | 6.40 | 11 | −12.27 | 14.57 | 15 | −12.67 | 12.68 |
All scores are age-adjusted.
Table 3.
Correlations Between Baseline Cognitive Scores and Outcome Measures
| Baseline Variable | N | r with PCL-5 Slope | 95% CI | P | N | r with CAPS-5 Slope | 95% CI | P | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Lower | Upper | Lower | Upper | |||||||
|
| ||||||||||
| RAVLT1–5 Z-Score* | 23 | −.05 | −.45 | .37 | .827 | 15 | −.39 | −.75 | .15 | .135 |
| RAVLT Trial 7 Z- Score* | 23 | −.25 | −.60 | .18 | .250 | 15 | −.31 | −.71 | .24 | .256 |
| WAIS Coding Normative Score* | 23 | .06 | −.36 | .46 | .769 | 15 | −.05 | −.55 | .47 | .855 |
| WAIS Letter-Number Normative Score* | 23 | .07 | −.35 | .47 | .732 | 15 | .05 | −.48 | .55 | .860 |
| D-KEFS Color-Word Inhibition Scaled Score* | 23 | .54 | .17 | .78 | .006 | 15 | .13 | −.41 | .60 | .628 |
| D-KEFS Inhibition/Switching Scaled Score* | 23 | .23 | −.20 | .59 | .276 | 15 | −.15 | −.61 | .40 | .595 |
| D-KEFS Letter Fluency Scaled Score* | 23 | −.13 | −.51 | .30 | .558 | 15 | −.39 | −.75 | .15 | .141 |
| D-KEFS Category Fluency Scaled Score* | 23 | −.14 | −.52 | .29 | .522 | 15 | −.12 | −.59 | .42 | .670 |
| ACS Test of Premorbid Functioning Standard Score* | 23 | .28 | −.15 | .62 | .191 | 15 | .19 | −.36 | .64 | .488 |
| Study Group: PTSD vs. PTSD+mTBI | 23 | .30 | −.13 | .63 | .163 | 15 | <.01 | −.51 | .51 | .998 |
|
| ||||||||||
| Baseline Variable | N | r with ΔPCL-5 | 95% CI | P | N | r with ΔPCL-5 | 95% CI | P | ||
|
|
|
|||||||||
| Lower | Upper | Lower | Upper | |||||||
|
| ||||||||||
| RAVLT1.5 Z-Score* | 22 | .03 | −.40 | .45 | .890 | 15 | −.20 | −.65 | .34 | .457 |
| RAVLT Trial 7 Z-Score* | 22 | −.13 | −.52 | .31 | .565 | 15 | .02 | −.50 | .53 | .936 |
| WAIS Coding Normative Score* | 22 | .05 | −.38 | .46 | .815 | 15 | .09 | −.44 | .58 | .733 |
| WAIS Letter-Number Normative Score* | 22 | .12 | −.32 | .51 | .601 | 15 | .20 | −.35 | .64 | .474 |
| D-KEFS Color-Word Inhibition Scaled Score* | 22 | −.02 | −.44 | .41 | .932 | 15 | .35 | −.19 | .73 | .184 |
| D-KEFS Inhibition/Switching Scaled Score* |
22 | −.03 | −.45 | .39 | .884 | 15 | .03 | −.49 | .53 | .914 |
| D-KEFS Letter Fluency Scaled Score* | 22 | −.06 | −.47 | .37 | .795 | 15 | .07 | −.45 | .57 | .788 |
| D-KEFS Category Fluency Scaled Score* | 22 | −.19 | −.57 | .25 | .380 | 15 | .15 | −.40 | .61 | .597 |
| ACS Test of Premorbid Functioning Standard Score* | 22 | .02 | −.41 | .43 | .942 | 15 | .29 | −.26 | .70 | .281 |
| Study Group: PTSD vs. PTSD+mTBI | 22 | .21 | −.23 | .58 | .333 | 15 | .05 | −.47 | .55 | .853 |
All scores are age-adjusted.
In order to address the issue of Type II error in the face of negative results, we averaged the lower (predicted direction) confidence limits of the correlations between each of the 9 cognitive predictors and the treatment outcome measures, which yielded: for PCL slope, −0.33; for delta PCL, −0.44; for CAPS slope, −0.58, for delta CAPS, −0.41. Cohen has proposed the following descriptors for effect sizes, as follows: r = .1 mild, r = .3 moderate, r = .5 strong {46; 47}.
Discussion
The current study investigated the effect of pre-treatment cognitive limitations on PTSD outcomes among veterans receiving evidence-based psychotherapies, i.e., PE or CPT, in a naturalistic treatment setting. Contrary to our prediction, pre-treatment cognitive limitations did not significantly predict poorer PTSD symptom response to CBT. Inspection of the correlations table (Table 3) does not reveal any meaningful patterns. For example, the only statistically significant finding was that D-KEFS color-word inhibition positively correlated with individual PCL-5 slope, thereby militating against the hypothesis. However, similar correlations were not evident with the other outcome measures (CAPS-5 slope, ΔPCL-5, ΔCAPS-5). There was a notable lack of consistent patterns for the remainder of the cognitive baseline variables. Additionally, we found no evidence that the presence of categorical mTBI correlates with poorer outcomes from CBT. Examination of confidence limits for the PCL outcome measure supports the conclusion that these results do not represent a Type II error with regard to the absence of a strong association, but they do not refute the possibility of a medium association.
Our findings are consistent with the portion of the literature that finds no association between cognition and CBT outcome {30}, and no association between the presence of mild TBI and ability to benefit from CBT for PTSD {31–33}. The similarity of our results to those in the above studies may be partially due to comparability of study populations, viz., OIF/OEF/OND veterans, and treatment approaches {31–33}. On the other hand, our findings differ from those of studies that found an association between cognition and CBT outcome {25–28}. These differences may be partially due to difference in study samples (veterans in our study vs. civilians in the Wild & Gur and Nijdam et al. studies; predominantly male veterans in our study vs. only female veterans in the Haaland et al. study) and difference in outcomes, e.g., PTSD symptoms in our study vs. nightmare distress and severity {28}.
The present study has several limitations. As noted above, results from a larger sample could have conveyed greater protection against Type II error. Additionally, our naturalistic treatment design does not allow us to rule out the contributions of multiple unmeasured factors, e.g., socioeconomic status, substance use, that may have confounded the true association between cognitive limitations and change in PTSD symptoms following CBT. The design did not incorporate a means for determining whether the objective cognitive limitations in any given subject in the PTSD+mTBI group were due to the mTBI or other factors (not an easy task)., Given our study’s limitations, independent replication of our results with larger study samples is warranted.
In contrast, our naturalistic design increases the generalizability of our results to other real-world PTSD populations encountered in veteran outpatient clinics. An additional strength of our study is that our main predictor construct (viz., cognition) was measured via objective and validated cognitive measures rather than via mere self-report.
The major clinical implication of our study is that, other things being equal, individuals with poorer neurocognitive abilities should not be assumed to benefit less from CBT for PTSD than those without such limitations. Our results discourage any notion of excluding PTSD patients with poorer cognitive abilities from CBT.
Acknowledgements:
The Home Base Program is supported philanthropically by the Wounded Warrior Project and is part of the Warrior Care Network™. This research was partly supported by a National Institute of Mental Health career development award grant 1K23MH097844-01A1 awarded to Kaloyan S. Tanev.
Footnotes
Previous Presentation: N/A
Location of Work: Home Base, A Red Sox Foundation and Massachusetts General Hospital Program, Massachusetts General Hospital, Boston, MA, USA
Disclosures: None of the authors have any disclosures of conflicts of interest.
Contributor Information
Kaloyan S. Tanev, Home Base, a Red Sox Foundation and Massachusetts General Hospital Program, Boston Department of Psychiatry, Massachusetts General Hospital, Boston.
Lydia E. Federico, Home Base, a Red Sox Foundation and Massachusetts General Hospital Program, Boston; Department of Psychiatry, Massachusetts General Hospital, Boston.
Mark S. Greenberg, Department of Psychiatry, Massachusetts General Hospital, Boston
Scott P. Orr, Department of Psychiatry, Massachusetts General Hospital, Boston
Elizabeth M. Goetter, Home Base, a Red Sox Foundation and Massachusetts General Hospital Program, Boston Department of Psychiatry, Massachusetts General Hospital, Boston.
Patricia A. Resick, Department of Psychiatry and Behavioral Sciences, Duke University Medical School, Durham, N.C.
Roger K. Pitman, Department of Psychiatry, Massachusetts General Hospital, Boston
References
- 1.Tanielian T, Jaycox LH, Schell TL, et al. : Invisible Wounds of War. Summary and Recommendations for Addressing Psychological and Cognitive Injuries, Santa Monica, CA, RAND Corporation, 2008. Available from: http://www.dtic.mil/dtic/tr/fulltext/u2/a480992.pdf [Google Scholar]
- 2.Ramchand R, Schell TL, Karney BR, et al. : Disparate prevalence estimates of PTSD among service members who served in Iraq and Afghanistan: Possible explanations. J. Trauma. Stress 2010; 23:59–68 [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Veterans Affairs: Report on VA Facility Specific Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans Diagnosed with Potential or Provisional PTSD, 2017. Available from: https://www.publichealth.va.gov/docs/epidemiology/ptsd-report-fy2015-qrt3.pdf
- 4.Lindquist LK, Love HC, Elbogen EB: Traumatic Brain Injury in Iraq and Afghanistan Veterans: New Results from a National Random Sample Study. J. Neuropsychiatry Clin. Neurosci 2017; 29:254–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiteneck GG, Cuthbert JP, Mellick DC: VA Traumatic Brain Injury Veterans Health Registry Report, Department of Veterans Affairs/Department of Defense, 2015. Available from: https://www.publichealth.va.gov/docs/epidemiology/TBI-report-fy2013-qtr4.pdf [Google Scholar]
- 6.Vasterling JJ, Verfaellie M, Sullivan KD: Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: Perspectives from cognitive neuroscience. Clin. Psychol. Rev 2009; 29:674–684 [DOI] [PubMed] [Google Scholar]
- 7.Vasterling JJ, Duke LM, Brailey K, et al. : Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology 2002; 16:5–14 [DOI] [PubMed] [Google Scholar]
- 8.Gilbertson MW, Gurvits TV, Lasko NB, et al. : Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J. Trauma. Stress 2001; 14:413–432 [DOI] [PubMed] [Google Scholar]
- 9.Qureshi SU, Long ME, Bradshaw MR, et al. : Does PTSD impair cognition beyond the effect of trauma? J. Neuropsychiatry Clin. Neurosci 2011; 23:16–28 [DOI] [PubMed] [Google Scholar]
- 10.Gurvits TV, Gilbertson MW, Lasko NB, et al. : Neurologic soft signs in chronic posttraumatic stress disorder. Arch. Gen. Psychiatry 2000; 57:181–186 [DOI] [PubMed] [Google Scholar]
- 11.Sullivan K, Krengel M, Bradford W, et al. : Neuropsychological functioning in military pesticide applicators from the Gulf War: Effects on information processing speed, attention and visual memory. Neurotoxicol. Teratol 2018; 65:1–13 [DOI] [PubMed] [Google Scholar]
- 12.Samuelson KW, Neylan TC, Metzler TJ, et al. : Neuropsychological functioning in posttraumatic stress disorder and alcohol abuse. Neuropsychology 2006; 20:716–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowell TA, Kieffer KM, Siders CA, et al. : Neuropsychological findings in combat-related posttraumatic stress disorder. Clin. Neuropsychol 2002; 16:310–321 [DOI] [PubMed] [Google Scholar]
- 14.Clark AL, Amick MM, Fortier C, et al. : Poor performance validity predicts clinical characteristics and cognitive test performance of OEF/OIF/OND Veterans in a research setting. Clin. Neuropsychol 2014; 28:802–825 [DOI] [PubMed] [Google Scholar]
- 15.Wisdom NM, Pastorek NJ, Miller BI, et al. : PTSD and cognitive functioning: Importance of including performance validity testing. Clin. Neuropsychol 2014; 28:128–145 [DOI] [PubMed] [Google Scholar]
- 16.Cooper DB, Vanderploeg RD, Armistead-Jehle P, et al. : Factors associated with neurocognitive performance in OIF/OEF servicemembers with postconcussive complaints in postdeployment clinical settings. J. Rehabil. Res. Dev 2014; 51:1023–1034 [DOI] [PubMed] [Google Scholar]
- 17.West LK, Curtis KL, Greve KW, et al. : Memory in traumatic brain injury: The effects of injury severity and effort on the Wechsler Memory Scale-III. J. Neuropsychol 2011; 5:114–125 [DOI] [PubMed] [Google Scholar]
- 18.Frencham KAR, Fox AM, Maybery MT: Neuropsychological studies of mild traumatic brain injury: A meta-analytic review of research since 1995. J. Clin. Exp. Neuropsychol 2005; 27:334–351 [DOI] [PubMed] [Google Scholar]
- 19.Carroll LJ, Cassidy JD, Peloso PM, et al. : Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med 2004; 84–105 [DOI] [PubMed] [Google Scholar]
- 20.McInnes K, Friesen CL, MacKenzie DE, et al. : Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PLoS One 2017; 12:e0174847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson LA, Yoash-Gantz RE, Pickett TC, et al. : Relationship between processing speed and executive functioning performance among OEF/OIF veterans: Implications for postdeployment rehabilitation. J. Head Trauma Rehabil 2009; 24:32–40 [DOI] [PubMed] [Google Scholar]
- 22.Foa EB, Hembree E, Rothbaum BO: Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences, Therapist Guide, New York, NY, Oxford University Press, 2007 [Google Scholar]
- 23.Resick PA, Monson CM, Chard KM: Cognitive Processing Therapy for PTSD: A Comprehensive Manual, New York, NY, Guilford Publications, 2016 [Google Scholar]
- 24.Foa EB, Keane TM, Friedman MJ, et al. (eds): Effective Treatments for PTSD: Practice Guidelines from the International Society for Traumatic Stress Studies (2nd Ed.), New York, NY, Guilford Press, 2009 [Google Scholar]
- 25.Wild J, Gur RC: Verbal memory and treatment response in post-traumatic stress disorder. Br. J. Psychiatry J. Ment. Sci 2008; 193:254–255 [DOI] [PubMed] [Google Scholar]
- 26.Haaland KY, Sadek JR, Keller JE, et al. : Neurocognitive Correlates of Successful Treatment of PTSD in Female Veterans. J. Int. Neuropsychol. Soc 2016; 22:643–651 [DOI] [PubMed] [Google Scholar]
- 27.Nijdam MJ, de Vries G, Gersons BP, et al. : Response to psychotherapy for posttraumatic stress disorder: The role of pretreatment verbal memory performance. J. Clin. Psychiatry 2015; 76:e1023–8 [DOI] [PubMed] [Google Scholar]
- 28.Scott JC, Harb G, Brownlow JA, et al. : Verbal memory functioning moderates psychotherapy treatment response for PTSD-Related nightmares. Behav. Res. Ther 2017; 91:24–32 [DOI] [PubMed] [Google Scholar]
- 29.Rizvi SL, Vogt DS, Resick PA: Cognitive and affective predictors of treatment outcome in Cognitive Processing Therapy and Prolonged Exposure for posttraumatic stress disorder. Behav. Res. Ther 2009; 47:737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueser KT, McGurk SR, Xie H, et al. : Neuropsychological predictors of response to cognitive behavioral therapy for posttraumatic stress disorder in persons with severe mental illness. Psychiatry Res. 2018; 259:110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chard KM, Schumm JA, McIlvain SM, et al. : Exploring the efficacy of a residential treatment program incorporating cognitive processing therapy-cognitive for veterans with PTSD and traumatic brain injury. J. Trauma. Stress 2011; 24:347–351 [DOI] [PubMed] [Google Scholar]
- 32.Walter KH, Kiefer SL, Chard KM: Relationship between posttraumatic stress disorder and postconcussive symptom improvement after completion of a posttraumatic stress disorder/traumatic brain injury residential treatment program. Rehabil. Psychol 2012; 57:13–17 [DOI] [PubMed] [Google Scholar]
- 33.Wolf GK, Strom TQ, Kehle SM, et al. : A Preliminary Examination of Prolonged Exposure Therapy With Iraq and Afghanistan Veterans With a Diagnosis of Posttraumatic Stress Disorder and Mild to Moderate Traumatic Brain Injury. J. Head Trauma Rehabil 2012; 27:26–32 [DOI] [PubMed] [Google Scholar]
- 34.Management of Concussion/mTBI Working Group: VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. J. Rehabil. Res. Dev 2009; 46:CP1–68 [PubMed] [Google Scholar]
- 35.Trivedi MH, Wisniewski SR, Morris DW, et al. : Concise Health Risk Tracking scale: A brief self-report and clinician rating of suicidal risk. J. Clin. Psychiatry 2011; 72:757–764 [DOI] [PubMed] [Google Scholar]
- 36.Weathers FW, Litz BT, Keane TM, et al. : The PTSD Checklist for DSM-5 (PCL-5), 2013. Available from: www.ptsd.va.gov
- 37.Bovin MJ, Marx BP, Weathers FW, et al. : Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychol. Assess 2016; 28:1379–1391 [DOI] [PubMed] [Google Scholar]
- 38.Weathers FW, Blake DD, Schnurr PP, et al. : The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) - Past Month [Measurement Instrument], 2013. Available from: http://www.ptsd.va.gov/ [Google Scholar]
- 39.Rey A: L’examen psychologique dans les cas d’encéphalopathie traumatique. (Les problems.). [The psychological examination in cases of traumatic encepholopathy. Problems.]. Arch. Psychol 1941; 28:215–285 [Google Scholar]
- 40.Schmidt M: Rey Auditory Verbal Learning Test: A Handbook, Los Angeles, CA, Western Psychological Services, 1996 [Google Scholar]
- 41.Wechsler D: Wechsler Adult Intelligence Scale–Fourth Edition (WAIS–IV), San Antonio, TX, The Psychological Corporation, 2008 [Google Scholar]
- 42.Delis DC, Kaplan E, Kramer JH: Delis-Kaplan Executive Function System: Examiner’s Manual, San Antonio, TX, The Psychological Corporation, 2001 [Google Scholar]
- 43.Strauss E, Sherman EMS, Spreen O: A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd Edition, New York, NY, Oxford University Press, 2006 [Google Scholar]
- 44.Hilsabeck RC, Gordon SN, Hietpas-Wilson T, et al. : Use of Trial 1 of the Test of Memory Malingering (TOMM) as a screening measure of effort: suggested discontinuation rules. Clin. Neuropsychol 2011; 25:1228–1238 [DOI] [PubMed] [Google Scholar]
- 45.Vanderploeg RD, Groer S, Belanger HG: Initial developmental process of a VA semistructured clinical interview for TBI identification. J. Rehabil. Res. Dev 2012; 49:545–556 [DOI] [PubMed] [Google Scholar]
- 46.Cohen J: Statistical Power Analysis for the Behavioral Sciences, New York, NY, Routledge, 1988 [Google Scholar]
- 47.Cohen J: A Power Primer. Psychol. Bull 1992; 112:155–159 [DOI] [PubMed] [Google Scholar]