Abstract
Approximately fifty percent of sunlight reaching the Earth’s surface is visible light (400–700 nm). Other sources of visible light include lasers, light-emitting diodes (LEDs), and flash lamps. Photons from visible light are absorbed by photoreceptive chromophores (e.g., melanin, heme, and opsins), altering skin function by activating and imparting energy to chromophores. Additionally, visible light can penetrate the full thickness of the skin and induce pigmentation and erythema. Clinically, lasers and light devices are used to treat skin conditions by utilizing specific wavelengths and treatment parameters. Red and blue light from LEDs and intense pulsed light (IPL) have been studied as anti-microbial and anti-inflammatory treatments for acne. Pulsed dye lasers are used to treat vascular lesions in adults and infants. Further research is necessary to determine the functional significance of visible light on skin health and wellness without confounding the influence of ultraviolet and infrared wavelengths.
Keywords: visible light, optical radiation, photobiomodulation, chromophores, lasers, photodermatitis, porphyria, phototherapy
1. Electromagnetic radiation
Optical radiation includes ultraviolet, visible, infrared radiation.
Most sunlight reaching the Earth’s surface is visible or infrared.
Electromagnetic radiation (EMR) includes gamma rays, X-rays, ultraviolet radiation (UVR), visible light (VL), infrared (IR), microwaves, and radio waves (Figure 1). UVR, VL, and IR are considered optical radiation (10 nm-1 mm) and defined as Light for clarity and convenience. The cutaneous effects of UVR and IR have been well studied; recently, advances in VL understanding on the skin have been made.1–10 VL is the narrow spectrum (400–700 nm) of EMR that the human eye can detect.3 VL accounts for ~50% of solar radiation reaching Earth’s surface and can be further divided by color and wavelength.3,11,12 UVR (10–400 nm) comprises ~5% of solar radiation reaching Earth’s surface.11,13,14 UVC and Extreme UV (EUV) are filtered by the atmosphere.11,13,14 IR (700 nm-1 mm) comprises the remaining 45% of solar radiation that reaches Earth’s surface.
Figure 1: Electromagnetic radiation spectrum.
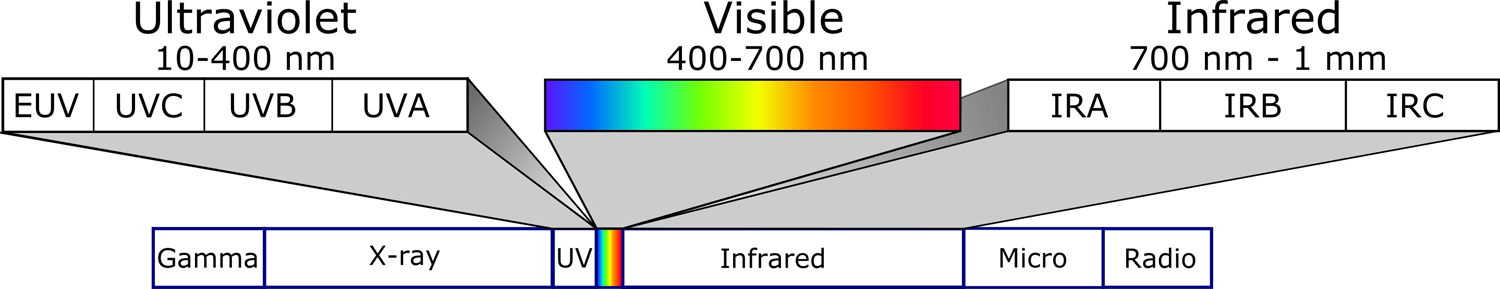
UVR, VL, and IR are optical radiation. VL can be divided by color: blue/violet (400–500 nm), green (500–565 nm), yellow (565–590 nm), orange (590–625 nm), or red (625–700 nm). Similarly, UVR is separated into separate spectra: UVA (320–400 nm), UVB (290–320 nm), UVC (200–290 nm), and extreme (EUV; 10–120 nm). IR can be subdivided into IRA (near-IR; 700–1440 nm), IRB (mid-IR; 1440–3000 nm), and IRC (far-IR; 3000 nm-1 mm) wavelengths. Spectral boundaries are not discrete, and there is an overlap in the biological effects between adjacent forms of EMR.
2. Parameters, devices, and safety
Natural and artificial sources emit VL.
Devices produce different forms of light based on operating principles.
Parameters.
Natural and artificial sources emit VL. Light exposure in a medical context can be described by specific terms and parameters (Table 1).15–20
Table 1:
Definition of light parameters.
| Terminology | Definition | Standard unit |
|---|---|---|
| Wavelength | Distance between two peaks of a wavefunction. | Meters |
| Irradiance (power density) | The power (energy/second) of light delivered to a unit of surface area. | Watts/meter2 |
| Fluence (dose) | The amount of energy delivered to a unit of surface area over a given time. Fluence = irradiance x time. | Joules/meter2 |
| Duration of exposure | The amount of time that the skin is exposed to light. | Seconds |
| Pulse width | The length of time that a pulsed device delivers light (i.e., “flashes” in a repetitive mode). | Seconds |
| Beamwidth | The diameter of the beam. The edge of the beam is defined by having an irradiance of at least 1/e2 of maximum irradiance. | Meters |
| Duty cycle | Percentage of time that the signal is active during one cycle. | Percentage of time |
| Luminance | The intensity of light at the source weighed by human perception of VL. | Lux |
| Photon flux | The number of photons delivered per unit of surface area at a given time. | Photons/meters2/seconds |
| Coherence | Light waves have an identical wavelength and constant phase. | N/A |
| Collimation | Light waves are parallel. | N/A |
Devices.
Multiple types of devices deliver VL as a therapeutic modality, including lasers, light-emitting diodes (LEDs), arc/flash lamps, halogen lamps, and fluorescent lights (Figure 2).3
Figure 2: Diagram of natural and artificial visible light sources.
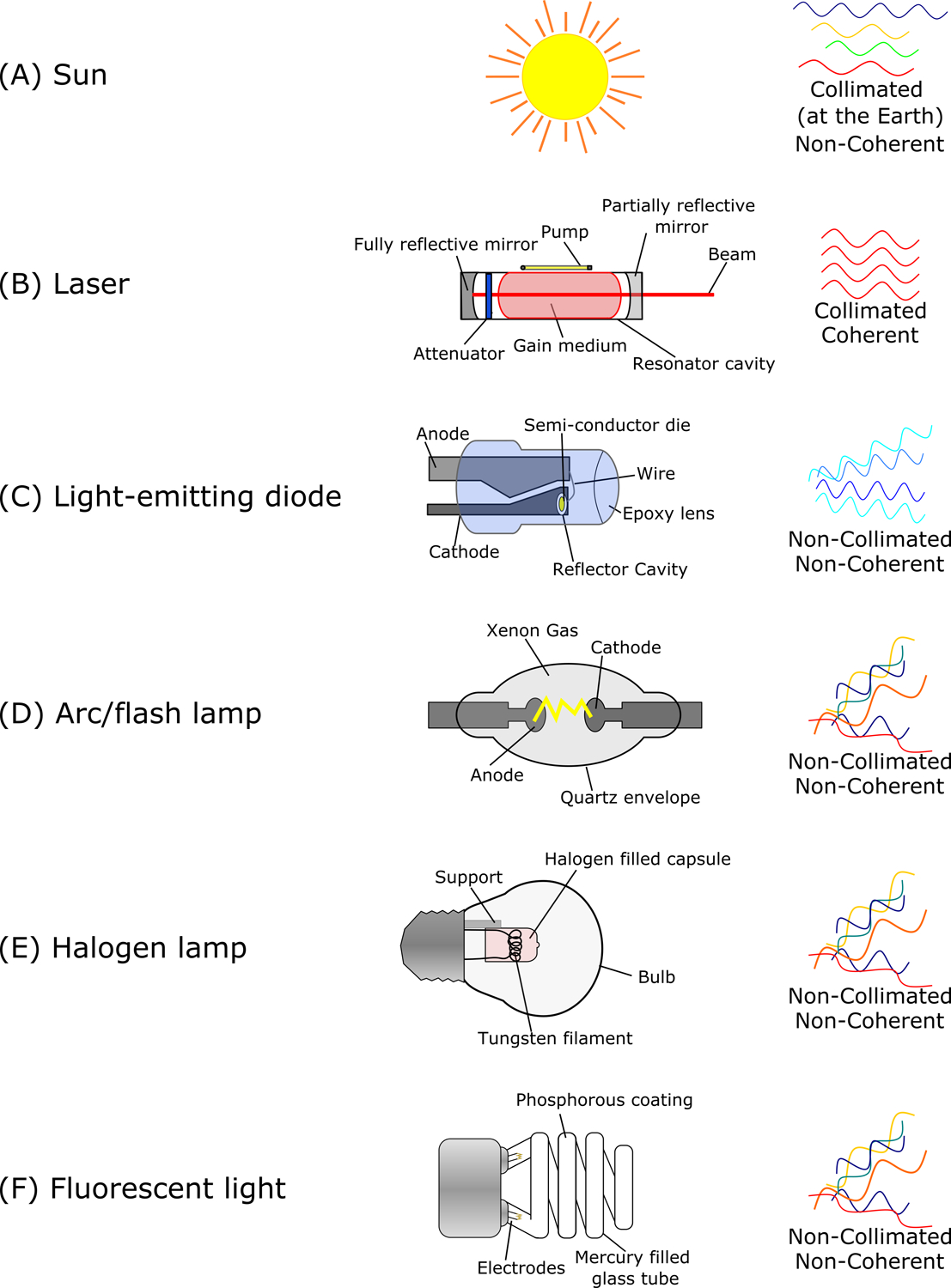
Devices are labeled with significant parts. Collimation (i.e., parallel), coherence (i.e., in-phase), and chromaticity are provided for each light source. (A) Sunlight is relatively collimated at the surface of the Earth. (B) Lasers pump energy through a gain medium (e.g., crystal or gas) to generate or amplify light between mirrors in the resonator cavity. The variable attenuator in the resonator cavity of Q-switched lasers allows for beam pulsing. Lasers are highly monochromatic, coherent, and collimated upon emission. (C) Light-emitting diodes (LEDs) pass an electrical current through a semiconductor. LEDs produce light in a narrow range to appear as a single color. Multiples LEDs can be placed in an array to generate white light or higher power densities. (D) Arc and flash lamps (e.g., intense pulsed light [IPL]) arc electricity through a mercury or xenon gas chamber and are optimized for continuous and pulsed operating conditions, respectively. Filters are applied to achieve specific wavelengths. (E) Halogen lamps heat a tungsten filament in a sealed chamber with small amounts of halogen gas. (F) Fluorescent lights excite electricity through mercury gas to produce UVR, which is converted to VL via the phosphorescent coating on the lamp’s inner surface.
Safety.
Solar irradiance at the Earth’s atmosphere is referred to as the solar constant (~136 mW/cm2).21,22 VL comprises ~53 mW/cm2 of the solar constant.21,22 The solar irradiance at the Earth’s surface (~100 mW/cm2) will vary depending on the latitude, time, and weather/atmospheric conditions.21,22 Commercially available VL sources used in dermatology practices, such as lasers and LEDs, may have power densities exceeding 100 mW/cm2.17,23 According to the United States (US) Occupational Safety and Health Administration (OSHA) regulatory standards, lasers in the VL domain can damage the retina. Therefore, wavelength-specific safety goggles are recommended for VL procedures. Concurrent US Federal Drug Administration (FDA) regulations for skin photoprotection are based on minimal erythema dose (MED) and critical wavelengths; these are discussed in Part 2.
3. Depth of penetration
Red light (625–700 nm) penetrates the skin deeper than blue light (400–500 nm)
Red light can penetrate the full skin thickness
Light propagation and penetration through the skin are dependent on reflection, scattering, and absorption.24 Approximately 4–7% of VL is reflected by the skin surface, regardless of incident wavelength, pigmentation, or structure.24,25 Keratins, collagen, melanin, and hemoglobin are the primary skin molecules responsible for the VL light penetration via scattering and absorption.24,25 Zinc, ion gated channels, NADH, bilirubin, and β-carotene also absorb and scatter VL. Most skin scattering occurs when photons are absorbed by filamentous proteins and re-emitted. Epidermal scattering may be greater than dermal scattering due to melanin in the epidermis.24–27 In the dermis, scattering occurs mainly in the direction of the incident beam as the diameters of collagen fibers are similar to VL wavelengths (i.e., Mie scattering), resulting in an enhanced depth of penetration.24–27 Chromophores, such as cytochrome c oxidase (COX), absorb specific light wavelengths, which excite electrons into a higher energy state. Subsequent activation of second messengers including, reactive oxygen species (ROS), ATP, nitric oxide, and cAMP, elicit modulation of inflammation, proliferation, and tissue repair.28 This process is called photobiomodulation or low-level light therapy. VL is not known to induce cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts directly, as DNA is a primary UVR chromophore.4–6,29,30 However, VL generates ROS, which can cause CPDs through a photochemical response.31–34 Water, comprising ~15–20% of the stratum cornea and ~70–75% of the remaining epidermis and dermis, has low VL absorption.3,35–39 However, water is the primary chromophore for IR (e.g., CO2) laser therapy.
Wavelength is directly proportional to the depth of penetration but inversely related to energy, according to Beer-Lambert and Planck’s laws, respectively.40,41 Consequently, blue light (BL) is higher energy than red light (RL) but has less penetration. Pathologic skin changes, including edema, erythema, pigmentation, and fibrosis, can alter light penetration by changing chromophore concentrations and tissue density.3 Along with demonstrating increased penetration depth with increasing wavelength, a custom Monte Carlo simulation of a multi-layered skin model demonstrated that increasing beamwidth for IPL enhances VL skin penetration.42 Monte Carlo computer simulations use skin and light parameters to model depth of penetration.43,44 Penetration depth increased markedly as beamwidth increased from 1–5 mm.42,45 Maximum penetration was achieved with a 10 mm beamwidth, and larger beamwidths did not further increase penetration depth.42,45
As depth of penetrations can vary based on beamwidth, wavelength, and power density, published measurements, and simulations of light penetration can vary substantially.25,27,46–49 RL penetrates 6–50 mm deep.25,27,47–50 BL likely has a maximum skin penetration of 0.5–1 mm.42,51 Epidermal thickness is ~30–100 μm, while epidermal plus dermal skin thickness is ~0.5–6 mm.48,52–54 VL when administered at the correct settings, can likely penetrate entirely through the epidermis. RL can likely reach most, if not all, dermal structures.
4. Skin chromophores
Melanin, heme, and opsins are chromophores receptive to VL.
Chromophores have specific absorption spectra.
The biological effects of VL are mediated through skin chromophores.
The primary VL skin chromophores are melanin, heme, and opsin (OPN) photoreceptors.3,11,28 Chromophores in the skin absorb discrete wavelengths at varying affinities (i.e., the absorption coefficient). It is possible to target chromophores using appropriate wavelengths and pulse widths selectively.55,56 According to the theory of selective photothermolysis, the pulse width must be shorter than the tissue’s thermal relaxation time, or non-specific thermal tissue damage occurs.56 Light energy parameters that do not cause photothermolysis or thermal damage but still alter biological function are considered photobiomodulatory.28,57 Photobiomodulation affects skin according to hormetic paradigms. Hormesis is the principle that an agent, substance, stimuli, or condition can induce a biphasic dose-response. VL’s lower fluences can have stimulatory effects, while higher fluences are inhibitory, cytotoxic, or destructive.58–61 The skin has an optical wavelength window (600–1300 nm) in which melanin, hemoglobin, and water absorption coefficients are the smallest.3,25 Within this optical window, RL and IRA light is absorbed by COX to trigger non-thermal photobiomodulatory effects.58–61
Melanin.
Human skin melanocytes produce two main types of melanin from dihydroxyphenylalanine (DOPA) precursors: yellow-red pheomelanin and black-brown eumelanin.62,63 BL, also called high energy visible light (HEVL), stimulates the expression and activity of tyrosinase, the rate-limiting enzyme in melanin production, via the OPN3 receptor present on keratinocytes and melanocytes.64,65 In melanocytes from skin of color (but not lighter skin types), BL activation of OPN3 leads to tyrosinase/tyrosinase-related protein complex formation.65 Therefore, BL may increase melanogenesis.64,65
Melanin has a poorly characterized structure and an absorption spectrum of 200–900 nm.3,62,63,66–69 Peak absorption varies depending on the melanin moiety, but light absorption is greatest in the UV spectrum and decreases into the VL and IR spectra.3,66–69 However, due to melanin’s broad absorption spectrum in the VL range, melanosomes may absorb light intended for other chromophores during phototherapy.70 Additionally, melanin has a thermal relaxation period of 70–250 ns.3,66–69 Patients with skin of color benefit from laser treatment with picosecond lasers, longer wavelengths, and lower power densities to limit thermal tissue damage.71–73 As a result, near-IR lasers (e.g., 1064 nm Nd:Yag) are considered safer than VL lasers for skin of color and pigmentation.74
In melanin-containing cells, ROS and reactive nitrogen species (RNS) generated by VL exposure can cause radiation-independent CPDs.34,67,75–77 Radiation-independent CPDs develop 3 hours after UVA exposure due to melanin interactions with ROS and RNS.34 In contrast, most CPDs are formed by direct absorption of UVB by DNA. However, potential VL-induced CPDs from ROS and RNS generation has been poorly studied.
Hemoglobin and heme derivatives.
Hemoglobin has a peak absorption in the blue (418 nm) and yellow/orange (542/577 nm) waveband.3,24 Erythrocyte concentration is the primary determinant of light absorption by hemoglobin.3,24 Porphyrins are tetrapyrrole macromolecules that serve as the intermediate for heme.78–80 Accumulation of porphyrins due to enzyme dysfunction causes cutaneous porphyrias. Porphyrins have an absorption spectra at 400–405 nm, known as the Soret band, and weaker absorption spectra of 500–750 nm.81,82 During photodynamic therapy (PDT), exogenous aminolevulinic acid (ALA), a non-photosensitive porphyrin precursor, is applied to the skin. ALA accumulates in cells and is converted to protoporphyrin IX (PP-IX).83 Subsequent irradiation with BL or RL from LEDs, fluorescent lights, or halogen lamps induces ROS generation and cell apoptosis.84–87 PDT, commonly used to treat actinic keratosis and skin aging, is considered a non-photothermolytic process.
COX is a heme and copper-containing protein in the mitochondrial electron transport chain that absorbs RL and IRA.28,57 RL and IRA absorption by COX is one of the primary drivers of photobiomodulation.28,57 RL and IRA activate the electron transport chain, which increases mitochondrial membrane potential and leads to intracellular accumulation of second messengers: ATP, ROS, cAMP, Ca2+, and nitric oxide.28,57 This can alter gene expression, protein activity, redox reactions, inflammation, and metabolism.28,30,31,57
Opsins.
OPN photoreceptors absorb specific wavelengths of light and are responsible for VL phototransduction (Table 2).88–90 Photoactivation of OPN1 and OPN2 in the retina’s cone and rod cells provides visual image signals.89,90 However, non-imaging forming OPNs have been identified in the skin.64,88,91–96 Skin OPNs can regulate circadian rhythms, epidermal barrier function, and melanogenesis.64,88,91–96 For example, BL activation of OPN3 may increase keratinocyte differentiation and tyrosinase activity in melanocytes.65,91
Table 2: Identified skin opsins.
Listed opsins, activation wavelengths, and identified expressed skin locations and cell types.
| Opsin | Activation Wavelength (nm) | Skin Expression |
|---|---|---|
| OPN1-SW | 420–425 | Epidermis, melanocytes, keratinocytes, and fibroblasts |
| OPN1-MW | 527–530 | Epidermis |
| OPN1-LW | 557–560 | Epidermis |
| OPN2 | 500–505 | Melanocytes, keratinocytes, fibroblasts, and hair follicle stem cells |
| OPN3 | 420–527 | Melanocytes, keratinocytes, fibroblasts, and hair follicle stem cells |
| OPN4 | 480 | Fibroblasts |
| OPN5 | 380 | Melanocytes, keratinocytes, and fibroblasts |
| Peropsin | 380–400 | Keratinocytes |
Abbreviations: OPN – opsin, nm – nanometer.
Skin reactions and pathologies
VL induces greater hyperpigmentation in skin of color than light skin.
VL triggers photodermatoses, including cutaneous porphyrias.
Hyperpigmentation.
Recent studies have examined the effects of pure VL and UVA1 on pigmentation and erythema, as the skin may not be protected from these spectra by standard sunscreen. Broad-spectrum and discrete VL wavelengths induce pigmentation with synergistic effects when combined with UVA exposure.97–104 Pigmentation, lasting at least 2 weeks, occurred following broad-spectrum VL irradiation (>97.5% VL, <0.2% UVA, 0.8–2.4% IRA) at 40–80 J/cm2 in skin types III-VI.98,105,106 40–80 J/cm2 is the equivalent of ~15–30 minutes of VL solar irradiation.21 BL at a fluence of 58±20 J/cm2 induced pigmentation in skin types III and IV, persisting at least 21 days.107 In another study, RL at 320 J/cm2 from LEDs induced hyperpigmentation in skin of color subjects, resolving 1 week to 3 months post-treatment.77 Pigmentation from VL is more intense and sustained than UVA1-induced pigmentation in dark-skinned subjects.77,97,98 VL causes a redistribution of melanin from the basal layer to the upper epidermis and activation of OPN3 in dark-skinned subjects, leading to Ca2+ and MITF dependent melanogenesis.64,65,98,102 Yellow light (YL) at a fluence of 5–20 J/cm2 inhibits melanogenesis in vitro.108
Photodermatoses.
Photodermatoses are diseases caused or exacerbated by light. Photodermatoses are classified as immunologically-mediated, photoaggrevated, or secondary to exogenous/endogenous agents and DNA-repair deficiencies.109–113 UVR is known to be the action spectra of most photodermatoses. However, VL is the action spectrum for solar urticaria and cutaneous porphyrias, and less commonly, in polymorphous (polymorphic) light eruption (PLE) and chronic actinic dermatitis.
Solar urticaria:
This is an uncommon mast cell-mediated photodermatosis.97,114,115 Symptoms of solar urticaria include erythema, pruritus, and whealing. Phototesting studies indicate that 14–90% of patients with solar urticaria reacted to VL alone or in combination with UVR.115–118 Fluorescent PDT light sources can induce urticaria and whealing.119,120 Phototesting for solar urticaria must be evaluated minutes post-exposure as urticaria resolves within a few hours. Management of solar urticaria includes photoprotection with broad-spectrum sunscreen, anti-histamine, UVA/UVA1 hardening, methotrexate, cyclosporine, and omalizumab.121–123 For those with action spectrum in the VL range, tinted sunscreen is necessary.
Porphyrias:
These are mostly autosomal dominant diseases caused by accumulations of porphyrins, known phototoxic agents. They are classified as cutaneous (i.e., skin blistering) or neurovisceral (i.e., abdominal pain, vomiting, and tachycardia).78,113 10 mW/cm2 of BL (405 nm) for 1000 seconds activates PP-IX.124,125 Management of porphyria cutanea tarda, the most common cutaneous porphyria, includes sun protection, avoidance of exacerbating factors (i.e., alcohol), phlebotomy, and low dose chloroquine.86,87 The FDA approved afamelanotide (Scenesse®), an α-melanocyte stimulating hormone (α-MSH) analog, in October 2019 as a new treatment for the second most common porphyria in the US, erythropoietic protoporphyria (EPP).126 Afamelanotide increases melanin production and serves as an antioxidant, hence down-regulates porphyrin-induced phototoxicity.
Polymorphous (polymorphic) light eruption (PLE):
This is the most common immunologically-mediated photodermatosis with multiple clinical presentations, including vesicular, papular, hemorrhagic, and eczematous; in dark-skinned individuals, it presents as a distinctive pin-head papular eruption.127 PLE is the most common photodermatosis worldwide and can affect all races and skin types.127 The majority of reactions occur due to UVA alone, but UVB and, uncommonly, VL may also responsible.127,128 In one study, 100 J/cm2 of VL (~40 minutes of VL sun exposure) induced PLE compared to 20–35 J/cm2 for UVA (~40–75 minutes of UVR sun exposure).21,129 Management of PLE include photoprotection, anti-histamines, and induction of tolerance (i.e., UVB hardening).115–118,127
Chronic actinic dermatitis:
This presents with eczematous and frequently lichenified skin lesions. UVB, UVA, and rarely VL have been identified as the action spectra, but the most severe reactions are associated with UVB exposure.130–132
Secondary photodermatoses:
141 to 313 J/cm2 of VL may induce skin reactions in some secondary photodermatoses, including systemic lupus erythematosus.133
Erythema and inflammation.
Exposure of subjects with skin phototypes I-III to VL with ≤2% UVA1 resulted in immediate erythema.134 In dark-skinned individuals (phototypes IV-VI), 480 J/cm2 of VL with 0.5% UVA, but not pure VL, induced immediate erythema assessed by investigator’s clinical inspection.106 In another study, VL and UVA1 light elicited immediate erythema lasting less than 24 hours in skin types II-IV patients.103 In two randomized controlled trials on the safety of LED RL (633 nm) on human skin, 320 J/cm2 and above induced prolonged erythema in some light-skinned patients, while RL at 480 J/cm2 and above caused blistering in some patients of various skin types.77 Erythema from visible light may be due to a combination of inefficient photoenergy transfer in the skin and inflammation; for some light sources, the device’s thermal effects might also contribute.77,102,135,136 Many laser and light protocols employ cooling devices to prevent thermal damage and minimize adverse events such as pain, erythema, blistering, scarring, and edema.77,137
5. Photomedical phototherapy
For photobiomodulation, light is delivered at parameters that do not induce thermal destructive processes
Treatment efficacy of VL is wavelength specific for each condition.
Lasers, IPL, LEDs, and fluorescent bulbs are commonly used in VL treatment protocols.
Acne.
In clinical trials, BL and RL from devices at 400–445 nm and 625–700 nm, respectively, treated mild to moderate acne by decreasing Cutibacterium acnes colonization, pore size, and inflammation (Table 3).17,138–140 Inflammatory lesions may respond better than non-inflammatory lesions.17,138–141 C. acnes directly produce porphyrins, which can absorb VL to produce ROS.17,140,142 Long-incubation PDT (>3-hours) with high-intensity RL activation may induce long-term acne remission but is associated with pain, inflammation, and photosensitivity.143
Table 3: Therapeutic applications of VL.
VL therapy efficacy depends on patient selection, disease severity, modality, and device settings.
| Condition | Spectrum | Wavelength (nm) | Device/Modality |
|---|---|---|---|
| Acne | Broad | 400–700+ | IPL |
| Blue | 405–420 | LED | |
| Blue | 405–420 | Fluorescent bulb | |
| Yellow | 585 or 595 | PDL | |
| Red | 630–670 | LED | |
| Hair Regrowth | Red | 633 or 660 | Laser Diode |
| Red | 630–660 | LED | |
| Neonatal Jaundice | Blue | 450–470 | LED |
| Blue | 400–550 | Halogen | |
| Blue | 400–550 | Fluorescent | |
| Tattoo Removal | Green | 532 | Frequency-doubled Nd:Yag |
| Red | 694.3 | Ruby | |
| Psoriasis | Blue | 405–420 | LED |
| Yellow | 585 or 595 | PDL | |
| Red | 625–670 | LED | |
| Skin Rejuvenation | Yellow | 570–590 | LED |
| Red | 625–670 | LED | |
| Red | 633 or 660 | Laser Diode | |
| Vascular Lesions | Broad | 400–700+ | IPL |
| Green | 532 | Frequency-doubled Nd:Yag | |
| Yellow | 578 | Copper Bromide | |
| Yellow | 585 or 595 | PDL | |
| Wound Healing | Blue | 405–420 | LED |
| Green | 515 or 520 | Laser Diode | |
| Yellow | 570–590 | LED | |
| Red | 625–660 | LED | |
| Red | 633 or 660 | Laser Diode |
Abbreviations: IPL – Intense pulsed light; LED – light-emitting diode, PDL – pulsed dye laser, Nd:Yag – neodymium-doped yttrium aluminum garnet, nm – nanometer.
Psoriasis.
Narrow-band UVB is commonly used to treat psoriasis, but researchers have also explored using VL.17,144 Blue and red LEDs may reduce erythema and improve the local psoriatic severity index (PASI).17,145,146 VL phototherapy inhibits keratinocyte and endothelial proliferation by increasing cellular differentiation.147,148 LED phototherapy may be better suited for reducing psoriatic erythema rather than improving desquamation.17,145,146 However, psoriatic recurrence may occur following cessation of treatment.
Wound healing.
Significant clinical research has examined the use of lasers, IPL, and LEDs for acute and chronic wound healing. Treatment parameters and patient selection vary considerably among publications.17,149–151 RL reduces wound healing time, lesion size, and erythema in diabetic foot ulcers.17,149–153 Venous ulcers may not respond to photobiomodulation.154,155 VL improves wound healing by reducing the expression of proinflammatory proteins and cytokines, increasing OPN3 mediated keratinocyte differentiation, and preventing bacterial growth.91,156–158
Hair growth.
Multiple devices, including caps, combs, and handheld units, are currently available to stimulate hair growth using LEDs or laser diodes (i.e., lasers with a semiconductor gain media). RL increases hair density, growth, and tensile strength in androgenic alopecia by stimulating the anagen growth phase.159 Clinical studies commonly use RL and near-IR photobiomodulation with fluences of 0.1–150 J/cm2 for hair regrowth.55,150
Rejuvenation and photodamage.
RL, YL, and BL have been studied as anti-aging therapeutics.17,140 The proposed mechanism of light-mediated anti-aging is photobiomodulatory activation of Ca2+, nitric oxide, matrix metalloproteinase, and collagen-stimulating pathways.17,140 PDT may improve photodamaged facial skin texture, wrinkles, and mottled pigmentation.160,161
6. Photothermolytic phototherapy
Lasers and IPL target chromophores and induce local tissue destruction via photothermolysis
Vascular lesions.
Pulsed dye laser (PDL; 585–600 nm) targets oxyhemoglobin and is the treatment of choice for vascular lesions, including port-wine stains (PWS), spider angiomas, and hemangiomas.3,162–164 In plaque and nail psoriasis, PDL induces photothermolysis of capillaries.147,165–169 PDL has been shown to have better efficacy than UVB excimer laser in treating nail bed and matrix lesions.147,165–169 Frequency-doubled Nd:Yag (532 nm) are also used for vascular lesions but do not penetrate as deeply as PDL.162
Special care is recommended when using PDL in skin of color and infants. The high density of melanin in individuals with skin of color results in absorption of VL, which produces heat generation and blistering. Asian patients have been safely treated with PDL and IPL for vascular lesions.74,170 Infants have thinner and less pigmentated skin, and long-pulsed treatment settings are recommended.171–174
Tattoo removal.
Q-switched frequency-doubled Nd:YAG (532 nm) and ruby (694 nm) lasers are used to remove tattoos.3 Ruby lasers target black and blue pigments, while 532 nm Nd:YAG lasers target yellow, orange, and red pigments. Typically, black tattoo pigments are easier to remove than colored pigments.175 532 nm picosecond pulsed lasers have enhanced safety and efficacy for tattoo removal compared to nanosecond lasers.176,177 Common adverse events include immediate erythema, hyper-/hypopigmentation, and hypersensitivity.
7. Future directions and conclusion
VL has the potential to treat a variety of skin diseases and affect skin health
Additional research is needed to determine the optimal treatment parameters for VL phototherapy.
VL is currently used for the treatment of skin conditions, and use may expand to new applications.98,178 Skin of color is more susceptible to high fluences of VL and requires conservative treatment parameters. Further research is necessary to determine VL’s functional significance on skin health without confounding UVR and IR wavelengths. A better understanding of VL therapeutic effects would improve treatment protocols and patient care. With the availability of light sources emitting broad but pure VL, these types of studies can now be performed.98,178 Comparative clinical trials can elucidate the optimal efficacy of specific wavelengths, fluences, and power densities.
Supplementary Material
Funding Source:
Dr. Jagdeo is supported by a National Institute of General Medical Sciences of the National Institute of Health (NIH) award (K23GM1173090).
Conflict of Interest Disclosure(s):
Henry W. Lim is an investigator for Incyte, L’Oreal, Pfizer, and PCORI, has served as a consultant for Pierre Fabre, ISDIN, Ferndale, Beiersdorf, and La Roche-Posay, and has participated as a speaker in general educational session for La Roche-Posay, and Cantabria labs. Indermeet Kohli is an investigator (grant funding received by the institution) for Ferndale, Estee Lauder, L’Oreal, Unigen, Johnson and Johnson, Allergan, and Bayer and is a consultant (fee and equipment received by the institution) for Pfizer, Johnson and Johnson, and Bayer. Jared Jagdeo is a member of the GlobalMed Scientific advisory board and a consultant for UV Biotek. Iltefat Hamzavi is an investigator for Estee Lauder, Ferndale Laboratories, Galderma, Bayer, Loreal, Lenicura, and Unigen.
ABBREVIATIONS
- ALA
Aminolevulinic acid
- BL
Blue light
- CME
Continuing medical education
- CPD
Cyclobutane pyrimidine dimer
- COX
Cytochrome c oxidase
- DOPA
Dihydroxyphenylalanine
- EMR
Electromagnetic radiation
- EPP
Erythropoietic protoporphyria
- EUV
Extreme UV
- FDA
Food and Drug Administration
- HEVL
High energy visible light
- IPL
Intense pulsed light
- IR
Infrared
- LED
Light-emitting diode
- MED
Minimal erythemal dose
- MITF
Microphthalmia-associated transcription factor
- OPN
Opsin
- OSHA
Occupation Safety and Health Administration
- PASI
Psoriasis severity index
- PDL
Pulsed dye laser
- PDT
Photodynamic therapy
- PLE
Polymorphic light eruptions
- PP-IX
Protoporphyrin IX
- PWS
Port Wine Stains
- RL
Red light
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- US
United States
- UVR
Ultraviolet radiation
- UV
Ultraviolet
- VL
Visible light
- YL
Yellow light
Footnotes
Prior presentation(s): The contents of this manuscript are not under consideration for publication elsewhere, have not been copyrighted or published previously, and will not be copyrighted, submitted, or published elsewhere while your journal’s acceptance is under consideration.
References
- 1.Narla S, Kohli I, Hamzavi IH, Lim HW. Visible light in photodermatology. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology. 2020;19(1):99–104. [DOI] [PubMed] [Google Scholar]
- 2.Sowa P, Rutkowska-Talipska J, Rutkowski K, Kosztyła-Hojna B, Rutkowski R. Optical radiation in modern medicine. Postepy dermatologii i alergologii. 2013;30(4):246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang MF, Tuchin VV, Yaroslavsky AN. Principles of light-skin interactions. In: Light-Based Therapies for Skin of Color. Springer; 2009:1–44. [Google Scholar]
- 4.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195(3):298–308. [DOI] [PubMed] [Google Scholar]
- 5.Alhasaniah A, Sherratt MJ, O’Neill CA. The Impact of Ultraviolet Radiation on Barrier Function in Human Skin: Molecular Mechanisms and Topical Therapeutics. Current medicinal chemistry. 2018;25(40):5503–5511. [DOI] [PubMed] [Google Scholar]
- 6.Christensen L, Suggs A, Baron E. Ultraviolet Photobiology in Dermatology. Advances in experimental medicine and biology. 2017;996:89–104. [DOI] [PubMed] [Google Scholar]
- 7.Barolet D, Christiaens F, Hamblin MR. Infrared and skin: Friend or foe. Journal of photochemistry and photobiology B, Biology. 2016;155:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muthusamy V, Piva TJ. The UV response of the skin: a review of the MAPK, NFκB and TNFα signal transduction pathways. Archives of dermatological research. 2010;302(1):5. [DOI] [PubMed] [Google Scholar]
- 9.Morita A Current developments in phototherapy for psoriasis. The Journal of dermatology. 2018;45(3):287–292. [DOI] [PubMed] [Google Scholar]
- 10.Cho S, Shin MH, Kim YK, et al. Effects of infrared radiation and heat on human skin aging in vivo. The journal of investigative dermatology Symposium proceedings. 2009;14(1):15–19. [DOI] [PubMed] [Google Scholar]
- 11.Diffey BL. What is light? Photodermatology, photoimmunology & photomedicine. 2002;18(2):68–74. [DOI] [PubMed] [Google Scholar]
- 12.Frederick J, Snell H, Haywood E. Solar ultraviolet radiation at the earth’s surface. Photochemistry and photobiology. 1989;50(4):443–450. [Google Scholar]
- 13.Diffey BL. Sources and measurement of ultraviolet radiation. Methods. 2002;28(1):4–13. [DOI] [PubMed] [Google Scholar]
- 14.Szenicer A, Fouhey DF, Munoz-Jaramillo A, et al. A deep learning virtual instrument for monitoring extreme UV solar spectral irradiance. Science advances. 2019;5(10):eaaw6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav RK. Definitions in laser technology. J Cutan Aesthet Surg. 2009;2(1):45–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farkas JP, Hoopman JE, Kenkel JM. Five parameters you must understand to master control of your laser/light-based devices. Aesthetic surgery journal. 2013;33(7):1059–1064. [DOI] [PubMed] [Google Scholar]
- 17.Jagdeo J, Austin E, Mamalis A, Wong C, Ho D, Siegel DM. Light‐emitting diodes in dermatology: A systematic review of randomized controlled trials. Lasers in surgery and medicine. 2018;50(6):613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuda E, Ogasawara H, Yoshida Y, Hayano J. Suppression of vagal cardiac modulation by blue light in healthy subjects. J Physiol Anthropol. 2016;35(1):24–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuesta M, Boudreau P, Cermakian N, Boivin DB. Skin Temperature Rhythms in Humans Respond to Changes in the Timing of Sleep and Light. J Biol Rhythms. 2017;32(3):257–273. [DOI] [PubMed] [Google Scholar]
- 20.Lok R, van Koningsveld MJ, Gordijn MCM, Beersma DGM, Hut RA. Daytime melatonin and light independently affect human alertness and body temperature. J Pineal Res. 2019;67(1):e12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gueymard CA. The sun’s total and spectral irradiance for solar energy applications and solar radiation models. Solar energy. 2004;76(4):423–453. [Google Scholar]
- 22.Kopp G, Lean JL. A new, lower value of total solar irradiance: Evidence and climate significance. Geophysical Research Letters. 2011;38(1). [Google Scholar]
- 23.Ilic S, Leichliter S, Streeter J, Oron A, DeTaboada L, Oron U. Effects of power densities, continuous and pulse frequencies, and number of sessions of low-level laser therapy on intact rat brain. Photomedicine and laser surgery. 2006;24(4):458–466. [DOI] [PubMed] [Google Scholar]
- 24.Lister T, Wright PA, Chappell PH. Optical properties of human skin. Journal of biomedical optics. 2012;17(9):090901. [DOI] [PubMed] [Google Scholar]
- 25.Anderson RR, Parrish JA. The optics of human skin. Journal of investigative dermatology. 1981;77(1):13–19. [DOI] [PubMed] [Google Scholar]
- 26.Jacques SL. Optical properties of biological tissues: a review. Physics in Medicine & Biology. 2013;58(11):R37. [DOI] [PubMed] [Google Scholar]
- 27.Wang EB, Kaur R, Fierro M, Austin E, Jones LR, Jagdeo J. Safety and penetration of light into the brain. In: Photobiomodulation in the Brain. Elsevier; 2019:49–66. [Google Scholar]
- 28.de Freitas LF, Hamblin MR. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE journal of selected topics in quantum electronics : a publication of the IEEE Lasers and Electro-optics Society. 2016;22(3):7000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffmann-Dörr S, Greinert R, Volkmer B, Epe B. Visible light (> 395 nm) causes micronuclei formation in mammalian cells without generation of cyclobutane pyrimidine dimers. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2005;572(1–2):142–149. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence KP, Douki T, Sarkany RPE, Acker S, Herzog B, Young AR. The UV/Visible Radiation Boundary Region (385–405 nm) Damages Skin Cells and Induces “dark” Cyclobutane Pyrimidine Dimers in Human Skin in vivo. Scientific reports. 2018;8(1):12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liebel F, Kaur S, Ruvolo E, Kollias N, Southall MD. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. The Journal of investigative dermatology. 2012;132(7):1901–1907. [DOI] [PubMed] [Google Scholar]
- 32.Austin E, Huang A, Adar T, Wang E, Jagdeo J. Electronic device generated light increases reactive oxygen species in human fibroblasts. Lasers in surgery and medicine. 2018:22794. [DOI] [PubMed] [Google Scholar]
- 33.Mamalis A, Garcha M, Jagdeo J. Light emitting diode-generated blue light modulates fibrosis characteristics: fibroblast proliferation, migration speed, and reactive oxygen species generation. Lasers in surgery and medicine. 2015;47(2):210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Premi S, Wallisch S, Mano CM, et al. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science (New York, NY). 2015;347(6224):842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lunkenheimer P, Emmert S, Gulich R, et al. Electromagnetic-radiation absorption by water. Physical Review E. 2017;96(6):062607. [DOI] [PubMed] [Google Scholar]
- 36.Gordon IE, Rothman LS, Gamache RR, et al. Current updates of the water-vapor line list in HITRAN: A new “Diet” for air-broadened half-widths. Journal of Quantitative Spectroscopy and Radiative Transfer. 2007;108(3):389–402. [Google Scholar]
- 37.Omi T, Numano K. The Role of the CO2 Laser and Fractional CO2 Laser in Dermatology. Laser Ther. 2014;23(1):49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagawa N, Matsumoto M, Sakai S. In vivo measurement of the water content in the dermis by confocal Raman spectroscopy. Skin Research and Technology. 2010;16(2):137–141. [DOI] [PubMed] [Google Scholar]
- 39.Boer M, Duchnik E, Maleszka R, Marchlewicz M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy dermatologii i alergologii. 2016;33(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kocsis L, Herman P, Eke A. The modified Beer–Lambert law revisited. Physics in Medicine & Biology. 2006;51(5):N91. [DOI] [PubMed] [Google Scholar]
- 41.Planck M On the law of the energy distribution in the normal spectrum. Ann Phys. 1901;4(553):1–11. [Google Scholar]
- 42.Ash C, Dubec M, Donne K, Bashford T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers in medical science. 2017;32(8):1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li T, Xue C, Wang P, Li Y, Wu L. Photon penetration depth in human brain for light stimulation and treatment: A realistic Monte Carlo simulation study. Journal of Innovative Optical Health Sciences. 2017;10(05):1743002. [Google Scholar]
- 44.Jarrah I, Radaideh MI, Kozlowski T. Determination and validation of photon energy absorption buildup factor in human tissues using Monte Carlo simulation. Radiation Physics and Chemistry. 2019;160:15–25. [Google Scholar]
- 45.Thaysen‐Petersen D, Bjerring P, Dierickx C, Nash J, Town G, Haedersdal M. A systematic review of light‐based home‐use devices for hair removal and considerations on human safety. Journal of the European Academy of Dermatology and Venereology. 2012;26(5):545–553. [DOI] [PubMed] [Google Scholar]
- 46.Lanzafame R Light Dosing and Tissue Penetration: It Is Complicated. In: Mary Ann Liebert, Inc., publishers; 140 Huguenot Street, 3rd Floor New: …; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu D, van Zeyl M, Valter K, Potas JR. Sex, but not skin tone affects penetration of red-light (660 nm) through sites susceptible to sports injury in lean live and cadaveric tissues. Journal of biophotonics. 2019;12(7):e201900010. [DOI] [PubMed] [Google Scholar]
- 48.Salehpour F, Cassano P, Rouhi N, et al. Penetration Profiles of Visible and Near-Infrared Lasers and Light-Emitting Diode Light Through the Head Tissues in Animal and Human Species: A Review of Literature. Photobiomodul Photomed Laser Surg. 2019;37(10):581–595. [DOI] [PubMed] [Google Scholar]
- 49.Clement M, Daniel G, Trelles M. Optimising the design of a broad‐band light source for the treatment of skin. Journal of Cosmetic and Laser Therapy. 2005;7(3–4):177–189. [DOI] [PubMed] [Google Scholar]
- 50.Niu T, Tian Y, Cai Q, Ren Q, Wei L. Red Light Combined with Blue Light Irradiation Regulates Proliferation and Apoptosis in Skin Keratinocytes in Combination with Low Concentrations of Curcumin. PloS one. 2015;10(9):e0138754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ankri R, Lubart R, Taitelbaum H. Estimation of the optimal wavelengths for laser-induced wound healing. Lasers in surgery and medicine. 2010;42(8):760–764. [DOI] [PubMed] [Google Scholar]
- 52.Lee Y, Hwang K. Skin thickness of Korean adults. Surgical and radiologic anatomy. 2002;24(3–4):183–189. [DOI] [PubMed] [Google Scholar]
- 53.Oltulu P, Ince B, Kökbudak N, Kılıç F. Measurement of epidermis, dermis, and total skin thicknesses from six different body regions with a new ethical histometric technique. Türk Plastik, Rekonstrüktif ve Estetik Cerrahi Dergisi (Turk J Plast Surg). 2018;26(2):56–61. [Google Scholar]
- 54.Chopra K, Calva D, Sosin M, et al. A comprehensive examination of topographic thickness of skin in the human face. Aesthet Surg J. 2015;35(8):1007–1013. [DOI] [PubMed] [Google Scholar]
- 55.Young AR. Chromophores in human skin. Phys Med Biol. 1997;42(5):789–802. [DOI] [PubMed] [Google Scholar]
- 56.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science (New York, NY). 1983;220(4596):524–527. [DOI] [PubMed] [Google Scholar]
- 57.Hamblin MR. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochemistry and photobiology. 2018;94(2):199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuffler DP. Photobiomodulation in promoting wound healing: a review. Regenerative medicine. 2016;11(1):107–122. [DOI] [PubMed] [Google Scholar]
- 59.Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy - an update. Dose Response. 2011;9(4):602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calabrese EJ. Hormesis: Path and Progression to Significance. International journal of molecular sciences. 2018;19(10):2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lev-Tov H, Mamalis A, Brody N, Siegel D, Jagdeo J. Inhibition of fibroblast proliferation in vitro using red light-emitting diodes. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al]. 2013;39(8):1167–1170. [DOI] [PubMed] [Google Scholar]
- 62.Micillo R, Panzella L, Koike K, Monfrecola G, Napolitano A, d’Ischia M. “Fifty Shades” of Black and Red or How Carboxyl Groups Fine Tune Eumelanin and Pheomelanin Properties. International journal of molecular sciences. 2016;17(5):746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hunt G, Kyne S, Ito S, Wakamatsu K, Todd C, Thody AJ. Eumelanin and phaeomelanin contents of human epidermis and cultured melanocytes. Pigment cell research. 1995;8(4):202–208. [DOI] [PubMed] [Google Scholar]
- 64.Setty SR. Opsin3-A Link to Visible Light-Induced Skin Pigmentation. The Journal of investigative dermatology. 2018;138(1):13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Regazzetti C, Sormani L, Debayle D, et al. Melanocytes Sense Blue Light and Regulate Pigmentation through Opsin-3. The Journal of investigative dermatology. 2018;138(1):171–178. [DOI] [PubMed] [Google Scholar]
- 66.Zonios G, Dimou A, Bassukas I, Galaris D, Tsolakidis A, Kaxiras E. Melanin absorption spectroscopy: new method for noninvasive skin investigation and melanoma detection. Journal of biomedical optics. 2008;13(1):014017. [DOI] [PubMed] [Google Scholar]
- 67.Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochemistry and photobiology. 2008;84(3):539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ou-Yang H, Stamatas G, Kollias N. Spectral responses of melanin to ultraviolet A irradiation. Journal of investigative dermatology. 2004;122(2):492–496. [DOI] [PubMed] [Google Scholar]
- 69.Xiao M, Chen W, Li W, et al. Elucidation of the hierarchical structure of natural eumelanins. Journal of The Royal Society Interface. 2018;15(140):20180045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson BA. Lasers in ethnic skin: a review. Journal of the American Academy of Dermatology. 2003;48(6):S134–S138. [DOI] [PubMed] [Google Scholar]
- 71.Shah S, Alster TS. Laser treatment of dark skin: an updated review. American journal of clinical dermatology. 2010;11(6):389–397. [DOI] [PubMed] [Google Scholar]
- 72.Randhawa M, Seo I, Liebel F, Southall MD, Kollias N, Ruvolo E. Visible Light Induces Melanogenesis in Human Skin through a Photoadaptive Response. PloS one. 2015;10(6):e0130949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhatt N, Alster TS. Laser surgery in dark skin. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al]. 2008;34(2):184–195. [DOI] [PubMed] [Google Scholar]
- 74.Woolery-Lloyd H, Ferguson N. Lasers in Skin of Color. In: Lasers in Dermatology and Medicine. Springer; 2018:437–448. [Google Scholar]
- 75.Cope FW, Sever RJ, Polis BD. Reversible free radical generation in the melanin granules of the eye by visible light. Archives of Biochemistry and Biophysics. 1963;100(2):171–177. [PubMed] [Google Scholar]
- 76.Chiarelli-Neto O, Ferreira AS, Martins WK, et al. Melanin photosensitization and the effect of visible light on epithelial cells. PloS one. 2014;9(11):e113266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jagdeo J, Nguyen JK, Ho D, et al. Safety of light emitting diode-red light on human skin: Two randomized controlled trials. Journal of biophotonics. 2020;13(3):e201960014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schulenburg-Brand D, Katugampola R, Anstey AV, Badminton MN. The cutaneous porphyrias. Dermatologic clinics. 2014;32(3):369–384, ix. [DOI] [PubMed] [Google Scholar]
- 79.Stein PE, Badminton MN, Rees DC. Update review of the acute porphyrias. British journal of haematology. 2017;176(4):527–538. [DOI] [PubMed] [Google Scholar]
- 80.Uttamlal M, Holmes-Smith AS. The excitation wavelength dependent fluorescence of porphyrins. Chemical Physics Letters. 2008;454(4–6):223–228. [Google Scholar]
- 81.Makarska-Bialokoz M Comparative study of binding interactions between porphyrin systems and aromatic compounds of biological importance by multiple spectroscopic techniques: A review. Spectrochimica acta Part A, Molecular and biomolecular spectroscopy. 2018;200:263–274. [DOI] [PubMed] [Google Scholar]
- 82.Giovannetti R The use of spectrophotometry UV-Vis for the study of porphyrins. Macro to nano spectroscopy. 2012;1:87–108. [Google Scholar]
- 83.Nielsen KP, Juzeniene A, Juzenas P, Stamnes K, Stamnes JJ, Moan J. Choice of optimal wavelength for PDT: the significance of oxygen depletion. Photochemistry and photobiology. 2005;81(5):1190–1194. [DOI] [PubMed] [Google Scholar]
- 84.LaRochelle EP, Marra K, LeBlanc RE, Chapman MS, Maytin EV, Pogue BW. Modeling PpIX effective light fluence at depths into the skin for PDT dose comparison. Photodiagnosis and photodynamic therapy. 2019;25:425–435. [DOI] [PubMed] [Google Scholar]
- 85.Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Scientific reports. 2018;8(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koo E, Austin E, Mamalis A, Jagdeo J. Efficacy of ultra short sub‐30 minute incubation of 5‐aminolevulinic acid photodynamic therapy in vitro. Lasers in surgery and medicine. 2017;49(6):592–598. [DOI] [PubMed] [Google Scholar]
- 87.Koo E, Austin E, Mamalis A, Jagdeo J. Thermal Ultra Short Photodynamic Therapy: Heating Fibroblasts During Sub–30-Minute Incubation of 5-Aminolevulinic Acid Increases Photodynamic Therapy–Induced Cell Death. Dermatologic Surgery. 2018;44(4):528–533. [DOI] [PubMed] [Google Scholar]
- 88.Suh S, Choi EH, Atanaskova Mesinkovska N. The expression of opsins in the human skin and its implications for photobiomodulation: A Systematic Review. Photodermatology, photoimmunology & photomedicine. 2020;36(5):329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Terakita A The opsins. Genome Biol. 2005;6(3):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olinski LE, Lin EM, Oancea E. Illuminating insights into opsin 3 function in the skin. Advances in Biological Regulation. 2020;75:100668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Castellano-Pellicena I, Uzunbajakava NE, Mignon C, Raafs B, Botchkarev VA, Thornton MJ. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing? Lasers in surgery and medicine. 2019;51(4):370–382. [DOI] [PubMed] [Google Scholar]
- 92.Olinski LE, Lin EM, Oancea E. Illuminating insights into opsin 3 function in the skin. Adv Biol Regul. 2020;75:100668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ozdeslik RN, Olinski LE, Trieu MM, Oprian DD, Oancea E. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(23):11508–11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsutsumi M, Ikeyama K, Denda S, et al. Expressions of rod and cone photoreceptor-like proteins in human epidermis. Experimental dermatology. 2009;18(6):567–570. [DOI] [PubMed] [Google Scholar]
- 95.Lamb TD, Collin SP, Pugh EN. Evolution of the vertebrate eye: opsins, photoreceptors, retina and eye cup. Nature Reviews Neuroscience. 2007;8(12):960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haltaufderhyde K, Ozdeslik RN, Wicks NL, Najera JA, Oancea E. Opsin expression in human epidermal skin. Photochemistry and photobiology. 2015;91(1):117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mahmoud BH, Hexsel CL, Hamzavi IH, Lim HW. Effects of visible light on the skin. Photochemistry and photobiology. 2008;84(2):450–462. [DOI] [PubMed] [Google Scholar]
- 98.Mahmoud BH, Ruvolo E, Hexsel CL, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. The Journal of investigative dermatology. 2010;130(8):2092–2097. [DOI] [PubMed] [Google Scholar]
- 99.Kollias N, Baqer A. An experimental study of the changes in pigmentation in human skin in vivo with visible and near infrared light. Photochemistry and photobiology. 1984;39(5):651–659. [DOI] [PubMed] [Google Scholar]
- 100.Rosen CF, Jacques SL, Stuart ME, Gange RW. Immediate pigment darkening: visual and reflectance spectrophotometric analysis of action spectrum. Photochemistry and photobiology. 1990;51(5):583–588. [DOI] [PubMed] [Google Scholar]
- 101.Ramasubramaniam R, Roy A, Sharma B, Nagalakshmi S. Are there mechanistic differences between ultraviolet and visible radiation induced skin pigmentation? Photochemical & Photobiological Sciences. 2011;10(12):1887–1893. [DOI] [PubMed] [Google Scholar]
- 102.Sklar LR, Almutawa F, Lim HW, Hamzavi I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology. 2013;12(1):54–64. [DOI] [PubMed] [Google Scholar]
- 103.Porges SB, Kaidbey KH, Grove GL. Quantification of visible light-induced melanogenesis in human skin. Photo-dermatology. 1988;5(5):197–200. [PubMed] [Google Scholar]
- 104.Cohen L, Brodsky MA, Zubair R, Kohli I, Hamzavi IH, Sadeghpour M. Cutaneous Interaction with Visible Light: What Do We Know. Journal of the American Academy of Dermatology. 2020;S0190–9622(20):30551–X. [DOI] [PubMed] [Google Scholar]
- 105.Soleymani T, Cohen DE, Folan LM, Okereke UR, Elbuluk N, Soter NA. Disparity in Cutaneous Pigmentary Response to LED vs Halogen Incandescent Visible Light: Results from a Single Center, Investigational Clinical Trial Determining a Minimal Pigmentary Visible Light Dose. Journal of drugs in dermatology : JDD. 2017;16(11):1105–1110. [PubMed] [Google Scholar]
- 106.Kohli I, Braunberger TL, Nahhas AF, et al. Long-wavelength Ultraviolet A1 and Visible Light Photoprotection: A Multimodality Assessment of Dose and Response. Photochemistry and photobiology. 2020;96(1):208–214. [DOI] [PubMed] [Google Scholar]
- 107.Duteil L, Cardot-Leccia N, Queille-Roussel C, et al. Differences in visible light-induced pigmentation according to wavelengths: a clinical and histological study in comparison with UVB exposure. Pigment cell & melanoma research. 2014;27(5):822–826. [DOI] [PubMed] [Google Scholar]
- 108.Chen L, Xu Z, Jiang M, Zhang C, Wang X, Xiang L. Light-emitting diode 585nm photomodulation inhibiting melanin synthesis and inducing autophagy in human melanocytes. Journal of dermatological science. 2018;89(1):11–18. [DOI] [PubMed] [Google Scholar]
- 109.Alvarez MS, Jacobs S, Jiang SB, Brancaccio RR, Soter NA, Cohen DE. Photocontact allergy to diallyl disulfide. American journal of contact dermatitis : official journal of the American Contact Dermatitis Society. 2003;14(3):161–165. [DOI] [PubMed] [Google Scholar]
- 110.Morikawa F, Fukuda M, Naganuma M, Nakayama Y. Phototoxic reaction to xanthene dyes induced by visible light. The Journal of dermatology. 1976;3(2):59–67. [DOI] [PubMed] [Google Scholar]
- 111.Cotterill JA. Severe phototoxic reaction to laser treatment in a patient taking St John’s Wort. Journal of cosmetic and laser therapy : official publication of the European Society for Laser Dermatology. 2001;3(3):159–160. [DOI] [PubMed] [Google Scholar]
- 112.Lehmann P, Schwarz T. Photodermatoses: diagnosis and treatment. Dtsch Arztebl Int. 2011;108(9):135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dawe R An overview of the cutaneous porphyrias. F1000Research. 2017;6:1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goetze S, Elsner P. Solar urticaria. J Dtsch Dermatol Ges. 2015;13(12):1250–1253. [DOI] [PubMed] [Google Scholar]
- 115.Kishimoto I, Uetsu N, Tanimura H, Fujii H, Okamoto H. Solar urticaria with a wide action spectrum from UVB to visible light complicated with UVA‐induced polymorphous light eruption. Photodermatology, photoimmunology & photomedicine. 2017;33(3):172–175. [DOI] [PubMed] [Google Scholar]
- 116.Pérez-Ferriols A, Barnadas M, Gardeazábal J, et al. Solar urticaria: epidemiology and clinical phenotypes in a Spanish series of 224 patients. Actas Dermo-Sifiliográficas (English Edition). 2017;108(2):132–139. [DOI] [PubMed] [Google Scholar]
- 117.Silpa-archa N, Wongpraparut C, Leenutaphong V. Analysis of solar urticaria in Thai patients. Asian Pacific journal of allergy and immunology. 2016;34(2):146–152. [DOI] [PubMed] [Google Scholar]
- 118.Monfrecola G, Masturzo E, Riccardo AM, Balato F, Ayala F, Di Costanzo MP. Solar urticaria: a report on 57 cases. American journal of contact dermatitis : official journal of the American Contact Dermatitis Society. 2000;11(2):89–94. [DOI] [PubMed] [Google Scholar]
- 119.Goetze S, Elsner P. Solar urticaria. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2015;13(12):1250–1253. [DOI] [PubMed] [Google Scholar]
- 120.Yokoyama S, Nakano H, Nishizawa A, Kaneko T, Harada K, Hanada K. A case of photocontact urticaria induced by photodynamic therapy with topical 5‐aminolaevulinic acid. The Journal of dermatology. 2005;32(10):843–847. [DOI] [PubMed] [Google Scholar]
- 121.Pinto Gouveia M, Gameiro A, Pinho A, Gonçalo M. Long‐term management of chronic spontaneous urticaria with omalizumab. Clinical and experimental dermatology. 2017;42(7):735–742. [DOI] [PubMed] [Google Scholar]
- 122.Badri T, Schlessinger J. Solar Urticaria. StatPearls Publishing, Treasure Island (FL); 2019. [Google Scholar]
- 123.Jiang AJ, Lim HW. Phototherapy in the Evaluation and Management of Photodermatoses. Dermatologic clinics. 2020;38(1):71–77. [DOI] [PubMed] [Google Scholar]
- 124.Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatologic Surgery. 2014;40(10):1094–1102. [DOI] [PubMed] [Google Scholar]
- 125.Maytin EV, Kaw U, Ilyas M, Mack JA, Hu B. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis and photodynamic therapy. 2018;22:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. New England Journal of Medicine. 2015;373(1):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Artz CE, Farmer CM, Lim HW. Polymorphous Light Eruption: a Review. Current dermatology reports. 2019;8(3):110–116. [Google Scholar]
- 128.van de Pas CB, Hawk JL, Young AR, Walker SL. An optimal method for experimental provocation of polymorphic light eruption. Archives of dermatology. 2004;140(3):286–292. [DOI] [PubMed] [Google Scholar]
- 129.Boonstra HE, van Weelden H, Toonstra J, van Vloten WA. Polymorphous light eruption: A clinical, photobiologic, and follow-up study of 110 patients. Journal of the American Academy of Dermatology. 2000;42(2 Pt 1):199–207. [DOI] [PubMed] [Google Scholar]
- 130.Quatrano NA, Shvartsbeyn M, Meehan SA, Soter NA, Cohen DE. Chronic actinic dermatitis occurring in an adult with atopic dermatitis. Dermatology online journal. 2015;21(12). [PubMed] [Google Scholar]
- 131.Lim HW, Morison WL, Kamide R, Buchness MR, Harris R, Soter NA. Chronic actinic dermatitis. An analysis of 51 patients evaluated in the United States and Japan. Archives of dermatology. 1994;130(10):1284–1289. [DOI] [PubMed] [Google Scholar]
- 132.Healy E, Rogers S. Photosensitivity dermatitis/actinic reticuloid syndrome in an Irish population: a review and some unusual features. Acta dermato-venereologica. 1995;75(1):72–74. [DOI] [PubMed] [Google Scholar]
- 133.van Weelden H, Velthuis PJ, Baart de la Faille H. Light-induced skin lesions in lupus erythematosus: photobiological studies. Archives of dermatological research. 1989;281(7):470–474. [DOI] [PubMed] [Google Scholar]
- 134.Kohli I, Zubair R, Lyons AB, et al. Impact of Long-Wavelength Ultraviolet A1 and Visible Light on Light-Skinned Individuals. Photochemistry and photobiology. 2019;95(6):1285–1287. [DOI] [PubMed] [Google Scholar]
- 135.Nardelli A, Deuschle E, de Azevedo LD, Pessoa JLN, Ghisi E. Assessment of Light Emitting Diodes technology for general lighting: A critical review. Renewable and Sustainable Energy Reviews. 2017;75:368–379. [Google Scholar]
- 136.Polefka TG, Meyer TA, Agin PP, Bianchini RJ. Effects of solar radiation on the skin. Journal of cosmetic dermatology. 2012;11(2):134–143. [DOI] [PubMed] [Google Scholar]
- 137.Das A, Sarda A, De A. Cooling Devices in Laser therapy. J Cutan Aesthet Surg. 2016;9(4):215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research. 2018;7:1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Scholz CFP, Kilian M. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int J Syst Evol Microbiol. 2016;66(11):4422–4432. [DOI] [PubMed] [Google Scholar]
- 140.Greaves AJ. The effects of narrowbands of visible light upon some skin disorders: a review. International journal of cosmetic science. 2016;38(4):325–345. [DOI] [PubMed] [Google Scholar]
- 141.Handler MZ, Bloom BS, Goldberg DJ. Energy-based devices in treatment of acne vulgaris. Dermatologic Surgery. 2016;42(5):573–585. [DOI] [PubMed] [Google Scholar]
- 142.Patwardhan SV, Richter C, Vogt A, Blume-Peytavi U, Canfield D, Kottner J. Measuring acne using Coproporphyrin III, Protoporphyrin IX, and lesion-specific inflammation: an exploratory study. Archives of dermatological research. 2017;309(3):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sakamoto FH, Torezan L, Anderson RR. Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practice: part II. Understanding parameters for acne treatment with photodynamic therapy. Journal of the American Academy of Dermatology. 2010;63(2):195–211. [DOI] [PubMed] [Google Scholar]
- 144.Zhang P, Wu MX. A clinical review of phototherapy for psoriasis. Lasers in medical science. 2018;33(1):173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pfaff S, Liebmann J, Born M, Merk HF, Von Felbert V. Prospective randomized long-term study on the efficacy and safety of UV-free blue light for treating mild psoriasis vulgaris. Dermatology (Basel, Switzerland). 2015;231(1):24–34. [DOI] [PubMed] [Google Scholar]
- 146.Ho D, Koo E, Mamalis A, Jagdeo J. A Systematic Review of Light Emitting Diode (LED) Phototherapy for Treatment of Psoriasis: An Emerging Therapeutic Modality. Journal of drugs in dermatology : JDD. 2017;16(5):482–488. [PubMed] [Google Scholar]
- 147.Kemény L, Varga E, Novak Z. Advances in phototherapy for psoriasis and atopic dermatitis. Expert review of clinical immunology. 2019;15(11):1205–1214. [DOI] [PubMed] [Google Scholar]
- 148.Liebmann J, Born M, Kolb-Bachofen V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. Journal of Investigative Dermatology. 2010;130(1):259–269. [DOI] [PubMed] [Google Scholar]
- 149.França CM, Anders JJ, Lanzafame RJ. Photobiomodulation in Wound Healing: What Are We Not Considering? Photomedicine and laser surgery. 2016;34(2):51–52. [DOI] [PubMed] [Google Scholar]
- 150.Mignon C, Botchkareva NV, Uzunbajakava NE, Tobin DJ. Photobiomodulation devices for hair regrowth and wound healing: a therapy full of promise but a literature full of confusion. Experimental dermatology. 2016;25(10):745–749. [DOI] [PubMed] [Google Scholar]
- 151.Tchanque‐Fossuo CN, Ho D, Dahle SE, et al. A systematic review of low‐level light therapy for treatment of diabetic foot ulcer. Wound Repair and Regeneration. 2016;24(2):418–426. [DOI] [PubMed] [Google Scholar]
- 152.Maiya AG, Kumar AS, Hazari A, et al. Photobiomodulation therapy in neuroischaemic diabetic foot ulcers: a novel method of limb salvage. J Wound Care. 2018;27(12):837–842. [DOI] [PubMed] [Google Scholar]
- 153.Dos Santos Mendes-Costa L, de Lima VG, Barbosa MPR, Dos Santos LE, de Siqueira Rodrigues Fleury Rosa S, Tatmatsu-Rocha JC. Photobiomodulation: systematic review and meta-analysis of the most used parameters in the resolution diabetic foot ulcers. Lasers in medical science. 2020. [DOI] [PubMed] [Google Scholar]
- 154.Siqueira CPCM, de Paula Ramos S, Gobbi CA, et al. Effects of weekly LED therapy at 625 nm on the treatment of chronic lower ulcers. Lasers in medical science. 2015;30(1):367–373. [DOI] [PubMed] [Google Scholar]
- 155.Vitse J, Bekara F, Byun S, Herlin C, Teot L. A double-blind, placebo-controlled randomized evaluation of the effect of low-level laser therapy on venous leg ulcers. The International Journal of Lower Extremity Wounds. 2017;16(1):29–35. [DOI] [PubMed] [Google Scholar]
- 156.Lipovsky A, Nitzan Y, Gedanken A, Lubart R. Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers in surgery and medicine. 2010;42(6):467–472. [DOI] [PubMed] [Google Scholar]
- 157.Taradaj J, Shay B, Dymarek R, et al. Effect of laser therapy on expression of angio- and fibrogenic factors, and cytokine concentrations during the healing process of human pressure ulcers. International journal of medical sciences. 2018;15(11):1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang P, Wang N, Wang C, et al. 460nm visible light irradiation eradicates MRSA via inducing prophage activation. Journal of photochemistry and photobiology B, Biology. 2017;166:311–322. [DOI] [PubMed] [Google Scholar]
- 159.Hamblin MR. Photobiomodulation for the management of alopecia: mechanisms of action, patient selection and perspectives. Clinical, cosmetic and investigational dermatology. 2019;12:669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sanclemente G, Ruiz-Cañas V, Miranda JM, Ferrín AP, Ramirez PA, Hernandez GN. Photodynamic Therapy Interventions in Facial Photodamage: A Systematic Review. Actas dermo-sifiliograficas. 2018;109(3):218–229. [DOI] [PubMed] [Google Scholar]
- 161.Huang A, Nguyen JK, Jagdeo J. Light-Emitting Diode-Based Photodynamic Therapy for Photoaging, Scars, and Dyspigmentation: A Systematic Review. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al]. 2020;Volume Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 162.Joo J, Michael D, Kilmer S. Lasers for Treatment of Vascular Lesions. In: Lasers in Dermatology and Medicine. Springer; 2018:49–61. [Google Scholar]
- 163.Chang YC, Lee SJ, Chung HJ. Treatment of post-pulsed dye laser purpura with pulsed dye laser. Journal of cosmetic and laser therapy : official publication of the European Society for Laser Dermatology. 2018;20(1):21–23. [DOI] [PubMed] [Google Scholar]
- 164.Brauer JA, Farhadian JA, Bernstein LJ, Bae YS, Geronemus RG. Pulsed Dye Laser at Subpurpuric Settings for the Treatment of Pulsed Dye Laser-Induced Ecchymoses in Patients With Port-Wine Stains. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al]. 2018;44(2):220–226. [DOI] [PubMed] [Google Scholar]
- 165.de Leeuw J, van Lingen RG, Both H, Tank B, Nijsten T, Martino Neumann H. A comparative study on the efficacy of treatment with 585 nm pulsed dye laser and ultraviolet B‐TL01 in plaque type psoriasis. Dermatologic surgery. 2009;35(1):80–91. [DOI] [PubMed] [Google Scholar]
- 166.Taibjee S, Cheung ST, Laube S, Lanigan S. Controlled study of excimer and pulsed dye lasers in the treatment of psoriasis. British Journal of Dermatology. 2005;153(5):960–966. [DOI] [PubMed] [Google Scholar]
- 167.Al-Mutairi N, Noor T, Al-Haddad A. Single blinded left-to-right comparison study of excimer laser versus pulsed dye laser for the treatment of nail psoriasis. Dermatology and therapy. 2014;4(2):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Treewittayapoom C, Singvahanont P, Chanprapaph K, Haneke E. The effect of different pulse durations in the treatment of nail psoriasis with 595-nm pulsed dye laser: a randomized, double-blind, intrapatient left-to-right study. Journal of the American Academy of Dermatology. 2012;66(5):807–812. [DOI] [PubMed] [Google Scholar]
- 169.Oram Y, Karincaoğlu Y, Koyuncu E, Kaharaman F. Pulsed dye laser in the treatment of nail psoriasis. Dermatologic Surgery. 2010;36(3):377–381. [DOI] [PubMed] [Google Scholar]
- 170.Lin MY, Lin CS, Hu S, et al. The application of 595-nm pulsed dye laser for vascular anomalies in a Chinese population: a 10-year experience. Journal of cosmetic and laser therapy : official publication of the European Society for Laser Dermatology. 2019;21(3):171–178. [DOI] [PubMed] [Google Scholar]
- 171.Alegre-Sánchez A, Pérez-García B, Boixeda P. Pulsed-Dye Laser Treatment of Port-Wine Stains in Children: Useful Tips to Avoid General Anesthesia. Pediatr Dermatol. 2017;34(5):619–621. [DOI] [PubMed] [Google Scholar]
- 172.Rizzo C, Brightman L, Chapas AM, et al. Outcomes of childhood hemangiomas treated with the pulsed-dye laser with dynamic cooling: a retrospective chart analysis. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al]. 2009;35(12):1947–1954. [DOI] [PubMed] [Google Scholar]
- 173.Chinnadurai S, Sathe NA, Surawicz T. Laser treatment of infantile hemangioma: A systematic review. Lasers in surgery and medicine. 2016;48(3):221–233. [DOI] [PubMed] [Google Scholar]
- 174.Chapas AM, Eickhorst K, Geronemus RG. Efficacy of early treatment of facial port wine stains in newborns: a review of 49 cases. Lasers in surgery and medicine. 2007;39(7):563–568. [DOI] [PubMed] [Google Scholar]
- 175.Torbeck RL, Schilling L, Khorasani H, Dover JS, Arndt KA, Saedi N. Evolution of the Picosecond Laser: A Review of Literature. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al]. 2019;45(2):183–194. [DOI] [PubMed] [Google Scholar]
- 176.Chaowattanapanit S, Silpa-Archa N, Kohli I, Lim HW, Hamzavi I. Postinflammatory hyperpigmentation: A comprehensive overview: Treatment options and prevention. Journal of the American Academy of Dermatology. 2017;77(4):607–621. [DOI] [PubMed] [Google Scholar]
- 177.Tafazzoli A, Rostan EF, Goldman MP. Q‐switched ruby laser treatment for postsclerotherapy hyperpigmentation. Dermatologic surgery. 2000;26(7):653–656. [DOI] [PubMed] [Google Scholar]
- 178.Kohli I, Zubair R, Lyons AB, et al. Impact of Long‐Wavelength Ultraviolet A1 and Visible Light on Light‐Skinned Individuals. Photochemistry and photobiology. 2019;95(6):1285–1287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


