Abstract
Background and Objectives:
EUS-guided drainage of pancreatic fluid collections (PFCs) has been increasingly performed using lumen-apposing metal stents (LAMS). However, recent data have suggested higher adverse event rates with LAMS compared to double pigtail plastic stents (DPS) alone. To decrease risks, there has been anecdotal use of placing DPS through the LAMS. We aimed to determine whether the placement of DPS through cautery-enhanced LAMS at time of initial placement decreases adverse events or need for reintervention.
Methods:
We performed a multicenter retrospective study between January 2015 and October 2017 examining patients who underwent EUS-guided drainage of pseudocysts (PP), walled-off necrosis (WON), and postsurgical fluid collection using a cautery enhanced LAMS with and without DPS.
Results:
There were 68 patients identified at 3 US tertiary referral centers: 44 PP (65%), 17 WON (25%), and 7 PFSC (10%). There were 35 patients with DPS placed through LAMS (Group 1) and 33 with LAMS alone (Group 2). Overall technical success was 100%, clinical success was 94%, and adverse events (bleeding, perforation, stent occlusion, and stent migration) occurred in 28% of patients. Subgroup analysis compared specific types of PFCs and occurrence of adverse events between each group with no significant difference detected in adverse event or reintervention rates.
Conclusion:
This multicenter study of various types of PFCs requiring EUS-guided drainage demonstrates that deployment of DPS across cautery-enhanced LAMS at the time of initial drainage does not have a significant effect on clinical outcomes, adverse events, or need for reinterventions.
Keywords: LAMS, double pigtail stents, pancreatic collections
INTRODUCTION
Pancreatic fluid collections (PFC) occur as a consequence of inflammatory pancreatitis, trauma, malignancy, and postsurgical ductal leakage.[1] PFCs generally form a well-defined wall after 4 weeks and evolve into either fluid-filled pancreatic pseudocysts (PP) or solid debris containing walled-off pancreatic necrosis (WON). Postsurgical fluid collections (PSFCs) are mainly fluid collections that occur within 30 days of surgery. Patients who develop symptoms (abdominal pain, infection, gastric outlet, or biliary obstruction) from their collections require drainage.
Since EUS-guided drainage has become first-line therapy for drainage of symptomatic PFCs, the technique and equipment used have evolved over time.[2,3] Historically, EUS-guided drainage involves needle puncture with passage of a guidewire into the collection followed by multiple equipment exchanges for tract dilation and placement of multiple 7-10Fr (2–3 mm diameter) double pigtail plastic stents (DPS) under fluoroscopic guidance. This technique can be time consuming and cumbersome, and importantly, complicates repeated entry into the cyst cavity for necrosectomy.[4,5]
Lumen-apposing metal stents (LAMS) have been developed to overcome these limitations and streamline deployment by obviating the need for multiple device exchanges and limiting the use of fluoroscopy. LAMS are bi-flanged, large diameter (10–20 mm diameter), fully covered stents with a unique dumbbell shape that promotes apposition between the walls of the collection and the lumen to stabilize the position of the stent.[6] They are removable and their wider diameter favor access to necrotic collections for debridement. LAMS (Cold Axios, Boston Scientific, Marlborough, MA) became the first stent type to be FDA approved in 2014, specifically for pseudocyst drainage.[7] Then, in 2015, a cautery enhanced LAMS (Hot Axios, Boston Scientific, Marlborough, MA) was introduced that allowed for direct puncture and stent deployment without dilation and solely under EUS guidance. Multiple noncomparative studies using LAMS have documented resolution rates of PP, WON, and PSFC as 91%, 87%, and 89%, respectively. While for PP, these rates are comparable to using DPS alone, LAMS has the advantage of providing a larger tract diameter to facilitate necrosectomy in heterogeneous collections such as WON and PSFC.[8,9,10]
Although initial studies demonstrated favorable safety data and shorter procedure times, more recent literature has reported an increased rate of adverse events (AEs) with LAMS used for drainage of PP compared to DPS alone. Two single-center studies have demonstrated higher bleeding rates and unplanned endoscopic reinterventions in the LAMS group compared to DPS alone.[11,12] Reported etiologies ranged from splenic artery aneurysm to intracavitary vessel bleeding in the bleeding group and stent occlusion by debris in the reintervention group.[12] Subsequently, two small retrospective, single-center studies demonstrated that adjunctive placement of DPS through a LAMS (both with and without cautery enhancement) into a PFC (combination of PP and WON) resulted in decreased adverse events, particularly bleeding and infection.[13,14]
We aimed to determine whether the placement of DPS through cautery-enhanced LAMS decreases adverse events or need for reintervention when used in various types of pancreatic collections: pancreatic pseudocyst (PP), walled-off necrosis (WON), and postsurgical fluid collections (PSFC) in a multicenter analysis.
METHODS
Consecutive patients with symptomatic PFCs (i.e. pain, infection, poor po intake, etc) from three U.S. academic tertiary care referral centers in whom EUS-guided drainage using a cautery enhanced LAMS was performed between January 2015 to October 2017 were included in the study. Institutional review board approval for the study and permission for data sharing was obtained by all participating centers. PP was defined as an encapsulated fluid-filled collection, greater than 4 weeks, after an episode of interstitial pancreatitis without evidence of debris or necrosis within the collection. WON was defined as a persistent collection with a well-defined wall and solid debris, greater than 4 weeks after a bout of necrotizing pancreatitis.[15] PSFC was defined as any collection in the abdomen or pelvis developing as a result of a surgical procedure within 30 days of surgery.[10]
The collections were initially identified by cross-sectional imaging study before endoscopic procedures. Data were collected on patient demographics, date LAMS placed, date removed, diameter of LAMS, site of collection, size and type of the collection, resolution of collection, use and type of DPS through the LAMS. In addition, data were collected on AEs both during and within 90 days after the procedure (bleeding, infections, perforation, mis-deployment of stent, and stent migration) and need for unplanned reintervention before collection resolution.
Technique
All procedures were performed with the patient under general anesthesia or monitored anesthesia care. Cautery-enhanced LAMS (Hot Axios; Boston Scientific, Marlborough, MA) was placed under EUS guidance. A therapeutic linear array echoendoscope (GF-UCT180; Olympus, Center Valley, PA) was used to perform the procedure.
If a guidewire was used, then the collection was initially punctured using an FNA needle (either 19 or 22-gauge) and a guidewire was passed through the needle and coiled inside the fluid collection under EUS or fluoroscopic guidance. The cautery-enhanced LAMS delivery system was advanced over the wire or with direct puncture and passed into the collection without the use of a dilator balloon.
The use of a guidewire versus direct puncture, use of fluoroscopy, the choice of LAMS stent diameter (10 vs. 15 mm), and DPS placement (if placed or not, one or two DPS placed, 7 or 10Fr) placed through the LAMS were all at the discretion of the endoscopist.
Patient follow-up
Follow-up cross-sectional imaging or EUS was performed to assess resolution of the PFC after LAMS placement. Imaging was performed between 4 and 8 weeks after initial placement of LAMS depending on the individual physician practice. If resolution of the collection was noted on cross-sectional imaging, repeat endoscopy was performed with removal of LAMS. If the collection had not resolved, repeat imaging was obtained between 2 and 4 weeks after subsequent imaging.
Study outcomes
Safety was measured by the intraprocedural and postprocedural (up to 3 months) adverse events. The primary outcome was the rate of adverse events seen with LAMS deployment with DPS versus LAMS without DPS. Adverse events included the following: bleeding, perforation, stent occlusion, and migration. We defined bleeding as hemorrhage necessitating blood transfusion and endoscopic confirmation that the bleeding source was related to LAMS placement. Perforation was defined as presence of air in the peritoneum or mediastinum with evidence of full-thickness defect in the gastric or duodenal lumen during or following the procedure. Migration was defined as movement of the LAMS stent out of the fluid collection and into the gastric cavity or vice versa. Bleeding and perforation were considered major adverse events. Secondary outcomes were unplanned reintervention rates after initial deployment of the LAMS, due to signs of infection, pain, or bleeding. Technical success was described as successfully placing the LAMS into the fluid collection and adjoining the two lumens. Clinical success was defined as complete resolution of PFC on follow-up cross-sectional imaging in association with clinical resolution of symptoms at 3-month follow-up.
Statistical analysis
Chi-square and Fisher exact test analysis were used for comparison of quantitative variables. The significance of differences between groups was tested by logistic regression analysis to identify factors. Results were considered significant at P < 0.05. All analyses were performed using SPSS for windows, version 15.0 (SPSS Inc., Chicago IL, USA). Linear regression was calculated using STATA software.
RESULTS
There were 68 patients identified at 3 US tertiary referral centers between 2015 and 2017 that underwent cautery enhanced LAMS placement for symptomatic PFCs. There were 44 patients with PPs (65%), 17 patients with WON (25%), and 7 with PFSC (10%). Most of the patients were male (64%) with an average age of 55 and average PFC size of 107.1 mm. Average duration of follow-up was 39 days. The 15 mm LAMS diameter was used in 43/68 (63%) and 10-mm LAMS in the remainder.
There were 35 patients that had DPS placed through their LAMS (Group 1) and 33 that had LAMS alone (Group 2). Overall technical success was 100%, clinical success was 94%, and adverse events were observed in 19/68 patients (28%). Overall rates of adverse events were bleeding 6/68 (9%), perforation 3/68 (4%), stent occlusion 2/68 (3%), and migration 8/68 (11%).
The breakdown of patients into each group and rates of clinical success and adverse events are shown in Figure 1. Baseline characteristics are shown in Table 1. Group 1 (DPS with LAMS) had more wire-guided placement of LAMS (24 vs. 12; P = 0.01) and dilations performed at time of placement (21 vs. 7; P = 0.01). Group 2 (LAMS alone) had more patients with PP (18 vs. 26; P = 0.04). Table 2 shows the breakdown in Group 1 with regard to DPS status. There were 27/35 (77%) that had one DPS placed and 23/35 (66%) of the DPS were 10 French in diameter. The majority of the DPS (63%) were removed at the same session as the LAMS removal.
Figure 1.
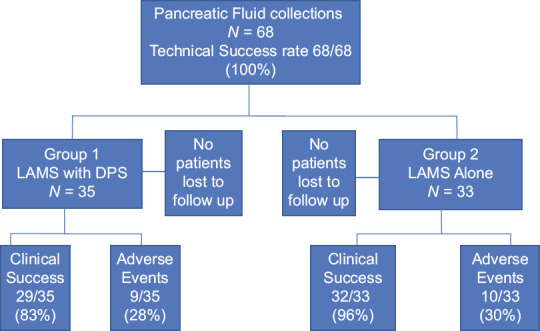
. Flow sheet of patients enrolled and adverse events
Table 1.
Patient and pancreatic fluid collections characteristics
| Group 1 LAMS with DPS (n=35) | Group 2 LAMS alone (n=33) | P | |
|---|---|---|---|
| Gender | |||
| Male | 21 | 23 | 0.45 |
| Female | 14 | 10 | |
| Age, mean±SD | 57.1±14 | 52.4±12 | 0.80 |
| Indication for drainage | |||
| PP | 18 | 26 | 0.04 |
| WON | 11 | 6 | 0.19 |
| PSFC | 6 | 1 | 0.06 |
| Etiology | |||
| Alcohol | 10 | 14 | 0.19 |
| Gallstones | 9 | 12 | 0.70 |
| Postsurgical | 6 | 1 | 0.06 |
| Other | 9 | 6 | 0.46 |
| Collection diameter (mm)±SD | 103.7±30.5 | 110.4±40 | 0.22 |
| Site | |||
| Head | 6 | 5 | 0.63 |
| Body | 18 | 17 | 0.72 |
| Tail | 5 | 10 | 0.08 |
| Surgical bed | 6 | 1 | 0.06 |
| Drainage site | |||
| Gastric | 32 | 30 | 0.78 |
| Duodenal | 3 | 3 | 0.43 |
| LAMS placement | |||
| Wire | 24 | 12 | 0.01 |
| Direct puncture | 11 | 21 | 0.02 |
| Dilation at placement | |||
| Yes | 21 | 7 | 0.01 |
| No | 14 | 26 |
LAMS: Lumen-apposing metal stents; DPS: Double pigtail plastic stents; SD: Standard deviation; PP: Pseudocysts; WON: Walled-off necrosis; PSFC: Postsurgical fluid collection
Table 2.
Procedure characteristics of Group 1: Lumen-apposing metal stents with double pigtail plastic stents
| n=35 | |
|---|---|
| Pigtail stent number | |
| 1 | 27 |
| 2 | 8 |
| Pigtail stent diameter | |
| 7 French | 12 |
| 10 French | 23 |
| Pigtails removed when Axios removed, n(%) | |
| Yes | 22/35 (63) |
| No | 11/35 (31) |
In terms of overall success rates (technical and clinical) and adverse events, there was no significant difference between Group 1 and Group 2 [Table 3]. Subgroup analysis compared specific types of PFCs and occurrence of adverse events between Group 1 and Group 2 [Table 4], and no significant difference in overall adverse events was seen. We further controlled for type of fluid collection using multivariate logistic regression analysis and there was no relationship between the use of pigtail stents and major adverse events (odds ratio [OR]: 0.7 [95% confidence interval [CI]: 0.2–3.0) or overall adverse events (OR: 0.8 [95% CI: 0.3–2.3]).
Table 3.
Outcomes
| Group 1 LAMS with DPS (%) | Group 2 LAMS alone (%) | P | |
|---|---|---|---|
| Technical success: Placement | 35 of 35 (100) | 33 of 33 (100) | 1.00 |
| Technical success: Removal | 31 of 35 (89) | 33 of 33 (100) | 0.20 |
| Clinical success at 3 months | 29 of 35 (83) | 32 of 33 (96) | 0.67 |
| Overall adverse events | 9 of 35 (26) | 10 of 33 (30) | 0.75 |
| Bleeding | 3 (8) | 3 (9) | 1.00 |
| Perforation | 1 (2) | 2 (6) | 0.78 |
| Stent occlusion | 0 | 2 (6) | 0.49 |
| Migration | 5 (15) | 3 (9) | 0.46 |
| Need for reintervention | |||
| Yes | 11 (31) | 7 (21) | 0.34 |
| No | 24 (69) | 26 (78) |
LAMS: Lumen-apposing metal stents; DPS: Double pigtail plastic stents
Table 4.
Adverse events related to pancreatic fluid collections type
| Type of collection | Group 1 LAMS with DPS (%) | Group 2 LAMS alone (%) | P |
|---|---|---|---|
| PP | 1 of 18 (5) | 6 of 26 (23) | 0.11 |
| Stent occlusion | 0 | 1 (4) | 1.00 |
| Major bleeding | 1 (5) | 1 (7) | 1.00 |
| Perforation | 0 | 1 (7) | 0.50 |
| Migration | 0 | 3 (11) | 0.25 |
| WON | 7 of 11 (64) | 3 of 6 (50) | 0.39 |
| Stent occlusion | 0 | 1 (14) | 0.42 |
| Major bleeding | 2 (18) | 1 (14) | 1.00 |
| Perforation | 1 (9) | 1 (14) | 1.00 |
| Migration | 4 (36) | 0 | 0.11 |
| PSFC | 1 of 6 (17) | 1 of 1 (33) | 1.00 |
| Stent occlusion | 0 | 0 | 1.00 |
| Major bleeding | 0 | 1 (33) | 0.40 |
| Perforation | 1 (17) | 0 | 0.40 |
| Migration | 0 | 0 | 1.00 |
PP: Pseudocysts; WON: Walled-off necrosis; PSFC: Postsurgical fluid collection; LAMS: Lumen-apposing metal stents; DPS: Double pigtail plastic stents
No difference was seen in adverse events based on number or diameter of DPS used in Group 1 (1-pigtail 23% vs. 2-pigtails 67%; P = 0.09) and using 7Fr vs. 10Fr pigtail (24% vs. 66%; P = 0.10). There was no difference in the rate of adverse events based on the length of time the LAMS was in place (52% adverse events <3 weeks, median 21 days (7–21 days) vs. 48% adverse events >3 weeks, median 35 days (28–77; P = 0.67).
DISCUSSION
EUS-guided transmural drainage is recognized as first-line therapy for the drainage of PFCs with high technical (>90%) and clinical success rates (>80%) for pseudocyst drainage, which exceeds other techniques.[16,17,18] With the advent of LAMS in 2014, the overall technical success of drainage of PFCs has risen to 95% regardless of the type of collection being drained and procedure times have been shown to decrease.[8,9,10] Our study similarly noted high technical success rates with LAMS placement in various PFC types (100%), with no difference in technical success with or without the use of DPS (100% vs. 100% P = 1.00). Furthermore, our data demonstrated a high clinical success rate with WON (90%) consistent with the current literature (86%).[9]
Two studies in the recent literature have suggested higher adverse events with LAMS compared to DPS alone for pseudocyst drainage. Bang et al. demonstrated in an interim analysis of a randomized controlled trial showing that LAMS had a 50% adverse event rate versus 0% in the DPS alone group.[11] Adverse events included delayed bleeding, buried stent syndrome, and obstructive jaundice due to stent-induced biliary stricture. Lang et al. demonstrated that LAMS had significantly more episodes of bleeding as compared to DPS alone (21% vs. 1%, respectively, P = 0.03).[12] Furthermore, this study also demonstrated that LAMS predisposed patients to more unplanned reinterventions; 26% in LAMS group versus only 10% in the DPS group (P = 0.07).
One proposed hypothesis for increased rates of bleeding or stent occlusion with LAMS is that a PFC may collapse quickly after drainage, causing the stent to disrupt regional vasculature or become obstructed by the back wall of the cyst.[3,13] In addition, a cause of increased rates of reintervention in this group may be from LAMS occlusion due to food and debris entering the larger caliber stents and leading to infection. Placing DPS through the LAMS could in theory prevent the increased friction between the back wall of the collection and the stent and has led some endoscopists to utilize this adjunctive technique. However, our study failed to demonstrate a significant reduction in bleeding or occlusion. This may be due to our overall lower rates of bleeding (9%) or occlusion (6%) than the above-mentioned studies.
This large multicenter study reported that deployment of pigtail stents across the LAMS did not significantly reduce overall adverse events when examining all types of PFCs, 26% with DPS (Group 1) versus 27% without DPS (Group 2); P = 0.88. Subgroup analysis failed to show a significant impact of DPS when each type of fluid collection was examined (PP vs. WON vs. PSFC). Although not focused specifically on the use of DPS, Fugazza et al.'s international retrospective data showed that DPS was not commonly placed at the time of LAMS (34/270 patients) for pseudocyst and WON and did not affect rates of clinical success or adverse events.[19]
Even when specific adverse events were examined, there was still no significant difference in rate of occurrence between groups. There also was no significant difference regarding necessity of endoscopic reintervention with the utilization of DPS (31% vs. 21%; P = 0.34). In the study by Bang et al., all adverse events were noted 3 weeks after placement of the LAMS in PPs.[11] Our data did not demonstrate a significant difference in adverse event rate when the LAMS was in place for less than or greater than 3 weeks.
Aburajab et al. published the first report demonstrating that the addition of DPS through LAMS in PPs would decrease the risk of infection requiring reintervention (0% in LAMS with DPS vs. 17% in LAMS alone).[13] This was a retrospective study of 47 patients undergoing LAMS drainage of PP. These findings were confirmed by Puga et al. in a similar retrospective study.[14] Although both studies are retrospective, our study has several strengths over these prior studies: larger sample size, multicenter, only cautery enhanced LAMS used, and a variety of PFC types were included. These aspects may make our results more applicable to clinical practice and suggest that the effort, time, and cost associated with the placement of DPS through LAMS may not confer any additional benefit.
There are limitations to this study. Its retrospective design, use of various EUS techniques, and different sized stents used are all factors that may add heterogeneity and impact our findings. Uniformity in size of LAMS and DPS placed would also have strengthened our results. Although our study has a larger sample size than prior studies of DPS through LAMS, it would have benefited from having more patients included to detect smaller differences in outcomes.
This multicenter study of various types of PFCs requiring EUS-guided drainage demonstrates that deployment of DPS across cautery-enhanced LAMS at the time of initial drainage does not improve clinical outcomes or decrease adverse events. Given the paucity of data and the variability in current clinical practice, our results should contribute to the existing literature on methods to optimize LAMS outcomes. Our study highlights the need for further prospective randomized controlled studies on this topic.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Vitas GJ, Sarr MG. Selected management of pancreatic pseudocysts: Operative versus expectant management. Surgery. 1992;111:123–30. [PubMed] [Google Scholar]
- 2.Varadarajulu S, Bang JY, Sutton BS, et al. Equal efficacy of endoscopic and surgical cystgastrostomy for pancreatic pseudocyst a randomized control trial. Gastroenterology. 2013;145:583–90. doi: 10.1053/j.gastro.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 3.Kahaleh M, Shami VM, Conaway MR, et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: A prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355–9. doi: 10.1055/s-2006-925249. [DOI] [PubMed] [Google Scholar]
- 4.Antillon MR, Shah RJ, Stiegmann G, et al. Single-step EUS-guided transmural drainage of simple and complicated pancreatic pseudocysts. Gastrointest Endosc. 2006;63:797–803. doi: 10.1016/j.gie.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 5.Azar RR, Oh YS, Janec EM, et al. Wire-guided pancreatic pseudocyst drainage by using a modified needle knife and therapeutic echoendoscope. Gastrointest Endosc. 2006;63:688–92. doi: 10.1016/j.gie.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Binmoeller K, Shah J. A novel lumen apposing stent for transluminal drainage of non-adherent extra-luminal fluid collections. Endoscopy. 2011;43:337–42. doi: 10.1055/s-0030-1256127. [DOI] [PubMed] [Google Scholar]
- 7.Itoi T, Binmoeller KF, Shah J, et al. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos) Gastrointest Endosc. 2012;75:870–6. doi: 10.1016/j.gie.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Shah RJ, Shah JN, Waxman I, et al. Safety and efficacy of EUS guided drainage of pancreatic fluid collections. Clin Gastroenterol Hepatol. 2015;13:747–52. doi: 10.1016/j.cgh.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 9.Sharaiha R, Tyberg A, Khashab M, et al. Endoscopic therapy with lumen apposing metal stents is safe and effective for patients with pancreatic walled-off necrosis. Clin Gastroenterol Hepatol. 2016;14:1797–803. doi: 10.1016/j.cgh.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Mudireddy P, Sethi A, Siddiqui AA, et al. EUS-guided drainage of postsurgical fluid collections using lumen apposing metal stents: A multi-center trial. Gastrointest Endosc. 2018;87:1256–62. doi: 10.1016/j.gie.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Bang JY, Hasan M, Navaneethan U, et al. Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: May not be business as usual. Gut. 2017;66:2054–6. doi: 10.1136/gutjnl-2016-312812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang G, Fritz C, Bhat T, et al. EUS-guided drainage of peri-pancreatic fluid collections with lumen apposing metal stents and plastic double-pigtail stents: Comparison of efficacy and adverse rates. Gastrointest Endosc. 2018;87:150–7. doi: 10.1016/j.gie.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Aburajab M, Smith Z, Khan A, et al. Safety and efficacy of lumen-apposing metal stents with and without simultaneous double-pigtail plastic stents for draining pancreatic pseudocyst. Gastrointest Endosc. 2018;87:1248–55. doi: 10.1016/j.gie.2017.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Puga M, Consiglieri C, Busquets J, et al. Safety of lumen-apposing stent with or without coaxial plastic stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: A retrospective study. Endoscopy. 2018;50:1022–6. doi: 10.1055/a-0582-9127. [DOI] [PubMed] [Google Scholar]
- 15.Banks P, Bollen T, Dervenis C, et al. Classificantion of acute pancreatitis-2012 revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 16.Sharaiha RZ, DeFilippis EM, Kedia P, et al. Metal versus plastic for pancreatic pseudocyst drainage: Clinical outcomes and success. Gastrointest Endosc. 2015;82:822–7. doi: 10.1016/j.gie.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 17.Sadik R, Kalaitzakis E, Thune A, et al. EUS-guided drainage is more successful in pancreatic pseudocysts compared with abscesses. Word J Gastroenterol. 2011;17:499–505. doi: 10.3748/wjg.v17.i4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gluck M, Ross A, Irani S, et al. Endoscopic and percutaneous drainage of symptomatic walled-off pancreatic necrosis reduces hospital stay and radiographic resources. Clin Gastroenterol Hepatol. 2010;8:1083–8. doi: 10.1016/j.cgh.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Fugazza A, Sethi A, Trindade AJ, et al. International multicenter comprehensive analysis of adverse events associated with lumen-apposing metal stent placement for pancreatic fluid collection drainage. Gastrointest Endosc. 2020;91:574–83. doi: 10.1016/j.gie.2019.11.021. [DOI] [PubMed] [Google Scholar]


