Abstract
Background and Objectives:
We initially reported EUS-guided lauromacrogol ablation (EUS-LA) to treat pancreatic cystic neoplasms (PCNs); however, its long-term effectiveness remains unknown. This study was performed to further determine the effectiveness of EUS-LA in a larger population with a long-term follow-up based on 5 years of experience with EUS-LA.
Materials and Methods:
From April 2015 to April 2020, 279 patients suspected of having PCNs were prospectively enrolled, and seventy patients underwent EUS-guided ablation using lauromacrogol alone. Fifty-five patients underwent follow-up, 35 of whom had a follow-up duration of at least 12 months. The effectiveness of ablation was determined based on volume changes.
Results:
Among the fifty female and twenty male patients with an overall mean age of 50.3 years, cysts were located in the head/neck of the pancreas in 37 patients (52.9%) and in the body/tail of the pancreas in 33 patients (47.1%). The adverse events rate was 3.6% (3/84), with 14 patients undergoing a second ablation. Among the 55 patients who underwent follow-up, the median cystic volume sharply decreased from 11,494.0 mm3 to 523.6 mm3 (P < 0.001), and the mean diameter decreased from 32.0 mm to 11.0 mm (P < 0.001). Postoperative imaging showed complete resolution (CR) in 26 patients (47.3%) and partial resolution (PR) in 15 (27.3%) patients. CR was observed in 18 (51.4%), and PR was observed in 9 (25.7%) patients among the 35 patients followed for at least 12 months.
Conclusions:
EUS-LA was effective and safe for the treatment of PCNs with stable effectiveness based on at least 12 months of follow-up.
Keywords: ablation, lauromacrogol, long-term follow-up, pancreatic cystic neoplasms
INTRODUCTION
Pancreatic cystic neoplasms (PCNs) have attracted attention in recent years due to their higher detection rate following the development and widespread use of radiological imaging. The majority of PCNs are detected incidentally, with a detection rate of 20% by abdominal imaging.[1] PCNs have a broad differential diagnosis and are mainly divided into serous cystic neoplasms (SCNs), mucinous cystic neoplasms (MCNs), intraductal papillary neoplasms (IPMNs), and other incidental types such as solid pseudopapillary neoplasms (SPNs) and cystic pancreatic neuroendocrine tumors (NETs). Malignancy varies with PCN type. SPNs and NETs are regarded as exhibiting low malignancy and requiring surgical resection. MCNs and IPMNs are related to malignancy or malignant potential, although most SCNs are benign.[2] The management of PCNs is challenging because differentiating the various PCN types is difficult although histological accuracy can be improved by the development of EUS-guided fine-needle biopsy, SpyGlass, and EUS-guided through-the-needle biopsy (EUS-TTNB). Resection of MCNs and IPMNs can prevent them from becoming malignant; however, surgical resection is associated with significant perioperative morbidity of 10%–40% and mortality rates as high as 2%,[3,4,5,6,7,8,9] and the operation can be challenging, especially for cysts located in the head or neck. Pancreaticoduodenectomy significantly reduces the quality of postoperative life. Follow-up seems to be another choice for these two types of cysts and SCNs; however, long-term surveillance not only adds to the financial burden and psychological stress of patients but also delays the diagnosis of malignancy and treatment. Therefore, a minimally invasive treatment is urgently required. EUS-guided ablation seems to be an attractive technique.
Inspired by studies of EUS-guided ethanol ablation with or without paclitaxel injection, which was demonstrated to be effective and safe for the treatment of PCNs,[1,6,8,9,10] we initially used lauromacrogol, which is typically used to treat esophageal variceal bleeding, as an ablative agent.[11] This new ablative method has been well accepted by the Asian EUS group and an international expert panel specialized in EUS-guided pancreatic cyst ablation.[12] Our previous study preliminarily demonstrated that EUS-guided lauromacrogol ablation (EUS-LA) was safe and efficacious; however, that study evaluated the treatment response based only on imaging examinations carried out 3 months after ablation, and only 29 patients were enrolled. There is no other study of EUS-guided ablation using lauromacrogol. The long-term effectiveness of EUS-LA remains unknown. Therefore, the aims of this study were to further determine the effectiveness of EUS-LA with a large population and a long-term follow-up based on our 5 years of experience with this new treatment for PCNs.
MATERIALS AND METHODS
Study design
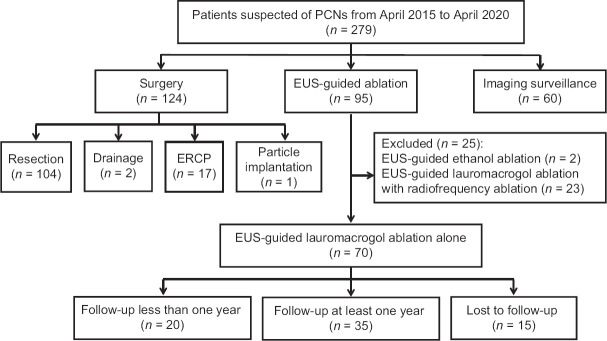
A total of 279 patients suspected of having PCNs were prospectively enrolled from April 2015 to April 2020. Among them, 95 patients underwent EUS-guided ablation. After 25 patients were excluded, we enrolled seventy patients to undergo EUS-guided ablation using lauromacrogol alone. Fourteen patients underwent two sessions of ablation among the seventy enrolled patients. Thirty-five patients were followed up for at least 12 months. The study flowchart is shown in Figure 1. This study was approved by the Institutional Review Board of the Chinese PLA General Hospital and registered in the Chinese Clinical Trials Registry (No. ChiCTR-OOC-15006118).
Figure 1.
Study flowchart
Patient selection
The patients who were enrolled to undergo EUS-LA were required to meet the following criteria: (1) patients identified as having PCNs by imaging with an age older than 18 years; (2) cysts without communication between the cyst and pancreatic duct; (3) SCNs in selected cases such as those with increasing size during radiological imaging surveillance, those causing symptoms or patients with a strong desire to undergo the procedure; (4) confirmed diagnosis of MCNs after malignancy had been ruled out; and (5) provision of informed consent. The exclusion criteria were as follows: (1) a recently identified episode of pancreatitis or suspected pseudocyst (PC) or pancreatic necrosis, as determined by imaging; (2) an inability to eliminate pancreatic cancer or signs of malignancy; (3) an inability to provide informed consent; (4) an inability to safely tolerate intravenous anesthesia; and (5) patients with conditions indicative of a high surgical risk such as pregnancy, coagulopathy, or severe cardiovascular disease. Considering that the communication between the cyst and the pancreatic duct of an IPMN may result in a poor treatment response and a higher risk of pancreatitis,[9] we excluded all types of IPMNs including main-duct IPMNs, branch-duct IPMNs (BD-IPMNs), and mixed IPMNs, despite previous studies demonstrating that EUS-guided ablation was safe in patients with BD-IPMNs.[6,8,13,14,15]
Endoscopic procedures
The instruments and equipment used were the following: linear-array echoendoscope (Prosound F75 [Aloka, Tokyo, Japan] and GF-UCT260 [Olympus, Tokyo, Japan]), a 22/19G Echotip needle (Cook, Limerick, Ireland), sulfur hexafluoride microbubbles for injection (Bracco Co. Ltd, Geneva, Switzerland), SpyGlass ([SpyGlass 4603, SpyGlass Lightsource 4619, and SpyGlass Camera 4610], Boston Scientific, Natick, Massachusetts, USA), and lauromacrogol for injection (Tianyu Pharmaceutical Co Ltd, Shanxi, China).
Patients suspected of having PCNs based on radiological imaging were subjected to EUS examination with a linear-array echoendoscope before ablation. The patients were sedated with intravenous anesthesia. The EUS-LA procedures have been described previously as follows.[11] (1) EUS-FNA was performed through transgastric or transduodenal puncture of the cyst with a needle. (2) The maximum possible volume of cyst fluid was aspirated until the cyst was collapsed. (3) The cystic fluid was sent for cytological and biochemical analyses after recording the cystic fluid characteristics, such as its color, viscosity, clarity, and volume. (4) A contrast agent, sulfur hexafluoride microbubbles, was injected into the cyst to evaluate the relationship between the pancreatic duct and the lesion when the presence of communication between the two structures could not be confirmed. SpyGlass was employed to obtain useful information for diagnosing PCNs in some cases.[16] EUS-TTNB was performed in some cases to improve diagnostic accuracy. (5) After evaluation, all of the imaging and test results including EUS-FNA lauromacrogol was injected into cysts meeting the inclusion criteria until the cystic wall was completely soaked in solution, followed by lavage (repetitive aspiration and reinjection of lauromacrogol) for 3–5 min to increase its concentration in the cyst. (6) Directly following lavage, half to two-thirds of the lauromacrogol was retrieved, leaving one-third to half of the lauromacrogol in the cyst. (7) Finally, the needle was carefully retracted. If there was a complete septum, needle puncture was performed to create a communication between locules through the septum. Lauromacrogol entered into each locule and soaked the wall of the locule. Each locule was ablated separately.
Patients were closely monitored after the procedure and were assessed for any adverse events (AEs) such as abdominal pain, fever, nausea, bleeding, pancreatitis, or an increase in serum amylase or lipase levels. Patients fasted for 2–3 days after the ablation. An intravenous proton pump inhibitor (PPI) and antibiotic were administered for 2–3 days, followed by oral PPI therapy for 3–7 days. Octreotide was intravenously administered for at least 1 day until the serum amylase levels returned to normal.
Follow-up after ablation
Follow-up imaging with either computed tomography (CT) or magnetic resonance imaging (MRI) was recommended at 3 months after ablation (regardless of whether the ablation was first or second). The next follow-ups were scheduled at an initial interval of 6 months and every year thereafter. Imaging surveillance was recommended if the maximum diameter of the cyst was <10.0 mm during follow-up. Reablation was suggested if the cyst was larger than 10.0 mm in diameter.
Definitions
The preoperative volume (original volume [OV]) and the most recent volume (final volume [FV]) of the lesion were calculated by constructing a three-dimensional image (MITK, Chinese Academy of Science, Beijing, China) based on CT or MRI images. Complete resolution (CR) was defined as an FV <5% of the OV; partial resolution (PR) was defined as an FV of 5%–25% of the OV; and persistent cyst (PeC) was defined as an FV >25% of the OV.
Outcome effectiveness was determined based on the most recent volume measured and the preoperative volume of the patient, unlike in our previous study, which evaluated the effectiveness of each session of ablation.[11] Previously, we defined the data recorded before the second examination as the baseline when documenting the results of the second ablation. In the present study, we regarded each patient as the unit instead of the ablation session.
After taking the imaging results, cystic fluid analysis results, cystic wall biopsy results (obtained using microforceps), and the patient's symptoms into consideration, the final diagnosis was made. The biopsy diagnosis was regarded as the gold standard, and the cytological result was regarded as the second most compelling result if it proved positive.
Statistical analysis
All statistical analyses were performed using SPSS 21.0 (IBM Corp, Armonk, NY, USA) and the statistical software packages R (http://www.R-project.org, The R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). Quantitative data, such as age, diameter, volume, cystic fluid analysis results, and follow-up period, are presented as means with standard deviations and as medians with ranges. Categorical variables, such as sex, location, locularity, and diagnosis, were expressed as simple proportions. Either Student's t-test or nonparametric tests were used to compare quantitative variables, whereas the Chi-square and Fisher's exact tests were performed to compare categorical variables between the two groups. A value of P < 0.05 was considered to indicate a significant difference.
RESULTS
A total of 279 patients (172 females and 107 males) suspected of having PCNs were prospectively enrolled from April 2015 to April 2020. After excluding 25 patients undergoing EUS radiofrequency ablation (EUS-RFA) with/without lauromacrogol ablation and ethanol ablation from the 95 patients who underwent EUS-guided ablation, we assigned seventy patients to undergo EUS-guided ablation using lauromacrogol alone.
The patient demographics and cyst characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of the patients and cysts
| Characteristics | Results |
|---|---|
| Age, mean±SD, years | 50.3±14.2 |
| Sex, n(%) | |
| Female | 50 (71.4) |
| Male | 20 (28.6) |
| Cyst location | |
| Head/neck | 37 (52.9) |
| Body/tail | 23 (47.1) |
| Diameter, median (range), mm | 35.5 (9.0-110.0) |
| Original volume of the cyst, median (range), mm3 | 13,123.1 (301.4-466, 468.9) |
| Septum, n(%) | |
| Yes | 55 (78.6) |
| No | 15 (21.4) |
| Volume of injected lauromacrogol, median (range), mL | 14.5 (0.5-50) |
| Follow-up period, median (range), months | 15 (2-55) |
| Presumptive diagnosis, n(%) | |
| SCN | 34 (48.6) |
| MCN | 27 (38.6) |
| Uncategorized cyst | 9 (12.8) |
SD: Standard deviation; SCN: Serous cystic neoplasm; MCN: Mucinous cystic neoplasms
The study population included fifty females and 20 males, with an overall mean age of 50.3 years. The mean PCN diameter was 35.5 mm (range 9.0–110.0 mm), and the median OV of the cyst was 13,123.1 mm3 (range: 301.4–466, 468.9 mm3). Cysts were located in the head/neck of the pancreas in 37 patients (52.9%) and in the body/tail of the pancreas in 33 patients (47.1%). A septum was detected in 55 patients (78.6%) and was absent in 15 patients (21.4%). The presumptive diagnosis was SCNs in 34 patients (48.6%), MCNs in 27 patients (38.6%), and uncategorized cysts in 9 patients (12.8%). Fifty-five patients participated in the follow-up, with a median follow-up period of 15 months (range: 2–55 months).
Effectiveness and safety outcomes
Among the 55 patients who underwent follow-up, the median cyst volume was sharply reduced from an OV of 11,494.0 mm3 (range: 301.4–466, 468.9) to an FV of 523.6 mm3 (range: 0–453,299.4) (P < 0.001), and the mean diameter decreased from 32.0 mm to 11.0 mm (P < 0.001). The postablation imaging results showed CR in 26 patients (47.3%) [Figure 2], PR in 15 patients (27.3%) [Figure 3], and PeC in 14 patients (25.4%).
Figure 2.
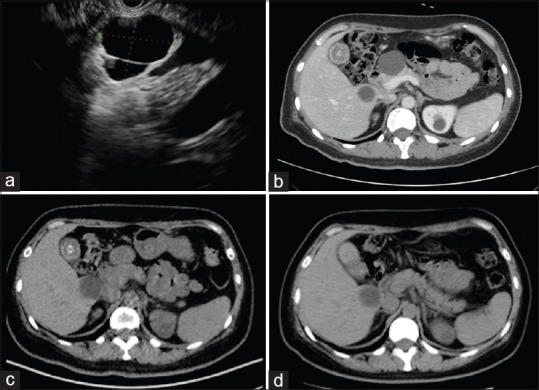
(a) EUS image before ablation, showing a cystic lesion in the head; (b) computed tomography of the same cyst before ablation, showing a 37.0 mm round cyst; (c) follow-up computed tomography at 5 months after ablation, showing complete resolution with the cyst disappearing; (d) follow-up computed tomography at 22 months after ablation, showing the cyst disappearing
Figure 3.
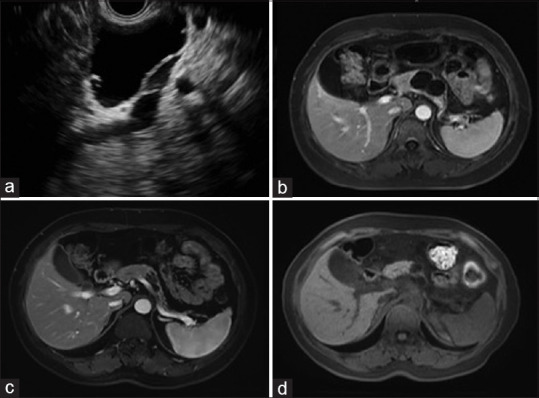
(a) EUS image before ablation, showing a cystic lesion in the body; (b) magnetic resonance imaging of the same cyst before ablation, showing a 41.0 mm × 27.0 mm cyst; (c) follow-up magnetic resonance imaging at 5 months after the first ablation, showing partial resolution with the cyst decreased to 24.0 mm × 11.0 mm. Then, a second ablation was performed; (d) follow-up magnetic resonance imaging at 17 months after the first ablation, showing that the cyst decreased to 15.0 mm × 12.0 mm
Fourteen of the seventy patients underwent a second ablation; therefore, 84 ablation sessions were carried out. EUS-guided lauromacrogol ablation was successfully performed in all 84 sessions. Two patients developed mild acute pancreatitis and recovered after consecutive treatment with prolonged octreotide with or without PPI and an antibiotic. One patient complaining of moderate fever recovered after injection of imipenem and cilastatin sodium. The major AEs rate was 3.6% (3/84). Minor AEs, such as mild abdominal pain and an increase in serum amylase levels, were noted in thirty cases; however, no special treatment was required for these patients. The treatment outcomes of EUS-guided lauromacrogol ablation were shown in Table 2.
Table 2.
Treatment outcomes of EUS-guided lauromacrogol ablation
| Characteristics | Results |
|---|---|
| Diameter, median (range), mm | |
| Initial | 32.0 (9.0-110.0) |
| Follow-up | 11.0 (107.0) |
| Volume, median (range), mm3 | |
| Initial | 11, 494.0 (301.4-466, 468.9) |
| Follow-up | 523.6 (0-453,299.4) |
| Treatment response, n(%) | |
| CR | 26 (47.3) |
| PR | 15 (27.3) |
| PeC | 14 (25.4) |
| AEs, n(%) | |
| Acute pancreatitis | 2 (2.4) |
| Fever | 1 (1.2) |
CR: Complete resolution; PR: Partial resolution; PeC: Persistent cyst
Comparison between the resolved group and the unresolved group
Univariate analysis was performed to examine the predictors for CR [Table 3]. The results showed that patient age and sex were not predictive. No significant differences were founded between the resolved group and the unresolved group with respect to location, initial diameter, OV, septum, and cyst subtype.
Table 3.
Comparison of EUS-guided lauromacrogol ablation between the resolved and unresolved groups
| Characteristics | Resolved groups (n=26) | Unresolved group (n=29) | P |
|---|---|---|---|
| Age, mean±SD, years | 48.6±13.1 | 54.2±14.9 | 0.244 |
| Sex, n | |||
| Female | 18 | 18 | 0.577 |
| Male | 8 | 11 | |
| Cyst location, n | |||
| Head/neck | 15 | 18 | 0.741 |
| Body/tail | 11 | 11 | |
| Initial diameter, median (range), mm | 26.5 (11.0-73.0) | 38.0 (9.0-110.0) | 0.341 |
| OV, median (range), mm3 | 9,489.5 (401.9-195,394.5) | 12,177.5 (301.4-466,468.9) | 0.590 |
| Septum, n | |||
| Yes | 20 | 23 | 0.831 |
| No | 6 | 6 | |
| Subtype, n† | |||
| SCN | 14 | 14 | 0.416 |
| MCN | 8 | 11 |
† Uncategorized cysts were not noted. SD: Standard deviation. OV: Original volume; SCN: Serous cystic neoplasm; MCN: Mucinous cystic neoplasms
Long-term outcomes
Among the 55 patients who participated in the follow-up, 35 patients were followed for more than 12 months. CR was observed in 18 patients (51.4%), PR in 9 patients (25.7%), and PeC in 8 patients (22.9%).
Outcomes of the second ablation session
Among the 14 patients who underwent a second ablation treatment, seven received imaging follow-up after the second ablation. The volumes of the cysts after the second ablation decreased in six patients, to 8.94%, 18.10%, 61.14%, 52.60%, 61.14%, and 66.80% of the volumes before the second ablation, while the volume was unchanged in one patient.
DISCUSSION
Ablative treatments, including ethanol ablation and RFA, have been reported as effective and safe treatments for PCNs.[1,6,8,9,10,13,15,17,18,19] Pai et al.[18] studied eight patients with pancreatic cystic lesions and found that the treatment response to EUS-RFA ranged from CR to a 50% reduction in size. A study using porcine cyst models tested a novel radiofrequency EUS-capable needle connected to a standard electrosurgical unit and showed that this method provided ablation in a temperature-dependent manner with a threshold of at least 60°C and a safe cyst margin below 97°C.[19]
Ethanol was the first ablative agent used to treat PCNs and is reported to be safe and feasible.[8] Ethanol is an inexpensive, widely available, low-viscosity agent that is easy to inject through a small-gauge needle.[20] Paclitaxel was injected after ethanol lavage to improve treatment responses.[1] Paclitaxel was reported to improve the CR rate from approximately 35%–60%.[1,6,8,9,10,14,15,17,21,22,23,24,25] A meta-analysis including seven studies describing ethanol ablation with/without paclitaxel to treat PCNs showed a CR rate of 56.20% (95% confidence interval [CI] = 48.16–64.08) and a PR rate of 23.72% (95% CI = 17.24–30.89).[26] However, the effectiveness of ethanol with/without paclitaxel was questioned, and more efficient agents were considered to be warranted.[27] Moyer et al.[28] demonstrated that ethanol was not required for effective EUS-guided pancreatic cyst ablation in a prospective, double-blind trial. Patients diagnosed with BD-IPMN were not excluded in previous studies; however, EUS-guided ethanol ablation therapy was reported to be effective in only 11% of IPMNs.[9] Therefore, all IPMNs were excluded in our study.
Considering that lauromacrogol, as a sclerosant, is safe even when injected into veins to treat esophageal variceal bleeding, we speculated that it would not cause severe AEs if injected into cysts. This agent can alter the surface tension around endothelial cells, causing vascular injury.[11] Abdominal pain is considered the most common complication after ethanol injection.[6,26] Unlike ethanol, lauromacrogol has a mild anesthetic effect that may reduce postoperative pain. In the present study, no patients complained of moderate or severe abdominal pain after therapy, although three patients experienced postoperative AEs without any need for surgical intervention. Acute pancreatitis was the second most common AE associated with ablation following abdominal pain and probably resulted from extravasation of the ablative agent from the cyst into either the parenchyma or pancreatic duct.[6] The 22G needle appeared safer than the 19Gg needle. However, the 19G needle performed better when used for aspiration than the 22G needle when the cystic fluid was viscous and can act as a tunnel for EUS-TNNB and SpyGlass. Needle choice should depend on the location, size, and fluid characteristics of the cyst.
The CR rate in the present study (47.3%), with a mean follow-up of 15 months, was higher than that observed in our previous study (37.9%) depending on the volume change 3 months after ablation.[11] The 3-month follow-up period did not appear sufficient for the ablative agent to achieve the best effect. Oh et al.[17] reported that cyst resolution required follow-up for at least 12 months because CR was achieved between 6 and 12 months after ablation in 57.1% of the patients. A study by Park et al. revealed that the majority of PCLs with CR or PR responded to the treatment within the first 6 months of therapy.[9] The CR rate in 35 patients followed for at least 12 months was similar to that of the full group of 55 patients who were followed up (51.4% vs. 47.3%, P = 0.926), indicating that the effectiveness of this treatment is stable. The present treatment response using lauromacrogol is higher than that associated with ablation using ethanol alone and similar to that observed in response to ethanol with paclitaxel injection.[9] However, the response evaluation criteria for PCNs have not yet been agreed upon. Determining which of the following evaluation criteria are optimal is difficult: volume change,[1,10,11,14,17,25,28,29] superficial area change,[6,15] or diameter change.[8,9]
Fourteen patients had PeC during the follow-up. Two patients shown to have cysts <10.0 mm in diameter were not recommended to underwent re-ablation because performing EUS-LA in cysts with a diameter <1 cm is challenging and dangerous. Six patients insisted on imaging surveillance and refused to undergo re-ablation or surgical resection. Five patients underwent a second ablation, one of whom had a cyst volume decreased to 61.14% of that before the second ablation, but the diagnosis remained PeC. The other four patients were lost to follow-up or had not yet reached the recommended follow-up interval after the second ablation. Among 14 cysts, the volume of only one cyst increased during the follow-up. This patient was pregnant 1 year after ablation; therefore, she refused to undergo any additional therapy except imaging surveillance. We speculated that pregnancy may have had an effect on the progression of the cyst.
Cyst volume after the second ablation decreased in six patients (but remained the same in one patient) based on imaging follow-up after the second ablation. No cyst was demonstrated to have increased in volume. We speculated that each cyst had different sensitivities to the ablative agent. Some cysts may not achieve CR after one ablative treatment; however, several sessions of ablation may result in CR. Multiple ethanol lavage sessions may result in a greater decrease in the size and surface area of pancreatic cysts.[17] Unfortunately, the upper limit of the number of ablation sessions remains unknown, and the circumstances under which patients should be recommended for surgical resection also remain unknown. Re-ablation appeared to decrease the volume of the cyst, even those that were demonstrated to be PC after the first ablation.
We compared the patients in the resolved group with those in the unresolved group, and no predictive factors for CR were found, in accordance with the results of DeWitt et al.[6] Park et al.[9] reported a study with the longest follow-up and the largest number of patients and showed that there were no significant differences between the resolved group and the unresolved group in terms of age, sex, location, and locularity. They demonstrated that PCNs of smaller size may easily achieve CR, consistent with several previous studies.[17,21] The ethanol concentration used in these three studies was 99%; however, 80% ethanol was used in the study by DeWitt et al.[6] This may be the reason underlying the nonconformity. Park et al.[9] also demonstrated that patients with IPMNs were less likely to achieve CR than those with other types, although this result was refuted by other researchers.[17,25,28] The results of Gan et al. demonstrated that the PeCs tended to be septated;[8] however, DeWitt et al.[6] reported that septation did not affect the treatment response. Choi et al.[25] found that a unilocular form was a predictor of CR, which was inconsistent with other studies.[9,22,28] Notably, the small sample in each group raises questions about our results regarding the predictive factors.
There are techniques that facilitate a higher CR rate. First, the maximum possible volume of cyst fluid should be aspirated to ensure the complete replacement of the cyst fluid with lauromacrogol. However, a small amount of fluid should be left around the tip of the needle within the cyst to prevent the possibility of pancreatic wall injury.[12] Second, septa should be torn between cysts such that lauromacrogol can be injected into every daughter cyst in multilocular lesions. Third, enough lauromacrogol should be injected to enable the cystic wall to be completely soaked in the solution.
This study had several limitations. First, the optimum concentration, injection volume, and remaining volume of lauromacrogol all remain uncertain. The injection was stopped while the cystic wall was completely soaked in solution in our study. However, we do not know whether a better treatment response would occur if more lauromacrogol were injected. The remaining volume was also determined based on the operators’ experience. Second, we did not use pancreatic cyst fluid DNA analysis to evaluate changes in cystic DNA. Postablation cyst fluid analysis has shown that new mutations were noted in 15.8% of cysts receiving ethanol ablation.[14] Whether lauromacrogol will eliminate all baseline mutations and/or new mutations or present no changes without a baseline mutation remains unknown. Furthermore, patients who achieve CR are more likely to be lost to follow-up than patients achieving PR or PC, which may lead to underestimation of the effectiveness of our therapy. Finally, we lacked control groups with which to compare the treatment outcomes between ethanol ablation and RFA. We are currently performing another study, in which lauromacrogol and RFA combined with lauromacrogol ablation are being compared.[30]
CONCLUSIONS
EUS-LA is effective and achieves a 47.3% rate of CR for the treatment of PCNs. The treatment is safe, with an AE rate of 3.6%. The long-term outcome of EUS-LA is stable with a CR rate of 51.4%.
Financial support and sponsorship
This study was supported by research grants from two National Key R&D Programs of China (2016YFC1303601 and 2020YFC2002705).
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank their colleagues in the Department of Gastroenterology and Hepatology of Chinese PLA General Hospital as well as all patients and participants involved in the data collection.
REFERENCES
- 1.Oh HC, Seo DW, Lee TY, et al. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636–42. doi: 10.1016/j.gie.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 2.DeWitt J. Endoscopic ultrasound-guided pancreatic cyst ablation. Gastrointest Endosc Clin N Am. 2012;22:291. doi: 10.1016/j.giec.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Goh BK, Tan YM, Cheow PC, et al. Cystic lesions of the pancreas: An appraisal of an aggressive resectional policy adopted at a single institution during 15 years. Am J Surg. 2006;192:148–54. doi: 10.1016/j.amjsurg.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Galanis C, Zamani A, Cameron JL, et al. Resected serous cystic neoplasms of the pancreas: A review of 158 patients with recommendations for treatment. J Gastrointest Surg. 2007;11:820–6. doi: 10.1007/s11605-007-0157-4. [DOI] [PubMed] [Google Scholar]
- 5.Kiely JM, Nakeeb A, Komorowski RA, et al. Cystic pancreatic neoplasms: Enucleate or resect? J Gastrointest Surg. 2003;7:890–7. doi: 10.1007/s11605-003-0035-7. [DOI] [PubMed] [Google Scholar]
- 6.DeWitt J, McGreevy K, Schmidt CM, et al. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: A randomized, double-blind study. Gastrointest Endosc. 2009;70:710–23. doi: 10.1016/j.gie.2009.03.1173. [DOI] [PubMed] [Google Scholar]
- 7.Nemeş R, Curcă T, Paraliov T, et al. Cystic tumors of the pancreas. Considerations upon 34 operated cases. Rom J Gastroenterol. 2002;11:303–8. [PubMed] [Google Scholar]
- 8.Gan SI, Thompson CC, Lauwers GY, et al. Ethanol lavage of pancreatic cystic lesions: Initial pilot study. Gastrointest Endosc. 2005;61:746–52. doi: 10.1016/s0016-5107(05)00320-2. [DOI] [PubMed] [Google Scholar]
- 9.Park JK, Song BJ, Ryu JK, et al. Clinical outcomes of endoscopic ultrasonography-guided pancreatic cyst ablation. Pancreas. 2016;45:889–94. doi: 10.1097/MPA.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 10.Oh HC, Seo DW, Kim SC, et al. Septated cystic tumors of the pancreas: Is it possible to treat them by endoscopic ultrasonography-guided intervention? Scand J Gastroenterol. 2009;44:242–7. doi: 10.1080/00365520802495537. [DOI] [PubMed] [Google Scholar]
- 11.Linghu E, Du C, Chai N, et al. A prospective study on the safety and effectiveness of using lauromacrogol for ablation of pancreatic cystic neoplasms with the aid of EUS. Gastrointest Endosc. 2017;86:872–80. doi: 10.1016/j.gie.2017.03.1525. [DOI] [PubMed] [Google Scholar]
- 12.Teoh AY, Seo DW, Brugge W, et al. Position statement on EUS-guided ablation of pancreatic cystic neoplasms from an international expert panel. Endosc Int Open. 2019;7:E1064–77. doi: 10.1055/a-0959-5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeWitt J, DiMaio CJ, Brugge WR. Long-term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest Endosc. 2010;72:862–6. doi: 10.1016/j.gie.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 14.DeWitt JM, Al-Haddad M, Sherman S, et al. Alterations in cyst fluid genetics following endoscopic ultrasound-guided pancreatic cyst ablation with ethanol and paclitaxel. Endoscopy. 2014;46:457–64. doi: 10.1055/s-0034-1365496. [DOI] [PubMed] [Google Scholar]
- 15.DiMaio CJ, DeWitt JM, Brugge WR. Ablation of pancreatic cystic lesions: The use of multiple endoscopic ultrasound-guided ethanol lavage sessions. Pancreas. 2011;40:664–8. doi: 10.1097/MPA.0b013e3182128d06. [DOI] [PubMed] [Google Scholar]
- 16.Chai N, Feng J, Guo Y, et al. Preliminary study of single-operator cholangioscopy for diagnosing pancreatic cystic lesions. Gastrointest Endosc. 2017;86:208–18. doi: 10.1016/j.gie.2017.01.038. [DOI] [PubMed] [Google Scholar]
- 17.Oh HC, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172–9. doi: 10.1053/j.gastro.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Pai M, Habib N, Senturk H, et al. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52–9. doi: 10.4240/wjgs.v7.i4.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moris M, Atar M, Kadayifci A, et al. Thermal ablation of pancreatic cyst with a prototype endoscopic ultrasound capable radiofrequency needle device: A pilot feasibility study. Endosc Ultrasound. 2017;6:123–30. doi: 10.4103/eus.eus_6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh HC, Brugge WR. EUS-guided pancreatic cyst ablation: A critical review (with video) Gastrointest Endosc. 2013;77:526–33. doi: 10.1016/j.gie.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 21.Caillol F, Poincloux L, Bories E, et al. Ethanol lavage of 14 mucinous cysts of the pancreas: A retrospective study in two tertiary centers. Endosc Ultrasound. 2012;1:48–52. doi: 10.7178/eus.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez V, Takahashi N, Levy MJ, et al. EUS-guided ethanol lavage does not reliably ablate pancreatic cystic neoplasms (with video) Gastrointest Endosc. 2016;83:914–20. doi: 10.1016/j.gie.2015.08.069. [DOI] [PubMed] [Google Scholar]
- 23.Kim KH, McGreevy K, La Fortune K, et al. Sonographic and cyst fluid cytologic changes after EUS-guided pancreatic cyst ablation. Gastrointest Endosc. 2017;85:1233–42. doi: 10.1016/j.gie.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 24.Oh HC, Seo DW, Kim SH, et al. Systemic effect of endoscopic ultrasonography-guided pancreatic cyst ablation with ethanol and paclitaxel. Dig Dis Sci. 2014;59:1573–7. doi: 10.1007/s10620-014-3037-2. [DOI] [PubMed] [Google Scholar]
- 25.Choi JH, Seo DW, Song TJ, et al. Long-term outcomes after endoscopic ultrasound-guided ablation of pancreatic cysts. Endoscopy. 2017;49:866–73. doi: 10.1055/s-0043-110030. [DOI] [PubMed] [Google Scholar]
- 26.Kandula M, Moole H, Cashman M, et al. Success of endoscopic ultrasound-guided ethanol ablation of pancreatic cysts: A meta-analysis and systematic review. Indian J Gastroenterol. 2015;34:193–9. doi: 10.1007/s12664-015-0575-2. [DOI] [PubMed] [Google Scholar]
- 27.Vazquez-Sequeiros E, Maluf-Filho F. Endosonography-guided ablation of pancreatic cystic tumors: Is it justified? Gastrointest Endosc. 2016;83:921–3. doi: 10.1016/j.gie.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 28.Moyer MT, Sharzehi S, Mathew A, et al. The safety and efficacy of an alcohol-free pancreatic cyst ablation protocol. Gastroenterology. 2017;153:1295–303. doi: 10.1053/j.gastro.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Moyer MT, Dye CE, Sharzehi S, et al. Is alcohol required for effective pancreatic cyst ablation? The prospective randomized CHARM trial pilot study. Endosc Int Open. 2016;4:E603–7. doi: 10.1055/s-0042-105431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng X, Linghu E, Chai N, et al. New treatment of the pancreatic cystic neoplasm: Endoscopic ultrasonography-guided radiofrequency ablation combined with lauromacrogol ablation. Turk J Gastroenterol. 2018;29:101–4. doi: 10.5152/tjg.2017.17340. [DOI] [PMC free article] [PubMed] [Google Scholar]