Abstract
EUS-guided biliary drainage (EUS-BD) and percutaneous transhepatic cholangiography biliary drainage (PTC) are the two alternate methods for biliary decompression in cases where ERCP fails. We conducted a systematic review and meta-analysis of studies to compare the efficacy and safety of endoscopic and percutaneous biliary drainage for malignant biliary obstruction in patients with failed ERCP. A total of ten studies were included, fulfilling the inclusion criteria, including four retrospective studies and six randomized controlled trials. We compared the technical and clinical success rates and the acute, delayed, and total adverse events of EUS-BD with PTC. The odds ratios (ORs) and confidence intervals (CIs) were calculated. There was no difference between technical (OR: 0.47 [95% CI: 0.20–1.07]; P = 0.27) and clinical (OR: 2.24 [95% CI: 1.10–4.55]; P = 0.51) success rates between EUS-PD and PTC groups. Procedural adverse events (OR: 0.17 [95% CI: 0.09–0.31]; P = 0.03) and total adverse events (OR: 0.09 [95% CI: 0.02–0.38]; P < 0.01) were significantly different between the two groups; however, delayed adverse events were nonsignificantly different (OR: 0.73 [95% CI: 0.34–1.57]; P = 0.97). This meta-analysis indicates that endoscopic biliary drainage (EUS-BD) is equally effective but safer in terms of acute and total adverse events than percutaneous transhepatic biliary drainage (PTC) for biliary decompression in patients with malignant biliary strictures who have failed an ERCP.
Keywords: confidence intervals, endoscopic retrograde cholangiopancreatography, EUS-guided biliary drainage, malignant biliary strictures, malignant obstructive jaundice, meta-analysis, odds ratios, percutaneous transhepatic cholangiography
INTRODUCTION
Obstructive jaundice due to malignant biliary strictures is usually secondary to pancreatic cancer, ampullary cancer, or cholangiocarcinoma.[1] Obstructive jaundice can lead to adverse events such as delayed tumor treatment, acute cholangitis, poor quality of life, and even death if not handled promptly. Successful biliary drainage in patients with malignant obstructive jaundice can significantly reduce these complications and improve overall prognosis.[2] ERCP is the most common biliary drainage method for palliation in patients with malignant biliary obstruction.[3] However, biliary decompression by ERCP can fail in 5%–10% of cases due to altered anatomy or concomitant bowel obstruction which prevents endoscopic access to the major papilla.[4,5] Percutaneous biliary drainage (PTC) and EUS-guided biliary drainage (EUS-BD) are the two generally applied alternative biliary drainage methods utilized if ERCP fails.[6] Percutaneous transhepatic biliary drainage (PTBC) is associated with adverse events in 20%–77% of cases, which include repeat intervention, recurrent infection, or spontaneous fistula formation.[7,8] It also requires the placement of long-term external catheter drainage, leading to longer recovery time and poor quality of life.[9] EUS-BD is considered a less invasive alternative approach following unsuccessful biliary cannulation. It allows visualization and access to the biliary tree by echoendoscopy and fluoroscopy.[3,10] The relatively low procedure cost along with the improved patient comfort and early recovery with fewer procedure-related adverse events are considered the perceived benefits of EUS-BD after failed ERCP.[11] However, differing opinions exist among the interventional radiologists and gastroenterologists about the efficacy and adverse event profile of both procedures.[12,13] For example, some studies have demonstrated that PTC has a similar or even better therapeutic success rate but a similar overall complications rate than EUS-BD for the management of malignant biliary tract obstruction.[12,13] It is extremely important to decide which procedure should be considered for those patients who have failed an initial ERCP approach for malignant biliary drainage. To date, only a few studies have been published that have compared the success rates and adverse events of EUS-BD and PTC after a failed ERCP. Therefore, we conducted a meta-analysis and sought to determine the efficacy and safety of PTC and EUS-BD in patients with malignant biliary obstruction who had a failed ERCP.[10,11,14,15,16,17,18,19,20,21]
MATERIALS AND METHODS
Literature search
The study methodology was designed and executed to adhere to the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) guidelines. A comprehensive search was conducted using the databases MEDLINE and EMBASE via Ovid, Cochrane Library via Wiley, LILACS, CINAHL via EBSCO, and Scopus for prospective or retrospective studies of biliary drainage by EUS-BD or percutaneous transhepatic biliary drainage (PTBD) in patients with malignant biliary obstruction from January 1, 2010, to July 1, 2019. Under MECIR guidelines, a combination of natural language and controlled vocabulary was employed to describe EUS-BD, PTC, and obstructive jaundice concepts. No limits were placed on the language of publication or study design within the initial search process itself. We only included studies that were published in English and which compared the effectiveness and adverse events of EUS-BD and PTC for malignant biliary obstruction after failed initial ERCP. To ensure that no potentially relevant items were overlooked, manual searching of reference lists of the included studies was also undertaken.
The search index terms used were (a) “cholangiocarcinoma” OR “pancreatic neoplasms” OR “malignant biliary strictures” and “malignant biliary obstruction,” (b) “biliary drainage” OR “percutaneous transhepatic biliary drainage (PTC)” OR “endoscopic retrograde cholangiopancreatography (ERCP)” along with (c) “adverse events” OR “complications” of PTBD and EUS-BD, and (d) “therapeutics” and “mortality” (subheading) related to both procedures. Moreover, we also identified additional articles by cross-checking the reference list of the already retrieved studies.
Study selection
The selection criteria used for included studies were (a) patients older than 18 years, (b) suspected malignant biliary stricture, and (c) reported procedural success for relief of obstructive jaundice after ERCP failure and procedure-related adverse events. The exclusion criteria used were (a) studies with population younger than 18 years, pregnant patients, unfit for either strategy, or lacking informed consent; (b) animal experiments, case reports, and studies that do not report original data, including reviews, editorials, or opinions; (c) incomplete literature data or information, incorporation of study definitions, or unclear descriptions of outcomes or adverse events; and (d) patients with benign distal biliary strictures. Some articles reported duplicate data sets, and we used one with the detailed reported datasets. All study titles and abstracts were independently reviewed by two authors to identify potentially eligible studies based on the abovementioned selection criteria. A full consensus was reached on the included studies, and any possible divergence was resolved by discussion with the senior author AS.
Data extraction
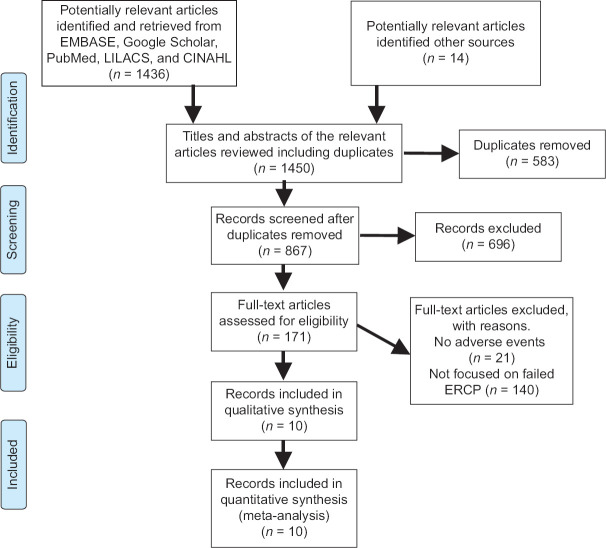
Title and abstract screenings were independently completed by two screeners who applied the previously defined inclusion and exclusion criteria. Conflicts were resolved through discussion or, where consensus could not be reached, by a discussion with the senior author, who was an expert in these procedures. Following title and abstract screening, full-text screening was undertaken. It was completed by two independent authors who resolved conflicts through discussion or, if necessary, by the senior author. Reasons for exclusion were recorded and reported in a PRISMA flowchart in accordance with best practices [Figure 1]. The data extraction was conducted independently by two authors, who then performed a risk of bias (quality) assessment using validated tools. Any disagreements were resolved by discussion between the authors. Data extraction forms were developed by one author and piloted by both for further revisions. Items that were found to be at a high risk of bias were excluded from further analysis or their results were qualified based on the type and extent of the potential bias.
Figure 1.
Flowchart of the studies included
The protocol of this meta-analysis and systemic review was registered in PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php) with an assigned ID: CRD42019141450. The primary outcome of this study was to compare the effectiveness of EUS-BD and PTC for the relief of distal malignant obstructive jaundice. Furthermore, the secondary outcomes were the technical success, adverse events of EUS-BD, and PTC procedures. Moreover, other outcome measures included were sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and area under the curve. Although, the included articles were missing the direct information and data for these outcome measures (sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and area under the curve). However, the necessary information about these outcomes (sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and area under the curve) could be extrapolated from the other outcomes included in the study such as the technical and clinical success rates along with the acute and delayed adverse event profile of the procedures.
Data points of interest included were trial characteristics, such as study design, duration of follow-up, study settings, number of clinical sites, primary outcomes in studies such as technical and clinical success rates along with acute and delayed adverse events of EUS-BD and PTC, and inclusion period. Information regarding participants was also extracted, including population characteristics, number of participants in EUS-BD or PTC groups, number of participants withdrawn or lost to follow-up, and number with missing outcome data, similarity of groups at baseline, and assessment of compliance. Moreover, the information regarding the etiology of bile duct obstruction and reason for failed ERCP were also collected.
The data were systemically collected from the absolute numbers that were directly provided or inferred through the information reported in manuscript or abstract selected. The meta-analysis only included studies that provided all the information necessary for at least one kind of analysis.
Quality assessment
The Newcastle–Ottawa Quality Assessment Scale was used to assess the quality of the cohort studies. Studies with a score of 5 or more out of 8 items on study selection, comparability, and outcome were interpreted as high quality and were included in the meta-analysis.[22] Similarly, the quality of the randomized controlled trials was assessed by using the Modified Jadad Score.[23] This scale includes 7 items on study population randomization, allocation concealment, population blinding, dropouts, and withdrawals. Those studies meeting the score criteria of 4 or more points were interpreted as high-quality RTCs and were included in the study. Quality assessment was done by three independent authors and all the articles were included after discussion to reach consensus [Table 1].
Table 1.
Main characteristics of the studies included in the meta-analysis
| Studies | Bories et al.[14] | Bapaye et al.[15] | Lu et al.[16] | Artifon et al.[17] | Bill et al.[18] |
|---|---|---|---|---|---|
| Study type | A multileft RCT (France) | A single-left retrospective cohort study (India) | Single-left comparative study (China) | Single-left prospective RCT (Brazil) | Single-left retrospective comparative study (USA) |
| Mean age of population (years)±SD | 62.5±8.5 versus58.8±9.95 | 59.9±13.3 versus 62.4±10.2 | 68±13.5 | 63.4±10 versus 71 | 66.5±12.6 |
| Male: female population ratio | NA | 1.08 versus 1.6 | NA | 2.25 versus2 | 1.2 versus 1.8 |
| Total preprocedure bilirubin (mean), mg/dl | NA | 7.11±7.6 versus 9.41±12.4 | NA | 16.4 versus 17.2 | 10.85 versus 13.24 |
| Mean bile duct diameter | NA | NA | NA | 13.7 versus 11.9 | NA |
| Bile duct obstruction etiology | |||||
| Ampullary adenocarcinoma | NA | 5 versus 3 | NA | 1 versus 0 | 3 versus 3 |
| Pancreatic carcinoma | NA | 15 versus 18 | NA | 10 versus 6 | 20 versus 20 |
| Cholangiocarcinoma | NA | 2 versus 2 | NA | 1 versus 1 | 2 versus 2 |
| Gallbladder cancer | NA | 0 | NA | 0 | 0 |
| Plasmacytoma | NA | 0 | NA | 1 versus 0 | 0 |
| Advanced lymphoma/liposarcoma | NA | 0 | NA | 0 versus 1 | 0 |
| Duodenal carcinoma | NA | 0 | NA | 0 | 0 |
| Gastric cancer | NA | 0 | NA | 0 versus 1 | 0 |
| Metastatic cancer | NA | 0 | NA | 0 versus 3 | 1 versus 5 |
| Reason for failed ERCP | |||||
| Altered anatomy | NA | 9 | NA | 1 | NA |
| Inability of cannulation | NA | 42 | NA | 16 | 16 versus 9 |
| Indwelling duodenal stent | NA | 16 | NA | 0 | NA |
| Stomach/duodenal invasion | NA | 32 | NA | 8 | 18 versus 7 |
| NOS/MJS score | 6 | 7 | 7 | 7 | 7 |
|
| |||||
| Studies | Khashab et al.[19] | Giovannini et al.[10] | Lee et al.[20] | Huang et al.[21] | Sharaiha et al.[11] |
|
| |||||
| Study type | Single-left retrospective cohort comparative study (USA) | Multileft randomized controlled phase II trial (France) | Multileft prospective randomized controlled phase II trial (South Korea) | Single-left retrospective comparative study (USA) | A single-left retrospective cohort study (USA) |
| Mean age of population (years)±SD | 64.9±12.5 versus66.9±12.5 | NA | 66.5 versus 68.4 | 68.9±4.62 versus 64±6.86 | 68.7±13.9 versus 58.8±13.6 |
| Male: female population ratio | 1.2 versus 1.31 | 0.91 versus 9 | 3.25 versus 3 | 2.27 versus 2 | 12 versus 1.47 |
| Total pre- and postprocedure bilirubin (mean), mg/dl | 15.8±11.3 versus 14.5±8.8 | NA | 10.4 versus 11.8 | 338.54±167.73 versus 142.43±65.64 | NA |
| Mean bile duct diameter | NA | NA | 11.22 versus 12.6 | NA | |
| Bile duct obstruction etiology | |||||
| Ampullary adenocarcinoma | 3 | NA | 1 versus 0 | 4 versus 2 | 3 |
| Pancreatic carcinoma | 43 | NA | 12 versus 12 | 10 versus 8 | 22 |
| cholangiocarcinoma | 12 | NA | 7 versus 14 | 22 versus 20 | 9 |
| Gallbladder cancer | 0 | NA | 5 versus 5 | NA | 0 |
| Plasmacytoma | 0 | NA | 0 | NA | 0 |
| Advanced lymphoma/liposarcoma | 1 | NA | 0 | NA | 0 |
| Duodenal carcinoma | 1 | NA | 3 versus 0 | NA | 5 |
| Gastric cancer | 1 | NA | 3 versus 2 | NA | 4 |
| Metastatic cancer | 12 | NA | 3 versus 1 | NA | 7 |
| Reason for failed ERCP | |||||
| Altered anatomy | 0 | NA | 12 versus. 10 | NA | NA |
| Inability of cannulation | 0 | NA | 0 | NA | NA |
| Indwelling duodenal stent | 0 | NA | 0 | NA | NA |
| Stomach/duodenal invasion | 0 | NA | 22 versus 22 | 2 versus 4 | NA |
| NOS/MJS score | 8 | 7 | 6 | 7 | 8 |
SD: Standard deviation; NOS: Newcastle–Ottawa Quality Assessment Scale; MJS: Modified Jadad Score; RCT: Randomized controlled trial; NA: Not applicable
The level of the quality of the evidence based on the Grading of Recommendations Assessment, Development and Evaluation Approach
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was employed to evaluate the level of the quality of the evidence and recommendation strength regarding main outcomes in this meta-analysis. Furthermore, the quality of the confidence of each outcome estimate was marked based on GRADE quality assessment results from all five domains (study limitations, inconsistency, indirectness, imprecision, and possible publication bias) [Table 2]. This procedure was implemented by using GRADEpro GDT software by two authors. Any disagreements were settled by discussion with the senior author AS.
Table 2.
Assessment of quality of evidence of outcomes EUS-biliary drainage versus percutaneous transhepatic cholangiography
| Certainty assessment | Number of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Intervention (%) | Comparison (%) | Relative (95% CI) | Absolute (95% CI) | ||
| Technical success rate | ||||||||||||
| 10 | Randomized trials and retrospective comparative studies | Seriousa | Not serious | Not serious | Not serious | None | 230/289 (79.6) | 270/284 (95.1) | OR: 0.47 (0.20–1.07) | 50 fewer per 1000 (from 157 fewer to 3 more) | ⨁⨁⨁◯ (moderate) | Critical |
| Clinical success rate | ||||||||||||
| 10 | Randomized trials and retrospective comparative studies | Seriousa | Not serious | Not serious | Not serious | None | 250/278 (89.9) | 248/280 (88.6) | OR: 0.73 (0.34–1.57) | 36 fewer per 1,000 (from 161 fewer to 38 more) | ⨁⨁⨁◯ (moderate) | Critical |
| Acute adverse events | ||||||||||||
| 10 | Randomized trials and retrospective comparative studies | Seriousa | Not serious | Not serious | Seriousb | None | 45/288 (15.6) | 140/285 (49.1) | OR: 0.17 (0.09–0.31) | 350 fewer per 1000 (from 411 fewer to 261 fewer) | ⨁⨁◯◯ (low) | Important |
| Chronic or delayed adverse events | ||||||||||||
| 10 | Randomized trials and retrospective comparative studies | Seriousa | Not serious | Not serious | Not seriousb | None | 12/288 (4.2) | 14/285 (4.9) | OR: 0.73 (0.34–1.57) | 13 fewer per 1000 (from 32 fewer to 26 more) | ⨁⨁⨁◯ (moderate) | Critical |
| Total adverse events | ||||||||||||
| 10 | Randomized trials and retrospective comparative studies | Seriousa | Not serious | Not serious | Seriousb | None | 54/288 (18.8) | 219/285 (76.8) | OR: 0.05 (0.01–0.20) | 626 fewer per 1000 (from 736 fewer to 370 fewer) | ⨁⨁◯◯ (low) | Important |
a The meta-analysis has RCTs and comparative studies, so there is a possible selection bias in comparative studies, b The included studies have few patients and thus have very few reported events. CI: Confidence interval; OR: Odds ratio; RCTs: Randomized controlled trials
Data analysis
Odds ratios (ORs) with confidence intervals (CIs) were calculated for all the outcomes. The Mantel–Haenszel test for random effects was used for the analysis because of the low heterogeneity from the fewer studies included. P < 0.05 was considered statistically significant for pooled ORs. Technical success of the PTC was defined as the successful catheter placement in the biliary tree. Technical success for EUS-BD was defined as successful stent placement in the biliary tract. Clinical success was defined as biochemical resolution of the obstructive jaundice with no immediate need for procedural re-intervention. Bleeding, subcapsular hematoma, hemobilia, perihepatic bile collection/biloma, recurrent abdominal pain, cholangitis, pancreatitis, pneumoperitoneum, sepsis/infection of drain site, perihepatic abscess, sheared guide wire, hepatic abscess, peritonitis/bile leak, tube malposition, venous fistula, and external biliary fistula were considered the acute procedural adverse events. Chronic or delayed adverse events included recurrent biliary obstruction, cholangitis, sepsis or bacteremia, and cholecystitis after the procedure. Moreover, the re-intervention rates of the procedures were also compared. Heterogeneity among studies was measured using Cochran's Q test and I2 statistics. Cochran's Q measures the heterogeneity between the studies. It is calculated as the weighted sum-of-squared differences between the individual study effects and the estimated pooled effect across all the included studies. At the same time, the I2 statistic describes the percentage of the variance between the included studies due to heterogeneity. A reference I2 value of 0%–25% shows that the heterogeneity is insignificant between studies. A value of 25%–50% represents low heterogeneity, and between 50% and 70% represents moderate. Any value above 75% mirrors a high heterogeneity between studies. All statistical analyses were done using Review Manager 4.0.
RESULTS
A total of 1450 articles were identified from the literature. After title and abstract screening, 171 articles were encompassed in the full text-read category. The articles removed included those that did not meet the inclusion and exclusion criteria, letters to the editor, case reports, and review articles. Furthermore, the studies without the outcomes of interest were also removed, leaving ten study articles that were incorporated in the meta-analysis. The manual review of the included studies’ references was also done, which did not yield additional studies.
Among the ten selected articles, six were randomized controlled trials, and four were retrospective studies. The total study population was comprised of 567 patients in the EUS-BD group and 564 patients in the PTC group. The mean age of the study population was 60 years ± 10. Four studies were published in the USA, two were in France, one in Korea, one in China, and one study in Brazil.[10,11,14,15,16,17,18,19,20,21] The main characteristics of the studies are depicted in Table 1.
Comparison of the technical and clinical success rates of EUS-guided biliary drainage and percutaneous transhepatic cholangiography
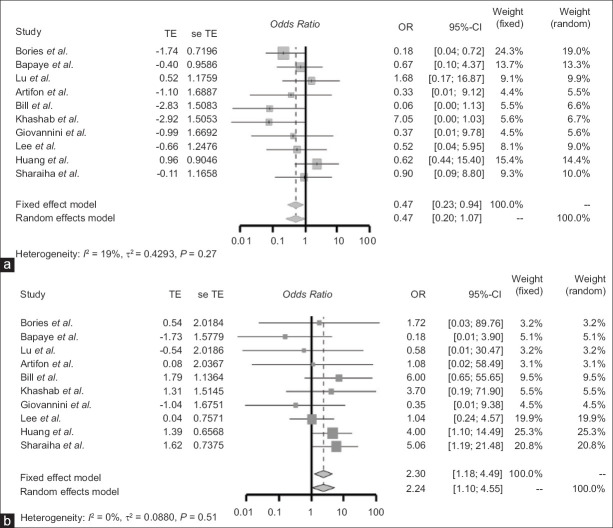
The technical and clinical success rates between the EUS-BD (n = 567) and PTC (n = 564) groups are shown in Table 3. The technical success rate for EUS-BD was 86.2%, and the technical success rate for PTC was 95% [Table 4]. There was no significant difference between both groups’ technical success rates (OR: 0.47 [95% CI: 0.20–1.07]; P = 0.27). Similarly, the clinical success rate for EUS-BD was 90%, and the clinical success rate for PTC was 88.6% [Table 4]. The clinical success rates were also not significantly different between the EUS-BD and PTC groups (OR: 2.24 [95% CI: 1.10–4.55]; P = 0.51) [Figure 2].
Table 3.
Technical and clinical success rates of the studies
| Study/subgroup | Technical success (events/total) | Clinical success (events/total) | ||
|---|---|---|---|---|
|
|
|
|||
| EUS-BD | PTC | EUS-BD | PTC | |
| Bories et al. | 16/33 | 16/19 | 33/33 | 19/19 |
| Bapaye et al. | 23/26 | 23/25 | 23/25 | 26/26 |
| Lu et al. | 32/33 | 57/60 | 33/33 | 57/57 |
| Artifon et al. | 12/13 | 12/12 | 13/13 | 12/12 |
| Bill et al. | 19/25 | 25/25 | 24/25 | 20/25 |
| Khashab et al. | 19/22 | 51/51 | 19/19 | 47/51 |
| Giovannini et al. | 19/20 | 17/17 | 18/19 | 17/17 |
| Lee et al. | 32/34 | 31/32 | 28/32 | 27/31 |
| Huang et al. | 34/36 | 26/30 | 32/36 | 20/30 |
| Sharaiha et al. | 43/47 | 12/13 | 27/43 | 3/12 |
PTC: Percutaneous transhepatic cholangiography; EUS-BD: EUS-biliary drainage
Table 4.
Safety and efficacy rates of outcomes of both procedures
| Events | EUS-BD versus PTC (%) | OR with 95% CI | P |
|---|---|---|---|
| Technical success rate | 86.2 versus95 | 0.47 (0.20–1.07) | 0.27 |
| Clinical success rate | 90 versus 88.6 | 2.24 (1.10–4.55) | 0.51 |
| Acute adverse events | 7.8 versus 24.8 | 0.17 (0.09–0.31) | 0.03 |
| Chronic or delayed adverse events | 2.1 versus2.5 | 0.73 (0.34–1.57) | 0.97 |
| Total adverse events | 10 versus27.3 | 0.09 (0.02–0.38) | 0.01 |
| Death rate | 1.4 versus 1.4 | 0.99 (0.37–0.266) | 0.99 |
| Re-intervention rate | 3.7 versus 13.8 | 0.99 (0.16–0.45) | 0.01 |
OR: Odds ratio; CI: Confidence interval; PTC: Percutaneous transhepatic cholangiography; EUS-BD: EUS-biliary drainage
Figure 2.
(a) Forest plot of the odds ratio of studies comparing the technical success rates of EUS-BD and PTC. (b) Forest plot of odds ratio of studies comparing the clinical success rates of EUS-BD and PTC. TE: Treatment effect; seTE: Standard error of treatment effect; OR: Odds ratio; CI: Confidence interval; PTC: Percutaneous transhepatic cholangiography; EUS-BD: EUS-biliary drainage
Comparison of the procedural adverse event rate of EUS-guided biliary drainage and percutaneous transhepatic cholangiography
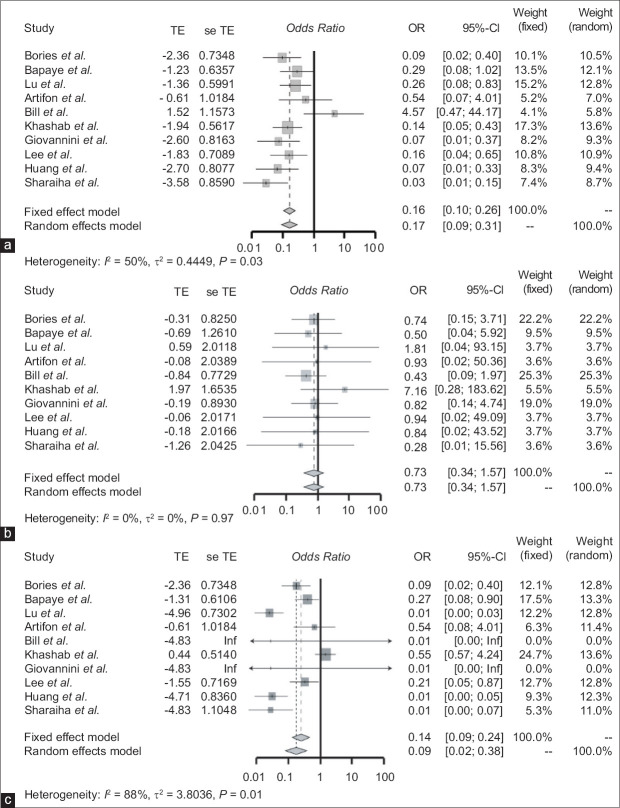
The acute procedural adverse event rate for the EUS-BD group was 7.8% and for the PTC group was 24.8% [Table 4]. The EUS-BD group had significantly fewer acute procedural adverse events compared to the PTC group (OR: 0.17 [95% CI: 0.09–0.31]; P = 0.03) [Figure 3]. Similarly, the total adverse event rate for EUS-BD was 10% and for PTC was 27.3%. The total procedural adverse event rate was also significantly lower in the EUS-BD group compared to the PTC group (OR: 0.09 [95% CI: 0.02–0.38]; P < 0.01) [Figure 3]. Finally, the long-term adverse event rate for EUS-BD was 2.1% and for PTC was 2.5%. There was no significant difference between the two groups in terms of delayed adverse events (OR: 0.73 [95% CI: 0.34–1.57]; P = 0.97) [Figure 3].
Figure 3.
(a) Forest plot of the odds ratio of studies comparing the acute adverse event of EUS-BD and PTC. (b) Forest plot of odds ratio of studies comparing the chronic or delayed adverse event of EUS-BD and PTC. (c) Forest plot of odds ratio of studies comparing the total adverse events of EUS-BD and PTC. TE: Treatment effect; seTE: Standard error of treatment effect; OR: Odds ratio; CI: Confidence interval; PTC: Percutaneous transhepatic cholangiography; EUS-BD: EUS-biliary drainage
Comparison of the re-intervention rate of EUS-guided biliary drainage and percutaneous transhepatic cholangiography
The re-intervention rate of the procedures was also reported in six studies. The re-intervention rate for the EUS-BD group was 3.7% compared to 13.8% for the PTC group [Table 4]. The re-intervention rates were significantly lower in the EUS-BD group compared to the PTC group (OR: 0.27 [95% CI: O.16–0.45]; P = 0.001) [Table 5]. The postprocedural death rate was 1.4% for both the groups and was not significantly different between the EUS-BD and PTC groups (OR: 0.99 [95% CI: 0.37–0.266]; P = 0.99) [Table 4].
Table 5.
Adverse events of the studies included EUS-biliary drainage versus percutaneous transhepatic cholangiography
| Study | Bories et al. | Bapaye et al. | Lu et al. | Artifon et al. | Bill et al. |
|---|---|---|---|---|---|
| Acute adverse events | |||||
| Bleeding | 3 versus5 | 0 versus 1 | 2 versus 5 | 1 versus 0 | 2 versus 1 |
| Subcapsular hematoma | – | – | – | – | – |
| Hemobilia | – | – | – | – | – |
| Perihepatic bile collection/biloma | – | – | – | 1 versus 0 | – |
| Recurrent abdominal pain | – | – | – | – | – |
| Cholangitis | 1 versus3 | 0 versus 2 | 2 versus 5 | – | – |
| Pancreatitis | – | – | – | – | 1 versus 0 |
| Pneumoperitoneum | – | – | – | – | – |
| Sepsis/infection of drain site | 5 versus 7 | 1 versus 2 | 0 versus 5 | – | – |
| Perihepatic abscess | – | – | – | – | – |
| Sheared guide wire | – | – | – | – | – |
| Hepatic abscess | – | – | – | 0 versus 2 | – |
| Peritonitis/bile leak | 1 versus1 | 4 versus0 | 0 versus 4 | 0 versus 1 | 1 versus 0 |
| Tube malposition | – | – | 0 versus2 | – | – |
| Venous fistula | – | – | – | – | – |
| External biliary fistula | 1 versus0 | 0 versus 7 | – | – | – |
| Chronic or delayed adverse events | |||||
| Recurrent biliary obstruction | – | – | – | – | 2 versus 5 |
| Deaths | 4 versus3 | 1 versus2 | – | – | – |
| Cholecystitis | – | – | – | – | 1 versus1 |
| Re-intervention/Repeat procedure | – | – | 2 versus17 | – | 4 versus15 |
| Overall cost of procedure (mean±SD) | NA | NA | NA | USD 5673 versus USD 7570 | NA |
|
| |||||
| Study | Khashab et al. | Lee et al. | Huang et al. | Giovannini et al. | Sharaiha et al. |
|
| |||||
| Acute adverse events | |||||
| Bleeding | 0 versus1 | – | 1 versus3 | 1 versus 4 | 2 versus1 |
| Subcapsular hematoma | 0 versus2 | – | 0 versus 2 | – | – |
| Hemobilia | 0 versus 5 | 0 versus1 | – | – | – |
| Perihepatic bile collection/biloma | 0 versus7 | – | – | – | 0 versus 1 |
| Recurrent abdominal pain | – | – | – | – | 0 versus 3 |
| Cholangitis | 0 versus3 | 0 versus5 | 1 versus0 | 1 versus 3 | – |
| Pancreatitis | 1 versus0 | 1 versus0 | – | – | 1 versus0 |
| Pneumoperitoneum | – | – | – | – | – |
| Sepsis/infection of drain site | 5 versus7 | 1 versus2 | 0 versus5 | – | – |
| Perihepatic abscess | 0 versus1 | – | – | – | 0 versus1 |
| Sheared guide wire | 1 versus0 | – | – | – | – |
| Hepatic abscess | – | – | – | 0 versus2 | – |
| Peritonitis/bile leak | 0 versus10 | 1 versus4 | 0 versus2 | 1 versus3 | 1 versus0 |
| Tube malposition | 0 versus2 | – | 0 versus2 | – | 0 versus4 |
| Venous fistula | 0 versus1 | – | – | – | – |
| External biliary fistula | – | – | – | – | – |
| Chronic or delayed adverse events | |||||
| Recurrent biliary obstruction | – | – | – | – | – |
| Deaths | – | – | – | 3 versus3 | – |
| Cholecystitis | 1 versus0 | – | – | – | – |
| Re-intervention/repeat procedure | 3 versus12 | 11 versus29 | – | – | 1 versus5 |
| Overall cost of procedure (mean±SD) | USD 9218±3772 versus USD 18.261±16.021 | NA | USD 15.35±5.51 versus USD 21.42±3.95 | NA | NA |
SD: Standard deviation; NA: Not applicable
The cost analysis was also reported in three studies. The overall cost of the EUS-BD procedure was significantly less compared to the PTC. For instance, Artifon et al. reported that the overall cost for the EUS-BD procedure was USD 5673 and for PTC was USD 7570.[17] Similarly, Khashab et al. reported that the overall cost for EUS-BD and PTC procedures was USD9218 ± 3772 and USD18,261 ± 16,021, respectively.[21]
Publication bias
The Egger regression test was conducted on the summary estimates to evaluate the possibility of any publication bias. P = 0.05 was considered statistically significant to identify publication bias. In the current meta-analysis, there was no evidence of publication bias as the Egger regression test demonstrated P = 0.01.
DISCUSSION
In patients with obstructive jaundice, ERCP is the standard technique to access the biliary tree with a success rate of greater than 90%.[19] Altered or variant anatomy, gastric outlet obstruction, an indwelling duodenal stent, and previous gastric bypass surgery are considered the most common reasons for the failed ERCP.[2,24,25] In such cases, percutaneous biliary drainage (PTC) or surgery has been the standard treatment for biliary drainage.[26,27,28,29,30] However, these procedures have a significantly high rate of adverse events. Long-term PTC procedure therapy is associated with multiple complications such as cholangitis, drain dislocations, and occlusion leading to frequent re-interventions and hospital stay.[9] Moreover, it leads to poorer quality of life, as patients remain with a long-term external drain for the rest of their lives.[31] EUS-BD is a novel technique and has been increasingly used as an alternative procedure to surgery or PTC for malignant biliary drainage in patients with a failed ERCP due to its minimal invasive nature.[3] It is now considered a safe, efficacious option compared to the surgery and radiology.[32,33,34,35,36,37]
While the global experience with EUS-BD has been rapidly growing, there are still limited data comparing its safety and efficacy to PTC. The availability of comparative data is essential to guide physicians about the optimal procedure (EUS-BD or PTC) for achieving biliary decompression in malignant biliary obstruction in patients with failed ERCP. Baniya et al. reported that EUS-BD is a safe and effective procedure and has the same technical and success rate compared to PTC.[38,39] Wang et al. also reported a high technical (94.7%) and clinical success rate of the EUS-BD (91.66%).[40] The current study used a larger number of articles with a relatively homogenous study population comparing the technical rates, clinical success rates, and adverse events of EUS-BD and PTC. This study is the first to weigh the acute and chronic (delayed) adverse events of these procedures in a single meta-analysis to the best of our knowledge.
The current study results demonstrate high technical and clinical success rates for both the procedures. There was no significant difference between the use of EUS-BD or PTC for biliary drainage. However, EUS-BD has significantly lower acute and total adverse event rates compared to PTC. This study shows that the PTC has a higher incidence of bleeding, sepsis/infection of the drain site, and formation of external biliary fistula compared with EUS-BD.[10,14,19,20] Furthermore, the rate of re-intervention after PTC is significantly higher compared to the EUS-BD.[11,16,18,19] However, the current study did not find any difference in the chronic (delayed) adverse events between these two procedures [Figure 3].
EUS-BD has been evolving and is now a feasible alternative to PTC in high-volume centers.[38] EUS-BD has certain advantages. First, it is a less invasive and a more physiologic procedure for biliary drainage. Furthermore, it prevents the need for a long-term external body drain, has a lower re-intervention rate, provides better nutrition absorption from gastrointestinal tract, and avoids excessive body electrolyte loss.[41] Furthermore, with a proper patient consent before ERCP, it can be done in the same endoscopic session if ERCP fails.[42] Our study demonstrated that EUS-BD is a safe and effective procedure for obstructive jaundice due to malignant biliary stricture compared to PTC with a lower adverse event profile. However, EUS-BD is also associated with certain problems. This procedure is technically diverse and complex and requires a great skill set and specialized training to perform.[43] Moreover, it is only feasible when performed by an experienced gastroenterologist at a high-volume medical center with appropriate surgical and interventional radiology backup to handle any acute complications such as bleeding, bile leaks, and pneumoperitoneum.[42,44,45] Furthermore, transmural puncture of the luminal side of the gastrointestinal tract to approach a sterile biliary tree can induce biliary infection.[46] Despite these issues, this procedure is increasingly being utilized as the rescue procedure of choice after ERCP fails due to its high success rate and overall lower adverse event profile demonstrated in recent studies compared to PTC.[47]
This study has several limitations. First, the study included both randomized trials and retrospective cohorts. Although randomized controlled trials produce high-level evidence, all of the six included randomized control trials were only comprised of a small population of patients comparing the EUS-BD with PTC. Therefore, the study included four high-quality retrospective studies with stratified analysis to increase the population size. Second, the study might have publication bias because of the inclusion of the small sample size studies favoring the results of EUS-BD. Third, the included studies lack homogeneity in terms of clinical success rate and acute and chronic (delayed) adverse events of both the procedures.
CONCLUSIONS
EUS-BD is a safer and more effective procedure compared to PTC in patients with malignant biliary obstruction who underwent unsuccessful ERCP. However, standardization of procedural technique, learning curve, and information regarding safety and technical success is needed from the community setting before it can be widely adopted.
Financial support and sponsorship
Nil.
Conflicts of interest
Douglas G. Adler is a Co-Editor-in-Chief of the journal and Ali A. Siddiqui is an Associate Editor. The article was subject to the journal's standard procedures, with peer review handled independently of these editors and their research groups.
REFERENCES
- 1.Tsuyuguchi T, Takada T, Miyazaki M, et al. Stenting and interventional radiology for obstructive jaundice in patients with unresectable biliary tract carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:69–73. doi: 10.1007/s00534-007-1282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurniawan J, Hasan I, Gani RA, et al. Mortality-related factors in patients with malignant obstructive jaundice. Acta Med Indones. 2016;48:282–8. [PubMed] [Google Scholar]
- 3.Khashab MA, Dewitt J. EUS-guided biliary drainage: Is it ready for prime time? Yes! Gastrointest Endosc. 2013;78:102–5. doi: 10.1016/j.gie.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Enochsson L, Swahn F, Arnelo U, et al. Nationwide, population-based data from 11,074 ERCP procedures from the Swedish registry for gallstone surgery and ERCP. Gastrointest Endosc. 2010;72:1175–84e1-3. doi: 10.1016/j.gie.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 5.Giovannini M, Bories E, Napoleon B, et al. 855 multicenter randomized phase II study: Percutaneous biliary drainage vs. EUS guided biliary drainage: Results of the intermediate analysis. Gastrointest Endosc. 2015;81:AB174. [Google Scholar]
- 6.Voegeli DR, Crummy AB, Weese JL. Percutaneous transhepatic cholangiography, drainage, and biopsy in patients with malignant biliary obstruction. An alternative to surgery. Am J Surg. 1985;150:243–7. doi: 10.1016/0002-9610(85)90129-1. [DOI] [PubMed] [Google Scholar]
- 7.Artifon EL, Sakai P, Cunha JE, et al. Surgery or endoscopy for palliation of biliary obstruction due to metastatic pancreatic cancer. Am J Gastroenterol. 2006;101:2031–7. doi: 10.1111/j.1572-0241.2006.00764.x. [DOI] [PubMed] [Google Scholar]
- 8.Laméris JS, Stoker J, Nijs HG, et al. Malignant biliary obstruction: Percutaneous use of self-expandable stents. Radiology. 1991;179:703–7. doi: 10.1148/radiology.179.3.2027978. [DOI] [PubMed] [Google Scholar]
- 9.Nennstiel S, Weber A, Frick G, et al. Drainage-related complications in percutaneous transhepatic biliary drainage: An analysis over 10 years. J Clin Gastroenterol. 2015;49:764–70. doi: 10.1097/MCG.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 10.Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 11.Sharaiha RZ, Kumta NA, Desai AP, et al. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: Predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg Endosc. 2016;30:5500–5. doi: 10.1007/s00464-016-4913-y. [DOI] [PubMed] [Google Scholar]
- 12.Inamdar S, Slattery E, Bhalla R, et al. Comparison of adverse events for endoscopic vs percutaneous biliary drainage in the treatment of malignant biliary tract obstruction in an inpatient national cohort. JAMA Oncol. 2016;2:112–7. doi: 10.1001/jamaoncol.2015.3670. [DOI] [PubMed] [Google Scholar]
- 13.Zhao XQ, Dong JH, Jiang K, et al. Comparison of percutaneous transhepatic biliary drainage and endoscopic biliary drainage in the management of malignant biliary tract obstruction: A meta-analysis. Dig Endosc. 2015;27:137–45. doi: 10.1111/den.12320. [DOI] [PubMed] [Google Scholar]
- 14.Bories E, Ratone JP, Caillol F, et al. Percutaneous Biliary Drainage Versus EUS Guided Biliary Drainage: A Multicenter Randomized Phase II Study. Gastrointest Endosc. 2017;85:AB469. [Google Scholar]
- 15.Bapaye A, Dubale N, Aher A. Comparison of endosonography-guided vs. percutaneous biliary stenting when papilla is inaccessible for ERCP. United European Gastroenterol J. 2013;1:285–93. doi: 10.1177/2050640613490928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu L, Jin H, Tang X, et al. A comparative study between EUS-guided biliary drainage and percutaneous biliary drainage in patients with malignant biliary obstruction and failed ERCP. United European Gastroenterology Journal. 2017;5:A247–8. [Google Scholar]
- 17.Artifon EL, Aparicio D, Paione JB, et al. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: Endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768–74. doi: 10.1097/MCG.0b013e31825f264c. [DOI] [PubMed] [Google Scholar]
- 18.Bill JG, Darcy M, Fujii-Lau LL, et al. A comparison between endoscopic ultrasound-guided rendezvous and percutaneous biliary drainage after failed ERCP for malignant distal biliary obstruction. Endosc Int Open. 2016;4:E980–5. doi: 10.1055/s-0042-112584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khashab MA, Valeshabad AK, Afghani E, et al. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557–65. doi: 10.1007/s10620-014-3300-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee TH, Choi JH, Park do H, et al. Similar efficacies of endoscopic ultrasound-guided transmural and percutaneous drainage for malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2016;14:1011–9e3. doi: 10.1016/j.cgh.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Huang P, Zhang H, Zhang XF, et al. Comparison of endoscopic ultrasonography guided biliary drainage and percutaneous transhepatic biliary drainage in the management of malignant obstructive jaundice after failed ERCP. Surg Laparosc Endosc Percutan Tech. 2017;27:e127–31. doi: 10.1097/SLE.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 22.Luchini C, Stubbs B, Solmi M, et al. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J Metaanal. 2017;5:80–4. [Google Scholar]
- 23.The Modified Jadad Scale to Assess the Quality of Randomized Controlled Trials (RCTs) [Last accessed on 2020 Dec 15]. Available from: https://figshare.com/articles/dataset/The_modified_Jadad_scale_to_assess_the_quality_of_randomized_controlled_trials_RCTs_/7561454/1 .
- 24.Püspök A, Lomoschitz F, Dejaco C, et al. Endoscopic ultrasound guided therapy of benign and malignant biliary obstruction: A case series. Am J Gastroenterol. 2005;100:1743–7. doi: 10.1111/j.1572-0241.2005.41806.x. [DOI] [PubMed] [Google Scholar]
- 25.Itoi T, Itokawa F, Sofuni A, et al. Endoscopic ultrasound-guided choledochoduodenostomy in patients with failed endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2008;14:6078–82. doi: 10.3748/wjg.14.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith AC, Dowsett JF, Russell RC, et al. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994;344:1655–60. doi: 10.1016/s0140-6736(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 27.Ferrucci JT, Jr, Mueller PR, Harbin WP. Percutaneous transhepatic biliary drainage: Technique, results, and applications. Radiology. 1980;135:1–13. doi: 10.1148/radiology.135.1.7360943. [DOI] [PubMed] [Google Scholar]
- 28.Harbin WP, Mueller PR, Ferrucci JT., Jr Transhepatic cholangiography: Complicatons and use patterns of the fine-needle technique: A multiinstitutional survey. Radiology. 1980;135:15–22. doi: 10.1148/radiology.135.1.6987704. [DOI] [PubMed] [Google Scholar]
- 29.Bahra M, Jacob D. Surgical palliation of advanced pancreatic cancer. Recent Results Cancer Res. 2008;177:111–20. doi: 10.1007/978-3-540-71279-4_13. [DOI] [PubMed] [Google Scholar]
- 30.Gupta K, Mallery S, Hunter D, et al. Endoscopic ultrasound and percutaneous access for endoscopic biliary and pancreatic drainage after initially failed ERCP. Rev Gastroenterol Disord. 2007;7:22–37. [PubMed] [Google Scholar]
- 31.Choi JH, Kim HW, Lee JC, et al. Percutaneous transhepatic versus EUS-guided gallbladder drainage for malignant cystic duct obstruction. Gastrointest Endosc. 2017;85:357–64. doi: 10.1016/j.gie.2016.07.067. [DOI] [PubMed] [Google Scholar]
- 32.Song TJ, Park DH, Eum JB, et al. EUS-guided cholecystoenterostomy with single-step placement of a 7F double-pigtail plastic stent in patients who are unsuitable for cholecystectomy: A pilot study (with video) Gastrointest Endosc. 2010;71:634–40. doi: 10.1016/j.gie.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 33.Súbtil JC, Betes M, Muñoz-Navas M. Gallbladder drainage guided by endoscopic ultrasound. World J Gastrointest Endosc. 2010;2:203–9. doi: 10.4253/wjge.v2.i6.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi JH, Lee SS, Choi JH, et al. Long-term outcomes after endoscopic ultrasonography-guided gallbladder drainage for acute cholecystitis. Endoscopy. 2014;46:656–61. doi: 10.1055/s-0034-1365720. [DOI] [PubMed] [Google Scholar]
- 35.Widmer J, Alvarez P, Gaidhane M, et al. Endoscopic ultrasonography-guided cholecystogastrostomy in patients with unresectable pancreatic cancer using anti-migratory metal stents: A new approach. Dig Endosc. 2014;26:599–602. doi: 10.1111/den.12163. [DOI] [PubMed] [Google Scholar]
- 36.Jang JW, Lee SS, Park DH, et al. Feasibility and safety of EUS-guided transgastric/transduodenal gallbladder drainage with single-step placement of a modified covered self-expandable metal stent in patients unsuitable for cholecystectomy. Gastrointest Endosc. 2011;74:176–81. doi: 10.1016/j.gie.2011.03.1120. [DOI] [PubMed] [Google Scholar]
- 37.Irani S, Baron TH, Grimm IS, et al. EUS-guided gallbladder drainage with a lumen-apposing metal stent (with video) Gastrointest Endosc. 2015;82:1110–5. doi: 10.1016/j.gie.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 38.Itoi T. Moving closer to developing an optimal algorithm for EUS-guided biliary drainage. Gastrointest Endosc. 2016;84:947–9. doi: 10.1016/j.gie.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 39.Baniya R, Upadhaya S, Madala S, et al. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage after failed endoscopic retrograde cholangiopancreatography: A meta-analysis. Clin Exp Gastroenterol. 2017;10:67–74. doi: 10.2147/CEG.S132004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang K, Zhu J, Xing L, et al. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest Endosc. 2016;83:1218–27. doi: 10.1016/j.gie.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 41.Holt BA, Hawes R, Hasan M, et al. Biliary drainage: Role of EUS guidance. Gastrointest Endosc. 2016;83:160–5. doi: 10.1016/j.gie.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 42.Kahaleh M, Artifon EL, Perez-Miranda M, et al. Endoscopic ultrasonography guided biliary drainage: Summary of consortium meeting, May 7th, 2011, Chicago. World J Gastroenterol. 2013;19:1372–9. doi: 10.3748/wjg.v19.i9.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James PD, Antonova L, Martel M, et al. Measures of trainee performance in advanced endoscopy: A systematic review. Best Pract Res Clin Gastroenterol. 2016;30:421–52. doi: 10.1016/j.bpg.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Nakai Y, Isayama H, Yamamoto N, et al. Safety and effectiveness of a long, partially covered metal stent for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Endoscopy. 2016;48:1125–8. doi: 10.1055/s-0042-116595. [DOI] [PubMed] [Google Scholar]
- 45.Kawakubo K, Isayama H, Kato H, et al. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci. 2014;21:328–34. doi: 10.1002/jhbp.27. [DOI] [PubMed] [Google Scholar]
- 46.Lakhtakia S. Complications of diagnostic and therapeutic endoscopic ultrasound. Best Pract Res Clin Gastroenterol. 2016;30:807–23. doi: 10.1016/j.bpg.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Dhir V, Itoi T, Khashab MA, et al. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest Endosc. 2015;81:913–23. doi: 10.1016/j.gie.2014.09.054. [DOI] [PubMed] [Google Scholar]