Abstract
Background and Objectives:
EUS-guided radiofrequency ablation (EUS-RFA) has been increasingly used for the treatment of pancreatic neoplasms. The role of EUS-RFA in the management of pancreatic cancer has not yet been elucidated. This study aimed to evaluate the survival impact of EUS-RFA in unresectable pancreatic cancer.
Methods:
Twenty-two patients (n = 14, locally advanced unresectable; n = 8, metastatic) with unresectable pancreatic cancer underwent EUS-RFA combined with subsequent chemotherapy between May 2016 and June 2019. Survival outcomes including overall survival (OS) and progression-free survival (PFS) were evaluated.
Results:
EUS-RFA was successful in all patients. The median number of RFA sessions was 5 (interquartile range, [IQR], 3.25–5.75). After successful EUS-RFA, subsequent gemcitabine-based chemotherapy was performed. Early procedure-related adverse events occurred in 4 out of 107 sessions (3.74%), including peritonitis (n = 1) and abdominal pain (n = 3). During follow-up over a median of 21.23 months (IQR, 10.73–27.1), the median OS and PFS were 24.03 months (95% confidence interval [CI], 16–35.8) and 16.37 months (95% CI, 8.87–19), respectively.
Conclusions:
EUS-RFA is technically feasible and safe for the management of unresectable pancreatic cancer. EUS-RFA combined with systemic chemotherapy may be associated with favorable survival outcomes. Further larger-scale prospective comparative study is required to confirm these findings.
Keywords: EUS, pancreatic neoplasms, radiofrequency ablation, treatment outcome
INTRODUCTION
Pancreatic cancer has a poor prognosis, with a 5-year overall survival (OS) rate of about 9%.[1] Surgery can provide long-term survival, with a 5-year OS rate of 18%–24%. However, most patients present with unresectable pancreatic cancer at the time of diagnosis because of locally advanced or distant metastasis. To date, clinical outcomes with chemotherapy or chemoradiation therapy are unsatisfactory for the management of unresectable pancreatic cancer.
Recently, EUS-guided radiofrequency ablation (RFA) has been applied for the management of pancreatic neoplasms. EUS-RFA can offer real-time imaging of the target lesion, and RFA may result in safe tissue ablation. Recently, several reports have demonstrated that EUS-RFA is effective and has an acceptable safety profile for the treatment of benign pancreatic tumors.[2,3,4] In our preliminary study, EUS-RFA combined with systemic chemotherapy was technically feasible and safe in patients with metastatic pancreatic cancer. However, despite encouraging results, the efficacy and long-term clinical outcomes of EUS-RFA have not been evaluated.[5,6]
This study aimed to evaluate the long-term survival outcomes of EUS-RFA in patients with unresectable pancreatic cancer.
METHODS
Patients
This study was a single-center, prospective observational study conducted between May 2016 and June 2019. The study was approved by the Institutional Review Board at Asan Medical Center (IRB No.: 2016-0108), and all patients signed a written informed consent form before enrollment. This study was registered with the Clinical Research Information Service at the Korea National Institute of Health, which is a registry in the World Health Organization Registry Network (KCT0002467). The inclusion criteria were as follows: (1) histopathologically confirmed pancreatic cancer and (2) at an unresectable stage due to locally advanced or metastatic disease. Exclusion criteria were as follows: (1) advanced heart or lung disease precluding adequate sedation, (2) surgically altered anatomies, (3) poor performance, (4) uncontrolled coagulopathy, and (5) informed consent not given.
EUS-radiofrequency ablation procedures
All patients were treated with EUS-RFA by an experienced endosonographer (D.W.S.) under conscious sedation using midazolam and meperidine. Prophylactic antibiotics were administered intravenously before each procedure.
EUS-RFA was performed using a 19-gauge RFA needle (140-cm long) and a VIVA RF generator (STARmed, Koyang, Korea). The RFA needle was inserted into the target lesion under EUS guidance to avoid intervening vessels. After puncturing the target lesion, the RF generator was activated to deliver 50 W of ablation power. Ablation was continued until the hyperechoic zone around the RFA needle tip sufficiently covered the tumor. The RFA needle was then repositioned to ablate another zone. RFA was usually started at the right distal portion of the tumor on the EUS image, while the RFA needle was withdrawn, after which the RFA needle was reinserted and RFA was repeated at the left side of the previous site.[5] After successful EUS-RFA, subsequent systemic chemotherapy was performed on the same day. If procedure-related adverse events occurred, systemic chemotherapy was delayed until the adverse events were resolved.
Simple abdominal radiograph and blood tests, including complete blood count, liver function tests, and serum amylase and/or lipase, were checked for adverse events on the following day. After the EUS-RFA, all patients were followed up at intervals of 2–3 months. At each follow-up, complete blood counts, biochemical profiles, tumor markers, and imaging studies were checked.
Outcome parameters and definitions
Unresectable locally advanced pancreatic cancer (LAPC) was defined as follows: (1) lesions of the pancreatic head/uncinate process including solid tumor contact with the superior mesenteric artery (SMA) >180°, solid tumor contact with the celiac axis (CA) >180°, solid tumor contact with the first jejunal SMA branch, an unreconstructible SMV/PV due to tumor involvement or occlusion, or contact with the most proximal jejunal branch draining into the SMV; and (2) lesions in the body and tail of the pancreas including solid tumor contact >180° with the SMA or CA, solid tumor contact with the CA and aortic involvement, or unreconstructible SMV/PV due to tumor involvement or occlusion.[7]
OS and progression-free survival (PFS) were estimated from the date of diagnosis of pancreatic cancer to the date of death or last follow-up examination and to the date of any site of tumor progression, respectively. Local control was defined as the absence of radiologic or clinical disease progression or recurrence within the treatment field. Freedom from local disease progression (FFLP) was calculated from the date of diagnosis to the date of local disease progression.[8] The following factors were evaluated for their impact on the different survival end points: age, sex, nodal metastasis, tumor size, tumor location, tumor extent (LAPC vs. metastatic), pre-EUS-RFA CA19-9 level, and chemotherapy. Procedure-related adverse events were classified and graded according to the American Society for Gastrointestinal Endoscopy Workshop reports.[9] Early procedural adverse events were defined as any procedure-related adverse event that occurred within 2 weeks, including bleeding, pancreatitis, and perforation. Late procedural adverse events were defined as those that occurred 2 weeks after EUS-RFA.
Statistical analysis
Statistical analyses were performed using SPSS Statistics 22.0 (SPSS Inc., Chicago, IL, USA). The results are expressed as means and standard deviations or medians and interquartile ranges (IQRs). The probability of cumulative survival was calculated using the Kaplan–Meier method. A P < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Baseline characteristics of the patients are summarized in Table 1. A total of 22 patients with unresectable pancreatic cancer (n = 14, locally advanced unresectable; n = 8, metastatic) underwent EUS-RFA. The median CA19-9 level before RFA was 200.8 U/mL (IQR, 15.9–901.3). Among these patients, CA19-9 levels were >200 U/mL in 11 patients (50%). Pancreatic cancer was located in the head of the pancreas in 14 patients (63.6%), in the pancreas body in 4 patients (18.2%), in the tail of the pancreas in 3 patients (13.6%), and in the resection margin in 1 patient (4.5%). The median size of the primary tumor was 38 mm (IQR, 32.75–45). Sixteen patients (72.7%) had nodal involvement. All patients underwent gemcitabine-based chemotherapy before (n = 19) and after (n = 3) EUS-RFA. Among these patients, 18 (81.8%) received induction chemotherapy [Figure 1].
Table 1.
Baseline characteristics of patients who underwent EUS-radiofrequency ablation
| Characteristics | Total (n=22), n(%) | Locally advanced (n=14), n(%) | Metastatic (n=8), n(%) |
|---|---|---|---|
| Age (years), median (IQR) | 60.5 (56.25-68.75) | 60 (56.75-69.5) | 60.5 (56-69.75) |
| Sex (male:female) | 13:9 | 10:4 | 3:5 |
| Location | |||
| Head | 14 (63.6) | 11 (78.6) | 3 (37.5) |
| Body | 4 (18.2) | 2 (14.3) | 2 (25) |
| Tail | 3 (13.6) | 1 (7.1) | 2 (25) |
| Distal pancreatectomy resection margin | 1 (4.5) | 0 | 1 (12.5) |
| Tumor size (mm), median (IQR) | 38 (32.75-45) | 35 (27.25-45) | 41.5 (35.25-75) |
| Initial CA19-9 (U/mL), median (IQR) | 200.8 (15.9-901.3) | 47.9 (6.8-489.55) | 619.45 (76.7–3489.75) |
| CA19-9>200 U/mL | 11 (50) | 5 (35.7) | 6 (75) |
| Nodal metastasis | 16 (72.7) | 8 (57.1) | 8 (100) |
| Sequential chemotherapy | |||
| Before EUS-RFA | 19 (86.4) | 14 (100) | 5 (62.5) |
| After EUS-RFA | 3 (13.6) | 0 | 3 (37.5) |
| Induction chemotherapy | 18 (81.8) | 10 (71.4) | 8 (100) |
IQR: Interquartile range; EUS-RFA: EUS-radiofrequency ablation; CA: Celiac axis
Figure 1.
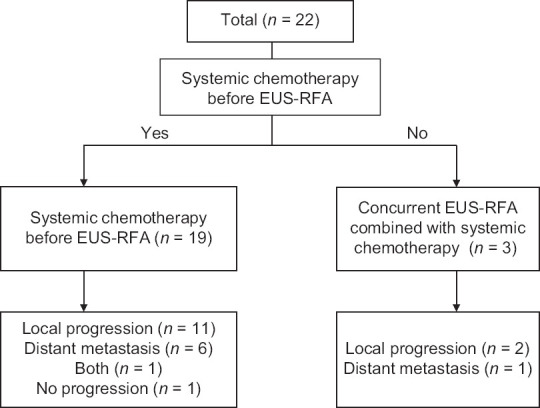
Flowchart of the study
Clinical outcomes
Clinical outcomes are summarized in Table 2. EUS-RFA was performed successfully in all patients [Figure 2]. The median number of RFA sessions was 5 (IQR, 3.25–5.75). Three patients underwent 1 session of RFA, 1 underwent 2 sessions, 2 underwent 3 sessions, 4 underwent 4 sessions, 6 underwent 5 sessions, 2 underwent 6 sessions, and the rest of the patients each underwent 8, 9, 10, and 11 sessions, respectively. The median time interval from diagnosis to EUS-RFA was 4.73 months (IQR, 2.66–9.65). Over a median follow-up period of 21.23 months (IQR, 10.73–27.1), 17 patients (77.3%) died due to disease progression. Twenty patients (95.5%) experienced treatment failure. Among these patients, treatment failure was first associated with local progression in 13 patients (59.1%), distant metastasis in 7 patients (31.8%), and both in one patient (4.5%).
Table 2.
Clinical outcomes of patients who underwent EUS-radiofrequency ablation combined with systemic chemotherapy
| Characteristics | Number of patients, n(%) |
|---|---|
| Number of RFA sessions, median (IQR) | 5 (3.25-5.75) |
| Time interval from diagnosis to EUS-RFA (months), median (IQR) | 4.73 (2.66-9.65) |
| Follow-up period (months), median (IQR) | 21.23 (10.73-27.1) |
| Treatment failure | 20 (95.5) |
| Local progression | 13 (59.1) |
| Distant metastasis | 7 (31.8) |
| Both | 1 (4.5) |
| Adverse events | 4/107 (3.74) |
| Abdominal pain | 3 |
| Peritonitis | 1 |
IQR: Interquartile range; EUS-RFA: EUS-radiofrequency ablation
Figure 2.
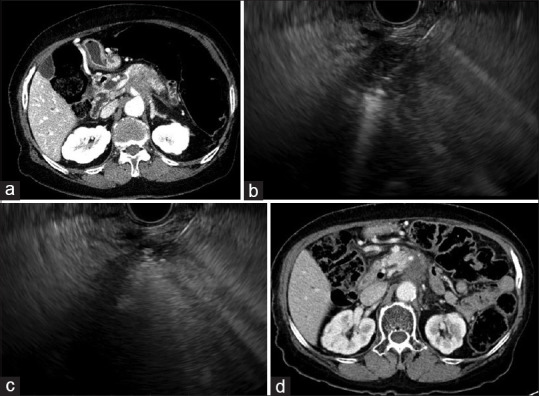
(a) Computed tomography showing a pancreatic body cancer before treatment. (b) EUS-guided radiofrequency ablation is performed. (c) Electrode was repositioned to ablate different areas. (d) At 14-months of follow-up, computed tomography showing necrosis of tumor without increase in size
Early procedure-related adverse events occurred in 4 out of 107 sessions (3.74%), including peritonitis (n = 1) and abdominal pain (n = 3). There were no severe adverse events, and the patients improved completely after conservative treatment. Subsequent systemic chemotherapy was performed within 2 days.
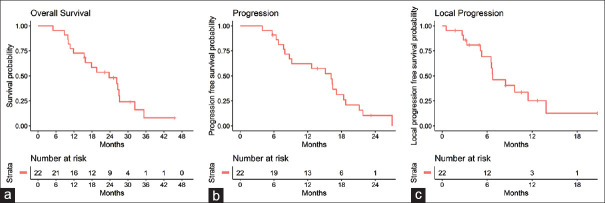
Univariate analysis results are summarized in Table 3. The median OS, PFS, and FFLP were 24.03 months (95% confidence interval [CI], 16–35.8), 16.37 months (95% CI, 8.87–19), and 6.83 months (95% CI, 6.6 – not estimable), respectively [Figure 3]. The 1-year OS and PFS rates were 72.7% (95% CI, 56.3%–93.9%) and 62.2% (95% CI, 44.6%–86.8%), respectively. The 1-year FFLP rate was 25.3% (95% CI, 10.5%–60.6%).
Table 3.
Univariate analysis of covariates associated with freedom-free local progression, progression-free survival, and overall survival
| Variables | FFLP | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age | 0.942 | 0.868-1.023 | 0.155 | 0.995 | 0.938-1.055 | 0.869 | 0.999 | 0.939-1.064 | 0.987 |
| Sex | 1.028 | 0.310-3408 | 0.964 | 0.449 | 0.167-1.205 | 0.112 | 0.477 | 0.180-1.261 | 0.136 |
| Tumor extent | 3.247 | 1.011-10.425 | 0.048 | 1.190 | 0.443-3.195 | 0.730 | 2.978 | 1.035-8.566 | 0.043 |
| Tumor size | 1.011 | 0.978-1.045 | 0.533 | 0.996 | 0.975-1.017 | 0.691 | 1.015 | 0.992-1.040 | 0.206 |
| Tumor location | 0.440 | 0.140-1.378 | 0.159 | 0.550 | 0.207-1.457 | 0.229 | 0.454 | 0.167-1.229 | 0.120 |
| Nodal metastasis | 1.102 | 0.338-3.592 | 0.872 | 0.437 | 0.150-1.278 | 0.131 | 0.535 | 0.178-1.612 | 0.266 |
| Distant metastasis | 1.998 | 0.645-6.196 | 0.23 | 0.610 | 0.212-1.756 | 0.359 | 2.498 | 0.852-7.326 | 0.095 |
| Pre-EUS-RFA CA19-9 | 1.521 | 0.514-4.501 | 0.449 | 1.825 | 0.712-4.677 | 0.210 | 2.021 | 0.759–5.382 | 0.159 |
| Time interval from the diagnosis to EUS-RFA | 1.001 | 0.997-1.005 | 0.526 | 0.993 | 0.988-0.998 | 0.004 | 0.999 | 0.997-1.002 | 0.513 |
| Number of EUS-RFA session | 1.158 | 0.961-1.396 | 0.123 | 1.188 | 0.999-1.412 | 0.051 | 1.094 | 0.953-1.257 | 0.202 |
| Induction chemotherapy | 5.277 | 0.671-41.486 | 0.114 | 1.074 | 0.300-3.846 | 0.913 | 3.269 | 0.737-14.500 | 0.119 |
FFLP: Freedom-free local progression; PSF: Progression-free survival; OS: Overall survival; HR: Hazard ratio; CI: Confidence interval; EUS-RFA: EUS-radiofrequency ablation; CA: Celiac axis
Figure 3.
Kaplan–Meier curves of (a) overall survival, (b) progression-free survival, and (c) freedom from local disease progression overall survival in patients underwent EUS-radiofrequency ablation with chemotherapy
On univariate analysis, the tumor extent was also associated with OS (P = 0.043). The time interval from diagnosis to EUS-RFA was associated with PFS (P = 0.019). Although statistically insignificant, the number of RFA sessions tended to be associated with PFS (P = 0.051). Tumor classification was also associated with FFLP (P = 0.048).
On subgroup analysis, the median OS (LAPC, 26.63 months [95% CI, 18.1 – not estimable] vs. metastatic, 15.05 months [95% CI, 10.13 – not estimable]), PFS (LAPC, 16.57 months [95% CI, 9.3 – not estimable] vs. metastatic, 10.86 months [95% CI, 6.9 – not estimable]), and FFLP (LAPC, 8.57 months [95% CI, 6.67 – not estimable] vs. metastatic, 5.17 months [95% CI, 2.7 – not estimable]) were longer in patients with LAPC than in patients with metastatic pancreatic cancer.
DISCUSSION
EUS-RFA has emerged as a promising treatment modality for various pancreatic tumors, including pancreatic cancer. Previous reports have shown that EUS-RFA can be applied for ablation of pancreatic tumors; however, the efficacy and safety of EUS-RFA still remain questionable with there being a potential risk of damage to the surrounding structures.[2,3,5,10] Our study demonstrated that EUS-RFA combined with subsequent systemic chemotherapy was technically feasible and had an acceptable range of adverse events in patients with unresectable pancreatic cancer. These results also suggested that EUS-RFA may increase survival outcomes by enhancing systemic chemotherapeutic effects.
In this series, a median of five sessions (IQR, 3.25–5.75) of EUS-RFA followed by chemotherapy within 2 days was performed successfully in all patients. Procedure-related adverse events occurred in 4 out of 107 (3.74%) sessions, including 1 episode of peritonitis and 3 episodes of abdominal pain. Except for one patient who had peritonitis, subsequent systemic chemotherapy was possible in patients who underwent EUS-RFA. In our previous study on benign solid pancreatic tumors, acute pancreatitis developed in one patient after ablation of a tumor that was close to the pancreatic duct.[3] In the current study, acute pancreatitis did not occur in any patient. As per experience accumulated through previous studies, EUS-RFA was performed while maintaining a minimum safety margin of 5 mm from the main pancreatic duct.[3] Furthermore, in patients with pancreatic cancer, chronic pancreatitis was also present at the time of presentation. Therefore, it is possible that postprocedural pancreatitis is less likely in a chronically scarred gland having severe fibrosis and atrophy.[11]
Local tumor control is an important issue; therefore, the current standard of care in patients with LAPC includes a combination of chemotherapy and radiotherapy.[12] However, 1-year FFLP rate was 25.3% (95% CI, 10.5%–60.6%). Considering the potential risk of thermal injury to adjacent organs and the relatively large size of tumors as compared with that reported in previous studies, the primary tumor was not completely ablated. Complete ablation of the tumor could increase the postprocedural adverse event; thus, we ablate only the primary tumor to minimize the adverse events. As a tradeoff for incomplete ablation of the primary tumor, the incidence of postprocedural adverse events was low (4 out of 107 sessions, 3.74%). On subgroup analysis, tumor extent (locally advanced vs. metastatic pancreatic cancer) was associated with local progression (LAPC, 8.57 months [IQR, 5.56–11.56] vs. metastatic, 5.16 months [IQR, 0.5–9.83], P = 0.222). With regard to local control of pancreatic cancer, EUS-RFA may be more helpful in patients with LAPC than in patients with metastatic pancreatic cancer.
In terms of PFS and OS, the time interval from the diagnosis to EUS-RFA (hazard ratio [HR] 1.001; 95% CI, 0.997–1.005; P = 0.004) and tumor extent (HR, 2.978; 95% CI, 1.035–8.566; P = 0.043) were statistically significant. In the current study, 86.4% of the patients underwent systemic chemotherapy before EUS-RFA. The median time interval from diagnosis to EUS-RFA was 4.73 months (IQR, 2.66–9.65). These results are thought to be due to the fact that EUS-RFA was not performed at the time of diagnosis and was additionally performed when the tumor did not decrease to systemic chemotherapy. The number of RFA sessions also tended to be associated with PFS (HR, 1.188; 95% CI, 0.999–1.412; P = 0.051). The median number of RFA sessions was 5 (IQR, 3.25–5.75). When the therapeutic effect of EUS-RFA was unsatisfactory, the procedure was performed repeatedly to reduce the tumor burden. These clinical practices may have affected the results.
Previously, we reported the clinical outcomes of systemic chemotherapy in patients with LAPC and metastatic pancreatic cancer. A total of 124 patients with LAPC underwent FOLFIRINOX treatment.[13] In these patients, the median OS and PFS were 17.1 months (95% CI, 13.2–20.9) and 10.1 months (95% CI, 8.4–11.8), respectively. A total of 308 patients with metastatic pancreatic cancer received nab-paclitaxel plus gemcitabine (n = 149) and FOLFIRINOX (n = 159).[14] In patients who were treated with nab-paclitaxel plus gemcitabine, the median OS and PFS were 11.4 months (95% CI, 9.7–13.0) and 6.8 months (95% CI, 5.7–7.9), respectively. In patients who received FOLFIRINOX, the median OS and PFS were 9.6 months (95% CI, 8.1–11.2) and 5.0 months (95% CI, 4.2–5.9), respectively. In this study, the median OS was 24.03 months in patients with unresectable pancreatic cancer, including LAPC and metastatic pancreatic cancer. In addition, the median OS was 26.63 months in patients with unresectable LAPC and 15.05 months in patients with metastatic pancreatic cancer. Considering the heterogeneity of enrolled patients, our results had relatively favorable survival outcomes compared with the median OS of 8.6–18.8 months in patients with LAPC and the median OS of 6.7–11.1 months in patients with metastatic pancreatic cancer from previous reports.[15,16,17,18,19] In a study by Haen et al., thermal ablation could induce an immune response toward the tumor, determined by the release of necrotic cell content in the extracellular space that stimulated the host's antitumor immunity.[20] A more recent study documented increased blood flow around the ablated area.[5] RFA combined with systemic chemotherapy may enhance tumor cell death at sublethal temperature in the peripheral or transition zone, which are the areas recovering from reversible injury. Apoptosis that is triggered by heat-induced cell injury is increased by the cytotoxic injury of chemotherapies.[21] Therefore, even suboptimal RFA treatment could affect these postprocedural tumor changes associated with systemic antitumor immune response.
As a local treatment for pancreatic cancer, stereotactic body radiation therapy (SBRT) and EUS-RFA can be used. Several studies have reported that SBRT may be helpful for improving patient survival.[8,22,23] However, radiation therapy is associated with late gastrointestinal toxicity of 6%–33%, including bowel perforation and bleeding.[24,25] Particularly, the duodenum is the main sources of potential toxicity of radiotherapy; thus, it may be difficult to apply radiotherapy for treating pancreatic cancer with duodenal invasion.[26] On the other hand, our results showed EUS-RFA is a safe treatment because it can be controlled by the endosonographer. When local ablation is suboptimal, repetitive RFA is possible for enhancing treatment effect. Thus, further prospective randomized trials comparing EUS-RFA and SBRT for patients with LAPC are required.
There are some limitations in this study. First, the number of enrolled patients was small and our study was a single-arm, noncomparative study. Therefore, large-scale randomized controlled studies comparing chemotherapy alone and EUS-RFA combined with chemotherapy are necessary to confirm our favorable results. Second, the systemic chemotherapy used in this study was inferior to that of current practice which uses a more effective regimen such as FOLFIRINOX and gemcitabine plus abraxane. In addition, there was a discrepancy between PFS/OS and FFLP. These discrepancies may be due to the median 4.73 months of time gap between the initial diagnosis and EUS-RFA. If EUS-RFA combined with systemic chemotherapy is initiated at the time of diagnosis, the results may change.
CONCLUSIONS
EUS-RFA is technically feasible and safe with favorable OS in concordance with systemic chemotherapy in patients with unresectable pancreatic cancer. Our results suggest that EUS-RFA combined with systemic chemotherapy is a promising treatment approach for patients with unresected pancreatic cancer. EUS-RFA with a more aggressive chemotherapy regimen may improve clinical outcomes and requires further investigation.
Financial support and sponsorship
This study was supported by a grant from the Korean Health Technology R and D project, Ministry of Health and Welfare, Republic of Korea (HI16C1163).
Conflicts of interest
Dong-Wan Seo and Do Hyun Park are Editorial Board Members of the journal. The article was subject to the journal's standard procedures, with peer review handled independently of these editors and their research groups.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Barthet M, Giovannini M, Lesavre N, et al. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: A prospective multicenter study. Endoscopy. 2019;51:836–42. doi: 10.1055/a-0824-7067. [DOI] [PubMed] [Google Scholar]
- 3.Choi JH, Seo DW, Song TJ, et al. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy. 2018;50:1099–104. doi: 10.1055/a-0583-8387. [DOI] [PubMed] [Google Scholar]
- 4.Lakhtakia S, Ramchandani M, Galasso D, et al. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos) Gastrointest Endosc. 2016;83:234–9. doi: 10.1016/j.gie.2015.08.085. [DOI] [PubMed] [Google Scholar]
- 5.Song TJ, Seo DW, Lakhtakia S, et al. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest Endosc. 2016;83:440–3. doi: 10.1016/j.gie.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 6.Scopelliti F, Pea A, Conigliaro R, et al. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg Endosc. 2018;32:4022–8. doi: 10.1007/s00464-018-6217-x. [DOI] [PubMed] [Google Scholar]
- 7.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1028–61. doi: 10.6004/jnccn.2017.0131. [DOI] [PubMed] [Google Scholar]
- 8.Jung J, Yoon SM, Park JH, et al. Stereotactic body radiation therapy for locally advanced pancreatic cancer. PLoS One. 2019;14:e0214970. doi: 10.1371/journal.pone.0214970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Crinò SF, D’Onofrio M, Bernardoni L, et al. EUS-guided radiofrequency ablation (EUS-RFA) of solid pancreatic neoplasm using an 18-gauge needle electrode: Feasibility, safety, and technical success. J Gastrointestin Liver Dis. 2018;27:67–72. doi: 10.15403/jgld.2014.1121.271.eus. [DOI] [PubMed] [Google Scholar]
- 11.Matsubayashi H, Fukutomi A, Kanemoto H, et al. Risk of pancreatitis after endoscopic retrograde cholangiopancreatography and endoscopic biliary drainage. HPB (Oxford) 2009;11:222–8. doi: 10.1111/j.1477-2574.2008.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balaban EP, Mangu PB, Khorana AA, et al. Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2654–68. doi: 10.1200/JCO.2016.67.5561. [DOI] [PubMed] [Google Scholar]
- 13.Yoo C, Hwang I, Song TJ, et al. FOLFIRINOX in borderline resectable and locally advanced unresectable pancreatic adenocarcinoma. Ther Adv Med Oncol. 2020;12:1758835920953294. doi: 10.1177/1758835920953294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang J, Hwang I, Yoo C, et al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: Retrospective analysis. Invest New Drugs. 2018;36:732–41. doi: 10.1007/s10637-018-0598-5. [DOI] [PubMed] [Google Scholar]
- 15.Philip PA, Lacy J, Portales F, et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): A multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5:285–94. doi: 10.1016/S2468-1253(19)30327-9. [DOI] [PubMed] [Google Scholar]
- 16.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–9. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 17.Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: The LAP07 randomized clinical trial. JAMA. 2016;315:1844–53. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 18.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 19.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haen SP, Pereira PL, Salih HR, et al. More than just tumor destruction: Immunomodulation by thermal ablation of cancer. Clin Dev Immunol. 2011;2011:160250. doi: 10.1155/2011/160250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu KF, Dupuy DE. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 22.Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–86. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 23.Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48–53. doi: 10.1016/j.radonc.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Moningi S, Dholakia AS, Raman SP, et al. The role of stereotactic body radiation therapy for pancreatic cancer: A single-institution experience. Ann Surg Oncol. 2015;22:2352–8. doi: 10.1245/s10434-014-4274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–21. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Hong JC, Czito BG, Willett CG, et al. A current perspective on stereotactic body radiation therapy for pancreatic cancer. Onco Targets Ther. 2016;9:6733–9. doi: 10.2147/OTT.S99826. [DOI] [PMC free article] [PubMed] [Google Scholar]